Effect of Foliar Application of Calcium and Salicylic Acid on Fruit Quality and Antioxidant Capacity of Sweet Pepper (Capsicum annuum L.) in Hydroponic Cultivation
Abstract
1. Introduction
2. Materials and Methods
2.1. Location of Research
2.2. Plant Material and Experimental Design
Experimental Factors
2.3. Evaluated Parameters
2.3.1. Physicochemical Analysis of the Fruit
2.3.2. Evaluation of Antioxidant Potential
DPPH Assay
ABTS Assay
TPC Assay
2.3.3. Sensory Evaluation of Pepper Fruit Quality
2.4. Statistical Methods
3. Results
3.1. Physicochemical and Sensory Characteristics, Yield and Antioxidant Potential of Pepper Fruits
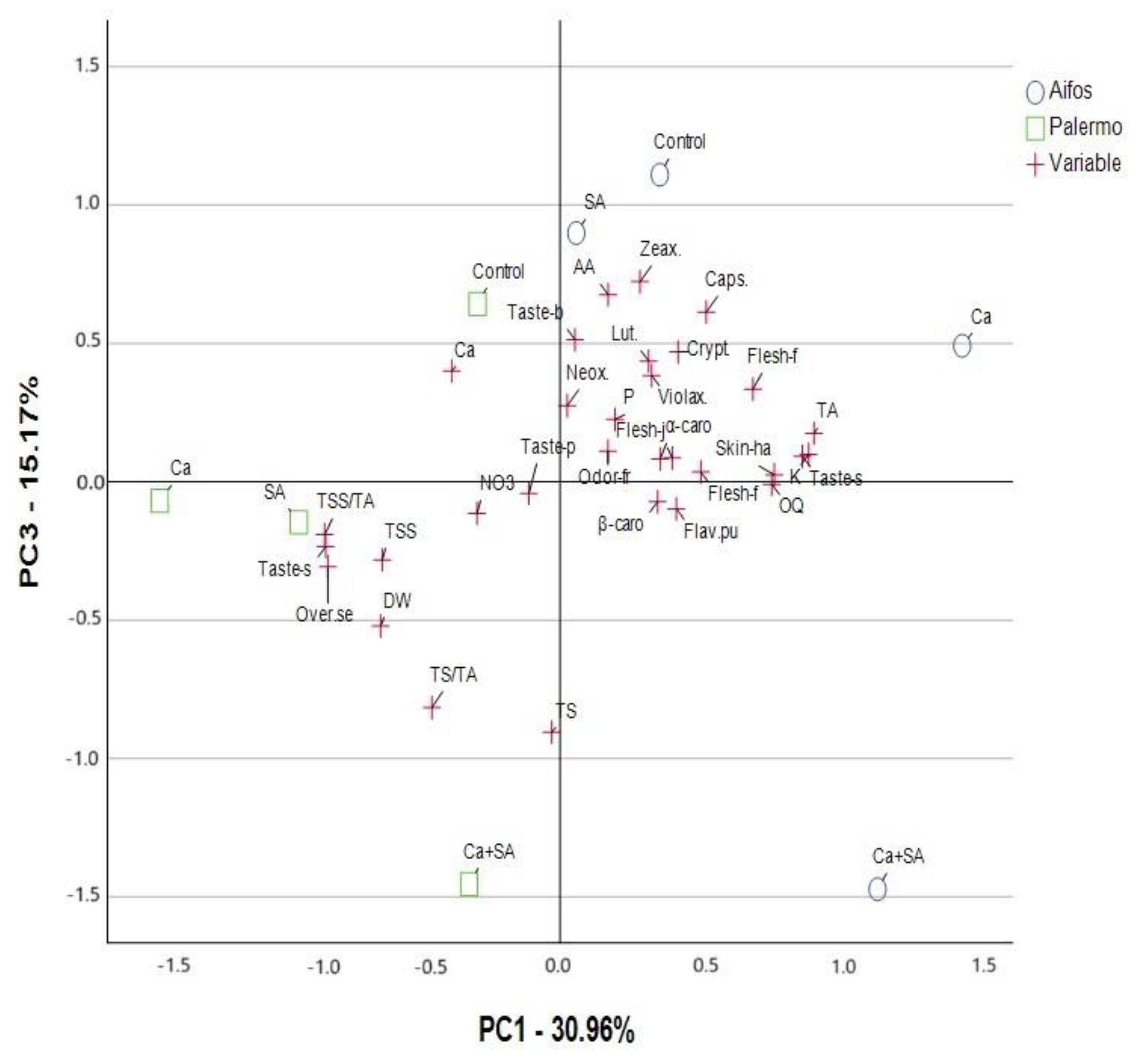
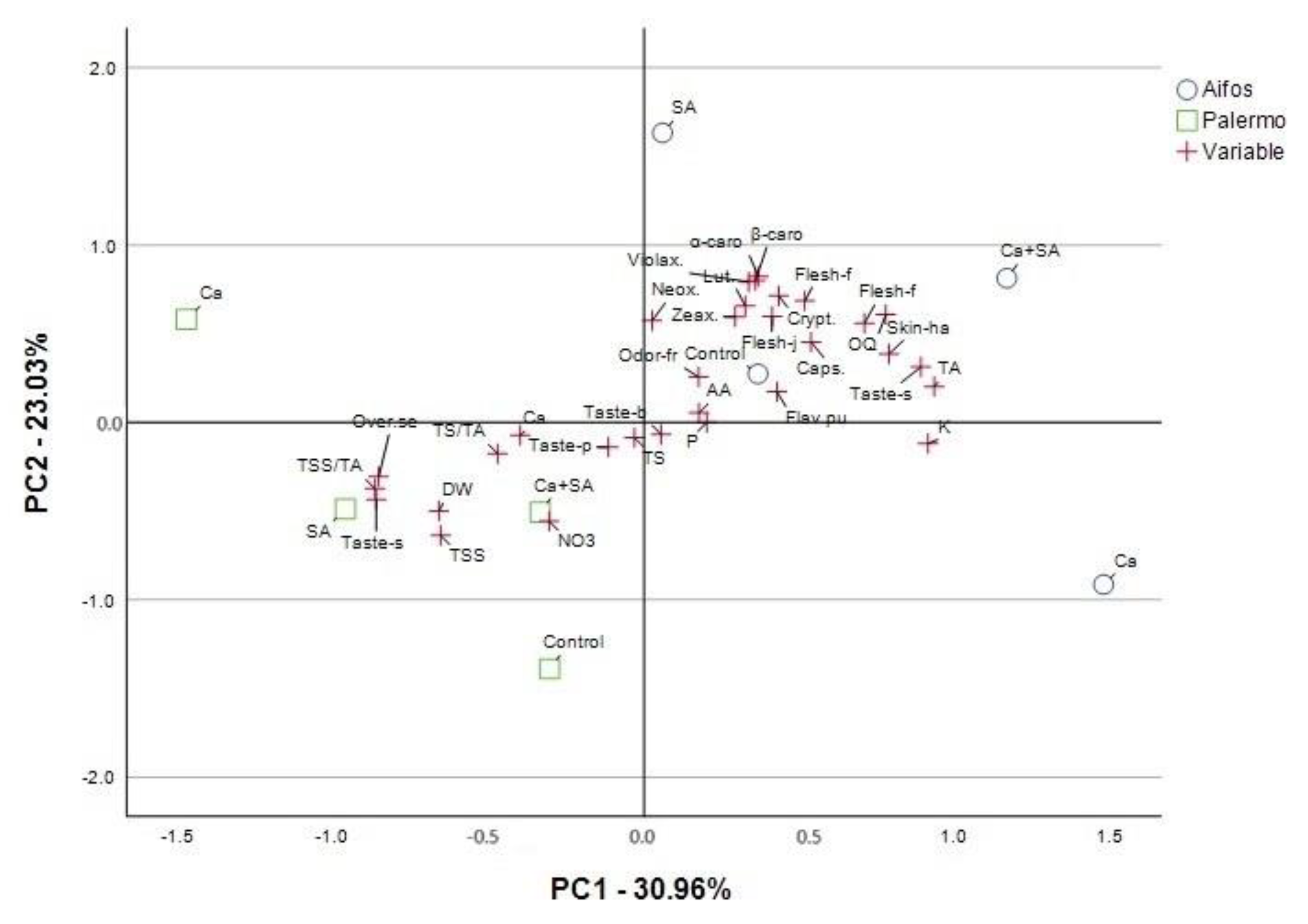
3.2. PCA Analysis for Physicochemical and Sensory Quality Parameters of Peppers Considering Foliar Treatment of Peppers with Calcium (Ca), Salicylic Acid (SA) and Calcium Combined with Salicylic Acid (Ca+SA)
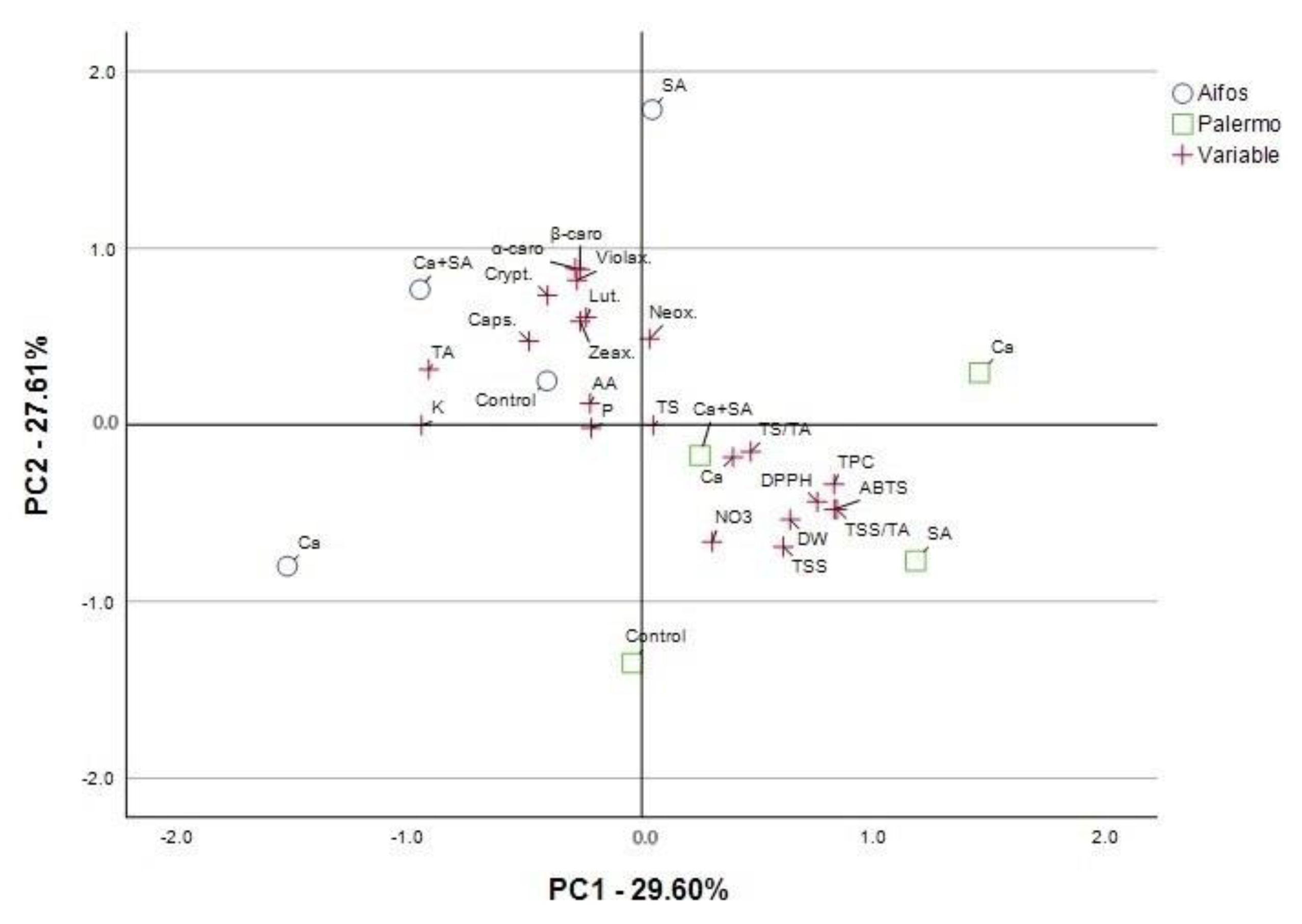
3.3. PCA Analysis for Physicochemical Parameters and Antioxidant Activity of Pepper Fruits Considering Foliar Treatment of Plants with Calcium (Ca), Salicylic Acid (SA) and Calcium Combined with Salicylic Acid (Ca+SA)
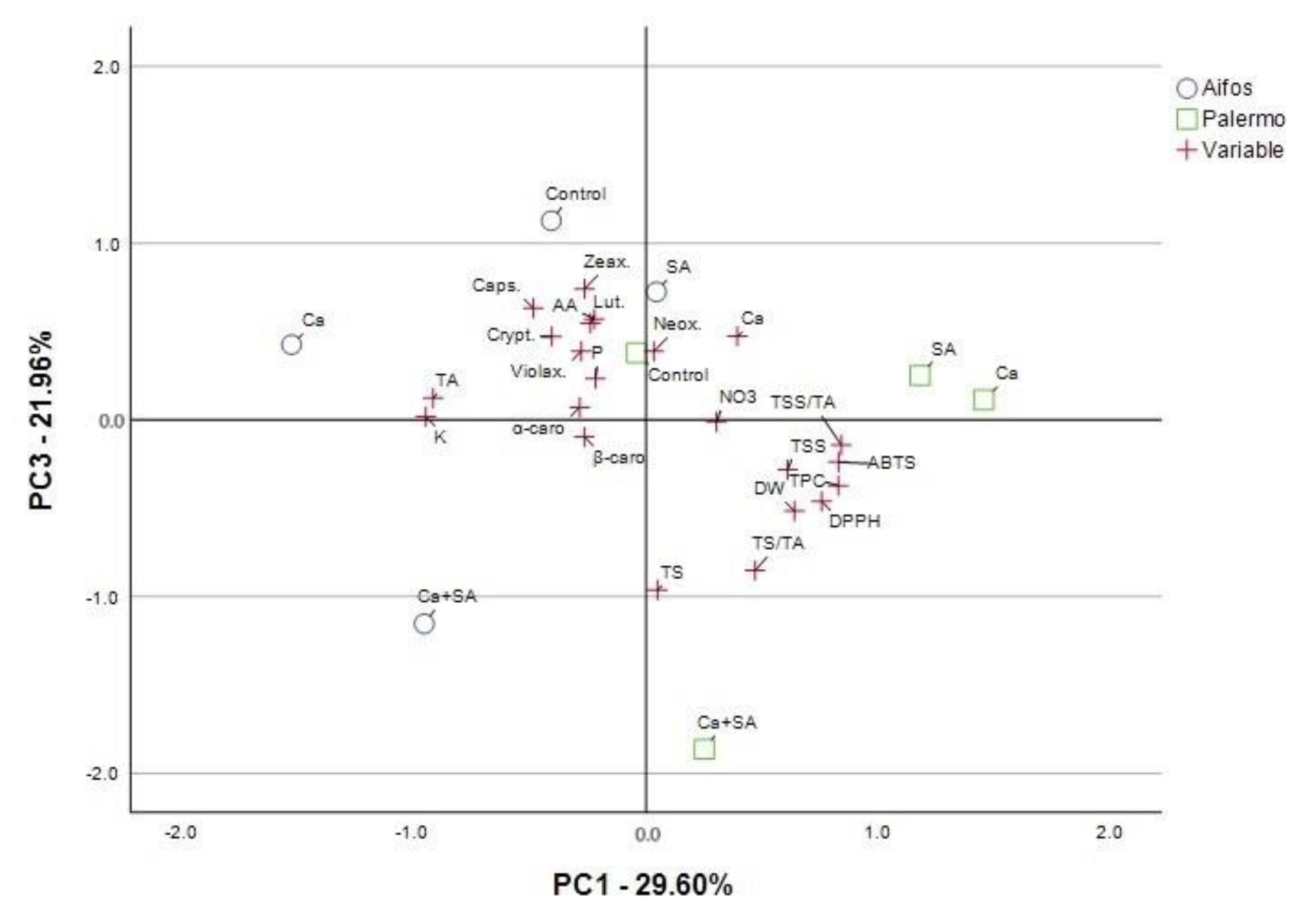
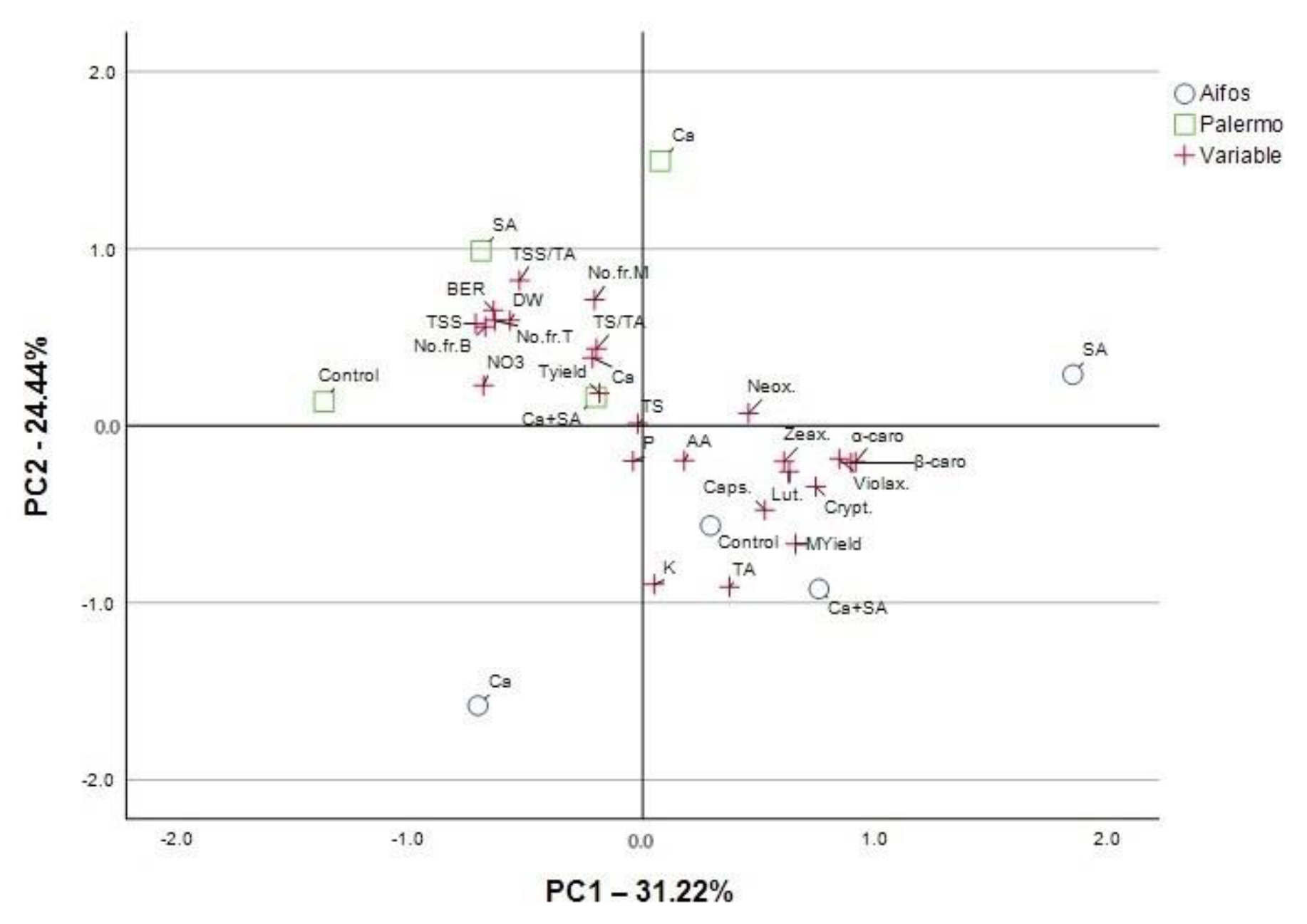
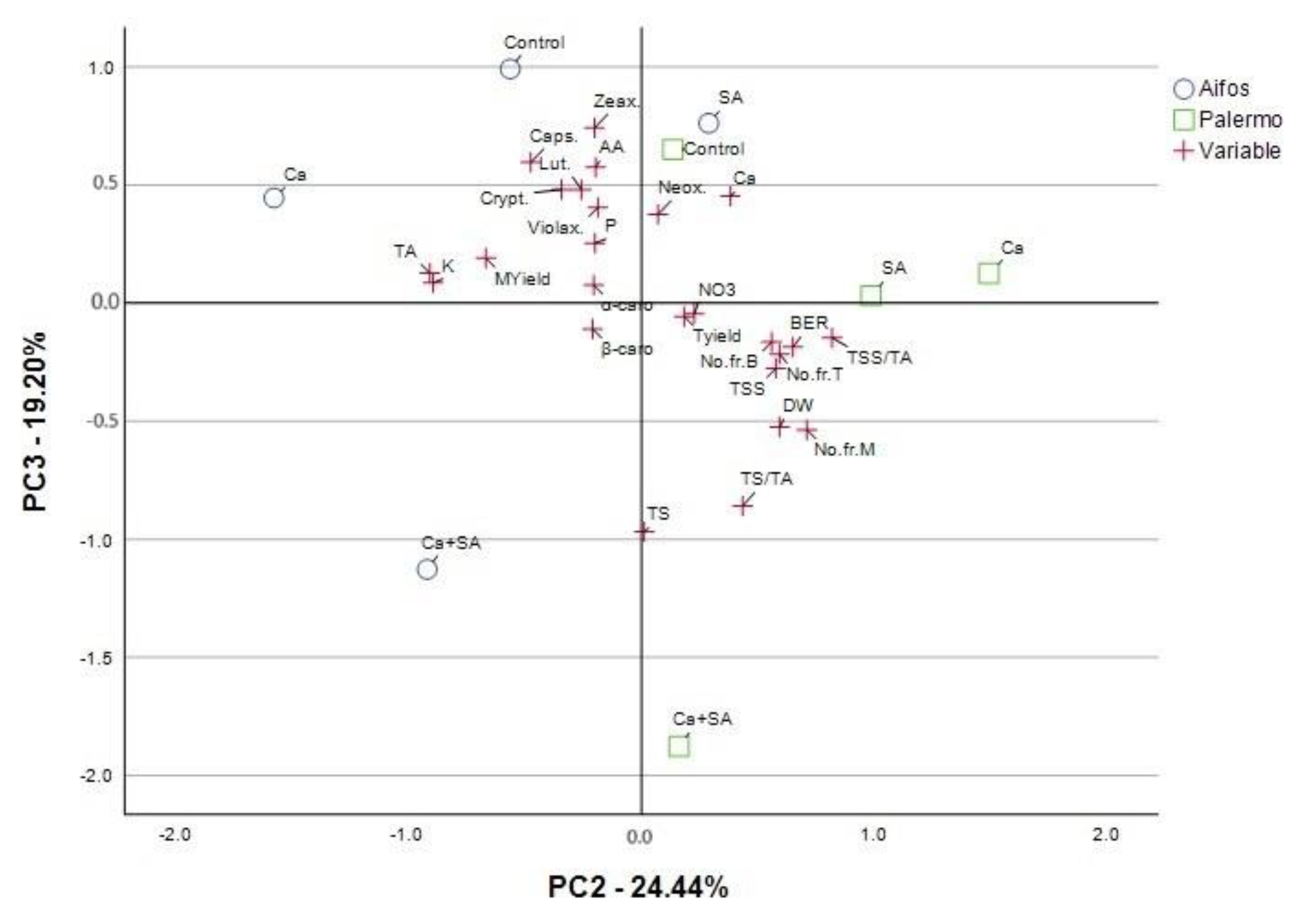
3.4. PCA Analysis for Physicochemical Parameters of Pepper Fruit and Yield Parameters Considering Foliar Treatment of Peppers with Calcium (Ca), Salicylic Acid (SA) and Calcium Combined with Salicylic Acid (Ca+SA)
4. Discussion
4.1. Quality Characteristics of Pepper Fruits Grown Hydroponically
4.2. Increase in Yield, Quality, and Antioxidant Potential of Pepper Fruit as a Result of CA and SA Application
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Carvalho Lemos, V.; Reimer, J.J.; Wormit, A. Color for life: Biosynthesis and distribution of phenolic compounds in pepper (Capsicum annuum). Agriculture 2019, 9, 81. [Google Scholar] [CrossRef]
- Tang, B.; Li, L.; Hu, Z.; Chen, Y.; Tan, T.; Jia, Y.; Xie, Q.; Chen, G. Anthocyanin accumulation and transcriptional regulation of anthocyanin biosynthesis in purple pepper. J. Agric. Food Chem. 2020, 68, 12152–12163. [Google Scholar] [CrossRef] [PubMed]
- Al-Snafi, A.E. The pharmacological importance of Capsicum species (Capsicum annuum and Capscicum frutescens) grown in Iraq. Pharm. Biol. 2015, 5, 124–142. [Google Scholar]
- Guil-Guerrero, J.L.; Martınez-Guirado, C.; Rebolloso-Fuentes, M.M.; Carrique-Perez, A. Nutrient composition and antioxidant activity of 10 pepper (Capsicum annuun) varieties. Eur. Food Res. Technol. 2006, 224, 1–9. [Google Scholar] [CrossRef]
- Paredes-Andrade, N.J.; Monteros-Altamirano, A.; Bastidas, C.G.T.; Sørensen, M. Morphological, sensorial and chemical characterization of chilli peppers (Capsicum spp.) from the CATIE Genebank. Agronomy 2020, 10, 1732. [Google Scholar] [CrossRef]
- Biratu, W.; Belew, D.; Ettissa, E. Evaluation of hot pepper (Capsicum annuum L.) cultivars for growth and dry pod yields against different blended fertilizer and nitrogen rates in raya Azebo, Southern Tigray. Int. J. Res. Agron. 2021, 4, 15–22. [Google Scholar] [CrossRef]
- Srivastava, N.; Sharma, V.; Saraf, K.; Dobriyal, A.K.; Kamal, B.; Jadon, V.S. In vitro antimicrobial activity of aerial parts extracts of Aconitum heterophyllum Wall. ex Royle. Indian J. Nat. Prod. Resour. 2011, 2, 504–507. [Google Scholar]
- Ademoyegun, O.T.; Fariyike, T.A.; Aminu-Taiwo, R.B. Effects of poultry droppings on the biologically active compounds in Capsicum annum L. (var. Nsukka yellow). Agric. Biol. J. N. Am. 2011, 2, 665–672. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Mizobuchi, T.; Kajikawa, R.; Kawashima, H.; Miyabe, F.; Terao, J.; Takamura, H.; Matoba, T. Radical-scavenging activity of vegetables and the effect of cooking on their activity. Food Sci. Technol. Res. 2001, 7, 250–257. [Google Scholar] [CrossRef]
- Simonne, A.H.; Simonne, E.H.; Eitenmiller, R.R.; Mills, H.A.; Green, N.R. Ascorbic acid and provitamine A contents in unusally colored bell peppers (Capsicum annum L.). J. Food Compos. Anal. 1997, 10, 299–311. [Google Scholar] [CrossRef]
- Nurzyńska-Wierdak, R. Capsicum annuum L. (bell pepper). A plant with unique bioactive compounds, nutraceutical and phytotherapeutic potential. A review. Ann. Hortic. 2022, 31, 4. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Bonesi, M.; Serio, A.; Chaves-López, C.; Falco, T.; Paparella, A.P.; Menichini, F.; Tundis, R. Application of nine air-dried Capsicum annum cultivars as food preservative: Micro-nutrient content, antioxidant activity, and foodborne pathogens inhibitory effects. Int. J. Food Prop. 2017, 20, 899–910. [Google Scholar] [CrossRef]
- Florkowska, K.; Duchnik, W.; Nowak, A.; Klimowicz, A. Właściwości antyoksydacyjne papryki ostrej odmiany Hungarian yellow. Pomeranian J. Life. Sci. 2018, 64, 126–131. [Google Scholar] [CrossRef][Green Version]
- Tzika, D.E.; Papadimitriou, V.; Sotiroudis, G.T.; Xenakis, A. Antioxidant properties of fruits and vegetables shots and juices: An electron paramagnetic study. Food Biophys. 2008, 1, 48–53. [Google Scholar] [CrossRef]
- Żurawik, A.; Jadczak, D.; Panayotov, N.; Żurawik, P. Antioxidant properties of pepper (Capsicum annuum L.) depending on its cultivar and fruit colouration. Plant Soil Environ. 2021, 67, 653–659. [Google Scholar] [CrossRef]
- Ghazemnezhad, M.; Sherafati, M.; Payvast, G.A. Variation in phenolic compounds, ascorbic acid and antioxidant activity of five coloured bell pepper (Capsicum annum) fruits at two different harvest times. J. Funct. Foods 2011, 1, 44–49. [Google Scholar] [CrossRef]
- Kaewseejan, N.; Sutthikhum, V.; Siriamornpun, S. Potential of Gynura procumbens leaves as source of flavonoid-enriched fractions with enhanced antioxidant capacity. J. Funct. Foods 2015, 12, 120–128. [Google Scholar] [CrossRef]
- Taher, M.A.; Tadros, L.K.; Dawood, D.H. Phytochemical constituents, antioxidant activity and safety evaluation of Kei-apple fruit (Dovyalis caffra). Food Chem. 2018, 265, 144–151. [Google Scholar] [CrossRef]
- Kulawik-Pióro, A. Marchew zwyczajna (Daucus carota L.) jako źródło substancji aktywnych i jej potencjał farmakologiczny i kosmetologiczny. Farm. Pol. 2022, 78, 536–548. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Apple peels as value-added food ingredient. J. Agric. Food Chem. 2003, 51, 1676–1683. [Google Scholar] [CrossRef]
- Maria do Socorro Rufino, M.; Alves, E.R.; de Brito, S.E.; Perez-Jimenez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef]
- Nurzyńska-Wierdak, R.; Papliński, R. Substancje bioaktywne i właściwości lecznicze fasoli zwykłej (Phaseolus vulgaris L.). Ann. Hortic. 2023, 32, 33–51. [Google Scholar] [CrossRef]
- Hayouni, E.A.; Abedrabba, M.; Bouix, M.; Hamdi, M. The effects of solvents and extraction method on the phenolic contents and biological activities in vitro of Tunisian Quercus coccifera L. and Juniperus phoenicea L. fruit extracts. Food Chem. 2007, 105, 1126–1134. [Google Scholar] [CrossRef]
- Alam, M.A.; Saleh, M.; Mohsin, G.M.; Nadirah, T.A.; Aslani, F.; Rahman, M.M.; Roy, S.K.; Juraimi, A.S.; Alam, M.Z. Evaluation of phenolics, capsaicinoids, antioxidant properties, and major macro-micro minerals of some hot and sweet peppers and ginger landraces of Malaysia. J. Food Process. Preserv. 2020, 44. [Google Scholar] [CrossRef]
- Lee, J.G.; Chae, Y.; Shin, Y.; Kim, Y.J. Chemical composition and antioxidant capacity of black pepper pericarp. Appl. Biol. Chem. 2020, 63, 35. [Google Scholar] [CrossRef]
- Salamatullah, A.M.; Hayat, K.; Husain, F.M.; Ahmed, M.A.; Arzoo, S.; Althbiti, M.M.; Alzahrani, A.; Al-Zaied, B.A.M.; Alyahya, H.K.; Albader, N.; et al. Effects of different solvent extractions on total polyphenol content, HPLC analysis, antioxidant capacity, and antimicrobial properties of peppers (red, yellow, and green (Capsicum annum L.). Evid.-Based Complement. Altern. Med. 2022, 2022, 7372101. [Google Scholar] [CrossRef]
- Ma, X.; Yu, H. Global burden of cancer. Yale J. Biol. Med. 2006, 79, 85–94. [Google Scholar]
- Matysiak, B.; Kaniszewski, S.; Mieszczakowska-Frąc, M. Growth and quality of leaf and romaine lettuce grown on a vertical farm in an aquaponics system: Results of farm research. Agriculture 2023, 13, 897. [Google Scholar] [CrossRef]
- Gruda, N.S. Increasing sustainability of growing media constituents and stand-alone substrates in soilless culture systems. Agronomy 2019, 9, 298. [Google Scholar] [CrossRef]
- Tzortzakis, N.; Massa, D.; Vandecasteele, B.J.A. The tripartite of soilless systems, growing media, and plants through an intensive crop production scheme. Agronomy 2022, 12, 1896. [Google Scholar] [CrossRef]
- Vandecasteele, B.; Van Loo, K.; Ommeslag, S.; Vierendeels, S.; Rooseleer, M.; Vandaele, E. Sustainable growing media blends with woody green composts: Optimizing the N release with organic fertilizers and interaction with microbial biomass. Agronomy 2022, 12, 422. [Google Scholar] [CrossRef]
- Gruda, N.S. Advances in soilless culture and growing media in today’s horticulture—An editorial. Agronomy 2022, 12, 2773. [Google Scholar] [CrossRef]
- Maher, M.; Prasad, M.; Raviv, M. Organic soilless media components. In Soilless Culture; Academic Press: Cambridge, MA, USA, 2008; pp. 459–504. [Google Scholar]
- Kraska, T.; Kleinschmidt, B.; Weinand, J.; Pude, R. Cascading use of Miscanthus as growing substrate in soilless cultivation of vegetables (Tomatoes, Cucumbers) and subsequent direct combustion. Sci. Hortic. 2018, 235, 205–213. [Google Scholar] [CrossRef]
- Kennard, N.; Stirling, R.; Prashar, A.; Lopez-Capel, E. Evaluation of Recycled Materials as Hydroponic Growing Media. Agronomy 2020, 10, 1092. [Google Scholar] [CrossRef]
- Saha, S.; Monroe, A.; Day, M.R. Growth, yield, plant quality and nutrition of basil (Ocimum basilicum L.) under soilless agricultural systems. Ann. Agric. Sci. 2016, 61, 181–186. [Google Scholar] [CrossRef]
- Ferby, V.; Kopta, T.; Komorowska, M.; Fidurski, M. Evaluation of alternative substrates for hydroponics based on biological parameters of leaf lettuce (Lactuca sativa L.) and its stress response. Folia Hortic. 2023, 35, 77–90. [Google Scholar] [CrossRef]
- Hepler, P.K. Calcium: A central regulator of plant growth and development. Plant Cell 2005, 17, 2142–2155. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Shankar, A.; Nalini Chandran, A.K.; Sharma, M.; Jung, K.H.; Suprasanna, P.; Pandey, G.K. Emerging concepts of potassium homeostasis in plants. J. Exp. Bot. 2020, 71, 608–619. [Google Scholar] [CrossRef]
- Ahn, J.; Park, M.; Kim, J.; Huq, E.; Kim, J.; Kim, D.-H. Physiological and transcriptomic analyses of healthy and blossom-end-rot (BER)-defected fruit of chili pepper (Capsicum annuum L.). Hortic. Environ. Biotechnol. 2024, 65, 971–980. [Google Scholar] [CrossRef]
- Manganaris, G.A.; Vasilakakis, M.; Diamantidis, G.; Mignani, I. Effect of calcium additives on physicochemical aspects of cell wall pectin and sensory attributes of canned peach (Prunus persica (L.) Batsch cv. ‘Andross’). J. Sci. Food Agric. 2005, 85, 1773–1778. [Google Scholar] [CrossRef]
- Saito, S.; Uozumi, N. Calcium-regulated phosphorylation systems controlling uptake and balance of plant nutrients. Front. Plant Sci. 2020, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Imenšek, N.; Sem, V.; Kolar, M.; Ivančič, A.; Kristl, J. The distribution of minerals in crucial plant parts of various elderberry (Sambucus spp.) interspecific hybrids. Plants 2021, 10, 653. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Li, H.; Ren, C.; Zhou, Y.; Zhu, W.; Zhang, S.; Liu, L. Calcium regulates growth and nutrient absorption in poplar seedlings. Front. Plant Sci. 2022, 13, 887098. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Ma, C.; Li, M.; Zhangzhong, L.; Song, P.; Li, Y. Interaction and adaptation of phosphorus fertilizer and calcium ion in drip irrigation systems: The perspective of emitter clogging. Agric. Water Manag. 2023, 282, 108269. [Google Scholar] [CrossRef]
- Kulbat, K. The role of phenolic compounds in plant resistance. Biotechnol. Food Sci. 2016, 80, 97–108. [Google Scholar] [CrossRef]
- Liu, J.; Liu, H.; Zhao, Z.; Wang, J.; Guo, D.; Liu, Y. Regulation of Actg1 and Gsta2 is possible mechanism by which capsaicin alleviates apoptosis in cell model of 6-OHDA-induced Parkinson’s disease. Biosci. Rep. 2020, 40, BSR20191796. [Google Scholar] [CrossRef]
- Wang, L.; Li, S. Role of salicylic acid in postharvest physiology. Fresh Prod. 2008, 2, 1–5. [Google Scholar]
- Vlot, A.C.; Dempsey, D.M.A.; Klessig, D.F. Salicylic acid, a multifaceted hormone to combat disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef]
- Jayakannan, M.; Bose, J.; Babourina, O.; Rengel, Z.; Shabala, S. Salicylic acid in plant salinity stress signalling and tolerance. Plant Growth Regul. 2015, 76, 25–40. [Google Scholar] [CrossRef]
- Sobczak, A.; Pióro-Jabrucka, E.; Gajc-Wolska, J.; Kowalczyk, K. Effect of salicylic acid and calcium on growth, yield, and fruit quality of pepper (Capsicum annuum L.) grown hydroponically. Agronomy 2024, 14, 329. [Google Scholar] [CrossRef]
- Sobczak, A.; Kućko, A.; Pióro-Jabrucka, E.; Gajc-Wolska, J.; Kowalczyk, K. Effect of salicylic acid on the growth and development of sweet pepper (Capsicum annum L.) under standard and high EC nutrient solution in aeroponic cultivation. Agronomy 2023, 13, 779. [Google Scholar] [CrossRef]
- Amin, G.H.; Abdel-Kader, D.Z.; Baraka, M.; Hassan, H.B. Role of salicylic acid in the machinery of acquired tolerance in two Capsicum species infected with powdery mildew disease. Aust. J. Basic Appl. Sci. 2009, 3, 2078–2096. [Google Scholar]
- Jiankang, C.; Kaifang, Z.; Weibo, J. Enhancement of post-harvest disease resistance in Ya Li pear (Pyrus bretschneideri) fruit by salicylic acid sprays on the trees during fruit growth. Eur. J. Plant Pathol. 2006, 114, 363–370. [Google Scholar]
- Yao, H.J.; Shiping, T. Effects of pre-and post-harvest application of salicylic acid or methyl jasmonate on inducing disease resistance of cherry fruit in storage. Postharvest Biol. Technol. 2005, 35, 253–262. [Google Scholar] [CrossRef]
- Xu, X.; Shiping, T. Salicylic acid alleviated pathogen-induced oxidative stress in harvested sweet cherry fruit. Postharvest Biol. Technol. 2008, 49, 379–385. [Google Scholar] [CrossRef]
- Vázquez-Hernández, M.C.; Parola-Contreras, I.; Montoya-Gómez, L.M.; Torres-Pacheco, I.; Schwarz, D.; Guevara-González, R.G. Eustressors: Chemical and physical stress factors used to enhance vegetables production. Sci. Hortic. 2019, 250, 223–229. [Google Scholar] [CrossRef]
- Yasmeen, T.; Ahmad, A.; Arif, M.S.; Mubin, M.; Rehman, K.; Shahzad, S.M.; Wijaya, L. Biofilm forming rhizobacteria enhance growth and salt tolerance in sunflower plants by stimulating antioxidant enzymes activity. Plant Physiol. Biochem. 2020, 156, 242–256. [Google Scholar] [CrossRef]
- Odriozola-Serrano, I.; Hernández-Jover, T.; Martín-Belloso, O. Comparative evaluation of UV-HPLC methods and reducing agents to determine vitamin C in fruits. Food Chem. 2007, 105, 1151–1158. [Google Scholar] [CrossRef]
- Grobelna, A.; Kalisz, S.; Kieliszek, M. The effect of the addition of blue honeysuckle berry juice to apple juice on the selected quality characteristics, anthocyanin stability, and antioxidant properties. Biomolecules 2019, 9, 744. [Google Scholar] [CrossRef]
- Polish Standard PN-90/A-75101/0; Determine the Content of Total Sugars as Well as Sacharose and Monosaccharides Using the Luff-Schoorl Method. Polish Committee for Standardization: Warsaw, Poland, 1990.
- Polish Standard PN-90/A-75101/04; Fruit and Vegetable Preserves. Preparation of Samples and Methods for Physicochemical Analysis. Determination of Total Acidity. Polish Committee for Standardization: Warsaw, Poland, 1990.
- Yen, G.C.; Chen, H.Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Grajek, W. Przeciwutleniacze w Żywności. Aspekty Zdrowotne, Technologiczne, Molekularne i Analityczne; WNT: Warszawa, Poland, 2007. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Koss-Mikołajczyk, I.; Baranowska, M.; Namieśnik, J.; Bartoszek, A. Determination of antioxidant activity of phytochemicals in cellular models by fluorescence/luminescence methods. Adv. Hyg. Exp. Med. 2017, 71, 602–617. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Hassan, N.M.; Yusof, N.A.; Yahaya, A.F.; Mohd Rozali, N.N.; Othman, R. Carotenoids of Capsicum fruits: Pigment profile and health-promoting functional attributes. Antioxidants 2019, 8, 469. [Google Scholar] [CrossRef]
- Deli, J.; Molnár, P.; Matus, Z.; Tóth, G. Carotenoid composition in the fruits of red paprika (Capsicum annuum var. Lycopersiciforme rubrum) during ripening; biosynthesis of carotenoids in red paprika. J. Agric. Food Chem. 2001, 49, 1517–1523. [Google Scholar] [CrossRef]
- Rodriguez-Uribe, L.; Guzman, I.; Rajapakse, W.; Richins, R.D.; O’Connell, M.A. Carotenoid accumulation in orange-pigmented Capsicum annuum fruit, regulated at multiple levels. J. Exp. Bot. 2012, 63, 517–526. [Google Scholar] [CrossRef]
- Cervantes-Paz, B.; Yahia, E.M.; Ornelas-Paz, J.J.; Victoria-Campos, C.I.; Ibarra-Junquera, V.; Pérez-Martínez, J.D.; Escalante-Minakata, P. Antioxidant activity and content of chlorophylls and carotenoids in raw and heat-processed Jalapeño peppers at intermediate stages of ripening. Food Chem. 2014, 146, 188–196. [Google Scholar] [CrossRef]
- Arimboor, R.; Natarajan, R.B.; Menon, K.R.; Chandrasekhar, L.P.; Moorkoth, V. Red pepper (Capsicum annuum) carotenoids as a source of natural food colors: Analysis and stability—A review. J. Food Sci. Technol. 2015, 52, 1258–1271. [Google Scholar] [CrossRef]
- Kennedy, L.E.; Abraham, A.; Kulkarni, G.; Shettigar, N.; Dave, T.; Kulkarni, M. Capsanthin, a plant-derived xanthophyll: A review of pharmacology and delivery strategies. AAPS PharmSciTech 2021, 22, 203. [Google Scholar] [CrossRef]
- Nowacka, M.; Fijalkowska, A.; Dadan, M.; Rybak, K.; Wiktor, A.; Witrowa-Rajchert, D. Effect of ultrasound treatment during osmotic dehydration on bioactive compounds of cranberries. Ultrasonics 2018, 83, 18–25. [Google Scholar] [CrossRef]
- Hallmann, E.; Marszałek, K.; Lipowski, J.; Jasińska, U.; Kazimierczak, R.; Średnicka-Tober, D.; Rembiałkowska, E. Polyphenols and carotenoids in pickled bell pepper from organic and conventional production. Food Chem. 2019, 278, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Mendoza, C.; Sánchez, E.; Muñoz-Márquez, E.; Sida-Arreola, J.P.; Flores-Córdova, M.A. Bioactive compounds and antioxidant activity in different grafted varieties of bell pepper. Antioxidants 2015, 4, 427–446. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y.; Koh, S.C. Fruit development and quality of hot pepper (Capsicum annuum L.) under various temperature regimes. Hortic. Sci. Technol. 2019, 37, 310–321. [Google Scholar] [CrossRef]
- Rodríguez-Ruiz, M.; Mateos, R.M.; Codesido, V.; Corpas, F.J.; Palma, J.M. Characterization of the galactono-1,4-lactone dehydrogenase from pepper fruits and its modulation in the ascorbate biosynthesis. Role of nitric oxide. Redox Biol. 2017, 12, 171–181. [Google Scholar] [CrossRef]
- Corpas, F.J.; Palma, J.M. Nitric oxide on/off in fruit ripening. Plant Biol. 2018, 20, 805–807. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, M.; Ren, L.; Li, A.; Chen, G.; Hu, Z. The SlFSR gene controls fruit shelf-life in tomato. J. Exp. Bot. 2018, 69, 2897–2909. [Google Scholar] [CrossRef]
- González-Gordo, S.; Bautista, R.; Claros, M.G.; Cañas, A.; Palma, J.M.; Corpas, F.J. Nitric oxide-dependent regulation of sweet pepper fruit ripening. J. Exp. Bot. 2019, 70, 4557–4570. [Google Scholar] [CrossRef]
- Cisternas-Jamet, J.; Salvatierra-Martínez, R.; Vega-Gálvez, A.; Stoll, A.; Uribe, E.; Goñi, M.G. Biochemical composition as a function of fruit maturity stage of bell pepper (Capsicum annuum) inoculated with Bacillus amyloliquefaciens. Sci. Hortic. 2020, 263, 109107. [Google Scholar] [CrossRef]
- Ribes-Moya, A.M.; Adalid, A.M.; Raigón, M.D.; Hellín, P.; Fita, A.; Rodríguez-Burruezo, A. Variation in flavonoids in a collection of peppers (Capsicum sp.) under organic and conventional cultivation: Effect of the genotype, ripening stage, and growing system. J. Sci. Food Agric. 2020, 100, 2208–2223. [Google Scholar] [CrossRef]
- Papathanasiou, T.; Gougoulias, N.; Karayannis, V.G.; Kamvoukou, C.A. Investigation of the total phenolic content and antioxidant capacity of three sweet pepper cultivars (Capsicum annuum L.) at different development and maturation stages. Period. Polytech. Chem. Eng. 2021, 65, 219–228. [Google Scholar] [CrossRef]
- Hamed, M.; Kalita, D.; Bartolo, M.E.; Jayanty, S.S. Capsaicinoids, polyphenols, and antioxidant activities of Capsicum annuum: Comparative study of the effect of ripening stage and cooking methods. Antioxidants 2019, 8, 364. [Google Scholar] [CrossRef] [PubMed]
- Chilczuk, B.; Marciniak, B.; Kontek, R.; Materska, M. Diversity of the chemical profile and biological activity of Capsicum annuum L. extracts in relation to their lipophilicity. Molecules 2021, 26, 5215. [Google Scholar] [CrossRef] [PubMed]
- Medina-Juárez, L.A.; Molina-Quijada, D.M.A.; Del Toro-Sánchez, C.L.; González-Aguilar, G.A.; Gámez-Meza, N. Antioxidant activity of peppers (Capsicum annuum L.) extracts and characterization of their phenolic constituents. Comm. Rep. Comm. 2012, 37, 588–593. [Google Scholar]
- Kowalczyk, K.; Gajc-Wolska, J.; Radzanowska, J.; Marcinkowska, M. Assessment of chemical composition and sensory quality of tomato fruit depending on cultivar and growing conditions. Acta Sci. Pol. Hortorum Cultus 2011, 10, 133–140. [Google Scholar]
- Felföldi, Z.; Ranga, F.; Roman, I.A.; Sestras, A.F.; Vodnar, D.C.; Prohens, J.; Sestras, R.E. Analysis of physico-chemical and organoleptic fruit parameters relevant for tomato quality. Agronomy 2022, 12, 1232. [Google Scholar] [CrossRef]
- Shehata, S.A.; Abdelrahman, S.Z.; Megahed, M.M.A.; Abdeldaym, E.A.; El-Mogy, M.M.; Abdelgawad, K.F. Extending shelf life and maintaining quality of tomato fruit by calcium chloride, hydrogen peroxide, chitosan, and ozonated water. Horticulturae 2021, 7, 309. [Google Scholar] [CrossRef]
- Abdelaal, K.A.; EL-Maghraby, L.M.; Elansary, H.; Hafez, Y.M.; Ibrahim, E.I.; El-Banna, M.; El-Esawi, M.; Elkelish, A. Treatment of Sweet Pepper with Stress Tolerance-Inducing Compounds Alleviates Salinity Stress Oxidative Damage by Mediating the Physio-Biochemical Activities and Antioxidant Systems. Agronomy 2020, 10, 26. [Google Scholar] [CrossRef]
- Ge, W.; Zhao, Y.; Kong, X.; Sun, H.; Luo, M.; Yao, M.; Wei, B.; Ji, S. Combining salicylic acid and trisodium phosphate alleviates chilling injury in bell pepper (Capsicum annuum L.) through enhancing fatty-acid desaturation efficiency and water retention. Food Chem. 2020, 327, 127057. [Google Scholar] [CrossRef]
- Dobón-Suárez, A.; Giménez, M.J.; García-Pastor, M.E.; Zapata, P.J. Salicylic acid preharvest treatment improves green pepper fruit quality and antioxidant capacity during postharvest storage. Acta Hortic. 2024, 1396, 471–476. [Google Scholar] [CrossRef]
- Yang, W.; Kang, J.; Liu, Y.; Guo, M.; Chen, G. Effect of salicylic acid treatment on antioxidant capacity and endogenous hormones in winter jujube during shelf life. Food Chem. 2022, 397, 133788. [Google Scholar] [CrossRef]
- Buczkowska, H.; Michalojc, Z.; Nurzynska-Wierdak, R. Yield and fruit quality of sweet pepper depending on foliar application of calcium. Turk. J. Agric. For. 2016, 40, 222–228. [Google Scholar] [CrossRef]
- Khazaei, Z.; Estaji, A. Impact of exogenous application of salicylic acid on the drought-stress tolerance in pepper (Capsicum annuum L.). J. Plant Physiol. Breed. 2021, 11, 33–46. [Google Scholar] [CrossRef]
- Khalid, M.F.; Elezz, A.A.; Jawaid, M.Z.; Ahmed, T. Salicylic acid restricts mercury translocation by activating strong antioxidant defense mechanisms in sweet pepper (Capsicum annuum L.). Environ. Technol. Innov. 2023, 32, 103283. [Google Scholar] [CrossRef]
- Dobón-Suárez, A.; Giménez, M.J.; García-Pastor, M.E.; Zapata, P.J. Salicylic acid foliar application increases crop yield and quality parameters of green pepper fruit during postharvest storage. Agronomy 2021, 11, 2263. [Google Scholar] [CrossRef]

| Parameter | Unit | Code | Cultivar | Average | |
|---|---|---|---|---|---|
| Aifos | Palermo | ||||
| Physicochemical composition (average of 48 values) | |||||
| Dry weight | % | DW | 8.36 ± 0.50 | 9.91 ± 1.69 | 9.14 ± 1.47 |
| Total soluble solids | °Brix | TSS | 8.40 ± 0.58 | 9.63 ± 0.99 | 9.01 ± 1.01 |
| Total sugars | g 100 g−1 FW | TS | 3.83 ± 1.68 | 4.35 ± 1.90 | 4.09 ± 1.80 |
| Titratable acidity | TA | 0.21 ± 0.02 | 0.17 ± 0.04 | 0.188 ± 0.03 | |
| TSS/TS ratio | i.u. | TSS/TA | 40.70 ± 3.99 | 58.82 ± 10.87 | 49.761 ± 12.22 |
| TS/TA ratio | i.u. | TS/TA | 18.59 ± 8.39 | 26.01 ± 10.91 | 22.301 ± 10.373 |
| Phosphorus | mg kg−1 FW | P | 223.92 ± 25.38 | 214.83 ± 49.60 | 219.38 ± 39.46 |
| Nitrates | NO3 | 108.14 ± 4.32 | 110.00 ± 3.81 | 109.07 ± 4.16 | |
| Calcium | Ca | 23.02 ± 4.08 | 24.09 ± 4.30 | 23.56 ± 4.20 | |
| Potassium | K | 2294.48 ± 122.82 | 2180.94 ± 216.45 | 2237.71 ± 184.12 | |
| Ascorbic acid | mg 100 g−1 FW | AA | 48.15 ± 10.07 | 47.15 ± 5.97 | 47.65 ± 8.25 |
| Neoxanthin | μg 100 g−1 FW | Neox. | 39.23 ± 27.05 | 36.53 ± 12.31 | 37.88 ± 20.95 |
| Lutein | Lut. | 234.36 ± 172.64 | 184.80 ± 74.89 | 209.58 ± 134.68 | |
| Zeaxanthin | Zeax. | 24.68 ± 12.97 | 19.50 ± 4.10 | 22.09 ± 9.91 | |
| Violaxanthin | Violax. | 6.46 ± 5.16 | 3.55 ± 2.98 | 5.00 ± 4.44 | |
| Capsanthin | Caps. | 1478.43 ± 1309.22 | 974.31 ± 528.73 | 1226.37 ± 1024.95 | |
| β-cryptoxanthin | Crypt. | 100.63 ± 56.74 | 76.11 ± 21.86 | 88.37 ± 44.51 | |
| α-carotene | α-carot. | 198.25 ± 121.44 | 138.09 ± 101.78 | 168.17 ± 115.48 | |
| β-carotene | β-carot. | 820.81 ± 458.92 | 650.26 ± 417.85 | 735.53 ± 444.89 | |
| Sensory quality (average of 48 values) | |||||
| Odour of fresh pepper | 0–10 point’s scale | Odor-fre.p. | 5.01 ± 1.93 | 4.78 ± 2.43 | 4.89 ± 2.19 |
| Skin hardness | Skin-hard. | 5.97 ± 2.08 | 5.00 ± 2.59 | 5.49 ± 2.39 | |
| Flesh fibrousness | Flesh-fibr. | 6.14 ± 1.88 | 5.24 ± 1.83 | 5.69 ± 1.91 | |
| Flesh juiciness | Flesh-juic. | 6.18 ± 1.90 | 4.86 ± 2.05 | 5.52 ± 2.081 | |
| Flesh firmness | Flesh-firmn. | 6.84 ± 1.98 | 3.02 ± 1.37 | 4.93 ± 2.555 | |
| Typical pepper taste | Taste-p. | 6.46 ± 1.27 | 6.65 ± 1.58 | 6.55 ± 1.43 | |
| Sour taste | Taste-so. | 3.74 ± 2.14 | 2.21 ± 1.66 | 2.98 ± 2.06 | |
| Sweet taste | Taste-swe. | 3.79 ± 2.11 | 6.40 ± 2.12 | 5.09 ± 2.48 | |
| Bitter taste | Taste-bit. | 0.51 ± 0.89 | 0.36 ± 1.08 | 0.44 ± 0.99 | |
| Pungent flavour | Flav.pung. | 0.97 ± 1.31 | 0.80 ± 1.02 | 0.88 ± 1.17 | |
| Off-flavor | Flav.of. | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| Overall quality | OQ | 7.18 ± 0.95 | 6.766 ± 1.22 | 6.973 ± 1.11 | |
| Overall sensory preference | Over.sense.pref. | 6.73 ± 1.40 | 7.29 ± 1.43 | 7.009 ± 1.44 | |
| Yield (average of 48 values) | |||||
| Total yield | g plant−1 | Tyield | 2844.07 ± 469.92 | 2955.05 ± 925.76 | 2899.56 ± 730.41 |
| Fruit with BER | BER | 738.48 ± 266.25 | 1714.22 ± 353.67 | 1226.35 ± 581.57 | |
| Marketable yield | MYield | 2105.58 ± 553.39 | 1240.82 ± 714.44 | 1673.20 ± 769.27 | |
| No. of Fruit Total yield | No. plant−1 | No.fr.Tyield | 17.69 ± 3.97 | 45.04 ± 11.63 | 31.37 ± 16.26 |
| Fruit with BER | No.fr.BER | 6.51 ± 2.92 | 30.70 ± 9.62 | 18.60 ± 14.09 | |
| Marketable yield | No.fr.Myield | 11.18 ± 3.47 | 14.34 ± 7.70 | 12.76 ± 6.13 | |
| Antioxidant activity of pepper fruits (average of 48 values) | |||||
| In vitro antioxidant DPPH assay | % RSC | DPPH | 64.12 ± 2.49 | 73.30 ± 2.92 | 68.71 ± 5.35 |
| ABTS assay | ABTS | 72.86 ± 2.47 | 83.43 ± 4.17 | 78.22 ± 6.32 | |
| Total phenolic content | mg CE 100 g−1 FW | TPC | 47.10 ± 3.50 | 60.80 ± 5.60 | 53.91 ± 8.31 |
| Parameter a | Principal Components (PCs) | ||||
|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC4 | PC5 | |
| DW | −0.661 b | −0.499 | −0.521 | 0.036 | −0.097 |
| TSS | −0.655 | −0.638 | −0.282 | 0.076 | 0.135 |
| TS | −0.031 | −0.086 | −0.905 | 0.165 | −0.283 |
| TS/TA | −0.472 | −0.176 | −0.816 | 0.138 | −0.153 |
| TA | 0.935 | 0.203 | 0.175 | 0.015 | −0.123 |
| TSS/TA | −0.867 | −0.373 | −0.191 | −0.047 | 0.171 |
| P | 0.202 | 0.000 | 0.225 | −0.050 | 0.916 |
| NO3 | −0.306 | −0.557 | −0.114 | −0.420 | 0.617 |
| Ca | −0.399 | −0.072 | 0.400 | −0.241 | 0.782 |
| K | 0.915 | −0.119 | 0.099 | 0.303 | −0.028 |
| AA | 0.177 | 0.054 | 0.676 | 0.195 | −0.671 |
| Neox. | 0.026 | 0.574 | 0.275 | −0.216 | 0.720 |
| Lut. | 0.326 | 0.657 | 0.436 | −0.495 | 0.030 |
| Zeax. | 0.294 | 0.596 | 0.723 | 0.014 | 0.183 |
| Violax. | 0.337 | 0.792 | 0.384 | 0.246 | −0.118 |
| Caps. | 0.538 | 0.453 | 0.613 | −0.149 | 0.157 |
| Crypt. | 0.434 | 0.713 | 0.469 | −0.008 | −0.053 |
| α-carot. | 0.368 | 0.823 | 0.082 | 0.334 | −0.197 |
| β-carot. | 0.359 | 0.800 | −0.072 | 0.315 | 0.190 |
| Odor-fre.p. | 0.176 | 0.256 | 0.111 | 0.912 | −0.233 |
| Skin-hard. | 0.789 | 0.386 | 0.025 | 0.017 | 0.089 |
| Flesh-fibr. | 0.518 | 0.685 | 0.035 | −0.46 | 0.123 |
| Flesh-juic. | 0.413 | 0.598 | 0.086 | −0.529 | 0.341 |
| Flesh-firmn. | 0.710 | 0.559 | 0.335 | −0.248 | 0.068 |
| Taste-p. | −0.115 | −0.139 | −0.042 | 0.900 | −0.105 |
| Taste-so. | 0.892 | 0.313 | 0.092 | 0.194 | −0.110 |
| Taste-swe. | −0.863 | −0.434 | −0.234 | −0.083 | 0.041 |
| Taste-bit. | 0.055 | −0.068 | 0.513 | −0.768 | 0.162 |
| Flav.pung. | 0.428 | 0.173 | −0.098 | 0.876 | −0.008 |
| OQ | 0.779 | 0.609 | −0.009 | −0.055 | −0.061 |
| Over.sense.pref. | −0.855 | −0.306 | −0.307 | 0.128 | −0.183 |
| Total variance explained-rotation sums of squared loadings | |||||
| Total | 9.598 | 7.138 | 4.704 | 4.624 | 3.410 |
| % of Variance | 30.961 | 23.026 | 15.174 | 14.916 | 11.001 |
| Cumulative % | 30.961 | 53.986 | 69.161 | 84.077 | 95.078 |
| Parameter a | Principal Components (PCs) | |||
|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC4 | |
| DW | 0.640 b | −0.537 | −0.516 | −0.134 |
| TSS | 0.611 | −0.69 | −0.282 | 0.035 |
| TS | 0.049 | −0.003 | −0.962 | −0.206 |
| TS/TA | 0.470 | −0.152 | −0.852 | −0.108 |
| TA | −0.918 | 0.314 | 0.123 | −0.066 |
| TSS/TA | 0.842 | −0.479 | −0.142 | 0.108 |
| P | −0.218 | −0.018 | 0.233 | 0.909 |
| NO3 | 0.304 | −0.662 | −0.011 | 0.636 |
| Ca | 0.394 | −0.183 | 0.473 | 0.753 |
| K | −0.949 | −0.003 | 0.017 | −0.097 |
| AA | −0.223 | 0.122 | 0.57 | −0.739 |
| Neox. | 0.034 | 0.487 | 0.387 | 0.762 |
| Lut. | −0.239 | 0.608 | 0.547 | 0.183 |
| Zeax. | −0.265 | 0.585 | 0.741 | 0.154 |
| Violax. | −0.279 | 0.819 | 0.389 | −0.168 |
| Caps. | −0.486 | 0.473 | 0.632 | 0.184 |
| Crypt. | −0.406 | 0.733 | 0.472 | 0.003 |
| α-carot. | −0.286 | 0.886 | 0.071 | −0.236 |
| β-carot. | −0.266 | 0.879 | −0.097 | 0.180 |
| DPPH | 0.757 | −0.436 | −0.461 | 0.000 |
| ABTS | 0.830 | −0.478 | −0.239 | 0.030 |
| TPC | 0.829 | −0.336 | −0.372 | 0.060 |
| Total variance explained: rotation sums of squared loadings | ||||
| Total | 6.511 | 6.073 | 4.831 | 3.236 |
| % of Variance | 29.597 | 27.606 | 21.959 | 14.708 |
| Cumulative % | 29.597 | 57.203 | 79.162 | 93.87 |
| Parameter a | Principal Components (PCs) | |||
|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC4 | |
| DW | −0.571 | 0.594 b | −0.525 | 0.146 |
| TSS | −0.719 | 0.578 | −0.276 | 0.012 |
| TS | −0.021 | 0.012 | −0.968 | 0.209 |
| TS/TA | −0.199 | 0.435 | −0.859 | 0.119 |
| TA | 0.374 | −0.910 | 0.127 | 0.054 |
| TSS/TA | −0.530 | 0.820 | −0.147 | −0.091 |
| P | −0.041 | −0.200 | 0.254 | −0.839 |
| NO3 | −0.685 | 0.227 | −0.045 | −0.649 |
| Ca | −0.216 | 0.383 | 0.453 | −0.744 |
| K | 0.049 | −0.896 | 0.086 | 0.190 |
| AA | 0.179 | −0.197 | 0.575 | 0.711 |
| Neox. | 0.456 | 0.071 | 0.376 | −0.767 |
| Lut. | 0.630 | −0.258 | 0.480 | −0.317 |
| Zeax. | 0.609 | −0.202 | 0.742 | −0.163 |
| Violax. | 0.846 | −0.187 | 0.405 | 0.176 |
| Caps. | 0.526 | −0.476 | 0.596 | −0.237 |
| Crypt. | 0.745 | −0.344 | 0.481 | −0.030 |
| α-carot. | 0.916 | −0.205 | 0.076 | 0.244 |
| β-carot. | 0.897 | −0.210 | −0.110 | −0.161 |
| Tyield | −0.186 | 0.184 | −0.057 | 0.885 |
| BER | −0.642 | 0.651 | −0.186 | 0.319 |
| MYield | 0.658 | −0.669 | 0.190 | −0.072 |
| No.fr.Tyield | −0.636 | 0.594 | −0.216 | 0.395 |
| No.fr.BER | −0.677 | 0.560 | −0.165 | 0.388 |
| No.fr.Myield | −0.207 | 0.711 | −0.537 | 0.355 |
| Total variance explained: rotation sums of squared loadings | ||||
| Total | 7.806 | 6.111 | 4.8 | 4.522 |
| % of Variance | 31.222 | 24.444 | 19.198 | 18.09 |
| Cumulative % | 31.222 | 55.666 | 74.864 | 92.954 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobczak-Samburska, A.; Pióro-Jabrucka, E.; Przybył, J.L.; Sieczko, L.; Kalisz, S.; Gajc-Wolska, J.; Kowalczyk, K. Effect of Foliar Application of Calcium and Salicylic Acid on Fruit Quality and Antioxidant Capacity of Sweet Pepper (Capsicum annuum L.) in Hydroponic Cultivation. Agriculture 2025, 15, 26. https://doi.org/10.3390/agriculture15010026
Sobczak-Samburska A, Pióro-Jabrucka E, Przybył JL, Sieczko L, Kalisz S, Gajc-Wolska J, Kowalczyk K. Effect of Foliar Application of Calcium and Salicylic Acid on Fruit Quality and Antioxidant Capacity of Sweet Pepper (Capsicum annuum L.) in Hydroponic Cultivation. Agriculture. 2025; 15(1):26. https://doi.org/10.3390/agriculture15010026
Chicago/Turabian StyleSobczak-Samburska, Anna, Ewelina Pióro-Jabrucka, Jarosław L. Przybył, Leszek Sieczko, Stanisław Kalisz, Janina Gajc-Wolska, and Katarzyna Kowalczyk. 2025. "Effect of Foliar Application of Calcium and Salicylic Acid on Fruit Quality and Antioxidant Capacity of Sweet Pepper (Capsicum annuum L.) in Hydroponic Cultivation" Agriculture 15, no. 1: 26. https://doi.org/10.3390/agriculture15010026
APA StyleSobczak-Samburska, A., Pióro-Jabrucka, E., Przybył, J. L., Sieczko, L., Kalisz, S., Gajc-Wolska, J., & Kowalczyk, K. (2025). Effect of Foliar Application of Calcium and Salicylic Acid on Fruit Quality and Antioxidant Capacity of Sweet Pepper (Capsicum annuum L.) in Hydroponic Cultivation. Agriculture, 15(1), 26. https://doi.org/10.3390/agriculture15010026







