Effect of Magnetic Field and UV-C Radiation on Postharvest Fruit Properties
Abstract
1. Introduction
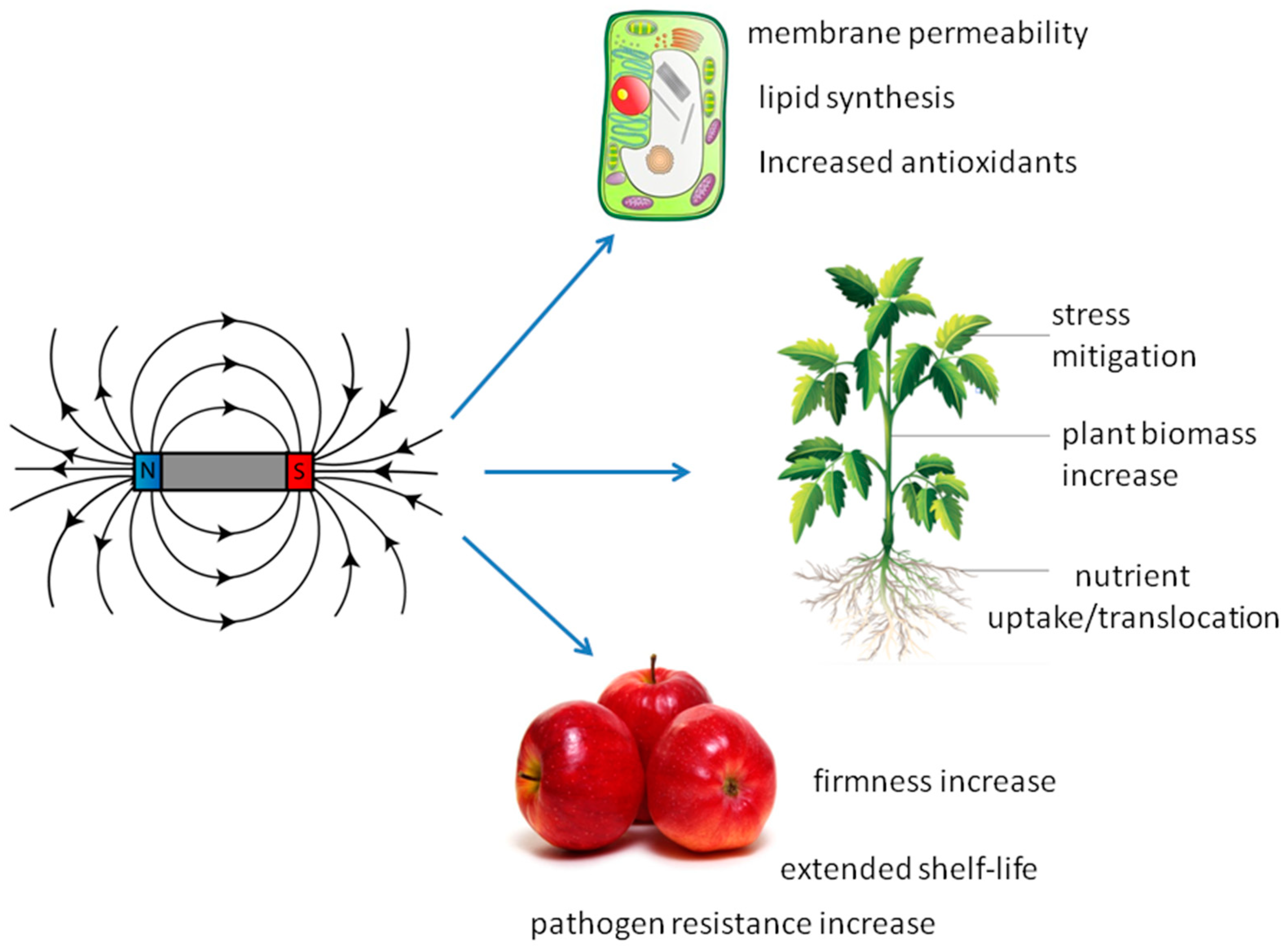
2. Magnetic Fields
2.1. Introduction to Magnetic Fields
2.2. MF and Plant Development
2.3. Plant Productivity
| Species | Dose | Parameter | References |
|---|---|---|---|
| Banana | 300 mT and 600 mT | Accelerated ripening, increased weight loss | [81] |
| Lime | 10 KHz EMF | Increase biomass of leaves, MDA, proline, and protein content Decrease H2O2 and carbohydrates, better health status reduction in phytoplasma in plant tissues (probably) | [82] |
| Passion fruit | Static MF 200 mT during germination test 14 days | increase in germination speed index, germination %, emergence speed index | [83] |
| Strawberry | PMF 5–100 mT, AMF 50–150 uT and 5–100 mT t = 5 min, 5 times | Firmness increase up to 30% (for 50–150 uT) | [84] |
| Strawberry | 0.096 T-0.384 T AMF | Increase fruit yield, N, K, Ca, Mg, Cu, Fe, Mn, Na, and Zn in plants Reduce P and S content | [49] |
| Tomato | Static MF 50, 100, 150 mT t = 1 h | Increase in plant height, shoot, and root weight, increased: number of leaves, flowers, and fruits per plant | [85] |
| Tomato | MF for 50 Hz 20, 40 and 60 mT t = 20 min | Seed germination, growth of young plant, size of fruit, stem length, weight of tomatoes, and earlier fruit setting | [73] |
| Tomato | 100 mT -170 mT SSMF | Enhance plant growth, pigments synthesis, and fruit yield | [86,87] |
2.4. Mineral Nutrition
2.5. Fruit Quality Properties
2.6. Fruit Protection
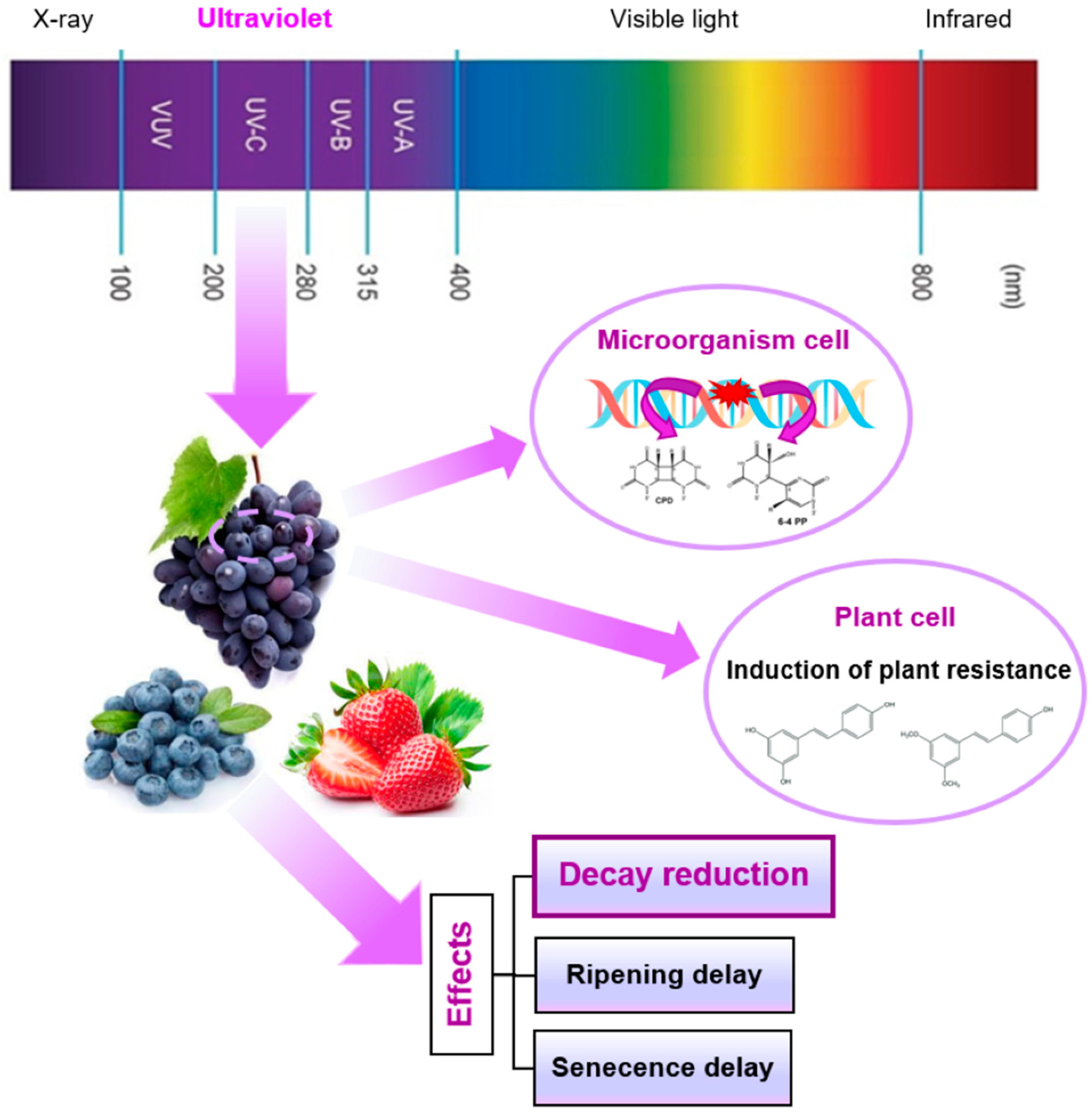
3. UV-C Radiation
3.1. Introduction to UV Radiation
3.2. Mechanism of Action of UV-C Radiation
3.3. Indirect Effects of UV Radiation
3.4. Bactericidal Effect and Application of UV-C Radiation
3.5. Factors Influencing the UV-C Efficiency
3.6. Reduction in Postharvest Diseases and Improvement of Fruit Quality as a Result of UV-C Treatment
| Fruit | MF/UV-C | Dose | Bioactive compounds | References |
|---|---|---|---|---|
| Apple | MF | 50–150 µT 10–100 Hz 5 min/week | Increase by 8% fructose and 25% glucose | [111] |
| Blackberry | MF | 50 Hz frequency magnetic field of 3 mT for up to 12 h | Increased anthocyanins (up to 6 h) Decreased after longer time | [105] |
| Blueberry | MF (pulsed) | 2 kV/cm 2–6 min | Anthocyanins and phenolic compounds increased by 10 and 25%, respectively | [92] |
| Cranberry | MF (pulsed) | 2–8 kV/cm | Lower respiration rate, no effect on SSC or color | [110] |
| Embolic fruit | MF | 430 kV/m | Increase in vitamin C content | [195] |
| Melon | MF | 2 mT 0–25 min | Reduced organic acid breakdown | [59] |
| Persimmon | MF (HEV) | 600 kV/m 30, 60, 90, 120 min | No impact on the number of total phenols | [95] |
| Blueberry | UV-C | 0.43 kJ∙m−2 | Increase in phenolic compounds | [174] |
| 2.15, 4.30 and 6.45 kJ∙m−2 | Higher antioxidant capacity | [174] | ||
| Grape | UV-C | 3.6 kJ m−2 | Increase in catechin and resveratrol content | [150] |
| Mango | UV-C | 2.46–4.93 kJ m−2 | Higher levels of total phenols and total flavonoids | [166] |
| Mandarin | UV-C | 1.5 and 3.0 kJ∙m−2 | Increase in flavonoids and total phenolic contents | [196] |
| Orange | UV-C | 0.5–3 kJ m−2 | Scopoletin and scoparone accumulation | [160] |
| Papaya | UV-C | 1.48 kJ m−2 | Increase in flavonoid content in peel | [167] |
| Peach | UV-C | 1.5–4.9 kJ m−2 | Increase in polyamines production | [197] |
| Pear | UV-C | 0.36 kJ∙m−2 | Increase in phenolic compounds | [153] |
| Sweet cherry | UV-C | 4 kJ m−2 | Increase in total phenolic content (21–36% in fruit grown under regulated deficit irrigation) | [198] |
| 1.05, 2.10, and 4.20 kJ m−2 | Increase in phenolics, flavonoids, and anthocyanins content | [170] | ||
| Strawberry | UV-C | 0.5–4 kJ m−2 | Increase in ethylene production | [162] |
| 0.43, 2.15, and 4.30 kJ m−2 | Higher antioxidant capacity | [169] | ||
| Tomato | UV-C | 4 and 8 kJ m−2 | Increase in total phenolic content | [173] |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Baky, N.A.; Amara, A.A. Recent approaches towards control of fungal diseases in plants: An updated review. J. Fungi 2021, 7, 900. [Google Scholar] [CrossRef]
- Available online: https://echa.europa.eu/regulations/biocidal-products-regulation/approval-of-active-substances/list-of-approved-active-substances (accessed on 8 February 2024).
- IFOAM. Available online: https://www.organicseurope.bio/about-us/organic-in-europe/ (accessed on 8 February 2024).
- Yemmireddy, V.; Adhikari, A.; Moreira, J. Effect of ultraviolet light treatment on microbiological safety and quality of fresh produce: An overview. Front. Nutr. 2022, 9, 871243. [Google Scholar] [CrossRef]
- Duarte-Sierra, A.; Charles, M.T.; Arul, J. UV-C hormesis. A means of controlling diseases and delaying senescence in fresh fruits and vegetables during storage. In Postharvest Pathology of Fresh Horticultural Produce; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Romanazzi, G.; Smilanick, J.L.; Feliziani, E.; Droby, S. Integrated management of postharvest gray mold on fruit crops. Post. Biol. Technol. 2016, 113, 69–76. [Google Scholar] [CrossRef]
- Guo, L.; Roknul, A.; Guo, Y.; Liu, D.; Ma, H. Germicidal efficacy of the pulsed magnetic field against pathogens and spoilage microorganisms in food processing: An overview. Food Control 2022, 136, 108496. [Google Scholar] [CrossRef]
- Darré, M.; Vicente, A.R.; Cisneros-Zevallos, L.; Artés-Hernández, F. Postharvest ultraviolet radiation in fruit and vegetables: Applications and factors modulating its efficacy on bioactive compounds and microbial growth. Foods 2022, 11, 653. [Google Scholar] [CrossRef]
- Regulation (EU) 2018/848 of the European Parliament and of the Council of 30 May 2018 on Organic Production and Labelling of Organic Products and Repealing Council Regulation (EC) No 834/2007. Available online: http://data.europa.eu/eli/reg/2018/848/oj (accessed on 8 February 2024).
- Belyavskaya, N.A.; Fomicheva, V.M.; Govorun, R.D.; Danilov, V. Structural-functional organization of the meristem cells of pea, lentil and flax roots in conditions of screening the geomagnetic field. Biophysics 1992, 37, 657–666. [Google Scholar]
- Harris, S.R.; Henbest, K.B.; Maeda, K.; Pannell, J.R.; Timmel, C.R.; Hore, P.J. Effect of magnetic fields on cryptochrome-dependent responses in Arabidopsis thaliana. J. R. Soc. Interface 2009, 6, 1193–1205. [Google Scholar] [CrossRef]
- Sarraf, M.; Kataria, S.; Taimourya, H.; Santos, L.; Menegatti, R.; Jain, M.; Ihtisham, M.; Liu, S. Magnetic field (MF) applications in plants: An overview. Plants 2020, 9, 1139. [Google Scholar] [CrossRef]
- Nyakane, N.E.; Markus, E.D.; Sedibe, M.M. The effects of magnetic fields on plants growth: A comprehensive review. Int. J. Food Eng. 2019, 5, 79–87. [Google Scholar] [CrossRef]
- Dhawi, F. Why magnetic fields are used to enhance a plant’s growth and productivity? Annu. Res. Rev. Biol. 2014, 4, 886–896. [Google Scholar] [CrossRef]
- Vashisth, A.; Nagarajan, S. Effect on germination and early growth characteristics in sunflower (Helianthus annuus) seeds exposed to static magnetic field. J. Plant Physiol. 2010, 167, 149–156. [Google Scholar] [CrossRef] [PubMed]
- McCann, J.; Dietrich, F.; Rafferty, C. The genotoxic potential of electric and magnetic fields: An update. Mutat. Res. 1998, 411, 45–86. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M. Magnetic field effect on plant growth, development, and evolution. Front. Plant Sci. 2014, 5, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Schenck, J.F. Physical interactions of static magnetic fields with living tissues. Prog. Biophys. Mol. Biol. 2005, 87, 185–204. [Google Scholar] [CrossRef] [PubMed]
- Tomska, A.; Wolny, L. Enhancement of biological wastewater treatment by magnetic field exposure. Desalination 2008, 222, 368–373. [Google Scholar] [CrossRef]
- Rosen, A.D. Mechanism of action of moderate-intensity static magnetic fields on biological systems. Cell Biochem. Biophys. 2003, 39, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Shawan, R. Electromagnetic Field; BUBT University: Dhaka, Bangladesh, 2012. [Google Scholar]
- Denegre, J.M.; Valles, J.M.; Lin, K.; Jordan, W.B.; Mowry, K.L. Cleavage planes in frog eggs are altered by strong magnetic fields. Proc. Natl. Acad. Sci. USA 1998, 95, 14729–14732. [Google Scholar] [CrossRef] [PubMed]
- Rosen, A.D. Effect of a 125 mT static magnetic field on the kinetics of voltage activated Na+ channels in GH3 cells. Bioelectromagnetics 2003, 24, 517–523. [Google Scholar] [CrossRef]
- Hughes, S.; El Haj, A.J.; Dobson, J.; Martinac, B. The influence of static magnetic fields on mechanosensitive ion channel activity in artificial liposomes. Eur. Biophys. J. 2005, 34, 461–468. [Google Scholar] [CrossRef]
- Baum, J.W.; Nauman, C.H. Influence of strong magnetic fields on genetic endpoints in Tradescantia tetrads and stamen hairs. Environ. Mutagen. 1984, 6, 49–58. [Google Scholar] [CrossRef]
- Novitskii, Y.I.; Novitskaya, G.V.; Serdyukov, Y.A. Lipid utilization in radish seedlings as affected by weak horizontal extremely low frequency magnetic field. Bioelectromagnetics 2014, 35, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Morrow, A.C.; Dunstan, R.H.; King, B.V.; Roberts, T.K. Metabolic effects of static magnetic fields on Streptococcus pyogenes. Bioelectromagnetics 2007, 28, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Huang, H.; Deng, A.; Pan, C. Effects of static magnetic fields on Escherichia coli. Micron 2009, 40, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Sun, D.W.; Han, Z. Applications of electromagnetic fields for nonthermal inactivation of microorganisms in foods: An overview. Trends Food Sci. Technol. 2017, 64, 13–22. [Google Scholar] [CrossRef]
- Sashikanth, R. Geomagnetism—A Historical Review. HAL 2020, hal-02901860. Available online: https://hal.science/hal-02901860/document (accessed on 5 March 2024).
- Buffett, B.A. Earth’s core and the geodynamo. Science 2000, 288, 2007–2012. [Google Scholar] [CrossRef] [PubMed]
- Finlay, C.C.; Maus, S.; Beggan, C.D.; Bondar, T.N.; Chambodut, A.; Chernova, T.A.; Zvereva, T.I. International geomagnetic reference field: The eleventh generation. Geophys. J. Int. 2010, 183, 1216–1230. [Google Scholar]
- Kobayashi, M.; Soda, N.; Miyo, T.; Ueda, Y. Effects of combined DC and AC magnetic fields on germination of hornwort seeds. Bioelectromagnetics 2004, 25, 552–559. [Google Scholar] [CrossRef]
- Belyavskaya, N.A. Biological effects due to weak magnetic field on plants. Adv. Space Res. 2004, 34, 1566–1574. [Google Scholar] [CrossRef]
- Aladjadjiyan, A. The use of physical methods for plant growing stimulation in Bulgaria. J. Cent. Eur. Agric. 2007, 8, 369–380. [Google Scholar]
- Wever, R. Einflußschwacherelektro-magnetischerfelder auf die Periodik des Menschen. Naturwissenschaften 1968, 55, 29–32. [Google Scholar] [CrossRef]
- Brown, F.A. Responses of the planarian, Dugesia, and the protozoan, Paramecium, to very weak horizontal magnetic fields. Biol. Bull. 1962, 123, 264–281. [Google Scholar] [CrossRef]
- Asashima, M.; Shimada, K.; Pfeiffer, C.J. Magnetic shielding induces early developmental abnormalities in the newt, Cynops pyrrhogaster. Bioelectromagnetics 1991, 12, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Galland, P.; Pazur, A. Magnetoreception in plants. J. Plant Res. 2005, 118, 371–389. [Google Scholar] [CrossRef] [PubMed]
- Thoradit, T.; Thongyoo, K.; Kamoltheptawin, K.; Tunprasert, L.; El-Esawi, M.A.; Aguida, B.; Jourdan, N.; Buddhachat, K.; Pooam, M. Cryptochrome and quantum biology: Unraveling the mysteries of plant magnetoreception. Front. Plant Sci. 2023, 14, 1266357. [Google Scholar] [CrossRef]
- Binhi, V.N. Theoretical concepts in magnetobiology. Electromagn. Biol. Med. 2001, 20, 43–58. [Google Scholar] [CrossRef]
- Lee, A.A.; Lau, J.C.S.; Hogben, H.J.; Biskup, T.; Katting, D.R.; Hore, P.J. Alternative radical pairs for cryptochrome-based magnetoreception. J. R. Soc. Interface 2014, 11, 20131063. [Google Scholar] [CrossRef] [PubMed]
- Ritz, T.; Yoshii, T.; Helfrich-Foester, C.; Ahmad, M. Cryptochrome: A photoreceptor with the properties of a magnetoreceptor? Commun. Integr. Biol. 2010, 3, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Solovyov, I.A.; Schulten, K. Reaction kinetics and mechanism of magnetic field effects in cryptochrome. J. Phys. Chem. B 2012, 116, 1089–1099. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Galland, P.; Ritz, T.; Wiltschko, P.; Wiltschko, W. Magnetic intensity affects cryptochrome-controlled response in Arabidopsis thaliana. Planta 2007, 225, 615–624. [Google Scholar] [CrossRef]
- Radhakrishnan, R. Magnetic field regulates plant functions, growth and enhances tolerance against environmental stresses. Physiol. Mol. Biol. Plants 2019, 25, 1107–1119. [Google Scholar] [CrossRef]
- Liedvogel, M.; Mouritsen, H. Cryptochromes-a potential magnetoreceptor: What do we know and what do we want to know? J. R. Soc. Interface 2010, 7, S147–S162. [Google Scholar] [CrossRef]
- Carbonell, M.V.; Martinez, E.; Amaya, J.M. Stimulation of germination in rise (Oryza savita L.) by a static magnetic field. Electro- Magnetobiol. 2000, 19, 121–128. [Google Scholar] [CrossRef]
- Eşitken, A.; Turan, M. Alternating magnetic field effects on yield and plant nutrient element composition of strawberry (Fragaria x ananassa cv. camarosa). Acta Agric. Scand. Sect. B—Soil Plant Sci. 2004, 54, 135–139. [Google Scholar] [CrossRef]
- Savostin, P.W. Magnetic growth relations in plants. Planta 1930, 12, 327. [Google Scholar]
- Murphy, J.D. The influence of magnetic fields on seed germination. Am. J. Bot. 1942, 29, 155. [Google Scholar]
- Audus, L.J. Magnetotropism: A new plant growth response. Nature 1960, 185, 132–134. [Google Scholar] [CrossRef]
- Pittman, U.J. Magnetism and plant growth. II. Effect on germination and early growth of corn and beans. Can. J. Plant Sci. 1965, 45, 549–555. [Google Scholar] [CrossRef]
- Mericle, R.P.; Mericle, L.W.; Smith, A.C.; Campbell, W.F.; Montgomery, D.J. Plant growth responses. In Biological Effects of Magnetic fields; Barnothy, M.F., Ed.; Plenum Press: New York, NY, USA, 1964; pp. 183–195. [Google Scholar]
- Pietruszewski, S.; Martínez, E. Magnetic field as a method of improving the quality of sowing material. Int. Agrophys. 2002, 29, 377–389. [Google Scholar] [CrossRef]
- Duarte Diaz, C.E.; Riquenes, J.A.; Sotolongo, B.; Portuondo, M.A.; Quintana, E.O.; Perez, R. Effects of magnetic treatment of irrigation water on the tomato crop. Hortic. Abst. 1997, 69, 494. [Google Scholar]
- Pieturszewski, S. Effect of magnetic biostimulation on wheat. Seeds Sci. Technol. 1993, 21, 621–626. [Google Scholar]
- Morar, R.; Iluga, A.; Dascalescu, L.; Munteanu, I. Electric field influence on the biological processes of seeds. In Proceedings of the International Symposium on High-Voltage Engineering, Yokohama, Japan, 23–27 August 1993; p. 286. [Google Scholar]
- Jia, J.; Wang, X.; Lv, J.; Gao, S.; Wang, G. Alternating magnetic field prior to cutting reduces wound response and maintains fruit quality of cut Cucumis melo L. cv. Hetao. Open Biotechnol. J. 2015, 9, 230–235. [Google Scholar] [CrossRef]
- Kurinobu, S.; Okazaki, Y. Dielectric constant and conductivity of one seed in the germination process. In Proceedings of the Annual Conference Record of IEEE/IAS, Orlando, FL, USA, 8–10 December 1995; pp. 1329–1334. [Google Scholar]
- Kato, R. Effects of magnetic fields on the growth of primary roots of Zea mays. Plant Cell Physiol. 1988, 29, 1215–1219. [Google Scholar]
- Mitrov, P.P.; Kroumova, Z.; Baidanova, V.D. Auxin content of corn and tomato plants following magnetic field treatments. Fiziol. No Rastjenja Rastenyata 1988, 14, 18–23. [Google Scholar]
- Reina, F.G.; Pascual, L.A.; Fundora, I.A. Influence of a stationary magnetic field on water relations in lettuce seeds. Part II: Experimental results. Bioelectromagnetics 2001, 22, 596–602. [Google Scholar] [CrossRef]
- Pieturszewski, S. Effect of alternating magnetic field on germination, growth and yield of plant seeds. Inz. Rol. 1999, 5, 209–215. [Google Scholar]
- Rochalska, M.; Orzeszko-Rywka, A. Magnetic field treatment improves seed performance. Sci. Technol. 2005, 33, 669–674. [Google Scholar] [CrossRef]
- Hussain, M.S.; Dastgeer, G.; Afzal, A.M.; Hussain, S.; Kanwar, R.R. Eco-friendly magnetic field treatment to enhance wheat yield and seed germination growth. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100299. [Google Scholar] [CrossRef]
- Liboff, A.R.; Cherng, S.; Jenrow, K.A.; Bull, A. Calmodulin dependent cyclic nucleotide phosphodiesterase activity is altered by 20 lT magnetostatic fields. Bioelectromagnetics 2003, 24, 2–38. [Google Scholar] [CrossRef]
- Nossol, B.; Buse, G.; Silny, J. Influence of weak static and 50 Hz magnetic fields on the redox activity of cytochrome-C oxidase. Bioelectromagnetics 1993, 14, 361–372. [Google Scholar] [CrossRef]
- Kato, R.; Kamada, H.; Asashma, M. Effects of high and very low magnetic fields on the growth of hairy roots of Daucus carota and Atropa belladonna. Plant Cell Physiol. 1989, 30, 605–608. [Google Scholar] [CrossRef]
- Jin, Y.; Guo, W.; Hu, X. Static magnetic field regulates Arabidopsis root growth via auxin signaling. Sci Rep. 2019, 9, 14384. [Google Scholar] [CrossRef]
- Matsuda, T.; Asou, H.; Kobayashi, M.; Yonekura, M. Influences of magnetic fields on growth and fruit production of strawberry. Acta Hortic. 1993, 348, 378–380. [Google Scholar] [CrossRef]
- Taimourya, H.; Oussible, M.; Baamal, L.; Harif, A.E.; Zaid, H.; Guedira, A.; Smouni, A. Magnetic treatment of culture medium enhance growth and minerals uptake of strawberry (Fragaria × ananassa Duch.) and tomato (Solanum lycopersicum) in Fe deficiency conditions. Int. J. Sci. Eng. Res. 2017, 8, 1414–1436. [Google Scholar]
- Jedlicka, J.; Paulen, O.; Ailer, S. Research of effect of low frequency magnetic field on germination, growth and fruiting of field tomatoes. Acta Hortic. Regiotect. 2015, 1, 1–4. [Google Scholar] [CrossRef]
- Esehaghbeygi, A.; Hajisadeghian, A.; Nasrabad, M.N. Role of a corona field application in the physicochemical properties of stored strawberries. Res. Agric. Eng. 2021, 67, 58–64. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, Y.; Yu, Y.; Li, Y.; Wei, S. Suppression of Arabidopsis flowering by near-null magnetic field is mediated by auxin. Bioelectromagnetics 2018, 39, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, S.I.; Baranskiy, P.I.; Litvinenko, L.G.; Shiyan, L.T. Barley growth in super weak magnetic field. Electron. Treat. Mater. 1977, 3, 71–73. [Google Scholar]
- Goodman, R.; Blank, M. Magnetic field stress induces expression of hsp70. Cell Stress Chaperones 1998, 3, 79–88. [Google Scholar] [CrossRef]
- Ruzic, R.; Jerman, I. Weak magnetic field decreases heat stress in cress seedlings. Electromagnetobiology 2002, 21, 69–80. [Google Scholar]
- Afzal, I.; Noor, M.A.; Bakhtavar, M.A.; Ahmad, A.; Haq, Z. Improvement of spring maize (Zea mays) performance through physical and physiological seed enhancements. Seed Sci. Technol. 2015, 43, 238–249. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Ranjitha-Kumari, B.D. Protective role of pulsed magnetic field against salt stress effects in soybean organ culture. Plant Biosyst. 2013, 147, 135–140. [Google Scholar] [CrossRef]
- Mahathaininwong, A. Effect of static magnetic field on ripening of Thai cavendish bananas. J. Phys. Conf. Ser. 2019, 1380, 012049. [Google Scholar] [CrossRef]
- Abdollahi, F.; Niknam, V.; Ghanati, F.; Masroor, F.; Noorbakhsh, S.N. Biological effects of weak electromagnetic field on healthy and infected lime (Citrus aurantifolia) trees with phytoplasma. Sci. World J. 2012. [Google Scholar] [CrossRef]
- Menegatti, R.D.; Oliveira de Oliveira, L.; Costa, A.; Braga, E.J.B.; Bianchi, V.J. Magnetic field and gibberlic acid as pre-germination treatment of passion fruit seeds. Ciênc. Agríc. 2019, 17, 5–22. [Google Scholar]
- Zaguła, G.; Puchalski, C.; Czernicka, M.; Bajcar, M.; Saletnik, B.; Woźny, M.; Szeregii, E. The magnetic field stimulation system applied on strawberry fruits. Econtechmod. Int. Q. 2017, 6, 117–122. [Google Scholar]
- Kutby, A.M.; Al-Zahrani, H.S.; Hakeem, K.R. Role of Magnetic Field and Brassinosteroids in Mitigating Salinity Stress in Tomato (Lycopersicon esculentum L.). Int. J. Eng. Res. Technol. 2020, 9, 306–319. [Google Scholar]
- Souza-Torres, E.; Porras-Leon, E.; Casate-Fernandez, R. Effects of magnetic treatment of tomato (Lycopersicon esculentum Mill) seeds on germination and seedling growth. Horic Abstr. 1999, 70, 6892. [Google Scholar]
- Souza-Torres, A.D.; Garcia, D.; Sueiro, L.; Gilart, F.; Porras, E.; Licea, L. Presowing magnetic treatments of tomato seeds increase the growth and yield of plants. Bioelectromagnetics 2006, 27, 247–257. [Google Scholar] [CrossRef]
- Savchenko, V.; Synyavskiy, O.; Dudnyk, A.; Nesvidomin, A.; Ramsh, V.; Bunko, V. The impact of a direct magnetic field on the cells. In Proceedings of the 2020 IEEE KhPI Week on Advanced Technology (KhPIWeek), Kharkiv, Ukraine, 5–10 October 2020; pp. 193–198. [Google Scholar] [CrossRef]
- Ercan, I.; Tombuloglu, H.; Alqahtani, N.; Alotaibi, B.; Bamhrez, M.; Alshumrani, R.; Ozcelik, S.; Kayed, T.S. Magnetic field effects on the magnetic properties, germination, chlorophyll fluorescence, and nutrient content of barley (Hordeum vulgare L.). Plant Phys. Biochem. 2022, 170, 36–48. [Google Scholar] [CrossRef]
- Chen, Y.P.; Li, R.; He, J.M. Magnetic field can alleviate toxicological effect induced by cadmium in mungbean seedlings. Ecotoxicology 2011, 20, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.E.; Tucker, G.A. Biochemistry of Fruit Ripening, 1st ed.; Springer: Dordrecht, The Netherlands, 1993; pp. 3–51. [Google Scholar]
- Jin, T.; Yu, Y.; Gurtler, J. Effects of pulsed electric field processing on microbial survival, quality change and nutritional characteristics of blueberries. LWT 2017, 77, 517–524. [Google Scholar] [CrossRef]
- Sun, P.; Liu, Y.; Wang, W.; Song, G.; Ren, Y. Regulation mechanism of magnetic field on pectinase and its preliminary application in postharvest sapodilla (Manilkara zapota). Food Chem. 2023, 409, 135300. [Google Scholar] [CrossRef] [PubMed]
- Leelapriya, T.; Dilip, K.S.; Sanker-Narayan, P.V. Effect of weak sinusoidal magnetic field on germination and yield of cotton (Gossypium sp.). Electromagn. Biol. Med. 2003, 22, 117–125. [Google Scholar] [CrossRef]
- Liu, C.; Chen, W.; Chang, C.; Li, P.; Lu, P.; Hsieh, C. Effect of a high voltage electrostatic field (HVEF) on the shelf life of persimmons (Diospyros kaki). LWT 2017, 75, 236–242. [Google Scholar] [CrossRef]
- Li, Z.; Yang, H.; Li, P.; Liu, J.; Wang, J.; Xu, Y. Fruit biomechanics based on anatomy: A review. Int. Agrophys. 2013, 27, 97–106. [Google Scholar] [CrossRef]
- Ng, J.K.; Schröder, R.; Sutherland, P.W.; Hallett, I.C.; Hall, M.I.; Prakash, R.; Smith, B.G.; Melton, L.D.; Johnston, J.W. Cell wall structures leading to cultivar differences in softening rates develop early during apple (Malus × domestica) fruit growth. BMC Plant Biol. 2013, 13, 183. [Google Scholar] [CrossRef]
- Szymańska-Chargot, M.; Chylińska, M.; Pieczywek, P.M.; Rösch, P.; Schmitt, M.; Popp, J.; Zdunek, A. Raman imaging of changes in polysaccharides distribution in the cell wall during apple fruit development and senescence. Planta 2016, 243, 935–945. [Google Scholar] [CrossRef]
- Volz, R.K.; Harker, F.R.; Lang, S. Firmness decline in gala apple during fruit development. J. Am. Soc. Hortic. Sci. 2003, 128, 797–802. [Google Scholar] [CrossRef]
- Cybulska, J.; Zdunek, A.; Kozioł, A. The self-assembled network and physiological degradation of pectins in carrot cell walls. Food Hydrocoll. 2015, 43, 41–50. [Google Scholar] [CrossRef]
- Puchalski, C. Methodological aspects of testing apple friction and firmness in terms of assessing their quality. Sci. J. Agric. Univ. Krakow 2001, 275, 1233–4189. [Google Scholar]
- Valentinuzzi, M. Rotational diffusion in a magnetic field and its possible magneto-biological implications. In Biological Effects of Magnetic Fields; Barnothy, M.F., Ed.; Springer: Boston, MA, USA, 1964. [Google Scholar]
- Boe, A.A.; Do, J.Y.; Salunke, D.K. Tomato ripening: Effects of light frequency, magnetic field, and chemical treatments. Econ. Bot. 1968, 22, 124–134. [Google Scholar] [CrossRef]
- Bourget, S.; Corcuff, R.; Angers, P.; Arul, J. Effect of the exposure to static magnetic field on the ripening and senescence of tomato fruits. Acta Hortic. 2012, 945, 129–134. [Google Scholar] [CrossRef]
- Răcuciu, M.; Oancea, S. Impact of 50 Hz magnetic field on the content of polyphenolic compounds from blackberries. Bulg. Chem. Commun. 2018, 50, 393–397. [Google Scholar]
- Zaguła, G.; Gorzelany, J.; Puchalski, C. Using a computer video system to examine the impact of magnetic and electromagnetic fields on quality of strawberries. Inż. Rol. 2010, 2, 293–300. [Google Scholar]
- Zaguła, G.; Puchalski, C.; Gorzelany, J. Spectroscopy method of evaluation of the influence of permanent and low-frequency magnetic fields during the increase and ripening on the balance of glucose and fructose of selected apple varieties. Inż. Rol. 2011, 9, 269–276. [Google Scholar]
- Wang, Y.; Wang, B.; Li, L. Keeping quality of tomato fruit by high electrostatic field pretreatment during storage. J. Sci. Food Agric. 2008, 88, 464–470. [Google Scholar] [CrossRef]
- Jaisue, N.; Setha, S.; Hamanaka, D.; Naradisorn, M. Impact of electric field on physicochemical properties and antioxidant activity of persimmon (Diospyros kaki L.). Eng. Agric. Environ. Food 2020, 13, 98–104. [Google Scholar] [CrossRef]
- Palanimuthu, V.; Rajkumar, P.; Orsat, V.; Gariépy, Y.; van Raghavan, V. Improving cranberry shelf-life using high voltage electric field treatment. J. Food Eng. 2009, 90, 365–371. [Google Scholar] [CrossRef]
- Zaguła, G.; Puchalski, C. Glucose-fructose changes in apples exposed to constant and slowly changing magnetic fields. Food Sci. Technol. Qual. 2013, 2, 162–172. [Google Scholar] [CrossRef]
- Yusuf, K.O.; Ogunlela, A.O. Effect of magnetically treated water on the quality of tomato. J. Sci. Eng. Technol. Katmandu 2016, 12, 29–33. [Google Scholar] [CrossRef]
- Sudarti, S.; Permatasari, E.; Ningtyias, F.W.; Mina, N.M.; Laksmiari, K. Analysis of vitamin C resistance in red grapes (Vitis vinifera) after exposure to extremely low frequency (ELF) magnetic fields intensity 700 uT and 900 uT. J. Penelit. Pendidik. IPA 2022, 8, 620–626. [Google Scholar] [CrossRef]
- Zaguła, G.; Tarapatskyy, M.; Bajcar, M.; Saletnik, B.; Puchalski, C.; Marczuk, A.; Andrejko, D.; Oszmiański, J. Near-null geomagnetic field as an innovative method of fruit storage. Processes 2020, 8, 262. [Google Scholar] [CrossRef]
- Atungulu, G.; Nishiyama, Y.; Koide, S. Respiration and climacteric patterns of apples treated with continuous and intermittent direct current electric field. J. Food Eng. 2004, 63, 1–8. [Google Scholar] [CrossRef]
- Van Loey, A.; Verachtert, B.; Hendrickx, M. Effects of high electric field pulses on enzymes. Trends Food Sci. Technol. 2002, 12, 94–102. [Google Scholar] [CrossRef]
- Novák, J.; Strašák, L.; Fojt, L.; Slaninová, I.; Vetterl, V. Effects of low-frequency magnetic fields on the viability of yeast Saccharomyces cerevisiae. Bioelectrochemistry 2007, 70, 115–121. [Google Scholar] [CrossRef]
- Resenburg, L.V.; Kruger, G.H.J.; Kruger, H. Proline accumulation as drought tolerance selection criterion: Its relationship to membrane integrity and chloroplast ultrastructure in Nicotiana tabacum L. J. Plant Physiol. 1993, 141, 188–194. [Google Scholar]
- Trebbi, G.; Borghini, F.; Lazzarato, L.; Torrigiani, P.; Calzoni, G.L.; Betti, L. Extremely low frequency weak magnetic fields enhance resistance of nn tobacco mosaic virus and elicit stress-related biochemical activities. Bioelectromagnetics 2007, 28, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Beltrán, J.A.; Barbosa-Cánovas, G.V. Review: Advantages and limitations on processing foods by UV light. Food Sci. Technol. Int. 2004, 10, 137–147. [Google Scholar] [CrossRef]
- Dai, T.; Vrahas, M.S.; Murray, C.K.; Hamblin, M.R. Ultraviolet C irradiation: An alternative antimicrobial approach to localized infections? Expert Rev. Anti-Infect. Ther. 2012, 10, 185–195. [Google Scholar] [CrossRef]
- Tchonkouang, R.D.; Lima, A.R.; Quintino, A.C.; Cristofoli, N.L.; Vieira, M.C. UV-C Light: A promising preservation technology for vegetable-based nonsolid food products. Foods 2023, 12, 3227. [Google Scholar] [CrossRef]
- Vanhaelewyn, L.; Van Der Straeten, D.; De Coninck, B.; Vandenbussche, F. Ultraviolet radiation from a plant perspective: The plant-microorganism context. Front. Plant Sci. 2020, 11, 597642. [Google Scholar] [CrossRef] [PubMed]
- Cutler, T.D.; Zimmerman, J.J. Ultraviolet irradiation and the mechanisms underlying its inactivation of infectious agents. Anim. Health Res. Rev. 2011, 12, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.P.; Häder, D.P. UV-induced DNA damage and repair: A review. Photochem. Photobiol. Sci. 2002, 1, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Banaś, A.K.; Zgłobicki, P.; Kowalska, E.; Bażant, A.; Dziga, D.; Strzałka, W. All you need is light. photorepair of UV-induced pyrimidine dimers. Genes 2020, 11, 1304. [Google Scholar] [CrossRef] [PubMed]
- Ploydaeng, M.; Rajatanavin, N.; Rattanakaemakorn, P. UV-C light: A powerful technique for inactivating microorganisms and the related side effects to the skin. Photodermatol. Photoimmunol. Photomed. 2021, 37, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Kim, S.J.; Kang, D.H. Bactericidal effect of 266 to 279 nm wavelength UVC-LEDs for inactivation of gram positive and gram negative foodborne pathogenic bacteria and yeasts. Food Res. Int. 2017, 97, 280–287. [Google Scholar] [CrossRef]
- Bintsis, T.; Litopoulou-Tzanetaki, E.; Robinson, R.K. Existing and potential applications of ultraviolet light in the food industry—A critical review. J. Sci. Food Agric. 2000, 80, 637–645. [Google Scholar] [CrossRef]
- Jildeh, Z.B.; Wagner, P.H.; Schöning, M.J. Sterilization of objects, products, and packaging surfaces and their characterization in different fields of industry: The status in 2020. Phys. Status Solidi A 2021, 218, 2000732. [Google Scholar] [CrossRef]
- Memarzadeh, F. A Review of recent evidence for utilizing ultraviolet irradiation technology to disinfect both indoor air and surfaces. Appl. Biosaf. 2021, 26, 52–56. [Google Scholar] [CrossRef]
- Tran, H.D.M.; Boivin, S.; Kodamatani, H.; Ikehata, K.; Fujioka, T. Potential of UV-B and UV-C irradiation in disinfecting microorganisms and removing N-nitrosodimethylamine and 1, 4-dioxane for potable water reuse: A review. Chemosphere 2021, 286, 131682. [Google Scholar] [CrossRef]
- Wang, C.P.; Chang, C.S.; Lin, W.C. Efficiency improvement of a flow-through water disinfection reactor using UV-C light emitting diodes. J. Water Process. Eng. 2021, 40, 101819. [Google Scholar] [CrossRef]
- Fan, X.; Huang, R.; Chen, H. Application of ultraviolet C technology for surface decontamination of fresh produce. Trends Food Sci. Technol. 2017, 70, 9–19. [Google Scholar] [CrossRef]
- Estrada-Beltrán, A.E.; Salas-Salazar, N.A.; Quintero-Ramos, A.; Parra-Quezada, R.A.; Soto-Caballero, M.C.; Rodríguez-Roque, M.J.; Chávez-Martínez, A.; Flores-Cordova, M.A. Effect of UV-C radiation and thermal treatment on volatile compounds, physicochemical, microbiological and phytochemical parameters on apple juice (Malus domestica) with raspberry (Rubus idaleus L.). Beverages 2024, 10, 7. [Google Scholar] [CrossRef]
- Sauceda-Gálvez, J.N.; Martinez-Garcia, M.; Hernández-Herrero, M.M.; Gervilla, R.; Roig-Sagués, A.X. Short Wave Ultraviolet Light (UV-C) Effectiveness in the inactivation of bacterial spores inoculated in turbid suspensions and in cloudy apple juice. Beverages 2021, 7, 11. [Google Scholar] [CrossRef]
- Gopisetty, V.V.S.; Patras, A.; Pendyala, B.; Kilonzo-Nthenge, A.; Ravi, R.; Pokharel, B.; Zhang, L.; Si, H.; Sasges, M. UV-C Irradiation as an alternative treatment technique: Study of its effect on microbial inactivation, cytotoxicity, and sensory properties in cranberry-flavored water. Innov. Food Sci. Emerg. Technol. 2019, 52, 66–74. [Google Scholar] [CrossRef]
- Pinto, E.P.; Perin, E.C.; Schott, I.B.; Düsman, E.; da Silva Rodrigues, R.; Lucchetta, L.; Manfroi, V.; Rombaldi, C.V. Phenolic compounds are dependent on cultivation conditions in face of UV-C radiation in ‘Concord’ grape juices (Vitis labrusca). LWT 2022, 154, 112681. [Google Scholar] [CrossRef]
- Gök, S.B. Effect of UV-C treatment on microbial population and bioactive compounds of orange juice using modified reactor based on dean vortex flow. J. Food 2021, 46, 634–646. [Google Scholar]
- Barut Gök, S. UV-C treatment of apple and grape juices by modified UV-C reactor based on dean vortex technology: Microbial, physicochemical and sensorial parameters evaluation. Food Bioprocess Technol. 2021, 14, 1055–1066. [Google Scholar] [CrossRef]
- Delorme, M.M.; Guimarães, J.T.; Coutinho, N.M.; Balthazar, C.F.; Rocha, R.S.; Silva, R.; Margalho, L.P.; Pimentel, T.L.; Silva, M.C.; Freitas, M.Q.; et al. Ultraviolet radiation: An interesting technology to preserve quality and safety of milk and dairy foods. Trends Food Sci. Technol. 2020, 102, 146–154. [Google Scholar] [CrossRef]
- Junqua, R.; Vinsonneau, E.; Ghidossi, R. Microbial stabilization of grape musts and wines using coiled UV-C reactor. Oeno One 2020, 54, 109–112. [Google Scholar] [CrossRef]
- Terry, L.A.; Joyce, D.C. Elicitors of induced disease resistance in postharvest horticultural crops: A brief review. Postharvest Biol. Technol. 2004, 32, 1–13. [Google Scholar] [CrossRef]
- Wenneker, M.; Joosten, N.; Luckerhoff, L. Use of (pulsed) UV-C light to control spore germination and mycelial growth of storage diseases causing fungi, and effect on control of storage rot in apples and pears. IOBC-WPRS Bull. 2013, 91, 389–393. [Google Scholar]
- Jijakli, M.H.; Lepoivre, P. State of the art and challenges of post-harvest disease management in apples. In Disease Management of Fruits and Vegetables; Mukerij, K.G., Ed.; Springer: Cham, The Netherlands, 2004; pp. 59–94. [Google Scholar]
- Stevens, C.; Wilson, C.L.; Lu, J.Y.; Khan, V.A.; Chalutz, E.; Droby, S.; Kabwe, M.K.; Haung, Z.; Adeyeye, O.; Pusey, L.P.; et al. Plant hormesis induced by ultraviolet light-C for controlling postharvest diseases of tree fruits. Crop Prot. 1996, 15, 129–134. [Google Scholar] [CrossRef]
- de Capdeville, G.; Wilson, C.L.; Beer, S.V.; Aist, J.R. Alternative disease control agents induce resistance to blue mold in harvested ‘red delicious’ apple fruit. Phytopathology 2002, 92, 900–908. [Google Scholar] [CrossRef] [PubMed]
- D’hallewin, G.; Schirra, M.; Pala, M.; Ben-Yehoshua, S. Ultraviolet C irradiation at 0.5 kJ·m−2 reduces decay without causing damage or affecting postharvest quality of Star Ruby grapefruit (C. paradisi Macf.). J. Agric. Food Chem. 2000, 48, 4571–4575. [Google Scholar] [CrossRef] [PubMed]
- Nigro, F.; Ippolito, A.; Lima, G. Use of UV-C light to reduce Botrytis storage rot of table grapes. Postharvest Biol. Technol. 1998, 13, 171–181. [Google Scholar] [CrossRef]
- Romanazzi, G.; Gabler, F.M.; Smilanick, J.L. Preharvest chitosan and postharvest UV-C irradiation treatments suppress gray mold of table grapes. Plant Dis. 2006, 90, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Kinay, P.; Yildiz, F.; Sen, F.; Yildiz, M.; Karacali, I. Integration of pre- and postharvest treatments to minimize Penicillium decay of satsuma mandarins. Postharvest Biol. Technol. 2005, 37, 31–36. [Google Scholar] [CrossRef]
- Stevens, C.; Khan, V.A.; Lu, J.Y.; Wilson, C.L.; Pusey, P.L.; Kabwe, M.K.; Igwegbe, E.C.K.; Chalutz, E.; Droby, S. The germicidal and hermetic effects of UV-C light on reducing brown rot disease and yeast microflora of peaches. Crop Prot. 1998, 17, 75–84. [Google Scholar] [CrossRef]
- Sun, T.; Ouyang, H.; Sun, P.; Zhang, W.; Wang, Y.; Cheng, S.; Chen, G. Postharvest UV-C irradiation inhibits blackhead disease by inducing disease resistance and reducing mycotoxin production in ‘Korla’fragrant pear (Pyrus sinkiangensis). Int. J. Food Microbiol. 2022, 362, 109485. [Google Scholar] [CrossRef]
- Baka, M.; Mercier, J.; Corcuff, R.; Castaigne, F.; Arul, J. Photochemical treatment to improve storability of fresh strawberries. J. Food Sci. 1999, 64, 1068–1072. [Google Scholar] [CrossRef]
- Marquenie, D.; Michiels, C.W.; Geeraerd, A.H.; Schenk, A.; Soontjen, C.; Van Impe, J.F.; Nicolaï, B.M. Using survival analysis to investigate the effect of UV-C and heat treatment on storage rot of strawberry and sweet cherry. Int. J. Food Microbiol. 2002, 73, 187–196. [Google Scholar] [CrossRef]
- Pan, J.; Vicente, A.R.; Martínez, G.A.; Chaves, A.R.; Civello, P.M. Combined use of UV-C irradiation and heat treatment to improve postharvest life of strawberry fruit. J. Sci. Food Agric. 2004, 84, 1831–1838. [Google Scholar] [CrossRef]
- Stevens, C.; Liu, J.; Khan, V.A.; Lu, J.Y.; Kabwe, M.K.; Wilson, C.L.; Igwegbe, E.C.K.; Chalutz, E.; Droby, S. The effects of low-dose ultraviolet light-C treatment on polygalacturonase activity, delay ripening and Rhizopus soft rot development of tomatoes. Crop Prot. 2004, 23, 551–554. [Google Scholar] [CrossRef]
- Luckey, T.D. Hormesis with Ionizing Radiation; CRC Press: Boca Raton, FL, USA, 1980; 222p. [Google Scholar]
- Sarig, P.; Zutkhi, Y.; Monjauze, A.; Lisker, N.; Ben-Arie, R. Phytoalexin elicitation in grape berries and their susceptibility to Rhizopus stolonifer. Physiol. Mol. Plant Pathol. 1997, 50, 337–347. [Google Scholar] [CrossRef]
- D’hallewin, G.; Schirra, M.; Manueddu, E.; Piga, A.; Ben-Yehoshua, S. Scoparone and scopoletin accumulation and ultraviolet-C induced resistance to postharvest decay in oranges as influenced by harvest date. J. Am. Soc. Hortic. Sci. 1999, 124, 702–707. [Google Scholar] [CrossRef]
- Kouassi, N.; Corcuff, R.; Arul, J.; Tweddell, R.J. Effect of storage temperature and age after harvest on the accumulation of the phytoalexin 6-methoxymellein in UV-C treated carrots. Acta Hortic. 2011, 945, 135–138. [Google Scholar] [CrossRef]
- Nigro, F.; Ippolito, A.; Lattanzio, V.; Di Venere, D.; Salerno, M. Effect of ultraviolet-c light on postharvest decay of strawberry. J. Plant Pathol. 2000, 82, 29–37. [Google Scholar]
- Pombo, M.A.; Rosli, H.G.; Martínez, G.A.; Civello, P.M. UV-C treatment affects the expression and activity of defense genes in strawberry fruit (Fragaria×ananassa, Duch.). Postharvest Biol. Technol. 2011, 59, 94–102. [Google Scholar] [CrossRef]
- Droby, S.; Chalutz, E.; Horev, B.; Cohen, L.; Gaba, V.; Wilson, C.L.; Wisniewski, M.E. Factors affecting UV-induced resistance in grapefruit against the green mould decay caused by Penicillium digitatum. Plant Pathol. 1993, 42, 418–424. [Google Scholar] [CrossRef]
- Maurer, L.H.; Bersch, A.M.; Santos, R.O.; Trindade, S.C.; Costa, E.L.; Peres, M.M.; Emanuelli, T. Postharvest UV-C irradiation stimulates the non-enzymatic and enzymatic antioxidant system of ‘Isabel’ hybrid grapes (Vitis labrusca × Vitis vinifera L.). Int. Food Res. J. 2017, 102, 738–747. [Google Scholar] [CrossRef]
- González-Aguilar, G.; Zavaleta-Gatica, R.; Tiznado-Hernández, M. Improving postharvest quality of mango ‘Haden’ by UV-C treatment. Postharvest Biol. Technol. 2007, 45, 108–116. [Google Scholar] [CrossRef]
- Rivera-Pastrana, D.M.; Gardea, A.A.; Yahia, E.M.; Martínez-Téllez, M.A.; González-Aguilar, G.A. Effect of UV-C irradiation and low temperature storage on bioactive compounds, antioxidant enzymes and radical scavenging activity of papaya fruit. J. Food Sci. Technol. 2014, 51, 3821–3829. [Google Scholar] [CrossRef][Green Version]
- El Ghaouth, A.; Wilson, C.L.; Callahan, A.M. Induction of chitinase, beta-1,3-glucanase, and phenylalanine ammonia lyase in peach fruit by UV-C treatment. Phytopathol. 2003, 93, 349–355. [Google Scholar] [CrossRef]
- Erkan, M.; Wang, S.Y.; Wang, C.Y. Effect of UV treatment on antioxidant capacity: Antioxidant enzyme activity and decay in strawberry fruit. Postharvest Biol. Technol. 2008, 48, 163–171. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, W.; Liu, J.; Liu, H.; Lv, Z.; Zhang, C.; Chen, D.; Jiao, Z. Postharvest UV-C irradiation increased the flavonoids and anthocyanins accumulation, phenylpropanoid pathway gene expression, and antioxidant activity in sweet cherries (Prunus avium L.). Postharvest Biol. Technol. 2021, 175, 111490. [Google Scholar] [CrossRef]
- Barka, E.A.; Kalantari, S.; Makhlouf, J.; Arul, J. Impact of UV-C irradiation on the cell wall-degrading enzymes during ripening of tomato (Lycopersicon esculentum L.) fruit. J. Agric. Food Chem. 2000, 48, 667–671. [Google Scholar] [CrossRef]
- Valero, A.; Begum, M.; Leong, S.L.; Hocking, A.D.; Ramos, A.J.; Sanchis, V.; Marín, S. Effect of germicidal UVC light on fungi isolated from grapes and raisins. Lett. Appl. Microbiol. 2007, 45, 238–243. [Google Scholar] [CrossRef]
- Liu, C.; Cai, L.; Lu, X.; Han, X.; Ying, T. Effect of postharvest UV-C irradiation on phenolic compound content and antioxidant activity of tomato fruit during storage. J. Integr. Agric. 2012, 11, 159–165. [Google Scholar] [CrossRef]
- Wang, C.Y.; Chen, C.T.; Wang, S.Y. Changes of flavonoid content and antioxidant capacity in blueberries after illumination with UV-C. Food Chem. 2009, 117, 426–431. [Google Scholar] [CrossRef]
- Ramos, B.; Miller, F.A.; Brandão, T.R.S.; Teixeira, P.; Silva, C.L.M. Fresh fruits and vegetables—An overview on applied methodologies to improve its quality and safety. Innov. Food Sci. Emerg. Technol. 2013, 20, 1–15. [Google Scholar] [CrossRef]
- Severo, J.; de Oliveira, I.R.; Bott, R.; Le Bourvellec, C.; Renard, C.M.G.; Page, D.; Chaves, F.C.; Rombaldi, C.V. Preharvest UV-C radiation impacts strawberry metabolite content and volatile organic compound production. LWT—Food Sci. Technol. 2017, 85, 390–393. [Google Scholar] [CrossRef]
- González-Aguilar, G.A.; Wang, C.Y.; Buta, J.G.; Krizek, D.T. Use of UV-C irradiation to prevent decay and maintain postharvest quality of ripe ‘Tommy Atkins’ mangoes. Int. J. Food Sci. Technol. 2001, 36, 767–773. [Google Scholar] [CrossRef]
- Pristijono, P.; Golding, J.B.; Bowyer, M.C. Postharvest UV-C treatment, followed by storage in a continuous low-level ethylene atmosphere, maintains the quality of ‘Kensington pride’ mango fruit stored at 20 °C. Horticulturae 2019, 5, 1. [Google Scholar] [CrossRef]
- Pristijono, P.; Bowyer, M.C.; Papoutsis, K.; Scarlett, C.J.; Vuong, Q.V.; Stathopoulos, C.E.; Golding, J.B. Improving the storage quality of Tahitian limes (Citrus latifolia) by pre-storage UV-C irradiation. J. Food Sci. Technol. 2019, 56, 1438–1444. [Google Scholar] [CrossRef]
- Birmpa, A.; Sfika, V.; Vantarakis, A. Ultraviolet light and ultrasound as non-thermal treatments for the inactivation of microorganisms in fresh ready-to-eat foods. Int. J. Food Microbiol. 2013, 167, 96–102. [Google Scholar] [CrossRef]
- Mercier, J.; Baka, M.; Reddy, B.; Corcuff, R.; Arul, J. Shortwave ultraviolet irradiation for control of decay caused by Botrytis cinerea in bell pepper: Induced resistance and germicidal effects. J. Am. Soc. Hortic. Sci. 2001, 126, 128–133. [Google Scholar] [CrossRef]
- Vicente, A.R.; Pineda, C.; Lemoine, L.; Civello, P.M.; Martinez, G.A.; Chaves, A.R. UV-C Treatments reduce decay, retain quality and alleviate chilling injury in pepper. Postharvest Biol. Technol. 2005, 35, 69–78. [Google Scholar] [CrossRef]
- Park, M.H.; Kim, J.G. Low-dose UV-C irradiation reduces the microbial population and preserves antioxidant levels in peeled garlic (Allium sativum L.) during storage. Postharvest Biol. Technol. 2015, 100, 109–112. [Google Scholar] [CrossRef]
- Allende, A.; Artés, F. UV-C Radiation as a novel technique for keeping quality of fresh processed “Lollo Rosso” lettuce. Food Res. Int. 2003, 36, 739–746. [Google Scholar] [CrossRef]
- Kim, Y.H.; Jeong, S.G.; Back, K.H.; Park, K.H.; Chung, M.S.; Kang, D.H. Effect of various conditions on Inactivation of Escherichia Coli O157:H7, Salmonella Typhimurium, and Listeria Monocytogenes in fresh-cut lettuce using ultraviolet radiation. Int. J. Food Microbiol. 2013, 166, 349–355. [Google Scholar] [CrossRef]
- Artés-Hernández, F.; Escalona, V.H.; Robles, P.A.; Martínez-Hernández, G.B.; Artés, F. Effect of UV-C radiation on quality of minimally processed spinach leaves. J. Sci. Food Agric. 2009, 89, 414–421. [Google Scholar] [CrossRef]
- Sun, T.; Xu, L.; Sun, H.; Yue, Q.; Zhai, H.; Yao, Y. VvVHP1; 2 is transcriptionally activated by VvMYBA1 and promotes anthocyanin accumulation of grape berry skins via glucose signal. Front. Plant Sci. 2017, 8, 811. [Google Scholar] [CrossRef]
- Henry-Kirk, R.A.; Plunkett, B.; Hall, M.; McGhie, T.; Allan, A.C.; Wargent, J.J.; Espley, R.V. Solar UV light regulates flavonoid metabolism in apple (Malus x domestica). Plant Cell Environ. 2018, 41, 675–688. [Google Scholar] [CrossRef]
- Assumpção, C.F.; Hermes, V.S.; Pagno, C.; Castagna, A.; Mannucci, A.; Sgherri, C.; de Oliveira Rios, A. Phenolic enrichment in apple skin following post-harvest fruit UV-B treatment. Postharvest Biol. Technol. 2018, 138, 37–45. [Google Scholar] [CrossRef]
- Josuttis, M.; Dietrich, H.; Treutter, D.; Will, F.; Linnemannstöns, L.; Krüger, E. Solar UVB response of bioactives in strawberry (Fragaria × ananassa Duch. L.): A comparison of protected and open-field cultivation. J. Agric. Food Chem. 2010, 58, 12692–12702. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Lim, S.; Lee, J.G.; Lee, E.J. VcBBX, VcMYB21, and VcR2R3MYB transcription factors are involved in UV–B-induced anthocyanin biosynthesis in the peel of harvested blueberry fruit. J. Agric. Food Chem. 2017, 65, 2066–2073. [Google Scholar] [CrossRef]
- Ruiz, V.E.; Cerioni, L.; Zampini, I.C.; Cuello, S.; Isla, M.I.; Hilal, M.; Rapisarda, V.A. UV-B radiation on lemons enhances antifungal activity of flavedo extracts against Penicillium digitatum. LWT 2017, 85, 96–103. [Google Scholar] [CrossRef]
- Jiang, Z.; Zheng, Y.; Qiu, R.; Yang, Y.; Xu, M.; Ye, Y.; Xu, M. Short UV-B exposure stimulated enzymatic and non-enzymatic antioxidants and reduced oxidative stress of cold-stored mangoes. J. Agric. Food Chem. 2015, 63, 10965–10972. [Google Scholar] [CrossRef]
- Abdipour, M.; Hosseinifarahi, M.; Naseri, N. Combination method of UV-B and UV-C prevents post-harvest decay and improves organoleptic quality of peach fruit. Sci. Hortic. 2019, 256, 108564. [Google Scholar] [CrossRef]
- Bajgai, T.; Hashinaga, F.; Isobe, S.; Vijaya, G.S.; Ngadi, M. Application of high electric field (HEF) on the shelf-life extension of emblic fruit (Phyllanthus emblica L.). J. Food Eng. 2006, 74, 308–313. [Google Scholar] [CrossRef]
- Shen, Y.; Sun, S.; Qiao, L.; Chen, J.; Liu, D.; Ye, X. Effect of UV-C treatments on phenolic compounds and antioxidant capacity of minimally processed Satsuma mandarin during refrigerated storage. Postharvest Biol. Technol. 2013, 76, 50–57. [Google Scholar] [CrossRef]
- González Aguilar, G.; Wang, C.Y.; Buta, G.J. UV-C irradiation reduces breakdown and chilling injury of peaches during cold storage. J. Sci. Food Agric. 2004, 84, 415–422. [Google Scholar] [CrossRef]
- Martínez-Hernández, G.B.; Blanco, V.; Blaya-Ros, P.J.; Torres, R.; Domingo, R.; Artés-Hernández, F. Effects of UV-C on bioactive compounds and quality changes during shelf life of sweet cherry grown under conventional or regulated deficit irrigation. Sci. Hortic. 2020, 269, 109398. [Google Scholar] [CrossRef]
- Forges, M.; Bardin, M.; Urban, L.; Aarrouf, J.; Charles, F. Impact of UV-C radiation applied during plant growth on pre-and postharvest disease sensitivity and fruit quality of strawberry. Plant Dis. 2020, 104, 3239–3247. [Google Scholar] [CrossRef]
- Syamaladevi, R.M.; Adhikari, A.; Lupien, S.L.; Dugan, F.; Bhunia, K.; Dhingra, A.; Sablani, S.S. Ultraviolet-C light inactivation of Penicillium expansum on fruit surfaces. Food Control 2015, 50, 297–303. [Google Scholar] [CrossRef]
- Butot, S.; Cantergiani, F.; Moser, M.; Jean, J.; Lima, A.; Michot, L.; Putallaz, T.; Stroheker, T.; Zuber, S. UV-C inactivation of foodborne bacterial and viral pathogens and surrogates on fresh and frozen berries. Int. J. Food Microbiol. 2018, 275, 8–16. [Google Scholar] [CrossRef]
- Ben-Yehoshua, S.; Rodov, V.; Peretz, J. Constitutive and induced resistance of citrus fruit against pathogens. ACIAR Proc. 1998, 80, 78–92. [Google Scholar]
- González-Barrio, R.; Salmenkallio-Marttila, M.; Tomás-Barberán, F.A.; Cantos, E.; Espín, J.C. Etiology of UV-C-induced browning in var. Superior white table grapes. J. Agric. Food Chem. 2005, 53, 5990–5996. [Google Scholar] [CrossRef]
- Costa, L.; Vicente, A.R.; Civello, P.M.; Chaves, A.R.; Martínez, G.A. UV-C treatment delays postharvest senescence in broccoli florets. Postharvest Biol. Technol. 2006, 39, 204–210. [Google Scholar] [CrossRef]
- Sonntag, F.; Liu, H.; Neugart, S. Nutritional and Physiological Effects of postharvest UV radiation on vegetables: A review. J. Agric. Food Chem. 2023, 71, 9951–9972. [Google Scholar] [CrossRef]
- Civello, P.M.; Vicente, A.R.; Martínez, G.A. UV-C technology to control postharvest diseases of fruits and vegetables. In Recent Advances in Alternative Postharvest Technologies to Control Fungal Diseases in Fruits and Vegetables; Troncoso-Rojas, R., Tiznado-Hernández, M.E., González-León, A., Eds.; Transworld Research Network: Trivandrum, Kerala, India, 2006; pp. 71–102. [Google Scholar]
- Bu, J.; Yu, Y.; Aisikaer, G.; Ying, T. Postharvest UV-C irradiation inhibits the production of ethylene and the activity of cell wall-degrading enzymes during softening of tomato (Lycopersicon esculentum L.) fruit. Postharvest Biol. Technol. 2013, 86, 337–345. [Google Scholar] [CrossRef]
- Pombo, M.A.; Dotto, M.C.; Martínez, G.A.; Civello, P.M. UV-C irradiation delays strawberry fruit softening and modifies the expression of genes involved in cell wall degradation. Postharvest Biol. Technol. 2009, 51, 141–148. [Google Scholar] [CrossRef]
- Dong, Q.; Manns, D.C.; Feng, G.; Yue, T.; Churey, J.J.; Worobo, R.W. Reduction of patulin in apple cider by UV radiation. J. Food Prot. 2010, 73, 69–74. [Google Scholar] [CrossRef]
- Elsinghorst, A.; Tikekar, R.V. Generation of oxidative species from ultraviolet light induced photolysis of fructose. Food Chem. 2014, 154, 276–281. [Google Scholar] [CrossRef]
- Fan, X.; Geveke, D.J. Furan formation in sugar solution and apple cider upon ultraviolet treatment. J. Agric. Food Chem. 2007, 55, 7816–7821. [Google Scholar] [CrossRef]
- Kharel, G.P.; Hasinaga, F.; Shintani, R. Effect of High Electric Fields on Some Fruits and Vegetables. J. Japan. Soc. Cold Preserv. Food 1996, 22, 17–22. [Google Scholar] [CrossRef]
- Zhang, H.; Hashinaga, F.; Zhang, H. Effect of high electric field on the quality of Satsuma mandarin fruits. J. Soc. High Technol. Agric. 1997, 9, 107–113. [Google Scholar] [CrossRef]
- Cantos, E.; Espin, J.C.; Tomas-Barberan, F.A. Postharvest stilbene-enrichment of red and white table grape varieties using UV-C irradiation pulses. J. Agric. Food Chem. 2002, 50, 6322–6329. [Google Scholar] [CrossRef]
- Pristijono, P.; Bowyer, M.C.; Scarlett, C.J.; Vuong, Q.V.; Stathopoulos, C.E.; Golding, J.B. Combined postharvest uv-c and 1-methylcyclopropene (1-mcp) treatment, followed by storage continuously in low level of ethylene atmosphere improves the quality of tahitian limes. J. Food Sci. Technol. 2018, 55, 2467–2475. [Google Scholar] [CrossRef]
- Maharaj, R.; Arul, J.; Nadeau, P. Effect of photochemical treatment in the preservation of fresh tomato (Lycopersicon esculentum cv. Capello) by delaying senescence. Postharvest Biol. Technol. 1999, 15, 13–23. [Google Scholar] [CrossRef]
- Wenneker, M.; Thomma, B.P.H.J. Latent postharvest pathogens of pome fruit and their management: From single measures to a systems intervention approach. Eur. J. Plant Pathol. 2020, 156, 663–681. [Google Scholar] [CrossRef]
- Hadjok, C.; Mittal, G.S.; Warriner, K. Inactivation of human pathogens and spoilage bacteria on the surface and internalized within fresh produce by using a combination of ultraviolet light and hydrogen peroxide. J. Appl. Microbiol. 2008, 104, 1014–1024. [Google Scholar] [CrossRef]
- Durak, M.Z.; Churey, J.J.; Worobo, R.W. Efficacy of UV, acidified sodium hypochlorite, and mild heat for decontamination of surface and infiltrated Escherichia coli O157:H7 on green onions and baby spinach. J. Food Prot. 2012, 75, 1198–1206. [Google Scholar] [CrossRef]
- Xu, F.; Wang, S.; Xu, J.; Liu, S.; Li, G. Effects of combined aqueous chlorine dioxide and UV-C on shelf-life quality of blueberries. Postharvest Biol. Technol. 2016, 117, 125–131. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Ukuku, D.; Fan, X.; Juneja, V.K. Efficacy of integrated treatment of UV light and low-dose gamma irradiation on inactivation of Escherichia coli O157:H7 and Salmonella enterica on grape tomatoes. J. Food Sci. 2013, 78, 1049–1056. [Google Scholar] [CrossRef]
- Vunnam, R.; Hussain, A.; Nair, G.; Bandla, R.; Gariepy, Y.; Donnelly, D.J.; Kubow, S.; Raghavan, G.S. Physico-chemical changes in tomato with modified atmosphere storage and UV treatment. J. Food Sci. Technol. 2014, 51, 2106–2112. [Google Scholar] [CrossRef]
- Gayan, E.; Manas, P.; Alvarez, I.; Condon, S. Mechanism of the synergistic inactivation of Escherichia coli by UV-C light at mild temperatures. Appl. Environ. Microbiol. 2013, 79, 4465–4473. [Google Scholar] [CrossRef]
- Esua, O.J.; Chin, N.L.; Yusof, Y.A.; Sukor, R. Combination of ultrasound and ultraviolet-C irradiation on kinetics of color, firmness, weight loss, and total phenolic content changes in tomatoes during storage. J. Food Process. Preserv. 2019, 43, e14161. [Google Scholar] [CrossRef]
- Esua, O.J.; Chin, N.L.; Yusof, Y.A.; Sukor, R. A Review on individual and combination technologies of UV-C radiation and ultrasound in postharvest handling of fruits and vegetables. Processes 2020, 8, 1433. [Google Scholar] [CrossRef]
- Zambrano-Zaragoza, M.L.; Quintanar-Guerrero, D.; González-Reza, R.M.; Cornejo-Villegas, M.A.; Leyva-Gómez, G.; Urbán-Morlán, Z. Effects of UV-C and edible nano-coating as a combined strategy to preserve fresh-cut cucumber. Polymers 2021, 13, 3705. [Google Scholar] [CrossRef] [PubMed]
| Fruit | Dose | Phytopathogen | References |
|---|---|---|---|
| Blueberry | 2 kV/cm 2–6 min | E. coli, Listeria and native microorganisms | [92] |
| Strawberry | 3.61, 4.56, and 5.13 kV/cm for 1 h | Botrytis cinerea | [74] |
| Fruit | Dose | Phytopathogen | References |
|---|---|---|---|
| Apple | 4.8 kJ m−2 | Alternaria 1 | [146] |
| 7.5 kJ m−2 | Colletotrichum gloeosporioides 1 | [146] | |
| 7.5 kJ m−2 | Monilinia 1 | [146] | |
| 7.5 kJ m−2 | Penicillium expansum 1 | [147] | |
| Grapefruit | 2.2 kJ m−2 | Penicillium digitatum 1 | [146] |
| 0.5 kJ m−2 | [148] | ||
| Grape | 0.12–0.25 kJ m−2 | Botrytis cinerea 1 | [149] |
| 3.6 kJ m−2 | [150] | ||
| Mandarin | 3.4 kJ m−2 | Penicillium digitatum 1 | [151] |
| Peach | 7.5 kJ m−2 | Monilinia fructicola 1 | [152] |
| Pear | 0.36 kJ∙m−2 | Alternaria alternata | [153] |
| Strawberry | 0.25–1.0 kJ m−2 | Botrytis cinerea | [154] |
| 0.05–1.5 kJ m−2 | [155] | ||
| 4.1 kJ m−2 | [156] | ||
| Tangerine | 1.3 kJ m−2 | Penicillium digitatum 1 | [146] |
| 0.84–3.6 kJ m−2 | Alternaria citri 1 | [146] | |
| 0.84–3.6 kJ m−2 | Geotrichum candidum 1 | [146] | |
| Tomato | 3.6 kJ m−2 | Rhizopus stolonifer 2 | [157] |
| Fruit | Dose | Effect | References |
|---|---|---|---|
| Persimmon | 600 kV/m 30, 60, 90, 120 min | Inhibited pectinesterase activity | [95] |
| Sapodilla | 3 mT, 0.5 h | Decreased activity of pectinase | [93] |
| Fruit | Dose | Effect | References |
|---|---|---|---|
| Grape | 0, 0.5, 1.0, 2.0, or 4.0 kJ m−2 | Increase in antioxidant enzymes activity (SOD and CAT) | [165] |
| Grapefruit | 1.6–6.4 kJ m−2 | Increase in phenylalanine ammonia-lyase and peroxidase activities | [164] |
| Mango | 2.46–4.93 kJ m−2 | Increase in phenylalanine ammonia-lyase activity Increase in lipoxygenase activity | [166] |
| Papaya | 1.48 kJ m−2 | Higher catalase (CAT) activity | [167] |
| Peach | 7.6 kJ m−2 | Induction of chitinase Induction of β-1,3-glucanase Increase in phenylalanine ammonia-lyase activity | [168] |
| Pear | 0.36 kJ∙m−2 | Increase in activities of chitinase, β-1,3-glucanase, peroxidase, superoxide dismutase, catalase, ascorbate peroxidase, and phenylalanine ammonia-lyase Decrease in activities of lipoxygenase and polyphenol oxidase | [153] |
| Strawberry | 0.5–4 kJ m−2 | Increase in phenylalanine ammonia-lyase activity | [162] |
| 0.43, 2.15, and 4.30 kJ m−2 | Increase in activities of glutathione peroxidase, glutathione reductase, superoxide dismutase, ascorbate peroxidase, guaiacol peroxidase, monodehydroascorbate reductase, and dehydroascorbate reductase | [169] | |
| Sweet cherry | 1.05, 2.10, and 4.20 kJ m−2 | Increase in activities of phenylalanine ammonia-lyase, cinnamate-4-hydroxylase, and 4-coumarate-CoA ligase | [170] |
| Tomato | 3.6 kJ m−2 | Reduction in cell wall-degrading enzyme activity | [171] |
| Fruit | MF/UV-C | Dose | Effects | References |
|---|---|---|---|---|
| Apple | MF | 200 mT 5 × 30 min | Change in firmness depending on cultivar (Melrose increase Šampion decrease, Cortland no change) | [101] |
| Banana | MF (HEV) | 430 kV/m | Respiration rate suppression in the pre-climacteric period | [212] |
| Blueberry | MF | 2 kV/cm 2–6 min | Increased softening, improved safety, quality, and nutritional value | [92] |
| Mandarin | MF (HEV) | 105 kV/m for 1 h | Delayed chlorophyll degradation | [213] |
| Pear | MF (HEV) | 430 kV/m | Respiration rate suppression in the pre-climacteric period | [212] |
| Persimmon | MF (HEV) | 600 kV/m 30, 60, 90, 120 min | Delay in weight loss, decreasing hardiness rate | [109] |
| Plum | MF (HEV) | 430 kV/m | Respiration rate suppression in the pre-climacteric period | [212] |
| Strawberry | MF (Alternate) | AMF for 50–150 uT | Increase in firmness up to 30% | [106] |
| Grape | UV-C | Delayed ripening and senescence | [214] | |
| Lime | UV-C | 7.2 kJ m−2 | Delayed ripening and senescence | [215] |
| Mandarin | UV-C | 3.4 kJ m−2 | Burning and browning on the fruit surface | [151] |
| Mango | UV-C | 4.0, 8.3, and 11.7 kJ∙m−2 | Delayed in ripening, reduced endogenous ethylene production, suppressed respiration rate, and lowered chlorophyll content | [178] |
| Oranges | UV-C | 0.5–3 kJ m−2 | Delayed ripening and senescence | [160] |
| Peach | UV-C | 1.5–4.9 kJ m−2 | Reduced breakdown and chilling injury | [197] |
| 0.72 kJ m−2 | Reduced weight loss, decay, TSS (total soluble solids) of fruits, improved physicochemical and sensory properties during storage | [194] | ||
| Pear | UV-C | 0.36 kJ∙m−2 | Reduction in accumulation of mycotoxins released by Alternaria alternata | [153] |
| Strawberry | UV-C | 0.25–1.0 kJ m−2 | Lower respiration rate, delayed softening, higher anthocyanin amount | [154] |
| UV-C | 4.1 kJ m−2 | Delayed softening and anthocyanin accumulation | [156] | |
| Tomato | MF (HEF) | 2 kV cm−1 | Delayed softening, respiration and color change | [108] |
| UV-C | 3.6 kJ m−2 | Delayed senescence | [216] | |
| Delayed fruit softening | [171] |
| Criterion | MF | UV-C | |
|---|---|---|---|
| Advantages | Effectiveness of microbial inactivation | Considerable for pulsed MF or at high MF induction level | Considerable on fruit surface |
| Toxicity/Risk of formation of toxic by-products | None | Non-toxic/no residues | |
| Cost | Low | Low | |
| Effect on the nutritional quality and organoleptic parameters | Minimal (sugar changes) | Minimal | |
| Invasiveness of the method | Non-invasive | Non-invasive | |
| Energy consumption | Low to medium | Low | |
| Limitations | Penetration capacity | Excellent | Low |
| Irregularities or wounds on the fruit surface | None | Low effectiveness |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gąstoł, M.; Błaszczyk, U. Effect of Magnetic Field and UV-C Radiation on Postharvest Fruit Properties. Agriculture 2024, 14, 1167. https://doi.org/10.3390/agriculture14071167
Gąstoł M, Błaszczyk U. Effect of Magnetic Field and UV-C Radiation on Postharvest Fruit Properties. Agriculture. 2024; 14(7):1167. https://doi.org/10.3390/agriculture14071167
Chicago/Turabian StyleGąstoł, Maciej, and Urszula Błaszczyk. 2024. "Effect of Magnetic Field and UV-C Radiation on Postharvest Fruit Properties" Agriculture 14, no. 7: 1167. https://doi.org/10.3390/agriculture14071167
APA StyleGąstoł, M., & Błaszczyk, U. (2024). Effect of Magnetic Field and UV-C Radiation on Postharvest Fruit Properties. Agriculture, 14(7), 1167. https://doi.org/10.3390/agriculture14071167






