Chemical and Biological Amendments and Crop Rotation Affect Soil Carbon and Nitrogen Sequestration by Influencing the Carbon and Nitrogen Contents of Soil Aggregates
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design and Field Management
2.3. Soil Sampling
2.4. Separation of Soil Aggregates and Determination of SOC and TN in Soil Aggregates
2.5. Contribution Rate of Aggregates of Different Particle Sizes to SOC and TN
2.6. Soil Enzyme Activities
2.7. Statistical Analysis
3. Results
3.1. Soil Physical Properties
3.2. Soil Aggregate Size Distribution
3.3. Soil Aggregates C and N Distribution
3.4. Soil Aggregate Enzyme Activities
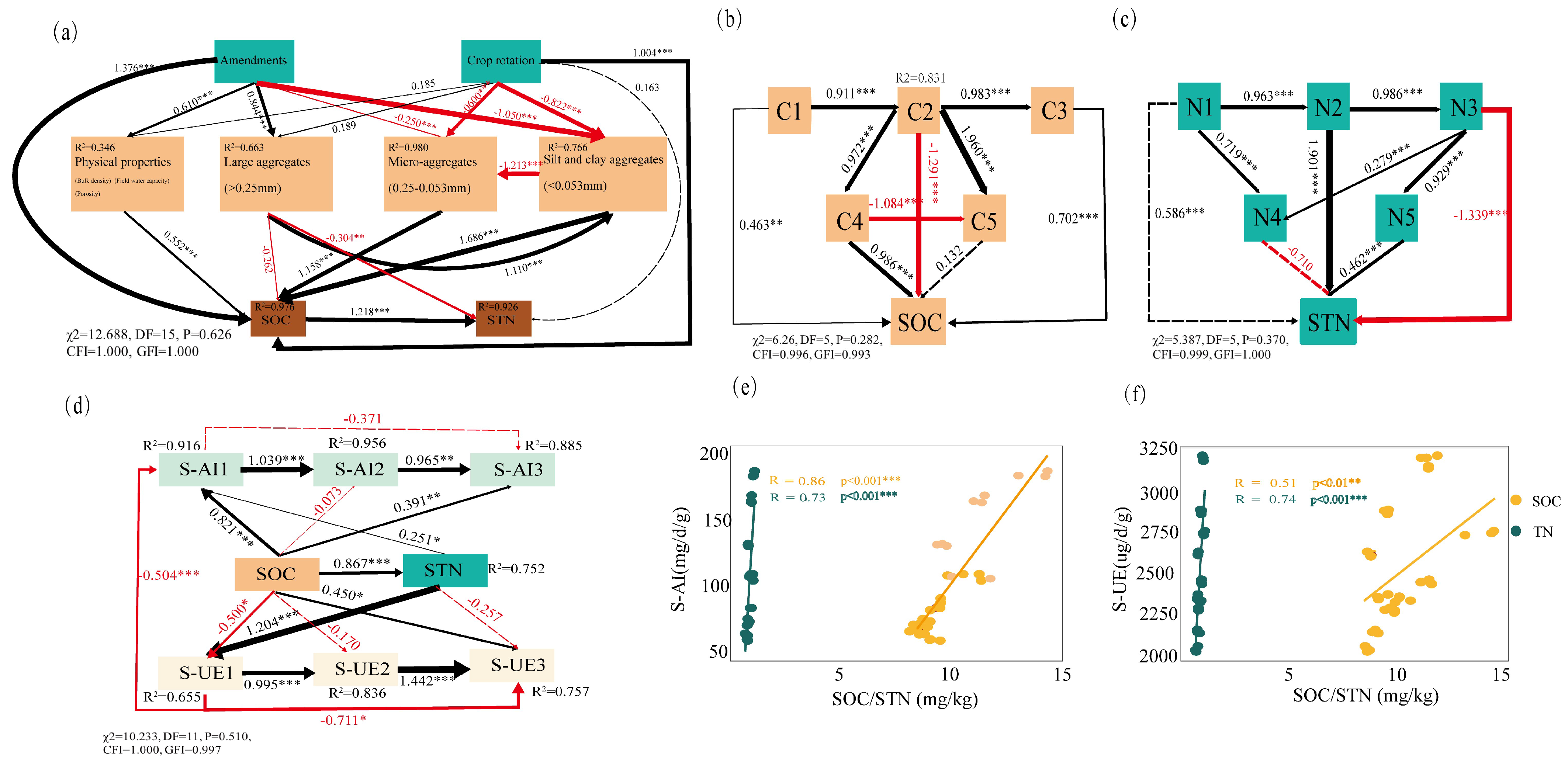
3.5. Relationship Between Soil Aggregate Size, Soil Aggregate C and N Contents, and Soil Aggregate Enzyme Activities
4. Discussion
4.1. Effect of Amendments and Crop Rotation on Soil Physical Properties, SOC, and TN
4.2. Effect of Crop Rotation and Amendments on Soil Aggregates
4.3. Effect of Crop Rotation and Amendments on Soil Enzyme Activity
4.4. Mechanisms of SOC, TN Content, and Enzyme Activity in Relation to Aggregate C, N, and Stability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Zhang, S.; Li, X.; Chen, K.; Shi, J.; Wang, Y.; Luo, P.; Yang, J.; Wang, Y.; Han, X. Long-term fertilization altered microbial community structure in an aeolian sandy soil in northeast China. Front. Microbiol. 2022, 13, 979759. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Cui, R.; Geng, G.; Dong, Y.; Xu, Y.; Sun, Y.; Stevanato, P.; Yu, L.; Liu, J.; Nurminsky, V.N.; et al. Sugar accumulation stage in sugar beets is a key stage in response to continuous cropping soil microbial community assembly. Plant Soil 2024, 504, 457–473. [Google Scholar] [CrossRef]
- Miao, S.; Qiao, Y.; Li, P.; Han, X.; Tang, C. Fallow associated with autumn-plough favors structure stability and storage of soil organic carbon compared to continuous maize cropping in Mollisols. Plant Soil 2017, 416, 27–38. [Google Scholar] [CrossRef]
- Zhao, X.; Hao, C.; Zhang, R.; Jiao, N.; Tian, J.; Lambers, H.; Liang, C.; Cong, W.-F.; Zhang, F. Intercropping increases soil macroaggregate carbon through root traits induced microbial necromass accumulation. Soil Biol. Biochem. 2023, 185, 109146. [Google Scholar] [CrossRef]
- Li, J.; Ma, Y.; Hu, M.; Zhao, Y.; Liu, B.; Wang, C.; Zhang, M.; Zhang, L.; Yang, X.; Mu, G. Multi-Omics and miRNA Interaction Joint Analysis Highlight New Insights Into Anthocyanin Biosynthesis in Peanuts (Arachis hypogaea L.). Front. Plant Sci. 2022, 13, 818345. [Google Scholar] [CrossRef]
- Cheng, S.-F.; Huang, C.-Y.; Lin, S.-C.; Chen, K.-L.; Lin, Y.-C. Feasibility of using peanut (Arachis hypogaea L.) for phytoattenuation on lead-contaminated agricultural land—An in situ study. Agric. Ecosyst. Environ. 2015, 202, 25–30. [Google Scholar] [CrossRef]
- Chen, W.; Teng, Y.; Li, Z.; Liu, W.; Ren, W.; Luo, Y.; Christie, P. Mechanisms by which organic fertilizer and effective microbes mitigate peanut continuous cropping yield constraints in a red soil of south China. Appl. Soil Ecol. 2018, 128, 23–34. [Google Scholar] [CrossRef]
- Hu, J.; Telenko, D.E.; Phipps, P.M.; Hills, H.; Grabau, E.A. Quantifying transgene flow rate in transgenic Sclerotinia-resistant peanut lines. Field Crops Res. 2015, 178, 69–76. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, J.; Liu, Z.; Hu, X.; Yu, Z.; Li, Y.; Chen, X.; Li, L.; Jin, J.; Wang, G. Chitin amendments eliminate the negative impacts of continuous cropping obstacles on soil properties and microbial assemblage. Front. Plant Sci. 2022, 13, 1067618. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, R.; Li, S.; Chen, Y.; Li, Z.; He, P.; Huang, X.; Huang, K. Effects of continuous cropping on soil, senescence, and yield of Tartary buckwheat. Agron. J. 2021, 113, 5102–5113. [Google Scholar] [CrossRef]
- Li, X.-G.; Ding, C.-F.; Zhang, T.-L.; Wang, X.-X. Fungal pathogen accumulation at the expense of plant-beneficial fungi as a consequence of consecutive peanut monoculturing. Soil Biol. Biochem. 2014, 72, 11–18. [Google Scholar] [CrossRef]
- Han, F.; Javed, T.; Hussain, S.; Guo, S.; Guo, R.; Yang, L.; Liu, X.; Cai, T.; Zhang, P.; Jia, Z.; et al. Maize/peanut rotation intercropping improves ecosystem carbon budget and economic benefits in the dry farming regions of China. J. Environ. Manag. 2024, 353, 120090. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yao, F.; Mi, G.; Wang, L.; Wu, H.; Wang, Y. Crop rotation increases root biomass and promotes the correlation of soil dissolved carbon with the microbial community in the rhizosphere. Front. Bioeng. Biotechnol. 2022, 10, 1081647. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Yu, F.; Yuan, M.; Wang, J.; Liu, C.; He, W.; Ge, Z.; Sun, Y.; Liu, Y. Responses of Rhizosphere Bacterial and Fungal Communities to the Long-Term Continuous Monoculture of Water Oat. Microorganisms 2022, 10, 2174. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Du, J.; Li, Y.; Tang, H.; Yin, Z.; Yang, L.; Ding, X. Evolutions and Managements of Soil Microbial Community Structure Drove by Continuous Cropping. Front. Microbiol. 2022, 13, 839494. [Google Scholar] [CrossRef]
- Haq, M.Z.U.; Yu, J.; Yao, G.; Yang, H.; Iqbal, H.A.; Tahir, H.; Cui, H.; Liu, Y.; Wu, Y. A Systematic Review on the Continuous Cropping Obstacles and Control Strategies in Medicinal Plants. Int. J. Mol. Sci. 2023, 24, 12470. [Google Scholar] [CrossRef]
- Kagawa, K.; Gonai, T.; Ichige, H.; Fujita, Y.; Terakado, I.; Shimizu, A.; Iimura, T. Effect of Hot Water Drip Irrigation Treatment of Continuous Cropping Soil Before Replanting on Growth and Yields of Young Japanese Pear Trees. Hortic. J. 2022, 91, 501–507. [Google Scholar] [CrossRef]
- Yang, L.; Wang, C.; He, X.; Liang, H.; Wu, Q.; Sun, X.; Liu, M.; Shen, P. Multi-year crop rotation and quicklime application promote stable peanut yield and high nutrient-use efficiency by regulating soil nutrient availability and bacterial/fungal community. Front. Microbiol. 2024, 15, 1367184. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.; Huang, D.; Li, X.; Gregorich, E.; McLaughlin, N.; Zhang, X.; Chen, X.; Zhang, S.; Liang, A.; et al. Effect of long-term tillage and cropping system on portion of fungal and bacterial necromass carbon in soil organic carbon. Soil Tillage Res. 2022, 218, 105307. [Google Scholar] [CrossRef]
- Akshit; Kumar, S.; Sheoran, N.; Devi, P.; Sharma, K.; Kamboj, E.; Kumar, P. Legumes in Cropping Systems: A Way Toward Agricultural Sustainability and Diversification. Commun. Soil Sci. Plant Anal. 2024, 55, 596–608. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Z.; Gao, F.; Wang, Y.; Lai, H.; Pan, X.; Yang, D.; Li, X. A 2-year study on the effects of tillage and straw management on the soil quality and peanut yield in a wheat–peanut rotation system. J. Soils Sediments 2021, 21, 1698–1712. [Google Scholar] [CrossRef]
- Nichols, V.A.; Osterholz, W.; Archontoulis, S.V.; Liebman, M. The roots of the rotation effect run deep. Field Crops Res. 2024, 319, 109640. [Google Scholar] [CrossRef]
- Huang, T. Imran. Mitigating cadmium contamination in soil using Biochar, sulfur-modified Biochar, and other organic amendments. Int. J. Phytoremediation 2025, 27, 874–887. [Google Scholar] [CrossRef]
- Ma, S.; Cao, Y.; Lu, J.; Ren, T.; Cong, R.; Lu, Z.; Zhu, J.; Li, X. Response of soil aggregation and associated organic carbon to organic amendment and its controls: A global meta-analysis. CATENA 2023, 237, 107774. [Google Scholar] [CrossRef]
- Rahman, M.; Guo, Z.; Zhang, Z.; Zhou, H.; Peng, X. Wetting and drying cycles improving aggregation and associated C stabilization differently after straw or biochar incorporated into a Vertisol. Soil Tillage Res. 2018, 175, 28–36. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, J.; Wang, K.; Wang, X.; Zhang, Z.; Xie, X.; Cai, J. Greater mineral and aggregate protection for organic carbon in the soil amended by weathered coal than by biochar: Based on a 3-year field experiment. Geoderma 2023, 438, 116639. [Google Scholar] [CrossRef]
- Mustafa, A.; Minggang, X.; Shah, S.A.A.; Abrar, M.M.; Nan, S.; Baoren, W.; Zejiang, C.; Saeed, Q.; Naveed, M.; Mehmood, K.; et al. Soil aggregation and soil aggregate stability regulate organic carbon and nitrogen storage in a red soil of southern China. J. Environ. Manag. 2020, 270, 110894. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Xu, Y.; Feng, W.; Zhang, X.; Yao, S.; Zhang, B. Modeling the dynamics of protected and primed organic carbon in soil and aggregates under constant soil moisture following litter incorporation. Soil Biol. Biochem. 2020, 151, 108039. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, Y.; Han, B.; Liu, B.; Wang, X.; Ma, L.; Chen, X.; Li, Z. Augmenting the stability of soil aggregate carbon with nutrient management in worldwide croplands. Agric. Ecosyst. Environ. 2024, 370, 109052. [Google Scholar] [CrossRef]
- Di Iorio, E.; Circelli, L.; Angelico, R.; Torrent, J.; Tan, W.; Colombo, C. Environmental implications of interaction between humic substances and iron oxide nanoparticles: A review. Chemosphere 2022, 303, 135172. [Google Scholar] [CrossRef]
- Chen, M.; Wu, L.; Ding, X.; Liu, L.; Li, Y.; Fei, C.; Zhang, S. Fe-modified biochar improved the stability of soil aggregates and organic carbon: Evidence from enzymatic activity and microbial composition. Land Degrad. Dev. 2024, 35, 732–743. [Google Scholar] [CrossRef]
- Xia, R.; Shi, D.; Ni, S.; Wang, R.; Zhang, J.; Song, G. Effects of soil erosion and soil amendment on soil aggregate stability in the cultivated-layer of sloping farmland in the Three Gorges Reservoir area. Soil Tillage Res. 2022, 223, 105447. [Google Scholar] [CrossRef]
- Rieke, E.L.; Bagnall, D.K.; Morgan, C.L.; Flynn, K.D.; Howe, J.A.; Greub, K.L.; Mac Bean, G.; Cappellazzi, S.B.; Cope, M.; Liptzin, D.; et al. Evaluation of aggregate stability methods for soil health. Geoderma 2022, 428, 116156. [Google Scholar] [CrossRef]
- Chen, Y.; Ren, K.; Su, J.; He, X.; Zhao, G.; Hu, B.; Chen, Y.; Xu, Z.; Jin, Y.; Zou, C. Rotation and Organic Fertilizers Stabilize Soil Water-Stable Aggregates and Their Associated Carbon and Nitrogen in Flue-Cured Tobacco Production. J. Soil Sci. Plant Nutr. 2020, 20, 192–205. [Google Scholar] [CrossRef]
- Dos Santos Canalli, L.B.; Dos Santos, J.B.; Benassi, D.A.; Oliveira de Francisco, A.L.; Benassi, C.; de Aguiar, A.N.; Cordeiro, E.; Mendes, R.S. Soil carbon and structural quality in crop rotations under no-tillage system. Braz. Arch. Biol. Technol. 2020, 63. [Google Scholar] [CrossRef]
- Tong, L.; Zhu, L.; Lv, Y.; Zhu, K.; Liu, X.; Zhao, R. Response of organic carbon fractions and microbial community composition of soil aggregates to long-term fertilizations in an intensive greenhouse system. J. Soils Sediments 2020, 20, 641–652. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, M.; Han, X.; Lu, X.; Chen, X.; Feng, H.; Wu, Z.; Liu, C.; Yan, J.; Zou, W. Evaluation of the soil aggregate stability under long term manure and chemical fertilizer applications: Insights from organic carbon and humic acid structure in aggregates. Agric. Ecosyst. Environ. 2024, 376, 109217. [Google Scholar] [CrossRef]
- Tahoun, A.M.M.A.; El-Enin, M.M.A.; Mancy, A.G.; Sheta, M.H.; Shaaban, A. Integrative Soil Application of Humic Acid and Foliar Plant Growth Stimulants Improves Soil Properties and Wheat Yield and Quality in Nutrient-Poor Sandy Soil of a Semiarid Region. J. Soil Sci. Plant Nutr. 2022, 22, 2857–2871. [Google Scholar] [CrossRef]
- Koga, N. Tillage, fertilizer type, and plant residue input impacts on soil carbon sequestration rates on a Japanese Andosol. Soil Sci. Plant Nutr. 2017, 63, 396–404. [Google Scholar] [CrossRef]
- Luo, X.; Bai, Y.-N.; Sun, K.; Zhang, W.; Dai, C.-C. Reduced pollen activity in peanut (Arachis hypogaea L.) by long-term monocropping is linked to flower water deficit. Plant Soil 2023, 482, 427–450. [Google Scholar] [CrossRef]
- Hu, R.; Zhang, H.; Sun, J.; Zhang, Y.; He, M.; Zhang, Z. Biocrusts enhance soil nitrogen mineralization and nitrification under experimental warming in a dryland ecosystem. Appl. Soil Ecol. 2024, 201, 105502. [Google Scholar] [CrossRef]
- Li, S.-X.; Wang, Z.-H.; Malhi, S.S.; Li, S.-Q.; Gao, Y.-J.; Tian, X.-H. Nutrient and water management effects on crop pro-duction, and nutrient and water use efficiency in dryland areas of China. Adv. Agron. 2009, 102, 223–265. [Google Scholar]
- Gao, M.; Yang, J.; Liu, C.; Gu, B.; Han, M.; Li, J.; Li, N.; Liu, N.; An, N.; Dai, J.; et al. Effects of long-term biochar and biochar-based fertilizer application on brown earth soil bacterial communities. Agric. Ecosyst. Environ. 2021, 309, 107285. [Google Scholar] [CrossRef]
- Yan, W.; Jiang, W.; Han, X.; Hua, W.; Yang, J.; Luo, P. Simulating and Predicting Crop Yield and Soil Fertility under Climate Change with Fertilizer Management in Northeast China Based on the Decision Support System for Agrotechnology Transfer Model. Sustainability 2020, 12, 2194. [Google Scholar] [CrossRef]
- Blake, B.R.; Hartge, K.H. Particle density. In Methods of Soil Analysis, Part 1, 2nd ed.; ASA and SSSA: Madison, WI, USA, 1986; Volume 9, pp. 377–382. [Google Scholar]
- Yang, Z.; Liu, Y.; Chen, M.; Wang, X.; Ye, C.; Li, X.; Chen, W.; Yang, Y.; Wang, B.; Li, C.; et al. Influence of Coupling Effects between Gravel Soil Porosity and Cement Grout Weight on Diffusion Laws and Morphologies of Penetration Grouting. Appl. Sci. 2022, 12, 7601. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, M.; Wu, W.; Tanveer, S.K.; Wen, X.; Liao, Y. The effects of conservation tillage practices on the soil water-holding capacity of a non-irrigated apple orchard in the Loess Plateau, China. Soil Tillage Res. 2013, 130, 7–12. [Google Scholar] [CrossRef]
- Elliott, E.T. Aggregate Structure and Carbon, Nitrogen, and Phosphorus in Native and Cultivated Soils. Soil Sci. Soc. Am. J. 1986, 50, 627–633. [Google Scholar] [CrossRef]
- Chi, Y.; Ma, X.; Zhang, X.; Wang, R.; Zhang, D.; Chu, S.; Zhao, T.; Zhou, P.; Zhang, D. Plant growth promoting endophyte modulates soil ecological characteristics during the enhancement process of cadmium phytoremediation. J. Environ. Manag. 2024, 369, 122206. [Google Scholar] [CrossRef]
- Wang, Y.L.; Jiang, F.H.; Xu, J.B.; Liu, Z.Y.; Gao, Z. Micro-aggregate associated organic carbon in red soil as affected by long-term application of combined organic-inorganic fertilizers. Chin. J. Soil Sci. 2018, 49, 377–384. [Google Scholar]
- Jiang, L.; Cheng, H.; Peng, Y.; Sun, T.; Gao, Y.; Wang, R.; Ma, Y.; Yang, J.; Yu, Q.; Zhang, H.; et al. Relative role of soil nutrients vs. carbon availability on soil carbon mineralization in grassland receiving long-term N addition. Soil Tillage Res. 2024, 235, 105864. [Google Scholar] [CrossRef]
- Li, Y.; Feng, H.; Dong, Q.; Xia, L.; Li, J.; Li, C.; Zang, H.; Andersen, M.N.; Olesen, J.E.; Jørgensen, U.; et al. Ammoniated straw incorporation increases wheat yield, yield stability, soil organic carbon and soil total nitrogen content. Field Crops Res. 2022, 284, 108558. [Google Scholar] [CrossRef]
- Getahun, G.T.; Kätterer, T.; Munkholm, L.J.; Parvage, M.M.; Keller, T.; Rychel, V.; Kirchmann, H. Short-term effects of loosening and incorporation of straw slurry into the upper subsoil on soil physical properties and crop yield. Soil Tillage Res. 2018, 184, 62–67. [Google Scholar] [CrossRef]
- Li, C.; Xiong, Y.; Qu, Z.; Xu, X.; Huang, Q.; Huang, G. Impact of biochar addition on soil properties and water-fertilizer productivity of tomato in semi-arid region of Inner Mongolia, China. Geoderma 2018, 331, 100–108. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, C.; Wang, R.; Li, J.; Wang, X. Conservation tillage rotation enhanced soil structure and soil nutrients in long-term dryland agriculture. Eur. J. Agron. 2021, 131, 126379. [Google Scholar] [CrossRef]
- Kim, B.-G.; Lee, G.-S.; Park, C.-L.; Jeon, H.-S. Modification of calcined clay and its physical properties for use as a subsidiary material for growing media. J. Plant Nutr. 2010, 33, 654–669. [Google Scholar] [CrossRef]
- Li, X.-G.; Zhang, T.-L.; Wang, X.-X.; Hua, K.; Zhao, L.; Han, Z.-M. The Composition of Root Exudates from Two Different Resistant Peanut Cultivars and Their Effects on the Growth of Soil-Borne Pathogen. Int. J. Biol. Sci. 2013, 9, 164–173. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, N.; Sun, D. Dry–wet cycles induce the decoupling of carbon and nitrogen mineralization at high temperatures in semi-arid grassland soil. Soil Biol. Biochem. 2024, 188, 109227. [Google Scholar] [CrossRef]
- Yudina, A.; Kuzyakov, Y. Dual nature of soil structure: The unity of aggregates and pores. Geoderma 2023, 434, 116478. [Google Scholar] [CrossRef]
- Six, J.; Paustian, K. Aggregate-associated soil organic matter as an ecosystem property and a measurement tool. Soil Biol. Biochem. 2014, 68, A4–A9. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, W.; Wang, X.; Liu, Y.; Yu, B.; Chen, X.; Zou, C. Life cycle assessment of a long-term multifunctional winter wheat-summer maize rotation system on the North China Plain under sustainable P management. Sci. Total. Environ. 2021, 783, 147039. [Google Scholar] [CrossRef]
- Wang, D.; Fonte, S.J.; Parikh, S.J.; Six, J.; Scow, K.M. Biochar additions can enhance soil structure and the physical stabilization of C in aggregates. Geoderma 2017, 303, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Cai, A.; Xu, M.; Wang, B.; Zhang, W.; Liang, G.; Hou, E.; Luo, Y. Manure acts as a better fertilizer for increasing crop yields than synthetic fertilizer does by improving soil fertility. Soil Tillage Res. 2019, 189, 168–175. [Google Scholar] [CrossRef]
- Zhang, A.; Cui, L.; Pan, G.; Li, L.; Hussain, Q.; Zhang, X.; Zheng, J.; Crowley, D. Effect of biochar amendment on yield and methane and nitrous oxide emissions from a rice paddy from Tai Lake plain, China. Agric. Ecosyst. Environ. 2010, 139, 469–475. [Google Scholar] [CrossRef]
- Chen, Z.; Geng, S.; Zhou, X.; Gui, H.; Zhang, L.; Huang, Z.; Wang, M.; Zhang, J.; Han, S. Nitrogen addition decreases soil aggregation but enhances soil organic carbon stability in a temperate forest. Geoderma 2022, 426, 116112. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, X.; Jing, Y.; Li, Q.; Zhang, J.; Huang, Q. Effects of biochar amendment on rapeseed and sweet potato yields and water stable aggregate in upland red soil. CATENA 2014, 123, 45–51. [Google Scholar] [CrossRef]
- Li, Y.; Lv, B.; Wu, L.; Xue, J.; He, X.; Li, B.; Huang, M.; Yang, L. Understanding the impact of soil components on the environmental existence of Nonylphenol: From the perspective of soil aggregates. Environ. Res. 2024, 261, 119750. [Google Scholar] [CrossRef]
- Sheng, M.-H.; Ai, X.-Y.; Huang, B.-C.; Zhu, M.-K.; Liu, Z.-Y.; Ai, Y.-W. Effects of biochar additions on the mechanical stability of soil aggregates and their role in the dynamic renewal of aggregates in slope ecological restoration. Sci. Total. Environ. 2023, 898, 165478. [Google Scholar] [CrossRef]
- Hu, F.; Xu, C.; Ma, R.; Tu, K.; Yang, J.; Zhao, S.; Yang, M.; Zhang, F. Biochar application driven change in soil internal forces improves aggregate stability: Based on a two-year field study. Geoderma 2021, 403, 115276. [Google Scholar] [CrossRef]
- Pituello, C.; Ferro, N.D.; Francioso, O.; Simonetti, G.; Berti, A.; Piccoli, I.; Pisi, A.; Morari, F. Effects of biochar on the dynamics of aggregate stability in clay and sandy loam soils. Eur. J. Soil Sci. 2018, 69, 827–842. [Google Scholar] [CrossRef]
- Rahman, M.; Zhu, Q.; Zhang, Z.; Zhou, H.; Peng, X. The roles of organic amendments and microbial community in the improvement of soil structure of a Vertisol. Appl. Soil Ecol. 2017, 111, 84–93. [Google Scholar] [CrossRef]
- Xu, H.; Qu, Q.; Li, G.; Liu, G.; Geissen, V.; Ritsema, C.J.; Xue, S. Impact of nitrogen addition on plant-soil-enzyme C–N–P stoichiometry and microbial nutrient limitation. Soil Biol. Biochem. 2022, 170, 108714. [Google Scholar] [CrossRef]
- Li, C.; Shi, Z.; Cai, J.; Wang, P.; Wang, F.; Ju, M.; Liu, J.; Yu, Q. Synthesis of Phenylboronic Acid-Functionalized Magnetic Nanoparticles for Sensitive Soil Enzyme Assays. Molecules 2022, 27, 6883. [Google Scholar] [CrossRef]
- Liang, Q.; Chen, H.; Gong, Y.; Yang, H.; Fan, M.; Kuzyakov, Y. Effects of 15 years of manure and mineral fertilizers on enzyme activities in particle-size fractions in a North China Plain soil. Eur. J. Soil Biol. 2014, 60, 112–119. [Google Scholar] [CrossRef]
- Li, W.; Xiao, Q.; Hu, C.; Liu, B.; Sun, R. A comparison of the efficiency of different urease inhibitors and their effects on soil prokaryotic community in a short-term incubation experiment. Geoderma 2019, 354, 113877. [Google Scholar] [CrossRef]
- Tian, S.; Zhu, B.; Yin, R.; Wang, M.; Jiang, Y.; Zhang, C.; Li, D.; Chen, X.; Kardol, P.; Liu, M. Organic fertilization promotes crop productivity through changes in soil aggregation. Soil Biol. Biochem. 2022, 165, 108533. [Google Scholar] [CrossRef]
- Jia, W.; Wang, B.; Wang, C.; Sun, H. Tourmaline and biochar for the remediation of acid soil polluted with heavy metals. J. Environ. Chem. Eng. 2017, 5, 2107–2114. [Google Scholar] [CrossRef]
- Santos, M.P.F.; Brito, M.J.P.; Junior, E.C.S.; Bonomo, R.C.F.; Veloso, C.M. Pepsin immobilization on biochar by adsorption and covalent binding, and its application for hydrolysis of bovine casein. J. Chem. Technol. Biotechnol. 2019, 94, 1982–1990. [Google Scholar] [CrossRef]
- Tu, C.; Wei, J.; Guan, F.; Liu, Y.; Sun, Y.; Luo, Y. Biochar and bacteria inoculated biochar enhanced Cd and Cu immobilization and enzymatic activity in a polluted soil. Environ. Int. 2020, 137, 105576. [Google Scholar] [CrossRef]
- Deng, L.; Wang, T.; Luo, W.; He, L.; Liang, Z. Effects of a compound microbial agent and plants on soil properties, enzyme activities, and bacterial composition of Pisha sandstone. Environ. Sci. Pollut. Res. 2021, 28, 53353–53364. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H. Physico-chemical properties and microbial responses in biochar-amended soils: Mechanisms and future directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Fu, S.; Méndez, A.; Gascó, G. Interactive effects of biochar and the earthworm Pontoscolex corethrurus on plant productivity and soil enzyme activities. J. Soils Sediments 2013, 14, 483–494. [Google Scholar] [CrossRef]
- Nahidan, S.; Nourbakhsh, F. Large macroaggregates determine distribution of soil amidohydrolase activities at different landscape positions. CATENA 2018, 170, 316–323. [Google Scholar] [CrossRef]
- Zhang, X.; Xin, X.; Zhu, A.; Zhang, J.; Yang, W. Effects of tillage and residue managements on organic C accumulation and soil aggregation in a sandy loam soil of the North China Plain. CATENA 2017, 156, 176–183. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, P.; Qiao, Y.; Li, Y.; Liu, S.; Gao, C.; Liu, C.; Shao, J.; Yu, H.; Zhao, Z.; et al. The potential for soil C sequestration and N fixation under different planting patterns depends on the carbon and nitrogen content and stability of soil aggregates. Sci. Total. Environ. 2023, 897, 165430. [Google Scholar] [CrossRef]
- Abdalla, K.; Gaiser, T.; Seidel, S.J.; Pausch, J. Soil organic carbon and nitrogen in aggregates in response to over seven decades of farmyard manure application. J. Plant Nutr. Soil Sci. 2023, 186, 253–258. [Google Scholar] [CrossRef]
- Cui, Y.; Moorhead, D.L.; Peng, S.; Sinsabaugh, R.L. New insights into the patterns of ecoenzymatic stoichiometry in soil and sediment. Soil Biol. Biochem. 2023, 177, 108910. [Google Scholar] [CrossRef]


| Soil Depth (cm) | pH | Organic Carbon (g/kg) | Total Nitrogen (g/kg) | Total Phosphorus (g/kg) | Total Potassium (g/kg) | Available Nitrogen (mg/kg) | Available Phosphorus (mg/kg) | Available Potassium (mg/kg) |
|---|---|---|---|---|---|---|---|---|
| 0–20 | 5.59 ± 0.10 | 6.95 ± 0.11 | 0.95 ± 0.01 | 0.41 ± 0.02 | 17.64 ± 1.14 | 78.30 ± 4.52 | 10.37 ± 1.25 | 52.56 ± 6.47 |
| 20–40 | 5.84 ± 0.21 | 8.52 ± 0.14 | 1.03 ± 0.02 | 0.49 ± 0.04 | 18.40 ± 1.24 | 120.45 ± 9.78 | 19.72 ± 2.35 | 71.23 ± 5.45 |
| Treatment | Nutrient Input (kg/hm2) | ||||
|---|---|---|---|---|---|
| N | P2O5 | K2O | Chemical Modifier | Biological Modifier | |
| MC | 225 | 75 | 75 | 0 | 0 |
| M-PR | 225 ①/60 ② | 75/82.5 | 75/112.5 | 0 | 0 |
| PC | 60 | 82.5 | 112.5 | 0 | 0 |
| PCCA | 58.1 | 79.4 | 110.3 | 750 | 0 |
| PCBA | 56.6 | 82.3 | 112.3 | 0 | 45 |
| Treatment | Physical Properties | |||||
|---|---|---|---|---|---|---|
| Bulk Density (g/cm3) | Field Water-Holding Capacity (%) | Porosity (%) | ||||
| 0–20 cm | 20–40 cm | 0–20 cm | 20–40 cm | 0–20 cm | 20–40 cm | |
| MC | 1.15 ± 0.02 c | 1.28 ± 0.03 c | 36.10 ± 0.24 d | 32.61 ± 0.42 b | 56.54 ± 0.85 a | 51.66 ± 1.16 b |
| M-PR | 1.09 ± 0.01 d | 1.20 ± 0.02 d | 45.14 ± 0.31 a | 39.25 ± 0.46 a | 58.80 ± 0.22 a | 54.88 ± 0.75 a |
| PC | 1.30 ± 0.01 a | 1.40 ± 0.02 ab | 29.60 ± 0.57 e | 27.17 ± 0.43 d | 51.11 ± 0.98 b | 47.08 ± 0.80 cd |
| PCCA | 1.23 ± 0.02 b | 1.36 ± 0.02 b | 41.33 ± 0.31 b | 32.40 ± 0.46 b | 53.57 ± 0.61 b | 48.86 ± 0.67 c |
| PCBA | 1.29 ± 0.03 a | 1.45 ± 0.02 a | 37.77 ± 0.49 c | 30.95 ± 0.33 c | 51.38 ± 1.06 b | 45.29 ± 0.79 d |
| Soil Depth | Treatment | Aggregate Size | ||||
|---|---|---|---|---|---|---|
| >1 mm | 1–0.5 mm | 0.5–0.25 mm | 0.25–0.053 mm | <0.053 mm | ||
| 0–20 cm | MC | 6.49 ± 0.30 a | 4.24 ± 0.36 bc | 13.13 ± 0.42 b | 31.05 ± 0.20 a | 45.09 ± 0.26 c |
| M-PR | 3.58 ± 0.18 b | 8.21 ± 0.30 a | 17.22 ± 0.26 a | 27.19 ± 0.62 b | 43.79 ± 0.76 c | |
| PC | 2.25 ± 0.15 cd | 3.99 ± 0.30 c | 13.01 ± 0.33 b | 22.24 ± 0.48 c | 58.51 ± 0.68 a | |
| PCCA | 1.93 ± 0.07 d | 4.77 ± 0.17 bc | 16.98 ± 0.42 a | 25.76 ± 0.33 b | 50.56 ± 0.92 b | |
| PCBA | 2.61 ± 0.20 c | 5.07 ± 0.26 b | 13.05 ± 0.36 b | 26.42 ± 0.48 b | 52.85 ± 1.12 b | |
| 20–40 cm | MC | 10.71 ± 0.38 b | 10.38 ± 0.38 b | 12.69 ± 1.61 b | 28.74 ± 1.66 bc | 37.49 ± 3.11 a |
| M-PR | 13.48 ± 0.82 a | 12.62 ± 0.79 a | 12.80 ± 0.59 b | 22.47 ± 3.37 c | 38.63 ± 5.23 a | |
| PC | 1.76 ± 0.08 c | 5.37 ± 0.62 cd | 20.55 ± 4.61 a | 34.01 ± 1.70 b | 38.31 ± 5.23 a | |
| PCCA | 2.67 ± 0.26 c | 6.40 ± 0.32 c | 10.66 ± 0.27 b | 47.31 ± 4.11 a | 32.96 ± 3.27 a | |
| PCBA | 2.64 ± 0.02 c | 4.19 ± 0.19 d | 12.46 ± 0.66 b | 36.56 ± 0.92 b | 44.15 ± 0.23 a | |
| Treatments | 0–20 cm | 20–40 cm | ||
|---|---|---|---|---|
| SOC (g/kg) | TN (g/kg) | SOC (g/kg) | TN (g/kg) | |
| MC | 9.11 ± 0.08 d | 1.05 ± 0.03 d | 8.75 ± 0.06 c | 0.98 ± 0.03 d |
| M-PR | 10.32 ± 0.21 c | 1.15 ± 0.02 c | 8.86 ± 0.06 c | 1.05 ± 001 c |
| PC | 9.77 ± 0.12 cd | 1.06 ± 0.01 d | 9.39 ± 0.15 b | 1.03 ± 0.01 cd |
| PCCA | 14.04 ± 0.42 a | 131 ± 0.02 a | 11.64 ± 0.15 a | 1.27 ± 0.02 a |
| PCBA | 11.48 ± 0.15 b | 1.21 ± 0.01 b | 9.64 ± 0.05 b | 1.18 ± 0.02 b |
| Soil Layer (cm) | Treatment | >1 mm | 1–0.5 mm | 0.5–0.25 mm | 0.25–0.053 mm | <0.053 mm | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C-CR% | N-CR% | C-CR% | N-CR% | C-CR% | N-CR% | C-CR% | N-CR% | C-CR% | N-CR% | ||
| 0–20 cm | MC | 7.56 ± 1.23 a | 7.42 ± 0.53 a | 9.95 ± 1.09 ab | 9.40 ± 0.54 ab | 15.46 ± 1.03 b | 14.16 ± 1.50 b | 37.21 ± 6.94 ab | 35.72 ± 5.31 b | 30.87 ± 6.50 c | 32.01 ± 7.29 c |
| M–PR | 6.71 ± 1.46 ab | 4.82 ± 0.94 b | 5.18 ± 0.93 c | 3.86 ± 0.70 c | 8.30 ± 0.15 c | 6.86 ± 0.06 c | 16.70 ± 1.48 d | 15.70 ± 0.77 c | 61.20 ± 2.04 a | 62.80 ± 2.85 a | |
| PC | 3.62 ± 0.42 c | 2.97 ± 0.38 c | 4.45 ± 0.52 c | 4.52 ± 0.43 c | 15.48 ± 1.91 b | 14.77 ± 2.3 b | 32.36 ± 3.14 bc | 35.52 ± 2.90 b | 36.80 ± 4.85 bc | 41.13 ± 4.43 b | |
| PCCA | 3.88 ± 0.32 c | 4.13 ± 022 bc | 8.74 ± 0.98 b | 8.46 ± 0.63 b | 24.70 ± 4.50 a | 24.92 ± 3.06 a | 30.12 ± 1.03 c | 37.75 ± 0.63 b | 41.16 ± 1.91 b | 47.47 ± 3.90 b | |
| PCBA | 5.17 ± 0.69 bc | 5.08 ± 0.84 b | 11.88 ± 2.43 a | 10.77 ± 2.25 a | 21.45 ± 4.25 a | 23.96 ± 4.10 a | 43.36 ± 1.82 a | 46.84 ± 2.38 a | 35.04 ± 2.21 bc | 38.88 ± 3.26 bc | |
| 20–40 cm | MC | 4.85 ± 0.19 a | 4.77 ± 0.19 a | 5.91 ± 0.61 c | 6.45 ± 0.20 b | 8.75 ± 0.10 c | 13.58 ± 0.21 c | 12.67 ± 0.63 d | 14.21 ± 1.04 b | 59.77 ± 1.10 b | 67.58 ± 5.41 a |
| M-PR | 3.42 ± 0.16 b | 3.44 ± 0.07 c | 4.34 ± 0.10 d | 4.27 ± 0.11 c | 8.86 ± 0.10 c | 15.52 ± 0.87 b | 15.65 ± 0.25 b | 17.14 ± 0.66 a | 51.77 ± 0.63 c | 61.03 ± 0.73 b | |
| PC | 2.84 ± 0.28 b | 2.39 ± 0.20 d | 6.59 ± 0.58 c | 5.77 ± 0.62 b | 9.39 ± 0.26 b | 11.62 ± 0.36 d | 11.65 ± 0.38 e | 12.96 ± 0.42 c | 59.90 ± 2.07 b | 66.49 ± 1.45 a | |
| PCCA | 5.05 ± 0.54 a | 4.30 ± 0.38 b | 10.40 ± 1.38 a | 9.70 ± 1.37 a | 11.64 ± 0.27 a | 14.55 ± 0.77 bc | 14.28 ± 0.64 c | 13.91 ± 0.23 bc | 47.95 ± 2.68 d | 57.84 ± 2.57 b | |
| PCBA | 4.80 ± 0.39 a | 3.57 ± 0.19 c | 8.71 ± 0.36 b | 6.85 ± 0.20 b | 9.64 ± 0.09 b | 17.24 ± 1.41 a | 16.73 ± 0.71 a | 15.02 ± 0.45 b | 69.11 ± 1.74 a | 66.60 ± 1.44 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Li, S.; Xu, K.; Wang, J.; Yang, J.; Han, X. Chemical and Biological Amendments and Crop Rotation Affect Soil Carbon and Nitrogen Sequestration by Influencing the Carbon and Nitrogen Contents of Soil Aggregates. Agriculture 2025, 15, 1051. https://doi.org/10.3390/agriculture15101051
Zhu Z, Li S, Xu K, Wang J, Yang J, Han X. Chemical and Biological Amendments and Crop Rotation Affect Soil Carbon and Nitrogen Sequestration by Influencing the Carbon and Nitrogen Contents of Soil Aggregates. Agriculture. 2025; 15(10):1051. https://doi.org/10.3390/agriculture15101051
Chicago/Turabian StyleZhu, Zefang, Shuangting Li, Kangbo Xu, Jing Wang, Jinfeng Yang, and Xiaori Han. 2025. "Chemical and Biological Amendments and Crop Rotation Affect Soil Carbon and Nitrogen Sequestration by Influencing the Carbon and Nitrogen Contents of Soil Aggregates" Agriculture 15, no. 10: 1051. https://doi.org/10.3390/agriculture15101051
APA StyleZhu, Z., Li, S., Xu, K., Wang, J., Yang, J., & Han, X. (2025). Chemical and Biological Amendments and Crop Rotation Affect Soil Carbon and Nitrogen Sequestration by Influencing the Carbon and Nitrogen Contents of Soil Aggregates. Agriculture, 15(10), 1051. https://doi.org/10.3390/agriculture15101051






