Abstract
Cowpea (Vigna unguiculata (L.) Walp.) is a vital legume crop recognized for its nutritional value and adaptability to various growing conditions. However, exposure of cowpea to drought stress during the early growth stages can significantly restrict growth and yield potential. Therefore, identifying cowpea genotypes tolerant to drought during early growth and development is essential for maintaining yield potential. This study characterized 15 diverse cowpea genotypes for various physiological, pigment, and morphological traits that may contribute to drought tolerance. At the V2 stage, the cowpea genotypes were subjected to two moisture regimes: control (100% irrigation) and drought (50% irrigation) for 22 days to assess trait responses and their relationship to drought tolerance. Drought-stressed plants decreased stomatal conductance by 79%, negatively correlating with a 2.9 °C increase in canopy temperature. Under drought, the photochemical reflectance index (PRI) was strongly associated with the quantum yield of PSII and electron transport rate. Shoot biomass decreased by 51% and root biomass by 32% under drought. Leaf area and shoot weight were correlated with root traits such as total length, surface area, and weight. Among all genotypes, 280785-11 and UCR 1004 demonstrated superior rooting vigor under drought, emphasizing their efficiency in resource utilization. These findings highlight the relevance of utilizing drought-adaptive traits to improve early-season drought tolerance.
1. Introduction
Various anthropogenic activities and an increase in extreme events like drought have significantly transformed crop-growing environments [1,2]. Drought caused over USD 37 billion in crop losses globally between 2008 and 2018 [3], while the United States (US) alone suffered an economic loss of USD 5.4 billion in 2024 [4]. Agriculture consumes more than 87% of global freshwater resources [5], and with a 50–60% increase in projected food demand by 2050 [6], the pressure on water resources is expected to intensify. Consequently, agricultural water demand is projected to double, and freshwater availability may drop by 50% in the next two decades [7,8]. Therefore, there is a growing need to look for crops that maintain or improve yields without compromising nutritional quality under these conditions. Legumes are a promising option among such crops due to their high protein content and adaptability to harsh environments [9]. Among legumes, cowpea is one of the versatile crops grown in the tropics and subtropics. It contains over 25% protein, 2.2% lipids composed of 70.7% unsaturated fatty acids, vitamin B (57–150% of the daily adult requirement per 100 g), and essential minerals like phosphorus, magnesium, iron, copper, and manganese (41–76% of daily requirements per 100 g) [10,11]. With the shift toward plant-based proteins, demand for cowpeas is expected to rise [12]. It also serves as fodder for livestock and aids in soil health enhancement, benefiting over 200 million farmers globally [13]. In the US, cowpea is grown in the Southern and Western regions due to its adaptability to poor soils [14]. However, cowpea growing regions are generally rainfed and experience substantial water-deficit conditions during crucial growth phases, restricting potential yields [15,16]. Although cowpeas are relatively tolerant to stress, high-yielding varieties that perform well under optimal irrigation are sensitive to drought stress, highlighting the need to enhance drought resilience [17].
Among the critical growth phases, the early vegetative growth stage is vital for establishing root systems and nodule formation [18,19]. Drought stress during this critical period can impair physiological functions and morphological development, negatively impacting yield and yield-attributing traits [20,21]. Roots are the first to perceive drought and synthesize the abscisic acid (ABA) hormone, sending a signal to guard cells for stomatal closure to reduce water loss through the canopy [22]. Studies show that cowpea rapidly adapts by declining in stomatal conductance under drought [20,21,23]. It was also observed that this stomatal limitation results in reduced transpirational cooling, leading to higher canopy temperatures, lower intercellular CO2 concentration, and thereby restricts assimilation [20,24].
Furthermore, these physiological changes cause oxidative stress due to an increase in reactive oxygen species (ROS) [25]. These short-term stress-induced changes damage chloroplasts and photosynthetic pigments in crops [26,27]. Eventually, the combination of physiology and biophysical changes at the cell level further extends to the leaf and whole-plant levels, resulting in decreased leaf numbers, leaf area, node numbers, plant height, and biomass, thereby negatively affecting growth and yield [28,29]. Further, advancements in non-invasive plant phenotyping using sensing offer greater advantages for quantifying physiological and biophysical changes in crops under stress [30]. Reflectance indices are used to understand plant health and pigment changes under stress. The photochemical reflectance index (PRI) linked to xanthophyll cycle activity decreased under drought in crops [31,32,33]. Chlorophyll and carotenoids indicate plant stress, with chlorophyll essential for light harvesting and carotenoids quenching reactive oxygen species (ROS) and acting as antioxidants [34,35,36]. Studies have shown a decline in chlorophyll index due to oxidative damage under drought [37,38], while carotenoid levels increase to protect the photosynthetic system [39]. Despite multiple efforts to use sensing tools to study stress-induced changes in leaf properties and plant health in legumes, little has been explored in cowpea.
Roots anchor plants, acquire soil resources, and sense environmental stimuli. Their plasticity is key to survival, as roots adapt morphologically to meet water demands at the canopy. Deeper roots with higher root prolificacy and thinner diameter have been shown to improve water uptake, biomass production, and water use efficiency by increasing total resource absorption area, thus increasing yield [40,41,42]. Previous studies on legumes have highlighted that root length, lateral root diameters, and root length density contribute to yield [42,43,44]. The reported genetic variability in root traits of cowpea highlights the opportunity to explore below-ground traits to improve performance under drought stress [45,46]. The early vegetative stage in legumes includes all root classes, with the mature root system evolving from this initial structure [47,48]. Thus, analyzing root traits at the early vegetative or nodulation stage provides insights into drought tolerance and resource allocation. This information helps identify genotypes with optimized root and shoot development and higher biomass. Studies in other crops have demonstrated the potential to enhance plants’ ability to withstand prolonged drought by modifying physiological and root traits [49,50]. Moreover, studies suggest water uptake and conservation traits help sustain metabolism and crop growth during drought [51,52]. Among the various traits identified, root characteristics have shown greater importance in maintaining tissue water relations, thus promoting high growth rates. Evidence suggests that genotypes with strong roots and efficient water use during vegetative growth show better drought tolerance during reproductive stages, resulting in higher yields and better pod-filling [52]. While much research has examined drought responses in aboveground traits, root system architecture changes are less studied in cowpea due to difficulties in phenotyping. Our study addresses this gap by integrating morpho-physiological and root architecture traits using diverse cowpea genotypes during the early vegetative stage. Thus, we hypothesize that cowpea genotypes with higher stomatal sensitivity or low canopy temperature and a higher root-to-shoot ratio facilitate maintaining higher growth rates or biomass. The specific objectives of this study are to (i) characterize morpho-physiological and spectral trait responses of cowpea genotypes under drought stress, (ii) assess the variability in root traits of cowpea genotypes, and (iii) identify cowpea genotypes with superior physiology and root traits under drought.
2. Materials and Methods
Fifteen genetically diverse cowpea genotypes, including cultivars, accessions, and breeding lines obtained from the USDA Germplasm Resource Information Network (GRIN), along with a check cultivar (Mississippi Pinkeye Purple Hull) from a local seed supplier (HOSS, Norman Park, GA, USA) were used in the study. These genotypes were selected based on their maturity of approximately 70 days (Table S1). The experiment was conducted in a greenhouse facility equipped with ventilated flaps and cooling pads to maintain controlled microclimatic conditions at the R.R. Foil Plant Science Center (33°28′9.96132″ N 88°47′3.13548″ W), Mississippi State University. The average day and night temperatures were maintained at 30.4 °C and 24 °C, respectively, while relative humidity inside the greenhouse was 79.8% throughout the experiment period.
2.1. Stress Treatment
Three uniform seeds were sown in each pot (40 cm height; 8 cm width) filled with a mixture of soil and sand in a 1:1 ratio by volume. After 7 days of emergence, one seedling per pot was maintained. 2 g of a slow-release Osmocote fertilizer (14:14:14 N:P:K; Specialty Fertilizers, Dublin, OH, USA) was added to the top layer of soil mixture before sowing, and an additional 2 g was applied 10 days after emergence to ensure optimal soil fertility. A total of 150 healthy plants (15 genotypes × 2 treatments × 5 replications) were grown under optimal moisture conditions. The plants were sprayed twice with a Garden Safe organic insecticide (Sarasota Green Group, Sarasota, FL, USA) to prevent the infestation of sucking pests and ants. At the V2 stage, the pots were divided into two batches (Figure 1). One batch was maintained at a volumetric water content (VWC) of ~0.11 m3 m−3, representing 100% irrigation (control, CNT). The other batch was kept at ~50% VWC of the control (drought stress, DS) for 22 days (Figure S1). Pots were watered manually using a measuring cup to maintain the desired soil moisture based on VWC across treatments. Daily soil moisture levels were monitored by recording VWC at 10 cm using a handheld moisture meter (ML3 ThetaKit; Delta-T Devices, Cambridge, UK) before irrigation. Each pot was measured three times, and the target VWC was maintained.

Figure 1.
Cowpea plants under control (red labels) and drought (white labels) conditions during the early vegetative stage.
2.2. Data Collection
2.2.1. Leaf Physiology, Pigments, and Spectral Reflectance Properties
All non-destructive measurements were taken on the same fully expanded topmost third trifoliate leaf. If the third trifoliate leaf was absent, measurements were taken from the upper fully expanded leaflet. After 14 days of DS, stomatal conductance (gs), transpiration rate (E), electron transport rate (ETR), and quantum efficiencies of photosystem II (PhiPS2) were recorded using a handheld LI-600 porometer (LI-COR Biosciences, Lincoln, NE, USA). Leaf pigment properties, such as the chlorophyll index (Chl) and nitrogen–flavonol index (NFI), were measured using MPM-100 (Opti-Sciences Inc., Hudson, NH, USA). All the physiological measurements were taken during the sunny day between 09:30 A.M. and 12:00 P.M. Additionally, variation in the effect of irrigation treatments on mid-day canopy temperatures (Ctemp) of cowpea genotypes was recorded using an infrared radiometer (Apogee Instruments Inc., Logan, UT, USA) between 1:00 P.M. and 2:00 P.M. The leaf-level spectral reflectance was recorded using Polypen RP410 (Photon Systems Instruments, Drásov, Czech Republic). The indices used in the study based on the spectral data were calculated using the following formulas (Table 1).

Table 1.
Reflectance/transmittance indices used in the study.
2.2.2. Aboveground Traits
At the end of the stress period (22 days after stress imposition), the shoot was cut at the root-to-shoot junction to record shoot length (SL), node number (NN), and leaf number (LN). Leaves were separated from the stems of each plant and scanned using an LI-3100C leaf area meter (LI-COR Biosciences, Lincoln, NE, USA) to record the total leaf area. Each plant’s leaves and stem parts were placed in the brown paper bag and dried at 70 °C for 72 h to obtain leaf weight (LW) and stem weight (StemW). The total shoot biomass was estimated as a summation of leaf and stem biomass per plant. Finally, the specific leaf area (SLA, cm2/g) was calculated as a ratio of leaf area to leaf dry weight.
2.2.3. Root Morphology
The pots were transferred to the root-washing area, rinsed, and washed to extract the root system. The harvested roots were washed with fresh water to eliminate residual soil before being scanned on a custom-designed plexiglass tray (40 cm × 30 cm × 2 cm). Scanning was performed using the EPSON Expression 13000 XL scanner (Epson America, Los Alamitos, CA, USA) at a resolution of 600 DPI with its proprietary EPSON Scan 2 software. In brief, roots were arranged in the tray, filled with 2 cm of water, and spread evenly using a paintbrush to ensure that all root structures were visible for imaging. Scanned images were processed with RhizoVision Explorer v2.0.3 (https://www.rhizovision.com/, accessed on 31 December 2024), utilizing appropriate image thresholding to set pixel intensity [56]. Non-root objects were filtered out, and root pruning was applied based on visual observations within the software to minimize analytical errors. The RhizoVision Explorer tool was used to extract root morphological traits, including total root length (TRL), root surface area (RSA), root volume (RV), and average root diameter (RD). The roots were further classified into three groups based on their diameter (D) range: fine roots (diameter < 0.3 mm) were classified as D1, medium roots (0.3–3 mm) as D2, and relatively larger roots (>3 mm) as D3. Root length (RL) and root surface area (RSA) were quantified for each root group using image analysis software, yielding metrics such as RLD1 and RSAD1 for the fine root group, with similar metrics obtained for the other groups. After scanning, roots were placed on paper towels to absorb excess moisture before being oven-dried at 70 °C for 72 h. Finally, root dry weight (RW) was recorded, and the root-to-shoot ratio (RW/SW) was calculated to estimate stress-induced variability in root traits.
2.2.4. Statistical Analysis
A split-plot randomized complete block design was utilized to set up the experiment, with moisture treatment as the main plot and genotypes as the subplot. ANOVA was performed using the “doebioresearch” package in RStudio (v.2023.12.0) to assess the impact of irrigation treatments on various attributes. The least significant difference (LSD) test was used for mean separation between the treatments. All graphs and visualizations were generated using R Studio (version 2024.12.0) and SigmaPlot 14.5 (Systat Software, San Jose, CA, USA). Principal component analysis (PCA) was performed with the “factoextra” package, and results were illustrated using the “ggplot2” package. The stress tolerance index (STI) was calculated by following the formula [57].
where
- Ys represents the phenotypic mean of a given genotype under drought for a given trait;
- Yc represents the phenotypic mean of a given genotype under control for a given trait;
- Xc represents the mean of all genotypes under control for a given trait.
3. Results
3.1. Physiological Traits
A significant effect of treatment (p < 0.01) and genotype (p < 0.05) was observed for gs and E, with no significant interaction noted (Table 2). Under control, the highest gs was recorded in TVu1977 (1.07 mol m−2 s−1) and the lowest in TVu1596 (0.23 mol m−2 s−1) (Figure 2a). On average, exposure of cowpea genotypes to drought reduced gs by 79%, with genotypes Bambey 28 (98%) and TVu 1910 (25%) experiencing the highest and lowest percentage reductions, respectively (Figure 2a). A maximum gs under drought was observed in TVu 1910 (0.31 mol m−2 s−1), while Early Ramshorn Blackeye exhibited the lowest gs (Figure 2a). Likewise, water loss through the canopy or E showed a similar response to gs under control, with TVu 1977 displaying the highest rate, while TVu1596 recorded the lowest rate (Figure 2b). Under DS, IFH 27-8, TVu 1910, and KVu 447 were the top three genotypes having the highest E (>2.59 mmol m−2 s−1), while Early Ramshorn Blackeye and Bambey 28 exhibited the lowest E (<0.36 mmol m−2 s−1). Ctemp was increased by 2.9 °C (p < 0.01) under DS (Table 2). However, IFH 27-8 maintained a canopy temperature similar to control with only a minimum increase of 0.5 °C. In contrast, Bambey 28, with the highest decrease in gs and E, experienced a maximum rise (5.2 °C) in Ctemp compared to the control (34.9 °C) (Figure 2a–c).

Table 2.
Analysis of variance of physiological, spectral, shoot, and root traits of 15 cowpea genotypes under control (CNT) and drought stress (DS).
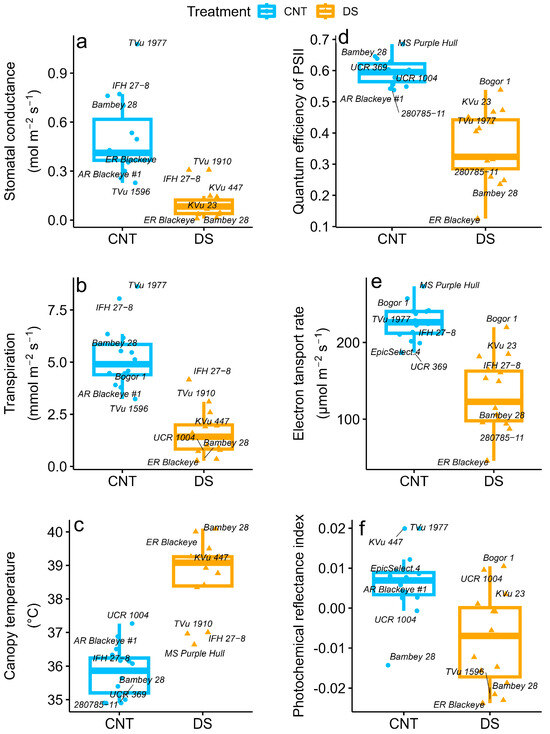
Figure 2.
Variability in (a) stomatal conductance (gs, mol m−2 s−1), (b) transpiration (E, mmol m−2 s−1), (c) canopy temperature (Ctemp, °C), (d) quantum efficiency of photosystem II (PhiPS2), (e) electron transport rate (ETR, μmol m−2 s−1), and (f) photochemical reflectance index (PRI) of 15 cowpea genotypes under control (CNT) and drought stress (DS). The whiskers indicate the interquartile range, and the outer dots are outliers. The middle line represents the mean of the 15 cowpea genotypes, and the box shows the range of the 25th–75th percentiles of the data. Genotype labels in each boxplot indicate the top three and bottom three performers for a given trait.
PhiPS2, the proportion of light absorbed by PSII for photochemistry, varied significantly between treatments (p < 0.01) and among genotypes (p < 0.05; Table 2). On average, drought reduced PhiPS2 by 40.5%. Genotypes Early Ramshorn Blackeye and Bambey 28, with the highest reductions in E, also suffered the highest drops in PhiPS2—78% and 63%, respectively (Figure 2b,d). Another chlorophyll fluorescence parameter, ETR, an indicator of photosynthetic activity that measures the rate of flow of electrons through PSII, was significantly affected by treatment (p < 0.001) and genotypes (p < 0.05; Table 2). On average, ETR decreased by 42% under DS. Genotype Early Ramshorn Blackeye, which suffered the highest reductions in E and PhiPS2, also recorded a 79% decrease in ETR (Figure 2). The photochemical reflectance index (PRI), an optical indicator of light-use efficiency, varied by treatment and genotype (p < 0.05; Table 2). On average, PRI decreased from 0.006 to −0.008, where genotype Bogor 1 and UCR 1004 had the highest PRI values (0.01) (Figure 2e).
3.2. Leaf Pigments and Reflectance
DS affected leaf pigments, chlorophyll index, and the NFI (p < 0.05; Table 2). However, no genotypic and interaction effects were observed for these traits. The chlorophyll index was reduced by 16% under drought (Figure 3a). Among all genotypes, Mississippi Pinkeye Purple Hull, TVu 1910, and 280785-11 exhibited the highest chlorophyll index (≥0.47) under control. Moreover, genotype 280785-11 under DS maintained a relatively high chlorophyll index (0.42), ranking among the top three genotypes (Figure 3a). The NFI was reduced significantly by 31% under drought (Figure 3b). One of the measures of stressed vegetation, CRI1, varied with treatment (p < 0.05) and genotype (p < 0.001) effect (Table 2). On average, CRI1 decreased by 7% under DS, with genotype Bambey 28 showing a 23% reduction. In contrast, genotypes Bogor 1, 280785-11, and Early Ramshorn Blackeye exhibited increased CRI1. TVu1977 maintained the highest CRI1 value under control (5.4) and drought (5.3). A vegetation index that estimates the amount of anthocyanins (ARI1) in leaves increased significantly by 120% under drought (p < 0.01). Among the genotypes, Bogor 1 recorded the lowest ARI1 value (−0.28), while TVu 1596 exhibited the highest value (0.53) under DS. (Figure 3d).
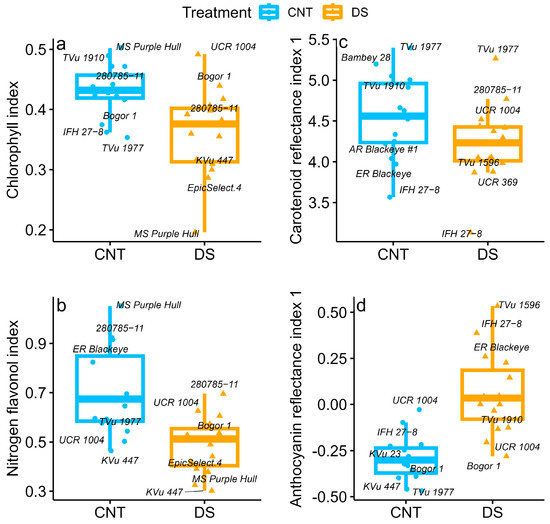
Figure 3.
Boxplots showing the differences in (a) chlorophyll index (Chl), (b) nitrogen–flavonol index (NFI), (c) carotenoid reflectance index 1 (CRI1), and (d) anthocyanin reflectance index 1 (ARI1) of 15 cowpea genotypes under control (CNT) and drought stress (DS). The whiskers indicate the interquartile range, and the outer dots are outliers. The middle line represents the mean of the 15 cowpea genotypes, and the box shows the range of the 25th–75th percentiles of the data. Genotype labels in each boxplot indicate the top three and bottom three performers for a given trait.
3.3. Shoot Traits
DS differentially affected node number, leaf number, leaf area, and specific leaf area, exhibiting varying phenotypic plasticity among cowpea genotypes (Table 2 and Table S2). There was no significant interaction between cowpea genotypes and irrigation treatment for all the morphological traits except SLA (p < 0.05). However, genotypes and irrigation treatment significantly affected NN and LN individually (p < 0.01; Table 2 and Table S2). In the control treatment, genotype TVu1977 had the highest node number of five, while under DS, the same genotype showed three nodes (Table S3). The minimum node reduction was observed in the genotype TVu1910 (13%), whereas Mississippi Pinkeye Purple Hull experienced the highest reduction at 56% under drought (Table S3). DS reduced LN by 29% after 22 days of stress. Genotypes IFH27-8 and Bambey 28 had the highest LN (~4) under drought, while Mississippi Pinkeye Purple Hull experienced the greatest reduction in LN (56%) (Figure 4a). Drought had no significant effect on shoot length (Table S2). However, the genotypes significantly varied in shoot length (p < 0.001). Reduction in nodes and leaves significantly reduced LA by 42% under drought (p < 0.05; Table 2), with no genotype and treatment interaction effect. The LA varied from 86 cm2 in IFH 27-8 to 187 cm2 in Bambey 28 under control and from 53 cm2 in Bogor 1 to 116 cm2 in Arkansas Blackeye #1 under drought (Figure 4b). Further, SLA exhibited a significant increase (29%) under drought (p < 0.01) and also varied with genotype (p < 0.001; Table 2). Early Ramshorn Blackeye had the highest SLA (231 g/cm2) under DS, and EpicSelect.4 showed the lowest reduction (3%) under drought, whereas Mississippi Pinkeye Purple Hull showed the lowest SLA (109 g/cm2) in both treatments (Figure 4c)
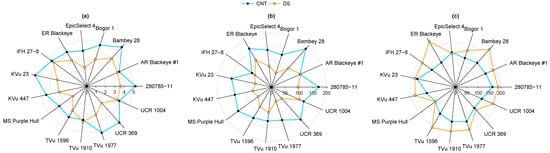
Figure 4.
Radar plot showing the variability in (a) leaf number (LN), (b) leaf area (LA), and (c) specific leaf area (SLA) of 15 cowpea genotypes under control (CNT) and drought stress (DS).
3.4. Shoot and Root Biomass
The root and shoot biomass traits of cowpeas differed significantly under irrigation treatments and genotypes. On average, DS reduced leaf weight by 56% and stem weight by 41% (p < 0.05; Table S2). This reduction in leaf weight and stem weight resulted in a 51% reduction in shoot weight as compared to the control (p < 0.01; Table 2). Genotype 280785-11 maintained the highest aboveground biomass (1.03 g/plant) under DS (Figure 5a). Genotype IFH 27-8, which had the shortest height at both treatments, also exhibited the lowest biomass at 0.90 g/plant under control conditions, while genotype TVu1977 had the lowest shoot biomass (0.45 g/plant) under DS. Significant treatment and genotype effects were observed in RW (p < 0.05 and p < 0.001, respectively; Table 2), with DS reducing the RW by 32% (Figure 5b). Genotypes Early Ramshorn Blackeye, UCR 1004, Arkansas Blackeye #1, and 280785-11 were able to maintain relatively higher RW across the treatments (>0.35 g/plant; Figure 5b). In contrast, genotypes UCR 369 and Bogor 1 exhibited poor root growth (>50% less compared to high root type) under drought. Additionally, exposure to DS increased the root-to-shoot ratio by 42% (Table 2, Figure 5c). However, the genotype and treatment interaction effect were not significant, indicating a consistent response across all genotypes.
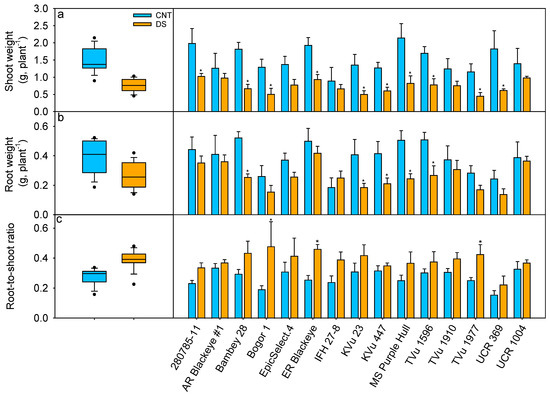
Figure 5.
Impact of drought stress on (a) shoot weight (SW), (b) root weight (RW), and (c) root-to-shoot ratio (RW/SW) during the vegetative stage. Blue represents control (CNT), and orange represents drought stress (DS). * indicates significant differences between treatments within the genotype at p < 0.05.
3.5. Root Traits
Twenty-two days of DS significantly influenced root morphological parameters such as TRL (−12%), RSA (−31%), RD (−20%), and RV (−52%) (Table 2). The TRL varied from 8633 mm (IFH 27-8) to 24,095 mm (Bambey 28) under control and from 9614 mm (UCR 369) to 22,863 mm (280785-11) under drought (Figure 6a). Genotypes, KVu 447 and UCR 369, showed a significant reduction of 42% in TRL under drought. While genotypes 280785-11 and Arkansas Blackeye #1 maintained relatively higher TRL during DS than the control, the differences were not statistically significant. DS led to an average reduction in RD of 20% (p < 0.001; Table 2), with IFH 27-8 exhibiting the highest (0.41 mm) and TVu 1977 the lowest (0.34 mm) under drought (Figure 6b). Further analysis of RD classifications revealed differential responses: root length diameter range 1 (RLD1) increased by 16% (p < 0.05), while RLD2 and RLD3 decreased by 29% (p < 0.01) and 63% (p < 0.05), respectively, compared to controls (Table S2). Under drought, 280785-11 had the highest RLD1 (11,835 mm), whereas UCR 369 had the lowest (4789 mm) (Table S3). Significant treatment and genotype effects (p < 0.01) were noted for RSA. The genotype 280785-11 had the maximum RSA under DS (256 cm2), while UCR 369 had the lowest (109 cm2). KVu 447 showed the highest reduction in RSA (60%) under drought, indicating a greater impact on fine and lateral roots. Among root classes, surface area diameter range 1 (SAD1) increased by 13% (p < 0.05), while SAD2 and SAD3 decreased by 36% (p < 0.001) and 66% (p < 0.01), respectively, highlighting variability in root plasticity (Table S2). 280785-11, with the highest TRL under drought, also had the highest SAD1 (70.46 cm2) and SAD2 (181.56 cm2). In SAD3, Early Ramshorn Blackeye had the highest value (23.2 cm2), while Bogor 1 had the lowest (0.95 cm2) (Table S3). Under DS, RV decreased by 52%, with Early Ramshorn Blackeye showing the highest volume (7.01 cm3) and Bogor 1 the lowest (1.6 cm3), indicating a strong water limitation response (Figure 6d).
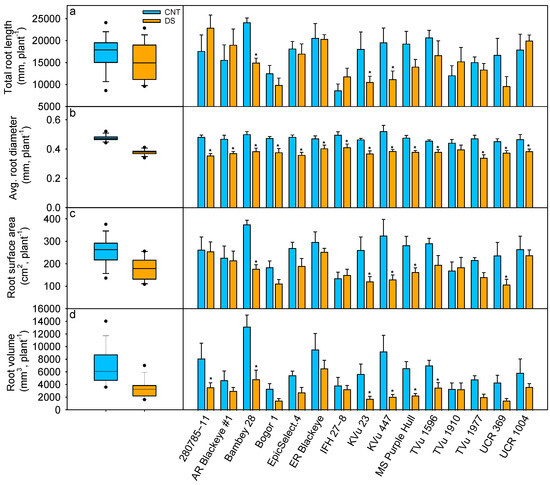
Figure 6.
Effect of drought stress on (a) total root length (TRL), (b) average root diameter (RD), (c) root surface area (RSA), and (d) root volume (RV) on cowpea genotypes. Blue represents control (CNT), and orange represents drought stress (DS). * indicates significant differences between treatments within the genotype at p < 0.05.
3.6. Principal Component Analysis and Drought Stress Tolerance
Biplots based on three principal components (PCs) were created to assess trait variation and genotype responses for control, DS, and STI. PC1 and PC2 cumulatively explained the variation in traits, accounting for 49.7%, 54.9%, and 54.8% of the total variation under control (Figure 7a), drought (Figure 7b), and STI, respectively (Figure 7c). Under control conditions, key traits influencing PC1 included SW, RW, LA, TRL, LW, and RSA, while PC2 was shaped by gs, LN, NN, E, and CRI1. Genotype 280785-11 and Bambey 28 strongly associated with shoot and root traits, indicating robust growth. PC1 indicated positive loadings for most morphological traits, Chl, and NFI, while Ctemp and PRI had negative loadings, reflecting the negative impact of higher canopy temperatures on growth. For PC2, physiological traits (gs, E), NN, and RD had positive loadings, whereas ARI1 and RW/SW were negative, suggesting a trade-off between physiological activity and assimilate allocation.
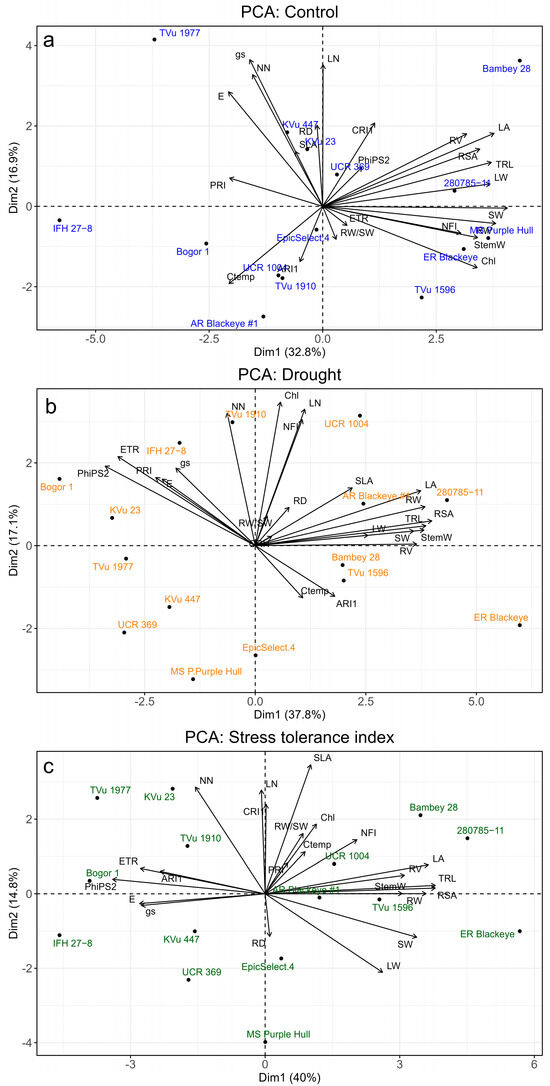
Figure 7.
Principal component analysis (PCA) of 15 cowpea genotypes with arrangement of physiological, spectral, and morphological traits in the first two principal components (PC1 and PC2) in (a) control, (b) drought stress, and (c) stress tolerance index. Acronyms are given in Table 1.
Under DS, PC1 showed positive loadings for root and shoot traits. At the same time, physiology and pigments influenced PC2, focusing on deeper root adaptations and downregulation of physiological processes to conserve water during drought (Figure 7b). Most physiological traits were placed in the second quadrant, where genotype IFH 27-8 was closely associated, showing reliance on physiological mechanisms for adaptation to drought. Genotype-specific responses were observed, with 280785-11, Arkansas Blackeye #1, and UCR 1004 displaying strong vegetative growth. In contrast, genotypes like Bogor 1, KVu 23, and UCR 369 showed negative loadings, indicating a higher susceptibility of morphological traits to drought. Based on the STI, Arkansas Blackeye #1 was replaced by Bambey 28 among the genotypes from the first quadrant in the PCA drought biplot (Figure 7c).
For STI, PC1 was influenced by TRL, RSA, LA, RW, and PhiPS2, indicating strong vegetative structure maintenance, while SLA, NN, LN, CRI1, and LW drove PC2. Bogor 1 was closely linked to physiological traits. Genotypes 280785-11, Bambey 28, and Early Ramshorn Blackeye maintained biomass under drought, whereas IFH 27-8, Bogor 1, and TVu 1977 showed weaker maintenance. TVu 1977 and KVu 23 excelled in traits like SLA and leaf pigments. Genotype 280785-11 performed well across irrigation treatments, while MS Purple Hull, UCR 369, and IFH 27-8 were drought-sensitive, clustering in the third quadrant with poor biomass traits.
4. Discussion
Drought adversely affects physiological, biochemical, and morphological processes in crops, leading to reduced yield [58]. In arid and semi-arid regions, decreased rainfall is projected to lower crop biomass and yield potential [59]. While cowpea is generally more tolerant to drought compared to other crops, drought during the vegetative stage can hinder growth and canopy establishment, negatively impacting yield [20,60]. Previous studies on cowpeas primarily focused on morpho-physiological and biochemical traits with only a few genotypes (2-3), mainly targeting shoot traits [17,20,28,29,61]. Drought tolerance is influenced by an interplay of various traits based on genetics [62,63]. This study used 15 genotypes to explore the variability of root and shoot traits during the early vegetative stage. Using physiological, biophysical, and morphological characteristics, findings led to the identification of genotypes with contrasting tolerance to drought.
4.1. Distinct Genotypic Response for Physiology and Pigments
Quantifying stomatal function and photosynthetic efficiency has been widely utilized to understand the response of drought in different crops [21,64]. Exposure of cowpea genotypes to DS led to a significant decrease in gs, indicating significant stomatal limitations as observed in other crops, including cowpea [20,50,65]. Genotypes exhibiting higher gs or E combined with lower canopy temperatures during DS generally enhance photosynthetic capacity and tolerance [66,67]. Among tested genotypes under stress, IFH 27-8 maintained higher gs, E, and ETR with lower canopy temperature, whereas Early Ramshorn Blackeye had the opposite trend (Figure 7b). The differences in gs suggest that IFH 27-8 may use an anisohydric strategy, maintaining gas exchange during drought, while Early Ramshorn Blackeye likely adopts an isohydric approach by quickly closing stomata [60].
The chlorophyll index, a proxy for leaf greenness closely linked to photosynthetic performance, has been extensively studied in stress tolerance studies [28,68]. While drought typically reduces chlorophyll, genotypes with higher chlorophyll content under drought sustain photosynthesis rates and greater yields [17,69]. Genotypes UCR 1004, Bogor 1, and 280785-11 maintained high chlorophyll levels, with 280785-11 showing the best performance in both treatment conditions. Under DS, these genotypes also had greater NN and LN, suggesting they may have adaptive mechanisms to maintain structural traits (Figure 7b). This performance could be partly attributed to their association with N-fixing bacteria, which enhances nitrogen accumulation and chlorophyll synthesis under stress, warranting future studies on this topic [70]. Previous studies have shown that higher chlorophyll positively correlates with photosynthetic rate and drought tolerance under stress, further supporting our speculation that these genotypes possess mechanisms to improve stress tolerance [28,50]. Even though genotype Bogor 1 has higher pigment and physiology, it showed poor root and shoot traits. Greater gas exchange and pigments were observed, likely due to shorter plants with smaller canopies that reduced water demand, allowing limited irrigation in the drought treatment sufficient to support metabolic function.
4.2. Leaf Biophysical Properties Association with Drought Tolerance
Vegetative indices (VIs) associated with xanthophylls, anthocyanins, and carotenoids have often been used in plant stress tolerance studies. These pigments are crucial in photoprotection due to oxidative stress. For instance, a low PRI value indicates stress-induced reduction in photosynthetic efficiency, whereas a higher PRI suggests enhanced xanthophyll cycle activity, reflecting improved photoprotection under stress [71]. A higher ARI1 suggests increased anthocyanin production, reflecting a protective response against the possible ROS activity [72]. In this study, PRI was closely associated with fluorescence traits (PhiPS2 and ETR), where genotypes Bogor 1 and UCR 1004 exhibited higher values (Figure 7b) for both the traits and VIs under drought. This strong correlation suggests that PRI can be used as a good indicator for differentiating stress-tolerant and sensitive genotypes in cowpea and other crops [73,74]. Carotenoids help protect chlorophyll from oxidative damage, particularly by quenching singlet oxygen and the triplet-excited state of chlorophylls, dissipating excess energy as heat [36,75,76]. Thus, higher CRI1 values signify increased carotenoid synthesis to maintain photosynthetic function. Some studies have observed an increase in carotenoid pigments under drought [23,77], while others have reported a decline [78,79,80], indicating a variable response depending on species, growth stage, and duration of stress. In this study, CRI1 levels decreased under drought, indicating oxidative stress. However, genotype 280785-11 maintained higher CRI1 and chlorophyll levels under drought, suggesting that carotenoids provide photoprotective mechanisms to reduce oxidative damage and enhance photosynthetic efficiency. Previous studies showed that ARI1 closely estimates anthocyanin content through reflectance measurements and is used to indicate abiotic stress tolerance [55,64,81]. Our results align with these findings, suggesting increased anthocyanin levels under DS. However, no consistent association was found between ARI1 and physiological traits, highlighting individual genotype variability. For example, IFH 27-8 showed better physiological performance and higher ARI1 values under drought, while Early Ramshorn Blackeye had high ARI1 but poor physiological performance. The early vegetative stage involves ongoing pigment accumulation and structural adaptations, which ARI1 may not fully capture. Anthocyanins often reflect transient stress responses linked to photoprotective shifts rather than direct physiological functions [82]. Therefore, using ARI1 alone may not be reliable for selecting tolerant genotypes at an early stage. Results suggest that some VIs can be utilized for large-scale phenotyping to identify tolerant and sensitive genotypes under stress, thus assisting in crop management and breeding for stress-tolerant varieties.
4.3. Response of Specific and Total Leaf Area Under Drought
Traits associated with LA, such as NN and LN, are important features that distinguish a tolerant genotype from a sensitive one under stress [83]. Previous studies have shown that drought significantly decreases these traits, resulting in reduced biomass and lower yield [20,84,85]. Canopy cover is an important trait associated with capturing photosynthetically active radiation and converting it into chemical energy via photosynthesis. As observed in cowpea, substantial evidence showed decreased LN and LA under drought in different legumes [20,86]. Reduction in LN can be explained by a reduction in nodes or branching with fewer internodes. Similarly, reduced LA can be attributed to restricted cell elongation and expansion triggered by ABA signaling and accelerated leaf senescence due to an increase in chlorophyll degradation rather than a decrease in chlorophyll synthesis [86]. Oxidative stress caused by drought increases ROS, which could have further promoted chlorophyll degradation and disrupted the photosynthetic machinery.
The observed reduction in LA in this study and in other crops indicates morphological adjustment to minimize water loss through transpiration under drought [50,87]. Genotype UCR 369 showed the most significant decrease in LN and LA, indicating a higher susceptibility to drought. This is further supported by its negative correlation with root traits like TRL, RW/SW ratio, and RSA, highlighting its shallow rooting and reduced drought tolerance (Figure 7b). Conversely, the Arkansas Blackeye #1 genotype maintained an LA comparable to the control, benefiting from a deeper root system that effectively accesses water. Consistent with previous studies [17,20], Arkansas Blackeye #1 emerged as drought-tolerant during the early vegetative stage (Figure 7b). Leaf area per unit weight indicates leaf thickness and structural investment; thicker leaves have lower SLA, reducing water loss through cell wall lignification and improving water use efficiency [88]. Under drought, due to reduced water relations, plants produce leaves with less structural tissue (reduced vascular tissues) to conserve resources; this potentially lowers photosynthetic area and rates under stress [89]. Similar responses were reported in cowpea under mild drought stress [90]. Overall, the reduction in leaf traits during drought led to decreased shoot weight, as it reduced carbon assimilation for biomass production [29,91,92]. Overall, genotypes UCR 1004, Arkansas Blackeye #1, and 280785-11 with higher LA and shoot weight exhibited drought tolerance.
4.4. Root Traits and Biomass Partitioning
Root traits have different adaptations to different environments, and traits that might help in efficient resource acquisition under irrigated conditions may differ in their performance under rainfed conditions [42,93]. The response also varies with genotypes, where different genotypes might have distinct mechanisms and trait combinations, differentiating them from each other [91,94]. Generally, root system architecture traits are associated with drought tolerance in grain legumes [95], such as TRL (tap + lateral + fine roots), RD, and RSA [42,96]. Our results show that TRL decreased under drought stress; however, significant differences were observed in Bambey 28, KVu 23, and KVu 447 (Figure 6a). This indicates a genotype-specific variability in root responses to drought and the ability of cowpea to maintain its root system under drought [97]. Additionally, differential responses were observed after RD classifications. Fine root length with diameter < 0.3 mm (RLD1) increased under drought stress. In contrast, it decreased in RLD2 (0.3–3 mm) and RLD3 (>3 mm), suggesting a shift in root architecture as an adaptive response to reduced soil moisture (Table S2). The fine roots with RLD1 had a higher proportion of TRL under drought (51%) than the control (39%), indicating fine root proliferation facilitating greater soil exploration under limited soil moisture availability. RSA and RV are the major architectural traits associated with proliferative rooting, which confers drought tolerance in legumes [95]. In this study, genotypes UCR 1004, 280785-11, and Early Ramshorn Blackeye with larger RSA and RV correlated with aboveground biomass, indicating that these root architectural traits help facilitate improved water and nutrition uptake (Figure 6 and Figure 7b).
The plasticity of the root system plays a critical role in maintaining plant physiological processes and productivity under drought conditions. In our study, DS decreased root weight and increased the root-to-shoot ratio due to a greater reduction in aboveground biomass compared to an increase in root biomass (Figure 5). The increase in the root-to-shoot ratio is consistent with those of previous studies [27,91,98], indicating a shift in resource allocation towards root development to enhance water uptake. Since carbon is one of the key resources for growth, cowpea plants might have focused on survival rather than shoot biomass accumulation, resulting in reduced shoot growth instead of an increase in root biomass. Observed decline in root biomass under drought indicates that instead of increasing total root biomass, plants may adjust root morphology, such as increasing fine root production or root-to-shoot ratio, rather than simply accumulating more root biomass. Plants with a higher root-to-shoot ratio adapt better to prolonged drought, allowing them to maintain photosynthesis and metabolic functions under stress compared to shallow-rooted genotypes [99,100,101,102]. Overall, genotypes (UCR 1004, 280785-11, and Arkansas Blackeye #1) with higher root biomass under drought exhibited better aboveground biomass, demonstrating greater drought tolerance compared to genotypes (UCR 369 and KVu 447) with lower root biomass, which indicates susceptibility (Figure 5b and Figure 6b).
5. Conclusions
Drought stress during the early vegetative stage of cowpea revealed considerable variation among diverse cowpea genotypes. Short-term early-season drought significantly affected physiology, biophysical, and morphology traits. gs reduced considerably under drought, leading to damage in photosynthetic machinery, as evidenced by reductions in PhiPS2 and ETR. The strong association of leaf reflectance or VIs with variability in chlorophyll fluorescence properties highlights the potential of using proximal sensing to distinguish genotypes with contrasting tolerance to drought. Based on the combined principal component and stress tolerance index analyses, genotypes 280785-11 and UCR 1004 exhibited superior performance under drought, as evidenced by higher root weight, root surface area, and shoot weight, highlighting their efficiency in resource utilization. These results open avenues for harnessing drought-adaptive traits and mapping genetic loci that can be used for molecular breeding programs to enhance cowpea drought tolerance. While our focus was primarily on morpho-physiological and spectral traits, future works exploring how root nodulation traits and the efficiency of symbiosis with N-fixing bacteria are affected under drought stress would shed light on complex metabolism and stress tolerance.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agriculture15101075/s1, Figure S1: Line graph showing the normalized daily volumetric water content (%) over 22 days of stress under control (CNT, blue) and drought stress (DS, orange) conditions; Table S1: A list of cowpea genotypes used in the experiment; Table S2: Analysis of variance of shoot and root morphological traits of 15 cowpea genotypes with mean values under control (CNT) and drought stress (DS); Table S3: Variation in shoot and root morphological traits of 15 cowpea genotypes subjected to control (CNT) and drought (DS) treatments.
Author Contributions
S.P.: data curation, formal analysis, data interpretation, writing—original draft, and visualization. L.V.S.: data curation and writing—review and editing. B.S.S.: data curation and writing—review and editing. V.H.: data curation. R.L.H.: writing—review and editing. R.B.: conceptualization, supervision, resources, project administration, methodology, investigation, funding acquisition, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Mississippi Agricultural and Forestry Experiment Station, Special Research Initiative (MAFES-SRI), USDA-Agricultural Research Service (USDA-ARS) (58-6064-3-007), and the National Institute of Food and Agriculture (MIS-430030).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The authors will make the data available on request.
Acknowledgments
We thank the Plant Stress Physiology Laboratory members for their assistance during the experimental set-up and the USDA Germplasm Resources Information Network (GRIN) for providing cowpea seeds.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare no conflicts of interest.
References
- Bolan, S.; Padhye, L.P.; Jasemizad, T.; Govarthanan, M.; Karmegam, N.; Wijesekara, H.; Amarasiri, D.; Hou, D.; Zhou, P.; Biswal, B.K.; et al. Impacts of Climate Change on the Fate of Contaminants Through Extreme Weather Events. Sci. Total Environ. 2024, 909, 168388. [Google Scholar] [CrossRef] [PubMed]
- Cook, B.I.; Mankin, J.S.; Anchukaitis, K.J. Climate Change and Drought: From Past to Future. Curr. Clim. Change Rep. 2018, 4, 164–179. [Google Scholar] [CrossRef]
- FAO. The Impact of Disasters and Crises on Agriculture and Food Security: 2021; FAO: Rome, Italy, 2021; ISBN 978-92-5-134071-4.
- NCEI U.S. Billion-Dollar Weather and Climate Disasters (2025); NCEI U.S.: Asheville, NC, USA, 2025.
- Wu, B.; Tian, F.; Zhang, M.; Piao, S.; Zeng, H.; Zhu, W.; Liu, J.; Elnashar, A.; Lu, Y. Quantifying Global Agricultural Water Appropriation with Data Derived from Earth Observations. J. Clean. Prod. 2022, 358, 131891. [Google Scholar] [CrossRef]
- Falcon, W.P.; Naylor, R.L.; Shankar, N.D. Rethinking Global Food Demand for 2050. Popul. Dev. Rev. 2022, 48, 921–957. [Google Scholar] [CrossRef]
- Chaturvedi, V.; Hejazi, M.; Edmonds, J.; Clarke, L.; Kyle, P.; Davies, E.; Wise, M. Climate Mitigation Policy Implications for Global Irrigation Water Demand. Mitig. Adapt. Strateg. Glob. Change 2015, 20, 389–407. [Google Scholar] [CrossRef]
- Gleick, P.H. The World’s Water 2000–2001: The Biennial Report on Freshwater Resources; Island Press: Washington, DC, USA, 2000; ISBN 978-1-59726-284-2. [Google Scholar]
- Dutta, A.; Trivedi, A.; Nath, C.P.; Gupta, D.S.; Hazra, K.K. A comprehensive review on grain legumes as climate-smart crops: Challenges and prospects. Environ. Chall. 2022, 7, 100479. [Google Scholar] [CrossRef]
- de Frota, K.M.G.; Soares, R.A.M.; Arêas, J.A.G. Chemical Composition of Cowpea (Vigna unguiculata L. Walp), Brs-Milênio Cultivar. Food Sci. Technol. 2008, 28, 470–476. [Google Scholar] [CrossRef]
- Iqbal, A.; Khalil, I.A.; Ateeq, N.; Sayyar Khan, M. Nutritional quality of important food legumes. Food Chem. 2006, 97, 331–335. [Google Scholar] [CrossRef]
- Singh, A.K.; Elango, D.; Raigne, J.; Van der Laan, L.; Rairdin, A.; Soregaon, C.; Singh, A. Plant-Based Protein Crops and Their Improvement: Current Status and Future Perspectives. Crop Sci. 2025, 65, e21389. [Google Scholar] [CrossRef]
- Singh, B.B.; Ajeigbe, H.A.; Tarawali, S.A.; Fernandez-Rivera, S.; Abubakar, M. Improving the Production and Utilization of Cowpea as Food and Fodder. Field Crops Res. 2003, 84, 169–177. [Google Scholar] [CrossRef]
- da Silva, A.C.; da Santos, D.C.; Junior, D.L.T.; da Silva, P.B.; Santos, R.C.D.; Siviero, A. Cowpea: A Strategic Legume Species for Food Security and Health. In Legume Seed Nutraceutical Research; IntechOpen: London, UK, 2018; ISBN 978-1-78985-398-8. [Google Scholar]
- Ezin, V.; Tosse, A.G.C.; Chabi, I.B.; Ahanchede, A. Adaptation of Cowpea (Vigna unguiculata L. Walp.) to Water Deficit During Vegetative and Reproductive Phases Using Physiological and Agronomic Characters. Int. J. Agron. 2021, 2021, 9665312. [Google Scholar] [CrossRef]
- Farooq, M.; Gogoi, N.; Barthakur, S.; Baroowa, B.; Bharadwaj, N.; Alghamdi, S.S.; Siddique, K.H.M. Drought Stress in Grain Legumes during Reproduction and Grain Filling. J. Agron. Crop Sci. 2017, 203, 81–102. [Google Scholar] [CrossRef]
- Ravelombola, W.; Shi, A.; Chen, S.; Xiong, H.; Yang, Y.; Cui, Q.; Olaoye, D.; Mou, B. Evaluation of Cowpea for Drought Tolerance at Seedling Stage. Euphytica 2020, 216, 123. [Google Scholar] [CrossRef]
- Anjum, S.; Ashraf, U.; Zohaib, A.; Tanveer, M.; Naeem, M.; Ali, I.; Tabassum, T.; Nazir, U. Growth and developmental responses of crop plants under drought stress: A review. Zemdirb.-Agric. 2017, 104, 267–276. [Google Scholar] [CrossRef]
- Tricot, F.; Crozat, Y.; Pellerin, S. Root System Growth and Nodule Establishment on Pea (Pisum sativum). J. Exp. Bot. 1997, 48, 1935–1941. [Google Scholar] [CrossRef]
- Olorunwa, O.J.; Shi, A.; Barickman, T.C. Varying Drought Stress Induces Morpho-Physiological Changes in Cowpea (Vigna unguiculata L.) Genotypes Inoculated with Bradyrhizobium japonicum. Plant Stress 2021, 2, 100033. [Google Scholar] [CrossRef]
- Singh, S.; Reddy, K.R. Regulation of Photosynthesis, Fluorescence, Stomatal Conductance and Water-Use Efficiency of Cowpea (Vigna unguiculata L. Walp.) Under Drought. J. Photochem. Photobiol. B 2011, 105, 40–50. [Google Scholar] [CrossRef]
- Aslam, M.M.; Waseem, M.; Jakada, B.H.; Okal, E.J.; Lei, Z.; Saqib, H.S.A.; Yuan, W.; Xu, W.; Zhang, Q. Mechanisms of Abscisic Acid-Mediated Drought Stress Responses in Plants. Int. J. Mol. Sci. 2022, 23, 1084. [Google Scholar] [CrossRef]
- Nunes, C.; Moreira, R.; Pais, I.; Semedo, J.; Simões, F.; Veloso, M.M.; Scotti-Campos, P. Cowpea Physiological Responses to Terminal Drought—Comparison between Four Landraces and a Commercial Variety. Plants 2022, 11, 593. [Google Scholar] [CrossRef]
- Biriah, N.; Chemining’wa, G.; Olubayo, F.; Saha, H. Effect of Drought Stress on Canopy Temperature, Growth and Yield Performance of Cowpea Varieties. Int. J. Plant Soil. Sci. 2016, 9, 1–12. [Google Scholar] [CrossRef]
- Lima, J.; Lobato, A. Brassinosteroids Improve Photosystem II Efficiency, Gas Exchange, Antioxidant Enzymes and Growth of Cowpea Plants Exposed to Water Deficit. Physiol. Mol. Biol. Plants 2017, 23, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Ritte, I.P.; Egnin, M.; Bernard, G.C.; Mortley, D.; Idehen, O.; Okoma, M.P.; Bonsi, C. Morpho-Physiological and Molecular Responses to Seedling-Stage Drought Stress in Different Cowpea Cultivars. Int. J. Plant Biol. 2025, 16, 25. [Google Scholar] [CrossRef]
- Walne, C.H.; Thenveettil, N.; Ramamoorthy, P.; Bheemanahalli, R.; Reddy, K.N.; Reddy, K.R. Unveiling Drought-Tolerant Corn Hybrids for Early-Season Drought Resilience Using Morpho-Physiological Traits. Agriculture 2024, 14, 425. [Google Scholar] [CrossRef]
- Cui, Q.; Xiong, H.; Yufeng, Y.; Eaton, S.; Imamura, S.; Santamaria, J.; Ravelombola, W.; Mason, R.E.; Wood, L.; Mozzoni, L.A.; et al. Evaluation of Drought Tolerance in Arkansas Cowpea Lines at Seedling Stage. HortScience 2020, 55, 1132–1143. [Google Scholar] [CrossRef]
- Ravelombola, W.; Shi, A.; Qin, J.; Weng, Y.; Bhattarai, G.; Zia, B.; Zhou, W.; Mou, B. Investigation on Various Aboveground Traits to Identify Drought Tolerance in Cowpea Seedlings. HortScience 2018, 53, 1757–1765. [Google Scholar] [CrossRef]
- Galieni, A.; D’Ascenzo, N.; Stagnari, F.; Pagnani, G.; Xie, Q.; Pisante, M. Past and Future of Plant Stress Detection: An Overview from Remote Sensing to Positron Emission Tomography. Front. Plant Sci. 2021, 11, 609155. [Google Scholar] [CrossRef]
- Ogawa, T.; Tamaki, M.; Usui, T.; Hikosaka, K. Hyperspectral Image Extraction to Evaluate the Photosynthetic and Stress Status of Plants, Using a Photochemical Reflectance Index (pri). Sci. Hortic. 2024, 336, 113349. [Google Scholar] [CrossRef]
- Bheemanahalli, R.; Krishnan, B.S.; Wijewardane, N.K.; Samiappan, S.; Reddy, K.R. Remote sensing algorithms and their applications in plant phenotyping. In Translating Physiological Tools to Augment Crop Breeding; Springer Nature: Singapore, 2023; pp. 337–353. [Google Scholar] [CrossRef]
- Poudel, S.; Vennam, R.R.; Shrestha, A.; Reddy, K.R.; Wijewardane, N.K.; Reddy, K.N.; Bheemanahalli, R. Resilience of soybean cultivars to drought stress during flowering and early-seed setting stages. Sci. Rep. 2023, 13, 1277. [Google Scholar] [CrossRef]
- Pintó-Marijuan, M.; Munné-Bosch, S. Photo-Oxidative Stress Markers as a Measure of Abiotic Stress-Induced Leaf Senescence: Advantages and Limitations. J. Exp. Bot. 2014, 65, 3845–3857. [Google Scholar] [CrossRef]
- Ranjan, S.; Singh, R.; Singh, M.; Pathre, U.; Shirke, P. Characterizing Photoinhibition and Photosynthesis in Juvenile-Red Versus Mature-Green Leaves of Jatropha curcas L. Plant Physiol. Biochem. 2014, 79, 48–59. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Zur, Y.; Chivkunova, O.B.; Merzlyak, M.N. Assessing Carotenoid Content in Plant Leaves with Reflectance Spectroscopy. Photochem. Photobiol. 2002, 75, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Tengey, T.K.; Gyamfi, R.A.; Sallah, E.K.; Issahaku, M.; Ndela, D.N.; Seidu, M.; Senyabor, A.F.; Affram, E.I.; Amoako, O.A.; Naapoal, C. Seedling Stage Drought Screening of Candidate Cowpea (Vigna unguiculata L. Walp.) Genotypes. Cogent Food Agric. 2023, 9, 2212463. [Google Scholar] [CrossRef]
- Awasthi, R.; Gaur, P.; Turner, N.; Vadez, V.; Siddique, K.; Nayyar, H. Effects of Individual and Combined Heat and Drought Stress During Seed Filling on the Oxidative Metabolism and Yield of Chickpea (Cicer arietinum) Genotypes Differing in Heat and Drought Tolerance. Crop Pasture Sci. 2017, 68, 823–841. [Google Scholar] [CrossRef]
- Chakravaram, A.; Sankarapillai, L.V.; Poudel, S.; Bheemanahalli, R. Interactive Effects of Drought and High Night Temperature on Physiology And Yield Components of Cowpea (Vigna unguiculata (L.) Walp.). J. Agric. Food Res. 2025, 21, 101844. [Google Scholar] [CrossRef]
- Kalra, A.; Goel, S.; Elias, A.A. Understanding Role of Roots in Plant Response to Drought: Way Forward to Climate-Resilient Crops. Plant Genome 2024, 17, e20395. [Google Scholar] [CrossRef]
- Ma, Z.; Guo, D.; Xu, X.; Lu, M.; Bardgett, R.D.; Eissenstat, D.M.; McCormack, M.L.; Hedin, L.O. Evolutionary History Resolves Global Organization of Root Functional Traits. Nature 2018, 555, 94–97. [Google Scholar] [CrossRef]
- Ramamoorthy, P.; Lakshmanan, K.; Upadhyaya, H.D.; Vadez, V.; Varshney, R.K. Root Traits Confer Grain Yield Advantages Under Terminal Drought in Chickpea (Cicer arietinum L.). Field Crops Res. 2017, 201, 146–161. [Google Scholar] [CrossRef]
- Amy Lydia, L. Evaluation of Root Traits Associated with Drought Tolerance in Dry Bean (Phaseolus vulgaris L.). Master’s Thesis, Michigan State University, East Lansing, MI, USA, 2013. [Google Scholar]
- Abdel-Haleem, H.; Lee, G.-J.; Boerma, R.H. Identification of QTL for Increased Fibrous Roots in Soybean. Theor. Appl. Genet. 2011, 122, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Adu, M.O.; Asare, P.A.; Yawson, D.O.; Dzidzienyo, D.K.; Nyadanu, D.; Asare-Bediako, E.; Afutu, E.; Tachie-Menson, J.W.; Amoah, M.N. Identifying Key Contributing Root System Traits to Genetic Diversity in Field-Grown Cowpea (Vigna unguiculata L. Walp.) Genotypes. Field Crops Res. 2019, 232, 106–118. [Google Scholar] [CrossRef]
- Nkomo, G.V.; Sedibe, M.M.; Mofokeng, M.A. Phenotyping cowpea accessions at the seedling stage for drought tolerance in controlled environments. Open Agric. 2022, 7, 433–444. [Google Scholar] [CrossRef]
- Strock, C.F.; Burridge, J.; Massas, A.S.F.; Beaver, J.; Beebe, S.; Camilo, S.A.; Fourie, D.; Jochua, C.; Miguel, M.; Miklas, P.N.; et al. Seedling root architecture and its relationship with seed yield across diverse environments in Phaseolus vulgaris. Field Crops Res. 2019, 237, 53–64. [Google Scholar] [CrossRef]
- Zhao, J.; Bodner, G.; Rewald, B.; Leitner, D.; Nagel, K.A.; Nakhforoosh, A. Root Architecture Simulation Improves the Inference from Seedling Root Phenotyping Towards Mature Root Systems. J. Exp. Bot. 2017, 68, 965–982. [Google Scholar] [CrossRef] [PubMed]
- Lilley, J.M.; Kirkegaard, J.A. Benefits of increased soil exploration by wheat roots. Field Crops Res. 2011, 122, 118–130. [Google Scholar] [CrossRef]
- Narayana, N.K.; Wijewardana, C.; Alsajri, F.A.; Reddy, K.R.; Stetina, S.R.; Bheemanahalli, R. Resilience of Soybean Genotypes to Drought Stress During the Early Vegetative Stage. Sci. Rep. 2024, 14, 17365. [Google Scholar] [CrossRef]
- Klein, S.P.; Schneider, H.M.; Perkins, A.C.; Brown, K.M.; Lynch, J.P. Multiple Integrated Root Phenotypes Are Associated with Improved Drought Tolerance. Plant Physiol. 2020, 183, 1011–1025. [Google Scholar] [CrossRef]
- Polania, J.; Poschenrieder, C.; Rao, I.; Beebe, S. Root traits and their potential links to plant ideotypes to improve drought resistance in common bean. Theor. Exp. Plant Physiol. 2017, 29, 143–154. [Google Scholar] [CrossRef]
- Franzoni, G.; Ferrante, A. Plant Extract Improves Quality Traits of Green and Red Lettuce Cultivars. Heliyon 2024, 10, e39224. [Google Scholar] [CrossRef]
- Gamon, J.A.; Serrano, L.; Surfus, J.S. The Photochemical Reflectance Index: An Optical Indicator of Photosynthetic Radiation Use Efficiency Across Species, Functional Types, and Nutrient Levels. Oecologia 1997, 112, 492–501. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N.; Chivkunova, O.B. Optical Properties and Nondestructive Estimation of Anthocyanin Content in Plant Leaves. Photochem. Photobiol. 2001, 74, 38–45. [Google Scholar] [CrossRef]
- Seethepalli, A.; Dhakal, K.; Griffiths, M.; Guo, H.; Freschet, G.T.; York, L.M. RhizoVsion Explorer: Open-Source Software for Root Image Analysis and Measurement Standardization. AoB PLANTS 2021, 13, plab056. [Google Scholar] [CrossRef]
- Fernandez, G.C.J. Stress Tolerance Index- a New Indicator of Tolerance. HortScience 1992, 27, 626d–626. [Google Scholar] [CrossRef]
- Anjum, S.; Xie, X.; Wang, L.; Saleem, M.; Man, C.; Lei, W. Morphological, Physiological and Biochemical Responses of Plants to Drought Stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar] [CrossRef]
- Asif, Z.; Chen, Z.; Sadiq, R.; Zhu, Y. Climate Change Impacts on Water Resources and Sustainable Water Management Strategies in North America. Water Resour. Manag. 2023, 37, 2771–2786. [Google Scholar] [CrossRef]
- Goufo, P.; Moutinho-Pereira, J.M.; Jorge, T.F.; Correia, C.M.; Oliveira, M.R.; Rosa, E.A.S.; António, C.; Trindade, H. Cowpea (Vigna unguiculata L. Walp.) Metabolomics: Osmoprotection as a Physiological Strategy for Drought Stress Resistance and Improved Yield. Front. Plant Sci. 2017, 8, 586. [Google Scholar] [CrossRef]
- Carvalho, M.; Castro, I.; Moutinho-Pereira, J.; Correia, C.; Egea-Cortines, M.; Matos, M.; Rosa, E.; Carnide, V.; Lino-Neto, T. Evaluating stress responses in cowpea under drought stress. J. Plant Physiol. 2019, 241, 153001. [Google Scholar] [CrossRef]
- Carvalho, M.; Lino-Neto, T.; Rosa, E.; Carnide, V. Cowpea: A legume crop for a challenging environment. J. Sci. Food Agric. 2017, 97, 4273–4284. [Google Scholar] [CrossRef]
- Golldack, D.; Li, C.; Mohan, H.; Probst, N. Tolerance to drought and salt stress in plants: Unraveling the signaling networks. Front. Plant Sci. 2014, 5, 151. [Google Scholar] [CrossRef] [PubMed]
- Mazur, M.; Matoša Kočar, M.; Jambrović, A.; Sudarić, A.; Volenik, M.; Duvnjak, T.; Zdunić, Z. Crop-Specific Responses to Cold Stress and Priming: Insights from Chlorophyll Fluorescence and Spectral Reflectance Analysis in Maize and Soybean. Plants 2024, 13, 1204. [Google Scholar] [CrossRef]
- Melo, A.S.; Costa, R.R.; da Sá, F.V.S.; Dias, G.F.; de Alencar, R.S.; de Viana, P.M.O.; Peixoto, T.D.C.; Suassuna, J.F.; Brito, M.E.B.; de Ferraz, R.L.S.; et al. Modulation of Drought-Induced Stress in Cowpea Genotypes Using Exogenous Salicylic Acid. Plants 2024, 13, 634. [Google Scholar] [CrossRef]
- Lotfi, R.; Eslami-Senoukesh, F.; Mohammadzadeh, A.; Zadhasan, E.; Abbasi, A.; Kalaji, H.M. Identification of Key Chlorophyll Fluorescence Parameters as Biomarkers for Dryland Wheat Under Future Climate Conditions. Sci. Rep. 2024, 14, 28699. [Google Scholar] [CrossRef]
- Prince, S.; Anower, M.R.; Motes, C.M.; Hernandez, T.D.; Liao, F.; Putman, L.; Mattson, R.; Seethepalli, A.; Shah, K.; Komp, M.; et al. Intraspecific Variation for Leaf Physiological and Root Morphological Adaptation to Drought Stress in Alfalfa (Medicago sativa L.). Front. Plant Sci. 2022, 13, 795011. [Google Scholar] [CrossRef] [PubMed]
- Monteoliva, M.; Guzzo, M.; Posada, G. Breeding for Drought Tolerance by Monitoring Chlorophyll Content. Gene Technol. 2021, 10, 10-35248. [Google Scholar]
- Kashiwagi, J.; Upadhyaya, H.D.; Krishnamurthy, L. Significance and Genetic Diversity of Spad Chlorophyll Meter Reading in Chickpea Germplasm in the Semi-Arid Environments. J. Food Legum. 2010, 23, 99–105. [Google Scholar]
- Staudinger, C.; Mehmeti-Tershani, V.; Gil-Quintana, E.; Gonzalez, E.M.; Hofhansl, F.; Bachmann, G.; Wienkoop, S. Evidence for a rhizobia-induced drought stress response strategy in Medicago truncatula. J. Proteomics 2016, 136, 202–213. [Google Scholar] [CrossRef]
- D’Odorico, P.; Schönbeck, L.; Vitali, V.; Meusburger, K.; Schaub, M.; Ginzler, C.; Zweifel, R.; Velasco, V.M.E.; Gisler, J.; Gessler, A.; et al. Drone-based physiological index reveals long-term acclimation and drought stress responses in trees. Plant Cell Environ. 2021, 44, 3552–3570. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Antioxidant Activity of Anthocyanins and Anthocyanidins: A Critical Review. Int. J. Mol. Sci. 2024, 25, 12001. [Google Scholar] [CrossRef] [PubMed]
- Sukhova, E.; Yudina, L.; Kior, A.; Kior, D.; Popova, A.; Zolin, Y.; Gromova, E.; Sukhov, V. Modified Photochemical Reflectance Indices as New Tool for Revealing Influence of Drought and Heat on Pea and Wheat Plants. Plants 2022, 11, 1308. [Google Scholar] [CrossRef]
- Yudina, L.; Sukhova, E.; Gromova, E.; Nerush, V.; Vodeneev, V.; Sukhov, V. A Light-Induced Decrease in the Photochemical Reflectance Index (PRI) Can Be Used to Estimate the Energy-Dependent Component of Non-Photochemical Quenching Under Heat Stress and Soil Drought in Pea, Wheat, and Pumpkin. Photosynth. Res. 2020, 146, 175–187. [Google Scholar] [CrossRef]
- Swapnil, P.; Meena, M.; Singh, S.K.; Dhuldhaj, U.P.; Harish; Marwal, A. Vital roles of carotenoids in plants and humans to deteriorate stress with its structure, biosynthesis, metabolic engineering and functional aspects. Curr. Plant Biol. 2021, 26, 100203. [Google Scholar] [CrossRef]
- Young, A.J. The Photoprotective Role of Carotenoids in Higher Plants. Physiol. Plant. 1991, 83, 702–708. [Google Scholar] [CrossRef]
- Gomes, A.M.F.; Rodrigues, A.P.; António, C.; Rodrigues, A.M.; Leitão, A.E.; Batista-Santos, P.; Nhantumbo, N.; Massinga, R.; Ribeiro-Barros, A.I.; Ramalho, J.C. Drought Response of Cowpea (Vigna unguiculata L. Walp.) Landraces at Leaf Physiological and Metabolite Profile Levels. Environ. Exp. Bot. 2020, 175, 104060. [Google Scholar] [CrossRef]
- Pantha, S.; Kilian, B.; Özkan, H.; Zeibig, F.; Frei, M. Physiological and Biochemical Changes Induced by Drought Stress During the Stem Elongation and Anthesis Stages in the Triticum Genus. Environ. Exp. Bot. 2024, 228, 106047. [Google Scholar] [CrossRef]
- Talebi, R.; Ensafi, M.H.; Baghebani, N.; Karami, E.; Mohammadi, K. Physiological Responses of Chickpea (Cicer arietinum) Genotypes to Drought Stress. Environ. Exp. Biol. 2013, 11, 9–15. [Google Scholar]
- Rizvi, A.H.; Dwivedi, V.K.; Sairam, R.K.; Yadav, S.S.; Bharadwaj, C.; Sarker, A.; Alam, A. Physiological Studies on Moisture Stress Tolerance in Chickpea (Cicer arietinum L.) Genotypes. Int. J. Sci. Res. Agric. Sci. 2014, 1, 23–31. [Google Scholar] [CrossRef]
- Iozia, L.M.; Varone, L. Short Range Shifts in Plant Physiological Responses to Induced Water Stress: Experimental Evidence of Intraspecific Trait Variability Differentiating Neighbouring Mediterranean Plant Populations. Plant Stress 2024, 13, 100556. [Google Scholar] [CrossRef]
- Chalker-Scott, L. Environmental Significance of Anthocyanins in Plant Stress Responses. Photochem. Photobiol. 1999, 70, 1–9. [Google Scholar] [CrossRef]
- Beebe, S.; Rao, I.; Blair, M.; Acosta, J. Phenotyping Common Beans for Adaptation to Drought. Front. Physiol. 2013, 4, 35. [Google Scholar] [CrossRef]
- Yahaya, D.; Denwar, N.; Blair, M.W. Effects of Moisture Deficit on the Yield of Cowpea Genotypes in the Guinea Savannah of Northern Ghana. Agric. Sci. 2019, 10, 577–595. [Google Scholar] [CrossRef]
- Fenta, B.A.; Beebe, S.E.; Kunert, K.J.; Burridge, J.D.; Barlow, K.M.; Lynch, J.P.; Foyer, C.H. Field Phenotyping of Soybean Roots for Drought Stress Tolerance. Agronomy 2014, 4, 418–435. [Google Scholar] [CrossRef]
- Akyeampong, E. Some Responses of Cowpea to Drought Stress. In Proceedings of the Potentials of Forage Legumes in Farming Systems of Sub-Saharan Africa, Addis Ababa, Ethiopia, 16–19 September 1985; pp. 141–159. [Google Scholar]
- Hayatu, M.; Mukhtar, F.B. Physiological Responses of Some Drought Resistant Cowpea Genotypes (Vigna unguiculata L. Walp) to Water Stress. Bayero J. Pure Appl. Sci. 2010, 3, 69–75. [Google Scholar]
- Craufurd, P.Q.; Wheeler, T.R.; Ellis, R.H.; Summerfield, R.J.; Williams, J.H. Effect of Temperature and Water Deficit on Water-Use Efficiency, Carbon Isotope Discrimination, and Specific Leaf Area in Peanut. Crop Sci. 1999, 39, 136–142. [Google Scholar] [CrossRef]
- Payne, W.A.; Wendt, C.W.; Hossner, L.R.; Gates, C.E. Estimating Pearl Millet Leaf Area and Specific Leaf Area. Agron. J. 1991, 83, 937–941. [Google Scholar] [CrossRef]
- Anyia, A.O.; Herzog, H. Water-Use Efficiency, Leaf Area and Leaf Gas Exchange of Cowpeas Under Mid-Season Drought. Eur. J. Agron. 2004, 20, 327–339. [Google Scholar] [CrossRef]
- Santos, R.; Carvalho, M.; Rosa, E.; Carnide, V.; Castro, I. Root and Agro-Morphological Traits Performance in Cowpea Under Drought Stress. Agronomy 2020, 10, 1604. [Google Scholar] [CrossRef]
- Wijewardana, C.; Alsajri, F.A.; Irby, J.T.; Krutz, L.J.; Golden, B.; Henry, W.B.; Gao, W.; Reddy, K.R. Physiological assessment of water deficit in soybean using midday leaf water potential and spectral features. J. Plant Interact. 2019, 14, 533–543. [Google Scholar] [CrossRef]
- Tardieu, F. Any Trait or Trait-Related Allele Can Confer Drought Tolerance: Just Design the Right Drought Scenario. J. Exp. Bot. 2012, 63, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.B.; Burridge, J.D.; Ishiyaku, M.F.; Boukar, O.; Lynch, J.P. Phenotyping Cowpea for Seedling Root Architecture Reveals Root Phenes Important for Breeding Phosphorus Efficient Varieties. Crop Sci. 2022, 62, 326–345. [Google Scholar] [CrossRef]
- Ye, H.; Roorkiwal, M.; Valliyodan, B.; Zhou, L.; Chen, P.; Varshney, R.K.; Nguyen, H.T. Genetic Diversity of Root System Architecture in Response to Drought Stress in Grain Legumes. J. Exp. Bot. 2018, 69, 3267–3277. [Google Scholar] [CrossRef]
- Vadez, V.; Rao, S.; Kholova, J.; Krishnamurthy, L.; Kashiwagi, J.; Ratnakumar, P.; Sharma, K.K.; Bhatnagar-Mathur, P.; Basu, P.S. Root Research for Drought Tolerance in Legumes: Quo Vadis? J. Food Legum. 2008, 21, 77–85. [Google Scholar]
- Hall, A. Phenotyping Cowpeas for Adaptation to Drought. Front. Physiol. 2012, 3, 155. [Google Scholar] [CrossRef]
- Kou, X.; Han, W.; Kang, J. Responses of Root System Architecture to Water Stress at Multiple Levels: A Meta-Analysis of Trials Under Controlled Conditions. Front. Plant Sci. 2022, 13, 1085409. [Google Scholar] [CrossRef] [PubMed]
- Dharmappa, P.M.; Doddaraju, P.; Malagondanahalli, M.V.; Rangappa, R.B.; Mallikarjuna, N.M.; Rajendrareddy, S.H.; Ramanjinappa, R.; Mavinahalli, R.P.; Prasad, T.G.; Udayakumar, M.; et al. Introgression of Root and Water Use Efficiency Traits Enhances Water Productivity: An Evidence for Physiological Breeding in Rice (Oryza sativa L.). Rice 2019, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Cui, K.; Xu, A.; Nie, L.; Huang, J.; Peng, S. Drought Stress Condition Increases Root to Shoot Ratio Via Alteration of Carbohydrate Partitioning and Enzymatic Activity in Rice Seedlings. Acta Physiol. Plant. 2015, 37, 9. [Google Scholar] [CrossRef]
- Bheemanahalli, R.; Narayanaswamy, B.R.; Mohankumar, M.V.; Sumanth, K.K.; Rajanna, M.P.; Mohanraju, B.; Udayakumar, M.; Sheshshayee, M.S. Root Traits and Cellular Level Tolerance Hold the Key in Maintaining Higher Spikelet Fertility of Rice Under Water Limited Conditions. Funct. Plant Biol. FPB 2014, 41, 930–939. [Google Scholar] [CrossRef]
- Gargallo-Garriga, A.; Sardans, J.; Pérez-Trujillo, M.; Rivas-Ubach, A.; Oravec, M.; Vecerova, K.; Urban, O.; Jentsch, A.; Kreyling, J.; Beierkuhnlein, C.; et al. Opposite Metabolic Responses of Shoots and Roots to Drought. Sci. Rep. 2014, 4, 6829. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).