Fate of Mycotoxins in Local-Race Populations of Maize Collected in the Southwest of France, from the Field to the Flour and Meal in Organic Farms
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Mycotoxin Analyses
2.3. Detection of Fusarium Species
2.4. Data Analyses
3. Results
3.1. Occurrence of Fusarium Mycotoxins in Maize Grains
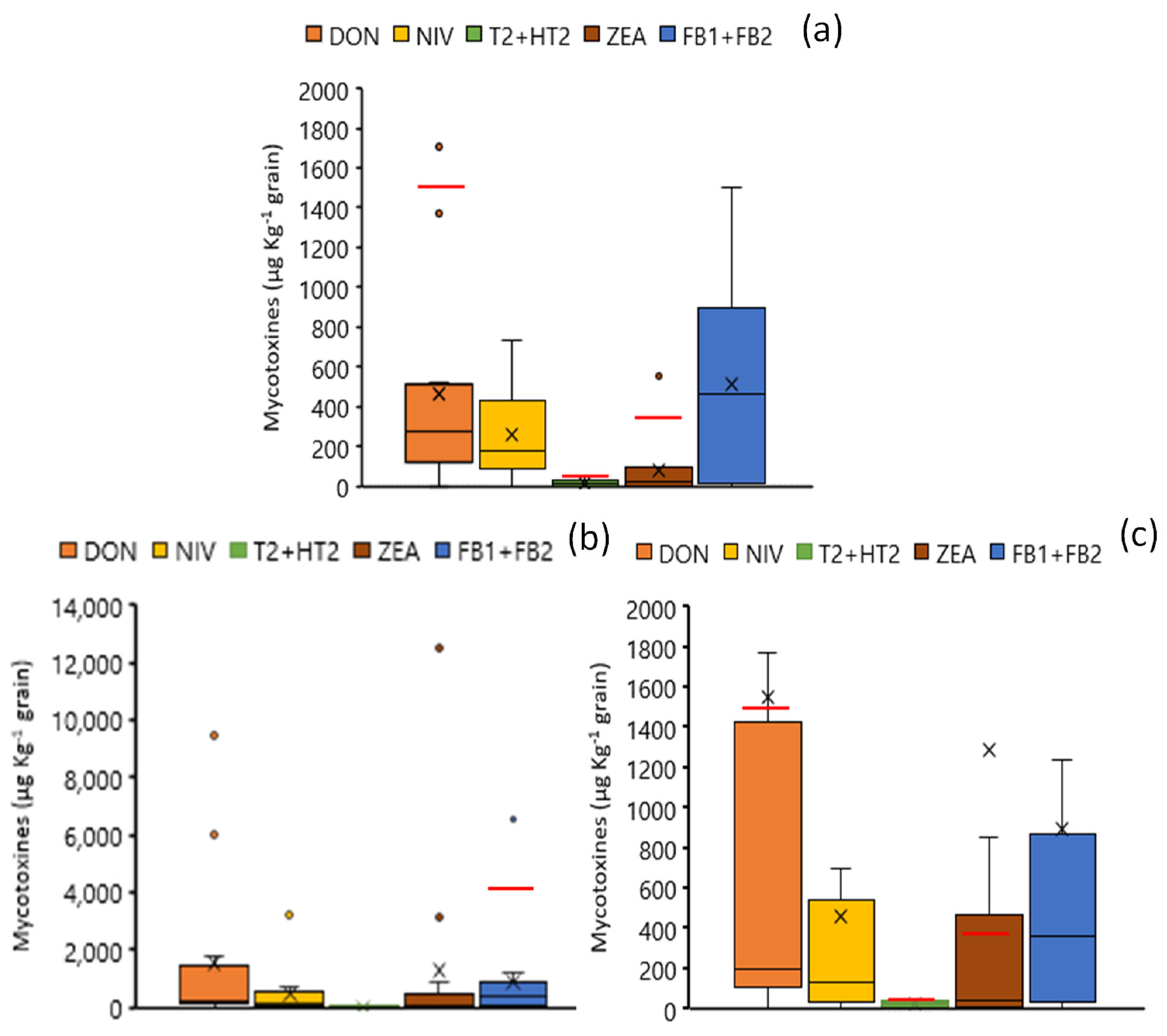
3.2. Levels of Grain Contamination by Fusarium Mycotoxins and Fusarium Species at Harvest
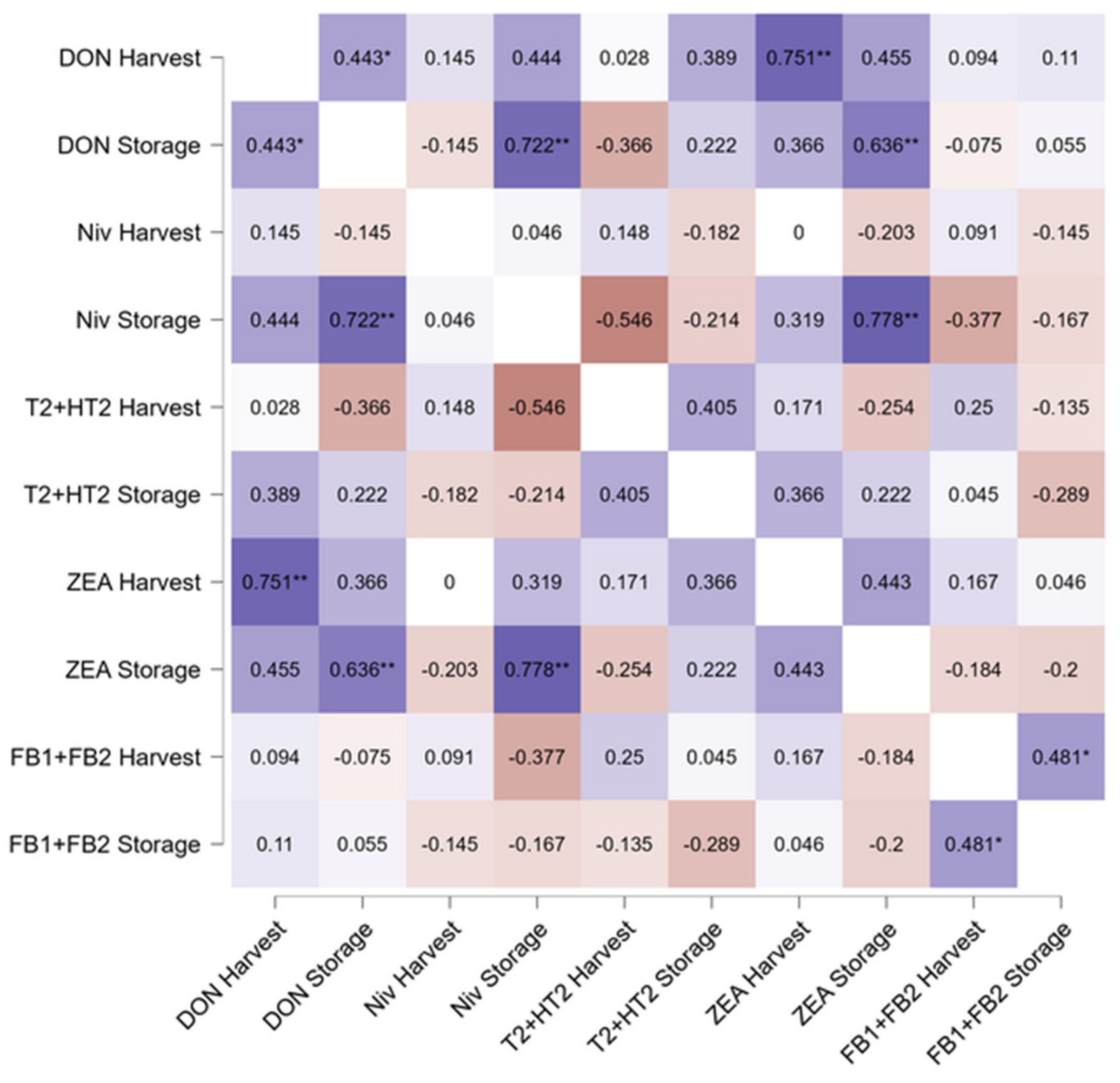
3.3. Fate of Fusarium Mycotoxins and Fusarium Species During Storage
3.4. Transfer of Mycotoxins into Milling Products
3.5. Ochratoxin A and Aflatoxins in Maize Grains and Millings
4. Discussion
4.1. Maize Grains Contamination by Mycotoxins and Fusarium Species
4.2. Fate of Mycotoxin at Storage and During Dry Milling
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harlan, J.R. Our vanishing genetic resources: Modern varieties replace ancient populations that have provided genetic variability for plant breeding programs. Science 1975, 188, 618–621. [Google Scholar] [CrossRef]
- Peres, J.C.; Aparecido, C.C.; Felicio, D.H.J.D.A.; Felicio, R.C.; Gonçalez, E. Fungal infection and mycotoxins contamination in organic and conventional maize. J. Agric. Environ. Sci. 2018, 7, 76–82. [Google Scholar] [CrossRef]
- Șonea, C.; Toader, M.; Năstase, I.P. The quality of maize grains in organic farming system. Rom. Biotechnol. Lett. 2020, 25, 1781–1789. [Google Scholar] [CrossRef]
- de Galarreta, J.I.R.; Butrón, A.; Ortiz-Barredo, A.; Malvar, R.A.; Ordás, A.; Landa, A.; Revilla, P. Mycotoxins in maize grains grown in organic and conventional agriculture. Food Control 2015, 52, 98–102. [Google Scholar] [CrossRef]
- Ariño, A.; Estopañan, G.; Juan, T.; Herrera, A. Estimation of dietary intakes of fumonisins B1 and B2 from conventional and organic corn. Food Control 2007, 18, 1058–1062. [Google Scholar] [CrossRef]
- Olson, M. Mycotoxins in organic and conventional foods and effects of the environment. In Health Benefits of Organic Food: Effects of the Environment; Givens, D.I., Baxter, S., Minihane, A.M., Shaw, E.H., Eds.; CABI Oxfordshire: Wallingford, UK, 2008; pp. 145–159. ISBN 978-1-84593-459-0. [Google Scholar]
- Tangni, E.K.; Pussemier, L.; Schneider, Y.J.; Larondelle, Y. Mycotoxines dans les céréales et produits dérivés: Revue de la littérature sur les filières biologiques et conventionnelles en Europe. Cah. Agric. 2013, 22, 152–164. [Google Scholar] [CrossRef]
- Dinis, I.; Santos, D.; Mendes-Moreira, P. Revitalising traditional cereals in Portugal: Challenges, opportunities, and strategies for value chain development. Sustainability 2025, 17, 2745. [Google Scholar] [CrossRef]
- Ortman, T.; Sandström, E.; Bengtsson, J.; Watson, C.A.; Bergkvist, G. Farmers’ motivations for landrace cereal cultivation in Sweden. Biol. Agric. Hortic. 2023, 39, 247–268. [Google Scholar] [CrossRef]
- Gwirtz, J.A.; Garcia-Casal, M.N. Processing maize flour and corn meal food products. Ann. N. Y. Acad. Sci. 2014, 1312, 66–75. [Google Scholar] [CrossRef]
- Aguado, A.; Savoie, J.-M.; Chéreau, S.; Ducos, C.; Aguilar, M.; Ferrer, N.; Aguilar, M.; Pinson-Gadais, L.; Richard-Forget, F. Priming to protect maize from Fusarium verticillioides and its fumonisin accumulation. J. Sci. Food Agric. 2019, 99, 64–72. [Google Scholar] [CrossRef]
- Nicholson, P.; Simpson, D.R.; Weston, G.; Rezanoor, H.N.; Lees, A.K.; Parry, D.W.; Joyce, D. Detection and quantification of Fusarium culmorum and Fusarium graminearum in cereals using PCR assays. Phys. Mol. Plant Pathol. 1998, 53, 17–37. [Google Scholar] [CrossRef]
- Wilson, A.; Simpson, D.; Chandler, E.; Jennings, P.; Nicholson, P. Development of PCR assays for the detection and differentiation of Fusarium sporotrichioides and Fusarium langsethiae. FEMS Microbiol. Let. 2004, 233, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global mycotoxin occurrence in feed: A ten-year survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, S.; Scarpino, V.; Lanzanova, C.; Romano, E.; Reyneri, A. Multi-mycotoxin long-term monitoring survey on North-Italian maize over an 11-year period (2011–2021): The co-occurrence of regulated, masked and emerging mycotoxins and fungal metabolites. Toxins 2022, 14, 520. [Google Scholar] [CrossRef] [PubMed]
- Mahdjoubi, C.K.; Arroyo-Manzanares, N.; Hamini-Kadar, N.; García-Campaña, A.M.; Mebrouk, K.; Gámiz-Gracia, L. Multi-mycotoxin occurrence and exposure assessment approach in foodstuffs from Algeria. Toxins 2020, 12, 194. [Google Scholar] [CrossRef]
- Tarazona, A.; Gómez, J.V.; Mateo, F.; Jimenez, M.; Romera, D.; Mateo, E.M. Study on mycotoxin contamination of maize kernels in Spain. Food Cont. 2020, 118, 107370. [Google Scholar] [CrossRef]
- Oliveira, C.A.F.; Cruz, J.V.S.; Rosim, R.E.; Bordin, K.; Kindermann, A.C.P.; Corassin, C.H. Simultaneous occurrence of aflatoxins and fumonisins in corn intended for the pet feed industry and for human consumption. J. Food Chem. Nanotechnol. 2016, 2, 1–5. [Google Scholar] [CrossRef]
- Weaver, A.C.; Weaver, D.M.; Adams, N.; Yiannikouris, A. Co-Occurrence of 35 mycotoxins: A seven-year survey of corn grain and corn silage in the United States. Toxins 2021, 13, 516. [Google Scholar] [CrossRef]
- Fusilier, K.; Chilvers, M.I.; Limay-Rios, V.; Singh, M.P. Mycotoxin co-occurrence in Michigan harvested maize grain. Toxins 2022, 14, 431. [Google Scholar] [CrossRef]
- Souza, M.L.M.; Sulyok, M.; Freitas-Silva, O.; Costa, S.S.; Brabet, C.; Machinski Junior, M.; Sekiyama, B.L.; Vargas, E.A.; Krska, R.; Schuhmacher, R. Cooccurrence of mycotoxins in maize and poultry feeds from Brazil by liquid chromatography/tandem mass spectrometry. Sci. World J. 2013, 14, 427369. [Google Scholar] [CrossRef]
- Tonial Simões, C.; Kobs Vidal, J.; da Rosa da Silva, C.; Alves Sarturi, J.; Fabris Laber, I.; Madalosso, T.; Mallmann, C.A. A two-year study on the occurrence and concentration of mycotoxins in corn varieties with different endosperm textures. J. Sci. Food Agric. 2023, 103, 7199–7206. [Google Scholar] [CrossRef]
- Camardo Leggieri, M.; Lanubile, A.; Dall’Asta, C.; Pietri, A.; Battilani, P. The impact of seasonal weather variation on mycotoxins: Maize crop in 2014 in northern Italy as a case study. World Mycotoxin J. 2020, 13, 25–36. [Google Scholar] [CrossRef]
- Leite, M.; Freitas, A.; Sanches Silva, A.; Barbosa, J.; Ramos, F. Maize food chain and mycotoxins: A review on occurrence studies. Trends Food Sci. Technol. 2021, 115, 307–331. [Google Scholar] [CrossRef]
- Munkvold, G.P.; Proctor, R.H.; Moretti, A. Mycotoxin production in Fusarium according to contemporary species concepts. Annu. Rev. Phytopathol. 2021, 59, 373–402. [Google Scholar] [CrossRef] [PubMed]
- Carbas, B.; Simões, D.; Soares, A.; Freitas, A.; Ferreira, B.; Carvalho, A.R.F.; Silva, A.S.; Pinto, T.; Diogo, E.; Andrade, E.; et al. Occurrence of Fusarium spp. in maize grain harvested in Portugal and accumulation of related mycotoxins during storage. Foods 2021, 10, 375. [Google Scholar] [CrossRef] [PubMed]
- Gargouri, S.; Masiello, M.; Somma, S.; Haidukowski, M.; Khaterchi, R.; Chekal, S.; Derouich, S.; Balmas, V.; Moretti, A. Maize–fusarium interactions: Tunisian insights into mycotoxin ecology. Fun. Biol. 2024, 128, 2460–2470. [Google Scholar] [CrossRef]
- Mesterhazy, A.; Toldine Toth, E.; Szel, S.; Varga, M.; Toth, B. Resistance of maize hybrids to Fusarium graminearum, F. culmorum, and F. verticillioides Ear Rots with toothpick and silk channel Inoculation, as well as their toxin production. Agronomy 2020, 10, 1283. [Google Scholar] [CrossRef]
- Boutigny, A.L.; Ward, T.J.; Ballois, N.; Iancu, G.; Ioos, R. Diversity of the Fusarium graminearum species complex on French cereals. Eur. J. Plant Pathol. 2014, 138, 133–148. [Google Scholar] [CrossRef]
- Meneely, J.; Greer, B.; Kolawole, O.; Elliott, C. T-2 and HT-2 Toxins: Toxicity, occurrence and analysis: A Review. Toxins 2023, 15, 481. [Google Scholar] [CrossRef]
- Arata, A.F.; Martínez, M.; Pesquero, N.V.; Cristos, D.; Dinolfo, M.I. Contamination of Fusarium spp. and mycotoxins at different ear physiological stages of maize in Argentina. Int. J. Food Microbiol. 2024, 410, 110493. [Google Scholar] [CrossRef]
- Battilani, P.; Toscano, P.; Van der Fels-Klerx, H.; Moretti, A.; Leggieri, M.C.; Brera, C.; Rortais, A.; Goumperis, T.; Robinson, T. Aflatoxin B1 contamination in maize in Europe increases due to climate change. Sci. Rep. 2016, 6, 24328. [Google Scholar] [CrossRef]
- Bailly, S.; Orlando, B.; Brustel, J.; Bailly, J.-D.; Levasseur-Garcia, C. Rapid detection of aflatoxins in ground maize using near infrared spectroscopy. Toxins 2024, 16, 385. [Google Scholar] [CrossRef] [PubMed]
- Focker, M.; van Eupen, M.; Verweij, P.; Liu, C.; van Haren, C.; van der Fels-Klerx, H.J. Effects of climate change on areas suitable for maize cultivation and aflatoxin contamination in Europe. Toxins 2023, 15, 599. [Google Scholar] [CrossRef] [PubMed]
- García-Díaz, M.; Gil-Serna, J.; Vázquez, C.; Botia, M.N.; Patiño, B. A comprehensive study on the occurrence of mycotoxins and their producing fungi during the Maize production cycle in Spain. Microorganisms 2020, 8, 141. [Google Scholar] [CrossRef]
- Orsi, R.B.; Corrêa, B.; Possi, C.R.; Schammass, E.A.; Nogueira, J.R.; Dias, S.M.; Malozzi, M.A. Mycoflora and occurrence of fumonisins in freshly harvested and stored hybrid maize. J. Stored Prod. Res. 2000, 36, 75–87. [Google Scholar] [CrossRef]
- Queiroz, V.A.V.; De Oliveira Alves, G.L.; Da Conceição, R.R.P.; Guimarães, L.J.M.; Mendes, S.M.; De Aquino Ribeiro, P.E.; Da Costa, R.V. Occurrence of fumonisins and zearalenone in maize stored in family farm in Minas Gerais, Brazil. Food Control 2012, 28, 83–86. [Google Scholar] [CrossRef]
- Atukwase, A.; Kaaya, A.N.; Muyanja, C. Dynamics of Fusarium and fumonisins in maize during storage–a case of the traditional storage structures commonly used in Uganda. Food Control 2012, 26, 200–205. [Google Scholar] [CrossRef]
- Fandohan, P.; Gnonlonfin, B.; Hell, K.; Marasas, W.F.O.; Wingfield, M.L. Natural occurrence of Fusarium and subsequent fumonisin contamination in preharvest and stored maize in Benin, West Africa. Int. J. Food Microbiol. 2005, 99, 173–183. [Google Scholar] [CrossRef]
- Walker, S.; Jaime, R.; Kagot, V.; Probst, C. Comparative effects of hermetic and traditional storage devices on maize grain: Mycotoxin development, insect infestation and grain quality. J. Stored Prod. Res. 2018, 77, 34–44. [Google Scholar] [CrossRef]
- Gaël, Y.V.; Biego, G.H.; Constant, K.K.; Leonce, N.G.; Coulibaly, A. Evolution of mycotoxins during maize grains storage in triple bags containing plants biopesticides (Lippia multiflora and Hyptis suaveolens). Asian Food Sci. J. 2020, 17, 22–33. [Google Scholar] [CrossRef]
- Venslovas, E.; Mankevičienė, A.; Kochiieru, Y.; Merkevičiūtė-Venslovė, L.; Janavičienė, S. Effect of storage conditions on the occurrence of mycotoxins and nutrient composition in maize grains. Zemdirb.-Agric. 2022, 109, 359–364. [Google Scholar] [CrossRef]
- Lee, H.J.; Ryu, D. Worldwide occurrence of mycotoxins in cereals and cereal-derived food products: Public health perspectives of their co-occurrence. J. Agric. Food Chem. 2017, 65, 7034–7051. [Google Scholar] [CrossRef] [PubMed]
- Schaarschmidt, S.; Fauhl-Hassek, C. The fate of mycotoxins during the primary food processing of maize. Food Control 2021, 121, 107651. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, W.; Sun, C.; Liu, H.; Dou, J. Occurrence and fate analysis of mycotoxins in maize during the post-harvest period. Toxins 2024, 16, 459. [Google Scholar] [CrossRef]
- Gomes, A.L.; Sousa, R.L.M.D.; das Neves, L.A.V.; da Gloria, E.M.; Burbarelli, M.F.D.C.; Seno, L.D.O.; Petrus, R.R.; Fernandes, A.M. Occurrence and Co-exposure of aflatoxins and fumonisins in conventional and organic corn. Food Control 2024, 165, 110628. [Google Scholar] [CrossRef]
- Oliveira, M.S.; Rocha, A.; Sulyok, M.; Krska, R.; Mallmann, C.A. Natural mycotoxin contamination of maize (Zea mays L.) in the south region of Brazil. Food Control 2017, 73, 127–132. [Google Scholar] [CrossRef]
- Pietri, A.; Bertuzzi, T.; Pallaroni, L.; Piva, G. Occurence of mycotoxins and ergosterol in maize harvested over 5 years in the northern Italy. Food Addit. Contam. 2004, 21, 479–487. [Google Scholar] [CrossRef]


| Maize Products | Mycotoxins | Commission Regulation (EU) Number | ||||
|---|---|---|---|---|---|---|
| 2006/1881 | 2007/1126 | 2023/915 | 2024/1022 | 2024/1038 | ||
| Unprocessed grains | DON | 1750 | 1750 | 1750 | 1500 | |
| ZEA | 200 | 350 | 350 | |||
| FB1 + FB2 | 2000 | 4000 | 4000 | |||
| T-2 + HT-2 | 100 | |||||
| Grains intended for direct human consumption | DON | 750 | 750 | |||
| ZEA | 200 | 100 | ||||
| FB1 + FB2 | 1000 | 100 | ||||
| T-2 + HT-2 | 50 | |||||
| Milling fractions of maize with at least 90% of the particles in the milling product have a size ≤500 μm; flour not placed on the market for the final consumer | DON | 750 | 750 | 750 | ||
| ZEA | 300 | 300 | ||||
| FB1 + FB2 | 2000 | 2000 | ||||
| T-2 + HT-2 | 20 | |||||
| Milling fractions of maize with less than 90% of the particles in the milling product have a size ≤500 μm; meal not placed on the market for the final consumer | DON | 1250 | 1250 | 750 | ||
| ZEA | 200 | 200 | ||||
| FB1 + FB2 | 1400 | 1400 | ||||
| T-2 + HT-2 | 20 | |||||
| Mycotoxins | Method | LoQ (3) (µg kg−1) |
|---|---|---|
| Deoxynivalenol (DON) | HPLC/MS/MS (1) | 50 |
| Nivalenol (Niv) | HPLC/MS/MS | 50 |
| 3-acetyl Deoxynivalenol (3-ADON) | HPLC/MS/MS | 100 |
| 15-acetyl Deoxynivalenol (15-ADON | HPLC/MS/MS | 100 |
| Fusarenone-X (Fx) | HPLC/MS/MS | 50 |
| DAS | HPLC/MS/MS | 10 |
| T2 toxin (T-2) | HPLC/MS/MS | 5 |
| HT2 toxin (HT-2) | HPLC/MS/MS | 5 |
| Zearalenone (ZEA) | HPLC/MS/MS | 10 |
| Alpha-Zearalenone | HPLC/MS/MS | 50 |
| Beta-Zearalenone | HPLC/MS/MS | 50 |
| Zearalanone | HPLC/MS/MS | 50 |
| Fumonisin B1 (FB1) | HPLC/MS/MS | 20 |
| Fumonisin B2 (FB2) | HPLC/MS/MS | 20 |
| Fumonisin B3 (FB3) | HPLC/MS/MS | 20 |
| Aflatoxin B1 (Afla-B1) | HPLC/FLD (2) | 0.1 |
| Aflatoxin B2 (Afla-B2) | HPLC/FLD | 0.1 |
| Aflatoxin G1 (Afla-G1) | HPLC/FLD | 0.1 |
| Aflatoxin G2 (Afla-G2) | HPLC/FLD | 0.1 |
| Ochratoxin A (OTA) | HPLC/FLD | 0.2 |
| Primer Names | Primer Sequences | Ta 1 | Fusarium Species | Reference |
|---|---|---|---|---|
| Fum1-656F Fum1-1158R | CGGTTGTTCATCATCTCTGA GCTCCCGATGTAGAGCTTCTT | 60 °C | F. verticillioides | [11] |
| Fp82F Fp82R | CAA GCA AAC AGG CTC TTC ACC TGT TCC ACC TCA GTG ACA GGT T | 60 °C | F. poae | [12] |
| Fg16N-F Fg16N-R | ACAGATGACAAGATTCAGGCACA TTCTTTGACATCTGTTCAACCCA | 60 °C | F. graminearum | [12] |
| FC01-F FC01-R | ATGGTGAACTCGTCGTGGC CCCTTCTTACGCCAATCTCG | 60 °C | F. culmorum | [12] |
| FspoF1 LanspoR1 | CGCACAACGCAAACTCATC TACAAGAAGACGTGGCGATAT | 60 °C | F. sporotrichioides | [13] |
| DON | 15-ADON | Niv | FX | ZEA | T-2 | HT-2 | FB1 | FB2 | FB3 | Afla | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| DON | 10–12 | 3 | 9 | 1 | 9 | 7 | 7 | 10 | 8 | 8 | 1 |
| 15-ADON | 5 | 5–3 | 3 | 0 | 3 | 3 | 3 | 3 | 3 | 3 | 0 |
| Niv | 6 | 3 | 7–10 | 0 | 9 | 6 | 6 | 8 | 7 | 7 | 1 |
| Fx | 3 | 2 | 3 | 3–1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| Zea | 7 | 4 | 4 | 3 | 7–10 | 7 | 7 | 8 | 7 | 7 | 1 |
| T-2 | 3 | 1 | 4 | 2 | 3 | 4–7 | 7 | 7 | 7 | 7 | 1 |
| HT-2 | 7 | 3 | 5 | 2 | 6 | 4 | 7–8 | 9 | 9 | 9 | 1 |
| FB1 | 7 | 2 | 5 | 2 | 5 | 4 | 7 | 9–11 | 9 | 9 | 1 |
| FB2 | 6 | 1 | 4 | 1 | 4 | 3 | 6 | 8 | 8–9 | 1 | |
| FB3 | 5 | 1 | 4 | 1 | 4 | 3 | 5 | 6 | 6 | 6–9 | 1 |
| Afla | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1–1 |
| Samples | Fusarium Species | |||||
|---|---|---|---|---|---|---|
| F. culmorum | F. graminearum | F. poae | F. sporotrichioides | F. verticillioides | ||
| C-24-VA-02 | Harvest | 0 | ++ | 0 | 0 | 0 |
| Storage | 0 | ++++ | ++ | ++ | ++ | |
| Flour | 0 | ++++ | ++ | ++ | ++ | |
| Meal | 0 | ++++ | ++ | ++ | ++ | |
| C-24-VA-01 | Harvest | 0 | ++ | 0 | 0 | 0 |
| Storage | + | ++++ | ++ | 0 | ++ | |
| Flour | 0 | ++++ | ++ | ++ | ++ | |
| Meal | 0 | ++++ | ++ | ++ | + | |
| C-24-VA-00 | Harvest | 0 | ++ | ++ | ++ | + |
| Storage | + | ++++ | ++++ | ++ | + | |
| Flour | + | ++++ | ++++ | + | 0 | |
| Meal | + | ++++ | ++++ | ++ | + | |
| C-24-SA-01 | Harvest | 0 | ++ | 0 | 0 | 0 |
| Storage | 0 | ++ | 0 | 0 | 0 | |
| Flour | 0 | ++ | ++ | ++ | + | |
| Meal | 0 | ++ | 0 | ++ | ++ | |
| C-24-SASM-01 | Harvest | 0 | ++ | 0 | 0 | +++ |
| Storage | 0 | ++ | ++ | 0 | + | |
| Flour | 0 | ++ | 0 | + | ++ | |
| Meal | 0 | ++ | 0 | 0 | ++ | |
| C-47-BR-01 | Harvest | + | +++ | ++ | 0 | 0 |
| Storage | 0 | +++ | ++ | + | ++ | |
| Flour | 0 | +++ | ++ | ++ | ++++ | |
| Meal | 0 | ++ | ++ | 0 | ++ | |
| Measure 1 | Measure 2 | W | z | p |
|---|---|---|---|---|
| DON Harvest | DON Storage | 19.000 | −1.569 | 0.129 |
| Niv Harvest | Niv Storage | 17.000 | −1.070 | 0.322 |
| T2 + HT2 Harvest | T2 + HT2 Storage | 11.000 | −1.362 | 0.193 |
| ZEA Harvest | ZEA Storage | 9.000 | −1.886 | 0.067 |
| FB1 + FB2 Harvest | FB1 + FB2 Storage | 30.000 | −0.706 | 0.519 |
| Origin of the Samples | DON + 15-ADON | Niv + Fx | T-2 + HT-2 | ZEA | FB1 + FB2 + FB3 |
|---|---|---|---|---|---|
| C-24-VA-02 | 3.36 * | 3.45 * | 14.28 * | 1.54 | S+ |
| C-24-VA-01 | 2.80 * | S++ | 5.26 * | 1.26 | 1.35 |
| C-24-VA-00 | 12.32 * | S++ | S+ | 490.20 * | S+ |
| C-24-SA-01 | 0.34 * | 0.43 * | 0.42 | 0.67 | 1.52 |
| C-24-SASM-01 | 1.44 | nd | 1.04 | nd | 2.92 * |
| C-47-BR-01 | 1.06 | nd | S+ | 0.46 | 4.38 * |
| C-64-JA-01 | 0.84 | 2.60 * | S+ | S+ | 0.86 |
| C-64-BE-01 | 0.12 * | 0.21 * | 0 | 0.71 | 0 |
| C64-AR-01 | S+ | nd | nd | nd | 0.27 |
| C-64-BE-02 | S+ | S+ | nd | S+ | nd |
| C-64-GA-01 | 0.34 * | 0.27 * | nd | S+ | nd |
| C-64-BU-1 | nd | 0.39 * | 0 | nd | 0.40 * |
| C-64-IB-1 | 1.29 | 6.51 * | 3.45 * | 31.42 * | 0.38 * |
| Ratio | Mycotoxins | |||||||
|---|---|---|---|---|---|---|---|---|
| Flour/Meal | DON | Niv | T-2 | HT-2 | ZEA | FB1 | FB2 | FB3 |
| C-24-VA-02 | 1.35 | 2.06 | 1.63 | 1.87 | 1.56 | 1.82 | 2.31 | 1.59 |
| C-24-VA-01 | 1.60 | 0.82 | 1.50 | 1.37 | 1.79 | 1.73 | 2.45 | 1.83 |
| C-24-VA-00 | 1.21 | 0.72 | 1.13 | 0.59 | 1.06 | 1.26 | 1.44 | 1.43 |
| C-24-SA-01 | 1.44 | 0.74 | 2.12 | 1.60 | 6.56 | 3.35 | 3.53 | 3.05 |
| C-24-SASM-01 | 1.48 | / | 1.91 | 1.65 | / | 2.15 | 2.24 | 2.35 |
| C-47-BR-01 | 1.22 | / | 2.36 | 1.04 | 1.37 | 1.76 | 2.11 | 2.15 |
| C-64-JA-01 | 0.63 | 1.22 | / | 0.00 | 0.43 | 0.56 | 0.55 | 0.68 |
| Afla-B1 | Afla-B2 | Afla-G1 | Afla-G2 | Sum | |
|---|---|---|---|---|---|
| Grain at harvest | <LoQ | <LoQ | <LoQ | <LoQ | <LoQ |
| Grain after storage | 99.6 (+/−43.8) | 5.5 (+/−2.4) | 70.0 (+/−30.8) | 4.0 (+/−1.7) | 179 (+/−74) |
| Flour | 80.1 (+/−35.3) | 4.1 (+/−1.8) | 38.2 (+/−16.8) | 2.5 (+/−1.1) | 125 (+/−55) |
| Meal | 134 (+/−58) | 5.8 (+/−2.5) | 54.5 (+/−24.0) | 3.1 (+/−1.4) | 197 (+/−81) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savoie, J.-M.; Pinson-Gadais, L.; Vidal, R.; Vindras-Fouillet, C. Fate of Mycotoxins in Local-Race Populations of Maize Collected in the Southwest of France, from the Field to the Flour and Meal in Organic Farms. Agriculture 2025, 15, 1064. https://doi.org/10.3390/agriculture15101064
Savoie J-M, Pinson-Gadais L, Vidal R, Vindras-Fouillet C. Fate of Mycotoxins in Local-Race Populations of Maize Collected in the Southwest of France, from the Field to the Flour and Meal in Organic Farms. Agriculture. 2025; 15(10):1064. https://doi.org/10.3390/agriculture15101064
Chicago/Turabian StyleSavoie, Jean-Michel, Laetitia Pinson-Gadais, Rodolphe Vidal, and Camille Vindras-Fouillet. 2025. "Fate of Mycotoxins in Local-Race Populations of Maize Collected in the Southwest of France, from the Field to the Flour and Meal in Organic Farms" Agriculture 15, no. 10: 1064. https://doi.org/10.3390/agriculture15101064
APA StyleSavoie, J.-M., Pinson-Gadais, L., Vidal, R., & Vindras-Fouillet, C. (2025). Fate of Mycotoxins in Local-Race Populations of Maize Collected in the Southwest of France, from the Field to the Flour and Meal in Organic Farms. Agriculture, 15(10), 1064. https://doi.org/10.3390/agriculture15101064






