Short-Term Effects of Wood Biochar on Soil Fertility, Heterotrophic Respiration and Organic Matter Composition
Abstract
1. Introduction
2. Materials and Methods
2.1. Biochar
2.2. Experimental Field and Microplot Preparation
2.3. Soil Sampling and Characterization
2.4. Soil Chemical Analysis
2.5. Carbon Mote System for Measuring Soil Respiration
2.6. Effects of Biochar on Soil Fertility
2.7. Extraction of Soil Organic Matter
2.8. FT-IR and CPMAS NMR Spectroscopies
2.9. Thermochemiolysis Followed by Gas Chromatography and Mass Spectrometry (Pyr-GC/MS)
3. Results and Discussion
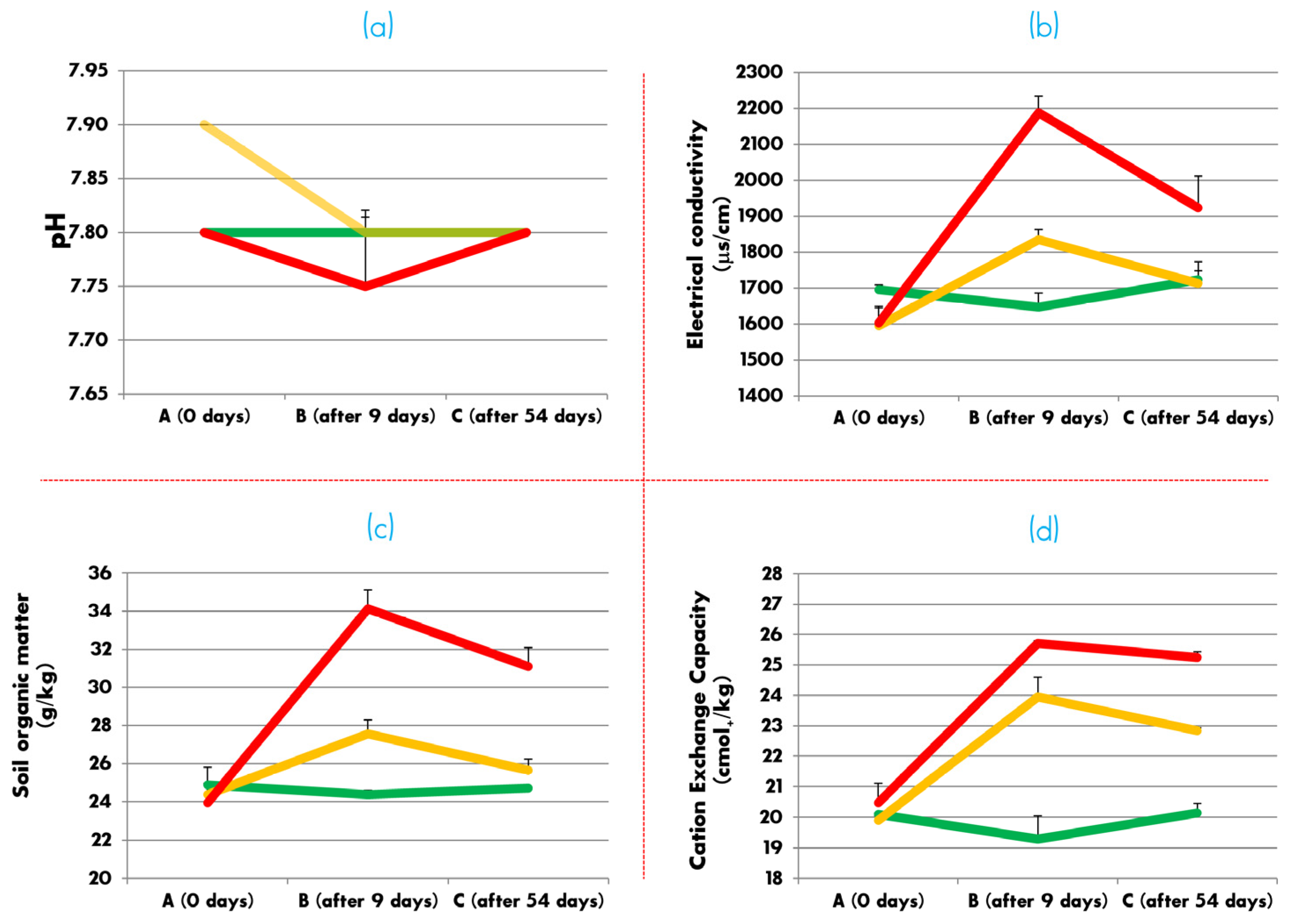
3.1. Soil Chemical Analyses
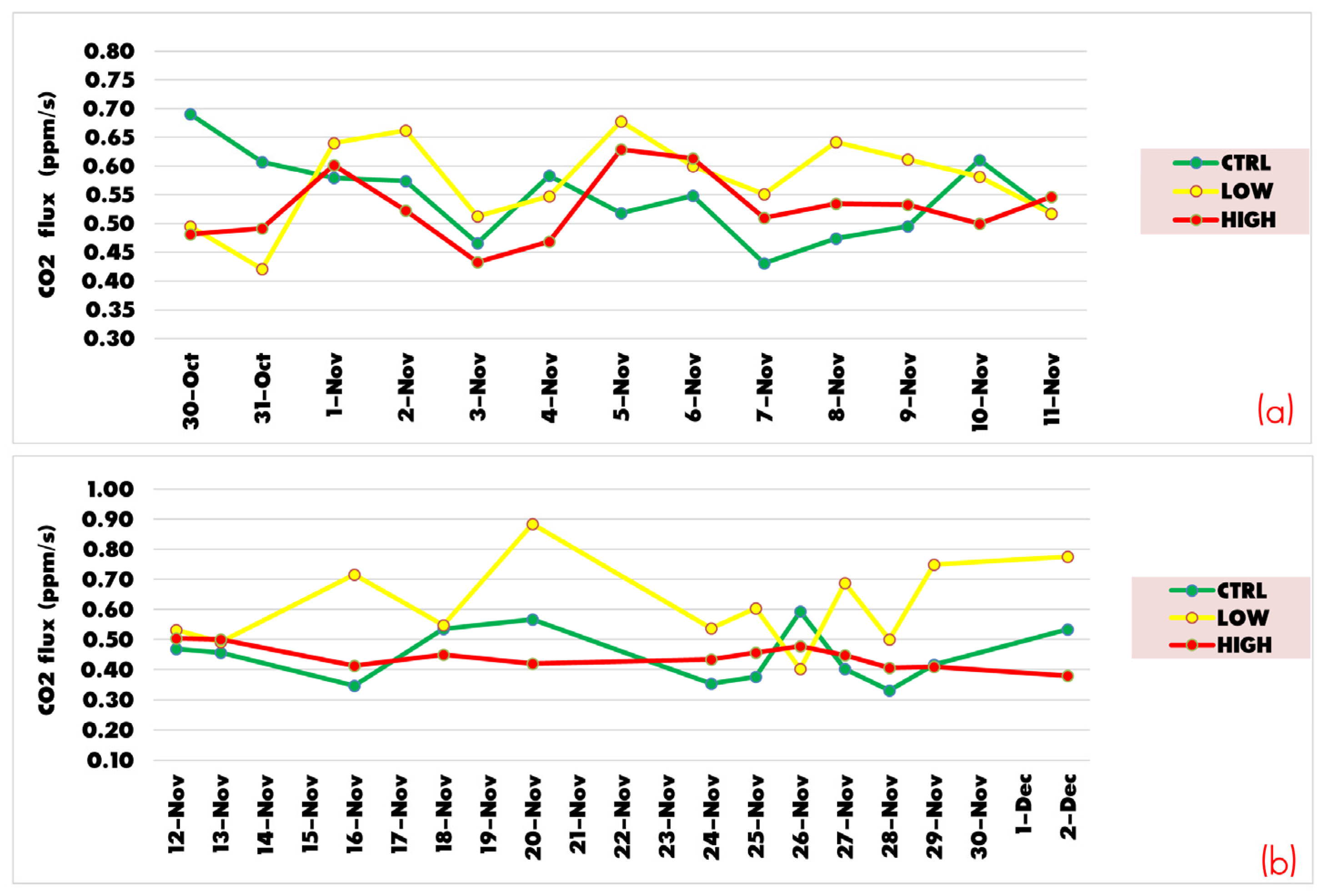
3.2. Soil Heterotrophic Respiration
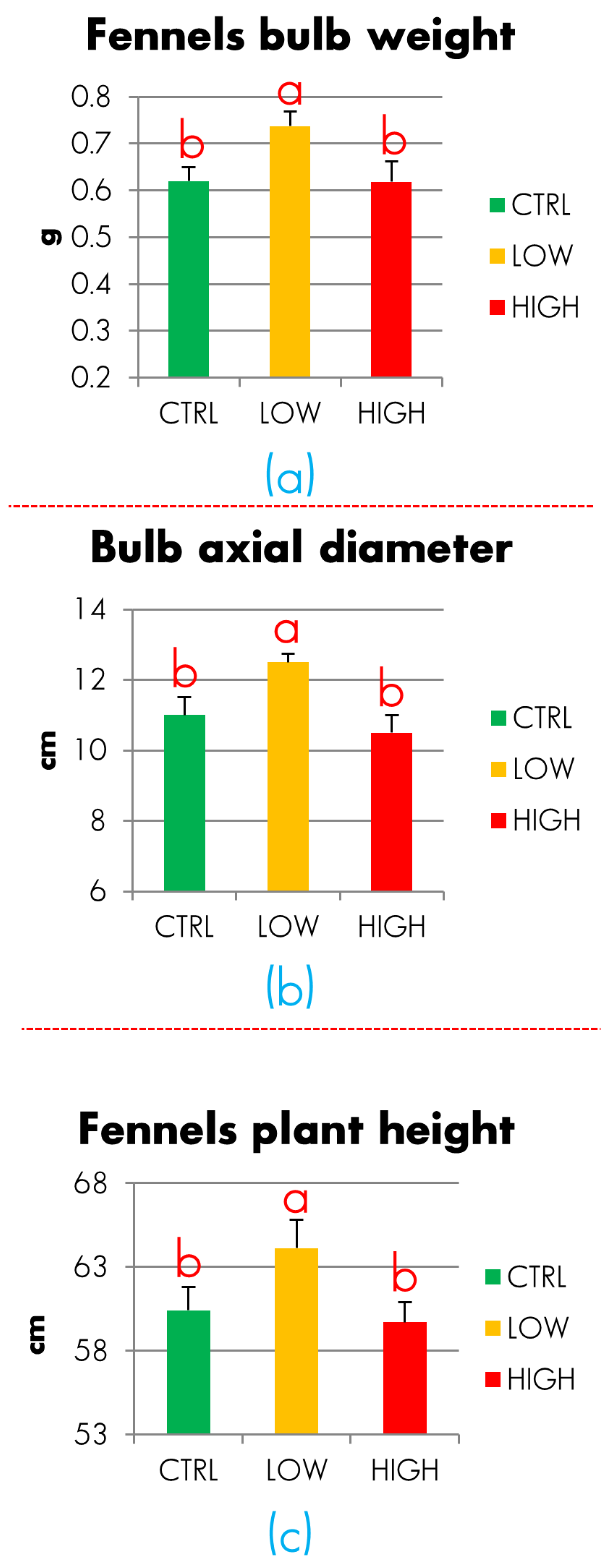
3.3. Fennel Experimentation
3.4. Characterization of Biochar and SOM Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Obalum, S.; Chibuike, G.; Peth, S.; Oyang, Y. Soil organic matter as sole indicator of soil degradation. Environ. Monit. Assess. 2017, 189, 176. [Google Scholar] [CrossRef] [PubMed]
- Bento, L.R.; Spaccini, R.; Cangemi, S.; Mazzei, P.; De Freitas, B.B.; de Sousa, A.E.O.; Moreira, A.B.; Ferreira, O.P.; Piccolo, A.; Bisinoti, M.C. Hydrochar obtained with by-products from the sugarcane industry: Molecular features and effects of extracts on maize seed germination. J. Environ. Manag. 2021, 281, 111878. [Google Scholar] [CrossRef] [PubMed]
- Kambo, H.S.; Dutta, A. A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Maczak, D.; Lejcuś, K.; Kulczycki, G.; Misiewicz, J. Towards circular economy: Sustainable soil additives from natural waste fibres to improve water retention and soil fertility. Sci. Total Environ. 2022, 844, 157169. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Senthil Kumar, P.; Varjani, S.; Saravanan, A. A critical review on the biochar production techniques, characterization, stability and applications for circular bioeconomy. Biotechnol. Rep. 2020, 28, e00570. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.; Steele, P. Pyrolysis of wood/biomass for biooil: A critical review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Zhang, A.F.; Bian, R.J.; Pan, G.X.; Cui, L.Q.; Hussain, Q.; Li, L.Q.; Zheng, J.W.; Zheng, J.F.; Zhang, X.H.; Han, X.J.; et al. Effects of biochar amendment on soil quality, crop yield and greenhouse gas emission in a chinese rice paddy: A field study of 2 consecutive rice growing cycles. Field Crops Res. 2012, 127, 153–160. [Google Scholar] [CrossRef]
- Alkharabsheh, H.M.; Seleiman, M.F.; Battaglia, M.L.; Shami, A.; Jalal, R.S.; Bushra, A.A.; Almutairi, K.F.; Al-Saif, A.M. Biochar and its broad impacts in soil quality and fertility, nutrient leaching and crop productivity: A review. Agronomy 2021, 11, 993. [Google Scholar] [CrossRef]
- Diatta, A.A.; Fike, J.H.; Battaglia, M.L.; Galbraith, J.M.; Baig, M.B. Effects of biochar on soil fertility and crop productivity in arid regions: A review. Arab. J. Geosci. 2020, 13, 595. [Google Scholar] [CrossRef]
- Amoah-Antwi, C.; Kwiatkowska-Malina, J.; Thornton, S.F.; Fenton, O.; Malina, G.; Szara, E. Restoration of soil quality using biochar and brown coal waste: A review. Sci. Total Environ. 2020, 722, 137852. [Google Scholar] [CrossRef]
- Xu, H.; Cai, A.; Wu, D.; Liang, G.; Xiao, J.; Xu, M.; Colinet, G.; Zhang, W. Effects of biochar application on crop productivity, soil carbon sequestration, and global warming potential controlled by biochar C:N ratio and soil pH: A global meta-analysis. Soil Tillage Res. 2021, 213, 105125. [Google Scholar] [CrossRef]
- Asai, H.; Samson, B.K.; Stephan, H.M.; Songyikhangsuthor, K.; Hommaa, K.; Kiyono, Y.; Inoue, Y.; Shiraiwa, T.; Horie, T. Biochar amendment techniques for upland rice production in Northern Laos 1. Soil physical properties, leaf SPAD and grain yield. Field Crops Res. 2009, 111, 81–84. [Google Scholar] [CrossRef]
- Blackwell, P.; Riethuller, G.; Collins, M. Biochar application to soil. In Biochar for Environmental Management; Lehmann, J., Joseph, S., Eds.; Earthscan: London, UK, 2009; pp. 207–227. [Google Scholar]
- Haefele, S.M.; Konboonc, Y.; Wongboon, W.; Amarante, S.; Maarifat, S.S.; Pfeiffer, E.M.; Knoblauch, C. Effects and fate of biochar from rice residues in rice based systems. Field Crops Res. 2011, 121, 430–440. [Google Scholar] [CrossRef]
- Parlavecchia, M.; Gattullo, R.; Perri, G.; Loffredo, E. Modulatory effects of biochar, hydrochar and vermicompost on the growth of horticultural plants and phytopathogenic fungi. In Proceedings of the VI International Symposium on Applications of Modelling as an Innovative Technology in the Horticultural Supply Chain Model-IT, Molfetta, Italy, 2–12 June 2019; Volume 1311, pp. 541–548. [Google Scholar]
- Agarwal, H.; Kashyap, V.H.; Mishra, A.; Bordoloi, S.; Singh, P.K.; Joshi, N.C. Biochar-based fertilizers and their applications in plant growth promotion and protection. 3 Biotech 2022, 12, 136. [Google Scholar] [CrossRef] [PubMed]
- Vannini, A.; Tarasconi, D.; Pietropoli, F.; Forte, T.A.G.W.; Grillo, F.; Carbognani, M.; Petraglia, A. Effects of Wood-Derived Biochar on Soil Respiration of a European Beech Forest Under Current Climate and Simulated Climate Change. Forests 2025, 16, 474. [Google Scholar] [CrossRef]
- Wang, Q.; Yuan, J.; Yang, X.; Han, X.; Lan, Y.; Cao, D.; Sun, Q.; Cui, X.; Meng, J.; Chen, W. Responses of soil respiration and C sequestration efficiency to biochar amendment in maize field of Northeast China. Soil Tillage Res. 2022, 223, 105442. [Google Scholar] [CrossRef]
- Zhang, R.; Qu, Z.; Liu, L.; Yang, W.; Wang, L.; Li, J.; Zhang, D. Soil respiration and organic carbon response to biochar and their influencing factors. Atmosphere 2022, 13, 2038. [Google Scholar] [CrossRef]
- Ojeda, G.; Gil, J.M.; Mattana, S.; Bachmann, J.; Quenea, K.; Sobral, A.J. Biochar ageing effects on soil respiration, biochar wettability and gaseous CO2 adsorption. Mitig. Adapt. Strateg. Glob. Change 2024, 29, 11. [Google Scholar] [CrossRef]
- Feng, Z.; Fan, Z.; Song, H.; Li, K.; Lu, H.; Liu, Y.; Cheng, F. Biochar induced changes of soil dissolved organic matter: The release and adsorption of dissolved organic matter by biochar and soil. Sci. Total Environ. 2021, 783, 147091. [Google Scholar] [CrossRef] [PubMed]
- Rombolà, A.G.; Torri, C.; Vassura, I.; Venturini, E.; Reggiani, R.; Fabbri, D. Effect of biochar amendment on organic matter and dissolved organic matter composition of agricultural soils from a two-year field experiment. Sci. Total Environ. 2022, 812, 151422. [Google Scholar] [CrossRef] [PubMed]
- Rombolà, A.G.; Greggio, N.; Fabbri, D.; Facchin, A.; Torri, C.; Pulcher, R.; Carlini, C.; Balugani, E.; Marazza, D.; Zannoni, D.; et al. Changes of labile, stable and water-soluble fractions of biochar after two years in a vineyard soil. Environ. Sci. Adv. 2023, 2, 1587–1599. [Google Scholar] [CrossRef]
- El-Naggar, A.; Zhou, R.; Tang, R.; Hur, J.; Cai, Y.; Chang, S.X. Rice husk and its biochar have contrasting effects on water-soluble organic matter and the microbial community in a bamboo forest soil. Land 2022, 11, 2265. [Google Scholar] [CrossRef]
- Bi, Y.; Kuzyakov, Y.; Cai, S.; Zhao, X. Accumulation of organic compounds in paddy soils after biochar application is controlled by iron hydroxides. Sci. Total Environ. 2021, 764, 144300. [Google Scholar] [CrossRef]
- Pospíšilová, L.; Horáková, E.; Fišera, M.; Jerzykiewicz, M.; Menšík, L. Effect of selected organic materials on soil humic acids chemical properties. Environ. Res. 2020, 187, 109663. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Dou, S.; Zheng, S. Effects of Corn Straw and Biochar Returning to Fields Every Other Year on the Structure of Soil Humic Acid. Sustainability 2022, 14, 15946. [Google Scholar] [CrossRef]
- DM of Ministry of Agricultural and Forestry Policies. Official Methods of Soil Chemical Analyses; Serie Generale; Gazzetta Ufficiale: Rome, Italy, 1999; p. 248.
- Lardo, E.; Palese, A.M.; Nuzzo, V.; Xiloyannis, C.; Celano, G. Variability of total soil. respiration in a Mediterranean vineyard. Soil Res. 2015, 53, 531–541. [Google Scholar] [CrossRef]
- Verrillo, M.; Salzano, M.; Cozzolino, V.; Spaccini, R.; Piccolo, A. Bioactivity and antimicrobial properties of chemically characterized compost teas from different green composts. Waste Manag. 2021, 120, 98–107. [Google Scholar] [CrossRef]
- Lee, X.; Yang, F.; Xing, Y.; Huang, Y.; Xu, L.; Liu, Z.; Holtzman, R.; Kan, I.; Li, Y.; Zhang, L.; et al. Use of biochar to manage soil salts and water: Effects and mechanisms. Catena 2022, 211, 106018. [Google Scholar] [CrossRef]
- Lu, H.L.; Li, K.W.; Nkoh Nkoh, J.; Shi, Y.X.X.; He, X.; Hong, Z.N.; Xu, R.K. Effects of the increases in soil pH and pH buffering capacity induced by crop residue biochars on available Cd contents in acidic paddy soils. Chemosphere 2022, 301, 134674. [Google Scholar] [CrossRef]
- Yu, H.; Zou, W.; Chen, J.; Chen, H.; Yu, Z.; Huang, J.; Tamg, H.; Wei, X.; Gao, B. Biochar amendment improves crop production in problem soils: A review. J. Environ. Manag. 2019, 232, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Shah, T.; Shah, S.; Shah, Z. Soil respiration, pH and EC as influenced by biochar. Soil Environ. 2017, 36, 77–83. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, A.; Ji, C.; Joseph, S.; Bian, R.; Li, L.; Pan, G.; Paz Ferreiro, J. Biochar’s effect on crop productivity and the dependence on experimental conditions a meta analysis of literature data. Plant Soil 2013, 373, 583–594. [Google Scholar] [CrossRef]
- Ding, X.; Li, G.; Zhao, X.; Lin, Q.; Wang, C. Biochar application significantly increases soil organic carbon under conservation tillage: An 11-year field experiment. Biochar 2023, 5, 28. [Google Scholar] [CrossRef]
- Bolan, S.; Sharma, S.; Mukherjee, S.; Kumar, M.; Rao, C.S.; Nataraj, K.C.; Singh, G.; Vinu, A.; Bhowmik, A.; Sharma, H.; et al. Biochar modulating soil biological health: A review. Sci. Total Environ. 2024, 914, 169585. [Google Scholar] [CrossRef] [PubMed]
- Domingues, R.R.; Sánchez-Monedero, M.A.; Spokas, K.A.; Melo, L.C.A.; Trugilho, P.F.; Valenciano, M.N.; Silva, C.A. Enhancing Cation Exchange Capacity of Weathered Soils Using Biochar: Feedstock, Pyrolysis Conditions and Addition Rate. Agronomy 2020, 10, 824. [Google Scholar] [CrossRef]
- Omara, P.; Singh, H.; Singh, K.; Sharma, L.; Otim, F.; Obia, A. Short-term effect of field application of biochar on cation exchange capacity, pH, and electrical conductivity of sandy and clay loam temperate soils. Technol. Agron. 2023, 3, 16. [Google Scholar] [CrossRef]
- Wu, H.; Guo, Z.; Peng, C. Land use induced changes of organic carbon storage in soils of China. Glob. Change Biol. 2003, 9, 305–315. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Y.; Li, W.; Zhao, R. Seasonal variation of soil respiration under different land use/land cover in arid region. Sci. China Ser. D Earth Sci. 2007, 50, 76–85. [Google Scholar] [CrossRef]
- El Naggar, A.; El Naggar, A.H.; Shaheen, S.M.; Sarkar, B.; Chang, S.X.; Tsang, D.C.W.; Rinklebe, J. Biochar composition dependent impacts on soil nutrient release, carbon mineralization, and potential environmental risk: A review. J. Environ. Manag. 2019, 241, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Jiao, Y.; Liu, T.; Gu, H.; Li, Z.; Wang, S.; Liu, J. Effects of biochar addition on soil fauna communities—A meta-analysis. Soil Use Manag. 2024, 40, e13096. [Google Scholar] [CrossRef]
- Jeffery, S.; Bezemer, T.M.; Cornelissen, G.; Kuyper, T.W.; Lehmann, J.; Mommer, L.; Sohi, S.H.; van de Voorde, T.F.J.; Wardle, D.A.; van Groenigen, J.W. The way forward in biochar research: Targeting trade-offs between the potential wins. GCB Bioenergy 2015, 7, 1–13. [Google Scholar] [CrossRef]
- Jeffery, S.; Abalos, D.; Prodana, M.; Bastos, A.C.; van Groenigen, J.W.; Hungate, B.A.; Verheijen, F. Biochar boosts tropical but not temperate crop yields. Environ. Res. Lett. 2017, 12, 5. [Google Scholar] [CrossRef]
- Karabay, U.; Toptas, A.; Yanik, J.; Aktas, L. Does Biochar Alleviate Salt Stress Impact on Growth of Salt-Sensitive Crop Common Bean. Commun. Soil Sci. Plant Anal. 2021, 52, 456–469. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Hu, L.; Wang, Y.; Wu, Z.; Meng, Y.; Liang, Z.; Yang, L.; Wei, G.; Huang, Y. Identification of lignans and quality assessment in Dendrobium officinale under different cultivation modes. Rap. Commun. Mass Spec. 2023, 37, e9541. [Google Scholar] [CrossRef]
- Machado, W.; Franchini, J.C.; Guimaraes, M.D.F.; Filho, J.T. Spectroscopic characterization of humic and fulvic acids in oil aggregates, Brasil. Heliyon 2020, 6, e04078. [Google Scholar] [CrossRef]
- Li, L.; Ma, L.; Yanan, L.; Wang, Y.; Sun, S. Spectroscopic analysis of the effects of alkaline extractants on humic acids isolated from herbaceous peat. Spectroscopy 2024, 39, 20. [Google Scholar] [CrossRef]
- Sun, P.; Huang, Y.; Yang, X.; Liao, A.; Wu, J. The role of indole derivative in the growth of plants: A review. Front. Plant Sci. 2023, 16, 1120613. [Google Scholar] [CrossRef]

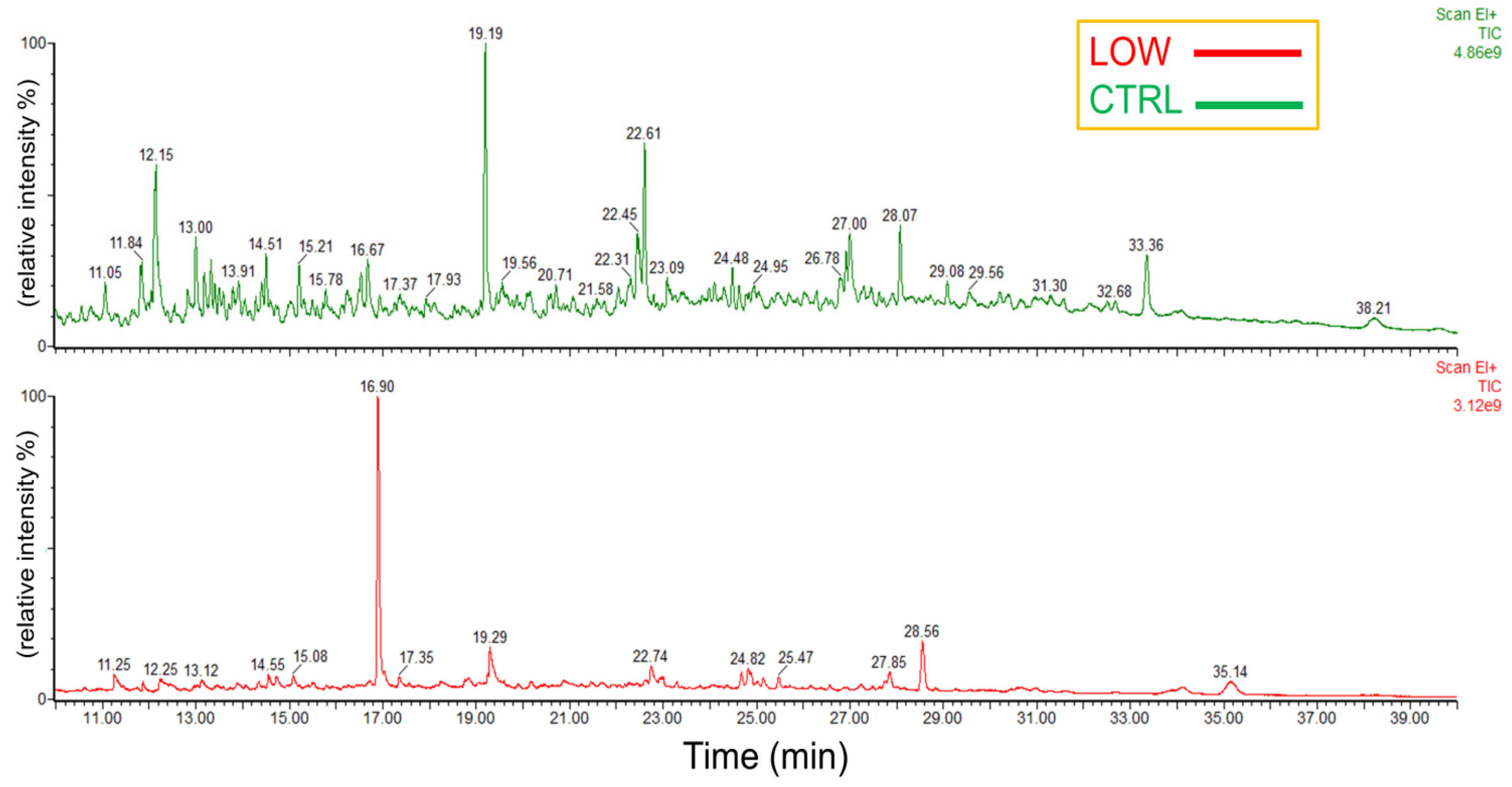
| C-CTRL | C-LOW | C-CTRL (%) | C-LOW (%) | |
|---|---|---|---|---|
| N-compounds | 94,314,231 | 13,212,714 | 3.62 | 1.64 |
| Fame | 10,168,751.3 | 18,439,959.5 | 0.39 | 2.28 |
| Branched fame and Hydroxyacids | 228,126,380 | 93,427,032.62 | 8.75 | 11.57 |
| Unsaturated branched fame | 0 | 47,709,987 | 0.00 | 5.91 |
| Aromatic | 233,967,191.2 | 212,946,751.5 | 8.98 | 26.37 |
| N-Aromatic | 349,283,102 | 84,987,775.75 | 13.41 | 10.52 |
| Lignin-G | 400,985,722 | 83,730,246.75 | 15.39 | 10.37 |
| Lignin-P | 28,572,688 | 0 | 1.10 | 0.00 |
| Lignin-S | 255,884,054 | 49,253,888.5 | 9.82 | 6.10 |
| Total Lignin | 685,442,464 | 132,984,135.3 | 26.31 | 16.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Curcio, R.; Bilotti, R.; Lia, C.; Compitiello, M.; Cangemi, S.; Verrillo, M.; Spaccini, R.; Mazzei, P. Short-Term Effects of Wood Biochar on Soil Fertility, Heterotrophic Respiration and Organic Matter Composition. Agriculture 2025, 15, 1091. https://doi.org/10.3390/agriculture15101091
Curcio R, Bilotti R, Lia C, Compitiello M, Cangemi S, Verrillo M, Spaccini R, Mazzei P. Short-Term Effects of Wood Biochar on Soil Fertility, Heterotrophic Respiration and Organic Matter Composition. Agriculture. 2025; 15(10):1091. https://doi.org/10.3390/agriculture15101091
Chicago/Turabian StyleCurcio, Rossella, Raffaele Bilotti, Carmine Lia, Michele Compitiello, Silvana Cangemi, Mariavittoria Verrillo, Riccardo Spaccini, and Pierluigi Mazzei. 2025. "Short-Term Effects of Wood Biochar on Soil Fertility, Heterotrophic Respiration and Organic Matter Composition" Agriculture 15, no. 10: 1091. https://doi.org/10.3390/agriculture15101091
APA StyleCurcio, R., Bilotti, R., Lia, C., Compitiello, M., Cangemi, S., Verrillo, M., Spaccini, R., & Mazzei, P. (2025). Short-Term Effects of Wood Biochar on Soil Fertility, Heterotrophic Respiration and Organic Matter Composition. Agriculture, 15(10), 1091. https://doi.org/10.3390/agriculture15101091







