Unveiling the Transformative Effects of Forest Restoration on the Soil Chemistry and Biology of Sandy Soils in Southern Nyírség, Hungary
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Sampling Sites
2.2. Soil Sampling and Analyses
2.3. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HP | Hosszúpályi |
| DB | Debrecen |
| K | stability coefficient |
| KA | Arany-type plasticity index |
| HU% | humus content of the soil |
| SOC | soil organic carbon |
| OM | organic matter |
| DOC | dissolved organic carbon |
References
- IUSS Working Group WRB. World Reference Base for Soil Resources 2022: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences: Vienna, Austria, 2022; ISBN 979-8-9862451-1-9. [Google Scholar]
- Hartemink, A.E.; Huting, J. Land Cover, Extent, and Properties of Arenosols in Southern Africa. Arid Land Res. Manag. 2008, 22, 134–147. [Google Scholar] [CrossRef]
- Szemerédy Miklós Erdőtörténelem, I. A Felső-Tisza-Vidék Erdeinek Másfél Évszázada (Hajdú-Szabolcs vm.) 1848-Tól 2000-Ig; Nyomdaipari SZKKT: Debrecen, Hungary, 2009. [Google Scholar]
- Erdozain, M.; Alberdi, I.; Aszalós, R.; Bollmann, K.; Detsis, V.; Diaci, J.; Đodan, M.; Efthimiou, G.; Gálhidy, L.; Haase, M.; et al. The Evolution of Forest Restoration in Europe: A Synthesis for a Step Forward Based on National Expert Knowledge. Curr. For. Rep. 2024, 11, 4. [Google Scholar] [CrossRef]
- Griffiths, P.; Müller, D.; Kuemmerle, T.; Hostert, P. Agricultural Land Change in the Carpathian Ecoregion after the Breakdown of Socialism and Expansion of the European Union. Environ. Res. Lett. 2013, 8, 45024. [Google Scholar] [CrossRef]
- Hansen, M.C.; Potapov, P.V.; Moore, R.; Hancher, M.; Turubanova, S.A.; Tyukavina, A.; Thau, D.; Stehman, S.V.; Goetz, S.J.; Loveland, T.R.; et al. High-Resolution Global Maps of 21st-Century Forest Cover Change. Science 2013, 342, 850–853. [Google Scholar] [CrossRef]
- Guo, L.B.; Gifford, R.M. Soil Carbon Stocks and Land Use Change: A Meta Analysis. Glob. Change Biol. 2002, 8, 345–360. [Google Scholar] [CrossRef]
- Vesterdal, L.; Ritter, E.; Gundersen, P. Change in Soil Organic Carbon Following Afforestation of Former Arable Land. For. Ecol. Manag. 2002, 169, 137–147. [Google Scholar] [CrossRef]
- Jandl, R.; Lindner, M.; Vesterdal, L.; Bauwens, B.; Baritz, R.; Hagedorn, F.; Johnson, D.W.; Minkkinen, K.; Byrne, K.A. How Strongly Can Forest Management Influence Soil Carbon Sequestration? Geoderma 2007, 137, 253–268. [Google Scholar] [CrossRef]
- Six, J.; Callewaert, P.; Lenders, S.; De Gryze, S.; Morris, S.J.; Gregorich, E.G.; Paul, E.A.; Paustian, K. Measuring and Understanding Carbon Storage in Afforested Soils by Physical Fractionation. Soil Sci. Soc. Am. J. 2002, 66, 1981–1987. [Google Scholar] [CrossRef]
- Wei, X.; Shao, M.; Gale, W.; Li, L. Global Pattern of Soil Carbon Losses Due to the Conversion of Forests to Agricultural Land. Sci. Rep. 2014, 4, 4062. [Google Scholar] [CrossRef]
- Mayer, M.; Prescott, C.E.; Abaker, W.E.A.; Augusto, L.; Cécillon, L.; Ferreira, G.W.D.; James, J.; Jandl, R.; Katzensteiner, K.; Laclau, J.-P.; et al. Tamm Review: Influence of Forest Management Activities on Soil Organic Carbon Stocks: A Knowledge Synthesis. For. Ecol. Manag. 2020, 466, 118127. [Google Scholar] [CrossRef]
- Bouwman, A.F.; Leemans, R. The Role of Forest Soils in the Global Carbon Cycle. In Carbon Forms and Functions in Forest Soils; McFee, W.W., Kelly, J.M., Eds.; Soil Science Society of America: Madison, WI, USA, 2006; pp. 503–525. ISBN 978-0-89118-869-8. [Google Scholar]
- Jégou, D.; Cluzeau, D.; Hallaire, V.; Balesdent, J.; Tréhen, P. Burrowing Activity of the Earthworms Lumbricus Terrestris and Aporrectodea Giardi and Consequences on C Transfers in Soil. Eur. J. Soil Biol. 2000, 36, 27–34. [Google Scholar] [CrossRef]
- Del Galdo, I.; Six, J.; Peressotti, A.; Francesca Cotrufo, M. Assessing the Impact of Land-use Change on Soil C Sequestration in Agricultural Soils by Means of Organic Matter Fractionation and Stable C Isotopes. Glob. Change Biol. 2003, 9, 1204–1213. [Google Scholar] [CrossRef]
- Kincses, I.; Filep, T.; Nagy, P.; Kovács, A. Water Soluble Nitrogen Forms on Two Different Soils as Affected by Biofertilization. Cereal Res. Commun. 2007, 35, 597–600. [Google Scholar] [CrossRef]
- Nagy, P.; Racskó, J.; Vágó, I.; Holb, I. Effect of Different Groundcover Matter on Nitrogen and Sulphur Content of Soil and Leaf in Apple Orchard in Eastern Hungary. Cereal Res. Commun. 2006, 34, 585–588. [Google Scholar] [CrossRef]
- Paul, K.I.; Polglase, P.J.; Nyakuengama, J.G.; Khanna, P.K. Change in Soil Carbon Following Afforestation. For. Ecol. Manag. 2002, 168, 241–257. [Google Scholar] [CrossRef]
- Leuschner, C.; Feldmann, E.; Pichler, V.; Glatthorn, J.; Hertel, D. Forest Management Impact on Soil Organic Carbon: A Paired-Plot Study in Primeval and Managed European Beech Forests. For. Ecol. Manag. 2022, 512, 120163. [Google Scholar] [CrossRef]
- Maes, J.; Bruzón, A.G.; Barredo, J.I.; Vallecillo, S.; Vogt, P.; Rivero, I.M.; Santos-Martín, F. Accounting for Forest Condition in Europe Based on an International Statistical Standard. Nat. Commun. 2023, 14, 3723. [Google Scholar] [CrossRef]
- Führer, E.; Gálos, B.; Rasztovics, E.; Jagodics, A.; Mátyás, C. Erdészeti Klímaosztályok Területének Változása. Erdészeti Lapok 2017, 152, 174–177. [Google Scholar]
- Kádár, I.; Szemes, I. A Nyírlugosi Tartamkísérlet 30 Éve; MTA Talajtani és Agrárkémiai Kut. Int.: Budapest, Hungary, 1994. [Google Scholar]
- Mayer, J.; Gunst, L.; Mäder, P.; Samson, M.-F.; Carcea, M.; Narducci, V.; Thomsen, I.K.; Dubois, D. Productivity, Quality and Sustainability of Winter Wheat under Long-Term Conventional and Organic Management in Switzerland. Eur. J. Agron. 2015, 65, 27–39. [Google Scholar] [CrossRef]
- Kong, Y.; Nagano, H.; Kátai, J.; Vágó, I.; Oláh, Á.Z.; Yashima, M.; Inubushi, K. CO2, N2O and CH4 Production/Consumption Potentials of Soils under Different Land-Use Types in Central Japan and Eastern Hungary. Soil Sci. Plant Nutr. 2013, 59, 455–462. [Google Scholar] [CrossRef]
- Demeter, I.; Makádi, M.; Tomócsik, A.; Aranyos, T.J.; Michéli, E.; Posta, K. Chemical and Microbiological Properties of Hungarian Sandy Soils under Different Management Practices. Appl. Ecol. Environ. Res. 2018, 16, 3473–3488. [Google Scholar] [CrossRef]
- Kristó, L. Erdészeti Szaporítóanyag-Termelés; Hermann Ottó Intézet Nonprofit Kft.: Budapest, Hungary, 2017. [Google Scholar]
- Rédei, K. Az Akác Termesztés-Fejlesztésének Biológiai Alapjai És Gyakorlata; Erdészeti Tudományos Intézet: Budapest, Hungary, 2006. [Google Scholar]
- Rezapour, S.; Alipour, O. Degradation of Mollisols Quality after Deforestation and Cultivation on a Transect with Mediterranean Condition. Environ. Earth Sci. 2017, 76, 755. [Google Scholar] [CrossRef]
- Christopoulou Olga, G. Deforestation/Reforestation in Mediterranean Europe: The Case of Greece. In Soil Erosion Studies; Godone, D., Ed.; InTech: Vienna, Austria, 2011; pp. 41–50. ISBN 978-953-307-710-9. [Google Scholar]
- Bruun, T.B.; Elberling, B.; De Neergaard, A.; Magid, J. Organic Carbon Dynamics in Different Soil Types After Conversion of Forest to Agriculture. Land Degrad. Dev. 2015, 26, 272–283. [Google Scholar] [CrossRef]
- Mäkipää, R.; Abramoff, R.; Adamczyk, B.; Baldy, V.; Biryol, C.; Bosela, M.; Casals, P.; Curiel Yuste, J.; Dondini, M.; Filipek, S.; et al. How Does Management Affect Soil C Sequestration and Greenhouse Gas Fluxes in Boreal and Temperate Forests?—A Review. For. Ecol. Manag. 2023, 529, 120637. [Google Scholar] [CrossRef]
- Achat, D.L.; Fortin, M.; Landmann, G.; Ringeval, B.; Augusto, L. Forest Soil Carbon Is Threatened by Intensive Biomass Harvesting. Sci. Rep. 2015, 5, 15991. [Google Scholar] [CrossRef]
- Johannesson, C.-F.; Ilvesniemi, H.; Kjønaas, O.J.; Larsen, K.S.; Lehtonen, A.; Nordén, J.; Paré, D.; Silvennoinen, H.; Stendahl, J.; Stupak, I.; et al. Decadal Decline in Forest Floor Soil Organic Carbon after Clear-Cutting in Nordic and Canadian Forests. For. Ecol. Manag. 2025, 586, 122668. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, X.; Bai, S.; Zhu, T.; Qiu, W.; You, Y.; Wu, M.; Berninger, F.; Sun, Z.; Zhang, H.; et al. Effects of Forest Regeneration Practices on the Flux of Soil CO2 after Clear-Cutting in Subtropical China. J. Environ. Manag. 2018, 212, 332–339. [Google Scholar] [CrossRef]
- Arunrat, N.; Sereenonchai, S.; Kongsurakan, P.; Iwai, C.B.; Yuttitham, M.; Hatano, R. Post-Fire Recovery of Soil Organic Carbon, Soil Total Nitrogen, Soil Nutrients, and Soil Erodibility in Rotational Shifting Cultivation in Northern Thailand. Front. Environ. Sci. 2023, 11, 1117427. [Google Scholar] [CrossRef]
- He, Z.; Xu, H.; He, S.; Liang, X.; Zheng, Z.; Luo, Z.; Wang, Y.; Zhang, Y.; Tan, B. Characteristics of Soil Organic Carbon (SOC) Loss with Water Erosion in Sloping Farmland of Southwestern China during Maize (Zea mays L.) Growth Stages. Agronomy 2023, 13, 738. [Google Scholar] [CrossRef]
- Bidló, A.; Horváth, A. Talajok Szerepe a Klímaváltozásban. Erdészettudományi Közlemények 2018, 8, 57–71. [Google Scholar]
- Stefanovits, P.; Filep, G.; Füleky, G. Talajtan; Mezőgazda Kiadó: Budapest, Hungary, 1999. [Google Scholar]
- Csubák, M.; Posta, J.; Zsuposné, O.Á. Összefüggések a Humuszsavak Kémiai Összetétele És a Talaj Biológiai Aktivitása Között; Food and Agriculture Organization of the United Nations: Rome, Italy, 2007; pp. 1–10. [Google Scholar]
- Kotroczó, Z.; Fekete, I. Significance of Soil Respiration from Biological Activity in the Degradation Processes of Different Types of Organic Matter. DRC Sustain. Future 2020, 1, 171–179. [Google Scholar] [CrossRef]
- Boncz, I. Kutatásmódszetani Alapismeretek; Pécsi Tudományegyetem: Pécs, Hungary, 2015; ISBN 978-963-642-826-6. [Google Scholar]
- Bodó, A.; Farkas, Á.; Nagy, D.U.; Rudolf, K.; Hoffmann, R.; Kocsis, M.; Morschhauser, T. Soil Humus, Iron, Sulphate and Magnesium Content Affect Nectar Traits of Wild Garlic (Allium ursinum L.). Plants 2021, 10, 597. [Google Scholar] [CrossRef] [PubMed]
- MSZ-08-0210:1977; Testing Organic Carbon Content. Hungarian Standards Institution: Budapest, Hungary, 1977.
- Bálint, S.; Füleky, G.; Győri, D.; Hargitai, L.; Kardos, J.; Lukács, A.; Molnár, E. Talaj-És Agrokémiai Vizsgálati Módszerkönyv 2; Mezőgazdasági Könyvkiadó Vállalat: Budapest, Hungary, 1988. [Google Scholar]
- Schinner, F.; Öhlinger, R.; Kandeler, E.; Margesin, R. Methods in Soil Biology; Springer: Berlin, Heidelberg, 1996; ISBN 978-3-642-64633-1. [Google Scholar]
- Kátai, J.; Zsuposné, Á.O.; Tállai, M.; Alshaal, T. Would Fertilization History Render the Soil Microbial Communities and Their Activities More Resistant to Rainfall Fluctuations? Ecotoxicol. Environ. Saf. 2020, 201, 110803. [Google Scholar] [CrossRef]
- Ragályi, P.; Lončarić, Z.; Rebekić, A.; Rékási, M.; Borsányi, B.; Molnár, S.; Szabó, A.; Draskovits, E.; Uzinger, N. The Effect of Sewage Sludge and Sludge Compost on Soil Fertility, Organic Matter Content and Yield of Perennial Ryegrass (Lolium perenne). Sveučilište u Zagrebu, Agronomski fakultet. In Proceedings of the 55th Croatian & 15th International Symposium on Agriculture, Vodice, Croatia, 16–21 February 2020; pp. 76–80. [Google Scholar]
- MSZ-08-0206/2:1978; Determination of the pH of Soil (in Hungarian) Hungarian Standard. Available online: http://szabvanykonyvtar.mszt.hu/ (accessed on 3 May 2025).
- Takamoto, A.; Takahashi, T.; Togami, K. Estimation Models from Soil pH with a Solid-to-Liquid Ratio of 1:2.5 to pH Measured by Other Methods Using Soils in Japan. Soil Sci. Plant Nutr. 2023, 69, 190–198. [Google Scholar] [CrossRef]
- Khan, R.; Jan, S.; Amin, R.; Haq, I.U.; Shoukat, A.; Khan, A.; Akbar, M.S.; Ali, T.; Younas, O.; Rasheed, S.M. Impact of Atrazine and Bromoxynil on the Colony Forming Units (Cfu) of Soil Bacteria. Agric. Sci. J. 2023, 5, 1–7. [Google Scholar] [CrossRef]
- Irving, D.; Bakhshandeh, S.; Tran, T.K.A.; McBratney, A.B. A Cost-Effective Method for Quantifying Soil Respiration. Soil Secur. 2024, 16, 100162. [Google Scholar] [CrossRef]
- Sándor, Z.; Kincses, I.; Tállai, M.; Lowy, D.A.; Melendez, J.R.; Guananga Diaz, N.I.; Guevara Iñiguez, L.E.; Cuenca Nevarez, G.; Talledo Solórzano, V.; Kátai, J. Effect of Herbicides on Soil Respiration: A Case Study Conducted at Debrecen-Látókép Plant Cultivation Experimental Station. F1000Res 2020, 9, 1348. [Google Scholar] [CrossRef]
- Obasi, G.O.P.; Töpfer, K. (Eds.) Land Use, Land-Use Change, and Forestry: A Special Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2000; ISBN 978-92-9169-114-2. [Google Scholar]
- Balláné Kovács, A.; Kremper, R.; Kincses, I.; Leviczky, Á. Influences of Different Organic Fertilizers on Nutrients of Humic Sandy Soil and on the Growth of Spinach (Spinacia oleracea L.). Acta Agrar. Debr. 2016, 70, 23–28. [Google Scholar] [CrossRef]
- Nagy, P.T.; Gonda, I.; Dremák, P.; Holb, I.J. Study on the Micronutrient Content of Soil and Leaf of an Organic Apple Orchard in Eastern Hungary. Int. J. Hortic. Sci. 2006, 12, 7–11. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, X.; Ren, T.; Luo, J.; Liang, J.; Wang, E.T.; Shi, F. Differentiation of Soil Metabolic Function and Microbial Communities between Plantations and Natural Reforestation. Front. Microbiol. 2025, 16, 1544641. [Google Scholar] [CrossRef]
- Libus, J.; Mauer, O.; Vavříček, D. Soil Preparation by Ploughing in the Floodplain Forest and Its Influence on Vegetation and Primary Soil Characteristics. J. For. Sci. 2010, 56, 183–196. [Google Scholar] [CrossRef]
- Zanella, A.; Jabiol, B.; Ponge, J.F.; Sartori, G.; De Waal, R.; Van Delft, B.; Graefe, U.; Cools, N.; Katzensteiner, K.; Hager, H.; et al. A European Morpho-Functional Classification of Humus Forms. Geoderma 2011, 164, 138–145. [Google Scholar] [CrossRef]
- Meurer, K.H.E.; Chenu, C.; Coucheney, E.; Herrmann, A.M.; Keller, T.; Kätterer, T.; Nimblad Svensson, D.; Jarvis, N. Modelling Dynamic Interactions between Soil Structure and the Storage and Turnover of Soil Organic Matter. Biogeosciences 2020, 17, 5025–5042. [Google Scholar] [CrossRef]
- Hicks, L.C.; Meir, P.; Nottingham, A.T.; Reay, D.S.; Stott, A.W.; Salinas, N.; Whitaker, J. Carbon and Nitrogen Inputs Differentially Affect Priming of Soil Organic Matter in Tropical Lowland and Montane Soils. Soil Biol. Biochem. 2019, 129, 212–222. [Google Scholar] [CrossRef]
- Kátai, J.; Oláh, Á.; Sándor, Z.; Tállai, M. Comparison of Soil Parameters of the Carbon and Nitrogen Cycles in a Long-Term Fertilization Field Experiment. Agrokémia és Talajtan 2014, 63, 129–138. [Google Scholar] [CrossRef]
- Szili-Kovács, T.; Kátai, J.; Takács, T. Mikrobiológiai Indikátorok Alkalmazása a Talajminőség Értékelésében. 1. Módszerek. Agrokémia és Talajtan 2011, 60, 273–286. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, J.; Zhang, J.; Xin, X.; Hao, X. How Different Long-term Fertilization Strategies Influence Crop Yield and Soil Properties in a Maize Field in the North China Plain. J. Plant Nutr. Soil Sci. 2013, 176, 99–109. [Google Scholar] [CrossRef]
- Tállai, M.; Kátai, J.; Zsuposné, O.Á.; Sándor, Z.; Balláné, K.A. Különböző Hasznosítású Talajtípusok Összehasonlítása Néhány Mikrobiológiai Jellemző Alapján; Talajvédelem Talajvédelmi Alapítvány, Magyar Talajtani Társaság, SZTE Mezőgazdasági Kar: Hódmezővásárhely, Hungary, 2023; pp. 204–217. ISBN 978-615-01-9177-5. [Google Scholar]
- Anderson, T.-H. Microbial Eco-Physiological Indicators to Asses Soil Quality. Agric. Ecosyst. Environ. 2003, 98, 285–293. [Google Scholar] [CrossRef]
- Buckeridge, K.M.; Mason, K.E.; McNamara, N.P.; Ostle, N.; Puissant, J.; Goodall, T.; Griffiths, R.I.; Stott, A.W.; Whitaker, J. Environmental and Microbial Controls on Microbial Necromass Recycling, an Important Precursor for Soil Carbon Stabilization. Commun. Earth Environ. 2020, 1, 36. [Google Scholar] [CrossRef]
- John Raphael, L.; Mromba, C. Reforestation for Mitigation and Adaptation to Climate Change in North-Eastern Highlands of Tanzania: Beyond Carbon Sequestration. Tanzan. J. Popul. Stud. Dev. 2024, 31, 1–15. [Google Scholar] [CrossRef]
- Varnagirytė-Kabašinskienė, I.; Survila, G.; Armolaitis, K. Deep Soil Ploughing for Afforestation: A Review of Potential Impacts on Soil and Vegetation. Balt. For. 2022, 27, 1–11. [Google Scholar] [CrossRef]
- Dolmatov, S.; Makunina, Y.; Voinash, S.; Tikhonov, E.; Aksenov, A.; Alekseeva, S.; Parfenopulo, G. Search for Reserves to Improve the Efficiency of Forest Soil Preparation Technology and Land Clearing during Reforestation by Reducing the Energy Intensity of the Process. E3S Web Conf. 2023, 390, 7045. [Google Scholar] [CrossRef]


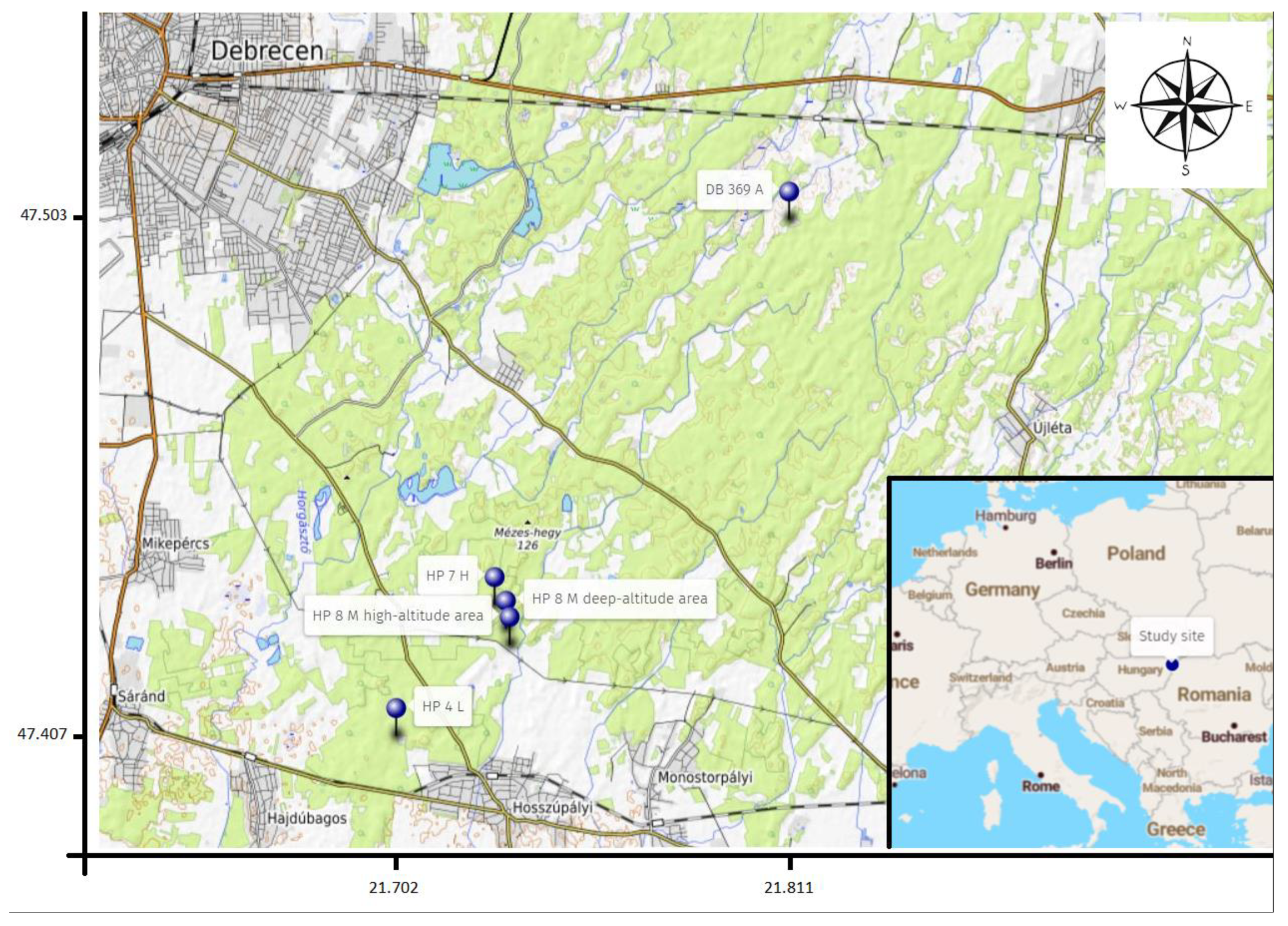
| Location of the Forest | Extracted Tree Species | Tree Species for Afforestation |
|---|---|---|
| Hosszúpályi 7 H | Scotch pine (Pinus sylvestris, L.) | Black locust (Robinia pseudoacacia, L.) |
| Hosszúpályi 8 M | Cotton wood (Populus x) | Black locust (Robinia pseudoacacia, L.) |
| Hosszúpályi 8 M | Cotton wood (Populus x) | Cottonwood (Populus x) |
| Hosszúpályi 4 L | Black locust (Robinia pseudoacacia, L.) | Scotch pine (Pinus sylvestris, L.) |
| Debrecen 369 A | Scotch pine (Pinus sylvestris, L.) | Black locust (Robinia pseudoacacia, L.) |
| Sampling Sites with GPS Coordinates (GPSWGS84) | Cultivation Methods | Depth (cm) | Sample No. |
|---|---|---|---|
| Forest I. Hosszúpályi 7 H 47.431, 21.730 | * HP 7 H: unplowed | 0–30 | 1 |
| HP 7 H: deep plowed upper layer | 0–30 | 2 | |
| HP 7 H: deep plowed lower layer | 30–70 | 3 | |
| HP 7 H: row of tree stumps with soil content | 0–15 | 4 | |
| Forest II. (high-altitude area) Hosszúpályi 8 M 47.424, 21.734 | HP 8 M: unplowed | 0–30 | 5 |
| HP 8 M: deep plowed upper layer | 0–30 | 6 | |
| HP 8 M: deep plowed lower layer | 30–70 | 7 | |
| HP 8 M: row of tree stumps with soil content | 0–15 | 8 | |
| Forest II. (deep-altitude area) Hosszúpályi 8 M 47.427, 21.733 | HP 8 M: unplowed | 0–30 | 9 |
| HP 8 M: deep plowed upper layer | 0–30 | 10 | |
| HP 8 M: deep plowed lower layer | 30–70 | 11 | |
| HP 8 M: row of tree stumps with soil content | 0–15 | 12 | |
| Forest III. Hosszúpályi 4 L 47.407, 21.702 | HP 4 L: unplowed | 0–30 | 13 |
| HP 4 L: deep plowed upper layer | 0–30 | 14 | |
| HP 4 L: deep plowed lower layer | 30–70 | 15 | |
| HP 4 L: row of trees stumps with soil content | 0–15 | 16 | |
| Forest IV. Debrecen 369 A 47.503, 21.811 | DB 369 A: unplowed | 0–30 | 17 |
| ** DB 369 A: deep plowed upper layer | 0–30 | 18 | |
| DB 369 A: deep plowed lower layer | 30–70 | 19 | |
| DB 369 A: row of tree stumps with soil content | 0–15 | 20 |
 | Horizon Designation | Horizon Depth (cm) | Description of the Soil Layer |
| Ah | 0–5 | Reddish gray (2.5YR 5/1) color, single-grain sand, slightly humic level | |
| AC | 5–50 | Light reddish gray (2.5YR 7/1) color, single-grain sand, slightly humus-like, roots interspersed | |
| Bw | 50–110 | Light red (2.5YR 7/6) color, single-grain sand, sparsely rooted | |
| Cl/Bt | 110–140 | Reddish brown (2.5YR 5/4) color, single-grain sand | |
| Cl/Bt | 140–170 | Pinkish gray (7.5YR 6/2) color, single-grain sand | |
| Clr | 170– | Gray (7.5YR 6/1) color, single-grain sand with reductive features |
| Sample | KA | HU% | Stability Coefficient (K) | pH (H2O) | Microscopic Fungi × 103 g−1 | Soil Bacteria × 106 g−1 | mg CO2 × 100 g−1 Soil | mg CO2 × 100 g−1 × 10 Day−1 |
|---|---|---|---|---|---|---|---|---|
| 1 | 19.80 ± 0.79 ab | 0.36 ± 0.28 a | 1.26 ± 0.14 a | 4.59 ± 0.20 a | 31.50 ± 5.27 ab | 0.95 ± 0.27 a | 12.97 ± 1.85 a | 13.67 ± 2.46 a |
| 2 | 18.20 ± 1.42 a | 0.22 ± 0.13 a | 7.06 ± 1.40 b | 5.41 ± 0.23 b | 51.50 ± 4.36 b | 0.41 ± 0.09 a | 12.47 ± 1.38 a | 13.27 ± 1.17 a |
| 3 | 18.40 ± 1.64 a | 0.10 ± 0.04 a | 0.53 ± 0.13 a | 5.22 ± 0.22 b | 10.50 ± 1.50 a | 0.68 ± 0.24 a | 13.45 ± 0.47 a | 16.78 ± 1.22 ab |
| 4 | 21.70 ± 0.70 b | 4.20 ± 0.53 b | 0.07 ± 0.03 a | 4.69 ± 0.12 a | 121.50 ± 21.78 c | 7.68 ± 0.64 b | 17.88 ± 1.14 b | 17.86 ± 1.11 c |
 | Horizon Designation | Horizon Depth (cm) | Description of the Soil Layer |
| Ah | 0–10 | Dark gray (2.5Y 4/1) color, sandy loam, strong humus level, interwoven with roots | |
| E1 | 10–15 | Grayish white (2.5Y 8/1) color, single-grain sand, slightly humus level, interwoven with roots | |
| A/E | 15–45 | Gray (2.5Y 6/2) color, single-grain sand, interspersed with roots, traces of soil mixing | |
| E2 | 45–50 | Light gray (2.5Y 7/1) color, single-grain sand, root interspersed | |
| Bw | 50–55 | Gray (7.5YR 6/1) color, single-grain sand, interspersed with roots, organic matter accumulation | |
| C | 55–85 | Pinkish gray (7.5YR 7/2) color, more compacted, single-grain sandy loam | |
| C/Bt | 85–140 | Dark reddish brown (2.5YR 3/3) color, compacted loam layer | |
| Clr | 140–150 | Grayish brown (2.5Y 5/2) color, rootless sand | |
| Clr | 150– | Gray (2.5Y 5/1) color single-grain sand with reductive features |
| Sample | KA | HU % | Stability Coefficient (K) | pH (H2O) | Microscopic Fungi × 103 g−1 | Soil Bacteria × 106 g−1 | mg CO2 × 100 g−1 Soil | mg CO2 × 100 g−1 × 10 Day−1 |
|---|---|---|---|---|---|---|---|---|
| 5 | 36.20 ± 1.49 c | 2.84 ± 0.24 b | 0.46 ± 0.14 a | 6.68 ± 0.27 b | 151.75 ± 36.52 b | 0.70 ± 0.21 a | 14.70 ± 2.39 a | 15.13 ± 2.05 ab |
| 6 | 22.00 ± 2.12 a | 1.70 ± 0.32 a | 0.99 ± 0.31 b | 5.66 ± 0.32 a | 28.00 ± 7.37 a | 1.55 ± 0.34 b | 12.56 ± 1.23 a | 13.36 ± 0.94 a |
| 7 | 28.60 ± 1.39 b | 1.27 ± 0.26 a | 0.38 ± 0.05 a | 6.31 ± 0.24 ab | 12.50 ± 4.92 a | 1.06 ± 0.26 ab | 12.05 ± 1.54 a | 17.51 ± 1.07 b |
| 8 | 31.50 ± 0.92 b | 1.41 ± 0.16 a | 1.39 ± 0.17 b | 7.23 ± 0.56 b | 15.00 ± 2.18 a | 5.55 ± 0.34 c | 13.08 ± 1.14 a | 16.47 ± 1.42 ab |
 | Horizon Designation | Horizon Depth (cm) | Description of the Soil Layer |
| Ah1 | 0–10 | Very dark gray (10YR 3/1) color, subangular blocky structure, clay loam, heavily humic level, heavily rooted. | |
| Ah2 | 10–35 | Dark grayish brown (10YR 4/21) color, subangular blocky structure, loamy loam, highly humic level, heavily rooted, slightly calcareous | |
| Blo | 35–55 | Red (2.5YR 4/6) color, single-grain structure loam, sparsely rooted | |
| Ab | 55–80 | Dark reddish gray (2.5YR 4/1) color, grain structure loam, less rooted | |
| C | 80–120 | Grayish white (2.5Y 8/1) color, single-grain sand | |
| Clo | 120–160 | Olive gray (2.5Y 5/2) color, single-grain sand | |
| Clr | 160– | Bluish-gray color (GLEY2 5/5B) loam |
| Sample | KA | HU % | Stability Coefficient (K) | pH (H2O) | Microscopic Fungi × 103 g−1 | Soil bacteria × 106 g−1 | mg CO2 × 100 g−1 Soil | mg CO2 × 100 g−1 × 10 Day−1 |
|---|---|---|---|---|---|---|---|---|
| 9 | 42.60 ± 1.59 c | 4.01 ± 0.16 b | 2.11 ± 0.22 a | 7.79 ± 0.36 a | 37.00 ± 5.41 bc | 10.73 ± 1.22 c | 14.54 ± 0.72 a | 15.81 ± 1.41 a |
| 10 | 30.60 ± 1.99 a | 3.24 ± 0.36 b | 2.26 ± 0.18 a | 7.52 ± 0.33 a | 24.00 ± 6.61 ab | 6.45 ± 0.28 b | 13.69 ± 1.28 a | 14.34 ± 1.33 a |
| 11 | 34.10 ± 1.04 ab | 1.03 ± 0.48 a | 2.33 ± 0.21 a | 7.92 ± 0.27 a | 11.50 ± 2.65 a | 0.73 ± 0.10 a | 15.69 ± 1.45 a | 17.88 ± 1.98 a |
| 12 | 38.40 ± 2.38 bc | 3.11 ± 1.03 b | 2.07 ± 0.13 a | 7.84 ± 0.28 a | 43.50 ± 8.35 c | 7.12 ± 0.35 b | 14.26 ± 1.01 a | 15.39 ± 0.92 a |
 | Horizon Designation | Horizon Depth (cm) | Description of the Soil Layer |
| Ah | 0–20 | Brown (7.5YR 5/2) color, single-grain sand, slightly humus-like, rooted | |
| A/C | 20–40 | Brown (7.5YR 4/4) color, single-grain sand, buried in a lightly humic layer, interspersed with roots. The shape of layer boundaries is irregular | |
| C | 40–90 | Light Brown (7.5YR 6/4) color, single-grain sand, sparsely rooted | |
| C/Bt | 90–180 | Brown (7.5YR 4/4) color, single-grain sand | |
| Cl | 180– | Gray (7.5YR 6/1) color, single-grain sand |
| Sample | KA | HU% | Stability Coefficient (K) | pH (H2O) | Microscopic Fungi × 103 g−1 | Soil Bacteria × 106 g−1 | mg CO2 × 100 g−1 Soil | mg CO2 × 100 g−1 × 10 Day−1 |
|---|---|---|---|---|---|---|---|---|
| 13 | 26.70 ± 1.39 c | 0.26 ± 0.09 a | 1.55 ± 0.38 b | 4.15 ± 0.12 a | 39.50 ± 8.35 b | 0.73 ± 0.18 a | 10.76 ± 0.44 a | 16.30 ± 1.21 b |
| 14 | 18.10 ± 1.25 a | 0.17 ± 0.07 a | 0.48 ± 0.10 a | 4.25 ± 0.22 a | 16.50 ± 2.50 a | 1.24 ± 0.26 a | 11.17 ± 2.50 a | 12.73 ± 1.39 a |
| 15 | 21.80 ± 1.14 b | 0.41 ± 0.10 a | 1.87 ± 0.18 b | 5.63 ± 0.34 b | 4.50 ± 1.00 a | 0.87 ± 0.19 a | 12.68 ± 1.57 a | 15.22 ± 0.75 ab |
| 16 | 28.90 ± 1.14 c | 0.96 ± 0.32 b | 0.32 ± 0.15 a | 5.02 ± 0.54 ab | 73.50 ± 13.43 c | 3.46 ± 0.17 b | 13.82 ± 0.49 a | 14.02 ± 0.27 ab |
 | Horizon Designation | Horizon Depth (cm) | Description of the Soil Layer |
| Ah | 0–3 | Gray (7.5YR 5/1) color, single-grain sand, slightly humus-like level, rooted | |
| E | 3–20 | Light gray (7.5YR 7/1) color, single-grain sand, slightly humus-like level, rooted | |
| Bw | 20–55 | Dark brown (7.5YR 3/3) color, single-grain sand, heavily rooted | |
| C | 55–90 | Reddish yellow (7.5YR 6/6) color, single-grain sand, lightly rooted | |
| Clr | 90–160 | Dark gray (7.5YR 4/1) color, single-grain sand |
| Sample | KA | HU % | Stability Coefficient (K) | pH (H2O) | Microscopic Fungi × 103 g−1 | Soil Bacteria × 106 g−1 | mg CO2 × 100 g−1 Soil | mg CO2 × 100 g−1 × 10 Day−1 |
|---|---|---|---|---|---|---|---|---|
| 17 | 19.70 ± 0.62 ab | 4.33 ± 0.28 c | 0.07 ± 0.03 a | 4.08 ± 0.19 a | 430.50 ± 30.43 d | 7.77 ± 1.60 c | 16.68 ± 3.98 a | 17.19 ± 2.25 a |
| 18 | 17.60 ± 1.73 a | 2.82 ± 0.22 b | 0.16 ± 0.07 a | 4.63 ± 0.29 b | 124.50 ± 17.68 b | 2.70 ± 0.63 ab | 12.54 ± 2.05 a | 15.72 ± 1.35 a |
| 19 | 17.90 ± 0.69 ab | 1.03 ± 0.33 a | 0.32 ± 0.25 a | 5.42 ± 0.22 c | 22.50 ± 4.58 a | 0.78 ± 0.28 a | 15.85 ± 5.19 a | 17.63 ± 1.73 a |
| 20 | 20.40 ± 082 b | 3.11 ± 0.48 b | 0.09 ± 0.03 a | 4.61 ± 0.10 ab | 184.50 ± 23.11 c | 4.11 ± 0.16 b | 17.62 ± 1.18 a | 17.81 ± 1.36 a |
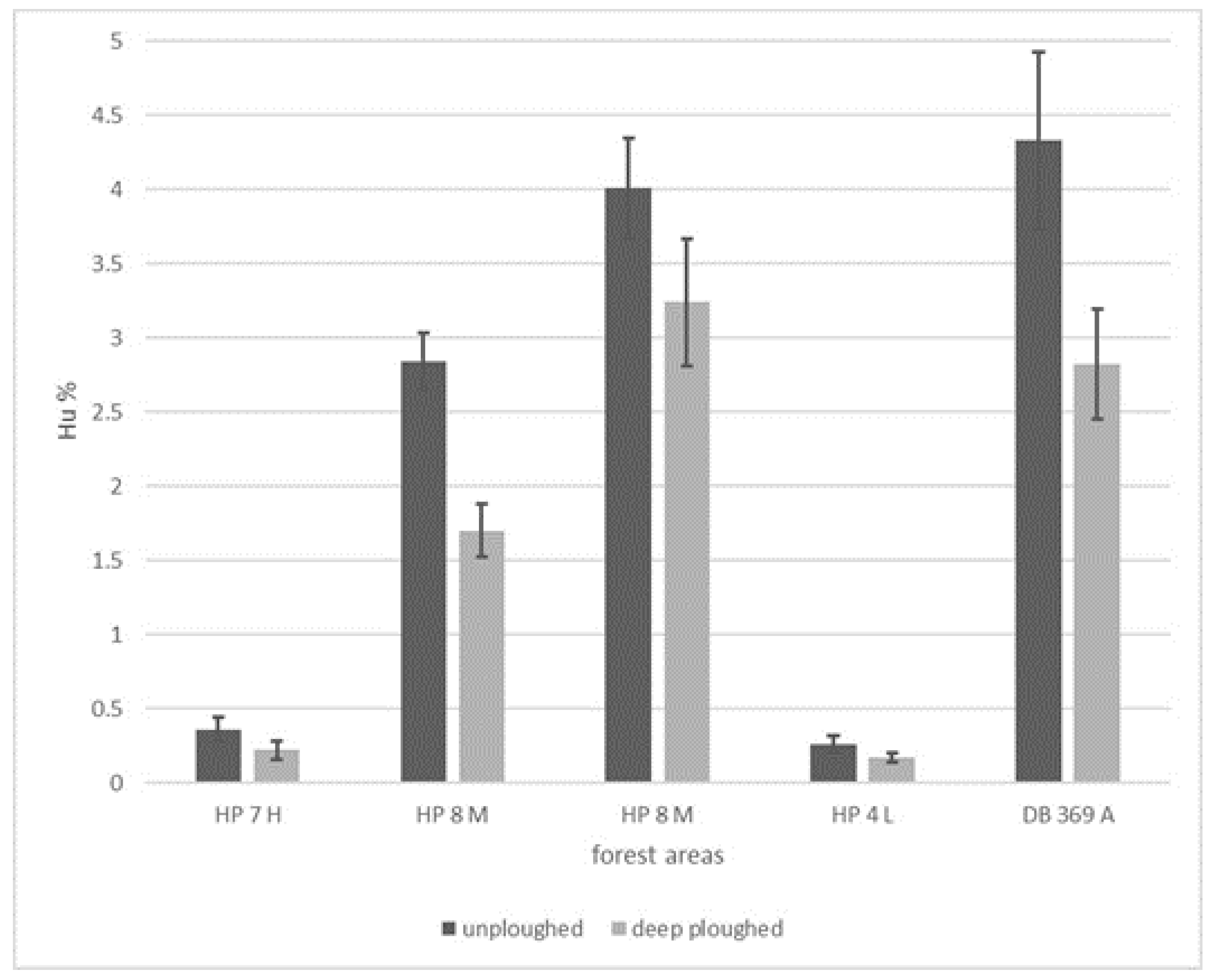
| Forest | Sample | HU (%) | HU (%) Loss |
|---|---|---|---|
| HP 7 H: unplowed | 1 | 0.36 | 38.89 |
| HP 7 H: deep plowed | 2 | 0.22 | |
| HP 8 M: upper lying unplowed | 5 | 2.84 | 40.14 |
| HP 8 M: upper lying deep plowed | 6 | 1.70 | |
| HP 8 M: deep-lying area unplowed | 9 | 4.01 | 19.20 |
| HP 8 M: deep-lying area deep plowed | 10 | 3.24 | |
| HP 4 L: unplowed | 13 | 0.26 | 34.62 |
| HP 4 L: deep plowed | 14 | 0.17 | |
| DB 369 A: unplowed | 17 | 4.33 | 34.87 |
| DB 369 A: deep plowed | 18 | 2.82 | |
| Average: 33.54% | |||
| Pearson Correlations | ||||||
|---|---|---|---|---|---|---|
| HU% | K-Coefficient | pH (H2O) | Microscopic Fungi | Total No. of Bacteria | CO2 Production | |
| HU% | 1 | |||||
| K coefficient | −0.330 | 1 | ||||
| pH (H2O) | 0.017 | 0.055 | 1 | |||
| Microscopic fungi | 0.806 ** | −0.254 | −0.360 | 1 | ||
| Total no. of bacteria | 0.648 ** | −0.248 | −0.155 | 0.469 * | 1 | |
| CO2 production | 0.607 ** | −0.364 | 0.122 | 0.238 | 0.223 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kocsis, I.A.; Tállai, M.; Zsuposné Oláh, Á.; László, Z.; Mokos, B.; Kincses, I.; Juhász, E.K.; Lowy, D.A.; Sándor, Z. Unveiling the Transformative Effects of Forest Restoration on the Soil Chemistry and Biology of Sandy Soils in Southern Nyírség, Hungary. Agriculture 2025, 15, 1030. https://doi.org/10.3390/agriculture15101030
Kocsis IA, Tállai M, Zsuposné Oláh Á, László Z, Mokos B, Kincses I, Juhász EK, Lowy DA, Sándor Z. Unveiling the Transformative Effects of Forest Restoration on the Soil Chemistry and Biology of Sandy Soils in Southern Nyírség, Hungary. Agriculture. 2025; 15(10):1030. https://doi.org/10.3390/agriculture15101030
Chicago/Turabian StyleKocsis, István Attila, Magdolna Tállai, Ágnes Zsuposné Oláh, Zoltán László, Béla Mokos, Ida Kincses, Evelin Kármen Juhász, Daniel A. Lowy, and Zsolt Sándor. 2025. "Unveiling the Transformative Effects of Forest Restoration on the Soil Chemistry and Biology of Sandy Soils in Southern Nyírség, Hungary" Agriculture 15, no. 10: 1030. https://doi.org/10.3390/agriculture15101030
APA StyleKocsis, I. A., Tállai, M., Zsuposné Oláh, Á., László, Z., Mokos, B., Kincses, I., Juhász, E. K., Lowy, D. A., & Sándor, Z. (2025). Unveiling the Transformative Effects of Forest Restoration on the Soil Chemistry and Biology of Sandy Soils in Southern Nyírség, Hungary. Agriculture, 15(10), 1030. https://doi.org/10.3390/agriculture15101030






