Modeling of the Physicochemical and Nutritional Composition of Musa paradisiaca (Williams Variety) at Different Ripening Stages in Ecuador
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- P1 (Stage 1): Dark green pericarp,
- P2 (Stage 2): Light green pericarp,
- P3 (Stage 3): Green pericarp with small yellow spots,
- P4 (Stage 4): Green pericarp with yellow streaks,
- P5 (Stage 5): Yellow pericarp with green margins,
- P6 (Stage 6): Fully yellow pericarp,
- P7 (Stage 7): Yellow pericarp with small, pigmented spots,
- P8 (Stage 8): Yellow pericarp with pronounced brown spots.
2.2. Banana Flour
2.3. Preparation of Aqueous Samples
2.4. Analysis of Basic Components
2.5. Determination of Starch and Free Sugar Content
2.5.1. Determination of Total Starch Content
- : Absorbance of the sample relative to the blank.
- Conversion factor for 100 µg of D-glucose absorbance.
- : Final volume.
- Weight of the sample in milligrams.
- 0.1: Volume of the sample analyzed (in mL).
- Adjustment factor for converting free D-glucose to anhydrous D-glucose.
2.5.2. Determination of Resistant Starch Content
- : Absorbance of the sample relative to the blank.
- Conversion factor for 100 µg of D-glucose absorbance.
- Correction for the volume (0.1 mL taken from 10 mL).
- : Volume of the sample analyzed (in mL).
- Adjustment factor for converting free D-glucose to anhydrous D-glucose.
2.5.3. Determination of Free Sugar Content
2.6. Soluble Solids Content (°Brix)
2.7. pH Measurement
2.8. Titratable Acidity
- V = Volume of the test sample (mL).
- V0 = Volume of the test portion (mL).
- V1 = Volume of the NaOH titrant solution (mL).
- C = Concentration of NaOH solution in moles per liter.
2.9. Determination of Vitamin Content via High-Performance Liquid Chromatography (HPLC)
2.9.1. Ascorbic Acid (Vitamin C)
2.9.2. Thiamine (Vitamin B1), Riboflavin (Vitamin B2), and Pyridoxine (Vitamin B6)
2.9.3. Peroxidase Activity
- ∆Abs: Change in absorbance.
- : Time interval in minutes.
- : Sample weight in grams.
2.9.4. Oxalic Acid and Tannin Content
2.10. Statistical Analysis
2.10.1. PERMANOVA Model
- : Number of groups.
- : Total number of observations.
2.10.2. HJ-Biplot
- U: A matrix whose columns are orthonormal eigenvectors of
- : A diagonal matrix of singular values of
- V: An orthogonal matrix whose columns are eigenvectors of .
3. Results
3.1. Characterization of Physicochemical and Nutritional Properties
3.1.1. Descriptive Analysis of the Physicochemical Composition of Banana
3.1.2. Descriptive Analysis of Nutritional Composition
3.2. Multivariate Analysis of Variance and Biplot Visualization of Physicochemical and Nutritional Differences During Ripening
3.2.1. PERMANOVA and HJ-Biplot Analysis to Assess Significant Differences and Associations
3.2.2. PERMANOVA of Nutritional Properties
4. Discussion
4.1. Physicochemical Properties and Their Evolution During Ripening
4.2. Nutritional and Antioxidant Composition
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martínez-Solórzano, G.E.; Rey-Brina, J.C. Bananos (Musa AAA): Importancia, Producción y Comercio En Tiempos de COVID-19. Agron. Mesoam. 2021, 32, 1034–1046. [Google Scholar] [CrossRef]
- Ruiz Medina, M.D.; Ruales, J. Post-Harvest Alternatives in Banana Cultivation. Agronomy 2024, 14, 2109. [Google Scholar] [CrossRef]
- INEC. Encuesta de Superficie y Producción Agropecuaria Continua (ESPAC). 2023. Available online: https://anda.inec.gob.ec/anda/index.php/catalog/1037 (accessed on 4 May 2024).
- Keivani Rad, N.; Mohri, M.; Seifi, H.A.; Haghparast, A. Supplementation of Overripe Pulp Extract and Green Peel Extract or Powder of Banana Fruit Peel (Musa. Cavendish) to Diets of Neonatal Dairy Calves: Effects on Haematological, Immunological and Performance Characteristics. Vet. Med. Sci. 2021, 7, 876–887. [Google Scholar] [CrossRef] [PubMed]
- Chamorro-Hernandez, L.M.; Herrera-Granda, E.P.; Rivas-Rosero, C. Selection of the Processing Method for Green Banana Chips from Barraganete and Dominico Varieties Using Multivariate Techniques. Appl. Sci. 2024, 14, 2682. [Google Scholar] [CrossRef]
- Assunção, A.G.L.; Cakmak, I.; Clemens, S.; González-Guerrero, M.; Nawrocki, A.; Thomine, S. Micronutrient Homeostasis in Plants for More Sustainable Agriculture and Healthier Human Nutrition. J. Exp. Bot. 2022, 73, 1789–1799. [Google Scholar] [CrossRef]
- Symmank, C.; Zahn, S.; Rohm, H. Visually Suboptimal Bananas: How Ripeness Affects Consumer Expectation and Perception. Appetite 2018, 120, 472–481. [Google Scholar] [CrossRef]
- Maduwanthi, S.D.T.; Marapana, R.A.U.J. Induced Ripening Agents and Their Effect on Fruit Quality of Banana. Int. J. Food Sci. 2019, 2019, 2520179. [Google Scholar] [CrossRef]
- Swinnen, J.; Maertens, M.; Colen, L. The Role of Food Standards in Trade and Development. In Food Safety, Market Organization, Trade and Development; Springer International Publishing: Cham, Switzerland, 2015; pp. 133–149. [Google Scholar] [CrossRef]
- Anderson, M.J. Permutation Tests for Univariate or Multivariate Analysis of Variance and Regression. Can. J. Fish. Aquat. Sci. 2001, 58, 626–639. [Google Scholar] [CrossRef]
- Garrido-Martín, D.; Calvo, M.; Reverter, F.; Guigó, R. A Fast Non-Parametric Test of Association for Multiple Traits. Genome Biol. 2023, 24, 230. [Google Scholar] [CrossRef]
- Galindo, M. Una Alternativa de Representación Simultánea: HJBiplot; Universitat Politècnica de Barcelona: Barcelona, Spain, 1986. [Google Scholar]
- Pilacuan-Bonete, L.; Galindo-Villardón, P.; Delgado-Álvarez, F. HJ-Biplot as a Tool to Give an Extra Analytical Boost for the Latent Dirichlet Assignment (LDA) Model: With an Application to Digital News Analysis about COVID-19. Mathematics 2022, 10, 2529. [Google Scholar] [CrossRef]
- Cheng, Y.; Huang, P.; Chan, Y.; Chiang, P.; Lu, W.; Hsieh, C.; Liang, Z.; Yan, B.; Wang, C.R.; Li, P. Investigate the Composition and Physicochemical Properties Attributes of Banana Starch and Flour during Ripening. Carbohydr. Polym. Technol. Appl. 2024, 7, 100446. [Google Scholar] [CrossRef]
- Chassagne-Berces, S.; Fonseca, F.; Citeau, M.; Marin, M. Freezing Protocol Effect on Quality Properties of Fruit Tissue According to the Fruit, the Variety and the Stage of Maturity. LWT-Food Sci. Technol. 2010, 43, 1441–1449. [Google Scholar] [CrossRef]
- Li, Z.; Thomas, C. Quantitative Evaluation of Mechanical Damage to Fresh Fruits. Trends Food Sci. Technol. 2014, 35, 138–150. [Google Scholar] [CrossRef]
- Guadalupe-Moyano, V.; Palacios-Ponce, A.S.; Rosell, C.M.; Cornejo, F. Impact of Drying Methods on Banana Flour in the Gluten-Free Bread Quality. LWT 2022, 168, 113904. [Google Scholar] [CrossRef]
- Martín Lorenzo, M.; Piedra-Buena Díaz, A.; Díaz Romero, C.; Rodríguez-Rodríguez, E.M.; Lobo, M.G. Physicochemical and Nutritional Characterization of Green Banana Flour from Discarded Cavendish Bananas. Sustainability 2024, 16, 6647. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2006. [Google Scholar]
- Valenzuela Cobos, J.D.; Guevara Viejó, F.; Grijalva Endara, A.; Vicente Galindo, P.; Galindo Villardón, P. Multivariate Statistical Techniques: A New Approach to Identify the Commercial Properties of Mixtures of Flours of Lentinula Edodes and Cocoa Pod Husk. J. Fungi 2023, 9, 991. [Google Scholar] [CrossRef]
- Adedayo, B.C.; Oboh, G.; Oyeleye, S.I.; Olasehinde, T.A. Antioxidant and Antihyperglycemic Properties of Three Banana Cultivars (Musa spp.). Scientifica 2016, 2016, 8391398. [Google Scholar] [CrossRef]
- Megazyme. Total Starch Assay Procedure (Amyloglucosidase/α-Amylase Method). AOAC Method 996.11; AACC Method 76.13. 2011. Available online: https://www.fts.co.nz/image/data/PDF/K-TSTA_1107_DATA.pdf (accessed on 18 February 2025).
- Huang, P.-H.; Cheng, Y.-T.; Lu, W.-C.; Chiang, P.-Y.; Yeh, J.-L.; Wang, C.-C.; Liang, Y.-S.; Li, P.-H. Changes in Nutrient Content and Physicochemical Properties of Cavendish Bananas Var. Pei Chiao during Ripening. Horticulturae 2024, 10, 384. [Google Scholar] [CrossRef]
- Scalisi, A.; O’Connell, M.G. Relationships between Soluble Solids and Dry Matter in the Flesh of Stone Fruit at Harvest. Analytica 2021, 2, 14–24. [Google Scholar] [CrossRef]
- Polak, N.; Kalisz, S.; Kruszewski, B. High-Temperature Short-Time and Ultra-High-Temperature Processing of Juices, Nectars and Beverages: Influences on Enzyme, Microbial Inactivation and Retention of Bioactive Compounds. Appl. Sci. 2024, 14, 8978. [Google Scholar] [CrossRef]
- Cohen, S.; Itkin, M.; Yeselson, Y.; Tzuri, G.; Portnoy, V.; Harel-Baja, R.; Lev, S.; Sa‘ar, U.; Davidovitz-Rikanati, R.; Baranes, N.; et al. The PH Gene Determines Fruit Acidity and Contributes to the Evolution of Sweet Melons. Nat. Commun. 2014, 5, 4026. [Google Scholar] [CrossRef]
- NTE INEN-ISO 750; Productos Vegetales Y DE Frutas–Determinación de La Acidez Titulable (IDT). ISO: Geneva, Switzerland, 2013.
- Gentili, A.; Dal Bosco, C. Analysis of Vitamins by Liquid Chromatography. In Liquid Chromatography; Elsevier: Amsterdam, The Netherlands, 2023; pp. 733–786. [Google Scholar] [CrossRef]
- Nelson, K.J.; Parsonage, D. Measurement of Peroxiredoxin Activity. Curr. Protoc. Toxicol. 2011, 49, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Karamad, D.; Khrosravi, K.; Hosseini, H.; Tavasoli, S. Analytical Procedures and Methods Validation for Oxalate Content Estimation. Biointerface Res. Appl. Chem. 2019, 9, 4305–4310. [Google Scholar] [CrossRef]
- Verzele, M.; Delahaye, P. Analysis of Tannic Acids by High-Performance Liquid Chromatography. J. Chromatogr. A 1983, 268, 469–476. [Google Scholar] [CrossRef]
- Mardia, K.V. Measures of Multivariate Skewness and Kurtosis with Applications. Biometrika 1970, 57, 519–530. [Google Scholar] [CrossRef]
- Tabachnick, B.G.; Fidell, L.S. Using Multivariate Statistics, 6th ed.; Pearson: Boston, MA, USA, 2013. [Google Scholar]
- Hair, J.; Babin, B.; Anderson, R.; Black, W. Multivariate Data Analysis, 8th ed.; Pearson Prentice: England, UK, 2019. [Google Scholar]
- Anderson, M.J. A New Method for Non-parametric Multivariate Analysis of Variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Zeileis, A.; Köll, S.; Graham, N. Various Versatile Variances: An Object-Oriented Implementation of Clustered Covariances in R. J. Stat. Softw. 2020, 95, 1–36. [Google Scholar] [CrossRef]
- Anderson, M.J. Distance-Based Tests for Homogeneity of Multivariate Dispersions. Biometrics 2006, 62, 245–253. [Google Scholar] [CrossRef]
- Bray, J.R.; Curtis, J.T. An Ordination of the Upland Forest Communities of Southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- Gabriel, K. The Biplot Graphic Display of Matrices with Application to Principal Component Analysis. Biometrika 1971, 58, 453–467. [Google Scholar] [CrossRef]
- Samanros, A.; Lin, J. Physicochemical Properties and in Vitro Digestibility of Starches from Different Taiwanese Banana Cultivars. Int. Food Res. J. 2021, 28, 1257–1267. [Google Scholar] [CrossRef]
- Li, M.-C.; Chou, C.-F.; Hsu, S.-C.; Lin, J.-S. Physicochemical Characteristics and Resistant Starch of Different Varieties of Banana from Taiwan. Int. J. Food Prop. 2020, 23, 1168–1175. [Google Scholar] [CrossRef]
- Biabiany, S.; Araou, E.; Cormier, F.; Martin, G.; Carreel, F.; Hervouet, C.; Salmon, F.; Efile, J.-C.; Lopez-Lauri, F.; D’Hont, A.; et al. Detection of Dynamic QTLs for Traits Related to Organoleptic Quality during Banana Ripening. Sci. Hortic. 2022, 293, 110690. [Google Scholar] [CrossRef]
- Pareek, S. Nutritional and Biochemical Composition of Banana (Musa spp.) Cultivars. In Nutritional Composition of Fruit Cultivars; Elsevier: Amsterdam, The Netherlands, 2015; pp. 49–81. [Google Scholar] [CrossRef]
- Fu, Y.; Meng, X.J.; Lu, N.N.; Jian, H.L.; Di, Y. Characteristics Changes in Banana Peel Coagulant during Storage Process. Int. J. Environ. Sci. Technol. 2019, 16, 7747–7756. [Google Scholar] [CrossRef]
- Addai, Z.R.; Abdullah, A.; Abd. Mutalib, S.; Musa, K.H.; Douqan, E.M.A. Antioxidant Activity and Physicochemical Properties of Mature Papaya Fruit (Carica papaya L. Cv. Eksotika). Adv. J. Food Sci. Technol. 2013, 5, 859–865. [Google Scholar] [CrossRef]
- Rop, O.; Sochor, J.; Jurikova, T.; Zitka, O.; Skutkova, H.; Mlcek, J.; Salas, P.; Krska, B.; Babula, P.; Adam, V.; et al. Effect of Five Different Stages of Ripening on Chemical Compounds in Medlar (Mespilus germanica L.). Molecules 2010, 16, 74–91. [Google Scholar] [CrossRef]
- Prabha, T.N.; Bhagyalakshmi, N. Carbohydrate Metabolism in Ripening Banana Fruit. Phytochemistry 1998, 48, 915–919. [Google Scholar] [CrossRef]
- Hardisson, A.; Rubio, C.; Baez, A.; Martin, M.; Alvarez, R.; Diaz, E. Mineral Composition of the Banana (Musa Acuminata) from the Island of Tenerife. Food Chem. 2001, 73, 153–161. [Google Scholar] [CrossRef]
- Mahmood, U.L.; Zora, S.; Hafiz, S.; Jashanpreet, K.; Andrew, W. Oxalic Acid: A Blooming Organic Acid for Postharvest Quality Preservation of Fresh Fruit and Vegetables. Postharvest Biol. Technol. 2023, 206, 112574. [Google Scholar] [CrossRef]
- Li, X.; Ouyang, W.; Jiang, Y.; Lin, Q.; Peng, X.; Hu, H.; Ye, Z.; Liu, G.; Cao, Y.; Yu, Y. Dextran-Sulfate-Sodium-Induced Colitis-Ameliorating Effect of Aqueous Phyllanthus emblica L. Extract through Regulating Colonic Cell Gene Expression and Gut Microbiomes. J. Agric. Food Chem. 2023, 71, 6999–7008. [Google Scholar] [CrossRef]
- Fernando, H.R.P.; Srilaong, V.; Pongpraser, N.; Boonyaritthongchai, P.; Jitareera, P. Changes in Antioxidant Properties and Chemical Composition during Ripening in Banana Variety ‘Hom Thong’ (AAA Group) and ‘Khai’ (AA Group). Int. Food Res. J. 2013, 21, 749–754. [Google Scholar]
- Freeland-Graves, J.H.; Sachdev, P.K.; Binderberger, A.Z.; Sosanya, M.E. Global Diversity of Dietary Intakes and Standards for Zinc, Iron, and Copper. J. Trace Elem. Med. Biol. 2020, 61, 126515. [Google Scholar] [CrossRef] [PubMed]
- Nguyễn, H.V.H.; Savage, G.P. Oxalate Content of New Zealand Grown and Imported Fruits. J. Food Compos. Anal. 2013, 31, 180–184. [Google Scholar] [CrossRef]
- Kalogiouri, N.P.; Aalizadeh, R.; Thomaidis, N.S. Application of an Advanced and Wide Scope Non-Target Screening Workflow with LC-ESI-QTOF-MS and Chemometrics for the Classification of the Greek Olive Oil Varieties. Food Chem. 2018, 256, 53–61. [Google Scholar] [CrossRef]
- Prasanna, V.; Prabha, T.N.; Tharanathan, R.N. Fruit Ripening Phenomena—An Overview. Crit. Rev. Food Sci. Nutr. 2007, 47, 1–19. [Google Scholar] [CrossRef]
- Holderbaum, D.F.; Kon, T.; Kudo, T.; Guerra, M.P. Enzymatic Browning, Polyphenol Oxidase Activity, and Polyphenols in Four Apple Cultivars: Dynamics during Fruit Development. HortScience 2010, 45, 1150–1154. [Google Scholar] [CrossRef]
- Wang, B.; Kang, J.; Wang, S.; Haider, F.U.; Zhong, Y.; Zhang, P. Variations in the End-Use Quality of Whole Grain Flour Are Closely Related to the Metabolites in the Grains of Pigmented Wheat (Triticum aestivum L.). Plants 2025, 14, 171. [Google Scholar] [CrossRef]
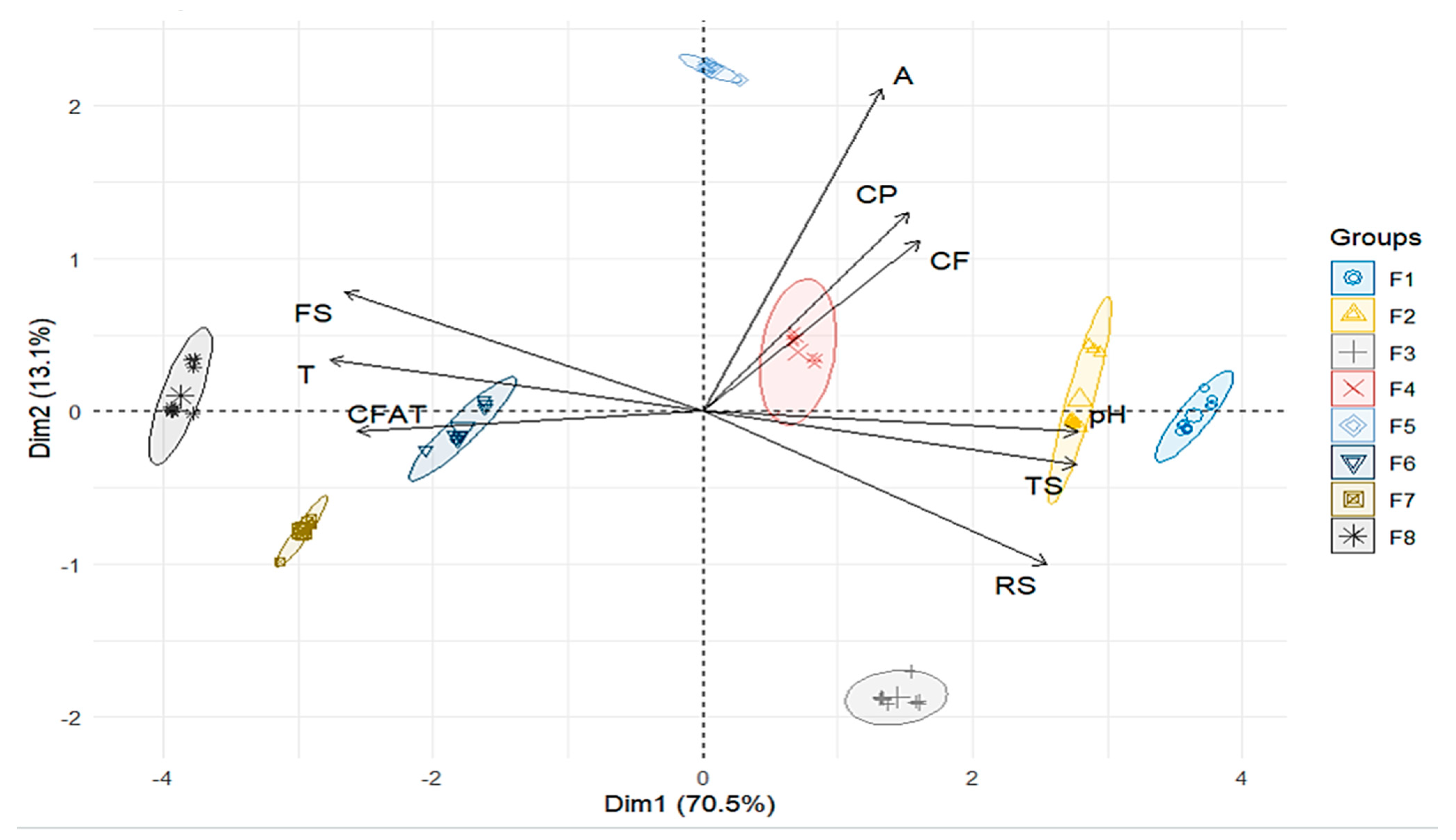
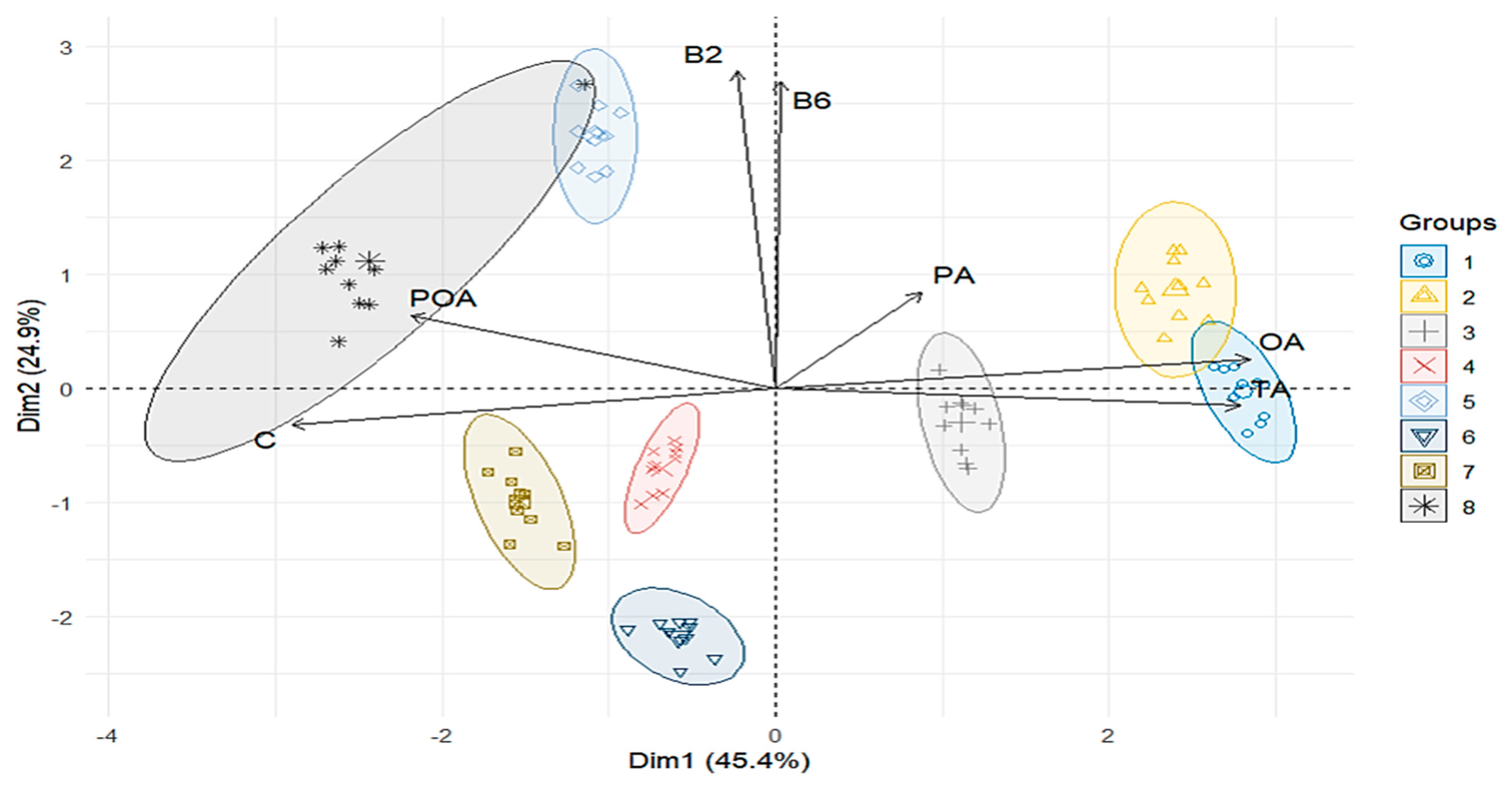
| Phases | pH | SS | TA | CF | CFAt | CP | A | TS | RS | FS |
|---|---|---|---|---|---|---|---|---|---|---|
| P1 | 5.48 a ± 0.03 | 15.20 h ± 0.10 | 0.29 h ± 0.01 | 0.64 bc ± 0.02 | 0.30 f ± 0.01 | 6.27 a ± 0.02 | 3.49 c ± 0.01 | 76.15 a ± 0.05 | 33.30 b ± 0.02 | 19.78 h ± 0.05 |
| P2 | 5.41 b ± 0.01 | 16.10 g ± 0.10 | 0.36 g ± 0.01 | 0.63 b ± 0.02 | 0.33 d ± 0.01 | 5.92 b ± 0.03 | 3.60 b ± 0.05 | 72.52 b ± 0.03 | 33.27 b ± 0.03 | 28.32 g ± 0.03 |
| P3 | 5.34 c ± 0.03 | 17.00 f ± 0.10 | 0.41 f ± 0.01 | 0.62 d ± 0.01 | 0.34 d ± 0.01 | 5.16 g ± 0.02 | 3.37 e ± 0.02 | 68.08 c ± 0.03 | 36.04 a ± 0.06 | 52.38 f ± 0.03 |
| P4 | 5.28 d ± 0.01 | 18.50 e ± 0.10 | 0.45 e ± 0.02 | 0.61 d ± 0.02 | 0.31 e ± 0.01 | 5.51 f ± 0.02 | 3.60 b ± 0.02 | 56.80 d ± 0.05 | 19.59 c ± 0.01 | 183.15 e ± 0.05 |
| P5 | 5.20 e ± 0.01 | 19.30 d ± 0.10 | 0.52 d ± 0.01 | 0.67 a ± 0.02 | 0.35 c ± 0.02 | 5.70 c ± 0.02 | 3.78 a ± 0.03 | 53.83 e ± 0.02 | 13.35 d ± 0.02 | 272.32 d ± 0.03 |
| P6 | 5.10 f ± 0.01 | 20.00 c ± 0.10 | 0.56 c ± 0.01 | 0.62 cd ± 0.01 | 0.36 c ± 0.02 | 5.61 e ± 0.07 | 3.24 f ± 0.03 | 45.05 f ± 0.05 | 9.24 e ± 0.03 | 316.62 c ± 0.04 |
| P7 | 5.02 g ± 0.02 | 21.00 b ± 0.10 | 0.62 b ± 0.01 | 0.61 d ± 0.01 | 0.40 b ± 0.01 | 5.20 g ± 0.02 | 3.20 g ± 0.02 | 41.33 g ± 0.02 | 9.12 e ± 0.03 | 323.91 b ± 0.04 |
| P8 | 4.95 h ± 0.04 | 21.73 a ± 0.12 | 0.68 a ± 0.01 | 0.53 e ± 0.01 | 0.41 a ± 0.02 | 5.65 d ± 0.03 | 3.42 d ± 0.03 | 33.92 h ± 0.03 | 5.78 f ± 0.03 | 361.85 a ± 0.06 |
| Phases | Vitamin (ug/g) | Bioactive Component | ||||||
|---|---|---|---|---|---|---|---|---|
| C | B1 | B2 | B6 | OA | TA | POA | PA | |
| P1 | 281.40 f ± 0.95 | 0.77 b ± 0.02 | 3.89 d ± 0.01 | 47.80 cd ± 0.20 | 22,905.33 a ± 56.54 | 459.18 b ± 3.31 | 2.56 g ± 0.01 | 3.88 a ± 0.02 |
| P2 | 289.58 e ± 0.83 | 0.82 a ± 0.02 | 4.23 bc ± 0.03 | 49.87 b ± 0.15 | 13,533.00 b ± 72.34 | 581.00 a ± 3.61 | 3.73 f ± 0.03 | 3.33 b ± 0.02 |
| P3 | 297.57 d ± 0.75 | 0.72 d ± 0.01 | 4.14 c ± 0.04 | 44.60 e ± 0.10 | 11,550.00 c ± 50.00 | 351.00 c ± 3.61 | 5.59 e ± 0.01 | 1.85 c ± 0.03 |
| P4 | 349.35 b ± 0.76 | 0.59 e ± 0.01 | 3.85 de ± 0.05 | 47.30 cd ± 0.20 | 335.00 d ± 5.00 | 312.33 d ± 2.52 | 6.05 d ± 0.05 | 1.87 c ± 0.02 |
| P5 | 331.28 c ± 0.94 | 0.72 cd ± 0.01 | 4.58 a ± 0.03 | 52.33 a ± 0.15 | 128.67 de ± 2.08 | 130.00 h ± 2.00 | 5.88 de ± 0.02 | 1.72 d ± 0.03 |
| P6 | 326.10 c ± 0.26 | 0.81 ab ± 0.01 | 3.71 e ± 0.04 | 41.67 f ± 0.15 | 100.17 e ± 0.76 | 241.07 e ± 1.01 | 6.39 c ± 0.01 | 1.52 e ± 0.03 |
| P7 | 354.88 a ± 1.40 | 0.80 bc ± 0.01 | 3.76 e ± 0.02 | 46.39 d ± 0.03 | 86.73 e ± 0.26 | 187.57 f ± 0.83 | 9.52 b ± 0.03 | 0.71 g ± 0.01 |
| P8 | 354.14 ab ± 1.42 | 0.82 a ± 0.01 | 4.30 b ± 0.05 | 48.28 c ± 0.03 | 14.00 e ± 0.18 | 157.53 g ± 1.11 | 24.53 a ± 0.03 | 0.80 f ± 0.02 |
| Df | SumOfSqs | R2 | F | Pr (>F) | |
|---|---|---|---|---|---|
| Phases | 7 | 1,357,021 | 0.9341 | 1105.70 | 0.001 *** |
| Residual | 72 | 95,729 | 0.0659 | ||
| Total | 79 | 1,452,750 | 10.000 |
| Df | SumOfSqs | R2 | F | Pr (>F) | |
|---|---|---|---|---|---|
| Phases | 1 | 4,067,951,730 | 0.7503 | 234.37 | 0.001 *** |
| Residual | 78 | 1,353,837,393 | 0.2497 | ||
| Total | 79 | 5,421,789,123 | 10.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcés-Moncayo, M.F.; Guevara-Viejó, F.; Valenzuela-Cobos, J.D.; Galindo-Villardón, P.; Vicente-Galindo, P. Modeling of the Physicochemical and Nutritional Composition of Musa paradisiaca (Williams Variety) at Different Ripening Stages in Ecuador. Agriculture 2025, 15, 1025. https://doi.org/10.3390/agriculture15101025
Garcés-Moncayo MF, Guevara-Viejó F, Valenzuela-Cobos JD, Galindo-Villardón P, Vicente-Galindo P. Modeling of the Physicochemical and Nutritional Composition of Musa paradisiaca (Williams Variety) at Different Ripening Stages in Ecuador. Agriculture. 2025; 15(10):1025. https://doi.org/10.3390/agriculture15101025
Chicago/Turabian StyleGarcés-Moncayo, María Fernanda, Fabricio Guevara-Viejó, Juan Diego Valenzuela-Cobos, Purificación Galindo-Villardón, and Purificación Vicente-Galindo. 2025. "Modeling of the Physicochemical and Nutritional Composition of Musa paradisiaca (Williams Variety) at Different Ripening Stages in Ecuador" Agriculture 15, no. 10: 1025. https://doi.org/10.3390/agriculture15101025
APA StyleGarcés-Moncayo, M. F., Guevara-Viejó, F., Valenzuela-Cobos, J. D., Galindo-Villardón, P., & Vicente-Galindo, P. (2025). Modeling of the Physicochemical and Nutritional Composition of Musa paradisiaca (Williams Variety) at Different Ripening Stages in Ecuador. Agriculture, 15(10), 1025. https://doi.org/10.3390/agriculture15101025








