Exploiting Chestnut Biochar as a Functional and Circular Ingredient in Weaned Piglet Diets
Abstract
1. Introduction
2. Materials and Methods
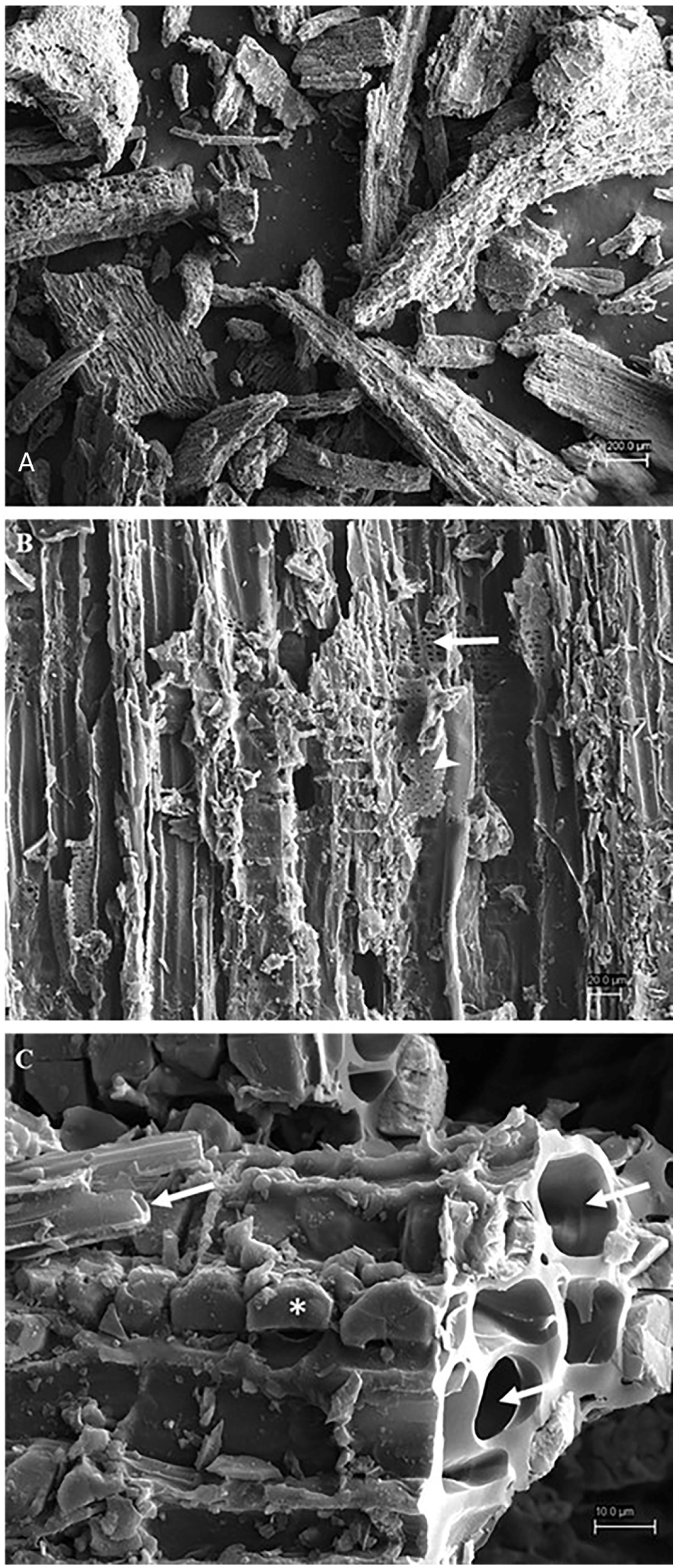
2.1. Chemical and Electron Microscopy Characterization of Chestnut Biochar
2.2. Animal Housing and Experimental Design
2.3. Sample Collection
2.4. Zootechnical Performance and Diarrhea Frequency
2.5. Microbiological Analysis of Fecal Samples and pH Determination
2.6. Apparent Total Tract Digestibility of Principal Nutrients
2.7. Mineral Content of Feces
2.8. Evaluation of Antioxidant Barrier in Serum Samples
2.9. Statistical Analysis
3. Results
3.1. Characterization of Chestnut Biochar Using Chemical and Electron Microscopy
3.2. Growth Performance
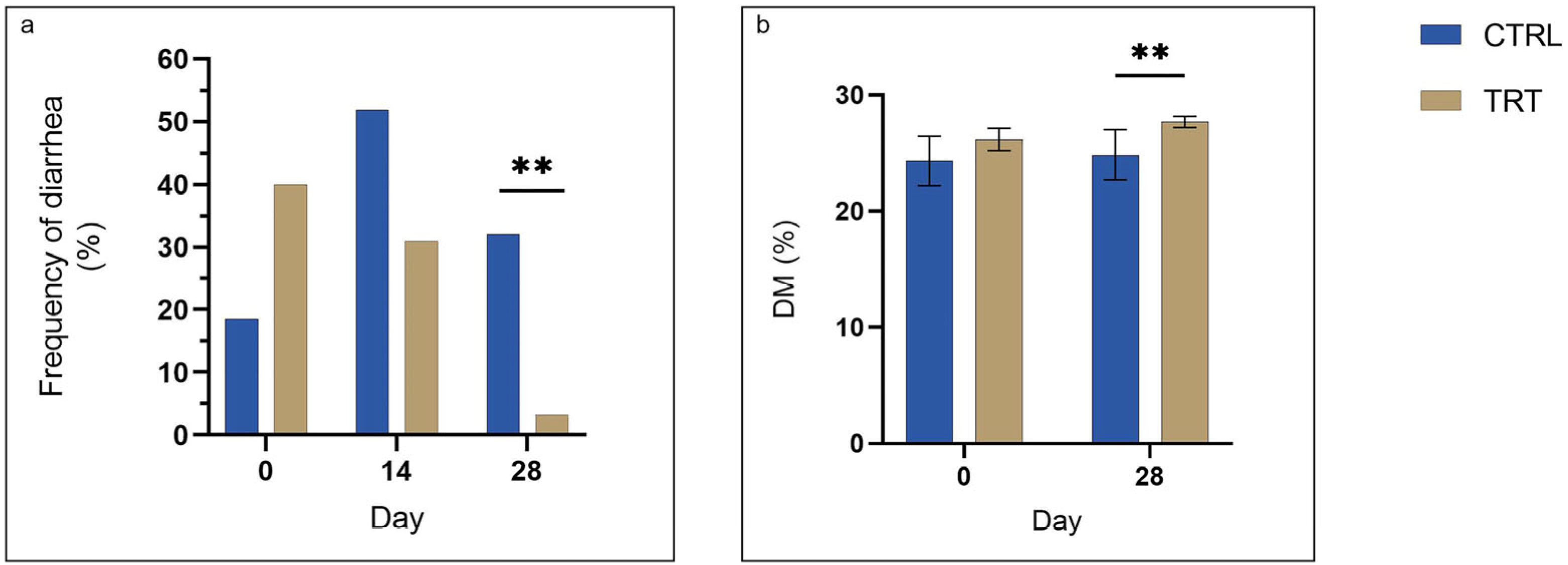
3.3. Diarrhea Frequency and Fecal Consistency
3.4. Microbiological Analysis of Fecal Samples
3.5. Nutrient Digestibility and Fecal Mineral Content
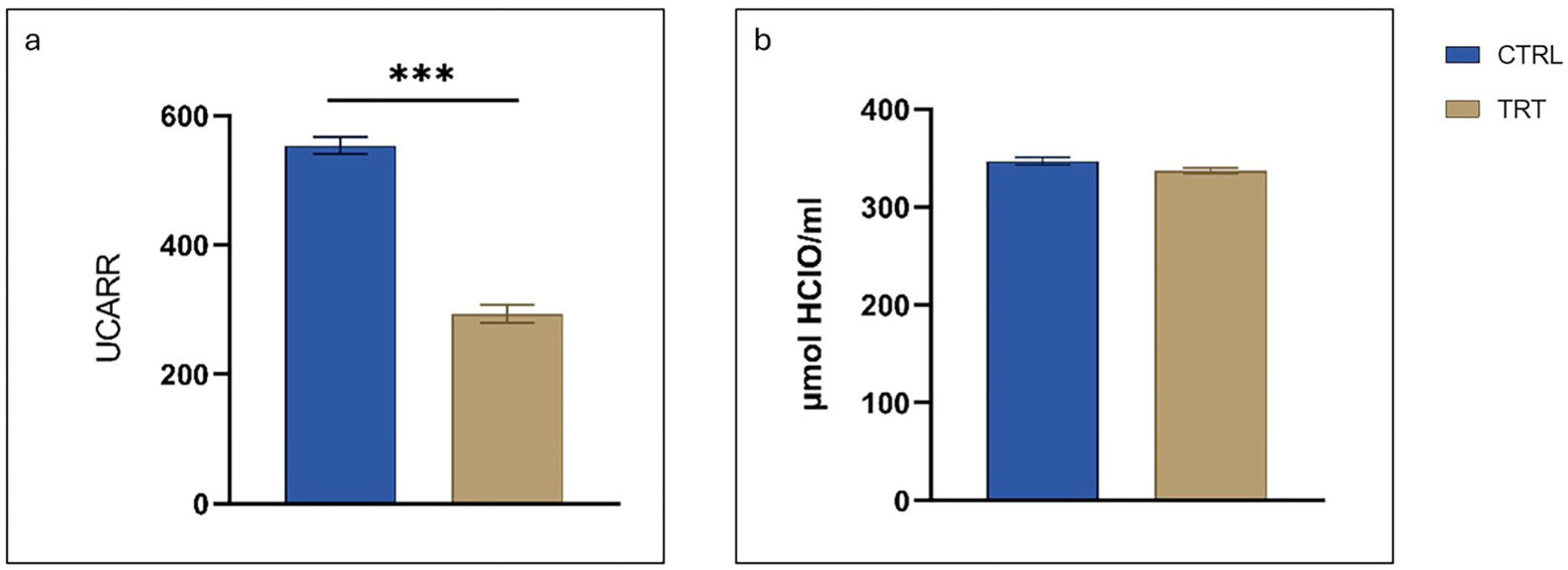
3.6. Serum Oxidative Status and Antioxidant Barrier
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. World Livestock: Transforming the Livestock Sector Through the Sustainable Development Goals; FAO: Quebec City, QC, Canada, 2019; ISBN 978-92-5-130883-7. [Google Scholar]
- UN. General Assembly UN Resolution 70/1: Transforming Our World: The 2030 Agenda for Sustainable Development. Available online: https://sdgs.un.org/2030agenda (accessed on 10 January 2025).
- Tretola, M.; Ottoboni, M.; Luciano, A.; Rossi, L.; Baldi, A.; Pinotti, L. Former Food Products Have No Detrimental Effects on Diet Digestibility, Growth Performance and Selected Plasma Variables in Post-Weaning Piglets. Ital. J. Anim. Sci. 2019, 18, 987–996. [Google Scholar] [CrossRef]
- Dell’Anno, M.; Reggi, S.; Caprarulo, V.; Hejna, M.; Sgoifo Rossi, C.; Callegari, M.; Baldi, A.; Rossi, L. Evaluation of Tannin Extracts, Leonardite and Tributyrin Supplementation on Diarrhoea Incidence and Gut Microbiota of Weaned Piglets. Animals 2021, 11, 1693. [Google Scholar] [CrossRef]
- Frazzini, S.; Reggi, S.; Dell’Anno, M.; Fifi, A.P.; Scaglia, E.; Ferri, I.; Rossi, L. Chemical-Functional Characterization of Ascophyllum nodosum and Phymatolithon calcareum and Dietary Supplementation in Post-Weaning Pigs. Front. Vet. Sci. 2024, 11, 1431091. [Google Scholar] [CrossRef]
- Ferraro, G.; Pecori, G.; Rosi, L.; Bettucci, L.; Fratini, E.; Casini, D.; Rizzo, A.M.; Chiaramonti, D. Biochar from Lab-Scale Pyrolysis: Influence of Feedstock and Operational Temperature. Biomass Convers. Biorefin. 2024, 14, 5901–5911. [Google Scholar] [CrossRef]
- Yuan, X.; Cao, Y.; Li, J.; Patel, A.K.; Dong, C.-D.; Jin, X.; Gu, C.; Yip, A.C.K.; Tsang, D.C.W.; Ok, Y.S. Recent Advancements and Challenges in Emerging Applications of Biochar-Based Catalysts. Biotechnol. Adv. 2023, 67, 108181. [Google Scholar] [CrossRef] [PubMed]
- Verdi, L.; Dalla Marta, A.; Orlandini, S.; Maienza, A.; Baronti, S.; Vaccari, F.P. Evaluation of Biochar Addition to Digestate, Slurry, and Manure for Mitigating Carbon Emissions. Agriculture 2024, 14, 162. [Google Scholar] [CrossRef]
- Premchand, P.; Demichelis, F.; Chiaramonti, D.; Bensaid, S.; Fino, D. Study on the Effects of Carbon Dioxide Atmosphere on the Production of Biochar Derived from Slow Pyrolysis of Organic Agro-Urban Waste. Waste Manag. 2023, 172, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Shoudho, K.N.; Khan, T.H.; Ara, U.R.; Khan, M.R.; Shawon, Z.B.Z.; Hoque, M.E. Biochar in Global Carbon Cycle: Towards Sustainable Development Goals. Curr. Res. Green Sustain. Chem. 2024, 8, 100409. [Google Scholar] [CrossRef]
- Qomariyah, N.; Ella, A.; Nurdin Ahmad, S.; Yusriani, Y.; Miftakhus Sholikin, M.; Rachmanto Prihambodo, T.; Retnani, Y.; Jayanegara, A.; Wina, E.; Permana, I.G. Dietary Biochar as a Feed Additive for Increasing Livestock Performance: A Meta-Analysis of In Vitro and In Vivo Experiment. Czech J. Anim. Sci. 2023, 68, 72–86. [Google Scholar] [CrossRef]
- Man, K.Y.; Chow, K.L.; Man, Y.B.; Mo, W.Y.; Wong, M.H. Use of Biochar as Feed Supplements for Animal Farming. Crit. Rev. Environ. Sci. Technol. 2021, 51, 187–217. [Google Scholar] [CrossRef]
- Reggi, S.; Frazzini, S.; Torresani, M.C.; Guagliano, M.; Cristiani, C.; Pilu, S.R.; Ghidoli, M.; Rossi, L. Metabolomic Profiling and Functional Characterization of Biochar from Vine Pruning Residues for Applications in Animal Feed. Animals 2024, 14, 3440. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, A.; Karuppiah, S.; Ebadi, M.; Shanley, R.; Khoruts, A.; Weisdorf, D.J.; Staley, C. A Dose-Finding Safety and Feasibility Study of Oral Activated Charcoal and Its Effects on the Gut Microbiota in Healthy Volunteers Not Receiving Antibiotics. PLoS ONE 2022, 17, e0269986. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EU) 2022/1104 of 1 July 2022 Amending Regulation (EU) No 68/2013 on the Catalogue of Feed Materials (Text with EEA Relevance). Off. J. Eur. Union 2022. [Google Scholar]
- Dell’Anno, M.; Frazzini, S.; Reggi, S.; Ferri, I.; Scaglia, E.; Schiasselloni, L.; Inglesi, A.; Riva, F.; Verdile, N.; Pasquariello, R.; et al. Evaluation of Dietary Supplementation of Ascophyllum nodosum and Lithothamnium calcareum as Functional Algae in F4+ Escherichia coli Challenged Piglets. Front. Vet. Sci. 2024, 11, 1430347. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 22nd ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2023. [Google Scholar]
- Hejna, M.; Moscatelli, A.; Onelli, E.; Baldi, A.; Pilu, S.; Rossi, L. Evaluation of concentration of heavy metals in animal rearing system. Ital. J. Anim. Sci. 2019, 18, 1372–1384. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Swine, 11th ed.; National Research Council: Ottawa, ON, Canada, 2020. [Google Scholar]
- Rossi, L.; Dell’Orto, V.; Vagni, S.; Sala, V.; Reggi, S.; Baldi, A. Protective Effect of Oral Administration of Transgenic Tobacco Seeds against Verocytotoxic Escherichia coli Strain in Piglets. Vet. Res. Commun. 2014, 38, 39–49. [Google Scholar] [CrossRef]
- Dell’Anno, M.; Frazzini, S.; Ferri, I.; Tuberti, S.; Bonaldo, E.; Botti, B.; Grossi, S.; Sgoifo Rossi, C.A.; Rossi, L. Effect of Dietary Supplementation of Chestnut and Quebracho Tannin Supplementation on Neonatal Diarrhoea in Preweaning Calves. Antioxidants 2024, 13, 237. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, R.T.; Ahmad, P.; Rafatullah, M. Insights into Biochar Applications: A Sustainable Strategy toward Carbon Neutrality and Circular Economy. In Catalytic Applications of Biochar for Environmental Remediation: Sustainable Strategies Towards a Circular Economy; American Chemical Society: Washington, DC, USA, 2024; pp. 1–30. [Google Scholar]
- Puglia, M.; Morselli, N.; Lumi, M.; Santunione, G.; Pedrazzi, S.; Allesina, G. Assessment of Hemp Hurd-Derived Biochar Produced through Different Thermochemical Processes and Evaluation of Its Potential Use as Soil Amendment. Heliyon 2023, 9, e14698. [Google Scholar] [CrossRef]
- Ottani, F.; Pedrazzi, S.; Morselli, N.; Puglia, M.; Allesina, G. Seeking the Synergistic Potential of Biochar Integration in Municipal Composting Plants for Techno-Economic and Environmental Leverage. Sustain. Energy Technol. Assess. 2024, 64, 103717. [Google Scholar] [CrossRef]
- Mosa, A.; Mansour, M.M.; Soliman, E.; El-Ghamry, A.; El Alfy, M.; El Kenawy, A.M. Biochar as a Soil Amendment for Restraining Greenhouse Gases Emission and Improving Soil Carbon Sink: Current Situation and Ways Forward. Sustainability 2023, 15, 1206. [Google Scholar] [CrossRef]
- Dell’Anno, M.; Scaglia, E.; Reggi, S.; Grossi, S.; Sgoifo Rossi, C.A.; Frazzini, S.; Caprarulo, V.; Rossi, L. Evaluation of Tributyrin Supplementation in Milk Replacer on Diarrhoea Occurrence in Preweaning Holstein Calves. Animal 2023, 17, 100791. [Google Scholar] [CrossRef] [PubMed]
- Restuccia, F.; Mašek, O.; Hadden, R.M.; Rein, G. Quantifying Self-Heating Ignition of Biochar as a Function of Feedstock and the Pyrolysis Reactor Temperature. Fuel 2019, 236, 201–213. [Google Scholar] [CrossRef]
- Yulis Setyawan, H.; Mayang Sabrina Sunyoto, N.; Sugiarto, Y.; Sue stining Dyah Dewanti, B.; Tri Widayanti, V.; Hakim, L.; Kurniawan, S.; Auliya Nugroho, G.b.; Ulandari, D.; Choirun, A.; et al. Characterisation of Biochar from Various Carbon Sources. BIO Web Conf. 2024, 90, 06003. [Google Scholar] [CrossRef]
- Amer, M.; Elwardany, A. Biomass Carbonization. In Renewable Energy—Resources, Challenges and Applications; IntechOpen: London, UK, 2020. [Google Scholar]
- European Commission. Commission Regulation (EU) No 1275/2013 of 6 December 2013 Amending Annex I to Directive 2002/32/EC of the European Parliament and of the Council as Regards Maximum Levels for Arsenic, Cadmium, Lead, Nitrites, Volatile Mustard Oil and Harmful Botanical Impurities Text with EEA Relevance. Off. J. Eur. Union 2013. [Google Scholar]
- Ertop, U.; Şevik, H.; Hendek Ertop, M. Mineral Composition and Heavy Metal Contents of Chestnut Honey Collected From Kastamonu Region. J. Apitherapy Nat. 2023, 6, 73–87. [Google Scholar] [CrossRef]
- Hejna, M.; Gottardo, D.; Baldi, A.; Dell’Orto, V.; Cheli, F.; Zaninelli, M.; Rossi, L. Review: Nutritional Ecology of Heavy Metals. Animal 2018, 12, 2156–2170. [Google Scholar] [CrossRef]
- Lu, X.; Gu, X. A Review on Lignin Pyrolysis: Pyrolytic Behavior, Mechanism, and Relevant Upgrading for Improving Process Efficiency. Biotechnol. Biofuels Bioprod. 2022, 15, 106. [Google Scholar] [CrossRef]
- Osman, A.I.; Fawzy, S.; Farghali, M.; El-Azazy, M.; Elgarahy, A.M.; Fahim, R.A.; Maksoud, M.I.A.A.; Ajlan, A.A.; Yousry, M.; Saleem, Y.; et al. Biochar for Agronomy, Animal Farming, Anaerobic Digestion, Composting, Water Treatment, Soil Remediation, Construction, Energy Storage, and Carbon Sequestration: A Review. Environ. Chem. Lett. 2022, 20, 2385–2485. [Google Scholar] [CrossRef]
- Franco, C.R.; Page-Dumroese, D.S.; Archuleta, J. Forest Management and Biochar for Continued Ecosystem Services. J. Soil. Water Conserv. 2022, 77, 60A–64A. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation (EU) 2018/1981 of 13 December 2018 Renewing the Approval of the Active Substances Copper Compounds, as Candidates for Substitution, in Accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council Concerning the Placing of Plant Protection Products on the Market, and Amending the Annex to Commission Implementing Regulation (EU) No 540/2011. Off. J. Eur. Union 2018. [Google Scholar]
- Graves, C.; Kolar, P.; Shah, S.; Grimes, J.; Sharara, M. Can Biochar Improve the Sustainability of Animal Production? Appl. Sci. 2022, 12, 5042. [Google Scholar] [CrossRef]
- Schmidt, H.-P.; Hagemann, N.; Draper, K.; Kammann, C. The Use of Biochar in Animal Feeding. PeerJ 2019, 7, e7373. [Google Scholar] [CrossRef] [PubMed]
- Lao, E.J.; Mbega, E.R. Biochar as a Feed Additive For Improving the Performance of Farm Animals. Malays. J. Sustain. Agric. 2020, 4, 86–93. [Google Scholar] [CrossRef]
- Komar, A.H.A.-J.; Yaser, K. Del Effect of Different Feed Additives on Growth Performance and Production in Livestock. Int. J. Agric. For. 2019, 9, 16–31. [Google Scholar]
- Chu, G.M.; Kim, J.H.; Kim, H.Y.; Ha, J.H.; Jung, M.S.; Song, Y.; Cho, J.H.; Lee, S.J.; Ibrahim, R.I.H.; Lee, S.S.; et al. Effects of Bamboo Charcoal on the Growth Performance, Blood Characteristics and Noxious Gas Emission in Fattening Pigs. J. Appl. Anim. Res. 2013, 41, 48–55. [Google Scholar] [CrossRef]
- Schubert, D.C.; Chuppava, B.; Witte, F.; Terjung, N.; Visscher, C. Effect of Two Different Biochars as a Component of Compound Feed on Nutrient Digestibility and Performance Parameters in Growing Pigs. Front. Anim. Sci. 2021, 2, 633958. [Google Scholar] [CrossRef]
- Rychen, G.; Aquilina, G.; Azimonti, G.; Bampidis, V.; de Lourdes Bastos, M.; Bories, G.; Chesson, A.; Cocconcelli, P.S.; Flachowsky, G.; Gropp, J.; et al. Guidance on the Assessment of the Safety of Feed Additives for the Target Species. EFSA J. 2017, 15, e05021. [Google Scholar] [CrossRef]
- Engelsmann, M.N.; Jensen, L.D.; van der Heide, M.E.; Hedemann, M.S.; Nielsen, T.S.; Nørgaard, J.V. Age-Dependent Development in Protein Digestibility and Intestinal Morphology in Weaned Pigs Fed Different Protein Sources. Animal 2022, 16, 100439. [Google Scholar] [CrossRef]
- Lim, M.Y.; Song, E.-J.; Kang, K.S.; Nam, Y.-D. Age-Related Compositional and Functional Changes in Micro-Pig Gut Microbiome. Geroscience 2019, 41, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, J.; Wang, G.; Cai, S.; Zeng, X.; Qiao, S. Advances in Low-Protein Diets for Swine. J. Anim. Sci. Biotechnol. 2018, 9, 60. [Google Scholar] [CrossRef]
- Pomar, C.; Andretta, I.; Remus, A. Feeding Strategies to Reduce Nutrient Losses and Improve the Sustainability of Growing Pigs. Front. Vet. Sci. 2021, 8, 742220. [Google Scholar] [CrossRef] [PubMed]
- Dourmad, J.-Y.; Jondreville, C. Impact of Nutrition on Nitrogen, Phosphorus, Cu and Zn in Pig Manure, and on Emissions of Ammonia and Odours. Livest. Sci. 2007, 112, 192–198. [Google Scholar] [CrossRef]
- Lu, L.; Liao, X.; Luo, X. Nutritional Strategies for Reducing Nitrogen, Phosphorus and Trace Mineral Excretions of Livestock and Poultry. J. Integr. Agric. 2017, 16, 2815–2833. [Google Scholar] [CrossRef]
- Rossi, L.; Turin, L.; Alborali, G.L.; Demartini, E.; Filipe, J.F.S.; Riva, F.; Riccaboni, P.; Scanziani, E.; Trevisi, P.; Dall’Ara, P.; et al. Translational Approach to Induce and Evaluate Verocytotoxic E. coli O138 Based Disease in Piglets. Animals 2021, 11, 2415. [Google Scholar] [CrossRef] [PubMed]
- Bava, R.; Castagna, F.; Lupia, C.; Poerio, G.; Liguori, G.; Lombardi, R.; Naturale, M.D.; Mercuri, C.; Bulotta, R.M.; Britti, D.; et al. Antimicrobial Resistance in Livestock: A Serious Threat to Public Health. Antibiotics 2024, 13, 551. [Google Scholar] [CrossRef]
- European Commission. Regulation (EU) 2019/6 of the European Parliament and of the Council of 11 December 2018 on Veterinary Medicinal Products and Repealing Directive 2001/82/EC. Off. J. Eur. Union 2019. [Google Scholar]
- Chandrasekaran, P.; Weiskirchen, S.; Weiskirchen, R. Effects of Probiotics on Gut Microbiota: An Overview. Int. J. Mol. Sci. 2024, 25, 6022. [Google Scholar] [CrossRef]
- Nordeste, R.; Tessema, A.; Sharma, S.; Kovač, Z.; Wang, C.; Morales, R.; Griffiths, M.W. Molecules Produced by Probiotics Prevent Enteric Colibacillosis in Pigs. BMC Vet. Res. 2017, 13, 335. [Google Scholar] [CrossRef]
- Naka, K.; Watarai, S.; Tana; Inoue, K.; Kodama, Y.; Oguma, K.; Yasuda, T.; Kodama, H. Adsorption Effect of Activated Charcoal on Enterohemorrhagic Escherichia coli. J. Vet. Med. Sci. 2001, 63, 281–285. [Google Scholar] [CrossRef]
- Aurelie, D.N.; Luciane Marlyse, M.; Samuel, E.J.; Ache Roland, N.; Moïse, N. Modification of The Bacterial Abundance Properties of Water by Immersed Non-Activated Charcoal. Clin. Med. Health Res. J. 2024, 4, 946–954. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free Radicals, Antioxidants in Disease and Health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar] [CrossRef]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Abdull Razis, A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative Stress, Free Radicals and Antioxidants: Potential Crosstalk in the Pathophysiology of Human Diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef]
- Menzel, A.; Samouda, H.; Dohet, F.; Loap, S.; Ellulu, M.S.; Bohn, T. Common and Novel Markers for Measuring Inflammation and Oxidative Stress Ex Vivo in Research and Clinical Practice—Which to Use Regarding Disease Outcomes? Antioxidants 2021, 10, 414. [Google Scholar] [CrossRef] [PubMed]
- Buchet, A.; Belloc, C.; Leblanc-Maridor, M.; Merlot, E. Effects of Age and Weaning Conditions on Blood Indicators of Oxidative Status in Pigs. PLoS ONE 2017, 12, e0178487. [Google Scholar] [CrossRef]
- Gorni, D. Valutazione Globale Dello Stress Ossidativo; Università Degli Studi di Parma: Parma, Italy, 2017. [Google Scholar]
- Lameirão, F.; Pinto, D.; Vieira, E.F.; Peixoto, A.F.; Freire, C.; Sut, S.; Dall’Acqua, S.; Costa, P.; Delerue-Matos, C.; Rodrigues, F. Green-Sustainable Recovery of Phenolic and Antioxidant Compounds from Industrial Chestnut Shells Using Ultrasound-Assisted Extraction: Optimization and Evaluation of Biological Activities In Vitro. Antioxidants 2020, 9, 267. [Google Scholar] [CrossRef]
- Reggi, S.; Frazzini, S.; Pedrazzi, S.; Ghidoli, M.; Torresani, M.C.; Puglia, M.; Morselli, N.; Guagliano, M.; Cristiani, C.; Pilu, S.R.; et al. Metabolomic Insights into the Potential of Chestnut Biochar as a Functional Feed Ingredient. Appl. Sci. 2025, 15, 1084. [Google Scholar] [CrossRef]
- Kunst, C.; Schmid, S.; Michalski, M.; Tümen, D.; Buttenschön, J.; Müller, M.; Gülow, K. The Influence of Gut Microbiota on Oxidative Stress and the Immune System. Biomedicines 2023, 11, 1388. [Google Scholar] [CrossRef]
- Baldelli, V.; Scaldaferri, F.; Putignani, L.; Del Chierico, F. The Role of Enterobacteriaceae in Gut Microbiota Dysbiosis in Inflammatory Bowel Diseases. Microorganisms 2021, 9, 697. [Google Scholar] [CrossRef]
- Gomez, S.A.; Abrey-Recalde, M.J.; Panek, C.A.; Ferrarotti, N.F.; Repetto, M.G.; Mejías, M.P.; Fernández, G.C.; Vanzulli, S.; Isturiz, M.A.; Palermo, M.S. The Oxidative Stress Induced in vivo by Shiga Toxin-2 Contributes to the Pathogenicity of Haemolytic Uraemic Syndrome. Clin. Exp. Immunol. 2013, 173, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Gobert, A.P.; Wilson, K.T. Polyamine- and NADPH-Dependent Generation of ROS during Helicobacter pylori Infection: A Blessing in Disguise. Free Radic. Biol. Med. 2017, 105, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, X.; Li, L.; Zhong, C.; Zhang, Y.; Yang, X.; Li, M.; Yang, C. The Role of Gut Microbiota in Intestinal Disease: From an Oxidative Stress Perspective. Front. Microbiol. 2024, 15, 1328324. [Google Scholar] [CrossRef] [PubMed]
| Ingredients, % as Fed Basis | CTRL | TRT |
|---|---|---|
| Barley | 21.525 | 21.525 |
| Soft wheat | 17.100 | 17.100 |
| Soybean meal (47% CP) | 9.300 | 9.300 |
| Maize | 8.000 | 8.000 |
| Biscuit meal | 8.000 | 8.000 |
| Flaked maize | 7.500 | 7.500 |
| Maize feed | 5.000 | 5.000 |
| Wheat bran | 5.000 | 4.000 |
| Soybean concentrate | 4.000 | 4.000 |
| Beet pulp | 2.500 | 2.500 |
| Breakfast products | 2.000 | 2.000 |
| Soybean oil | 1.800 | 1.800 |
| Whey powder | 1.500 | 1.500 |
| Fishmeal (72% CP) | 1.500 | 1.500 |
| Biochar | - | 1.000 |
| l-lysine sulfate | 0.790 | 0.790 |
| Organic acid mix 1 | 0.750 | 0.750 |
| Calcium formate | 0.650 | 0.650 |
| Monocalcium phosphate | 0.600 | 0.600 |
| Salt | 0.500 | 0.500 |
| Vitamin mineral premix 2 | 0.400 | 0.400 |
| Medium chain fatty acid 3 | 0.350 | 0.350 |
| Sucrose | 0.300 | 0.300 |
| l-threonine | 0.220 | 0.220 |
| Natural extract 4 | 0.200 | 0.200 |
| Dl-methionine | 0.170 | 0.170 |
| l-valine | 0.130 | 0.130 |
| Pellet binder 5 | 0.100 | 0.100 |
| l-tryptophan | 0.060 | 0.060 |
| Flavors | 0.045 | 0.045 |
| l-isoleucine | 0.010 | 0.010 |
| Chemical Composition (% as Fed Basis) | ||
| Dry matter 6 | 91.6 ± 0.70 | 91.4 ± 0.93 |
| Crude protein 6 | 15.5 ± 0.23 | 15.4 ± 0.09 |
| Ether extract 6 | 4.4 ± 0.18 | 4.3 ± 0.28 |
| Crude fiber 6 | 3.4 ± 0.07 | 3.6 ± 0.14 |
| Ash 6 | 5.4 ± 0.08 | 5.1 ± 0.19 |
| Calcium (Ca) 7 | 0.599 | 0.599 |
| Phosphorus (P) 7 | 0.622 | 0.622 |
| Ileal digestible lysine (SID) 7 | 1.05 | 1.05 |
| Metabolizable energy (ME) 7 | 13.475 MJ/kg | 13.475 MJ/kg |
| Nutrient Composition (as Fed Basis) | |||||
| Analyte | Content (%) | ||||
| Dry Matter | 59.8 ± 0.94 | ||||
| Ash | 4.5 ± 0.02 | ||||
| Crude Fiber | 49.1 ± 0.53 | ||||
| Crude Protein | 1.0 ± 0.21 | ||||
| Ether Extract | ND | ||||
| Mineral Composition (as Fed Basis) | |||||
| Mineral | Content (g kg−1) | Mineral | Content (mg kg−1) | Mineral | Content (mg kg−1) |
| Na | 0.1 ± 0.01 | Cr | 2.2 ± 0.53 | Zn | ND |
| Mg | 1.7± 0.16 | Mn | 257.3 ± 26.77 | As | 0.9± 0.13 |
| Al | 0.4 ± 0.08 | Fe | 1.4 ± 0.24 | Se | 0.02 ± 0.01 |
| P | 0.5 ± 0.11 | Co | 0.6 ± 0.12 | Mo | ND |
| K | 3.6 ± 0.24 | Ni | 7.4 ± 0.48 | Cd | 0.1 ± 0.02 |
| Ca | 2.0 ± 0.25 | Cu | 11.6 ± 3.59 | Pb | 2.7 ± 0.38 |
| Period | Group | p-Values | ||||
|---|---|---|---|---|---|---|
| CTRL | TRT | Time | Treatment | Treatment × Time | ||
| BW (kg) | <0.001 | 0.837 | 0.967 | |||
| d0 | 9.4 ± 1.24 | 9.5 ± 1.31 | ||||
| d7 | 11.5 ± 1.61 | 11.6 ± 1.84 | ||||
| d14 | 14.2 ± 2.21 | 14.1 ± 2.56 | ||||
| d21 | 18.1 ± 2.90 | 18.1 ± 3.54 | ||||
| d28 | 22.0 ± 3.82 | 22.1 ± 4.59 | ||||
| ADG (g/day) | <0.001 | 0.732 | 0.4192 | |||
| d0–d7 | 292.9 ± 24.88 | 304.8 ± 35.68 | ||||
| d7–d14 | 383.9 ± 43.73 | 354.6 ± 32.29 | ||||
| d14–d21 | 564.2 ± 32.40 | 575.3 ± 54.46 | ||||
| d21–d28 | 553.4 ± 63.73 | 575.7 ± 55.42 | ||||
| ADFI (g/day) | <0.001 | 0.435 | 0.652 | |||
| d0–d7 | 440.4 ± 15.86 | 416.8 ± 38.05 | ||||
| d7–d14 | 558.9 ± 39.88 | 527.3 ± 70.93 | ||||
| d14–d21 | 655.7 ± 27.73 | 656.2 ± 40.36 | ||||
| d21–d28 | 881.1 ± 48.06 | 893.7 ± 92.20 | ||||
| <0.001 | 0.522 | 0.217 | ||||
| FCR (g feed/g gain) | d0–d7 | 1.5 ± 0.10 | 1.4 ± 0.13 | |||
| d7–d14 | 1.5 ± 0.13 | 1.5 ± 0.24 | ||||
| d14–d21 | 1.2 ± 0.11 | 1.1 ± 0.10 | ||||
| d21–d28 | 1.6 ± 0.21 | 1.6 ± 0.15 | ||||
| Period | Group | p-Value | ||||
|---|---|---|---|---|---|---|
| CTRL | TRT | Time | Treatment | Time × Treatment | ||
| Total bacteria | 0.132 | 0.779 | 0.449 | |||
| d0 | 5.5 ± 0.87 | 5.7 ± 0.85 | ||||
| d14 | 5.5 ± 0.68 | 5.3 ± 0.81 | ||||
| d28 | 5.9 ± 0.38 | 5.7 ± 0.33 | ||||
| Lactic acid bacteria | <0.0001 | 0.206 | 0.019 | |||
| d0 | 6.2 ± 0.61 | 5.9 ± 0.73 | ||||
| d14 | 5.4 ± 0.81 a | 6.0 ± 0.47 b | ||||
| d28 | 5.0 ± 0.51 | 4.5 ± 0.48 | ||||
| Coliform bacteria | 0.003 | 0.055 | 0.079 | |||
| d0 | 2.6 ± 1.38 | 3.4 ± 1.41 | ||||
| d14 | 4.0 ± 0.72 a | 3.0 ± 0.80 b | ||||
| d28 | 4.5 ± 0.70 | 4.0 ± 0.74 | ||||
| Lactic acid bacteria/coliform bacteria | 0.0003 | 0.068 | 0.035 | |||
| d0 | 1.95 ± 0.59 | 1.89 ± 0.64 | ||||
| d14 | 1.40 ± 0.33 | 2.27 ± 1.23 | ||||
| Period | Diet (%) | p-Value | ||||
|---|---|---|---|---|---|---|
| CTRL | TRT | Time | Treatment | Treatment × Time | ||
| OM (on DM) | 0.03 | 0.88 | 0.90 | |||
| d0 | 66.4 ± 0.36 | 66.5 ± 0.37 | ||||
| d28 | 72.6 ± 9.65 | 73.4 ± 3.12 | ||||
| CP (on DM) | <0.001 | 0.004 | 0.005 | |||
| d0 | 72.5 ± 0.67 | 72.5 ± 0.58 | ||||
| d28 | 75.0 ± 2.05 a | 79.5 ± 1.74 b | ||||
| EE (on DM) | 0.02 | 0.94 | 0.15 | |||
| d0 | 71.8 ± 0.85 | 70.4 ± 2.27 | ||||
| d28 | 72.7 ± 1.77 | 74.0 ± 2.44 | ||||
| Mineral Content (in Dry Matter) | CTRL | TRT | p-Value |
|---|---|---|---|
| Na (g kg−1) | 1.3 ± 0.91 | 2.2 ± 0.73 | 0.546 |
| Mg (g kg−1) | 8.2 ± 0.99 | 8.9 ± 0.34 | 0.407 |
| Al (g kg−1) | 0.9 ± 0.04 | 1.0 ± 0.06 | 0.442 |
| P (g kg−1) | 10.1 ± 0.79 | 10.2 ± 0.81 | 0.909 |
| K (g kg−1) | 8.2 ± 0.99 | 8.9 ± 0.34 | 0.407 |
| Ca (g kg−1) | 1.9 ± 0.22 | 1.6 ± 0.19 | 0.219 |
| Cr (mg kg−1) | 12.5 ± 0.92 | 15.2 ± 0.701 | 0.187 |
| Mn (mg kg−1) | 566.0 ± 59.08 | 664.8 ± 29.23 | 0.304 |
| Fe (mg kg−1) | 1.9 ± 0.25 | 2.4 ± 0.27 | 0.098 |
| Co (mg kg−1) | 0.9 ± 0.27 | 1.4 ± 0.48 | 0.543 |
| Ni (mg kg−1) | 6.6 ± 0.96 | 7.2 ± 0.71 | 0.444 |
| Cu (mg kg−1) | 637.7 ± 62.07 | 640.1 ± 44.67 | 0.982 |
| Zn (mg kg−1) | 833.9 ± 20.48 | 770.1 ± 22.02 | 0.362 |
| As (mg kg−1) | 0.7 ± 0.14 | 0.4 ± 0.08 | 0.122 |
| Se (mg kg−1) | 0.7 ± 0.06 | 1.3 ± 0.06 | 0.236 |
| Mo (mg kg−1) | 4.9 ± 0.76 | 6.0 ± 0.35 | 0.054 |
| Cd (mg kg−1) | 0.3 ± 0.14 | 0.4 ± 0.08 | 0.242 |
| Pb (mg kg−1) | 1.6 ± 0.39 | 1.0 ± 0.82 | 0.334 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, L.; Frazzini, S.; Santoru, M.; Canala, B.; Ferri, I.; Moscatelli, A.; Onelli, E.; Dell’Anno, M.; Pilu, S.; Reggi, S. Exploiting Chestnut Biochar as a Functional and Circular Ingredient in Weaned Piglet Diets. Agriculture 2025, 15, 1082. https://doi.org/10.3390/agriculture15101082
Rossi L, Frazzini S, Santoru M, Canala B, Ferri I, Moscatelli A, Onelli E, Dell’Anno M, Pilu S, Reggi S. Exploiting Chestnut Biochar as a Functional and Circular Ingredient in Weaned Piglet Diets. Agriculture. 2025; 15(10):1082. https://doi.org/10.3390/agriculture15101082
Chicago/Turabian StyleRossi, Luciana, Sara Frazzini, Matteo Santoru, Benedetta Canala, Irene Ferri, Alessandra Moscatelli, Elisabetta Onelli, Matteo Dell’Anno, Salvatore Pilu, and Serena Reggi. 2025. "Exploiting Chestnut Biochar as a Functional and Circular Ingredient in Weaned Piglet Diets" Agriculture 15, no. 10: 1082. https://doi.org/10.3390/agriculture15101082
APA StyleRossi, L., Frazzini, S., Santoru, M., Canala, B., Ferri, I., Moscatelli, A., Onelli, E., Dell’Anno, M., Pilu, S., & Reggi, S. (2025). Exploiting Chestnut Biochar as a Functional and Circular Ingredient in Weaned Piglet Diets. Agriculture, 15(10), 1082. https://doi.org/10.3390/agriculture15101082








