Research on Nondestructive Inspection of Fruits Based on Spectroscopy Techniques: Experimental Scenarios, ROI, Number of Samples, and Number of Features
Abstract
1. Introduction
2. Spectral Imaging Technique
2.1. Principles of Spectroscopy
2.2. Spectral Data Acquisition and Processing
3. Experimental Environment
3.1. Laboratory Environment
3.2. Orchard Environment
3.2.1. Berries
3.2.2. Drupes
4. Selection of Regions of Interest
4.1. Threshold Segmentation
4.2. Manual Selection
5. Selection of the Number of Samples and the Number of Spectral Features
5.1. Quantitative Analysis
5.2. Qualitative Analysis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richa, R.; Kohli, D.; Vishwakarma, D.; Mishra, A.; Kabdal, B.; Kothakota, A.; Richa, S.; Sirohi, R.; Kumar, R.; Naik, B. Citrus fruit: Classification, value addition, nutritional and medicinal values, and relation with pandemic and hidden hunger. J. Agric. Food Res. 2023, 14, 100718. [Google Scholar] [CrossRef]
- Zhao, H.; Dong, Z.; Liu, B.; Xiong, H.; Guo, C.; Lakshmanan, P.; Wang, X.; Chen, X.; Shi, X.; Zhang, F. Can citrus production in China become carbon-neutral? A historical retrospect and prospect. Agric. Ecosyst. Environ. 2023, 348, 108412. [Google Scholar] [CrossRef]
- National Bureau of Statistics Home Page. Available online: https://data.stats.gov.cn/easyquery.htm?cn=C01 (accessed on 27 May 2024).
- Çetiner, H. Citrus disease detection and classification using based on convolution deep neural network. Microprocess. Microsyst. 2022, 95, 104687. [Google Scholar] [CrossRef]
- Chakraborty, S.K.; Subeesh, A.; Dubey, K.; Jat, D.; Chandel, N.S.; Potdar, R.; Rao, N.G.; Kumar, D. Development of an optimally designed real-time automatic citrus fruit grading–sorting machine leveraging computer vision-based adaptive deep learning model. Eng. Appl. Artif. Intell. 2023, 120, 105826. [Google Scholar] [CrossRef]
- Valencia-Chamorro, S.A.; Palou, L.; Del Río, M.A.; Pérez-Gago, M.B. Antimicrobial edible films and coatings for fresh and minimally processed fruits and vegetables: A review. Crit. Rev. Food Sci. Nutr. 2011, 51, 872–900. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liang, Y.; He, L.; Kaliaperumal, K.; Tan, H.; Jiang, Y.; Zhong, B.; Zhang, J. Effects of storage time and temperature on the chemical composition and organoleptic quality of Gannan navel orange (Citrus sinensis Osbeck cv. Newhall). J. Food Meas. Charact. 2022, 16, 935–944. [Google Scholar] [CrossRef]
- Manso, M.; Carvalho, M.L. Application of spectroscopic techniques for the study of paper documents: A survey. Spectrochim. Acta Part B At. Spectrosc. 2009, 64, 482–490. [Google Scholar] [CrossRef]
- Masithoh, R.; Pahlawan, M.; Wati, R. Non-destructive determination of SSC and pH of banana using a modular Vis/NIR spectroscopy: Comparison of Partial Least Square (PLS) and Principle Component Regression (PCR). In Proceedings of the IOP Conference Series: Earth and Environmental Science, Jakarta, Indonesia, 25–26 September 2021; p. 012047. [Google Scholar]
- Yang, Y. Prediction of pear sugar content based on near infrared spectroscopy. In Proceedings of the International Conference on Agri-Photonics and Smart Agricultural Sensing Technologies (ICASAST 2022), Zhengzhou, China, 4–6 August 2022; pp. 182–188. [Google Scholar]
- Malvandi, A.; Feng, H.; Kamruzzaman, M. Application of NIR spectroscopy and multivariate analysis for Non-destructive evaluation of apple moisture content during ultrasonic drying. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 269, 120733. [Google Scholar] [CrossRef]
- Lazim, S.; Nawi, M.; Bejo, S.; Shariff, A.; Abdullah, N. Prediction and classification of soluble solid contents to determine the maturity level of watermelon using visible and shortwave near infrared spectroscopy. Int. Food Res. J. 2022, 29, 1372–1379. [Google Scholar] [CrossRef]
- Legner, R.; Voigt, M.; Servatius, C.; Klein, J.; Hambitzer, A.; Jaeger, M. A four-level maturity index for hot peppers (Capsicum annum) using non-invasive automated mobile Raman spectroscopy for on-site testing. Appl. Sci. 2021, 11, 1614. [Google Scholar] [CrossRef]
- Ar, N.H.; Purwanto, Y.A.; Budiastra, I.W. Prediction of soluble solid content, vitamin C, total acid and firmness in astringent persimmon (Diospyros kaki L.) cv. Rendeu using NIR spectroscopy. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Melbourne, Australia, 12–13 October 2019; p. 012086. [Google Scholar]
- Qi, C.; Sandroni, M.; Westergaard, J.C.; Sundmark, E.H.R.; Bagge, M.; Alexandersson, E.; Gao, J. In-field classification of the asymptomatic biotrophic phase of potato late blight based on deep learning and proximal hyperspectral imaging. Comput. Electron. Agric. 2023, 205, 107585. [Google Scholar] [CrossRef]
- Badea, M.G.; Brand, L. [17] Time-resolved fluorescence measurements. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1979; Volume 61, pp. 378–425. [Google Scholar]
- Ma, F.; Yuan, M.; Kozak, I. Multispectral imaging: Review of current applications. Surv. Ophthalmol. 2023, 68, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Fernández Pierna, J.A.; Vermeulen, P.; Eylenbosch, D.; Burger, J.; Bodson, B.; Dardenne, P.; Baeten, V. Chemometrics in NIR Hyperspectral Imaging: Theory and Applications in the Agricultural Crops and Products Sector. In Comprehensive Chemometrics, 2nd ed.; Brown, S., Tauler, R., Walczak, B., Eds.; Elsevier: Oxford, UK, 2020; pp. 361–379. [Google Scholar]
- Antony, M.M.; Sandeep, C.S.; Lim, H.-T.; Matham, M.V. High-Resolution Ultra-Spectral Imager for Advanced Imaging in Agriculture and Biomedical Applications. J. Biomed. Photonics Eng. 2023, 9, 030304. [Google Scholar] [CrossRef]
- Abdelsalam, A.M.; Sayed, M.S. Real-time defects detection system for orange citrus fruits using multi-spectral imaging. In Proceedings of the 2016 IEEE 59th International Midwest Symposium on Circuits and Systems (MWSCAS), Abu Dhabi, United Arab Emirates, 16–19 October 2016; pp. 1–4. [Google Scholar]
- Gaikwad, S.; Tidke, S. Multi-spectral imaging for fruits and vegetables. Int. J. Adv. Comput. Sci. Appl. 2022, 13, 743–760. [Google Scholar] [CrossRef]
- Sun, Y.; Xiao, H.; Tu, S.; Sun, K.; Pan, L.; Tu, K. Detecting decayed peach using a rotating hyperspectral imaging testbed. LWT 2018, 87, 326–332. [Google Scholar] [CrossRef]
- Fan, S.; Li, C.; Huang, W.; Chen, L. Detection of blueberry internal bruising over time using NIR hyperspectral reflectance imaging with optimum wavelengths. Postharvest Biol. Technol. 2017, 134, 55–66. [Google Scholar] [CrossRef]
- Munera, S.; Blasco, J.; Amigo, J.M.; Cubero, S.; Talens, P.; Aleixos, N. Use of hyperspectral transmittance imaging to evaluate the internal quality of nectarines. Biosyst. Eng. 2019, 182, 54–64. [Google Scholar] [CrossRef]
- ElMasry, G.; Sun, D.-W. Principles of Hyperspectral Imaging Technology. In Hyperspectral Imaging for Food Quality Analysis and Control; Sun, D.-W., Ed.; Academic Press: San Diego, CA, USA, 2010; Chapter 1; pp. 3–43. [Google Scholar]
- Kravets, V.; Kondrashov, P.; Stern, A. Compressive ultraspectral imaging using multiscale structured illumination. Appl. Opt. 2019, 58, F32–F39. [Google Scholar] [CrossRef] [PubMed]
- Garini, Y.; Young, I.T.; McNamara, G. Spectral imaging: Principles and applications. Cytom. Part A 2006, 69A, 735–747. [Google Scholar] [CrossRef]
- Othman, N. IR spectroscopy in qualitative and quantitative analysis. In Infrared Spectroscopy-Perspectives and Applications; IntechOpen: London, UK, 2022. [Google Scholar]
- Zhang, D.; Xu, L.; Liang, D.; Xu, C.; Jin, X.; Weng, S. Fast prediction of sugar content in Dangshan pear (Pyrus spp.) using hyperspectral imagery data. Food Anal. Methods 2018, 11, 2336–2345. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, Y.; Huang, W.; Tian, X.; Xia, Y.; Xu, L.; Fan, S. Nondestructive measurement of soluble solids content in apple using near infrared hyperspectral imaging coupled with wavelength selection algorithm. Infrared Phys. Technol. 2019, 98, 297–304. [Google Scholar] [CrossRef]
- Pu, H.; Liu, D.; Wang, L.; Sun, D.-W. Soluble solids content and pH prediction and maturity discrimination of lychee fruits using visible and near infrared hyperspectral imaging. Food Anal. Methods 2016, 9, 235–244. [Google Scholar] [CrossRef]
- Patton, M.Q. Qualitative Research & Evaluation Methods: Integrating Theory and Practice; Sage Publications: Thousand Oaks, CA, USA, 2014. [Google Scholar]
- Ma, T.; Xia, Y.; Inagaki, T.; Tsuchikawa, S. Non-destructive and fast method of mapping the distribution of the soluble solids content and pH in kiwifruit using object rotation near-infrared hyperspectral imaging approach. Postharvest Biol. Technol. 2021, 174, 111440. [Google Scholar] [CrossRef]
- Wu, S.; Li, M.; Zhang, C.; Tan, Q.; Yang, X.; Sun, X.; Pan, Z.; Deng, X.; Hu, C. Effects of phosphorus on fruit soluble sugar and citric acid accumulations in citrus. Plant Physiol. Biochem. 2021, 160, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Borba, K.R.; Spricigo, P.C.; Aykas, D.P.; Mitsuyuki, M.C.; Colnago, L.A.; Ferreira, M.D. Non-invasive quantification of vitamin C, citric acid, and sugar in ‘Valência’oranges using infrared spectroscopies. J. Food Sci. Technol. 2021, 58, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Brereton, R.G. Chemometrics: Data Analysis for the Laboratory and Chemical Plant; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Chu, X.; Huang, Y.; Yun, Y.-H.; Bian, X. Chemometric Methods in Analytical Spectroscopy Technology; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Phillips, T.R.; Heaney, C.E.; Benmoufok, E.; Li, Q.; Hua, L.; Porter, A.E.; Chung, K.F.; Pain, C.C. Multi-Output Regression with Generative Adversarial Networks (MOR-GANs). Appl. Sci. 2022, 12, 9209. [Google Scholar] [CrossRef]
- Baker, M.J.; Trevisan, J.; Bassan, P.; Bhargava, R.; Butler, H.J.; Dorling, K.M.; Fielden, P.R.; Fogarty, S.W.; Fullwood, N.J.; Heys, K.A. Using Fourier transform IR spectroscopy to analyze biological materials. Nat. Protoc. 2014, 9, 1771–1791. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.; Guo, M.; Li, C.; Chen, S.; Liu, G.; He, J.; Wan, G.; Fan, N. Detection of early bruises in jujubes based on reflectance, absorbance and Kubelka-Munk spectral data. Postharvest Biol. Technol. 2022, 185, 111810. [Google Scholar] [CrossRef]
- Khakimov, B.; Gürdeniz, G.; Engelsen, S.B. Trends in the application of chemometrics to foodomics studies. Acta Aliment. 2015, 44, 4–31. [Google Scholar] [CrossRef]
- Skov, T.; Honoré, A.H.; Jensen, H.M.; Næs, T.; Engelsen, S.B. Chemometrics in foodomics: Handling data structures from multiple analytical platforms. TrAC Trends Anal. Chem. 2014, 60, 71–79. [Google Scholar] [CrossRef]
- Li, J.; Chen, L. Comparative analysis of models for robust and accurate evaluation of soluble solids content in ‘Pinggu’ peaches by hyperspectral imaging. Comput. Electron. Agric. 2017, 142, 524–535. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, C.; Yang, H.; Jiang, H.; Li, L.; Yang, G. Non-destructive and in-site estimation of apple quality and maturity by hyperspectral imaging. Comput. Electron. Agric. 2022, 195, 106843. [Google Scholar] [CrossRef]
- Shang, M.; Xue, L.; Zhang, Y.; Liu, M.; Li, J. Full-surface defect detection of navel orange based on hyperspectral online sorting technology. J. Food Sci. 2023, 88, 2488–2495. [Google Scholar] [CrossRef]
- Li, J.; Rao, X.; Ying, Y. Detection of common defects on oranges using hyperspectral reflectance imaging. Comput. Electron. Agric. 2011, 78, 38–48. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, S.; Dong, W.; Luo, W.; Huang, Y.; Zhan, B.; Liu, X. Detection of common defects on mandarins by using visible and near infrared hyperspectral imaging. Infrared Phys. Technol. 2020, 108, 103341. [Google Scholar] [CrossRef]
- Li, P.; Su, G.; Du, G.; Jiang, L.; Dong, Y.; Shan, Y. Portable LWNIR and SWNIR spectroscopy with pattern recognition technology for accurate and nondestructive detection of hidden mold infection in citrus. Microchem. J. 2023, 193, 109203. [Google Scholar] [CrossRef]
- Lorente, D.; Escandell-Montero, P.; Cubero, S.; Gómez-Sanchís, J.; Blasco, J. Visible–NIR reflectance spectroscopy and manifold learning methods applied to the detection of fungal infections on citrus fruit. J. Food Eng. 2015, 163, 17–24. [Google Scholar] [CrossRef]
- Luo, W.; Zhang, J.; Liu, S.; Huang, H.; Zhan, B.; Fan, G.; Zhang, H. Prediction of soluble solid content in Nanfeng mandarin by combining hyperspectral imaging and effective wavelength selection. J. Food Compos. Anal. 2024, 126, 105939. [Google Scholar] [CrossRef]
- Wang, A.; Xie, L. Technology using near infrared spectroscopic and multivariate analysis to determine the soluble solids content of citrus fruit. J. Food Eng. 2014, 143, 17–24. [Google Scholar] [CrossRef]
- Wei, X.; He, J.-C.; Ye, D.-P.; Jie, D.-F. Navel orange maturity classification by multispectral indexes based on hyperspectral diffuse transmittance imaging. J. Food Qual. 2017, 2017, 1023498. [Google Scholar] [CrossRef]
- Li, P.; Li, S.; Du, G.; Jiang, L.; Liu, X.; Ding, S.; Shan, Y. A simple and nondestructive approach for the analysis of soluble solid content in citrus by using portable visible to near-infrared spectroscopy. Food Sci. Nutr. 2020, 8, 2543–2552. [Google Scholar] [CrossRef]
- Qi, H.; Shen, C.; Chen, G.; Zhang, J.; Chen, F.; Li, H.; Zhang, C. Rapid and non-destructive determination of soluble solid content of crown pear by visible/near-infrared spectroscopy with deep learning regression. J. Food Compos. Anal. 2023, 123, 105585. [Google Scholar] [CrossRef]
- Gao, S.; Xu, J.-h. Hyperspectral image information fusion-based detection of soluble solids content in red globe grapes. Comput. Electron. Agric. 2022, 196, 106822. [Google Scholar] [CrossRef]
- Moghimi, A.; Aghkhani, M.H.; Sazgarnia, A.; Sarmad, M. Vis/NIR spectroscopy and chemometrics for the prediction of soluble solids content and acidity (pH) of kiwifruit. Biosyst. Eng. 2010, 106, 295–302. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.; Xiao, H.; Gu, X.; Pan, L.; Tu, K. Hyperspectral imaging detection of decayed honey peaches based on their chlorophyll content. Food Chem. 2017, 235, 194–202. [Google Scholar] [CrossRef]
- Rong, D.; Wang, H.; Ying, Y.; Zhang, Z.; Zhang, Y. Peach variety detection using VIS-NIR spectroscopy and deep learning. Comput. Electron. Agric. 2020, 175, 105553. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Y.; Pan, L.; Abbas, A.; Jiang, Y.; Wang, X. Authentication of the geographic origin of Yangshan region peaches based on hyperspectral imaging. Postharvest Biol. Technol. 2021, 171, 111320. [Google Scholar] [CrossRef]
- Gai, Z.; Sun, L.; Bai, H.; Li, X.; Wang, J.; Bai, S. Convolutional neural network for apple bruise detection based on hyperspectral. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 279, 121432. [Google Scholar] [CrossRef]
- Rivera, N.V.; Gómez-Sanchis, J.; Chanona-Pérez, J.; Carrasco, J.J.; Millán-Giraldo, M.; Lorente, D.; Cubero, S.; Blasco, J. Early detection of mechanical damage in mango using NIR hyperspectral images and machine learning. Biosyst. Eng. 2014, 122, 91–98. [Google Scholar] [CrossRef]
- Ardila, C.E.C.; Ramirez, L.A.; Ortiz, F.A.P. Spectral analysis for the early detection of anthracnose in fruits of Sugar Mango (Mangifera indica). Comput. Electron. Agric. 2020, 173, 105357. [Google Scholar] [CrossRef]
- Munawar, A. Rapid and non-destructive detection of insect infestations on intact mango by means of near infrared spectroscopy. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Yinchuan, China, 19–21 September 2019; p. 012037. [Google Scholar]
- Chu, X.; Zhang, K.; Wei, H.; Ma, Z.; Fu, H.; Miao, P.; Jiang, H.; Liu, H. A Vis/NIR spectra-based approach for identifying bananas infected with Colletotrichum musae. Front. Plant Sci. 2023, 14, 1180203. [Google Scholar] [CrossRef]
- Jie, D.; Zhou, W.; Wei, X. Nondestructive detection of maturity of watermelon by spectral characteristic using NIR diffuse transmittance technique. Sci. Hortic. 2019, 257, 108718. [Google Scholar] [CrossRef]
- Lu, R. Multispectral imaging for predicting firmness and soluble solids content of apple fruit. Postharvest Biol. Technol. 2004, 31, 147–157. [Google Scholar] [CrossRef]
- Liu, D.; Sun, D.-W.; Zeng, X.-A. Recent advances in wavelength selection techniques for hyperspectral image processing in the food industry. Food Bioprocess Technol. 2014, 7, 307–323. [Google Scholar] [CrossRef]
- Cubero, S.; Aleixos, N.; Moltó, E.; Gómez-Sanchis, J.; Blasco, J. Advances in machine vision applications for automatic inspection and quality evaluation of fruits and vegetables. Food Bioprocess Technol. 2011, 4, 487–504. [Google Scholar] [CrossRef]
- Kumar, S.; Baghel, M.; Yadav, A.; Dhakar, M.K. Postharvest biology and technology of berries. In Postharvest Biology and Technology of Temperate Fruits; Springer: Berlin/Heidelberg, Germany, 2018; pp. 349–370. [Google Scholar]
- Benelli, A.; Cevoli, C.; Ragni, L.; Fabbri, A. In-field and non-destructive monitoring of grapes maturity by hyperspectral imaging. Biosyst. Eng. 2021, 207, 59–67. [Google Scholar] [CrossRef]
- Tsakiridis, N.L.; Samarinas, N.; Kokkas, S.; Kalopesa, E.; Tziolas, N.V.; Zalidis, G.C. In situ grape ripeness estimation via hyperspectral imaging and deep autoencoders. Comput. Electron. Agric. 2023, 212, 108098. [Google Scholar] [CrossRef]
- Gao, Z.; Shao, Y.; Xuan, G.; Wang, Y.; Liu, Y.; Han, X. Real-time hyperspectral imaging for the in-field estimation of strawberry ripeness with deep learning. Artif. Intell. Agric. 2020, 4, 31–38. [Google Scholar] [CrossRef]
- Usall, J.; Casals, C.; Sisquella, M.; Palou, L.; De Cal, A. Alternative technologies to control postharvest diseases of stone fruits. Stewart Postharvest Rev. 2015, 11, 1–6. [Google Scholar]
- Wendel, A.; Underwood, J.; Walsh, K. Maturity estimation of mangoes using hyperspectral imaging from a ground based mobile platform. Comput. Electron. Agric. 2018, 155, 298–313. [Google Scholar] [CrossRef]
- Wang, H.; Mei, M.; Li, J. Research Progress on Non-Destructive Detection of Internal Quality of Fruits with Large Size and Thick Peel: A Review. Agriculture 2023, 13, 1838. [Google Scholar] [CrossRef]
- Tian, X.; Li, J.; Wang, Q.; Fan, S.; Huang, W.; Zhao, C. A multi-region combined model for non-destructive prediction of soluble solids content in apple, based on brightness grade segmentation of hyperspectral imaging. Biosyst. Eng. 2019, 183, 110–120. [Google Scholar] [CrossRef]
- Tian, P.; Meng, Q.; Wu, Z.; Lin, J.; Huang, X.; Zhu, H.; Zhou, X.; Qiu, Z.; Huang, Y.; Li, Y. Detection of mango soluble solid content using hyperspectral imaging technology. Infrared Phys. Technol. 2023, 129, 104576. [Google Scholar] [CrossRef]
- Rungpichayapichet, P.; Nagle, M.; Yuwanbun, P.; Khuwijitjaru, P.; Mahayothee, B.; Müller, J. Prediction mapping of physicochemical properties in mango by hyperspectral imaging. Biosyst. Eng. 2017, 159, 109–120. [Google Scholar] [CrossRef]
- Liu, D.; Wang, E.; Wang, G.; Ma, G. Nondestructive determination of soluble solids content, firmness, and moisture content of “Longxiang” pears during maturation using near-infrared spectroscopy. J. Food Process. Preserv. 2022, 46, e16332. [Google Scholar] [CrossRef]
- Xu, M.; Sun, J.; Yao, K.; Cai, Q.; Shen, J.; Tian, Y.; Zhou, X. Developing deep learning based regression approaches for prediction of firmness and pH in Kyoho grape using Vis/NIR hyperspectral imaging. Infrared Phys. Technol. 2022, 120, 104003. [Google Scholar] [CrossRef]
- Cevoli, C.; Iaccheri, E.; Fabbri, A.; Ragni, L. Data fusion of FT-NIR spectroscopy and Vis/NIR hyperspectral imaging to predict quality parameters of yellow flesh “Jintao” kiwifruit. Biosyst. Eng. 2024, 237, 157–169. [Google Scholar] [CrossRef]
- Xuan, G.; Gao, C.; Shao, Y. Spectral and image analysis of hyperspectral data for internal and external quality assessment of peach fruit. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 272, 121016. [Google Scholar] [CrossRef]
- Aozora, W.D.; Tantinantrakun, A.; Thompson, A.K.; Teerachaichayut, S. Near infrared hyperspectral imaging for predicting water activity of dehydrated pineapples. Res. Mil. 2022, 12, 1127–1133. [Google Scholar]
- Singh, H.K.; Tomar, S.K.; Maurya, P.K. Thresholding Techniques applied for Segmentation of RGB and multispectral images. In Proceedings of the International Journal of Computer Applications®, Online, 7–8 April 2012, ISSN 0975-8887. [Google Scholar]
- Çetin, N.; Karaman, K.; Kavuncuoğlu, E.; Yıldırım, B.; Jahanbakhshi, A. Using hyperspectral imaging technology and machine learning algorithms for assessing internal quality parameters of apple fruits. Chemom. Intell. Lab. Syst. 2022, 230, 104650. [Google Scholar] [CrossRef]
- Li, S.; Song, Q.; Liu, Y.; Zeng, T.; Liu, S.; Jie, D.; Wei, X. Hyperspectral imaging-based detection of soluble solids content of loquat from a small sample. Postharvest Biol. Technol. 2023, 204, 112454. [Google Scholar] [CrossRef]
- Sharma, S.; Sumesh, K.; Sirisomboon, P. Hyperspectral imaging system for maturity stage classification of durian pulp using bayesian optimized machine learning algorithms. Horticulture 2021, 65, 236–242. [Google Scholar]
- Abdullah, S.L.S.; Jamil, N.; Harun, H. Intelligent segmentation of fruit images using an integrated thresholding and adaptive K-means method (TsNKM). J. Teknol. 2016, 78. [Google Scholar]
- Bhargavi, K.; Jyothi, S. A survey on threshold based segmentation technique in image processing. Int. J. Innov. Res. Dev. 2014, 3, 234–239. [Google Scholar]
- Wang, T.; Li, G.; Dai, C. Soluble Solids Content prediction for Korla fragrant pears using hyperspectral imaging and GsMIA. Infrared Phys. Technol. 2022, 123, 104119. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Q.; Huang, J.; Zhu, J.; Liu, J. Nondestructive determination of SSC in Korla fragrant pear using a portable near-infrared spectroscopy system. Infrared Phys. Technol. 2021, 116, 103785. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, P.; Niu, T.; He, D.; Wang, M.; Yang, H.; Zhao, X. Soluble solid content and firmness index assessment and maturity discrimination of Malus micromalus Makino based on near-infrared hyperspectral imaging. Food Chem. 2022, 370, 131013. [Google Scholar] [CrossRef]
- Garillos-Manliguez, C.A.; Chiang, J.Y. Multimodal deep learning and visible-light and hyperspectral imaging for fruit maturity estimation. Sensors 2021, 21, 1288. [Google Scholar] [CrossRef]
- Mishra, P.; Woltering, E.; El Harchioui, N. Improved prediction of ‘Kent’mango firmness during ripening by near-infrared spectroscopy supported by interval partial least square regression. Infrared Phys. Technol. 2020, 110, 103459. [Google Scholar] [CrossRef]
- Cihan, M.; Ceylan, M. Hyperspectral imaging-based cutaneous wound classification using neighbourhood extraction 3D convolutional neural network. Biomed. Eng. Biomed. Tech. 2023, 68, 427–435. [Google Scholar] [CrossRef]
- Riccioli, C.; Pérez-Marín, D.; Garrido-Varo, A. Optimizing spatial data reduction in hyperspectral imaging for the prediction of quality parameters in intact oranges. Postharvest Biol. Technol. 2021, 176, 111504. [Google Scholar] [CrossRef]
- Zou, H.; Hastie, T. Regularization and variable selection via the elastic net. J. R. Stat. Soc. Ser. B Stat. Methodol. 2005, 67, 301–320. [Google Scholar] [CrossRef]
- Baştanlar, Y.; Özuysal, M. Introduction to machine learning. In miRNomics: MicroRNA Biology and Computational Analysis; Springer: Berlin/Heidelberg, Germany, 2014; pp. 105–128. [Google Scholar]
- Li, L.; Li, C.; Fang, J.; Chen, X.; Qin, W.; Zhang, H.; Xu, J.; Jia, B.; Heng, W.; Jin, X. Quantitatively Determine the Iron Content in the Fruit of ‘Huangguan’ Pear Using Near-Infrared Spectroscopy. Horticulturae 2023, 9, 773. [Google Scholar] [CrossRef]
- Pratiwi, E.Z.D.; Pahlawan, M.F.; Rahmi, D.N.; Amanah, H.Z.; Masithoh, R.E. Non-destructive evaluation of soluble solid content in fruits with various skin thicknesses using visible–shortwave near-infrared spectroscopy. Open Agric. 2023, 8, 20220183. [Google Scholar] [CrossRef]
- Maniwara, P.; Nakano, K.; Ohashi, S.; Boonyakiat, D.; Seehanam, P.; Theanjumpol, P.; Poonlarp, P. Evaluation of NIRS as non-destructive test to evaluate quality traits of purple passion fruit. Sci. Hortic. 2019, 257, 108712. [Google Scholar] [CrossRef]
- Xu, S.; Lu, H.; Wang, X.; Ference, C.M.; Liang, X.; Qiu, G. Nondestructive detection of internal flavor in ‘Shatian’ pomelo fruit based on visible/near infrared spectroscopy. Hortscience 2021, 56, 1325–1330. [Google Scholar] [CrossRef]
- Serna-Escolano, V.; Giménez, M.J.; Zapata, P.J.; Cubero, S.; Blasco, J.; Munera, S. Non-destructive assessment of ‘Fino’ lemon quality through ripening using NIRS and chemometric analysis. Postharvest Biol. Technol. 2024, 212, 112870. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, M.; Agyekum, A.A.; Wu, J.; Chen, Q.; Zuo, M.; El-Seedi, H.R.; Tao, F.; Shi, J.; Ouyang, Q. Quantitative detection of apple watercore and soluble solids content by near infrared transmittance spectroscopy. J. Food Eng. 2020, 279, 109955. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, X.; Liu, Y.; Ni, X.; Wang, H.; Liu, Y. Research on hyperspectral regression method of soluble solids in green plum based on one-dimensional deep convolution network. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 303, 123151. [Google Scholar] [CrossRef]
- Yao, Y.-n.; Ma, K.; Zhu, J.; Huang, F.; Kuang, L.; Wang, X.; Li, S. Non-destructive determination of soluble solids content in intact apples using a self-made portable NIR diffuse reflectance instrument. Infrared Phys. Technol. 2023, 132, 104714. [Google Scholar] [CrossRef]
- Hu, W.; Sun, D.-W.; Blasco, J. Rapid monitoring 1-MCP-induced modulation of sugars accumulation in ripening ‘Hayward’ kiwifruit by Vis/NIR hyperspectral imaging. Postharvest Biol. Technol. 2017, 125, 168–180. [Google Scholar] [CrossRef]
- Chen, J.; Bai, T.; Zhang, N.; Zhu, L.; Zhang, X. Hyperspectral detection of sugar content for sugar-sweetened apples based on sample grouping and SPA feature selecting methods. Infrared Phys. Technol. 2022, 125, 104240. [Google Scholar] [CrossRef]
- Huang, C.; Cai, J.; Zhou, Y.; El-Seedi, H.R.; Guo, Z. Fusion models for detection of soluble solids content in mandarin by Vis/NIR transmission spectroscopy combined external factors. Infrared Phys. Technol. 2022, 124, 104233. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Y.; Li, S.; Zeng, S.; Chi, Q.; Guo, W. Calibration transfer of cross soluble solids content of different kiwifruit cultivars based on Two-stage TrAdaBoost. R2. Postharvest Biol. Technol. 2024, 210, 112783. [Google Scholar] [CrossRef]
- Shao, Y.; Ji, S.; Xuan, G.; Wang, K.; Xu, L.; Shao, J. Soluble solids content monitoring and shelf life analysis of winter jujube at different maturity stages by Vis-NIR hyperspectral imaging. Postharvest Biol. Technol. 2024, 210, 112773. [Google Scholar] [CrossRef]
- Ma, T.; Zhao, J.; Inagaki, T.; Su, Y.; Tsuchikawa, S. Rapid and nondestructive prediction of firmness, soluble solids content, and pH in kiwifruit using Vis–NIR spatially resolved spectroscopy. Postharvest Biol. Technol. 2022, 186, 111841. [Google Scholar] [CrossRef]
- Huang, F.-H.; Liu, Y.-H.; Sun, X.; Yang, H. Quality inspection of nectarine based on hyperspectral imaging technology. Syst. Sci. Control Eng. 2021, 9, 350–357. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Jiang, X.; Liu, H. Detection of the quality of juicy peach during storage by visible/near infrared spectroscopy. Vib. Spectrosc. 2020, 111, 103152. [Google Scholar] [CrossRef]
- Lu, B.; Liu, N.; Li, H.; Yang, K.; Hu, C.; Wang, X.; Li, Z.; Shen, Z.; Tang, X. Quantitative determination and characteristic wavelength selection of available nitrogen in coco-peat by NIR spectroscopy. Soil Tillage Res. 2019, 191, 266–274. [Google Scholar] [CrossRef]
- Dalponte, M.; Bruzzone, L.; Vescovo, L.; Gianelle, D. The role of spectral resolution and classifier complexity in the analysis of hyperspectral images of forest areas. Remote Sens. Environ. 2009, 113, 2345–2355. [Google Scholar] [CrossRef]
- Balabin, R.M.; Smirnov, S.V. Variable selection in near-infrared spectroscopy: Benchmarking of feature selection methods on biodiesel data. Anal. Chim. Acta 2011, 692, 63–72. [Google Scholar] [CrossRef]
- Cocchi, M.; Biancolillo, A.; Marini, F. Chemometric methods for classification and feature selection. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; Volume 82, pp. 265–299. [Google Scholar]
- Du, X.-l.; Li, X.-y.; Liu, Y.; Zhou, W.-h.; Li, J.-l. Genetic algorithm optimized non-destructive prediction on property of mechanically injured peaches during postharvest storage by portable visible/shortwave near-infrared spectroscopy. Sci. Hortic. 2019, 249, 240–249. [Google Scholar] [CrossRef]
- Lee, W.-H.; Kim, M.S.; Lee, H.; Delwiche, S.R.; Bae, H.; Kim, D.-Y.; Cho, B.-K. Hyperspectral near-infrared imaging for the detection of physical damages of pear. J. Food Eng. 2014, 130, 1–7. [Google Scholar] [CrossRef]
- Sankaran, S.; Mishra, A.; Maja, J.M.; Ehsani, R. Visible-near infrared spectroscopy for detection of Huanglongbing in citrus orchards. Comput. Electron. Agric. 2011, 77, 127–134. [Google Scholar] [CrossRef]
- Ghooshkhaneh, N.G.; Golzarian, M.R.; Mollazade, K. VIS-NIR spectroscopy for detection of citrus core rot caused by Alternaria alternata. Food Control 2023, 144, 109320. [Google Scholar] [CrossRef]
- Xie, C.; Lee, W.S. Detection of citrus black spot symptoms using spectral reflectance. Postharvest Biol. Technol. 2021, 180, 111627. [Google Scholar] [CrossRef]
- Tian, X.; Fan, S.; Huang, W.; Wang, Z.; Li, J. Detection of early decay on citrus using hyperspectral transmittance imaging technology coupled with principal component analysis and improved watershed segmentation algorithms. Postharvest Biol. Technol. 2020, 161, 111071. [Google Scholar] [CrossRef]
- Lemaître, G.; Nogueira, F.; Aridas, C.K. Imbalanced-learn: A python toolbox to tackle the curse of imbalanced datasets in machine learning. J. Mach. Learn. Res. 2017, 18, 1–5. [Google Scholar]
- Bunkhumpornpat, C.; Sinapiromsaran, K.; Lursinsap, C. DBSMOTE: Density-based synthetic minority over-sampling technique. Appl. Intell. 2012, 36, 664–684. [Google Scholar] [CrossRef]
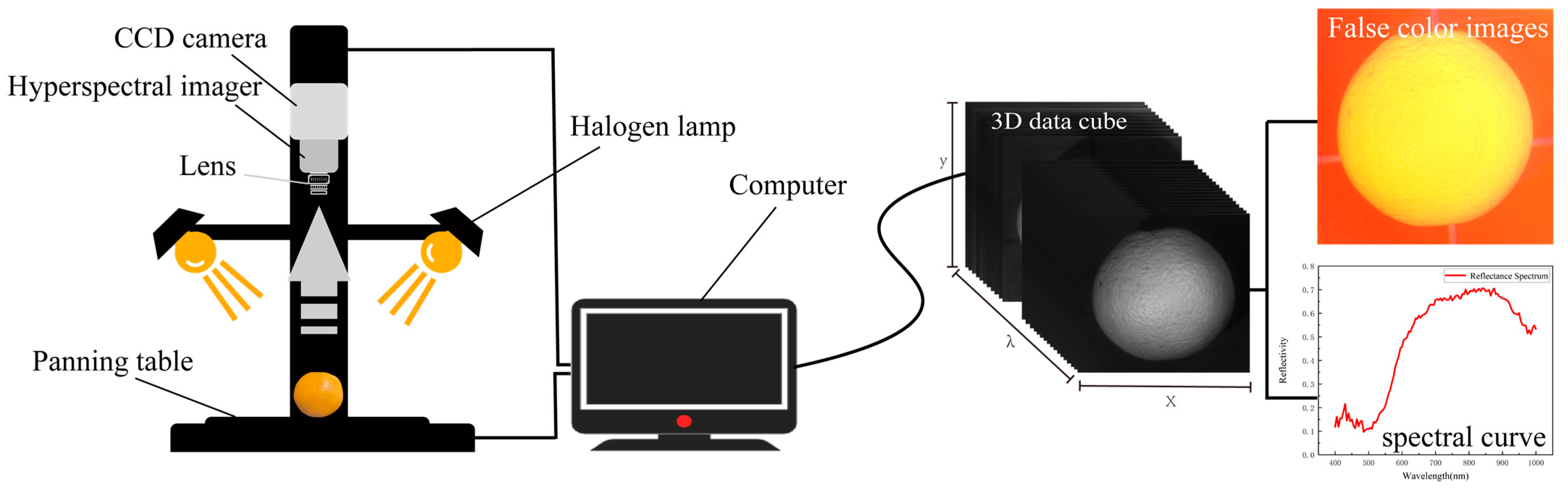
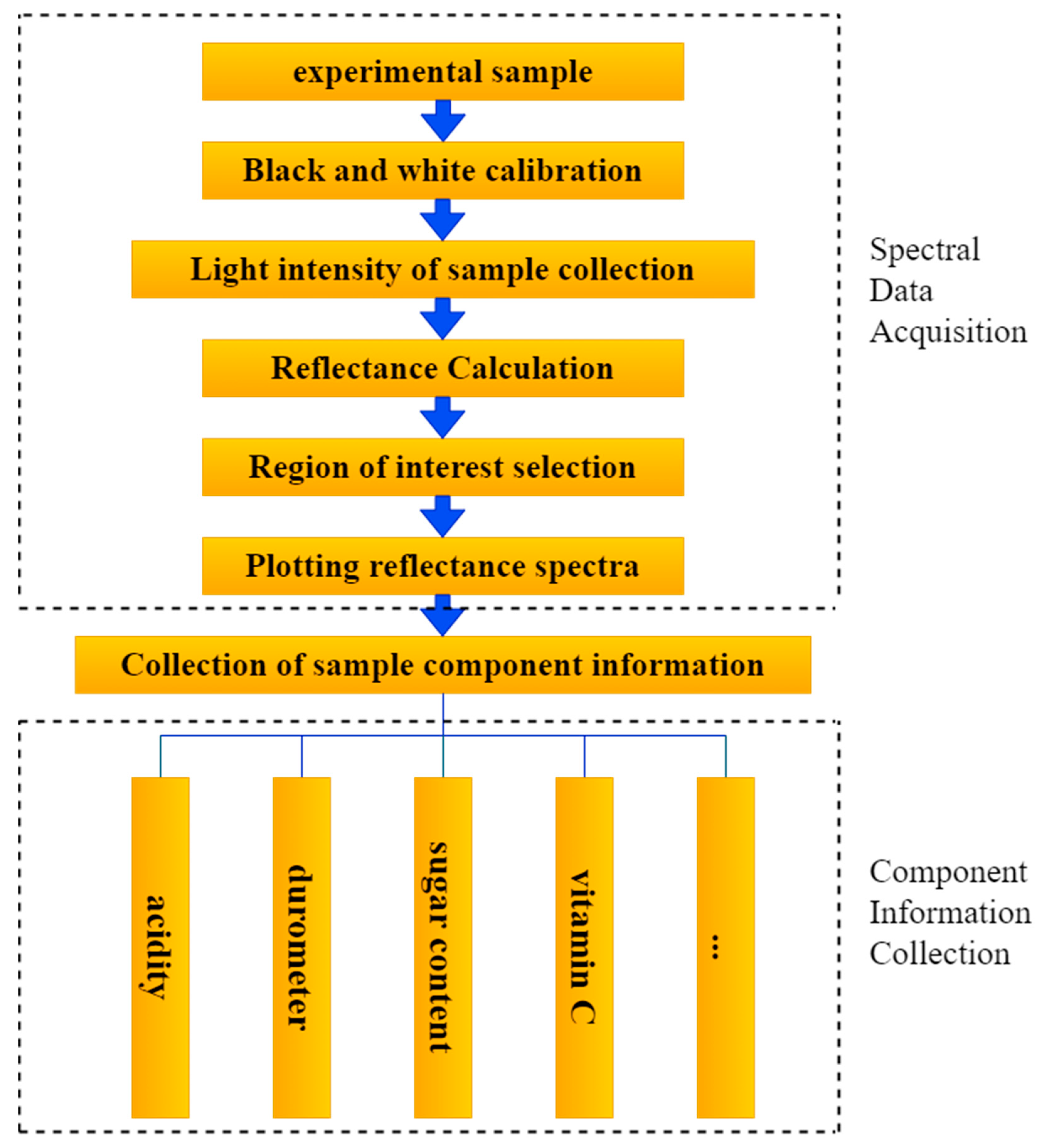



| Ref. | Fruit (No. of Samples) | Quality Parameters | Wavelength Range (nm) | Light Source | Data Analysis | Performance |
|---|---|---|---|---|---|---|
| [45] | Navel oranges (126 samples) | Defects | 975.15–2196.2 | Two 500 W halogen lamps | QCF | Accuracy = 100% |
| [46] | Oranges (460 samples) | Defects | 400–1000 | Two 150 W halogen lamps | BR + PCA | Accuracy = 93.7% |
| [47] | Citrus (606 samples) | Defects | 400–1000 | Two 100 W tungsten halogen lamps | PCA | Accuracy = 96.63% |
| [48] | Citrus (426 samples) | Mold infection | 900–1700 | 20 W halogen lamp | PLS-DA | Accuracy = 100% |
| [49] | Citrus (117 samples) | Detection of fungal infections | 1000–1700 | 10 W halogen light source | FA | Accuracy = 97.8% |
| [50] | Nanfeng mandarin (160 samples) | SSC | 380–1030 | Two 150 W halogen lamps | PLSR | = 0.956 |
| [51] | Citrus (150 samples) | SSC | 200–1100 | - | MLR | = 0.893 |
| [52] | Navel orange (150 samples) | Maturity | 390–1055 | Four 50 W halogen lamps | KNN | Accuracy = 96% |
| [53] | Citrus (105 samples) | SSC | 600–1100 | Light source of 12 V/20 W | PLS | = 0.82 |
| Ref. | Fruit (No. of Samples) | Experimental Environment | Quality Parameters | Wavelength Range (nm) | Light Source | Data Analysis | Performance |
|---|---|---|---|---|---|---|---|
| [54] | Pear (279 samples) | Laboratory | SSC | 397–1187 | 150 W halogen tungsten lamp | MLP-CNN-TCN | = 0.956 |
| [55] | Grape (360 samples) | Laboratory | SSC | 450–1000 | Four 50 W halogen lamps | PLSR | = 0.9762 |
| [56] | Kiwifruit (100 samples) | Laboratory | SSC, PH | 400–1000 | A tungsten halogen lamp 300 W | PLSR | = 0.93, 0.943 |
| [57] | Honey peach (300 samples) | Laboratory | Chlorophyll content | 400–1000 | Regulated halogen tungsten lamp (0–150 W) | PLS | = 0.904 |
| [58] | Peach (500 samples) | Laboratory | Five peach varieties | 350–820 | Tungsten halogen lamp (3.5 W) | CNN | Accuracy = 100% |
| [59] | Peach (200 samples) | Laboratory | Two peach varieties | 400–2500 | tungsten halogen lamp (400 W) | GSR | Accuracy = 99.3% |
| [60] | Apple (60 samples) | Laboratory | Bruises | 381–1037 | Four 75 W tungsten halogen lamps | CNN | Accuracy = 95.79% |
| [61] | mango | Laboratory | Mechanically induced damage | 650–1100 | Twelve halogen lamps (20 W) | KNN | Accuracy = 97.9% |
| [62] | Mango (161 samples) | Laboratory | Anthracnose | 350 –1900 | Two halogen bulbs (120 V 400 W) | LDA | Accuracy = 91–100% |
| [63] | Mango (40 samples) | Laboratory | Disease | 1000–2500 | - | PCA | Accuracy = 99% |
| [64] | Banana (330 samples) | Laboratory | Colletotrichum species | 339–1019 | - | CNN | Accuracy = 97.37% |
| [65] | Watermelon (200 samples) | Laboratory | Maturity | 200–1110 | Ten tungsten halogen lamps | Corrected-RPP (C-RPP) | CRR = 88.1% |
| Ref. | Fruit (No. of Samples) | Quality Parameters | Wavelength Range (nm) | Light Source | Data Analysis | Performance |
|---|---|---|---|---|---|---|
| [70] | Grapes (429 samples) | SSC | 400–1000 | Clear sky conditions (10:30 a.m. to 12:00 p.m.) | PLS | = 0.77 |
| [71] | Grapes (90 trees) | Sugar content | 450–1000 | Clear sky conditions (9:00 a.m. to 14:00 p.m.) | Novel autoencoder-based framework | = 0.7 |
| [72] | Strawberry (120 samples) | Maturity | 370–1015 | Overcast sky | CNN | Accuracy = 98.6% |
| Ref. | Fruit (No. of Samples) | Quality Parameters | Wavelength Range (nm) | Light Source | Data Analysis | Performance |
|---|---|---|---|---|---|---|
| [44] | Apple (100 samples) | Firmness | 500–900 | Natural light | PLSR | = 0.783 |
| [44] | Apple (100 samples) | SSC | 500–900 | Natural light | PLSR | = 0.901 |
| [44] | Apple (100 samples) | Starch pattern index | 500–900 | Natural light | PLSR | = 0.834 |
| [74] | Mango (78 samples) | Maturity | 390.9–887.4 | Clear sky conditions | CNN | = 0.64 |
| Ref. | Fruit (No. of Samples) | Quality Parameters | Wavelength Range (nm) | Selection Method (ROI) | Geometry/Value | Data Analysis | Performance |
|---|---|---|---|---|---|---|---|
| [78] | Mango (160 samples) | Firmness, TSS, TA | 450–998 | Manual framing | Square | PLS | = 0.81, 0.81, 0.5 |
| [79] | Apple (118 samples) | SSC | 450–1100 | Manual framing | Point | MNLR | = 0.953 |
| [80] | Grape (240 samples) | Firmness, pH | 400.68–1001.61 | Threshold segmentation | 0.04 | LSSVM, PLS | = 0.9232, 0.9005 |
| [81] | Kiwifruit (230 samples) | SSC, FF | 401–1000 | K-means clustering | - | PLS | = 0.914, 0.843 |
| [82] | Kiwifruit (150 samples) | SSC, Firmness | 400–1000 | Threshold segmentation | 0.1 | MLR | = 0.841, 0.826 |
| [83] | Pineapple | Water activity | 935–1720 | PCA | - | PLSR | = 0.72 |
| Ref. | Fruit (No. of Samples) | Sample Size | Quality Parameters | Wavelength Range (nm) | Number of Features | Data Analysis | Performance |
|---|---|---|---|---|---|---|---|
| [99] | Pear | 65 | Iron content | 900–1700 | 228 | PLSR | = 0.753 |
| [100] | Dragon fruit, banana | 100 | SSC | 400–1000 | 650 | PLS | = 0.59, 0.88 |
| [101] | Passion fruit | 240 | TSS, TA, pulp content (PC) | 800–1098 | 298 | PLSR | = 0.84, 0.91, 0.99 |
| [102] | Pomelo | 100 | TSS, acidity detection | 400–1700 | 1300 | PLSR | = 0.72, 0.55 |
| [103] | Lemon | 70 | TSS, TA | 950–1700 | 212 | PLSR | = 0.84, 0.72 |
| [104] | Apple | 663 | SSC | 590–1200 | 203 | PLS | = 0.9808 |
| [79] | Pear | 185 | SSC, firmness, MC | 833–2500 | 2073 | LSSVM | = 0.880, 0.826, 0.872 |
| [105] | Greengage fruit | 366 | sugar content | 400–1000 | 256 | PLSR | = 0.793 |
| [106] | Apple | 118 | SSC | 450–1100 | 650 | MNLR | = 0.953 |
| [107] | Kiwifruit | 210 | glucose | 400–1000 | 600 | MLP | = 0.934 |
| [108] | Apple | 174 | sugar content | 900–1750 | 850 | PLS | = 0.916667 |
| [109] | Citrus | 116 | SSC | 600–950 | 350 | PLS | = 0.987 |
| [110] | Kiwifruit | 800 | SSC | 950–1650 | 434 | PLSR | RPD = 4.386 |
| [111] | Winter jujube | 400 | SSC | 400–1000 | 600 | SVR | = 0.837 |
| [112] | Kiwifruit | 120 | SSC | 600–1100 | 111 | PLS | = 0.81 |
| [113] | Nectarine | 480 | SSC | 420–1000 | 580 | LSSVM | = 0.8146 |
| [114] | Peach | 150 | SSC | 350–1150 | 360 | PLS | = 0.819 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Lu, J.; Wang, Y.; Gao, J. Research on Nondestructive Inspection of Fruits Based on Spectroscopy Techniques: Experimental Scenarios, ROI, Number of Samples, and Number of Features. Agriculture 2024, 14, 977. https://doi.org/10.3390/agriculture14070977
Wang Q, Lu J, Wang Y, Gao J. Research on Nondestructive Inspection of Fruits Based on Spectroscopy Techniques: Experimental Scenarios, ROI, Number of Samples, and Number of Features. Agriculture. 2024; 14(7):977. https://doi.org/10.3390/agriculture14070977
Chicago/Turabian StyleWang, Qi, Jinzhu Lu, Yuanhong Wang, and Junfeng Gao. 2024. "Research on Nondestructive Inspection of Fruits Based on Spectroscopy Techniques: Experimental Scenarios, ROI, Number of Samples, and Number of Features" Agriculture 14, no. 7: 977. https://doi.org/10.3390/agriculture14070977
APA StyleWang, Q., Lu, J., Wang, Y., & Gao, J. (2024). Research on Nondestructive Inspection of Fruits Based on Spectroscopy Techniques: Experimental Scenarios, ROI, Number of Samples, and Number of Features. Agriculture, 14(7), 977. https://doi.org/10.3390/agriculture14070977





