Analysis of the Environmental Impact of Botanical Pesticides in Soil
Abstract
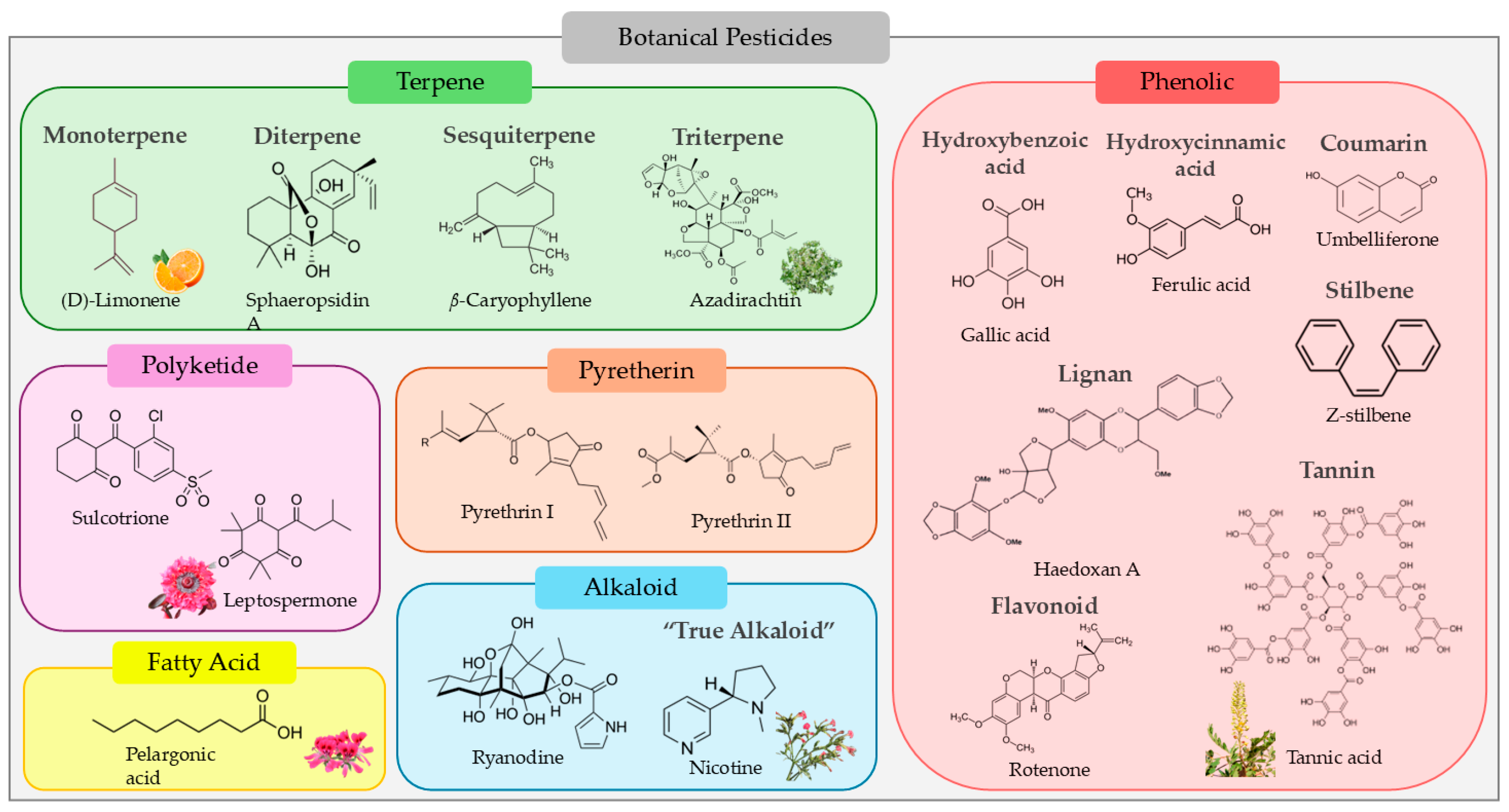
:1. Introduction
2. Degradation Rate, Metabolites, and Routes
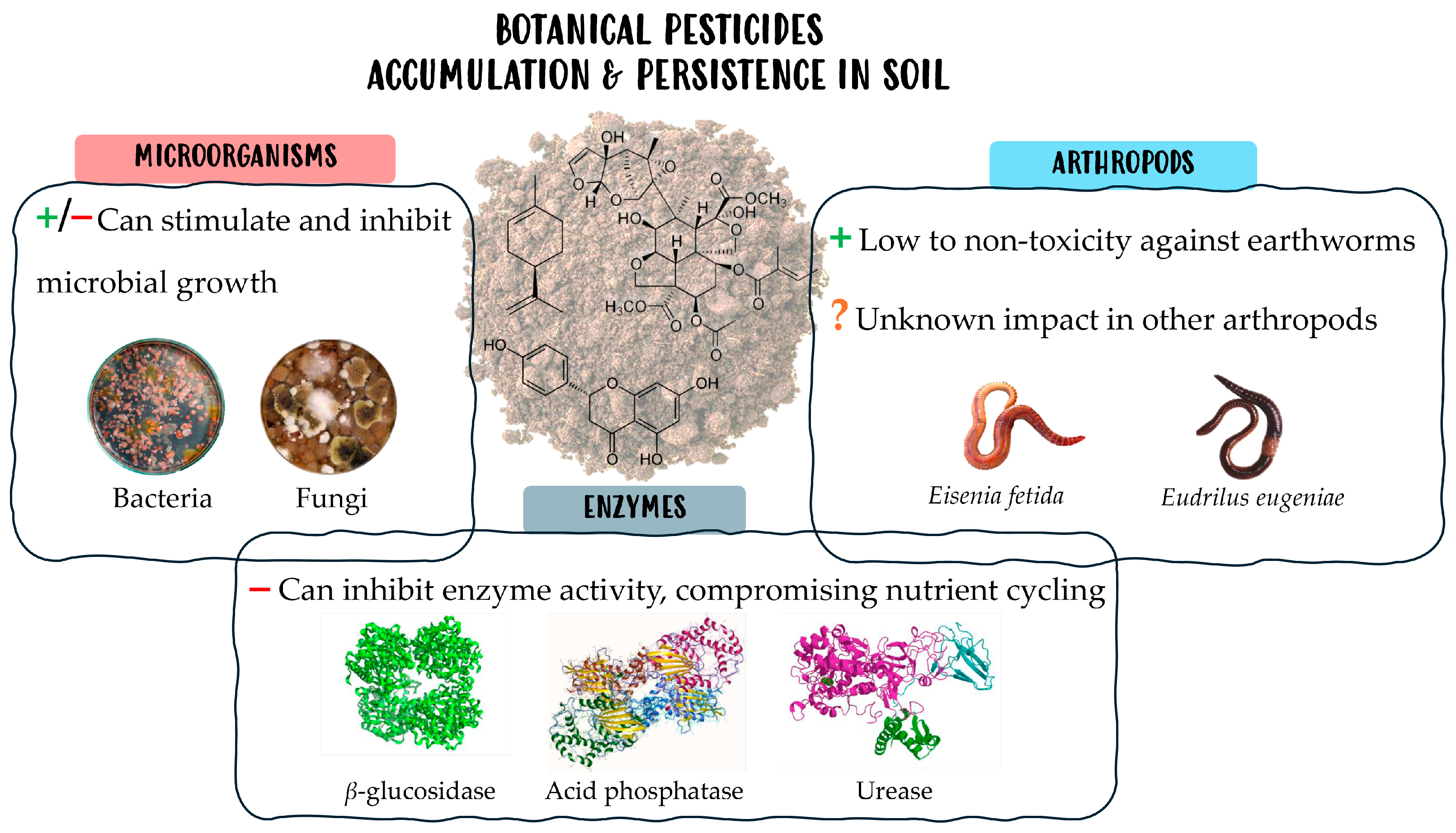
3. Impact in Soil Biology and Biochemistry
- Effects on Microorganisms
- b.
- Effects on Arthropods
- c.
- Impact in Soil Enzymes
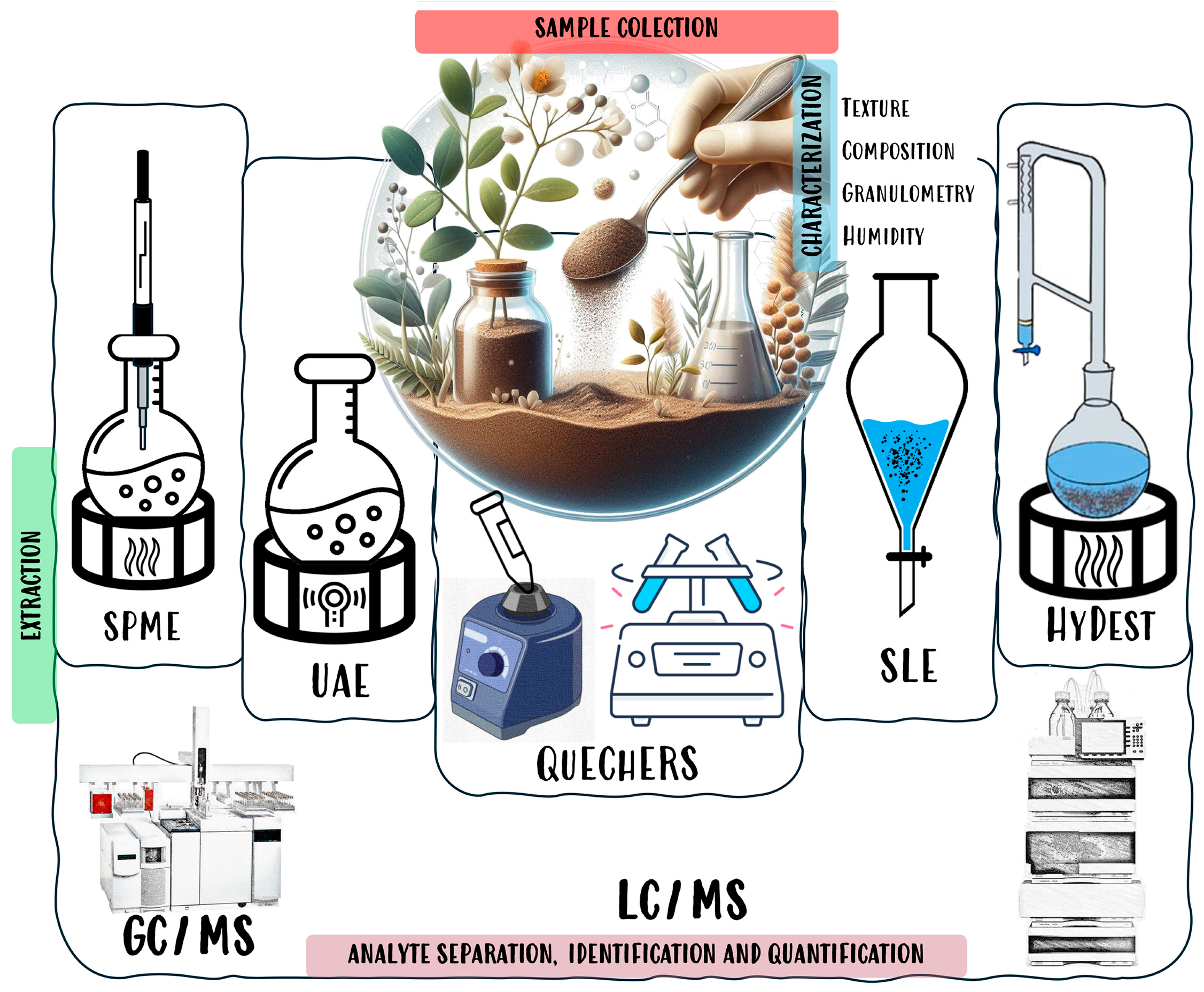
4. Analysis of Botanical Pesticides in Soils
5. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lehmann, J.; Bossio, D.A.; Kogel-Knabner, I.; Rillig, M.C. The concept and future prospects of soil health. Nat. Rev. Earth Environ. 2020, 1, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Siddique, K.H.M.; Liu, K. Cropping systems in agriculture and their impact on soil health-A review. Glob. Ecol. Conserv. 2020, 23, e01118. [Google Scholar] [CrossRef]
- Bhaduri, D.; Sihi, D.; Bhowmik, A.; Verma, B.C.; Munda, S.; Dari, B. A review on effective soil health bio-indicators for ecosystem restoration and sustainability. Front. Microbiol. 2022, 13, 938481. [Google Scholar] [CrossRef]
- Tataridas, A.; Kanatas, P.; Chatzigeorgiou, A.; Zannopoulos, S.; Travlos, I. Sustainable Crop and Weed Management in the Era of the EU Green Deal: A Survival Guide. Agronomy 2022, 12, 589. [Google Scholar] [CrossRef]
- Acheuk, F.; Basiouni, S.; Shehata, A.A.; Dick, K.; Hajri, H.; Lasram, S.; Yilmaz, M.; Emekci, M.; Tsiamis, G.; Spona-Friedl, M.; et al. Status and Prospects of Botanical Biopesticides in Europe and Mediterranean Countries. Biomolecules 2022, 12, 311. [Google Scholar] [CrossRef]
- Marrone, P.G. Status of the biopesticide market and prospects for new bioherbicides. Pest. Manag. Sci. 2024, 80, 81–86. [Google Scholar] [CrossRef]
- Pereira, V.; Figueira, O.; Castilho, P.C. Flavonoids as Insecticides in Crop Protection—A Review of Current Research and Future Prospects. Plants 2024, 13, 776. [Google Scholar] [CrossRef]
- Verified Market Reports. Global Botanical Native Pesticide Market by Type (Phytotoxin, Phytogenous Insect Hormone), by Application (Agriculture, Forestry), by Geographic Scope and Forecast. Available online: https://www.verifiedmarketreports.com/product/botanical-native-pesticide-market/ (accessed on 29 November 2024).
- Chandler, D.; Bailey, A.S.; Tatchell, G.M.; Davidson, G.; Greaves, J.; Grant, W.P. The development, regulation and use of biopesticides for integrated pest management. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 1987–1998. [Google Scholar] [CrossRef] [PubMed]
- Villaverde, J.J.; Sevilla-Morán, B.; Sandín-España, P.; López-Goti, C.; Alonso-Prados, J.L. Challenges of Biopesticides Under the European Regulation (EC) No. 1107/2009: An overview of new trends in residue analysis. Stud. Nat. Prod. Chem. 2014, 43, 437–482. [Google Scholar] [CrossRef]
- FAO; WHO. International Code of Conduct on Pesticide Management; FAO: Rome, Italy, 2017. [Google Scholar]
- Souto, A.L.; Sylvestre, M.; Tolke, E.D.; Tavares, J.F.; Barbosa-Filho, J.M.; Cebrian-Torrejon, G. Plant-Derived Pesticides as an Alternative to Pest Management and Sustainable Agricultural Production: Prospects, Applications and Challenges. Molecules 2021, 26, 4835. [Google Scholar] [CrossRef]
- Schnarr, L.; Segatto, M.L.; Olsson, O.; Zuin, V.G.; Kummerer, K. Flavonoids as biopesticides-Systematic assessment of sources, structures, activities and environmental fate. Sci. Total Environ. 2022, 824, 153781. [Google Scholar] [CrossRef] [PubMed]
- Šunjka, D.; Mechora, Š. An Alternative Source of Biopesticides and Improvement in Their Formulation—Recent Advances. Plants 2022, 11, 3172. [Google Scholar] [CrossRef] [PubMed]
- Karakoti, H.; Mahawer, S.K.; Kabdal, T.; Kumar, R.; Prakash, O. Alkaloids as Botanical Pesticides for Plants Protection. In The Essential Guide to Alkaloids; Semwal, D.K., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2023; pp. 219–236. [Google Scholar]
- Guleria, S.; Tiku, A.K. Botanicals in Pest Management: Current Status and Future Perspectives. In Integrated Pest Management: Innovation-Development Process; Springer: Dordrecht, The Netherlands, 2009; pp. 317–329. [Google Scholar] [CrossRef]
- Li, Y.; Yin, Y.M.; Wang, X.Y.; Wu, H.; Ge, X.Z. Evaluation of berberine as a natural fungicide: Biodegradation and antimicrobial mechanism. J. Asian Nat. Prod. Res. 2018, 20, 148–162. [Google Scholar] [CrossRef]
- Kui, W.; Chao, L.; Hao, L.; Jianmei, X.; Weibo, S.; Ligang, Z. Nematicidal activity of the alkaloids from Macleaya cordata against certain nematodes. Afr. J. Agric. Res. 2012, 7, 5925–5929. [Google Scholar] [CrossRef]
- Petchidurai, G.; Sahayaraj, K.; Al-Shuraym, L.A.; Albogami, B.Z.; Sayed, S.M. Insecticidal Activity of Tannins from Selected Brown Macroalgae against the Cotton Leafhopper Amrasca devastans. Plants 2023, 12, 3188. [Google Scholar] [CrossRef]
- Sarwar, M.H. The Killer Chemicals as Controller of Agriculture Insect Pests: The Conventional Insecticides. Int. J. Chem. Biomol. Sci. 2015, 1, 141–147. [Google Scholar]
- Golawska, S.; Sprawka, I.; Lukasik, I.; Golawski, A. Are naringenin and quercetin useful chemicals in pest-management strategies? J. Pest Sci. 2014, 87, 173–180. [Google Scholar] [CrossRef]
- da Silva, D.F.; Amaral, J.C.; Carlos, R.M.; Ferreira, A.G.; Forim, M.R.; Fernandes, J.B.; da Silva, M.; Filho, H.D.C.; de Souza, A.A. Octahedral ruthenium and magnesium naringenin 5-alkoxide complexes: NMR analysis of diastereoisomers and in-vivo antibacterial activity against Xylella fastidiosa. Talanta 2021, 225, 122040. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Ngoc, H.; Nguyen, C.Q.; Vo, K.A.T.; Nguyen, T.T.T.; Nghiem, D.T.; Ha, N.T.; Nguyen, V.; Choi, G.J.; Ardiansyah, A.G.; Nguyen, C.T.; et al. Insight into the role of phytoalexin naringenin and phytohormone abscisic acid in defense against phytopathogens Phytophthora infestans and Magnaporthe oryzae: In vitro and in silico approaches. Physiol. Mol. Plant Pathol. 2023, 127, 102123. [Google Scholar] [CrossRef]
- López, M.D.; Pascual-Villalobos, M.J. Mode of inhibition of acetylcholinesterase by monoterpenoids and implications for pest control. Ind. Crops Prod. 2010, 31, 284–288. [Google Scholar] [CrossRef]
- Garg, A.; Sharma, R.; Dey, P.; Kundu, A.; Kim, H.S.; Bhakta, T.; Kumar, A. Analysis of triterpenes and triterpenoids. In Recent Advances in Natural Products Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 393–426. [Google Scholar] [CrossRef]
- Wylie, M.R.; Merrell, D.S. The Antimicrobial Potential of the Neem Tree Azadirachta indica. Front. Pharmacol. 2022, 13, 891535. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Yang, Y.; Zhao, T.; Zou, K.; Peng, C.; Cai, H.; Wan, X.; Hou, R. Insecticidal Activity and Insecticidal Mechanism of Total Saponins from Camellia oleifera. Molecules 2019, 24, 4518. [Google Scholar] [CrossRef]
- Goujon, E.; Sta, C.; Trivella, A.; Goupil, P.; Richard, C.; Ledoigt, G. Genotoxicity of sulcotrione pesticide and photoproducts on Allium cepa root meristem. Pestic. Biochem. Physiol. 2014, 113, 47–54. [Google Scholar] [CrossRef]
- Mallet, C.; Romdhane, S.; Loiseau, C.; Beguet, J.; Martin-Laurent, F.; Calvayrac, C.; Barthelmebs, L. Impact of Leptospermone, a Natural beta-Triketone Herbicide, on the Fungal Composition and Diversity of Two Arable Soils. Front. Microbiol. 2019, 10, 1024. [Google Scholar] [CrossRef] [PubMed]
- Loddo, D.; Jagarapu, K.K.; Strati, E.; Trespidi, G.; Nikolic, N.; Masin, R.; Berti, A.; Otto, S. Assessing Herbicide Efficacy of Pelargonic Acid on Several Weed Species. Agronomy 2023, 13, 1511. [Google Scholar] [CrossRef]
- Cavoski, I.; Caboni, P.; Sarais, G.; Cabras, P.; Miano, T. Photodegradation of rotenone in soils under environmental conditions. J. Agric. Food. Chem. 2007, 55, 7069–7074. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, K.; Yan, C.; Li, W.S.; Li, H.; Zhang, N.; Zhang, Z.X. Effect of two formulations on the decline curves and residue levels of rotenone in cabbage and soil under field conditions. Ecotoxicol. Environ. Saf. 2014, 104, 23–27. [Google Scholar] [CrossRef]
- Ibarra-Gutierrez, M.T.; Serrano-Garcia, N.; Orozco-Ibarra, M. Rotenone-Induced Model of Parkinson’s Disease: Beyond Mitochondrial Complex I Inhibition. Mol. Neurobiol. 2023, 60, 1929–1948. [Google Scholar] [CrossRef]
- Cavoski, I.; Caboni, P.; Sarais, G.; Miano, T. Degradation and persistence of rotenone in soils and influence of temperature variations. J. Agric. Food. Chem. 2008, 56, 8066–8073. [Google Scholar] [CrossRef]
- US Department of Health and Human Services. Toxicological Profile for Pyrethrins and Pyrethroids. Atlanta (GA): Agency for Toxic Substances and Disease Registry (US); 2003. HEALTH EFFECTS. Available online: https://www.ncbi.nlm.nih.gov/books/NBK600322/ (accessed on 29 November 2024).
- Dalefield, R. Insecticides and Acaricides. In Veterinary Toxicology for Australia and New Zealand; Elsevier: Amsterdam, The Netherlands, 2017; pp. 87–109. [Google Scholar] [CrossRef]
- Penefsky, Z.J. Studies on mechanism of inhibition of cardiac muscle contractile tension by ryanodine. Mechanical response. Pflug. Arch. 1974, 347, 173–184. [Google Scholar] [CrossRef]
- Fairhurst, A.S.; Hamamoto, V.; Macri, J. Modification of ryanodine toxicity by dantrolene and halothane in a model of malignant hyperthermia. Anesthesiology 1980, 53, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, G. Ryanodine. In xPharm: The Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier: New York, NY, USA, 2009; pp. 1–4. [Google Scholar] [CrossRef]
- Mitra, S.; Saran, R.K.; Srivastava, S.; Rensing, C. Pesticides in the environment: Degradation routes, pesticide transformation products and ecotoxicological considerations. Sci. Total Environ. 2024, 935, 173026. [Google Scholar] [CrossRef]
- Liu, B.B.; Chen, B.; Zhang, J.; Wang, P.; Feng, G. The environmental fate of thymol, a novel botanical pesticide, in tropical agricultural soil and water. Toxicol. Environ. Chem. 2017, 99, 223–232. [Google Scholar] [CrossRef]
- Gámiz, B.; Hermosín, M.C.; Celis, R. Appraising factors governing sorption and dissipation of the monoterpene carvone in agricultural soils. Geoderma 2018, 321, 61–68. [Google Scholar] [CrossRef]
- Gámiz, B.; Facenda, G.; Celis, R. Modulating the persistence and bioactivity of allelochemicals in the rhizosphere: Salicylic acid, a case of study. Arch. Agron. Soil. Sci. 2018, 65, 581–595. [Google Scholar] [CrossRef]
- Wirsching, J.; Rodriguez, L.C.; Ditterich, F.; Pagel, H.; He, R.; Uksa, M.; Zwiener, C.; Kandeler, E.; Poll, C. Temperature and soil moisture change microbial allocation of pesticide-derived carbon. Eur. J. Soil. Sci. 2023, 74, e13417. [Google Scholar] [CrossRef]
- Khouni, M.; Hammecker, C.; Grunberger, O.; Chaabane, H. Effect of salinity on the fate of pesticides in irrigated systems: A first overview. Environ. Sci. Pollut. Res. Int. 2023, 30, 90471–90488. [Google Scholar] [CrossRef]
- Rodríguez-Cruz, M.S.; Jones, J.E.; Bending, G.D. Field-scale study of the variability in pesticide biodegradation with soil depth and its relationship with soil characteristics. Soil. Biol. Biochem. 2006, 38, 2910–2918. [Google Scholar] [CrossRef]
- Reyes-Avila, A.; Garrido Frenich, A.; Romero-Gonzalez, R. Degradation of limonene and trans-cinnamaldehyde in soil, and detection of their metabolites by UHPLC and GC-HRMS. Environ. Sci. Pollut. Res. Int. 2024, 31, 33058–33068. [Google Scholar] [CrossRef]
- Karamanoli, K.; Ainalidou, A.; Bouzoulda, F.; Vokou, D. Decomposition profiles of leaf essential oils in the soil environment. Ind. Crops Prod. 2018, 124, 397–401. [Google Scholar] [CrossRef]
- Bravetti, M.M.D.; Carpinella, M.C.; Palacios, S.M. Phytotoxicity of Cortaderia speciosa extract, active principles, degradation in soil and effectiveness in field tests. Chemoecology 2020, 30, 15–24. [Google Scholar] [CrossRef]
- Weidenhamer, J.D.; Romeo, J.T. Allelochemicals of Polygonella myriophylla: Chemistry and soil degradation. J. Chem. Ecol. 2004, 30, 1067–1082. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.P.; Li, C.R.; Wang, Q.K.; Wang, H.T.; Duan, B.L.; Zhang, G.C. Environmental behaviors of phenolic acids dominated their rhizodeposition in boreal poplar plantation forest soils. J. Soils Sed. 2016, 16, 1858–1870. [Google Scholar] [CrossRef]
- Furbo, S.; Mortensen, A.G.; Laursen, B.; Christophersen, C.; Fomsgaard, I.S. Degradation of biochanin A in soil. Chemoecology 2011, 21, 59–66. [Google Scholar] [CrossRef]
- Gámiz, B.; Celis, R. S-Carvone Formulation Based on Granules of Organoclay to Modulate Its Losses and Phytotoxicity in Soil. Agronomy 2021, 11, 1593. [Google Scholar] [CrossRef]
- Macias, F.A.; Oliveros-Bastidas, A.; Marin, D.; Castellano, D.; Simonet, A.M.; Molinillo, J.M. Degradation studies on benzoxazinoids. Soil degradation dynamics of 2,4-dihydroxy-7-methoxy-(2H)-1,4-benzoxazin-3(4H)-one (DIMBOA) and its degradation products, phytotoxic allelochemicals from gramineae. J. Agric. Food. Chem. 2004, 52, 6402–6413. [Google Scholar] [CrossRef]
- Shaw, L.J.; Hooker, J.E. The fate and toxicity of the flavonoids naringenin and formononetin in soil. Soil. Biol. Biochem. 2008, 40, 528–536. [Google Scholar] [CrossRef]
- Chinchilla, N.; Marín, D.; Oliveros-Bastidas, A.; Molinillo, J.M.G.; Macías, F. Soil biodegradation of a benzoxazinone analog proposed as a natural products-based herbicide. Plant Soil. 2015, 393, 207–214. [Google Scholar] [CrossRef]
- Huang, C.; Bian, C.; Wang, L.; Zhou, W.; Li, Y.; Li, B. Development and validation of a method for determining d-limonene and its oxidation products in vegetables and soil using GC–MS. Microchem. J. 2022, 179, 107470. [Google Scholar] [CrossRef]
- Wang, H.W.; Tang, M.J.; Su, C.L.; Zhang, W.; Xu, R.S.; Guan, Y.X.; Dai, C.C. The Alleopathic Compound Luteolin from Peanut Litter Affects Peanut Nodule Formation and the Rhizosphere Microbial Community. Agron. J. 2018, 110, 2587–2595. [Google Scholar] [CrossRef]
- Galan-Perez, J.A.; Gamiz, B.; Pavlovic, I.; Celis, R. Enantiomer-Selective Characterization of the Adsorption, Dissipation, and Phytotoxicity of the Plant Monoterpene Pulegone in Soils. Plants 2022, 11, 1296. [Google Scholar] [CrossRef] [PubMed]
- Galán-Pérez, J.A.; Gámiz, B.; Celis, R. Determining the effect of soil properties on the stability of scopoletin and its toxicity to target plants. Biol. Fertil. Soils 2021, 57, 643–655. [Google Scholar] [CrossRef]
- Real, M.; Gamiz, B.; Lopez-Cabeza, R.; Celis, R. Sorption, persistence, and leaching of the allelochemical umbelliferone in soils treated with nanoengineered sorbents. Sci. Rep. 2019, 9, 9764. [Google Scholar] [CrossRef] [PubMed]
- Real, M.; Facenda, G.; Celis, R. Sorption and dissipation of the allelochemicals umbelliferone and salicylic acid in a Mediterranean soil environment: Effect of olive-mill waste addition. Sci. Total Environ. 2021, 774, 145027. [Google Scholar] [CrossRef]
- Mączka, W.; Twardawska, M.; Grabarczyk, M.; Wińska, K. Carvacrol—A Natural Phenolic Compound with Antimicrobial Properties. Antibiotics 2023, 12, 824. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Liang, Y.; Shi, S.; Wu, C.; Shi, Z. Improving predictions and understanding of primary and ultimate biodegradation rates with machine learning models. Sci. Total Environ. 2023, 904, 166623. [Google Scholar] [CrossRef]
- Brydon, L.; Zhang, K.; Dobbie, G.; Taskova, K.; Wicker, J.S. Predictive modeling of biodegradation pathways using transformer architectures. J. Cheminform 2025, 17, 21. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; He, Y.; Ji, R. Biodegradation of phenolic pollutants and bioaugmentation strategies: A review of current knowledge and future perspectives. J. Hazard. Mater. 2024, 469, 133906. [Google Scholar] [CrossRef]
- Malla, M.A.; Dubey, A.; Kumar, A.; Vennapu, D.R.; Upadhyay, N.; Pradhan, D.; Pradhan, R.C.; Yadav, S. Process optimization of cypermethrin biodegradation by regression analysis and parametric modeling along with biochemical degradation pathway. Environ. Sci. Pollut. Res. Int. 2022, 29, 77418–77427. [Google Scholar] [CrossRef]
- Jenner, K.J.; Kreutzer, G.; Racine, P. Persistency assessment and aerobic biodegradation of selected cyclic sesquiterpenes present in essential oils. Environ. Toxicol. Chem. 2011, 30, 1096–1108. [Google Scholar] [CrossRef]
- Shi, Y.; Jiao, B.; Guo, P.; Pan, X.; Wu, X.; Xu, J.; Xiang, W.; Dong, F.; Wang, X.; Zheng, Y. Toxicity assessment of a novel biopesticide guvermectin and identification of its transformation products in soils. Sci. Total Environ. 2023, 903, 166113. [Google Scholar] [CrossRef] [PubMed]
- Al-Kaisi, M.M.; Lal, R. Conservation Agriculture Systems to Mitigate Climate Variability Effects on Soil Health. In Soil Health and Intensification of Agroecosytems; Elsevier: Amsterdam, The Netherlands, 2017; pp. 79–107. [Google Scholar] [CrossRef]
- Adomako, M.O.; Roiloa, S.; Yu, F.H. Potential Roles of Soil Microorganisms in Regulating the Effect of Soil Nutrient Heterogeneity on Plant Performance. Microorganisms 2022, 10, 2399. [Google Scholar] [CrossRef]
- Wang, G.; Ren, Y.; Bai, X.; Su, Y.; Han, J. Contributions of Beneficial Microorganisms in Soil Remediation and Quality Improvement of Medicinal Plants. Plants 2022, 11, 3200. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Cui, H.; Fu, C.; Li, R.; Qi, F.; Liu, Z.; Yang, G.; Xiao, K.; Qiao, M. Unveiling the crucial role of soil microorganisms in carbon cycling: A review. Sci. Total Environ. 2024, 909, 168627. [Google Scholar] [CrossRef]
- Xie, J.; Wicaksono, W.A.; Lv, Z.; Berg, G.; Cernava, T.; Ge, B. Rhizosphere bacteria show a stronger response to antibiotic-based biopesticide than to conventional pesticides. J. Hazard. Mater. 2023, 458, 132035. [Google Scholar] [CrossRef] [PubMed]
- Jouini, A.; Verdeguer, M.; Pinton, S.; Araniti, F.; Palazzolo, E.; Badalucco, L.; Laudicina, V.A. Potential Effects of Essential Oils Extracted from Mediterranean Aromatic Plants on Target Weeds and Soil Microorganisms. Plants 2020, 9, 1289. [Google Scholar] [CrossRef]
- Thiele-Bruhn, S.; Shikuku, V.; Dittrich, F.; Torjir, D.N.; Saini, M.; Getenga, Z. Soil sorption and effects on soil microorganisms of thymol and carvacrol monoterpenes from essential oils of aromatic plants. Front. Environ. Sci. 2024, 12, 1379018. [Google Scholar] [CrossRef]
- Pedrinho, A.; Karas, P.A.; Kanellopoulos, A.; Feray, E.; Korman, I.; Wittenberg, G.; Ramot, O.; Karpouzas, D.G. The effect of natural products used as pesticides on the soil microbiota: OECD 216 nitrogen transformation test fails to identify effects that were detected via q-PCR microbial abundance measurement. Pest. Manag. Sci. 2024, 80, 2563–2576. [Google Scholar] [CrossRef]
- Merino, D.; Casalongué, C.; Alvarez, V.A. Polysaccharides as Eco-nanomaterials for Agricultural Applications. In Handbook of Ecomaterials; Martínez, L.M.T., Kharissova, O.V., Kharisov, B.I., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 2709–2730. [Google Scholar] [CrossRef]
- Chmiel, M.; Drzymala, G.; Bocianowski, J.; Komnenic, A.; Baran, A.; Synowiec, A. Maltodextrin-Coated Peppermint and Caraway Essential Oils Effects on Soil Microbiota. Plants 2022, 11, 3343. [Google Scholar] [CrossRef]
- Bagyaraj, D.J.; Nethravathi, C.J.; Nitin, K.S. Soil Biodiversity and Arthropods: Role in Soil Fertility. In Economic and Ecological Significance of Arthropods in Diversified Ecosystems; Springer: Singapore, 2016; pp. 17–51. [Google Scholar] [CrossRef]
- Al-Maliki, S.; Al-Taey, D.K.A.; Al-Mammori, H.Z. Earthworms and eco-consequences: Considerations to soil biological indicators and plant function: A review. Acta Ecol. Sin. 2021, 41, 512–523. [Google Scholar] [CrossRef]
- De Bernardi, A.; Marini, E.; Casucci, C.; Tiano, L.; Marcheggiani, F.; Ciani, M.; Comitini, F.; Taskin, E.; Puglisi, E.; Vischetti, C. Ecotoxicological effects of a synthetic and a natural insecticide on earthworms and soil bacterial community. Environ. Adv. 2022, 8, 100225. [Google Scholar] [CrossRef]
- Vasantha-Srinivasan, P.; Senthil-Nathan, S.; Ponsankar, A.; Thanigaivel, A.; Chellappandian, M.; Edwin, E.S.; Selin-Rani, S.; Kalaivani, K.; Hunter, W.B.; Duraipandiyan, V.; et al. Acute toxicity of chemical pesticides and plant-derived essential oil on the behavior and development of earthworms, Eudrilus eugeniae (Kinberg) and Eisenia fetida (Savigny). Environ. Sci. Pollut. Res. Int. 2018, 25, 10371–10382. [Google Scholar] [CrossRef]
- Vivekanandhan, P.; Alahmadi, T.A.; Ansari, M.J.; Subala, S.P. Biocontrol efficacy of cajeput oil against Anopheles stephensi L. mosquito and its effect on non-target species. Front. Physiol. 2024, 15, 1357411. [Google Scholar] [CrossRef]
- Zabka, M.; Pavela, R.; Kovarikova, K.; Triska, J.; Vrchotova, N.; Bednar, J. Antifungal and Insecticidal Potential of the Essential Oil from Ocimum sanctum L. against Dangerous Fungal and Insect Species and Its Safety for Non-Target Useful Soil Species Eisenia fetida (Savigny, 1826). Plants 2021, 10, 2180. [Google Scholar] [CrossRef] [PubMed]
- Murfadunnisa, S.; Vasantha-Srinivasan, P.; Ganesan, R.; Senthil-Nathan, S.; Kim, T.J.; Ponsankar, A.; Kumar, S.D.; Chandramohan, D.; Krutmuang, P. Larvicidal and enzyme inhibition of essential oil from Spheranthus amaranthroids (Burm.) against lepidopteran pest Spodoptera litura (Fab.) and their impact on non-target earthworms. Biocatal. Agric. Biotechnol. 2019, 21, 101324. [Google Scholar] [CrossRef]
- Iperti, G. Biodiversity of predaceous coccinellidae in relation to bioindication and economic importance. Agric. Ecosyst. Environ. 1999, 74, 323–342. [Google Scholar] [CrossRef]
- Lami, F.; Burgio, G.; Magagnoli, S.; Depalo, L.; Lanzoni, A.; Frassineti, E.; Marotti, I.; Alpi, M.; Mercatante, D.; Rodriguez-Estrada, M.T.; et al. The Effects of Natural Insecticides on the Green Peach Aphid Myzus persicae (Sulzer) and Its Natural Enemies Propylea quatuordecimpunctata (L.) and Aphidius colemani Viereck. Insects 2024, 15, 556. [Google Scholar] [CrossRef]
- Britto, I.O.; Araujo, S.H.C.; Toledo, P.F.S.; Lima, G.D.A.; Salustiano, I.V.; Alves, J.R.; Mantilla-Afanador, J.G.; Kohlhoff, M.; Oliveira, E.E.; Leite, J.P.V. Potential of Ficus carica extracts against Euschistus heros: Toxicity of major active compounds and selectivity against beneficial insects. Pest. Manag. Sci. 2021, 77, 4638–4647. [Google Scholar] [CrossRef] [PubMed]
- Toledo, P.F.S.; da Cruz Araujo, S.H.; Mantilla Afanador, J.G.; Silva, A.C.F.; Machado, F.P.; Rocha, L.M.; Oliveira, E.E. Potential of Ocotea indecora Essential Oil for Controlling Drosophila suzukii: Molecular Predictions for Toxicity and Selectivity to Beneficial Arthropods. Neotrop. Entomol. 2024, 53, 189–199. [Google Scholar] [CrossRef]
- Silva, D.E.; Nascimento, J.M.d.; Meira, A.d.A.; Costa Corrêa, L.L.; Johann, L.; Rodrigues, R.; Ferla, N.J. Intraspecific variation of Typhlodromus (Typhlodromus) pyri Scheuten in vineyards of Northwest Portugal. Int. J. Acarol. 2019, 46, 60–62. [Google Scholar] [CrossRef]
- Duso, C.; Pozzebon, A.; Lorenzon, M.; Fornasiero, D.; Tirello, P.; Simoni, S.; Bagnoli, B. The Impact of Microbial and Botanical Insecticides on Grape Berry Moths and Their Effects on Secondary Pests and Beneficials. Agronomy 2022, 12, 217. [Google Scholar] [CrossRef]
- Paspati, A.; Karakosta, E.; Balanza, V.; Rodríguez-Gómez, A.; Grávalos, C.; Cifuentes, D.; Koukaki, A.; Stavrakaki, M.; Roditakis, E.; Bielza, P.; et al. Effects of novel and commercial phytochemicals on beneficial arthropods. Crop. Protect. 2023, 174, 106381. [Google Scholar] [CrossRef]
- Tian, Y.; Chen, Z.; Huang, X.; Zhang, L.; Zhang, Z. Evaluation of Botanicals for Management of Piercing-Sucking Pests and the Effect on Beneficial Arthropod Populations in Tea Trees Camellia sinensis (L.) O. Kuntze (Theaceae). J. Insect Sci. 2020, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sprunger, C.D.; Martin, T.K. An integrated approach to assessing soil biological health. Adv. Agron. 2023, 182, 132–160. [Google Scholar] [CrossRef]
- Liao, Z.; Fan, J.; Lai, Z.; Bai, Z.; Wang, H.; Cheng, M.; Zhang, F.; Li, Z. Response network and regulatory measures of plant-soil-rhizosphere environment to drought stress. Adv. Agron. 2023, 180, 93–169. [Google Scholar] [CrossRef]
- Adamczyk, S.; Adamczyk, B.; Kitunen, V.; Smolander, A. Monoterpenes and higher terpenes may inhibit enzyme activities in boreal forest soil. Soil. Biol. Biochem. 2015, 87, 59–66. [Google Scholar] [CrossRef]
- Papatheodorou, E.M.; Margariti, C.; Vokou, D. Effects of the two carvone enantiomers on soil enzymes involved in the C, P, and N cycles. J. Biol. Res. 2014, 21, 7. [Google Scholar] [CrossRef]
- Campolo, O.; Puglisi, I.; Barbagallo, R.N.; Cherif, A.; Ricupero, M.; Biondi, A.; Palmeri, V.; Baglieri, A.; Zappala, L. Side effects of two citrus essential oil formulations on a generalist insect predator, plant and soil enzymatic activities. Chemosphere 2020, 257, 127252. [Google Scholar] [CrossRef]
- Galhardi, J.A.; de Oliveira, J.L.; Ghoshal, S.; Fraceto, L.F. Soil Enzyme Responses to Polymeric Nanopesticides: An Ecological Risk Analysis Approach to Promote Sustainable Agriculture. ACS Agric. Sci. Technol. 2022, 2, 443–452. [Google Scholar] [CrossRef]
- Adamczyk, B.; Karonen, M.; Adamczyk, S.; Engström, M.T.; Laakso, T.; Saranpää, P.; Kitunen, V.; Smolander, A.; Simon, J. Tannins can slow-down but also speed-up soil enzymatic activity in boreal forest. Soil. Biol. Biochem. 2017, 107, 60–67. [Google Scholar] [CrossRef]
- Liu, X.; Yao, Z.; Cheng, L.; Gao, Z.; Ren, L.; Wang, Y.; Liu, Y.; Hu, Y. Condensed tannin addition decreased soil nitrate but increased soil enzyme activities in subtropical forest soil. J. Soils Sed. 2023, 24, 537–551. [Google Scholar] [CrossRef]
- Tava, A.; Biazzi, E.; Fornasier, F.; D’Addabbo, T.; Avato, P.; Scotti, C. Saponins in soil, their degradation and effect on soil enzymatic activities. J. Environ. Sci. 2024, 154, 713–728. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Ramlal, A.; Mallick, D.; Mishra, V. An Overview of Some Biopesticides and Their Importance in Plant Protection for Commercial Acceptance. Plants 2021, 10, 1185. [Google Scholar] [CrossRef]
- Ayilara, M.S.; Adeleke, B.S.; Akinola, S.A.; Fayose, C.A.; Adeyemi, U.T.; Gbadegesin, L.A.; Omole, R.K.; Johnson, R.M.; Uthman, Q.O.; Babalola, O.O. Biopesticides as a promising alternative to synthetic pesticides: A case for microbial pesticides, phytopesticides, and nanobiopesticides. Front. Microbiol. 2023, 14, 1040901. [Google Scholar] [CrossRef]
- Huang, C.; Zhou, W.; Bian, C.; Wang, L.; Li, Y.; Li, B. Degradation and Pathways of Carvone in Soil and Water. Molecules 2022, 27, 2415. [Google Scholar] [CrossRef]
- López-Serna, R.; Ernst, F.; Wu, L. Analysis of cinnamaldehyde and diallyl disulfide as eco-pesticides in soils of different textures—A laboratory-scale mobility study. J. Soils Sed. 2016, 16, 566–580. [Google Scholar] [CrossRef]
- Liu, X.; Tian, Y.; Dong, F.; Xu, J.; Li, Y.; Liang, X.; Wang, Y.; Zheng, Y. Simultaneous Determination of Matrine and Berberine in Fruits, Vegetables, and Soil Using Ultra-Performance Liquid Chromatography/Tandem Mass Spectrometry. J. AOAC Int. 2019, 97, 218–224. [Google Scholar] [CrossRef]
- Hui, X.; Chen, H.; Shen, S.; Zhi, H.; Li, W. Establishment of Residual Methods for Matrine in Quinoa Plants and Soil and the Effect on Soil Bacterial Community and Composition. Foods 2023, 12, 1337. [Google Scholar] [CrossRef]
- Feng, X.; Pan, L.; Wang, C.; Zhang, H. Residue analysis and risk assessment of pyrethrins in open field and greenhouse turnips. Environ. Sci. Pollut. Res. Int. 2018, 25, 877–886. [Google Scholar] [CrossRef]
- Adak, T.; Mukherjee, I. Dissipation kinetics of spinosad from tomato under sub-tropical agro-climatic conditions. Environ. Monit. Assess. 2016, 188, 299. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, H.; Wang, S. Dissipation and residue of spinosad in zucchini under field conditions. Bull. Environ. Contam. Toxicol. 2013, 91, 256–259. [Google Scholar] [CrossRef] [PubMed]
- El-Saeid, M.H.; Alghamdi, A.G. Identification of Pesticide Residues and Prediction of Their Fate in Agricultural Soil. Water Air Soil. Pollut. 2020, 231, 284. [Google Scholar] [CrossRef]
- Ghosson, H.; Raviglione, D.; Salvia, M.V.; Bertrand, C. Online Headspace-Solid Phase Microextraction-Gas Chromatography-Mass Spectrometry-based untargeted volatile metabolomics for studying emerging complex biopesticides: A proof of concept. Anal. Chim. Acta 2020, 1134, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Brinco, J.; Guedes, P.; Gomes da Silva, M.; Mateus, E.P.; Ribeiro, A.B. Analysis of pesticide residues in soil: A review and comparison of methodologies. Microchem. J. 2023, 195, 109465. [Google Scholar] [CrossRef]
- Gonzalez-Curbelo, M.A.; Varela-Martinez, D.A.; Riano-Herrera, D.A. Pesticide-Residue Analysis in Soils by the QuEChERS Method: A Review. Molecules 2022, 27, 4323. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, P.; Porto-Figueira, P.; Pereira, J.A.M.; Silva, C.; Medina, S.; Camara, J.S. QuEChERS-Fundamentals, relevant improvements, applications and future trends. Anal. Chim. Acta 2019, 1070, 1–28. [Google Scholar] [CrossRef]
- Prestes, O.D.; Padilla-Sanchez, J.A.; Romero-Gonzalez, R.; Lopez Grio, S.; Garrido Frenich, A.; Martinez-Vidal, J.L. Comparison of several extraction procedures for the determination of biopesticides in soil samples by ultrahigh pressure LC-MS/MS. J. Sep. Sci. 2012, 35, 861–868. [Google Scholar] [CrossRef]
- Ngegba, P.M.; Cui, G.; Khalid, M.Z.; Zhong, G. Use of Botanical Pesticides in Agriculture as an Alternative to Synthetic Pesticides. Agriculture 2022, 12, 600. [Google Scholar] [CrossRef]
- Damalas, C.; Koutroubas, S. Botanical Pesticides for Eco-Friendly Pest Management: Drawbacks and Limitations; Wiley: Hoboken, NJ, USA, 2020; pp. 181–193. [Google Scholar] [CrossRef]
- Rainio, J.; Niemelä, J. Ground beetles (Coleoptera: Carabidae) as bioindicators. Biodivers. Conserv. 2003, 12, 487–506. [Google Scholar] [CrossRef]
- Zhao, J.; Liang, D.; Li, W.; Yan, X.; Qiao, J.; Caiyin, Q. Research Progress on the Synthetic Biology of Botanical Biopesticides. Bioengineering 2022, 9, 207. [Google Scholar] [CrossRef]
- Xu, L.; Abd El-Aty, A.M.; Eun, J.B.; Shim, J.H.; Zhao, J.; Lei, X.; Gao, S.; She, Y.; Jin, F.; Wang, J.; et al. Recent Advances in Rapid Detection Techniques for Pesticide Residue: A Review. J. Agric. Food. Chem. 2022, 70, 13093–13117. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.; Salahshoor, Z.; Ho, K.V.; Lin, C.H.; Errea, M.I.; Fidalgo, M.M. Detection of chlorantraniliprole residues in tomato using field-deployable MIP photonic sensors. Mikrochim. Acta 2021, 188, 70. [Google Scholar] [CrossRef] [PubMed]
- Jara, M.D.L.; Alvarez, L.A.C.; Guimarães, M.C.C.; Antunes, P.W.P.; de Oliveira, J.P. Lateral flow assay applied to pesticides detection: Recent trends and progress. Environ. Sci. Pollut. Res. 2022, 29, 46487–46508. [Google Scholar] [CrossRef] [PubMed]
- Dhamu, V.N.; Sukumar, S.; Kadambathil, C.S.; Muthukumar, S.; Prasad, S. Targeted On-Demand Screening of Pesticide Panel in Soil Runoff. Front. Chem. 2021, 9, 782252. [Google Scholar] [CrossRef]
- Lin, Z.; Fu, X.; Zheng, K.; Han, S.; Chen, C.; Ye, D. Cellulose Surface Nanoengineering for Visualizing Food Safety. Nano Lett. 2024, 24, 10016–10023. [Google Scholar] [CrossRef]
| Botanical Pesticide | Mode of Action | Drawbacks | Reference |
|---|---|---|---|
Nicotine sulfate (from Nicotiana tabacum leaves)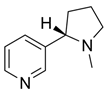 | Disrupts the insect’s nervous system, resulting in death due to convulsions and/or paralysis. | Highly toxic to mammals by inhalation and skin contact, to blooded animals and other insects. | [16] |
Pyrethrins (from Chrysanthemum genus) | Act on sodium channels of axonal membranes by altering their permeability, resulting in a decrease of sodium and potassium effluxes. The physiological effects are excitation, lack of coordination, and paralysis. They can also inhibit ATPases. | The pyrethrum crude extract may cause diverse complications in humans. Toxic to bees and fish. | [16,35,36] |
Rotenone (from Derris, Lonchocarpus, and Tephrosia genus)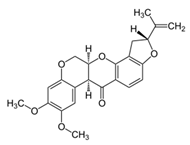 | Forms a complex with NADH dehydrogenase, inhibiting the oxidation of NADH to NAD and therefore, blocks the oxidation by NAD of several substrates. Inhibits mitochondrial electron transport and mitosis. | Toxic to mammals by inhalation, ingestion, and skin contact. Induces Parkinson’s disease in rats. Highly toxic to fish. | [16,32,33,36] |
Ryanodine (from Ryania speciosa)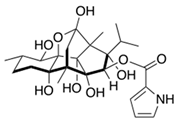 | Acts at the level of the sarcoplasmic reticulum membrane by binding to ryanodine receptors in muscle cells, leading to insect muscle contractions or paralysis depending on the concentration. | Produces irreversible contractures in vertebrate skeletal muscle and negative inotropic responses in mammalian cardiac muscle. | [37,38,39] |
Veratrine (mixture of alkaloids from Schoenocaulon officinale)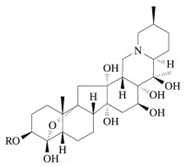 | Affects the nerve cells, resulting in loss of nerve function, paralysis, and death. Acts as a contact and stomach poison to insects. | Irritating to humans if inhaled and by skin contact. | [16] |
| Botanical Pesticide | Soil Type/Texture | Decomposition Profile | Reference |
|---|---|---|---|
| Essential Oils (EOs) | |||
| Cinnamomum verum | Sandy clay and clay loams |
| [47] |
| Citrus sinensis | Sandy clay and clay loams |
| [47] |
| Mentha piperita | Fallow land |
| [48] |
| Mentha spicata | Fallow land |
| [48] |
| Rosmarinus officinalis | Fallow land |
| [48] |
| Plant-Based Extracts | |||
| Cortaderia speciosa | Experimental field |
| [49] |
| Pure/Isolated Compounds | |||
| Arbutin | Scrub soil of Polygonella shrubs |
| [50] |
| Benzoic acid | Bulk soil |
| [51] |
| Benzoquinone | Scrub soil of Polygonella shrubs |
| [50] |
| Biochanin A | Sandy loam |
| [52] |
| Carvone | Agricultural soil |
| [42] |
| Agricultural soil |
| [53] | |
| Cinnamic acid | Bulk soil |
| [51] |
| 2,4-Dihydroxy-7-methoxy-(2H)-1,4-benzoxazin-3(4H)-one | Wheat crop soil |
| [54] |
| Formononetin | Modified agricultural soil |
| [55] |
| Gallic acid | Scrub soil of Polygonella shrubs |
| [50] |
| Hydroquinone | Scrub soil of Polygonella shrubs |
| [50] |
| p-hydroxybenzoic acid | Bulk soil |
| [51] |
| 4-hydroxy-(2H)-1,4 benzoxazin-3(4H)-one | Cultivated crop soil |
| [56] |
| Limonene | Silty, silty clay, and sandy clay loams |
| [57] |
| Luteolin | Experimental field |
| [58] |
| Naringenin | Modified agricultural soil |
| [55] |
| Pulegone | Agricultural soil |
| [59] |
| Salicylic acid | Sandy loam |
| [43] |
| Scopoletin | Sandy, clay, and sandy clay loams |
| [60] |
| Umbelliferone | Sandy and clay loams |
| [61] |
| Calcic Cambisol soil |
| [62] | |
| Vanillin acid | Bulk soil |
| [51] |
| Botanical Pesticide | Soil Type (Amount) | Methodology | LODs/LOQs (μg/kg) | Rec (%) | Ref |
|---|---|---|---|---|---|
| Arbutin, gallic acid, benzoquinone, hydroquinone | Scrub soil (sandy) of Polygonella shrubs | SLE: 1 g sieved air-dried soil sample (1.5 mm); 2 mL water extraction (60 min, 3× vortex); brief centrifugation, 0.2 µm nylon filtration; HPLC-UV | 1 (µg g–1) | --- | [50] |
| Biochanin A | Sandy loam | ASE: 15 g pulverized dried sample poured into a 33-mL extraction cell, diluted to 50 mL with 70% MeOH (5 min preheat, 5 min heat, 3 min static; flush, 80%; purge, 60 s; cycles, 4; 1500 psi; 40 °C); HPLC-MS/DAD | --- | --- | [48] |
| Carvone | Sand, sandy clay loam, silty loam and loam | QuEChERS: 5 g sample + 10 mL n-hexane + 5 mL water (50 mL tube); 2 min vortex; 2 g MgSO4 + 1 g NaCl; 1 min vortex; 7000 rpm 5 min; 1 mL supernatant + 40 mg C18 + 100 mg Na2SO4 (2.5 mL tube); 2 min vortex; 5000 rpm 5 min; 0.5 mL supernatant filtration (0.2 μm); GC-MS | 10–50 | 94.4–97.9 | [106] |
| Cinnamaldehyde and diallyl disulfide | fine sand, sandy loam, and silty clay loam soils | UAE: 6 g soil + 3 mL EtOAC, 1 min vortex, 20 min UAE extraction (RT); 1 min vortex, 3000 rpm 10 min; filtrate supernatant; repeat extraction twice; pool supernatants; GC-MS | 15–83 (ng g–1) | 61–70 | [107] |
| Limonene & trans-cinnamaldehyde | Sandy clay loam and clay loams (5 g) | SLE: soil samples + IS were shaken with 10 mL EtOAC (1 h), 5 min 5000 rpm, filtered and injected; GC-HRMS or UHPLC-HRMS | 1–10 | 83–106 | [44] |
| Limonene | Silty, silty clay, and sandy clay loams | QuEChERS: 5 g air-dried sieved (0.9-mm) sample; 10 mL n-hexane + 5 mL water (50 mL tube); 2 min vortex; 2 g MgSO4 + 1 g NaCl, 1 min vortex; 5 min 7000 rpm; 1 mL supernatant + 40 mg C18 + 5 mg GCB; 2 min vortex, 5 min 5000 rpm; 0.5 mL supernatant filtration (0.2 μm filter); GC-MS | 1–16/4–48 | 71–114 | [50] |
| Matrine and Berberine | Farming areas (China) | SLE: 10 g sample + 1 mL standard solution (50 mL tube), 1 min vortex; 30 min resting; 1% ammonia–ACN (20 mL), 5 min 1200 strokes/min shaker; 2 g NaCl, 1 min vortex; 5 min 3500 rpm; 2 mL upper layer + 25 mg PSA (PTFE centrifuge tube); vortex, 5 min 3500 rpm; supernatant filtration (0.22 μm); UHPLC-MS/MS | 0.34–1.07/1.12–3.58 | 73.1–109.3 | [108] |
| Matrine | Farming areas (China) | QuEChERS: 10 g soil + 2 mL 25% ammonia (50 mL tube); vortex, rest 10 min; 20 mL ACN, 3 min vortex; 1 g NaCl + 4 g anhydrous MgSO4; 1 min vortex; 5000 rpm 5 min; 1.5 mL supernatant + 100 mg PSA + 100 mg anhydrous MgSO4 (2 mL tube), 1 min vortex; 12,000 rpm 2 min; filter supernatant (0.22 µm); HPLC-MS/MS | 5–10 | 74.4–98.4 | [109] |
| Phenolic acids (p-coumaric acid, ferulic acid, vanillic acid, caffeic acid, p-hydroxybenzoic acid, and gallic acid) | Experimental fields (Catholic University of Córdoba) | SLE: MeOH extraction (3 × 50 mL); solvent evaporation; HPLC-UV | --- | --- | [46] |
| Phenolic acids (p-hydroxybenzoic, acid, benzoic acid, cinnamic acid, and vanillin acid | Bulk soil | SLE: 5 g air-dried, sieved (2-mm) and grounded soil samples; extractions with Mehlich III solution + NaOH (25 g sample) at 25 °C (24 h incubation); 10 min 4000 rpm; supernatant pH adjustment (2.5); 0.22 µm filtration; HPLC-UV | --- | [47] | |
| peppermint, spearmint and rosemary leaf EOs | Fallow land | Hydrodistillation (Clevenger apparatus, 3 h); GC-MC (1 µL injection) | --- | --- | [45] |
| Pyrethrins (pyrethrin I and II, cinerin I and II, and jasmolin I and II) | Farming areas (China) | SLE: 20 g sample + 20 mL ACN + 3 g NaCl, 2 min vortex mixer extraction (50-mL tubes); 5 min 3800 rpm; 10 mL supernatant (100-mL flask) rotary concentrated almost-dry state (one drop of liquid), dried (N2 steam); SPE cleanup: residue redissolved 1 mL acetone + n-hexane (1 + 9; v + v) loaded in activated SPE cartridge (1 g anhydrous Na2SO4 conditioned with 10 mL n-hexane); 10 mL acetone + n-hexane (1 + 9; v + v) elution; evaporation to dryness; 1 mL acetone redissolution, 0.22 µm filtration; GC-MS | 12–24/50 | 88.1–104 | [110] |
| Spinosad | Sandy loam | SLE: 25 g sample + 25 mL acetone (250 mL conical flask), 10 min horizontal shaker; supernatant filtration (Whatman No. 42 filter paper); repeat thrice; extract clean-up with liquid-liquid partitioning (3 × 30 mL DCM); pooled DCM evaporation (rotary evaporator); HPLC-UV | 50 | 80–82 | [111] |
| Farming areas (China) | SLE: 20 g sample + 5 mL water + 40 mL ACN (100 mL tube); 1 min vortex; + 7 g NaCl, 1 min vortex, 10 min 4000 rpm; 20 mL supernatant concentrated to dryness (vacuum rotary evaporator); + 2 mL ACN/EtOAC (3:1, v/v); SPE: ACN/ EtOAC (3:1, v/v) preconditioning (5 mL) and elution (25 mL); eluent evaporation (N2 stream); redissolution (2 mL MeOH); filtration (0.22 µm); UHPLC-MS/MS | 1/5 | 83.4–85.3 | [112] | |
| aridisol and entisol along with saline and calcareous soils | QuEChERS: 10 g soil + 7 mL water (50 mL); 25–30 min vortex; 10 mL ACN extraction (5–6 min); 1.5 mL supernatant clean-up (2 mL C-18 SPE tube), 2 min vortex, 2 min centrifugation (≥5000 g); GC-MS/MS | --- | 98–102% | [113] | |
| Myrica gale methanolic extract | Silt loam soil | SPME: Soil samples homogenized, passed through a 2 mm sieve, dark storage 4 °C); 6 g soil (crimped 20 mL HS-SPME vials) + 7.2 mg dry Myrica gale methanolic extract; automatic HS-SPME: 50/30 μm DVB/CAR/PDMS fibre, 5 min incubation, 30 min extraction, at 40 °C; GC-MS | --- | --- | [114] |
| 2,4-Dihydroxy-7-methoxy-(2H)-1,4-benzoxazin-3(4H)-one | Cultivated crop soil | US MeOH extraction (10 mL, 15 min, 5 °C); 5 min, 13000 rpm; repeat using EtOAC as extraction solvent (10 mL); combine extracts, distillation (reduced pressure); 2 mL MeOH + 1% acetic acid dissolution; filtration; HPLC-UV | --- | --- | [54,56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, V.; Castilho, P.C.; Pereira, J.A.M. Analysis of the Environmental Impact of Botanical Pesticides in Soil. Agriculture 2025, 15, 1053. https://doi.org/10.3390/agriculture15101053
Pereira V, Castilho PC, Pereira JAM. Analysis of the Environmental Impact of Botanical Pesticides in Soil. Agriculture. 2025; 15(10):1053. https://doi.org/10.3390/agriculture15101053
Chicago/Turabian StylePereira, Verónica, Paula C. Castilho, and Jorge A. M. Pereira. 2025. "Analysis of the Environmental Impact of Botanical Pesticides in Soil" Agriculture 15, no. 10: 1053. https://doi.org/10.3390/agriculture15101053
APA StylePereira, V., Castilho, P. C., & Pereira, J. A. M. (2025). Analysis of the Environmental Impact of Botanical Pesticides in Soil. Agriculture, 15(10), 1053. https://doi.org/10.3390/agriculture15101053








