Germination Under Temperature Stress Facilitates Invasion in Indehiscent Lepidium Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Sources
2.2. Microscopy of Lepidium Fruit Morphology and Germinating Units
2.3. Seed and Fruit Material: Collection and Processing
2.4. Dormancy Characterization and Diaspore Types of the Three Lepidium Species
2.5. Germination Assessment of Isolated Seeds and Fruits Across Temperature Gradients
2.6. Data Analysis
3. Results
3.1. The Indehiscent Fruit-Producing L. appelianum and L. draba Germinate Under a Broad Temperature Amplitude
3.2. Impact of Temperature Stress on Germination Success of the Invasive Dehiscent Fruit-Producing L. campestre
3.3. Germination Characteristics Reveal Temperature Stress Tolerance in Indehiscent Fruit-Producing Lepidium Species
4. Discussion
4.1. Linking Indehiscent Fruit Development and Enhanced Temperature Stress Tolerance
4.2. Wide Temperature Germination Range in L. appelianum and L. draba: An Adaptation to Extreme Climates
4.3. Distribution Patterns and Invasive Status: Implications for Management of Lepidium Species
4.4. Invasive Plant Traits, Temperature Stress, and the Success of Indehiscent-Fruited Lepidium Species
4.4.1. Germination Ecology and Dormancy Mechanisms as Drivers of Indehiscent-Fruited Lepidium Invasiveness Under Temperature Stress
4.4.2. Indehiscent Fruits in Lepidium Species with Broad Temperature Tolerance Enhance Invasiveness
4.4.3. High Competitiveness in Indehiscent Fruit-Producing Lepidium Species Increase Germination Success Under a Wider Range of Temperature Suggests Invasiveness
4.4.4. Secondary Metabolites Could Promote Germination Success of Indehiscent Fruit-Producing Lepidium Species Under Temperature Stress Suggesting Invasiveness
4.4.5. Long-Distance Dispersal in Indehiscent Fruit-Producing Lepidium Species Could Indicate Invasiveness Under a Warming Climate
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Kurtar, E.S. Modelling the effect of temperature on seed germination in some cucurbits. AJB 2010, 9, 1343–1353. [Google Scholar]
- Kirmizi, S. Dormancy and germination requirements of five species from Brassicaceae. JIST 2017, 7, 27–35. [Google Scholar] [CrossRef]
- Pham, D.H.; Wu, C.A. Seed longevity and germination of the emerging invasive species wavyleaf basketgrass (Oplismenus undulatifolius) under varied light regimes. Invasive Plant Sci. Manag. 2023, 16, 225–232. [Google Scholar] [CrossRef]
- Beckmann, M.; Bruelheide, H.; Erfmeier, A. Germination responses of three grassland species differ between native and invasive origins. Ecol. Res. 2011, 26, 763–771. [Google Scholar] [CrossRef]
- Heidari, Z.; Kamkar, B.; Sinaki, J.M. Influence of temperature on seed germination response of fennel. Adv. Plants Agric. Res. 2014, 1, 207–213. [Google Scholar]
- Weitbrecht, K.; Müller, K.; Leubner-Metzger, G. First off the mark: Early seed germination. J. Exp. Bot. 2011, 62, 3289–3309. [Google Scholar] [CrossRef]
- Channaoui, S.; El Idrissi, I.S.; Mazouz, H.; Nabloussi, A. Reaction of some rapeseed (Brassica napus L.) genotypes to different drought stress levels during germination and seedling growth stages. OCL 2019, 26, 23. [Google Scholar] [CrossRef]
- Lei, Y.H.; Chen, Z.P.; Li, Y.; Yu, Y.; Zhang, F.G.; Zhou, K.J.; Zhu, Z.H. Combined influence of low temperatureand drought on different varieties of rapeseed (Brassica napus L.). S. Afr. J. Bot. 2022, 147, 400–414. [Google Scholar]
- Chen, A.; Wang, Y.; Sui, X.; Jin, G.; Wang, K.; An, S. Effects of drought and temperature on the germination of seeds of Seriphidium transiliense, a desert xerophytic subshrub of Xinjiang, China. SST 2020, 48, 355–365. [Google Scholar] [CrossRef]
- Bhatt, A.; Chen, X.; Pompelli, M.F.; Jamal, A.; Mancinelli, R.; Radicetti, E. Characterizationof invasiveness, thermo tolerance and light requirement of nine invasive species in China. Plants 2023, 12, 1192. [Google Scholar] [CrossRef] [PubMed]
- Wen, B. Effects of high temperature and water stress on seed germination of the invasive species Mexican Sunflower. PLoS ONE 2015, 10, e0141567. [Google Scholar] [CrossRef] [PubMed]
- Al-Shehbaz, I.A. Lepidostemon (Brassicaceae) is no longer monotypic. Novon 2000, 10, 329–333. [Google Scholar] [CrossRef]
- Darbyshire, S.J.; Favreau, M.; Murray, M. Common and Scientific Names of Weeds in Canada; Publication 1397/B; Agriculture and Agri-Food Canada: Ottawa, ON, Canada, 2000; p. 132. [Google Scholar]
- Darbyshire, S.J. Inventory of Canadian Agricultural Weeds; Agriculture and Agri-Food Canada: Ottawa, ON, Canada, 2003; p. 396. [Google Scholar]
- Geleta, M.; Gustafsson, C.; Glaubitz, J.C.; Ortiz, R. High-density genetic linkage mappingof Lepidium based on genotyping-by-sequencing SNPs and segregating contig tag haplotypes. Front. Plant Sci. 2020, 11, 448. [Google Scholar] [CrossRef]
- Czerepanov, S.K. Vascular Plants of Russia and Adjacent States; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Francis, A.; Warwick, S.I. The biology of Canadian weeds. 3. Lepidium draba L., L. chalepense L., and L. appelianum Al-Shehbaz (updated). Can. J. Plant Sci. 2008, 88, 379–401. [Google Scholar]
- Mulligan, G.A.; Findlay, J.N. The biology of Canadian weeds. 3. Cardaria draba, C. chalepensis, and C. pubescens. Can. J. Plant Sci. 1974, 54, 149–160. [Google Scholar]
- Gaskin, J.F.; Zhang, D.; Bon, M.C. Invasion of Lepidium draba (Brassicaceae) in the western United States: Distributions and origins of chloroplast DNA haplotypes. Mol. Ecol. 2005, 14, 2331–2341. [Google Scholar] [CrossRef]
- Gaskin, J.F. Clonal structure of invasive hoary cress (Lepidium draba) infestations. Weed Sci. 2006, 54, 428–434. [Google Scholar] [CrossRef]
- Mulligan, G.A.; Frankton, C. Taxonomy of the genus Cardaria with particular reference to the species introduced into North America. Can. J. Bot. 1962, 40, 1411–1425. [Google Scholar] [CrossRef]
- Ball, P.W. Cardaria. In Flora Europeae; Tutin, T.G., Heywood, V.H., Burges, N.A., Moore, D.M., Valentine, D.H., Walters, S.M., Webb, D.A., Eds.; Cambridge University Press: Cambridge, UK, 1964; p. 333. [Google Scholar]
- Goodwin, K. Biology, Ecology and Management of Whitetop (Cardaria spp.); Montana State University, MSU Extension: Bozeman, MT, USA, 2003. [Google Scholar]
- Hinz, H.L.; Cristofaro, M. Prospects for the Biological Control of Perennial Weeds; A Joint Report Prepared by CABI Bioscience Switzerland Centre and BBCA; CABI Bioscience Switzerland Centre and BBCA: Rome, Italy, 2005; p. 23. [Google Scholar]
- Scurfield, G. Cardaria draba (L.) Desv. (Lepidium draba L.). J. Ecol. 1962, 50, 489–499. [Google Scholar] [CrossRef]
- USDA. Hoary Cress Consortium; USDA ARS Northern Plains Agricultural Research Laboratory: Sidney, Australia, 2002.
- USDA. The Plants Database; National Plant Data Center: Baton Rouge, LA, USA, 2004.
- Rice, P. Invaders Database System. 2017. Available online: https://invader.dbs.umt.edu/ (accessed on 8 February 2025).
- Schroeder, F.G. Lehrbuch der Pflanzengeographie; Quelle and Meyer: Wiesbaden, Germany, 1998. [Google Scholar]
- Partzsch, M. Zur Keimungsbiologie acht ausgewählter kurzlebiger Ruderal und Segetalarten. Hercynia NF 2010, 43, 149–166. [Google Scholar]
- Al-Shehbaz, I.A. The genera of Lepidieae (Cruciferae; Brassicaceae), the southeastern United States. J. Arnold Arbor. 1986, 67, 265–311. [Google Scholar] [CrossRef]
- Mummenhoff, K.; Franzke, A. Gone with the bird: Late tertiary and quaternary intercontinental long-distance dispersal and allopolyploidization in plants. Syst. Biodivers. 2007, 5, 255–260. [Google Scholar] [CrossRef]
- Mohammed, S.; Turečková, V.; Tarkowská, D.; Strnad, M.; Mummenhoff, K.; Leubner-Metzger, G. Pericarp-mediated chemical dormancy controls the fruit germination of the invasive hoary cress (Lepidium draba), but not of hairy whitetop (Lepidium appelianum). Weed Sci. 2019, 67, 560–571. [Google Scholar] [CrossRef]
- Mohammed, S.; Bhattacharya, S.; Gesing, M.A.; Klupsch, K.; Theißen, G.; Mummenhoff, K.; Müller, C. Morphologically and physiologically diverse fruits of two Lepidium species differ in allocation of glucosinolates into immature and mature seed and pericarp. PLoS ONE 2020, 15, e0227528. [Google Scholar] [CrossRef]
- Mohammed, S.; Mummenhoff, K. Ecological Adaptations for Success: Indehiscence Fruits Promote Drought-Tolerant Germination in Invasive Lepidium Species. SSRN 2025. [Google Scholar] [CrossRef]
- Mohammed, S.; Mummenhoff, K. Functional divergence exists in mucilage-mediated seed dispersal, but not in germination of myxospermic Lepidium campestre and Lepidium draba (Brassicaceae). Acta Oecol. 2024, 125, 104042. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography and Evolution of Dormancy and Germination; Academic Press: San Diego, CA, USA, 2014. [Google Scholar]
- Lu, J.J.; Tan, D.Y.; Baskin, J.M.; Baskin, C.C. Fruit and seed heteromorphism in the cold desert annual ephemeral Diptychocarpus strictus (Brassicaceae) and possible adaptive significance. Ann. Bot. 2010, 105, 999–1014. [Google Scholar] [CrossRef]
- Sun, Y.; Tan, D.Y.; Baskin, C.C.; Baskin, J.M. Role of mucilage in seed dispersal and germination of the annual ephemeral Alyssum minus (Brassicaceae). Aust. J. Bot. 2012, 60, 439–449. [Google Scholar] [CrossRef]
- Mamut, J.; Tan, D.Y.; Baskin, C.C.; Baskin, J.M. Role of trichomes and pericarp in the seed biology of the desert annual Lachnoloma lehmannii (Brassicaceae). Ecol. Res. 2014, 29, 33–44. [Google Scholar] [CrossRef]
- Tang, A.J.; Tian, M.H.; Long, C.L. Dormancy and germination in short-lived Lepidium perfoliatum L. (Brassicaceae) seeds. Pak. J. Bot. 2010, 42, 201–211. [Google Scholar]
- Zhou, Y.M.; Lu, J.J.; Tan, D.Y.; Baskin, C.C.; Baskin, J.M. Seed germination ecology of the colddesert annual Isatisviolascens (Brassicaceae): Two levels of physiologicaldormancy and role of the pericarp. PLoS ONE 2015, 10, e0140983. [Google Scholar] [CrossRef] [PubMed]
- Soltani, A.; Robertson, M.; Torabi, B.; Yousefi-Daz, M.; Sarparast, R. Modelling seedling emergence in chickpea as influenced by temperature and sowing depth. Agric.For. Meteorol. 2006, 138, 156–167. [Google Scholar] [CrossRef]
- Alvarado, V.; Bradford, K.J. A hydrothermal time model explains the cardinal temperatures for seed germination. Plant Cell Environ. 2022, 25, 1061–1069. [Google Scholar] [CrossRef]
- Mummenhoff, K.; Polster, A.; Mühlhausen, A.; Theißen, G. Lepidium as a model systemfor studying the evolution of fruit development in Brassicaceae. J. Exp. Bot. 2009, 60, 1503–1513. [Google Scholar] [CrossRef]
- Mühlhausen, A.; Lenser, T.; Mummenhoff, K.; Theißen, G. Evidence that an evolutionary transition from dehiscent to indehiscent fruits in Lepidium (Brassicaceae) was caused by a change in the control of valve margin identity genes. Plant J. 2013, 73, 824–835. [Google Scholar] [CrossRef]
- Cousens, R.D.; Young, K.R.; Tadayyon, A. The role of the persistent fruit wall in seed water regulation in Raphanus raphanistrum (Brassicaceae). Ann. Bot. 2010, 105, 101–108. [Google Scholar] [CrossRef]
- Montana Natural Heritage Program. Lepidium campestre (Field Pepper-Grass) Predicted Suitable Habitat Model; Montana Natural Heritage Program: Helena, MT, USA, 2024; p. 17. [Google Scholar]
- Francis, A.; Lujan-Toro, B.E.; Warwick, S.I.; Macklin, J.A.; Martin, S.L. Update on the Brassicaceae species checklist. Biodivers. Data. J. 2021, 9, e58773. [Google Scholar] [CrossRef]
- Mulligan, G.A. Weedy introduced mustards (Brassicaceae) of Canada. Can. Field-Nat. 2002, 116, 623–631. [Google Scholar] [CrossRef]
- Al-Shehbaz, I.A.; Mummenhoff, K.; Appel, O. Cardaria, Coronopus, and Stroganowia are united with Lepidium (Brassicaceae). Novon 2002, 12, 5–11. [Google Scholar] [CrossRef]
- Jalas, J.; Suominen, J.; Lampinen, R. (Eds.) Atlas Florae Europaeae distribution of vascular plants in Europe. Vol. 11: Cruciferae (Ricotia to Raphanus); Helsinki University Printing House: Helsinki, Finland, 1996; p. 310. [Google Scholar]
- Skinner, K.; Smith, L.; Rice, P. Using noxious weed lists to prioritize targets for developing weed management strategies. Weed Sci. 2000, 48, 640–644. [Google Scholar] [CrossRef]
- Invaders Database System. Noxious Weeds in the US and Canada; University of Montana: Missoula, MT, USA, 2006. [Google Scholar]
- Mulligan, G.A. Chromosome numbers determined from Canadian and Alaskan material of native and naturalized mustards, Brassicaceae (Cruciferae). Can. Field-Nat. 2002, 116, 611–622. [Google Scholar] [CrossRef]
- Baker, H.G. The evolution of weeds. Annu. Rev. Ecol. Syst. 1974, 5, 1–24. [Google Scholar] [CrossRef]
- Daehler, C.C. Performance comparisons of co-occurring native and alien invasive plants: Implications for conservation and restoration. Ann. Rev. Ecol. Evol. Syst. 2003, 34, 183–211. [Google Scholar] [CrossRef]
- Hellmann, J.J.; Byers, J.E.; Bierwagen, B.G.; Dukes, J.S. Five potential consequences of climate change for invasive species. Biol. Conserv. 2008, 22, 534–543. [Google Scholar] [CrossRef]
- Whitney, K.D.; Gabler, C.A. Rapid evolution in introduced species, ‘invasive traits’ and recipient communities: Challenges for predicting invasive potential. Divers. Distrib. 2008, 14, 569–580. [Google Scholar] [CrossRef]
- van Kleunen, M.; Dawson, W.; Schlaepfer, D.; Jeschke, J.M. Are invaders different? A conceptual framework of comparative approaches for assessing determinants of invasiveness. Ecol. Lett. 2010, 13, 947–958. [Google Scholar] [CrossRef]
- Clements, D.R.; DiTommaso, A. Climate change and weed adaptation: Can evolution of invasive plants lead to greater range expansion than forecasted? Weed Res. 2011, 51, 227–240. [Google Scholar] [CrossRef]
- Clements, D.R.; DiTommaso, A. Predicting weed invasion in Canada under climate change: Evaluating evolutionary potential. Can. J. Plant Sci. 2012, 92, 1013–1020. [Google Scholar] [CrossRef]
- Wolkovich, E.M.; Cleland, E.E. Phenological niches and the future of invaded ecosystems with climate change. AoB Plants 2014, 31, plu013. [Google Scholar] [CrossRef]
- Walck, J.L.; Hidayati, S.; Dixon, K.W.; Thompson, K.; Poschlod, P. Climate change and plant regeneration from seed. Glob. Change Biol. 2011, 17, 2145–2161. [Google Scholar] [CrossRef]
- Moravcova, L.; Pyšek, P.; Pergl, J.; Perglova, I.; Jarošík, V. Seasonal pattern of germination and seed longevity in the invasive species Heracleum mantegazzianum. Preslia 2006, 78, 287–301. [Google Scholar]
- Gioria, M.; Pyšek, P. Early bird catches the worm: Germination as a critical step in plant invasion. Biol. Invasions 2017, 19, 1055–1080. [Google Scholar] [CrossRef]
- Cunze, S.; Leiblein, M.C.; Tackenberg, O. Range expansion of Ambrosia artemisiifolia in Europe is promoted by climate change. Int. Sch. Res. 2013, 2013, 610126. [Google Scholar] [CrossRef]
- Allen, J.M.; Bradley, B.M. Out of the weeds? Reduced plant invasion risk with climate change in the continental United States. Biol. Conserv. 2016, 203, 306–312. [Google Scholar] [CrossRef]
- Panda, R.M.; Behera, M.D.; Roy, P.S. Assessing distributions of two invasive species of contrasting habits in future climate. J. Environ. Manage. 2018, 213, 478–488. [Google Scholar] [CrossRef]
- Bennett, A.E.; Thomsen, M.; Strauss, S.Y. Multiple mechanisms enable invasive speciesto suppress native species. Am. J. Bot. 2011, 98, 1086–1094. [Google Scholar] [CrossRef]
- Allstadt, A.; Caraco, T.; Molnár, F.; Korniss, G. Interference competition and invasion: Spatial structure, novel weapons and resistance zones. J. Theor. Biol. 2012, 306, 46–60. [Google Scholar] [CrossRef]
- Gioria, M.; Osborne, B.A. Resource competition in plant invasions: Emerging patterns and research needs. Front. Plant Sci. 2014, 5, 501. [Google Scholar] [CrossRef]
- Le Louarn, M.; Couillens, B.; Deschamps-Cottin, M.; Clergeau, P. Interference competition between an invasive parakeet and native bird species at feeding sites. J. Ethol. 2016, 34, 291–298. [Google Scholar] [CrossRef]
- Weidlich, E.W.A.; Flórido, F.G.; Sorrini, T.B.; Brancalion, P.H.S. Controlling invasive plant species in ecological restoration: A global review. J. Appl. Ecol. 2020, 57, 1806–1817. [Google Scholar] [CrossRef]
- Salehin, M.; Li, B.; Tang, M.; Katz, E.; Song, L.; Ecker, J.R.; Kliebenstein, D.J.; Estelle, M. Auxin sensitiveAux/IAA proteins mediate drought tolerance in Arabidopsis by regulating glucosinolate levels. Nat. Commun. 2019, 10, 4021. [Google Scholar] [CrossRef] [PubMed]
- Eom, S.H.; Baek, S.A.; Kim, J.K.; Hyun, T.K. Transcriptome analysis in chinese cabbage (Brassica rapa ssp. Pekinensis) provides the role of glucosinolate metabolism in response to drought stress. Molecules 2018, 23, 1186. [Google Scholar] [PubMed]
- Kiemnec, G.L.; McInnis, M.L. Hoary cress (Cardaria draba) root extract reduces germination and root growth of five plant species. Weed Technol. 2002, 16, 231–234. [Google Scholar] [CrossRef]
- Mack, R.N.; Simberloff, D.; Lonsdale, W.M.; Evans, H.; Clout, M.; Bazzaz, F.A. Biotic invasions: Causes, epidemiology, global consequences, and control. Ecol. Appl. 2000, 10, 689–710. [Google Scholar] [CrossRef]
- Pejchar, L.; Mooney, H.A. Invasive species, ecosystem services and human well-being. Trends Ecol. Evol. 2009, 24, 497–504. [Google Scholar] [CrossRef]
- Blois, J.L.; Zarnetske, P.L.; Fitzpatrick, M.C.; Finnegan, S. Climate change and the past, present, and future of biotic interactions. Science 2013, 341, 499–504. [Google Scholar] [CrossRef]
- Tilman, D.; Lehman, C. Human-caused environmental change: Impacts on plant diversity and evolution. Proc. Natl. Acad. Sci. USA 2001, 98, 5433–5440. [Google Scholar] [CrossRef]
- Mohammed, S.; Steinbrecher, T.; Leubner-Metzger, G.; Mummenhoff, K. Differential Primary Seed and Fruit Dispersal Mechanisms and Dispersal Biomechanics in Invasive Dehiscent and Indehiscent-Fruited Lepidium Species. Plants 2025, 14, 446. [Google Scholar] [CrossRef]
- Thiede, D.A.; Augspurger, C.K. Intraspecific variation in seed dispersion of Lepidium campestre (Barassicaceae). Am. J. Bot. 1996, 83, 856–866. [Google Scholar] [CrossRef]


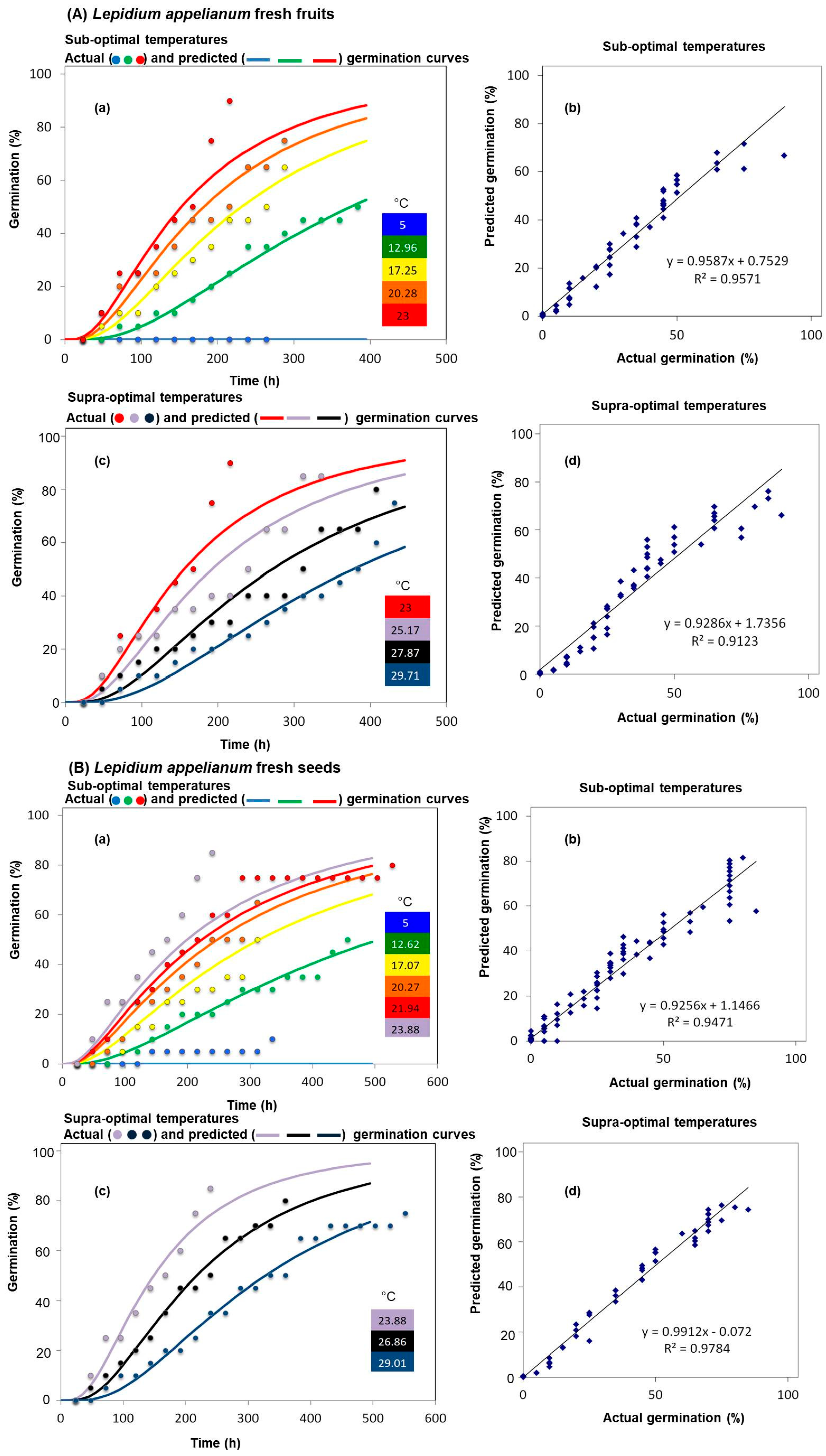
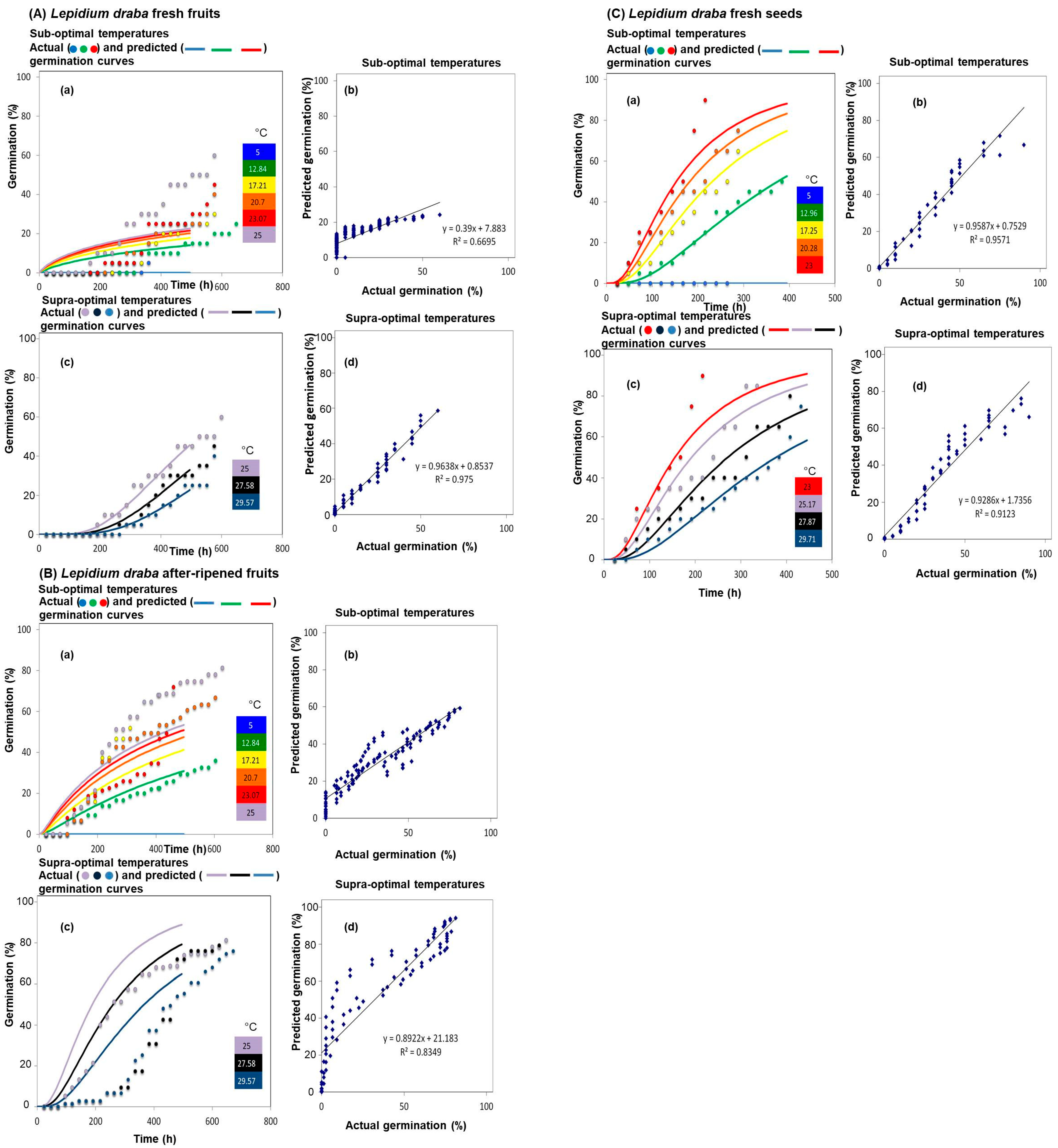

| Fruit Types | Species | Diaspore Types | Sub-Optimal Temperatures | Supra-Optimal Temperatures | ||
|---|---|---|---|---|---|---|
| Tb | (σTb) | Tc(50) | (σTc) | |||
| Indehiscent fruits | Lepidium appelianum | Fresh fruits | 1.0 | 0.35 | 39.8 | 0.34 |
| Fresh seeds | 2.5 | 0.33 | 35 | 0.32 | ||
| Lepidium draba | Fresh fruits | 5.3 | 1.28 | 33.8 | 0.25 | |
| After-ripened fruits | 4.0 | 1.0 | 34.4 | 0.34 | ||
| Fresh seeds | 5.3 | 0.25 | 34.4 | 0.32 | ||
| Dehiscent fruits | Lepidium campestre | Fresh seeds | 6.0 | 0.26 | 27.4 | 1.8 |
| After-ripened seeds | 5.8 | 0.19 | 33.3 | 0.26 | ||
| Species | Seed/Fruit Type | Temperature (°C) | Germination (%) Mean ± S.E. |
|---|---|---|---|
| Lepidium appelianum | Fresh fruits | 5.00 | 0 ± 0.0 b |
| 12.96 | 50 ± 2.8 c | ||
| 17.25 | 65 ± 2.8 cd | ||
| 20.28 | 75 ± 5.7 d | ||
| 23.00 | 88 ± 1.6 a | ||
| 25.17 | 90 ± 2.7 a | ||
| 27.87 | 80 ± 5.7 ad | ||
| 29.71 | 75 ± 2.8 ad | ||
| Fresh seeds | 5.00 | 10.5 ± 2.4 b | |
| 12.62 | 50 ± 2.7 c | ||
| 17.07 | 65 ± 5.6 ac | ||
| 20.27 | 75 ± 5.4 ad | ||
| 21.94 | 85 ± 2.8 ad | ||
| 23.88 | 91 ± 5.7 d | ||
| 26.86 | 85 ± 5.4 d | ||
| 29.01 | 80 ± 2.5 ad | ||
| Lepidium draba | Fresh fruits | 5.00 | 4 ± 0.5 b |
| 12.84 | 25 ± 2.6 a | ||
| 17.21 | 35 ± 2.4 ac | ||
| 20.70 | 40 ± 2.3 ac | ||
| 23.07 | 45 ± 2.5 c | ||
| 25.00 | 60 ± 5.4 cd | ||
| 27.58 | 45 ± 5.5 cd | ||
| 29.57 | 40 ± 5.3 acd | ||
| After-ripened fruits | 5.00 | 0 ± 0.0 b | |
| 12.84 | 36 ± 5.6 c | ||
| 17.21 | 52 ± 5.4 ac | ||
| 20.70 | 66 ± 3.7 a | ||
| 23.07 | 72 ± 4.0 ad | ||
| 25.00 | 81 ± 3.1 d | ||
| 27.58 | 78 ± 5.0 d | ||
| 29.57 | 76 ± 3.4 d | ||
| Fresh seeds | 5.00 | 0 ± 0.0 | |
| 12.96 | 50 ± 2.3 b | ||
| 17.25 | 65 ± 2.7 cd | ||
| 20.28 | 78 ± 4.4 a | ||
| 23.00 | 91 ± 1.0 e | ||
| 25.17 | 85 ± 2.8 ae | ||
| 27.87 | 80 ± 2.4 ae | ||
| 29.71 | 75 ± 1.7 ad | ||
| Lepidium campestre | Fresh seeds | 5.00 | 2.6 ± 0.3 b |
| 9.00 | 41 ± 1.6 c | ||
| 16.00 | 57 ± 1.4 d | ||
| 19.00 | 37 ± 1.4 c | ||
| 22.00 | 31 ± 1.0 e | ||
| 25.00 | 21 ± 1.6 a | ||
| 27.00 | 19 ± 0.8 a | ||
| 30.00 | 17 ± 1.4 a | ||
| After-ripened seeds | 5.00 | 20 ± 2.8 b | |
| 12.05 | 50 ± 5.5 c | ||
| 14.65 | 90 ± 2.6 ae | ||
| 18.44 | 100 ± 0.0 ae | ||
| 22.68 | 85 ± 0.0 ae | ||
| 25.13 | 80 ± 2.7 a | ||
| 27.67 | 75 ± 2.7 ac | ||
| 29.98 | 60 ± 5.5 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, S.; Mummenhoff, K. Germination Under Temperature Stress Facilitates Invasion in Indehiscent Lepidium Species. Agriculture 2025, 15, 1078. https://doi.org/10.3390/agriculture15101078
Mohammed S, Mummenhoff K. Germination Under Temperature Stress Facilitates Invasion in Indehiscent Lepidium Species. Agriculture. 2025; 15(10):1078. https://doi.org/10.3390/agriculture15101078
Chicago/Turabian StyleMohammed, Said, and Klaus Mummenhoff. 2025. "Germination Under Temperature Stress Facilitates Invasion in Indehiscent Lepidium Species" Agriculture 15, no. 10: 1078. https://doi.org/10.3390/agriculture15101078
APA StyleMohammed, S., & Mummenhoff, K. (2025). Germination Under Temperature Stress Facilitates Invasion in Indehiscent Lepidium Species. Agriculture, 15(10), 1078. https://doi.org/10.3390/agriculture15101078







