Organic Amendments Enhance the Remediation Potential of Economically Important Crops in Weakly Alkaline Heavy Metal-Contaminated Bauxite Residues
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
2.2. Methods
2.2.1. Experimental Design
2.2.2. BR Sampling and Analysis
2.2.3. Plant Sampling and Analysis
2.2.4. Analysis of Heavy Metal Content in Plants and BRs
2.2.5. Statistical Analyses
3. Results
3.1. BR pH, SOM, CEC, and Enzyme Activity
3.2. HM Content in BR
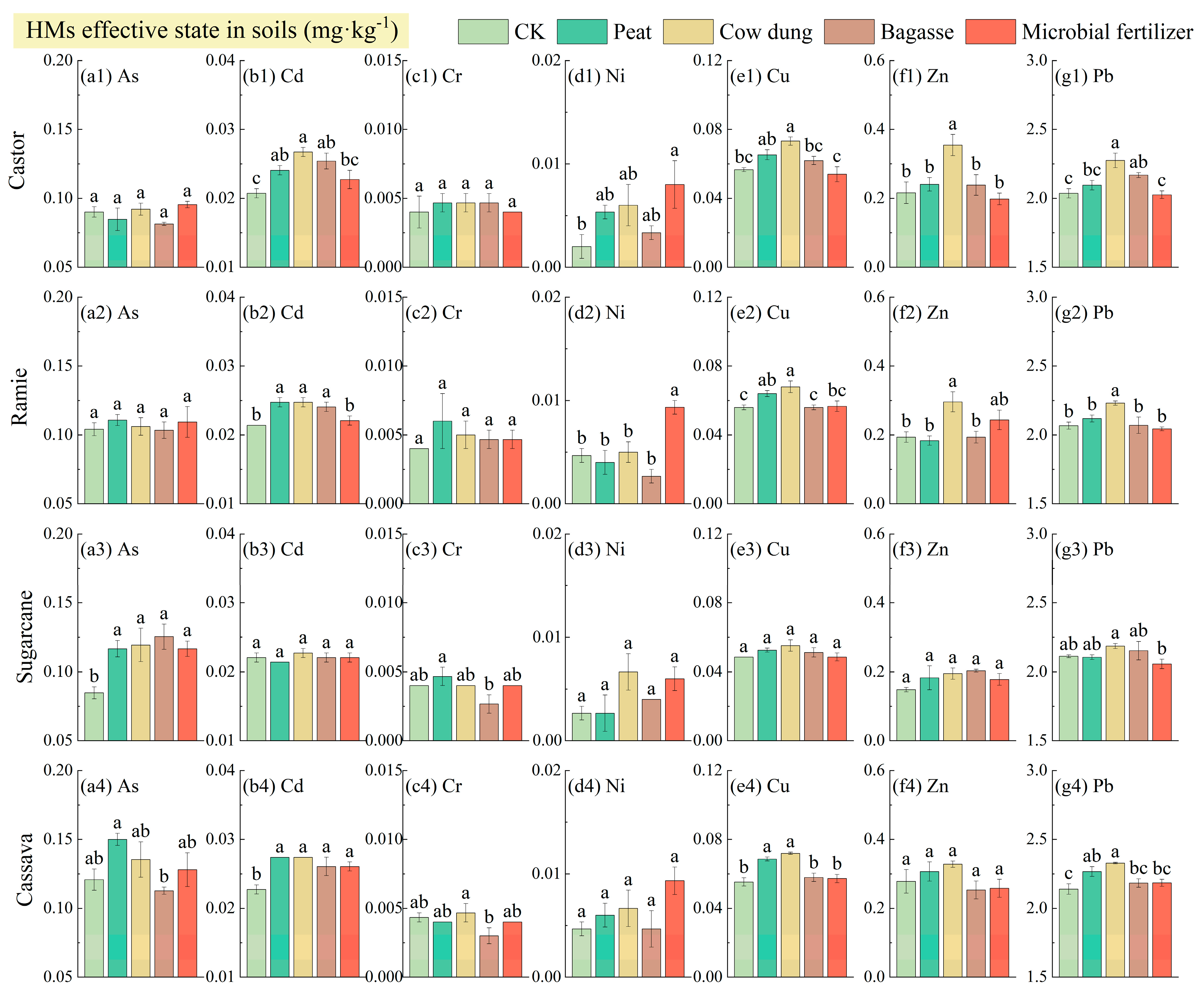
3.3. HM Effective State Content in BRs
3.4. Plant Growth
3.5. Plant Physiological Parameters
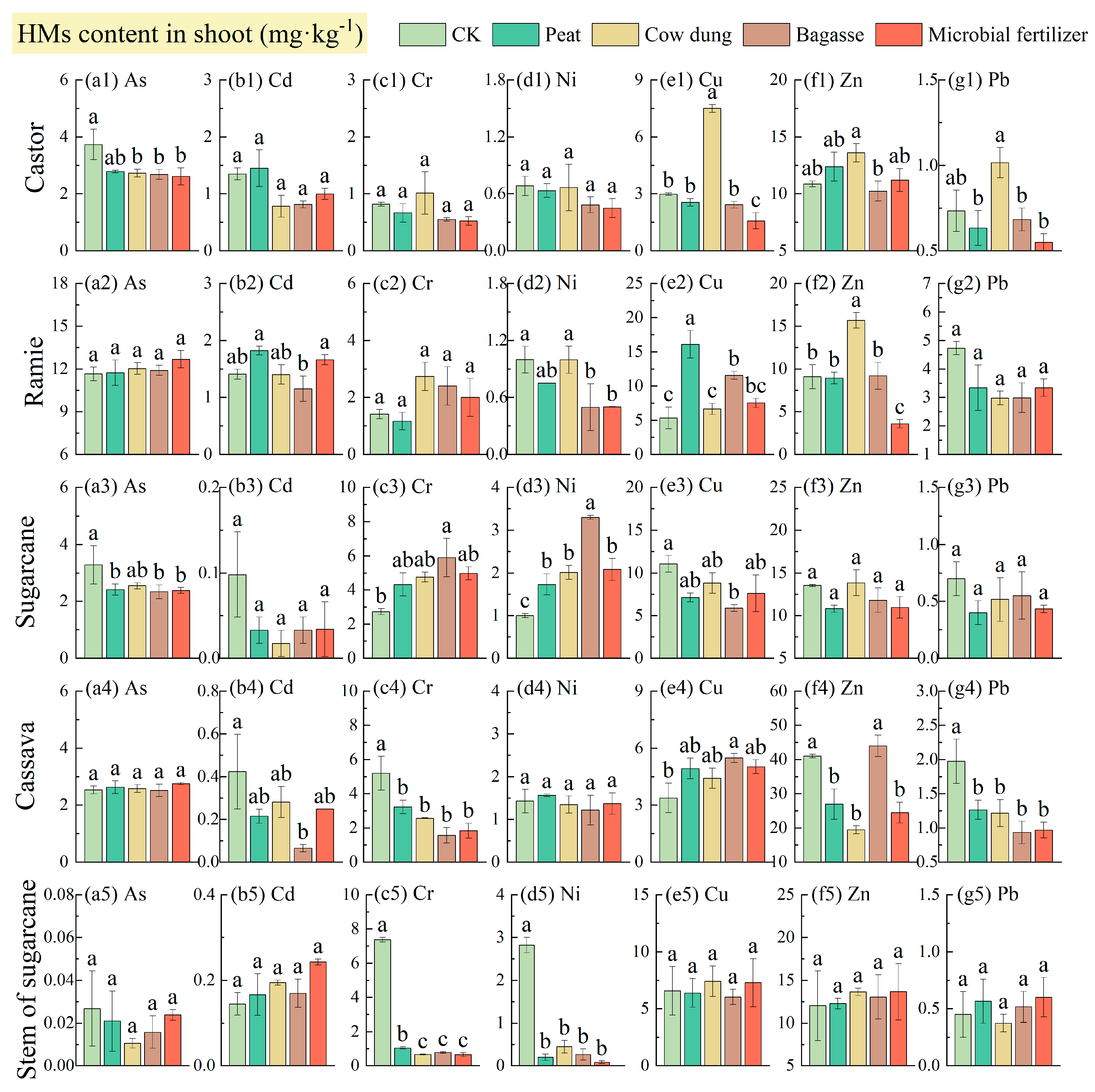
3.6. Plant HM Content
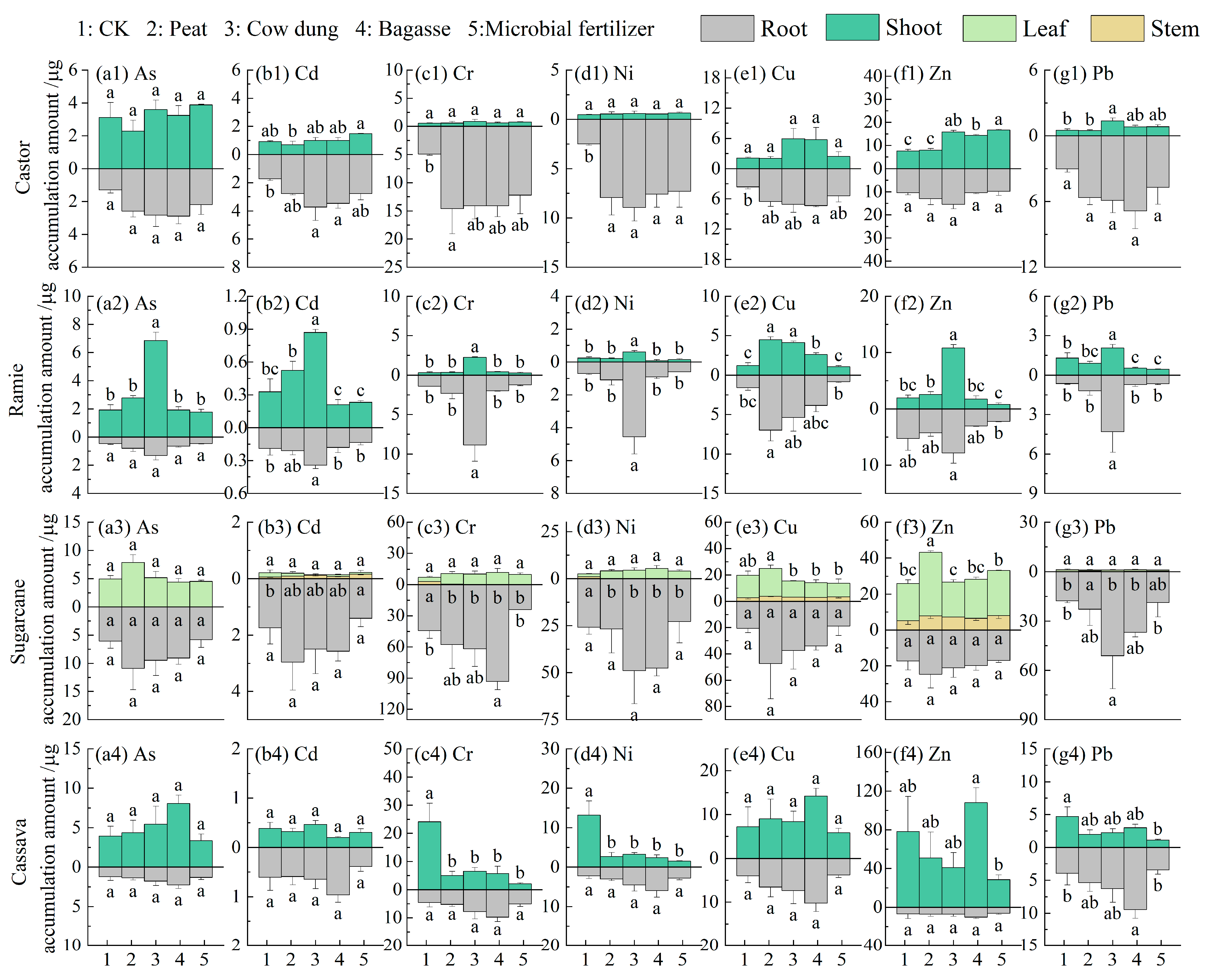
3.7. The Ability of Plants to Accumulate and Transport Heavy Metals
3.8. Plant HM Accumulation
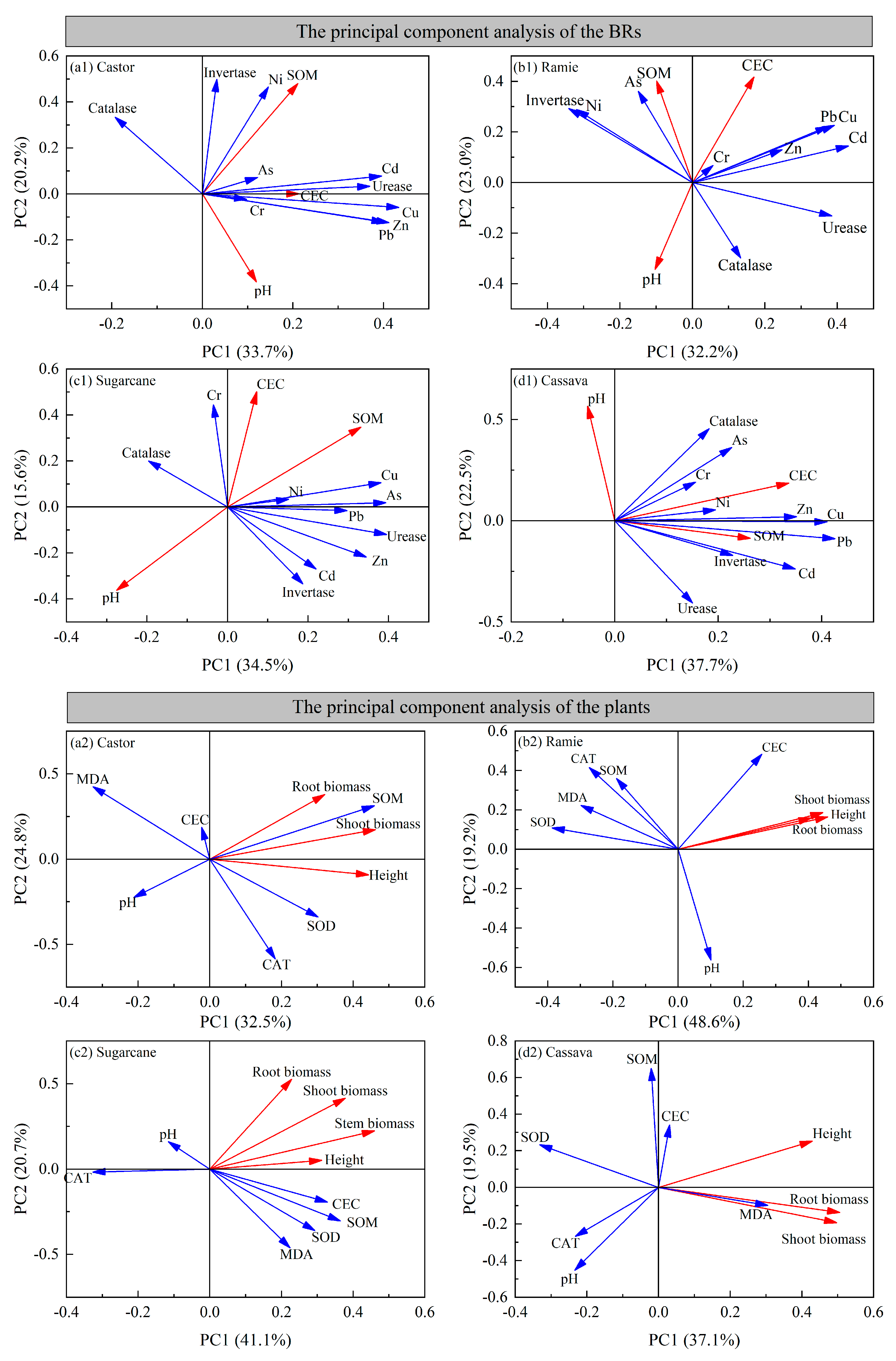
3.9. The Principal Component Analysis
4. Discussion
4.1. Organic Amendment Effects on Physical and Chemical Properties in BRs
4.2. Organic Amendment Effects on BR and Plant Enzyme Activity
4.3. Organic Amendment Effects on BR HM Content
4.4. Organic Amendment Effects on Plant Growth and HM Content
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Santini, T.C.; Fey, M.V. Spontaneous Vegetation Encroachment upon Bauxite Residue (Red Mud) As an Indicator and Facilitator of In Situ Remediation Processes. Environ. Sci. Technol. 2013, 47, 12089–12096. [Google Scholar] [CrossRef]
- Xue, S.; Qin, X.; Jiang, Y.; Guo, Y.; Chen, W.; Zhu, F. Advances in Microbe-Driven Ecological Restoration on Bauxite Residue Disposal Areas. Rev. Environ. Contam. Toxicol. 2023, 262, 3. [Google Scholar] [CrossRef]
- Xue, S.; Kong, X.; Zhu, F.; Hartley, W.; Li, X.; Li, Y. Proposal for Management and Alkalinity Transformation of Bauxite Residue in China. Environ. Sci. Pollut. Res. 2016, 23, 12822–12834. [Google Scholar] [CrossRef] [PubMed]
- Power, G.; Gräfe, M.; Klauber, C. Bauxite Residue Issues: I. Current Management, Disposal and Storage Practices. Hydrometallurgy 2011, 108, 33–45. [Google Scholar] [CrossRef]
- Upadhyay, N.; Verma, S.; Pratap Singh, A.; Devi, S.; Vishwakarma, K.; Kumar, N.; Pandey, A.; Dubey, K.; Mishra, R.; Kumar Tripathi, D.; et al. Soil Ecophysiological and Microbiological Indices of Soil Health: A Study of Coal Mining Site in Sonbhadra, Uttar Pradesh. J. Soil Sci. Plant Nutr. 2016, 16, 778–800. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, J.; Bai, Z.; Reading, L. Effects of Surface Coal Mining and Land Reclamation on Soil Properties: A Review. Earth Sci. Rev. 2019, 191, 12–25. [Google Scholar] [CrossRef]
- Fourie, A. Preventing Catastrophic Failures and Mitigating Environmental Impacts of Tailings Storage Facilities. Procedia Earth Planet. Sci. 2009, 1, 1067–1071. [Google Scholar] [CrossRef]
- Lockwood, C.L.; Stewart, D.I.; Mortimer, R.J.G.; Mayes, W.M.; Jarvis, A.P.; Gruiz, K.; Burke, I.T. Leaching of Copper and Nickel in Soil-Water Systems Contaminated by Bauxite Residue (Red Mud) from Ajka, Hungary: The Importance of Soil Organic Matter. Environ. Sci. Pollut. Res. 2015, 22, 10800–10810. [Google Scholar] [CrossRef] [PubMed]
- Kinnarinen, T.; Holliday, L.; Häkkinen, A. Dissolution of Sodium, Aluminum and Caustic Compounds from Bauxite Residues. Miner. Eng. 2015, 79, 143–151. [Google Scholar] [CrossRef]
- Xu, Y.; An, S.; Chen, Y.; Yuan, C.; Tao, P. Effect of Biomass Improvement Method on Reclaimed Soil of Mining Wasteland. Adv. Civ. Eng. 2022, 2022, 8375918. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Wei, Y.; Meng, H.; Cao, Y.; Lead, J.R.; Hong, J. Effects of Fertilization and Reclamation Time on Soil Bacterial Communities in Coal Mining Subsidence Areas. Sci. Total Environ. 2020, 739, 139882. [Google Scholar] [CrossRef]
- Wu, H.; Tang, T.; Zhu, F.; Wei, X.; Hartley, W.; Xue, S. Long Term Natural Restoration Creates Soil-Like Microbial Communities in Bauxite Residue: A 50-year Filed Study. Land Degrad. Dev. 2021, 32, 1606–1617. [Google Scholar] [CrossRef]
- Li, Z.; Ma, Z.; van der Kuijp, T.J.; Yuan, Z.; Huang, L. A Review of Soil Heavy Metal Pollution from Mines in China: Pollution and Health Risk Assessment. Sci. Total Environ. 2014, 468–469, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Liang, Y.; Chen, Y.a.; Fu, S.; Huang, Y.; Chen, Z.; Chang, X. Biomonitoring Trace Metal Contamination in Guangzhou Urban Parks Using Asian Tramp Snails (Bradybaena similaris). Chemosphere 2023, 334, 138960. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Yao, H.; Li, Y.; Zhu, Y. Microplastic Addition Alters the Microbial Community Structure and Stimulates Soil Carbon Dioxide Emissions in Vegetable-Growing Soil. Environ. Toxicol. Chem. 2021, 40, 352–365. [Google Scholar] [CrossRef]
- MEP. The National Soil Pollution Condition Investigation Communique; Ministry of Environmental Protection: Beijing, China, 2014. (In Chinese) [Google Scholar]
- Yang, Q.; Li, Z.; Lu, X.; Duan, Q.; Huang, L.; Bi, J. A Review of Soil Heavy Metal Pollution from Industrial and Agricultural Regions in China: Pollution and Risk Assessment. Sci. Total Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef]
- Ullah, H.; Khan, N.U.; Ali, F.; Shah, Z.A.; Ullah, Q. Health Risk of Heavy Metals from Vegetables Irrigated with Sewage Water in Peri-Urban of Dera Ismail Khan, Pakistan. Int. J. Environ. Sci. Technol. 2018, 15, 309–322. [Google Scholar] [CrossRef]
- Zhang, W. Characterization and Evaluation of Heavy Metal Pollution in Soil Wheat System around Coal Mines in Pingdingshan, China. Appl. Ecol. Environ. Res. 2019, 17, 5435–5447. [Google Scholar] [CrossRef]
- Huang, L.; Wu, H.; van der Kuijp, T.J. The Health Effects of Exposure to Arsenic-Contaminated Drinking Water: A Review by Global Geographical Distribution. Int. J. Environ. Health Res. 2015, 25, 432–452. [Google Scholar] [CrossRef]
- Garbisu, C.; Alkorta, I. Phytoextraction: A Cost-Effective Plant-Based Technology for the Removal of Metals from the Environment. Bioresour. Technol. 2001, 77, 229–236. [Google Scholar] [CrossRef]
- Yu, Q.; Gao, B.; Wu, P.; Chen, M.; He, C.; Zhang, X. Effects of Microplastics on the Phytoremediation of Cd, Pb, and Zn Contaminated Soils by Solanum photeinocarpum and Lantana camara. Environ. Res. 2023, 231, 116312. [Google Scholar] [CrossRef] [PubMed]
- Mahar, A.; Wang, P.; Ali, A.; Awasthi, M.K.; Lahori, A.H.; Wang, Q.; Li, R.; Zhang, Z. Challenges and Opportunities in the Phytoremediation of Heavy Metals Contaminated Soils: A Review. Ecotoxicol. Environ. Saf. 2016, 126, 111–121. [Google Scholar] [CrossRef]
- Zhang, X.; Xia, H.; Li, Z.; Zhuang, P.; Gao, B. Potential of Four Forage Grasses in Remediation of Cd and Zn Contaminated soils. Bioresour. Technol. 2010, 101, 2063–2066. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ali, S.; Yang, R.; Tao, J.; Ren, B. A newly Discovered Cd-Hyperaccumulator Lantana camara L. J. Hazard. Mater. 2019, 371, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Cretescu, I.; Caraiman, P.; Pohonțu, C.M.; Soreanu, G.; Macoveanu, M. Optimization Process of Cadmium and Zinc Removal from Soil by Phytoremediation Using Brassica napus and Triticales sp. Environ. Eng. Manag. J. 2012, 11, 271–278. [Google Scholar] [CrossRef]
- Ruiz Olivares, A.; Carrillo-González, R.; González-Chávez, M.d.C.A.; Soto Hernández, R.M. Potential of Castor Bean (Ricinus communis L.) for Phytoremediation of Mine Tailings and Oil Production. J. Environ. Manag. 2013, 114, 316–323. [Google Scholar] [CrossRef]
- Bauddh, K.; Singh, R.P. Cadmium Tolerance and Its Phytoremediation by Two Oil Yielding Plants Ricinus communis (L.) and Brassica juncea (L.) From the Contaminated Soil. Int. J. Phytoremediat. 2012, 14, 772–785. [Google Scholar] [CrossRef]
- Mirza, N.; Mubarak, H.; Chai, L.-Y.; Yang, Z.-H.; Mahmood, Q.; Yong, W.; Tang, C.-J.; Fahad, S.; Nasim, W. Constitutional Tolerance and Chlorophyll Fluorescence of Boehmeria nivea L in Response to the Antimony (Sb) and Arsenic (As) Co-Contamination. Toxicol. Environ. Chem. 2017, 99, 265–272. [Google Scholar] [CrossRef]
- Chen, K.; Chen, P.; Qiu, X.; Chen, J.; Gao, G.; Wang, X.; Zhu, A.; Yu, C. Regulating Role of Abscisic Acid on Cadmium Enrichment in Ramie (Boehmeria nivea L.). Sci. Rep. 2021, 11, 22045. [Google Scholar] [CrossRef]
- Huang, C.; Zhou, J.; Jie, Y.; Xing, H.; Zhong, Y.; She, W.; Wei, G.; Yu, W.; Ma, Y. A Ramie (Boehmeria nivea) bZIP Transcription Factor BnbZIP3 Positively Regulates Drought, Salinity and Heavy Metal Tolerance. Mol. Breed. 2016, 36, 120. [Google Scholar] [CrossRef]
- Yang, W.; Li, Z.; Wang, J.; Wu, P.; Zhang, Y. Crop Yield, Nitrogen Acquisition and Sugarcane Quality as Affected by Interspecific Competition and Nitrogen Application. Field Crops Res. 2013, 146, 44–50. [Google Scholar] [CrossRef]
- Xu, G.; Deng, C.; Guo, W.; Zhu, H.; Wang, X.; Zhu, K.; Yin, J.; Sun, Z. Accumulation Characteristics, Tolerance Differences and Risk Assessment of Heavy Metals in Sugarcane. J. Biobased Mater. Bioenergy 2020, 14, 420–429. [Google Scholar] [CrossRef]
- Shen, S.; Chen, J.; Chang, J.; Xia, B. Using Bioenergy Crop Cassava (Manihot esculenta) for Reclamation of Heavily Metal-Contaminated Land. Int. J. Phytoremediat. 2020, 22, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.P.; Luong, A.D.; Van, A.D.; Nguyen, T.T.A. Energy Crop as an Environmentally Sustainable Reclamation Option for Post-Mining Sites: A Life Cycle Assessment of Cassava Planting in Vietnam. Environ. Sci. Pollut. Res. 2022, 29, 6722–6732. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Cheng, P.; Zhang, S.; Zhang, S.; Sun, Y. Contribution of Arbuscular Mycorrhizal Fungi and Soil Amendments to Remediation of a Heavy Metal-Contaminated Soil Using Sweet Sorghum. Pedosphere 2022, 32, 844–855. [Google Scholar] [CrossRef]
- Baruah, N.; Gogoi, N.; Farooq, M. Influence of Biochar and Organic Soil Amendments on Bioavailability and Immobilization of Copper and Lead to Common Cocklebur in Acidic Sandy Loam Soil. J. Environ. Chem. Eng. 2020, 8, 104480. [Google Scholar] [CrossRef]
- Sharma, A.; Nagpal, A.K. Soil Amendments: A Tool to Reduce Heavy Metal Uptake in Crops for Production of Safe Food. Rev. Environ. Sci. Bio/Technol. 2018, 17, 187–203. [Google Scholar] [CrossRef]
- Meier, I.C.; Finzi, A.C.; Phillips, R.P. Root Exudates Increase N Availability by Stimulating Microbial Turnover of Fast-Cycling N Pools. Soil Biol. Biochem. 2017, 106, 119–128. [Google Scholar] [CrossRef]
- Cheng, Y.; Bu, X.; Li, J.; Ji, Z.; Wang, C.; Xiao, X.; Li, F.; Wu, Z.-H.; Wu, G.; Jia, P.; et al. Application of Biochar and Compost Improved Soil Properties and Enhanced Plant Growth in a Pb–Zn Mine Tailings Soil. Environ. Sci. Pollut. Res. 2023, 30, 32337–32347. [Google Scholar] [CrossRef]
- Hao, X.H.; Liu, S.L.; Wu, J.S.; Hu, R.G.; Tong, C.L.; Su, Y.Y. Effect of Long-Term Application of Inorganic Fertilizer and Organic Amendments on Soil Organic Matter and Microbial Biomass in Three Subtropical Paddy Soils. Nutr. Cycl. Agroecosyst. 2008, 81, 17–24. [Google Scholar] [CrossRef]
- Lei, N.; Han, J.; Mu, X.; Sun, Z.; Wang, H. Effects of Improved Materials on Reclamation of Soil Properties and Crop Yield in Hollow Villages in China. J. Soils Sediments 2019, 19, 2374–2380. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, C.; Zhang, J.; Liu, C.; Wu, Q. Fertilizer Impacts on Soil Aggregation and Aggregate-Associated Organic Components. Plant Soil Environ. 2018, 64, 338–343. [Google Scholar] [CrossRef]
- Lima, I.M.; Beacorn, J.A. Targeting a Sustainable Sugar Crops Processing Industry: A Review (Part I)—By-Product Applications. Sugar Tech 2022, 24, 970–991. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Kheir, A.M.S. Saline Soil Properties, Quality and Productivity of Wheat Grown with Bagasse Ash and Thiourea in Different Climatic Zones. Chemosphere 2018, 193, 538–546. [Google Scholar] [CrossRef]
- Mello, B.L.; Alessi, A.M.; McQueen-Mason, S.; Bruce, N.C.; Polikarpov, I. Nutrient Availability Shapes the Microbial Community Structure in Sugarcane Bagasse Compost-Derived Consortia. Sci. Rep. 2016, 6, 38781. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Gong, T.; Wang, J.; Li, G.; Liu, Y.; Zhen, J.; Ning, M.; Yue, D.; Du, Z.; Chen, G. Effects of Compound Microbial Fertilizer on Soil Characteristics and Yield of Wheat (Triticum aestivum L.). J. Soil Sci. Plant Nutr. 2020, 20, 2740–2748. [Google Scholar] [CrossRef]
- Zaborowska, M.; Kucharski, J.; Wyszkowska, J. Remediation of Soil Contaminated with Cadmium. J. Elem. 2015, 20, 769–784. [Google Scholar] [CrossRef]
- Correia, A.G.; da Silva, R.J.N.B.; Pedra, F.; Nunes, M.J. Assessment of the Determination of Heavy Metals in Organic Soil Improvers by ICP–OES. Accredit. Qual. Assur. 2014, 19, 87–97. [Google Scholar] [CrossRef]
- Wang, X.; Yu, H.-Y.; Li, F.; Liu, T.; Wu, W.; Liu, C.; Liu, C.; Zhang, X. Enhanced Immobilization of Arsenic and Cadmium in a Paddy Soil by Combined Applications of Woody Peat and Fe(NO3)3: Possible Mechanisms and Environmental Implications. Sci. Total Environ. 2019, 649, 535–543. [Google Scholar] [CrossRef]
- Liu, G.; Dai, Z.; Tang, C.; Xu, J. The Immobilization, Plant Uptake and Translocation of Cadmium in a Soil-Pakchoi (Brassica chinensis L.) System Amended with Various Sugarcane Bagasse-Based Materials. Environ. Pollut. 2022, 311, 119946. [Google Scholar] [CrossRef] [PubMed]
- Courtney, R.; Harrington, T.; Byrne, K.A. Indicators of Soil Formation in Restored Bauxite Residues. Ecol. Eng. 2013, 58, 63–68. [Google Scholar] [CrossRef]
- GB 15618-2018; Soil Environmental Quality—Risk Control Standard for Soil Contamination of Agricultural Land. CMEE (Ministry of Ecology and Environment of the People’s Republic of China): Beijing, China, 2018.
- Lu, R.K. Soil and Agro-Chemical Chemistry Analytical Methods; China Agricultural Science and Technology Press: Beijing, China, 1999. [Google Scholar]
- Tabatabai, M.A.; Bremner, J.M. Assay of Urease Activity in Soils. Soil Biol. Biochem. 1972, 4, 479–487. [Google Scholar] [CrossRef]
- Cang, L.; Zhou, D.-M.; Wang, Q.-Y.; Wu, D.-Y. Effects of Electrokinetic Treatment of a Heavy Metal Contaminated Soil on Soil Enzyme Activities. J. Hazard. Mater. 2009, 172, 1602–1607. [Google Scholar] [CrossRef] [PubMed]
- Trasar-Cepeda, C.; Camiña, F.; Leirós, M.C.; Gil-Sotres, F. An Improved Method to Measure Catalase Activity in Soils. Soil Biol. Biochem. 1999, 31, 483–485. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplasts: I. Kinetics and Stoichiometry of Fatty Acid Peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Gupta, A.S.; Webb, R.P.; Holaday, A.S.; Allen, R.D. Overexpression of Superoxide Dismutase Protects Plants from Oxidative Stress (Induction of Ascorbate Peroxidase in Superoxide Dismutase-Overexpressing Plants). Plant Physiol. 1993, 103, 1067–1073. [Google Scholar] [CrossRef]
- Kar, M.; Mishra, D. Catalase, Peroxidase, and Polyphenoloxidase Activities during Rice Leaf Senescence. Plant Physiol. 1976, 57, 315–319. [Google Scholar] [CrossRef] [PubMed]
- U.S. EPA. Method 3050B: Acid Digestion of Sediments, Sludges, and Soils; Revision 2; U.S. EPA: Washington, DC, USA, 1996. [Google Scholar]
- Zhang, X.; Xia, H.; Li, Z.A.; Zhuang, P.; Gao, B. Identification of a New Potential Cd-Hyperaccumulator Solanum photeinocarpum by Soil Seed Bank-Metal Concentration Gradient Method. J. Hazard. Mater. 2011, 189, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Gould, I.J.; Quinton, J.N.; Weigelt, A.; De Deyn, G.B.; Bardgett, R.D. Plant Diversity and Root Traits Benefit Physical Properties Key to Soil Function in Grasslands. Ecol. Lett. 2016, 19, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Azeez, L.; Oyedeji, A.O.; Aremu, H.K.; Busari, H.K.; Adekale, I.; Olabode, O.A. Silver Nanoparticles-Cow Dung Combination Disrupts Physiology, Enzyme Activities with Corresponding Increased Oxidative Stress and Heavy Metal Accumulation in Abelmoschus esculentus. Bull. Environ. Contam. Toxicol. 2022, 109, 893–899. [Google Scholar] [CrossRef]
- Fu, W.; Fan, J.; Wang, S.; Wang, H.; Dai, Z.; Zhao, X.; Hao, M. Woody Peat Addition Increases Soil Organic Matter but Its Mineralization Is Affected by Soil Clay in the Four Degenerated Erodible Soils. Agric. Ecosyst. Environ. 2021, 318, 107495. [Google Scholar] [CrossRef]
- Amoah, A.A.; Senge, M.; Miyagawa, S.; Itou, K. Effects of Soil Fertility Management on Growth, Yield, and Water-Use Efficiency of Maize (Zea mays L.) and Selected Soil Properties. Commun. Soil Sci. Plant Anal. 2012, 43, 924–935. [Google Scholar] [CrossRef]
- Lin, Y.; Ye, G.; Kuzyakov, Y.; Liu, D.; Fan, J.; Ding, W. Long-Term Manure Application Increases Soil Organic Matter and Aggregation, and Alters Microbial Community Structure and Keystone Taxa. Soil Biol. Biochem. 2019, 134, 187–196. [Google Scholar] [CrossRef]
- Mace, J.E.; Amrhein, C.; Oster, J.D. Comparison of Gypsum and Sulfuric Acid for Sodic Soil Reclamation. Arid. Soil Res. Rehabil. 1999, 13, 171–188. [Google Scholar] [CrossRef]
- Arif, M.; Ilyas, M.; Riaz, M.; Ali, K.; Shah, K.; Ul Haq, I.; Fahad, S. Biochar Improves Phosphorus Use Efficiency of Organic-Inorganic Fertilizers, Maize-Wheat Productivity and Soil Quality in a Low Fertility Alkaline Soil. Field Crops Res. 2017, 214, 25–37. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, C.; Haris, M.; Chen, C.; Wang, H.; Guo, J.; Meng, H.; Wu, X.; Liu, X.; Hu, W.; et al. Study of Ceramsite-Supported Iron and Manganese Oxides for Enhancing Soil Immobilization and Reducing Rice Plants Uptake of Cadmium. J. Environ. Chem. Eng. 2024, 12, 111938. [Google Scholar] [CrossRef]
- Han, X.; Liu, S.; Xie, Z.; Ma, X.; Wang, Y.; Peng, C. Dynamic Changes of Humic Acids in Chicken Manure Composting. Pol. J. Environ. Stud. 2022, 31, 1637–1644. [Google Scholar] [CrossRef]
- Sánchez-Monedero, M.A.; Cegarra, J.; García, D.; Roig, A. Chemical and Structural Evolution of Humic Acids During Organic Waste Composting. Biodegradation 2002, 13, 361–371. [Google Scholar] [CrossRef]
- de Souza, C.d.C.B.; García, A.C.; Lima, E.S.A.; do Amaral Sobrinho, N.M.B. Humic Substances Formation During Poultry Litter Composting and Its Influence on the Structural Characteristics of the Compost. J. Mater. Cycles Waste Manag. 2023, 25, 2232–2244. [Google Scholar] [CrossRef]
- Ren, J.; Ren, X.; Chen, J.; Guo, W.; Yang, B.; Du, P. Humic-Mineral Interactions Modulated by pH Conditions in Bauxite Residues—Implications in Stable Aggregate Formation. Geoderma 2021, 385, 114856. [Google Scholar] [CrossRef]
- Xue, S.; Ke, W.; Zhu, F.; Ye, Y.; Liu, Z.; Fan, J.; Hartley, W. Effect of Phosphogypsum and Poultry Manure on Aggregate-Associated Alkaline Characteristics in Bauxite Residue. J. Environ. Manag. 2020, 256, 109981. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Ye, Y.; Zhu, F.; Wang, Q.; Jiang, J.; Hartley, W. Changes in Distribution and Microstructure of Bauxite Residue Aggregates Following Amendments Addition. J. Environ. Sci. 2019, 78, 276–286. [Google Scholar] [CrossRef] [PubMed]
- You, F.; Zhang, L.; Ye, J.; Huang, L. Microbial Decomposition of Biomass Residues Mitigated Hydrogeochemical Dynamics in Strongly Alkaline Bauxite Residues. Sci. Total Environ. 2019, 663, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, M.; Wu, Y.; Núñez-Delgado, A.; Kuzyakov, Y.; Peng, Q.-A.; Lin, S.; Hu, R. Enzyme Activities and Organic Matter Mineralization in Response to Application of Gypsum, Manure and Rice Straw in Saline and Sodic Soils. Environ. Res. 2023, 224, 115393. [Google Scholar] [CrossRef]
- Li, Y.; Niu, W.; Wang, J.; Liu, L.; Zhang, M.; Xu, J. Effects of Artificial Soil Aeration Volume and Frequency on Soil Enzyme Activity and Microbial Abundance when Cultivating Greenhouse Tomato. Soil Sci. Soc. Am. J. 2016, 80, 1208–1221. [Google Scholar] [CrossRef]
- Xu, H.; Liu, G.; Wu, X.; Smoak, J.M.; Mu, C.; Ma, X.; Zhang, X.; Li, H.; Hu, G. Soil Enzyme Response to Permafrost Collapse in the Northern Qinghai-Tibetan Plateau. Ecol. Indic. 2018, 85, 585–593. [Google Scholar] [CrossRef]
- Ren, J.; Liu, X.; Yang, W.; Yang, X.; Li, W.; Xia, Q.; Li, J.; Gao, Z.; Yang, Z. Rhizosphere Soil Properties, Microbial Community, and Enzyme Activities: Short-Term Responses to Partial Substitution of Chemical Fertilizer with Organic Manure. J. Environ. Manag. 2021, 299, 113650. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Gu, T.; Wang, W.; Zhang, B.; Lin, X.; Huang, Q.; Shen, W. The Effects of Mineral Fertilizer and Organic Manure on Soil Microbial Community and Diversity. Plant Soil 2010, 326, 511–522. [Google Scholar] [CrossRef]
- Hu, J.; Lin, X.; Wang, J.; Dai, J.; Chen, R.; Zhang, J.; Wong, M.H. Microbial Functional Diversity, Metabolic Quotient, and Invertase Activity of a Sandy Loam Soil as Affected by Long-Term Application of Organic Amendment and Mineral Fertilizer. J. Soils Sediments 2011, 11, 271–280. [Google Scholar] [CrossRef]
- Chen, J.; Chen, D.; Xu, Q.; Fuhrmann, J.J.; Li, L.; Pan, G.; Li, Y.; Qin, H.; Liang, C.; Sun, X. Organic Carbon Quality, Composition of Main Microbial Groups, Enzyme Activities, and Temperature Sensitivity of Soil Respiration of an Acid Paddy Soil Treated with Biochar. Biol. Fertil. Soils 2019, 55, 185–197. [Google Scholar] [CrossRef]
- Orwin, K.H.; Wardle, D.A.; Greenfield, L.G. Ecological Consequences of Carbon Substrate Identity and Diversity in a Laboratory Study. Ecology 2006, 87, 580–593. [Google Scholar] [CrossRef]
- Lu, Y.; Li, X.; He, M.; Zhao, X.; Liu, Y.; Cui, Y.; Pan, Y.; Tan, H. Seedlings Growth and Antioxidative Enzymes Activities in Leaves under Heavy Metal Stress Differ Between Two Desert Plants: A Perennial (Peganum harmala) and an Annual (Halogeton glomeratus) Grass. Acta Physiol. Plant. 2010, 32, 583–590. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Ghani, M.I.; Ali, A.; Atif, M.J.; Ali, M.; Amin, B.; Anees, M.; Cheng, Z. Soil Amendment with Raw Garlic Stalk: A Novel Strategy to Stimulate Growth and the Antioxidative Defense System in Monocropped Eggplant in the North of China. Agronomy 2019, 9, 89. [Google Scholar] [CrossRef]
- Nadgórska-Socha, A.; Ptasiński, B.; Kita, A. Heavy Metal Bioaccumulation and Antioxidative Responses in Cardaminopsis arenosa and Plantago lanceolata Leaves from Metalliferous and Non-Metalliferous Sites: A Field Study. Ecotoxicology 2013, 22, 1422–1434. [Google Scholar] [CrossRef]
- Smaoui-Jardak, M.; Kriaa, W.; Maalej, M.; Zouari, M.; Kamoun, L.; Trabelsi, W.; Ben Abdallah, F.; Elloumi, N. Effect of the Phosphogypsum Amendment of Saline and Agricultural Soils on Growth, Productivity and Antioxidant Enzyme Activities of Tomato (Solanum lycopersicum L.). Ecotoxicology 2017, 26, 1089–1104. [Google Scholar] [CrossRef] [PubMed]
- Schützendübel, A.; Polle, A. Plant Responses to Abiotic Stresses: Heavy Metal-Induced Oxidative Stress and Protection by Mycorrhization. J. Exp. Bot. 2002, 53, 1351–1365. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Lin, J.-G. Bioleaching of Heavy Metals from Sediment: Significance of pH. Chemosphere 2001, 44, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Yang, H.; Jiang, M.; Zhang, Q. Effects of Four Cost-Effective Amendments on the Synchronous Stabilization of As, Zn, Cu and Cd in Contaminated Mixture of Residue and Soil from an Arsenic Smelting Site. J. Environ. Chem. Eng. 2022, 10, 107845. [Google Scholar] [CrossRef]
- Zuo, W.; Xu, K.; Zhang, W.; Wang, Y.; Gu, C.; Bai, Y.; Shan, Y.; Dai, Q. Heavy Metal Distribution and Uptake by Maize in a Mudflat Soil Amended by Vermicompost Derived from Sewage Sludge. Environ. Sci. Pollut. Res. 2019, 26, 30154–30166. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Wang, X.; Zhang, Y.; Zhang, M.; Wang, K.; Xie, P.; Ji, H. The Optimum pH and Eh for Simultaneously Minimizing Bioavailable Cadmium and Arsenic Contents in Soils under the Organic Fertilizer Application. Sci. Total Environ. 2020, 711, 135229. [Google Scholar] [CrossRef]
- Kashem, M.A.; Singh, B.R. Metal Availability in Contaminated Soils: I. Effects of Floodingand Organic Matter on Changes in Eh, pH and Solubility of Cd, Ni andZn. Nutr. Cycl. Agroecosyst. 2001, 61, 247–255. [Google Scholar] [CrossRef]
- Bai, Y.; Yan, Y.; Zuo, W.; Gu, C.; Guan, Y.; Wang, X.; Zhao, H.; Shan, Y.; Shao, H.; Feng, K. Distribution of Cadmium, Copper, Lead, and Zinc in Mudflat Salt-Soils Amended with Sewage Sludge. Land Degrad. Dev. 2018, 29, 1120–1129. [Google Scholar] [CrossRef]
- Soria, R.I.; Rolfe, S.A.; Betancourth, M.P.; Thornton, S.F. The Relationship Between Properties of Plant-Based Biochars and Sorption of Cd(II), Pb(II) and Zn(II) in Soil Model Systems. Heliyon 2020, 6, e05388. [Google Scholar] [CrossRef]
- Chen, B.; Zhou, D.; Zhu, L. Transitional Adsorption and Partition of Nonpolar and Polar Aromatic Contaminants by Biochars of Pine Needles with Different Pyrolytic Temperatures. Environ. Sci. Technol. 2008, 42, 5137–5143. [Google Scholar] [CrossRef] [PubMed]
- Zanin Lima, J.; Monici Raimondi Nauerth, I.; Ferreira da Silva, E.; José Pejon, O.; Guimarães Silvestre Rodrigues, V. Competitive Sorption and Desorption of Cadmium, Lead, and Zinc onto Peat, Compost, and Biochar. J. Environ. Manag. 2023, 344, 118515. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Gao, L.-Y.; Wu, R.-R.; Wang, H.; Xiao, R.-B. Qualitative and Quantitative Characterization of Adsorption Mechanisms for Cd2+ by Silicon-Rich Biochar. Sci. Total Environ. 2020, 731, 139163. [Google Scholar] [CrossRef]
- Raimondi, I.M.; Rodrigues, V.G.S.; Lima, J.Z.; Marques, J.P.; Vaz, L.A.A. The Potential Use of Pressmud as Reactive Material for Cd2+ Removal: Adsorption Equilibrium, Kinetics, Desorption, and Bioaccessibility. Water Air Soil Pollut. 2020, 231, 365. [Google Scholar] [CrossRef]
- Kang, J.; Zhang, Z.; Wang, J.J. Influence of Humic Substances on Bioavailability of Cu and Zn During Sewage Sludge Composting. Bioresour. Technol. 2011, 102, 8022–8026. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, A.; Xiang, Y.; Ejaz, A.; Niu, J. Self-Template Bagasse-Based Porous Carbons for High Performance Supercapacitors. Ind. Crops Prod. 2022, 176, 114291. [Google Scholar] [CrossRef]
- Li, H.; Hou, R.; Chen, Y.; Chen, H. Removal of Hexavalent Chromium from Aqueous Solutions Using Sulfonated Peat. Water 2019, 11, 1980. [Google Scholar] [CrossRef]
- Almås, Å.R.; Lofts, S.; Mulder, J.; Tipping, E. Solubility of Major Cations and Cu, Zn and Cd in Soil Extracts of Some Contaminated Agricultural Soils near a Zinc Smelter in Norway: Modelling with a Multisurface Extension of WHAM. Eur. J. Soil Sci. 2007, 58, 1074–1086. [Google Scholar] [CrossRef]
- Fahmi, A.H.; Samsuri, A.W.; Jol, H.; Singh, D. Bioavailability and Leaching of Cd and Pb from Contaminated Soil Amended with Different Sizes of Biochar. R. Soc. Open Sci. 2018, 5, 181328. [Google Scholar] [CrossRef] [PubMed]
- Antoniadis, V.; Zanni, A.A.; Levizou, E.; Shaheen, S.M.; Dimirkou, A.; Bolan, N.; Rinklebe, J. Modulation of Hexavalent Chromium Toxicity on Origanum vulgare in an Acidic Soil Amended with Peat, Lime, and Zeolite. Chemosphere 2018, 195, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, X.; Li, R.; Wang, G.; Jin, Y.; Xu, W.; Wang, H.; Qu, J. Organic Amendment Improves Rhizosphere Environment and Shapes Soil Bacterial Community in Black and Red Soil under Lead Stress. J. Hazard. Mater. 2021, 416, 125805. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.-J.; Su, J.-Q.; Sun, G.-X.; Wu, J.-S.; Wei, W.-X. Increased Microbial Functional Diversity under Long-Term Organic and Integrated Fertilization in a Paddy Soil. Appl. Microbiol. Biotechnol. 2018, 102, 1969–1982. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Zheng, C.; Zhang, Y.; Sun, Z. Organic Amendment Application Influence Soil Organism Abundance in Saline Alkali Soil. Eur. J. Soil Biol. 2013, 54, 32–40. [Google Scholar] [CrossRef]
- Wenger, K.; Gupta, S.K.; Furrer, G.; Schulin, R. The Role of Nitrilotriacetate in Copper Uptake by Tobacco. J. Environ. Qual. 2003, 32, 1669–1676. [Google Scholar] [CrossRef]
- Hsu, J.-H.; Lo, S.-L. Characterization and Extractability of Copper, Manganese, and Zinc in Swine Manure Composts. J. Environ. Qual. 2000, 29, 447–453. [Google Scholar] [CrossRef]
- Versini, A.; Poultney, D.; Bachir, H.; Février, A.; Paillat, J. Effect of Nitrogen Fertilisation on Sugarcane Root Development and Nitrogen Accumulation in Ratoon Crops Reunion Island. Sugar Tech 2020, 22, 1110–1121. [Google Scholar] [CrossRef]
- Dushenkov, V.; Kumar, P.B.A.N.; Motto, H.; Raskin, I. Rhizofiltration: The Use of Plants to Remove Heavy Metals from Aqueous Streams. Environ. Sci. Technol. 1995, 29, 1239–1245. [Google Scholar] [CrossRef]
- Zakari, S.; Jiang, X.; Zhu, X.; Liu, W.; Allakonon, M.G.B.; Singh, A.K.; Chen, C.; Zou, X.; Akponikpè, P.B.I.; Dossa, G.G.O.; et al. Influence of Sulfur Amendments on Heavy Metals Phytoextraction from Agricultural Contaminated Soils: A Meta-Analysis. Environ. Pollut. 2021, 288, 117820. [Google Scholar] [CrossRef] [PubMed]





| BR | Peat | Cow Dung | Bagasse | Microbial Fertilizer | ||
|---|---|---|---|---|---|---|
| Physicochemical properties | pH | 7.68 ± 0.03 | 5.93 ± 0.13 | 7.72 ± 0.10 | 7.24 ± 0.11 | 6.72 ± 0.08 |
| SOM (%) | 0.54 ± 0.08 | 12.66 ± 0.42 | 11.32 ± 0.16 | 11.47 ± 0.10 | 14.11 ± 0.57 | |
| CEC (cmol+·kg−1) | 3.20 ± 0.14 | / | / | / | / | |
| Available P (mg·kg−1) | 3.59 ± 0.92 | 136.28 ± 11.01 | 242.16 ± 14.74 | 5.35 ± 0.70 | 2.32 ± 1.47 | |
| Available K (mg·kg−1) | 17.59 ± 0.35 | 732.70 ± 44.13 | 3310.10 ± 263.07 | 260.23 ± 0.62 | 93.27 ± 8.88 | |
| Hydrolytic N (mg·kg−1) | 91.01 ± 29.73 | 406.25 ± 79.77 | 637.03 ± 55.58 | 84.01 ± 19.80 | 657.93 ± 19.70 | |
| Heavy metal content (mg·kg−1) | Total As | 109.74 ± 3.45 | 0.48 ± 0.02 | 2.06 ± 0.24 | 0.79 ± 0.53 | 0.87 ± 0.05 |
| Total Cd | 1.10 ± 0.28 | 0.06 ± 0.02 | 0.54 ± 0.02 | 0.14 ± 0.04 | 0.47 ± 0.02 | |
| Total Cr | 185.44 ± 9.14 | 1.23 ± 0.13 | 24.57 ± 1.28 | 3.35 ± 0.63 | 13.31 ± 1.06 | |
| Total Ni | 76.96 ± 6.93 | 0.55 ± 0.001 | 14.22 ± 1.07 | 1.42 ± 1.11 | 26.30 ± 0.75 | |
| Total Cu | 44.53 ± 1.43 | 21.71 ± 2.04 | 26.51 ± 2.01 | 4.30 ± 2.38 | 31.04 ± 0.34 | |
| Total Zn | 233.64 ± 13.44 | 13.22 ± 1.68 | 45.26 ± 1.53 | 8.91 ± 0.55 | 40.92 ± 0.50 | |
| Total Pb | 54.87 ± 1.81 | 4.93 ± 0.37 | 10.65 ± 0.69 | 4.25 ± 3.17 | 27.55 ± 3.67 | |
| Available As | 0.15 ± 0.01 | / | / | / | / | |
| Available Cd | 0.02 ± 0.001 | / | / | / | / | |
| Available Cr | 0.007 ± 0.001 | / | / | / | / | |
| Available Ni | 0.003 ± 0.001 | / | / | / | / | |
| Available Cu | 0.06 ± 0.003 | / | / | / | / | |
| Available Zn | 0.27 ± 0.004 | / | / | / | / | |
| Available Pb | 1.94 ± 0.03 | / | / | / | / |
| Plant | Treatment | Plant Height /cm | Root Biomass /g·plant−1 | Shoot Biomass /g·plant−1 | Stem Biomass /g·plant−1 | Total Biomass /g·plant−1 |
|---|---|---|---|---|---|---|
| Castor | CK | 20.00 ± 1.00 b | 0.62 ± 0.09 b | 0.70 ± 0.07 a | / | 1.32 ± 0.15 b |
| Peat | 19.83 ± 1.42 b | 0.86 ± 0.12 ab | 0.82 ± 0.24 a | / | 1.68 ± 0.36 ab | |
| Cow dung | 28.00 ± 2.65 a | 1.11 ± 0.25 a | 1.31 ± 0.16 a | / | 2.42 ± 0.34 a | |
| Bagasse | 20.17 ± 1.01 b | 0.92 ± 0.08 ab | 1.20 ± 0.19 a | / | 2.12 ± 0.11 ab | |
| Microbial fertilizer | 25.67 ± 2.09 ab | 0.92 ± 0.09 ab | 1.27 ± 0.25 a | / | 2.20 ± 0.30 ab | |
| Ramie | CK | 19.00 ± 6.00 bc | 0.07 ± 0.02 b | 0.23 ± 0.07 b | / | 0.30 ± 0.09 b |
| Peat | 28.67 ± 1.45 b | 0.09 ± 0.02 ab | 0.29 ± 0.03 b | / | 0.38 ± 0.06 b | |
| Cow dung | 48.83 ± 5.29 a | 0.15 ± 0.02 a | 0.63 ± 0.06 a | / | 0.78 ± 0.08 a | |
| Bagasse | 13.33 ± 1.86 c | 0.08 ± 0.012 b | 0.19 ± 0.03 b | / | 0.27 ± 0.04 b | |
| Microbial fertilizer | 11.67 ± 0.33 c | 0.06 ± 0.004 b | 0.14 ± 0.02 b | / | 0.20 ± 0.02 b | |
| Sugarcane | CK | 73.00 ± 6.11 a | 1.08 ± 0.27 a | 1.45 ± 0.12 a | 0.38 ± 0.04 a | 2.91 ± 0.32 a |
| Peat | 80.33 ± 5.04 a | 1.74 ± 0.59 a | 2.52 ± 0.64 a | 0.66 ± 0.14 a | 4.92 ± 1.32 a | |
| Cow dung | 75.67 ± 6.12 a | 1.43 ± 0.36 a | 2.06 ± 0.49 a | 0.65 ± 0.12 a | 4.14 ± 0.96 a | |
| Bagasse | 77.67 ± 3.48 a | 1.38 ± 0.19 a | 1.90 ± 0.25 a | 0.50 ± 0.02 a | 3.78 ± 0.46 a | |
| Microbial fertilizer | 82.83 ± 6.42 a | 1.04 ± 0.13 a | 1.91 ± 0.15 a | 0.60 ± 0.03 a | 3.55 ± 0.22 a | |
| Cassava | CK | 24.67 ± 2.05 a | 0.57 ± 0.27 a | 1.63 ± 0.60 a | / | 2.03 ± 0.96 a |
| Peat | 29.00 ± 3.22 a | 0.59 ± 0.21 a | 1.69 ± 0.66 a | / | 2.27 ± 0.87 a | |
| Cow dung | 30.33 ± 1.76 a | 0.63 ± 0.17 a | 2.08 ± 0.81 a | / | 2.70 ± 0.96 a | |
| Bagasse | 31.67 ± 1.45 a | 0.97 ± 0.16 a | 3.29 ± 0.61 a | / | 4.25 ± 0.73 a | |
| Microbial fertilizer | 29.33 ± 1.33 a | 0.50 ± 0.14 a | 1.22 ± 0.30 a | / | 1.72 ± 0.43 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Yu, Q.; Gao, B.; Hu, M.; Chen, H.; Liang, Y.; Yi, H. Organic Amendments Enhance the Remediation Potential of Economically Important Crops in Weakly Alkaline Heavy Metal-Contaminated Bauxite Residues. Agriculture 2025, 15, 15. https://doi.org/10.3390/agriculture15010015
Zhang X, Yu Q, Gao B, Hu M, Chen H, Liang Y, Yi H. Organic Amendments Enhance the Remediation Potential of Economically Important Crops in Weakly Alkaline Heavy Metal-Contaminated Bauxite Residues. Agriculture. 2025; 15(1):15. https://doi.org/10.3390/agriculture15010015
Chicago/Turabian StyleZhang, Xingfeng, Qiankui Yu, Bo Gao, Maosheng Hu, Hongxu Chen, Yexi Liang, and Haifeng Yi. 2025. "Organic Amendments Enhance the Remediation Potential of Economically Important Crops in Weakly Alkaline Heavy Metal-Contaminated Bauxite Residues" Agriculture 15, no. 1: 15. https://doi.org/10.3390/agriculture15010015
APA StyleZhang, X., Yu, Q., Gao, B., Hu, M., Chen, H., Liang, Y., & Yi, H. (2025). Organic Amendments Enhance the Remediation Potential of Economically Important Crops in Weakly Alkaline Heavy Metal-Contaminated Bauxite Residues. Agriculture, 15(1), 15. https://doi.org/10.3390/agriculture15010015






