The Potential of Grape Polyphenols Additive in Pig Nutrition: Chemical Structure, Bioavailability and Their Effect on Intestinal Health of Pigs
Abstract
1. Introduction
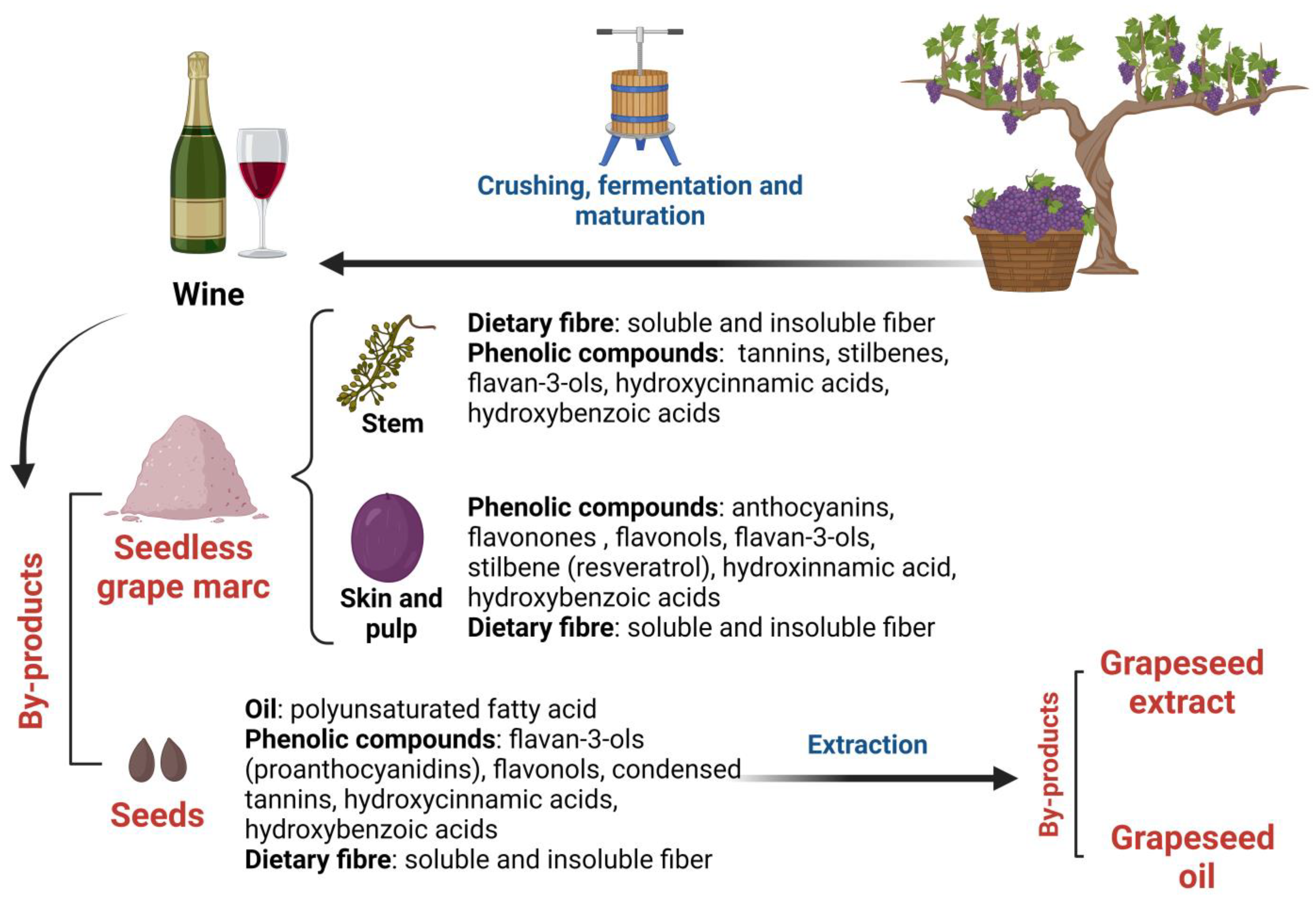
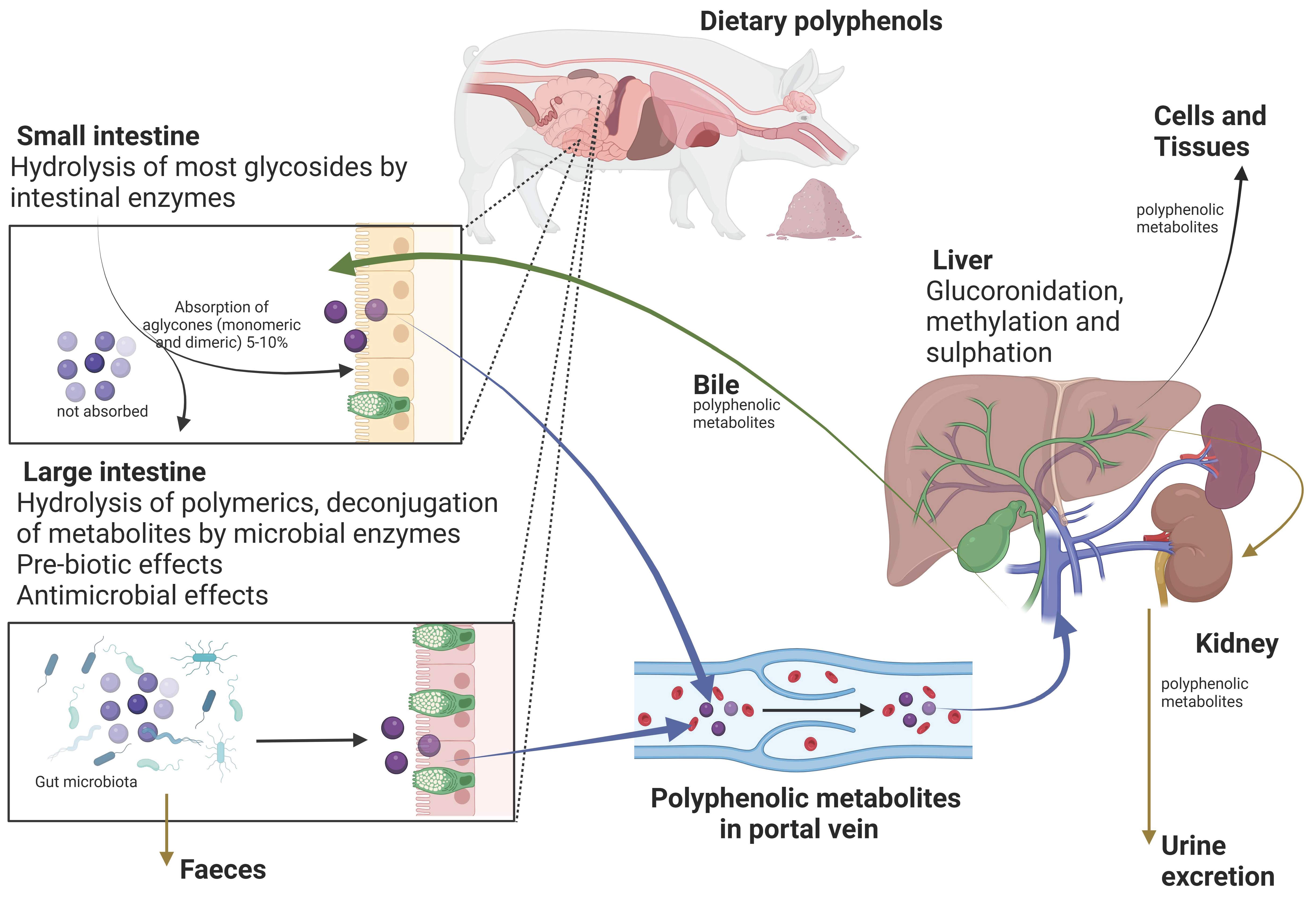
2. Grape Polyphenols: Their Chemical Structure and Bioavailability in the Gut
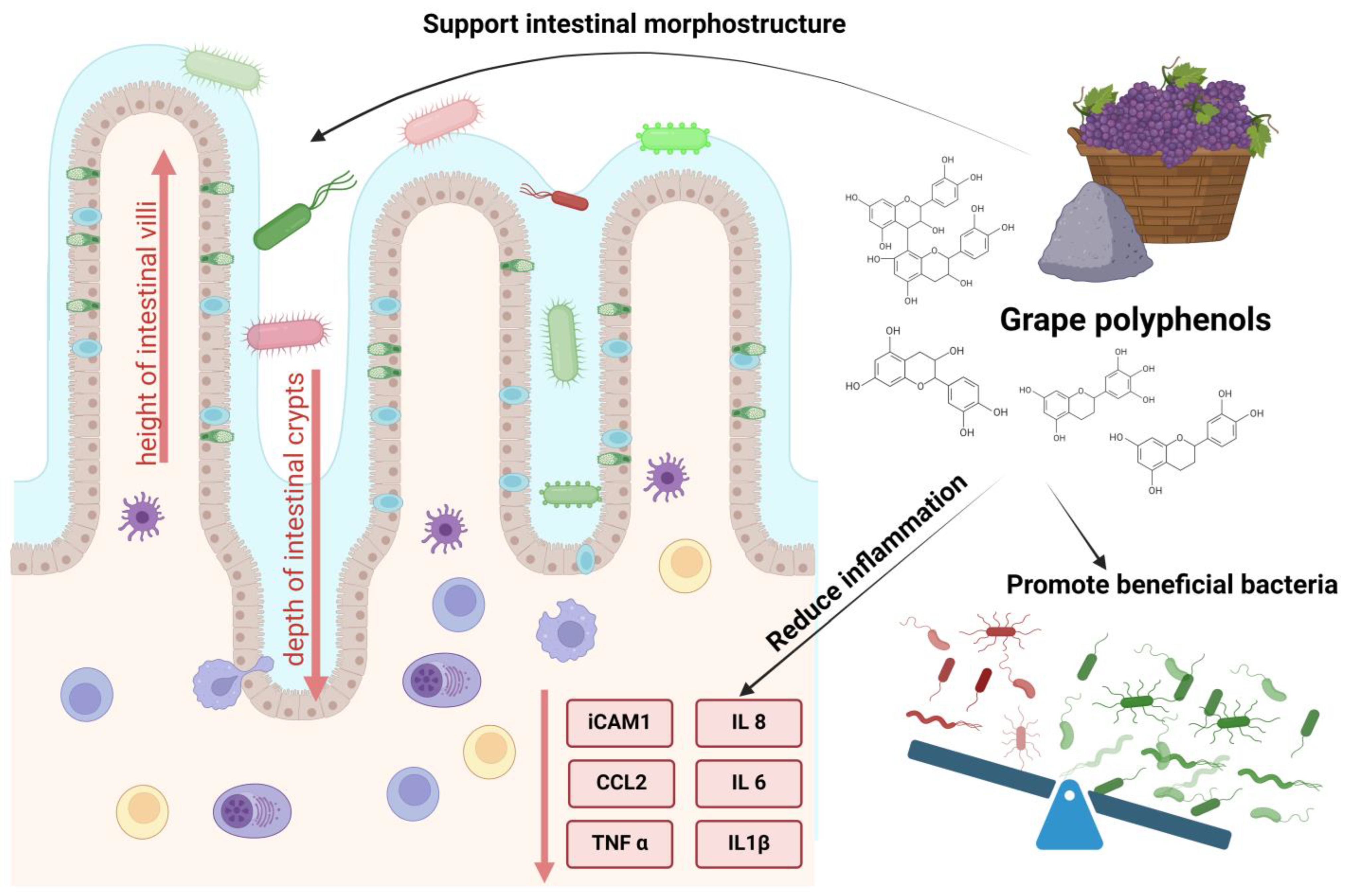
3. Antioxidant and Anti-Inflammatory Effects of Grape Polyphenols in Pig Feed
| Grape By-Product | Dose | Effect | References |
|---|---|---|---|
| Fermented grape pomace | 3% | ADFI, final bodyweight and ADG were not affected. FCR decreased | [248] |
| Grapeseed cake | 5% | ADG and ADFI were not affected Elevated plasma IgA levels and TBARS were significantly reduced | [242] |
| Complex polyphenpol extracts, including grape seeds | 1% | Reduced the level of plasma MDA | [246] |
| Grapeseed extract (procyanidins) | 0.04% | ADG increased and FCR decreased Increased expression of CAT, SOD and GSH-Px genes associated with antioxidant activity in the liver and could reduce MDA levels in muscle tissue, liver and serum | [39] |
| Grape pomace | 5% | Higher jejunal villus height and villus height/crypt depth ratio ADG, ADFI and FCR were not affected | [39] |
| Grapeseed extract (procyanidins) | 250 mg/kg | Improving the barrier function and morphology of the intestinal mucosa Enhanced the biodiversity of the gut ecosystem | [249] |
| Grapeseed and grape marc extract | 1% | Increased small intestine villus height/crypt depth ratio Gain/feed ratio improved Duodenal mucosal inflammation inhibition | [234] |
| Grape pomace | 9% | Increased ADG and final body weight Enhancement of antioxidant mechanisms and prevention of oxidative stress damage to lipids and proteins Enhances intestinal barrier function and health | [250] |
| Resveratrol | 0,2% | Antimicrobial effect: E. coli and Salmonella Bacteria growth promoting activity: Lactobacillus spp. | [251] |
| Grapeseed extract | 1% | Reducing E. coli-induced diarrhoea in weaned pigs | [252] |
| Grape seeds | 8% | There has been an increase in Bacteroidetes phylum and a significant decrease in Firmicutes phylum | [253] |
| Grapeseed extract | 1% | Microbiome ecological shift | [254] |
| Grapeseed procyanidins | 0.5, 1, and 1.5% | No significant effect on growth performance, increased antioxidant capacity, improved humoral and cellular immune responses, reduced incidence of diarrhoea | [15] |
| Grape seeds and grape marc | 1% | Modifies intestinal microbiota and reduces inflammation | [235] |
4. The Antimicrobial and Prebiotic Effects of Grape Polyphenols in the Intestine of Pigs
5. The Effects of Grape Polyphenols on the Production of Pigs
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Henchion, M.; Moloney, A.P.; Hyland, J.; Zimmermann, J.; McCarthy, S. Review: Trends for Meat, Milk and Egg Consumption for the next Decades and the Role Played by Livestock Systems in the Global Production of Proteins. Animal 2021, 15, 100287. [Google Scholar] [CrossRef]
- Cheng, Y.; Song, M.; Zhu, Q.; Azad, M.A.K.; Gao, Q.; Kong, X. Dietary Betaine Addition Alters Carcass Traits, Meat Quality, and Nitrogen Metabolism of Bama Mini-Pigs. Front. Nutr. 2021, 8, 728477. [Google Scholar] [CrossRef]
- Matarneh, S.K.; Silva, S.L.; Gerrard, D.E. New Insights in Muscle Biology That Alter Meat Quality. Annu. Rev. Anim. Biosci. 2021, 9, 355–377. [Google Scholar] [CrossRef] [PubMed]
- Erinle, T.J.; Adewole, D.I. Fruit Pomaces—Their Nutrient and Bioactive Components, Effects on Growth and Health of Poultry Species, and Possible Optimization Techniques. Anim. Nutr. 2022, 9, 357–377. [Google Scholar] [CrossRef] [PubMed]
- Reckmann, K.; Blank, R.; Traulsen, I.; Krieter, J. Comparative Life Cycle Assessment (LCA) of Pork Using Different Protein Sources in Pig Feed. Arch. Anim. Breed. 2016, 59, 27–36. [Google Scholar] [CrossRef]
- Altmann, B.; Neumann, C.; Velten, S.; Liebert, F.; Mörlein, D. Meat Quality Derived from High Inclusion of a Micro-Alga or Insect Meal as an Alternative Protein Source in Poultry Diets: A Pilot Study. Foods 2018, 7, 34. [Google Scholar] [CrossRef]
- Spigno, G.; Marinoni, L.; Garrido, G.D. State of the Art in Grape Processing By-Products. In Handbook of Grape Processing By-Products; Galanakis, C.M., Ed.; Academic Press: New York, NY, USA, 2017; pp. 1–27. ISBN 978-0-12-809870-7. [Google Scholar] [CrossRef]
- Shi, J.; Yu, J.; Pohorly, J.E.; Kakuda, Y. Polyphenolics in grape seeds—Biochemistry and functionality. J. Med. Food 2003, 6, 291–299. [Google Scholar] [CrossRef]
- Terra, X.; Pallarés, V.; Ardèvol, A.; Bladé, C.; Fernández-Larrea, J.; Pujadas, G.; Salvadó, J.; Arola, L.; Blay, M. Modulatory effect of grape-seed procyanidins on local and systemic inflammation in diet-induced obesity rats. J. Nutr. Biochem. 2011, 22, 380–387. [Google Scholar] [CrossRef]
- Park, S.-S.; Kim, J.-M.; Kim, E.-J.; Kim, H.-S.; An, B.-K.; Kang, C.-W. Effects of Dietary Turmeric Powder on Laying Performance and Egg Qualities in Laying Hens. Korean J. Poult. Sci. 2012, 39, 27–32. [Google Scholar] [CrossRef]
- Chu, H.; Tang, Q.; Huang, H.; Hao, W.; Wei, X. Grape-seed proanthocyanidins inhibit the lipopolysaccharide-induced inflammatory mediator expression in RAW264. 7 macrophages by suppressing MAPK and NF-κb signal pathways. Environ. Toxicol. Pharmacol. 2016, 41, 159–166. [Google Scholar]
- Dinh, J.; Angeloni, J.T.; Pederson, D.B.; Wang, X.; Cao, M.; Dong, Y. Cranberry extract standardized for proanthocyanidins promotes the immune response of Caenorhabditis elegans to Vibrio cholerae through the p38 MAPK pathway and HSF-1. PLoS ONE 2014, 9, e103290. [Google Scholar] [CrossRef] [PubMed]
- Lau, D.W.; King, A.J. Pre-and post-mortem use of grape seed extract in dark poultry meat to inhibit development of thiobarbituric acid reactive substances. J. Agric. Food Chem. 2003, 51, 1602–1607. [Google Scholar] [CrossRef] [PubMed]
- Rababah, T.; Ereifej, K.; Al-Mahasneh, M.; Al-Rababah, M. Effect of plant extracts on physicochemical properties of chicken breast meat cooked using conventional electric oven or microwave. Poult. Sci. 2006, 85, 148–154. [Google Scholar]
- Hao, R.; Li, Q.; Zhao, J.; Li, H.; Wang, W.; Gao, J. Effects of Grape Seed Procyanidins on Growth Performance, Immune Function and Antioxidant Capacity in Weaned Piglets. Livest. Sci. 2015, 178, 237–242. [Google Scholar] [CrossRef]
- Ali, K.; Maltese, F.; Choi, Y.H.; Verpoorte, R. Metabolic Constituents of Grapevine and Grape-Derived Products. Phytochem. Rev. 2010, 9, 357–378. [Google Scholar] [CrossRef] [PubMed]
- OIV. The OIV Provides Sound, Timely and Comparable Data and Statistics on the Vine and Wine Sector to Inform Policy Makers, Analysts, and Members of Civil Society around the World. Available online: https://www.oiv.int/what-we-do/statistics (accessed on 28 May 2024).
- Vivier, M.; Pretorius, I.S. Genetic Improvement of Grapevine: Tailoring Grape Varieties for the Third Millennium—A Review. S. Afr. J. Enol. Vitic. 2000, 21, 5–26. [Google Scholar] [CrossRef]
- Cotoras, M.; Vivanco, H.; Melo, R.; Aguirre, M.; Silva, E.; Mendoza, L. In Vitro and In Vivo Evaluation of the Antioxidant and Prooxidant Activity of Phenolic Compounds Obtained from Grape (Vitis vinifera) Pomace. Molecules 2014, 19, 21154–21167. [Google Scholar] [CrossRef]
- Ky, I.; Lorrain, B.; Kolbas, N.; Crozier, A.; Teissedre, P.-L. Wine By-Products: Phenolic Characterization and Antioxidant Activity Evaluation of Grapes and Grape Pomaces from Six Different French Grape Varieties. Molecules 2014, 19, 482–506. [Google Scholar] [CrossRef]
- Bail, S.; Stuebiger, G.; Krist, S.; Unterweger, H.; Buchbauer, G. Characterization of Various Grape Seed Oils by Volatile Compounds, Triacylglycerol Composition, Total Phenols and Antioxidant Capacity. Food Chem. 2008, 108, 1122–1132. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.N.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.; Cruz, A.P.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.; Freitas, S.P. Towards Integral Utilization of Grape Pomace from Winemaking Process: A Review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef]
- Yu, J.; Ahmedna, M. Functional Components of Grape Pomace: Their Composition, Biological Properties and Potential Applications. Int. J. Food Sci. Tech. 2013, 48, 221–237. [Google Scholar] [CrossRef]
- Viveros, A.; Chamorro, S.; Pizarro, M.; Arija, I.; Centeno, C.; Brenes, A. Effects of Dietary Polyphenol-Rich Grape Products on Intestinal Microflora and Gut Morphology in Broiler Chicks. Poult. Sci. 2011, 90, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Llobera, A.; Canellas, J. Dietary fibres content and antioxidant activity of Manto Negro red grape (Vitis vinifera): Pomace and stem. Food Chem. 2007, 101, 659–666. [Google Scholar] [CrossRef]
- Vlaicu, P.A.; Panaite, T.D.; Cornescu, M.G.; Ropota, M.; Olteanu, M.; Drăgotoiu, D. The influence of by-products on the production parameters and nutrient digestibility in fattening pig diet (60–100 kg). AgroLife Sci. J. 2019, 8, 261–269. [Google Scholar]
- Erinle, T.J.; Oladokun, S.; MacIsaac, J.; Rathgeber, B.; Adewole, D. Dietary grape pomace–effects on growth performance, intestinal health, blood parameters, and breast muscle myopathies of broiler chickens. Poult. Sci. 2022, 101, 101519. [Google Scholar]
- Skuras, D.; Psaltopoulos, D. A Broad Overview of the Main Problems Derived from Climate Change That Will Affect Agricultural Production in the Mediterranean Area. In Building Resilience for Adaptation to Climate Change in the Agriculture Sector; FAO: Rome, Italy; Volume 23, pp. 217–260. Available online: https://api.semanticscholar.org/CorpusID:55083905 (accessed on 10 May 2024).
- Lu, Y.; Foo, L.Y. The Polyphenol Constituents of Grape Pomace. Food Chem. 1999, 65, 1–8. [Google Scholar] [CrossRef]
- Brenes, A.; Viveros, A.; Chamorro, S.; Arija, I. Use of Polyphenol-Rich Grape by-products in Monogastric Nutrition. A Review. Anim. Feed Sci. Tech. 2016, 211, 1–17. [Google Scholar] [CrossRef]
- Gungor, E.; Altop, A.; Erener, G. Effect of Raw and Fermented Grape Seed on Growth Performance, Antioxidant Capacity, and Cecal Microflora in Broiler Chickens. Animal 2021, 15, 100194. [Google Scholar] [CrossRef]
- Costa, M.M.; Alfaia, C.M.; Lopes, P.A.; Pestana, J.M.; Prates, J.A. Grape By-Products as Feedstuff for Pig and Poultry Production. Animals 2022, 12, 2239. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Toledo, R.T. Major Flavonoids in Grape Seeds and Skins: Antioxidant Capacity of Catechin, Epicatechin, and Gallic Acid. J. Agric. Food Chem. 2004, 52, 255–260. [Google Scholar] [CrossRef]
- Felhi, S.; Baccouch, N.; Ben Salah, H.; Smaoui, S.; Allouche, N.; Gharsallah, N.; Kadri, A. Nutritional Constituents, Phytochemical Profiles, in Vitro Antioxidant and Antimicrobial Properties, and Gas Chromatography–Mass Spectrometry Analysis of Various Solvent Extracts from Grape Seeds (Vitis vinifera L.). Food Sci. Biotech. 2016, 25, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Igartuburu, J.M.; Pando, E.; Rodríguez-Luis, F.; Gil-Serrano, A. Structure of a Hemicellulose A Fraction in Dietary Fiber from the Seed of Grape Variety Palomino (Vitis vinifera Cv. Palomino). J. Nat. Prod. 1998, 61, 876–880. [Google Scholar] [CrossRef] [PubMed]
- Nardoia, M.; Romero, C.; Brenes, A.; Arija, I.; Viveros, A.; Ruiz-Capillas, C.; Chamorro, S. Addition of Fermented and Unfermented Grape Skin in Broilers’ Diets: Effect on Digestion, Growth Performance, Intestinal Microbiota and Oxidative Stability of Meat. Animal 2020, 14, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- Prozil, S.O.; Costa, E.V.; Evtuguin, D.V.; Lopes, L.P.C.; Domingues, M.R.M. Structural Characterization of Polysaccharides Isolated from Grape Stalks of Vitis vinifera L. Carbohydr. Res. 2012, 356, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Li, M.; Zhao, L.; Han, S.; Li, Y.; Xiong, B.; Jiang, L. Dietary Grape Seed Procyanidins Suppressed Weaning Stress by Improving Antioxidant Enzyme Activity and MRNA Expression in Weanling Piglets. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yu, H.; Fang, H.; Jin, Y.; Zhao, Y.; Shen, J.; Zhou, C.; Li, R.; Wang, J.; Fu, Y. Effects of Dietary Grape Pomace on the Intestinal Microbiota and Growth Performance of Weaned Piglets. Arch. Anim. Nutr. 2020, 74, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Alonso, Á.M.; Guillén, D.A.; Barroso, C.G.; Puertas, B.; García, A. Determination of Antioxidant Activity of Wine Byproducts and Its Correlation with Polyphenolic Content. J. Agric. Food Chem. 2002, 50, 5832–5836. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.L.; Varela, B.; García, M.T.; Carilla, J.; Matito, C.; Centelles, J.J.; Cascante, M.; Sort, X.; Bobet, R. Valorization of Grape (Vitis vinifera) Byproducts. Antioxidant and Biological Properties of Polyphenolic Fractions Differing in Procyanidin Composition and Flavonol Content. J. Agric. Food Chem. 2002, 50, 7548–7555. [Google Scholar] [CrossRef] [PubMed]
- Chedea, V.S.; Drăgulinescu, A.-M.; Tomoiagă, L.L.; Bălăceanu, C.; Iliescu, M.L. Climate Change and Internet of Things Technologies—Sustainable Premises of Extending the Culture of the Amurg Cultivar in Transylvania—A Use Case for Târnave Vineyard. Sustainability 2021, 13, 8170. [Google Scholar] [CrossRef]
- Iuga, M.; Mironeasa, S. Potential of Grape Byproducts as Functional Ingredients in Baked Goods and Pasta. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2473–2505. [Google Scholar] [CrossRef]
- de la Cerda-Carrasco, A.; López-Solís, R.; Nuñez-Kalasic, H.; Peña-Neira, Á.; Obreque-Slier, E. Phenolic Composition and Antioxidant Capacity of Pomaces from Four Grape Varieties (Vitis vinifera L.). J. Sci. Food Agric. 2015, 95, 1521–1527. [Google Scholar] [CrossRef]
- Muncaciu, M.L.; Marin, F.Z.; Nastasia, P.; Babeș, A.C. Comparative Polyphenolic Content of Grape Pomace Flours from ‘Fetească Neagră’and ‘Italian Riesling’Cultivars. Not. Bot. Horti Agrobo. 2017, 45, 532–539. [Google Scholar] [CrossRef][Green Version]
- Vermerris, W.; Nicholson, R. The Role of Phenols in Plant Defense. In Phenolic Compound Biochemistry; Springer: Dordrecht, Germany, 2006; pp. 211–234. ISBN 978-1-4020-5164-7. [Google Scholar] [CrossRef]
- Ozcan, T.; Akpinar-Bayizit, A.; Yilmaz-Ersan, L.; Delikanli, B. Phenolics in Human Health. Int. J. Chem. Eng. Appl. 2014, 5, 393–396. [Google Scholar] [CrossRef]
- Hrazdina, G. Biosynthesis of Flavonoids. In Plant Polyphenols: Synthesis, Properties, Significance; Springer: Boston, MA, USA, 1992; pp. 61–72. [Google Scholar]
- Winkel-Shirley, B. It Takes a Garden. How Work on Diverse Plant Species Has Contributed to an Understanding of Flavonoid Metabolism. Plant Physiol. 2001, 127, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.; Xie, D.; Sharma, S.B. Proanthocyanidins—A Final Frontier in Flavonoid Research? New Phytol. 2005, 165, 9–28. [Google Scholar] [CrossRef]
- Bravo, L. Polyphenols: Chemistry, Dietary Sources, Metabolism, and Nutritional Significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef]
- Seigler, D.S. Plant Secondary Metabolism; Springer Science & Business Media: New York, NY, USA, 1998; ISBN 978-1-4613-7228-8. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Pietta, P.; Minoggio, M.; Bramati, L. Plant Polyphenols: Structure, Occurrence and Bioactivity. Stud. Nat. Prod. Chem. 2003, 28, 257–312. [Google Scholar]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A Concise Overview on the Chemistry, Occurrence, and Human Health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [PubMed]
- Hichri, I.; Barrieu, F.; Bogs, J.; Kappel, C.; Delrot, S.; Lauvergeat, V. Recent Advances in the Transcriptional Regulation of the Flavonoid Biosynthetic Pathway. J. Exp. Bot. 2011, 62, 2465–2483. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Morand, C.; Manach, C.; Rémésy, C. Absorption and Metabolism of Polyphenols in the Gut and Impact on Health. Biomed. Pharmacother. 2002, 56, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.-G.; AG Koffas, M. Bioavailability and Recent Advances in the Bioactivity of Flavonoid and Stilbene Compounds. Curr. Org. Chem. 2010, 14, 1727–1751. [Google Scholar] [CrossRef]
- Motohashi, N. Bioactive Heterocycles VI: Flavonoids and Anthocyanins in Plants, and Latest Bioactive Heterocycles I; Springer: Leipzig, Germany, 2008; Volume 15, ISBN 978-3-540-79217-8. [Google Scholar] [CrossRef]
- Aron, P.M.; Kennedy, J.A. Flavan-3-ols: Nature, Occurrence and Biological Activity. Mol. Nutr. Food Res. 2008, 52, 79–104. [Google Scholar] [CrossRef] [PubMed]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Nassiri-Asl, M.; Hosseinzadeh, H. Review of the Pharmacological Effects of Vitis vinifera (Grape) and its Bioactive Compounds. Phytother. Res. 2009, 23, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Conde, C.; Silva, P.; Fontes, N.; Dias, A.C.P.; Tavares, R.M.; Sousa, M.J.; Agasse, A.; Delrot, S.; Gerós, H. Biochemical Changes throughout Grape Berry Development and Fruit and Wine Quality. Food 2007, 1, 1–22. [Google Scholar]
- Cho, M.J.; Howard, L.R.; Prior, R.L.; Clark, J.R. Flavonoid Glycosides and Antioxidant Capacity of Various Blackberry, Blueberry and Red Grape Genotypes Determined by High-performance Liquid Chromatography/Mass Spectrometry. J. Sci. Food Agric. 2004, 84, 1771–1782. [Google Scholar] [CrossRef]
- Xia, E.-Q.; Deng, G.-F.; Guo, Y.-J.; Li, H.-B. Biological Activities of Polyphenols from Grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef] [PubMed]
- Narayana, K.R.; Reddy, M.S.; Chaluvadi, M.; Krishna, D. Bioflavonoids Classification, Pharmacological, Biochemical Effects and Therapeutic Potential. Indian J. Pharmacol. 2001, 33, 2–16. [Google Scholar]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid Antioxidants: Chemistry, Metabolism and Structure-Activity Relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Shen, T.; Lou, H. Dietary Polyphenols and Their Biological Significance. Int. J. Mol. Sci. 2007, 8, 950–988. [Google Scholar] [CrossRef]
- Alzand, K.I.; Mohamed, M.A. Flavonoids: Chemistry, Biochemistry and Antioxidant Activity. J. Pharm. Res. 2012, 5, 37. [Google Scholar]
- Song, P.; Zhang, R.; Wang, X.; He, P.; Tan, L.; Ma, X. Dietary Grape-Seed Procyanidins Decreased Postweaning Diarrhea by Modulating Intestinal Permeability and Suppressing Oxidative Stress in Rats. J. Agric. Food Chem. 2011, 59, 6227–6232. [Google Scholar] [CrossRef]
- Mayer, R.; Stecher, G.; Wuerzner, R.; Silva, R.C.; Sultana, T.; Trojer, L.; Feuerstein, I.; Krieg, C.; Abel, G.; Popp, M. Proanthocyanidins: Target Compounds as Antibacterial Agents. J. Agric. Food Chem. 2008, 56, 6959–6966. [Google Scholar] [CrossRef]
- Goodrich, K.M.; Fundaro, G.; Griffin, L.E.; Hulver, M.W.; Ponder, M.A.; Neilson, A.P. Chronic Administration of Dietary Grape Seed Extract Increases Colonic Expression of Gut Tight Junction Protein Occludin and Reduces Fecal Calprotectin: A Secondary Analysis of Healthy Wistar Furth Rats. Nutr. Res. 2012, 32, 787–794. [Google Scholar] [CrossRef]
- Wang, H.; Xue, Y.; Zhang, H.; Huang, Y.; Yang, G.; Du, M.; Zhu, M. Dietary Grape Seed Extract Ameliorates Symptoms of Inflammatory Bowel Disease in IL 10-deficient Mice. Mol. Nutr. Food Res. 2013, 57, 2253–2257. [Google Scholar] [CrossRef]
- Bagchi, D.; Bagchi, M.; Stohs, S.J.; Das, D.K.; Ray, S.D.; Kuszynski, C.A.; Joshi, S.S.; Pruess, H.G. Free Radicals and Grape Seed Proanthocyanidin Extract: Importance in Human Health and Disease Prevention. Toxicology 2000, 148, 187–197. [Google Scholar] [CrossRef]
- Yamakoshi, J.; Saito, M.; Kataoka, S.; Tokutake, S. Procyanidin-Rich Extract from Grape Seeds Prevents Cataract Formation in Hereditary Cataractous (ICR/f) Rats. J. Agric. Food Chem. 2002, 50, 4983–4988. [Google Scholar] [CrossRef]
- Sato, M.; Bagchi, D.; Tosaki, A.; Das, D.K. Grape Seed Proanthocyanidin Reduces Cardiomyocyte Apoptosis by Inhibiting Ischemia/Reperfusion-Induced Activation of JNK-1 and C-JUN. Free Radic. Biol. Med. 2001, 31, 729–737. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A. Advances in Flavonoid Research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Crozier, A. Classification and Biosynthesis of Secondary Plant Products: An Overview. In Plants: Diet and Health. The Report of a British Nutrition Foundation Task Force; Goldberg, G., Ed.; Willey: London, UK, 2003; pp. 27–48. ISBN 9780632059621. [Google Scholar] [CrossRef]
- Zhao, J.; Pang, Y.; Dixon, R.A. The Mysteries of Proanthocyanidin Transport and Polymerization. Plant Physiol. 2010, 153, 437–443. [Google Scholar] [CrossRef]
- Santhakumar, A.B.; Battino, M.; Alvarez-Suarez, J.M. Dietary Polyphenols: Structures, Bioavailability and Protective Effects against Atherosclerosis. Food Chem. Toxicol. 2018, 113, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Verries, C.; Guiraud, J.-L.; Souquet, J.-M.; Vialet, S.; Terrier, N.; Olle, D. Validation of an Extraction Method on Whole Pericarp of Grape Berry (Vitis vinifera L. Cv. Shiraz) to Study Biochemical and Molecular Aspects of Flavan-3-Ol Synthesis during Berry Development. J. Agric. Food Chem. 2008, 56, 5896–5904. [Google Scholar] [CrossRef]
- Su, C.T.; Singleton, V. Identification of Three Flavan-3-Ols from Grapes. Phytochemistry 1969, 8, 1553–1558. [Google Scholar]
- Souquet, J.-M.; Labarbe, B.; Le Guernevé, C.; Cheynier, V.; Moutounet, M. Phenolic Composition of Grape Stems. J. Agric. Food Chem. 2000, 48, 1076–1080. [Google Scholar] [CrossRef]
- Cantos, E.; Espin, J.C.; Tomás-Barberán, F.A. Varietal Differences among the Polyphenol Profiles of Seven Table Grape Cultivars Studied by LC–DAD–MS–MS. J. Agric. Food Chem. 2002, 50, 5691–5696. [Google Scholar] [CrossRef]
- Gođevac, D.; Tešević, V.; Veličković, M.; Vujisić, L.; Vajs, V.; Milosavljević, S. Polyphenolic Compounds in Seeds from Some Grape Cultivars Grown in Serbia. J. Serb. Chem. Soc. 2010, 75, 1641–1652. [Google Scholar] [CrossRef]
- Freudenberg, K.; Blümmel, F. Hamameli-tannin. III. 17. Mitteilung Über Gerbstoffe Und Ähnliche Verbindungen. Liebigs Ann. Chem. 1924, 440, 45–59. [Google Scholar] [CrossRef]
- Singleton, V.; Draper, D.E.; Rossi, J.A. Paper Chromatography of Phenolic Compounds from Grapes, Particularly Seeds, and Some Variety-Ripeness Relationships. Am. J. Enol. Vitic. 1966, 17, 206–217. [Google Scholar] [CrossRef]
- Czochanska, Z.; Foo, L.Y.; Newman, R.H.; Porter, L.J. Polymeric Proanthocyanidins. Stereochemistry, Structural Units, and Molecular Weight. J. Chem. Soc. Perkin Trans. 1 1980, 12, 2278–2286. [Google Scholar] [CrossRef]
- Romeyer, F.M.; Macheix, J.-J.; Sapis, J.-C. Changes and Importance of Oligomeric Procyanidins during Maturation of Grape Seeds. Phytochemistry 1985, 25, 219–221. [Google Scholar] [CrossRef]
- Meyer, B.J.; Hernandez, R. Seed Tannin Extraction in Cabernet Sauvignon. Am. J. Enol. Vitic. 1970, 21, 184–188. [Google Scholar] [CrossRef]
- Oszmianski, J.; Romeyer, F.M.; Sapis, J.; Macheix, J. Grape Seed Phenolics: Extraction as Affected by Some Conditions Occurring during Wine Processing. Am. J. Enol. Vitic. 1986, 37, 7–12. [Google Scholar] [CrossRef]
- Khanbabaee, K.; Van Ree, T. Tannins: Classification and Definition. Nat. Prod. Rep. 2001, 18, 641–649. [Google Scholar] [PubMed]
- Tsang, C.; Auger, C.; Mullen, W.; Bornet, A.; Rouanet, J.-M.; Crozier, A.; Teissedre, P.-L. The Absorption, Metabolism and Excretion of Flavan-3-Ols and Procyanidins Following the Ingestion of a Grape Seed Extract by Rats. Br. J. Nutr. 2005, 94, 170–181. [Google Scholar] [CrossRef]
- Bogs, J.; Downey, M.O.; Harvey, J.S.; Ashton, A.R.; Tanner, G.J.; Robinson, S.P. Proanthocyanidin Synthesis and Expression of Genes Encoding Leucoanthocyanidin Reductase and Anthocyanidin Reductase in Developing Grape Berries and Grapevine Leaves. Plant Physiol. 2005, 139, 652–663. [Google Scholar] [CrossRef]
- Foo, L.Y.; Porter, L.J. The Phytochemistry of Proanthocyanidin Polymers. Phytochemistry 1980, 19, 1747–1754. [Google Scholar] [CrossRef]
- Foo, L.Y.; Porter, L.J. The Structure of Tannins of Some Edible Fruits. J. Sci. Food Agric. 1981, 32, 711–716. [Google Scholar] [CrossRef]
- Edwin, H. In Vino Veritas: Oligomeric Procyanidins and the Ageing of Red Wines. Phytochemistry 1980, 19, 2577–2582. [Google Scholar] [CrossRef]
- Lin, L.-Z.; Sun, J.; Chen, P.; Monagas, M.J.; Harnly, J.M. UHPLC-PDA-ESI/HRMS n Profiling Method to Identify and Quantify Oligomeric Proanthocyanidins in Plant Products. J. Agric. Food Chem. 2014, 62, 9387–9400. [Google Scholar] [CrossRef] [PubMed]
- Geny, L.; Saucier, C.; Bracco, S.; Daviaud, F.; Glories, Y. Composition and Cellular Localization of Tannins in Grape Seeds during Maturation. J. Agric. Food Chem. 2003, 51, 8051–8054. [Google Scholar] [CrossRef] [PubMed]
- Constabel, P.; Yoshida, K.; Walker, V. Diverse Ecological Roles of Plant Tannins: Plant Defense and Beyond. Recent Advances in Polyphenol Research; Willey: London, UK, 2014; pp. 115–142. [Google Scholar] [CrossRef]
- Li, M.; Hagerman, A.E. Interactions between Plasma Proteins and Naturally Occurring Polyphenols. Curr. Drug Met. 2013, 14, 432–445. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and Hydrolysable Tannins: Occurrence, Dietary Intake and Pharmacological Effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef]
- Prior, R.L.; Gu, L. Occurrence and Biological Significance of Proanthocyanidins in the American Diet. Phytochemistry 2005, 66, 2264–2280. [Google Scholar] [CrossRef]
- Terrier, N.; Ollé, D.; Verriès, C.; Cheynier, V. Biochemical & Molecular Aspects of Flavan-3-Ol Synthesis during Berry Development. In Grapevine Molecular Physiology & Biotechnology; Roubelakis-Angelakis, K.A., Ed.; Springer: Heidelberg, Germany, 2009; pp. 365–388. ISBN 978-90-481-2304-9. [Google Scholar] [CrossRef]
- Hanlin, R.L.; Kelm, M.A.; Wilkinson, K.L.; Downey, M.O. Detailed Characterization of Proanthocyanidins in Skin, Seeds, and Wine of Shiraz and Cabernet Sauvignon Wine Grapes (Vitis vinifera). J. Agric. Food Chem. 2011, 59, 13265–13276. [Google Scholar] [CrossRef]
- Pinelo, M.; Arnous, A.; Meyer, A.S. Upgrading of Grape Skins: Significance of Plant Cell-Wall Structural Components and Extraction Techniques for Phenol Release. Trends Food Sci. Technol. 2006, 17, 579–590. [Google Scholar] [CrossRef]
- Creasy, L.; Swain, T. Structure of Condensed Tannins. Nature 1965, 208, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Weinges, K.; Kaltenhäuser, W.; Marx, H.; Nader, E.; Nader, F.; Perner, J.; Seiler, D. Zur Kenntnis Der Proanthocyanidine, X Procyanidine Aus Früchten. Liebigs Ann. Chem. 1968, 711, 184–204. [Google Scholar] [CrossRef] [PubMed]
- Serrano, J.; Puupponen-Pimiä, R.; Dauer, A.; Aura, A.; Saura-Calixto, F. Tannins: Current Knowledge of Food Sources, Intake, Bioavailability and Biological Effects. Mol. Nutr. Food Res. 2009, 53, S310–S329. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Micaelo, N.; González-Abuín, N.; Ardevol, A.; Pinent, M.; Blay, M.T. Procyanidins and Inflammation: Molecular Targets and Health Implications. Biofactors 2012, 38, 257–265. [Google Scholar] [CrossRef]
- Pinent, M.; Castell-Auví, A.; Genovese, M.I.; Serrano, J.; Casanova, A.; Blay, M.; Ardévol, A. Antioxidant Effects of Proanthocyanidin-rich Natural Extracts from Grape Seed and Cupuassu on Gastrointestinal Mucosa. J. Sci. Food Agric. 2016, 96, 178–182. [Google Scholar] [CrossRef]
- Salvadó, M.J.; Casanova, E.; Fernández-Iglesias, A.; Arola, L.; Bladé, C. Roles of Proanthocyanidin Rich Extracts in Obesity. Food Funct. 2015, 6, 1053–1071. [Google Scholar] [CrossRef]
- Jin, Q.; O’Hair, J.; Stewart, A.C.; O’Keefe, S.F.; Neilson, A.P.; Kim, Y.-T.; McGuire, M.; Lee, A.; Wilder, G.; Huang, H. Compositional Characterization of Different Industrial White and Red Grape Pomaces in Virginia and the Potential Valorization of the Major Components. Foods 2019, 8, 667. [Google Scholar] [CrossRef]
- He, F.; Mu, L.; Yan, G.-L.; Liang, N.-N.; Pan, Q.-H.; Wang, J.; Reeves, M.J.; Duan, C.-Q. Biosynthesis of Anthocyanins and Their Regulation in Colored Grapes. Molecules 2010, 15, 9057–9091. [Google Scholar] [CrossRef]
- Montealegre, R.R.; Peces, R.R.; Vozmediano, J.C.; Gascueña, J.M.; Romero, E.G. Phenolic Compounds in Skins and Seeds of Ten Grape Vitis vinifera Varieties Grown in a Warm Climate. J. Food Compos. Anal. 2006, 19, 687–693. [Google Scholar] [CrossRef]
- Zhang, Z.-Z.; Che, X.-N.; Pan, Q.-H.; Li, X.-X.; Duan, C.-Q. Transcriptional Activation of Flavan-3-Ols Biosynthesis in Grape Berries by UV Irradiation Depending on Developmental Stage. Plant Sci. 2013, 208, 64–74. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, Y.; Lu, J. Phenolic Contents and Compositions in Skins of Red Wine Grape Cultivars among Various Genetic Backgrounds and Originations. Int. J. Mol. Sci. 2012, 13, 3492–3510. [Google Scholar] [CrossRef] [PubMed]
- Pasteur, L. Études Sur Le Vin: Ses Maladies, Causes Qui Les Provoquent, Procédés Nouveaux Pour Le Conserver et Pour Le Vieillir; Simon Raçou et Comp.: Paris, France, 1873. [Google Scholar]
- Willstätter, R.; Everest, A.E. Untersuchungen Über Die Anthocyane. I. Über Den Farbstoff Der Kornblume. Liebigs Ann. Chem. 1913, 401, 189–232. [Google Scholar] [CrossRef]
- Levy, L.F.; Posternack, T.; Robinson, R. Experiments on the Synthesis of the Anthocyanins. Part VIII. A Synthesis of Œnin Chloride. J. Chem. Soc. 1931, 2701–2715. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P. Recherches Sur Les Anthocyannes Des Végétaux: Application Au Genere Vitis; Librairie Générale de L’Enseignement: Paris, France, 1959. [Google Scholar]
- Rankine, B.C.; Kepner, R.E.; Webb, A.D. Comparison of Anthocyan Pigments of Vinifera Grapes. Am. J. Enol. Vitic. 1958, 9, 105–110. [Google Scholar] [CrossRef]
- Puissant, A.; Léon, H. La Matière Colorante Des Grains de Raisins de Certains Cépages Cultivés En Anjou En 1965. Ann. Technol. Agric. 1967, 3, 217–226, hal-02731988. [Google Scholar]
- Fong, R.A.; Kepner, R.E.; Webb, A.D. Acetic-Acid-Acylated Anthocyanin Pigments in the Grape Skins of a Number of Varieties of Vitis vinifera. Am. J. Enol. Vitic. 1971, 22, 150–155. [Google Scholar] [CrossRef]
- Castillo-Muñoz, N.; Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I. Flavonol Profiles of Vitis vinifera Red Grapes and Their Single-Cultivar Wines. J. Agric. Food Chem. 2007, 55, 992–1002. [Google Scholar] [CrossRef]
- Chedea, V.S.; Macovei, Ș.O.; Bocsan, I.C.; Măgureanu, D.C.; Levai, A.M.; Buzoianu, A.D.; Pop, R.M. Grape Pomace Polyphenols as a Source of Compounds for Management of Oxidative Stress and Inflammation—A Possible Alternative for Non-Steroidal Anti-Inflammatory Drugs? Molecules 2022, 27, 6826. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, J.d.S.; Guimarães, R.d.C.A.; Zorgetto-Pinheiro, V.A.; Fernandes, C.D.P.; Marcelino, G.; Bogo, D.; Freitas, K.d.C.; Hiane, P.A.; de Pádua Melo, E.S.; Vilela, M.L.B.; et al. Natural Antioxidant Evaluation: A Review of Detection Methods. Molecules 2022, 27, 3563. [Google Scholar] [CrossRef]
- Kammerer, D.; Claus, A.; Carle, R.; Schieber, A. Polyphenol Screening of Pomace from Red and White Grape Varieties (Vitis vinifera L.) by HPLC-DAD-MS/MS. J. Agric. Food Chem. 2004, 52, 4360–4367. [Google Scholar] [CrossRef]
- Baranowski, E.S.; Nagel, C.W. Kinetics of Malvidin-3-glucoside Condensation in Wine Model Systems. J. Food Sci. 1983, 48, 419–421. [Google Scholar] [CrossRef]
- Cheynier, V.F.; Trousdale, E.K.; Singleton, V.L.; Salgues, M.J.; Wylde, R. Characterization of 2-S-Glutathionyl Caftaric Acid and Its Hydrolysis in Relation to Grape Wines. J. Agric. Food Chem. 1986, 34, 217–221. [Google Scholar] [CrossRef]
- Carbonell-Capella, J.M.; Buniowska, M.; Barba, F.J.; Esteve, M.J.; Frígola, A. Analytical Methods for Determining Bioavailability and Bioaccessibility of Bioactive Compounds from Fruits and Vegetables: A Review. Compr. Rev. Food Sci. Food Sci. Food Saf. 2014, 13, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Courraud, J.; Charnay, C.; Cristol, J.-P.; Berger, J.; Avallone, S. In Vitro Lipid Peroxidation of Intestinal Bile Salt-Based Nanoemulsions: Potential Role of Antioxidants. Free Radic. Res. 2013, 47, 1076–1087. [Google Scholar] [CrossRef] [PubMed]
- Palafox-Carlos, H.; Ayala-Zavala, J.F.; González-Aguilar, G.A. The Role of Dietary Fiber in the Bioaccessibility and Bioavailability of Fruit and Vegetable Antioxidants. J. Food Sci. 2011, 76, R6–R15. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, M.F.; Kroon, P.A.; Williamson, G.; Garcia-Conesa, M.-T. Esterase Activity Able to Hydrolyze Dietary Antioxidant Hydroxycinnamates Is Distributed along the Intestine of Mammals. J. Agric. Food Chem. 2001, 49, 5679–5684. [Google Scholar] [CrossRef] [PubMed]
- Bunzel, M.; Ralph, J.; Lu, F.; Hatfield, R.D.; Steinhart, H. Lignins and Ferulate−Coniferyl Alcohol Cross-Coupling Products in Cereal Grains. J. Agric. Food Chem. 2004, 52, 6496–6502. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.; Fernandes, I.; Norberto, S.; Mateus, N.; Calhau, C. Interplay between Anthocyanins and Gut Microbiota. J. Agric. Food Chem. 2014, 62, 6898–6902. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, T.A.F.; Rogero, M.M.; Hassimotto, N.M.A.; Lajolo, F.M. The Two-Way Polyphenols-Microbiota Interactions and Their Effects on Obesity and Related Metabolic Diseases. Front. Nutr. 2019, 6, 188. [Google Scholar] [CrossRef]
- Monagas, M.; Urpi-Sarda, M.; Sánchez-Patán, F.; Llorach, R.; Garrido, I.; Gómez-Cordovés, C.; Andres-Lacueva, C.; Bartolomé, B. Insights into the Metabolism and Microbial Biotransformation of Dietary Flavan-3-Ols and the Bioactivity of Their Metabolites. Food Funct. 2010, 1, 233–253. [Google Scholar] [CrossRef]
- Cermak, R.; Landgraf, S.; Wolffram, S. The Bioavailability of Quercetin in Pigs Depends on the Glycoside Moiety and on Dietary Factors. J. Nutr. 2003, 133, 2802–2807. [Google Scholar] [CrossRef] [PubMed]
- Zubik, L.; Meydani, M. Bioavailability of Soybean Isoflavones from Aglycone and Glucoside Forms in American Women. The Am. J. Clin. Nutr. 2003, 77, 1459–1465. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eriz, G.; Sanhueza, V.; Roeckel, M.; Fernández, K. Inhibition of the Angiotensin-Converting Enzyme by Grape Seed and Skin Proanthocyanidins Extracted from Vitis vinífera L. Cv. País. LWT-Food Sci. Tech. 2011, 44, 860–865. [Google Scholar] [CrossRef]
- Ou, K.; Gu, L. Absorption and Metabolism of Proanthocyanidins. J. Funct. Foods 2014, 7, 43–53. [Google Scholar] [CrossRef]
- Kuhnle, G.; Spencer, J.P.; Schroeter, H.; Shenoy, B.; Debnam, E.S.; Srai, S.K.S.; Rice-Evans, C.; Hahn, U. Epicatechin and Catechin Are O-Methylated and Glucuronidated in the Small Intestine. Biochem. Biophys. Res. Commun. 2000, 277, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, J.B.; Walle, T. Glucuronidation and Sulfation of the Tea Flavonoid (−)-Epicatechin by the Human and Rat Enzymes. Drug Metab. Disp. 2002, 30, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Hackman, R.M.; Polagruto, J.A.; Zhu, Q.Y.; Sun, B.; Fujii, H.; Keen, C.L. Flavanols: Digestion, Absorption and Bioactivity. Phytochem. Rev. 2008, 7, 195–208. [Google Scholar] [CrossRef]
- Dangles, O.; Dufour, C.; Manach, C.; Morand, C.; Remesy, C. Binding of Flavonoids to Plasma Proteins. In Methods in Enzymology; Academic Press: New York, NY, USA, 2001; Volume 335, pp. 319–333. [Google Scholar] [CrossRef]
- Axling, U.; Olsson, C.; Xu, J.; Fernandez, C.; Larsson, S.; Ström, K.; Ahrné, S.; Holm, C.; Molin, G.; Berger, K. Green Tea Powder and Lactobacillus Plantarum Affect Gut Microbiota, Lipid Metabolism and Inflammation in High-Fat Fed C57BL/6J Mice. Nutr. Metab. 2012, 9, 105. [Google Scholar] [CrossRef]
- China, R.; Mukherjee, S.; Sen, S.; Bose, S.; Datta, S.; Koley, H.; Ghosh, S.; Dhar, P. Antimicrobial Activity of Sesbania Grandiflora Flower Polyphenol Extracts on Some Pathogenic Bacteria and Growth Stimulatory Effect on the Probiotic Organism Lactobacillus Acidophilus. Microbiol. Res. 2012, 167, 500–506. [Google Scholar] [CrossRef]
- Queipo-Ortuño, M.I.; Boto-Ordóñez, M.; Murri, M.; Gomez-Zumaquero, J.M.; Clemente-Postigo, M.; Estruch, R.; Diaz, F.C.; Andres-Lacueva, C.; Tinahones, F.J. Influence of Red Wine Polyphenols and Ethanol on the Gut Microbiota Ecology and Biochemical Biomarkers. Am. J. Clin. Nutr. 2012, 95, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Etxeberria, U.; Fernández-Quintela, A.; Milagro, F.I.; Aguirre, L.; Martínez, J.A.; Portillo, M.P. Impact of Polyphenols and Polyphenol-Rich Dietary Sources on Gut Microbiota Composition. J. Agric. Food Chem. 2013, 61, 9517–9533. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Indias, I.; Sánchez-Alcoholado, L.; Pérez-Martínez, P.; Andrés-Lacueva, C.; Cardona, F.; Tinahones, F.; Queipo-Ortuño, M.I. Red Wine Polyphenols Modulate Fecal Microbiota and Reduce Markers of the Metabolic Syndrome in Obese Patients. Food Funct. 2016, 7, 1775–1787. [Google Scholar] [CrossRef] [PubMed]
- Parada, J.; Aguilera, J. Food Microstructure Affects the Bioavailability of Several Nutrients. J. Food Sci. 2007, 72, R21–R32. [Google Scholar] [CrossRef] [PubMed]
- Galvano, F.; La Fauci, L.; Vitaglione, P.; Fogliano, V.; Vanella, L.; Felgines, C. Bioavailability, Antioxidant and Biological Properties of the Natural Free-Radical Scavengers Cyanidin and Related Glycosides. Ann. Ist. Super. Sanita 2007, 43, 382–393. [Google Scholar] [PubMed]
- Charron, C.S.; Kurilich, A.C.; Clevidence, B.A.; Simon, P.W.; Harrison, D.J.; Britz, S.J.; Baer, D.J.; Novotny, J.A. Bioavailability of Anthocyanins from Purple Carrot Juice: Effects of Acylation and Plant Matrix. J. Agric. Food Chem. 2009, 57, 1226–1230. [Google Scholar] [CrossRef] [PubMed]
- Mugford, C.A.; Kedderis, G.L. Sex-Dependent Metabolism of Xenobiotics. Drug Metab. Rev. 1998, 30, 441–498. [Google Scholar] [CrossRef] [PubMed]
- Renaud, H.J.; Cui, J.Y.; Khan, M.; Klaassen, C.D. Tissue Distribution and Gender-Divergent Expression of 78 Cytochrome P450 MRNAs in Mice. Tox. Sci. 2011, 124, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Kulkarni, K.; Hu, M. Gender-Dependent Differences in Uridine 5′-Diphospho-Glucuronosyltransferase Have Implications in Metabolism and Clearance of Xenobiotics. Expert Opin. Drug Metab. Toxicol. 2013, 9, 1555–1569. [Google Scholar] [CrossRef]
- Ruiz, M.L.; Mottino, A.D.; Catania, V.A.; Vore, M. Hormonal Regulation of Hepatic Drug Biotransformation and Transport Systems. Compr. Physiol. 2013, 3–4, 1721–1740. [Google Scholar]
- Dellinger, R.W.; Garcia, A.M.G.; Meyskens Jr, F.L. Differences in the Glucuronidation of Resveratrol and Pterostilbene: Altered Enzyme Specificity and Potential Gender Differences. Drug Metab. Pharmacok. 2014, 29, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Matal, J.; Jancova, P.; Šiller, M.; Masek, V.; Anzenbacherova, E.; Anzenbacher, P. Interspecies Comparison of the Glucuronidation Processes in the Man, Monkey, Pig, Dog and Rat. Neuro Endocrinol. Lett. 2008, 29, 738. [Google Scholar]
- Yamazaki, K.; Suzuki, M.; Itoh, T.; Yamamoto, K.; Kanemitsu, M.; Matsumura, C.; Nakano, T.; Sakaki, T.; Fukami, Y.; Imaishi, H. Structural Basis of Species Differences between Human and Experimental Animal CYP1A1s in Metabolism of 3,3′,4,4′,5-Pentachlorobiphenyl. J. Biochem. 2011, 149, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Helke, K.L.; Swindle, M.M. Animal Models of Toxicology Testing: The Role of Pigs. Expert Opin. Drug Metab. Toxicol. 2013, 9, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Biochemistry of Oxidative Stress. Biochem. Soc. Trans. 2007, 35, 1147–1150. [Google Scholar] [CrossRef] [PubMed]
- Frankič, T.; Salobir, J.; Rezar, V. The Effect of Vitamin E Supplementation on Reduction of Lymphocyte DNA Damage Induced by T-2 Toxin and Deoxynivalenol in Weaned Pigs. Anim. Feed Sci. Tech. 2008, 141, 274–286. [Google Scholar] [CrossRef]
- Lin, H.; Decuypere, E.; Buyse, J. Acute Heat Stress Induces Oxidative Stress in Broiler Chickens. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2006, 144, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, N.; Koinarski, V.; Gadjeva, V. Antioxidant Status during the Course of Eimeria tenella Infection in Broiler Chickens. Vet. J. 2006, 172, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased Oxidative Stress in Obesity and Its Impact on Metabolic Syndrome. J. Clin. Investig. 2017, 114, 1752–1761. [Google Scholar] [CrossRef]
- Vuolo, M.M.; Lima, V.S.; Junior, M.R.M. Phenolic Compounds: Structure, Classification, and Antioxidant Power. In Bioactive Compounds; Segura-Campos, M.R., Ed.; Health Benefits and Potential Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 33–50. ISBN 9780128147740. [Google Scholar] [CrossRef]
- Van Hung, P. Phenolic Compounds of Cereals and Their Antioxidant Capacity. Crit. Rev. Food Sci. Nutr. 2016, 56, 25–35. [Google Scholar] [CrossRef]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and Prooxidant Properties of Flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Khlebnikov, A.I.; Schepetkin, I.A.; Domina, N.G.; Kirpotina, L.N.; Quinn, M.T. Improved Quantitative Structure–Activity Relationship Models to Predict Antioxidant Activity of Flavonoids in Chemical, Enzymatic, and Cellular Systems. Bioorg. Med. Chem. 2007, 15, 1749–1770. [Google Scholar] [CrossRef] [PubMed]
- Pietta, P.-G. Flavonoids as Antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Puiggròs, F.; Llópiz, N.; Ardévol, A.; Bladé, C.; Arola, L.; Salvadó, M.J. Grape Seed Procyanidins Prevent Oxidative Injury by Modulating the Expression of Antioxidant Enzyme Systems. J. Agric. Food Chem. 2005, 53, 6080–6086. [Google Scholar] [CrossRef] [PubMed]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic Compounds in Plants and Agri-Industrial by-Products: Antioxidant Activity, Occurrence, and Potential Uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Foti, M.; Piattelli, M.; Amico, V.; Ruberto, G. Antioxidant Activity of Phenolic Meroditerpenoids from Marine Algae. J. Photochem. Photobiol. B Biol. 1994, 26, 159–164. [Google Scholar] [CrossRef]
- Figueroa-Espinoza, M.-C.; Villeneuve, P. Phenolic Acids Enzymatic Lipophilization. J. Agric. Food Chem. 2005, 53, 2779–2787. [Google Scholar] [CrossRef] [PubMed]
- Bendary, E.; Francis, R.; Ali, H.; Sarwat, M.; El Hady, S. Antioxidant and Structure–Activity Relationships (SARs) of Some Phenolic and Anilines Compounds. Ann. Agric. Sci. 2013, 58, 173–181. [Google Scholar] [CrossRef]
- Wang, T.; Li, Q.; Bi, K. Bioactive Flavonoids in Medicinal Plants: Structure, Activity and Biological Fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
- Jin, L.-Z.; Dersjant-Li, Y.; Giannenas, I. Application of Aromatic Plants and Their Extracts in Diets of Broiler Chickens. In Feed Additives; Academic Press: Cambridge, MA, USA, 2020; pp. 159–185. [Google Scholar] [CrossRef]
- Giannenas, I.; Bonos, E.; Christaki, E.; Florou-Paneri, P. Oregano: A Feed Additive with Functional Properties. In Therapeutic Foods, 8th ed.; Holban, A.M., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 179–208. ISBN 978-0-12-811517-6. [Google Scholar] [CrossRef]
- Valenzuela-Grijalva, N.V.; Pinelli-Saavedra, A.; Muhlia-Almazan, A.; Domínguez-Díaz, D.; González-Ríos, H. Dietary Inclusion Effects of Phytochemicals as Growth Promoters in Animal Production. J. Anim. Sci. Technol. 2017, 59, 8. [Google Scholar] [CrossRef]
- Pantano, C.; Reynaert, N.L.; Vliet, A.V.D.; Janssen–Heininger, Y.M. Redox-Sensitive Kinases of the Nuclear Factor-ΚB Signaling Pathway. Antiox. Redox Signal. 2006, 8, 1791–1806. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; Glass, C.K. Anti-Inflammatory Therapy in Chronic Disease: Challenges and Opportunities. Science 2013, 339, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Hiscott, J.; Marois, J.; Garoufalis, J.; D’Addario, M.; Roulston, A.; Kwan, I.; Pepin, N.; Lacoste, J.; Nguyen, H.; Bensi, G. Characterization of a Functional NF-ΚB Site in the Human Interleukin 1β Promoter: Evidence for a Positive Autoregulatory Loop. Moll. Cell. Biol. 1993, 13, 6231–6240. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Nuclear Factor-ΚB. Int. J. Biochem. Cell. Biol. 1997, 29, 867–870. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B. Nuclear Factor-ΚB: The Enemy within. Cancer Cell 2004, 6, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Shishodia, S.; Sandur, S.K.; Pandey, M.K.; Sethi, G. Inflammation and Cancer: How Hot Is the Link? Biochem. Pharmacol. 2006, 72, 1605–1621. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C. Biologic Basis for Interleukin-1 in Disease. Blood 1996, 87, 2095–2147. [Google Scholar] [CrossRef]
- Dinarello, C.A. Proinflammatory Cytokines. Chest 2000, 118, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Pillarisetti, S.; Saxena, U. Role of Oxidative Stress and Inflammation in the Origin of Type 2 Diabetes—A Paradigm Shift. Expert Opin. Ther. Targets 2004, 8, 401–408. [Google Scholar] [CrossRef]
- Opal, S.M.; DePalo, V.A. Anti-Inflammatory Cytokines. Chest 2000, 117, 1162–1172. [Google Scholar] [CrossRef]
- Ciz, M.; Denev, P.; Kratchanova, M.; Vasicek, O.; Ambrozova, G.; Lojek, A. Flavonoids Inhibit the Respiratory Burst of Neutrophils in Mammals. Oxid. Med. Cell. Longev. 2012, 2012, 181295. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; O’connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From Inflammation to Sickness and Depression: When the Immune System Subjugates the Brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef]
- Dinarello, C.A. Anti-Inflammatory Agents: Present and Future. Cell 2010, 140, 935–950. [Google Scholar] [CrossRef]
- Sharma, M. Chemokines and Their Receptors: Orchestrating a Fine Balance between Health and Disease. Crit. Rev. Biotech. 2010, 30, 1–22. [Google Scholar] [CrossRef]
- Rubio-Perez, J.M.; Morillas-Ruiz, J.M. A Review: Inflammatory Process in Alzheimer’s Disease, Role of Cytokines. Sci. World J. 2012, 1–15. [Google Scholar] [CrossRef]
- Lykkesfeldt, J.; Svendsen, O. Oxidants and Antioxidants in Disease: Oxidative Stress in Farm Animals. Vet. J. 2007, 173, 502–511. [Google Scholar] [CrossRef]
- Lipiński, K.; Mazur, M.; Antoszkiewicz, Z.; Purwin, C. Polyphenols in Monogastric Nutrition—A Review. Ann. Anim. Sci. 2017, 17, 41–58. [Google Scholar] [CrossRef]
- Rubió, L.; Motilva, M.-J.; Romero, M.-P. Recent Advances in Biologically Active Compounds in Herbs and Spices: A Review of the Most Effective Antioxidant and Anti-Inflammatory Active Principles. Crit. Rev. Food Sci. Nutr. 2013, 53, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Sung, B.; Prasad, S.; Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Regulation of Inflammation-Mediated Chronic Diseases by Botanicals. In Recent Trends in Medicinal Plants Research; Shyur, L.F., Lau, A.S.Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 62, pp. 57–132. ISBN 978-0-12-394591-4. [Google Scholar] [CrossRef]
- Panico, A.; Cardile, V.; Avondo, S.; Garufi, F.; Gentile, B.; Puglia, C.; Bonina, F.; Santagati, N.; Ronsisvalle, G. The In Vitro Effect of a Lyophilized Extract of Wine Obtained from Jacquez Grapes on Human Chondrocytes. Phytomedicine 2006, 13, 522–526. [Google Scholar] [CrossRef]
- Vendrame, S.; Klimis-Zacas, D. Anti-Inflammatory Effect of Anthocyanins via Modulation of Nuclear Factor-ΚB and Mitogen-Activated Protein Kinase Signaling Cascades. Nutr. Rev. 2015, 73, 348–358. [Google Scholar] [CrossRef]
- Na, H.-K.; Surh, Y.-J. Modulation of Nrf2-Mediated Antioxidant and Detoxifying Enzyme Induction by the Green Tea Polyphenol EGCG. Food Chem. Toxicol. 2008, 46, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.-C.; McIntosh, M.K. Potential Mechanisms by Which Polyphenol-Rich Grapes Prevent Obesity-Mediated Inflammation and Metabolic Diseases. Ann. Rev. Nutr. 2011, 31, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Cornelius, C.; Dinkova-Kostova, A.T.; Iavicoli, I.; Di Paola, R.; Koverech, A.; Cuzzocrea, S.; Rizzarelli, E.; Calabrese, E.J. Cellular Stress Responses, Hormetic Phytochemicals and Vitagenes in Aging and Longevity. BBA-Mol. Basis Dis. 2012, 1822, 753–783. [Google Scholar] [CrossRef]
- Son, T.G.; Camandola, S.; Mattson, M.P. Hormetic Dietary Phytochemicals. Neuromol. Med. 2008, 10, 236–246. [Google Scholar] [CrossRef]
- Ishii, T.; Itoh, K.; Yamamoto, M. Roles of Nrf2 in Activation of Antioxidant Enzyme Genes via Antioxidant Responsive Elements. In Protein Sensors and Reactive Oxygen Species—Part B: Thiol Enzymes and Proteins Series Methods in Enzymology; Sies, H., Packer, L., Eds.; Academic Press: New York, NY, USA, 2002; Volume 348, pp. 182–190. [Google Scholar] [CrossRef]
- Lee, J.; Giordano, S.; Zhang, J. Autophagy, Mitochondria and Oxidative Stress: Cross-Talk and Redox Signalling. Biochem. J. 2012, 441, 523–540. [Google Scholar] [CrossRef]
- Li, W.-G.; Zhang, X.-Y.; Wu, Y.; Tian, X. Anti-Inflammatory Effect and Mechanism of Proanthocyanidins from Grape Seeds. Acta Pharm. Sin. 2001, 22, 1117–1120. [Google Scholar]
- Terra, X.; Valls, J.; Vitrac, X.; Mérrillon, J.-M.; Arola, L.; Ardèvol, A.; Bladé, C.; Fernández-Larrea, J.; Pujadas, G.; Salvadó, J. Grape-Seed Procyanidins Act as Antiinflammatory Agents in Endotoxin-Stimulated RAW 264.7 Macrophages by Inhibiting NFkB Signaling Pathway. J. Agric. Food Chem. 2007, 55, 4357–4365. [Google Scholar] [CrossRef]
- Bitzer, Z.T.; Glisan, S.L.; Dorenkott, M.R.; Goodrich, K.M.; Ye, L.; O’Keefe, S.F.; Lambert, J.D.; Neilson, A.P. Cocoa Procyanidins with Different Degrees of Polymerization Possess Distinct Activities in Models of Colonic Inflammation. J. Nutr. Biochem. 2015, 26, 827–831. [Google Scholar] [CrossRef]
- Williams, A.R.; Klaver, E.J.; Laan, L.C.; Ramsay, A.; Fryganas, C.; Difborg, R.; Kringel, H.; Reed, J.D.; Mueller-Harvey, I.; Skov, S. Co-operative Suppression of Inflammatory Responses in Human Dendritic Cells by Plant Proanthocyanidins and Products from the Parasitic Nematode Trichuris Suis. Immunology 2017, 150, 312–328. [Google Scholar] [CrossRef] [PubMed]
- Midttun, H.L.; Ramsay, A.; Mueller-Harvey, I.; Williams, A.R. Cocoa Procyanidins Modulate Transcriptional Pathways Linked to Inflammation and Metabolism in Human Dendritic Cells. Food Funct. 2018, 9, 2883–2890. [Google Scholar] [CrossRef]
- Skyberg, J.A.; Robison, A.; Golden, S.; Rollins, M.F.; Callis, G.; Huarte, E.; Kochetkova, I.; Jutila, M.A.; Pascual, D.W. Apple Polyphenols Require T Cells to Ameliorate Dextran Sulfate Sodium-Induced Colitis and Dampen Proinflammatory Cytokine Expression. J. Leukoc. Biol. 2011, 90, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, Y.; Akiyama, H.; Nakano, M.; Shoji, T.; Kanda, T.; Ohtake, Y.; Takita, T.; Matsuda, R.; Maitani, T. Orally Administered Apple Procyanidins Protect against Experimental Inflammatory Bowel Disease in Mice. Int. Immunopharmacol. 2008, 8, 1802–1807. [Google Scholar] [CrossRef]
- Park, M.-K.; Park, J.-S.; Cho, M.-L.; Oh, H.-J.; Heo, Y.-J.; Woo, Y.-J.; Heo, Y.-M.; Park, M.-J.; Park, H.-S.; Park, S.-H. Grape Seed Proanthocyanidin Extract (GSPE) Differentially Regulates Foxp3+ Regulatory and IL-17+ Pathogenic T Cell in Autoimmune Arthritis. Immunol. Lett. 2011, 135, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Daughenbaugh, K.F.; Holderness, J.; Graff, J.C.; Hedges, J.F.; Freedman, B.; Graff, J.W.; Jutila, M.A. Contribution of Transcript Stability to a Conserved Procyanidin-Induced Cytokine Response in Γδ T Cells. Genes Immun. 2011, 12, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Holderness, J.; Jackiw, L.; Kimmel, E.; Kerns, H.; Radke, M.; Hedges, J.F.; Petrie, C.; McCurley, P.; Glee, P.M.; Palecanda, A. Select Plant Tannins Induce IL-2Rα up-Regulation and Augment Cell Division in Γδ T Cells. J. Immunol. 2007, 179, 6468–6478. [Google Scholar] [CrossRef] [PubMed]
- Witherden, D.A.; Havran, W.L. Cross-Talk between Intraepithelial Γδ T Cells and Epithelial Cells. J. Leukoc. Biol. 2013, 94, 69–76. [Google Scholar] [CrossRef]
- Casanova-Martí, À.; González-Abuín, N.; Serrano, J.; Blay, M.T.; Terra, X.; Frost, G.; Pinent, M.; Ardevol, A. Long Term Exposure to a Grape Seed Proanthocyanidin Extract Enhances L-Cell Differentiation in Intestinal Organoids. Mol. Nutr. Food Res. 2020, 64, 2000303. [Google Scholar] [CrossRef] [PubMed]
- Linden, S.; Sutton, P.; Karlsson, N.; Korolik, V.; McGuckin, M. Mucins in the Mucosal Barrier to Infection. Mucosal Immunol. 2008, 1, 183–197. [Google Scholar] [CrossRef]
- Georgiades, P.; Pudney, P.D.; Rogers, S.; Thornton, D.J.; Waigh, T.A. Tea Derived Galloylated Polyphenols Cross-Link Purified Gastrointestinal Mucins. PLoS ONE 2014, 9, e105302. [Google Scholar] [CrossRef]
- Martins, E.; Ferreira, A.C.F.; Skorupa, A.L.; Afeche, S.C.; Cipolla-Neto, J.; Costa Rosa, L.F.B.P. Tryptophan Consumption and Indoleamines Production by Peritoneal Cavity Macrophages. J. Leukoc. Biol. 2004, 75, 1116–1121. [Google Scholar] [CrossRef]
- Rosillo, M.; Sanchez-Hidalgo, M.; Cárdeno, A.; De La Lastra, C.A. Protective Effect of Ellagic Acid, a Natural Polyphenolic Compound, in a Murine Model of Crohn’s Disease. Biochem. Pharm. 2011, 82, 737–745. [Google Scholar] [CrossRef]
- Zhang, G.; Ross, C.; Blecha, F. Porcine Antimicrobial Peptides: New Prospects for Ancient Molecules of Host Defense. Vet. Res. 2000, 31, 277–296. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhang, G.; Wu, H.; Ross, C.; Blecha, F.; Ganz, T. Porcine Epithelial β-Defensin 1 Is Expressed in the Dorsal Tongue at Antimicrobial Concentrations. Infect. Immun. 1999, 67, 3121–3127. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Patil, A.A.; Zhang, G.; Ross, C.R.; Blecha, F. Bioinformatic and Expression Analysis of Novel Porcine β-Defensins. Mamm. Genome 2006, 17, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.L.; Ling, K.; Wang, M.; El-Nezami, H. Green Tea Polyphenol Epigallocatechin-3-gallate Improves Epithelial Barrier Function by Inducing the Production of Antimicrobial Peptide PBD-1 and PBD-2 in Monolayers of Porcine Intestinal Epithelial IPEC-J2 Cells. Mol. Nutr. Food Res. 2016, 60, 1048–1058. [Google Scholar] [CrossRef] [PubMed]
- Gessner, D.K.; Fiesel, A.; Most, E.; Dinges, J.; Wen, G.; Ringseis, R.; Eder, K. Supplementation of a Grape Seed and Grape Marc Meal Extract Decreases Activities of the Oxidative Stress-Responsive Transcription Factors NF-ΚB and Nrf2 in the Duodenal Mucosa of Pigs. Acta Vet. Scand. 2013, 55, 18. [Google Scholar] [CrossRef]
- Fiesel, A.; Gessner, D.K.; Most, E.; Eder, K. Effects of Dietary Polyphenol-Rich Plant Products from Grape or Hop on pro-Inflammatory Gene Expression in the Intestine, Nutrient Digestibility and Faecal Microbiota of Weaned Pigs. BMC Vet. Res. 2014, 10, 196. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Verma, I.M. NF-ΚB Regulation in the Immune System. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Rogler, G.; Brand, K.; Vogl, D.; Page, S.; Hofmeister, R.; Andus, T.; Knuechel, R.; Baeuerle, P.A.; Schölmerich, J.; Gross, V. Nuclear Factor ΚB Is Activated in Macrophages and Epithelial Cells of Inflamed Intestinal Mucosa. Gastroenterology 1998, 115, 357–369. [Google Scholar] [CrossRef]
- Stadnyk, A.W. Intestinal Epithelial Cells as a Source of Inflammatory Cytokines and Chemokines. Can. J. Gastroenterol. 2002, 16, 241–246. [Google Scholar] [CrossRef]
- Jung, H.C.; Eckmann, L.; Yang, S.; Panja, A.; Fierer, J.; Morzycka-Wroblewska, E.; Kagnoff, M. A Distinct Array of Proinflammatory Cytokines Is Expressed in Human Colon Epithelial Cells in Response to Bacterial Invasion. J. Clin. Investig. 1995, 95, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Son, D.O.; Satsu, H.; Shimizu, M. Histidine Inhibits Oxidative Stress-and TNF-α-Induced Interleukin-8 Secretion in Intestinal Epithelial Cells. FEBS Lett. 2005, 579, 4671–4677. [Google Scholar] [CrossRef]
- Ramiro-Puig, E.; Pérez-Cano, F.; Ramírez-Santana, C.; Castellote, C.; Izquierdo-Pulido, M.; Permanyer, J.; Franch, A.; Castell, M. Spleen Lymphocyte Function Modulated by a Cocoa-Enriched Diet. Clin. Exp. Immunol. 2007, 149, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Taranu, I.; Habeanu, M.; Gras, M.; Pistol, G.; Lefter, N.; Palade, M.; Ropota, M.; Sanda Chedea, V.; Marin, D. Assessment of the Effect of Grape Seed Cake Inclusion in the Diet of Healthy Fattening-finishing Pigs. J. Anim. Physiol. Anim. Nutr. 2018, 102, e30–e42. [Google Scholar] [CrossRef]
- Okazaki, Y.; Kakehi, S.; Xu, Y.; Tsujimoto, K.; Sasaki, M.; Ogawa, H.; Kato, N. Consumption of Sericin Reduces Serum Lipids, Ameliorates Glucose Tolerance and Elevates Serum Adiponectin in Rats Fed a High-Fat Diet. Biosci. Biotechnol. Biochem. 2010, 74, 1534–1538. [Google Scholar] [CrossRef] [PubMed]
- Phalipon, A.; Cardona, A.; Kraehenbuhl, J.-P.; Edelman, L.; Sansonetti, P.J.; Corthésy, B. Secretory Component: A New Role in Secretory IgA-Mediated Immune Exclusion In Vivo. Immunity 2002, 17, 107–115. [Google Scholar] [CrossRef]
- Mantis, N.J.; Rol, N.; Corthésy, B. Secretory IgA’s Complex Roles in Immunity and Mucosal Homeostasis in the Gut. Mucosal Immunol. 2011, 4, 603–611. [Google Scholar] [CrossRef]
- Zhang, H.J.; Jiang, X.R.; Mantovani, G.; Lumbreras, A.E.V.; Comi, M.; Alborali, G.; Savoini, G.; Dell’Orto, V.; Bontempo, V. Modulation of Plasma Antioxidant Activity in Weaned Piglets by Plant Polyphenols. Ital. J. Anim. Sci. 2014, 13, 3242. [Google Scholar] [CrossRef]
- Gessner, D.; Ringseis, R.; Eder, K. Potential of Plant Polyphenols to Combat Oxidative Stress and Inflammatory Processes in Farm Animals. J. Anim. Physiol. Anim. Nutr. 2017, 101, 605–628. [Google Scholar] [CrossRef]
- Yan, L.; Kim, I. Effect of Dietary Grape Pomace Fermented by Saccharomyces Boulardii on the Growth Performance, Nutrient Digestibility and Meat Quality in Finishing Pigs. Asian-Australas. J. Anim. Sci. 2011, 24, 1763–1770. [Google Scholar] [CrossRef]
- Han, M.; Song, P.; Huang, C.; Rezaei, A.; Farrar, S.; Brown, M.A.; Ma, X. Dietary Grape Seed Proanthocyanidins (GSPs) Improve Weaned Intestinal Microbiota and Mucosal Barrier Using a Piglet Model. Oncotarget 2016, 7, 80313. [Google Scholar] [CrossRef] [PubMed]
- Kafantaris, I.; Stagos, D.; Kotsampasi, B.; Hatzis, A.; Kypriotakis, A.; Gerasopoulos, K.; Makri, S.; Goutzourelas, N.; Mitsagga, C.; Giavasis, I. Grape Pomace Improves Performance, Antioxidant Status, Fecal Microbiota and Meat Quality of Piglets. Animal 2018, 12, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Hossain, M.; Kim, G.; Hwang, J.; Ji, H.; Yang, C. Effects of Resveratrol and Essential Oils on Growth Performance, Immunity, Digestibility and Fecal Microbial Shedding in Challenged Piglets. Asian-Australas. J. Anim. Sci. 2013, 26, 683. [Google Scholar] [CrossRef]
- Verhelst, R.; Schroyen, M.; Buys, N.; Niewold, T. Dietary Polyphenols Reduce Diarrhea in Enterotoxigenic Escherichia coli (ETEC) Infected Post-Weaning Piglets. Livest. Sci. 2014, 160, 138–140. [Google Scholar] [CrossRef]
- Grosu, I.A.; Pistol, G.C.; Taranu, I.; Marin, D.E. The Impact of Dietary Grape Seed Meal on Healthy and Aflatoxin B1 Afflicted Microbiota of Pigs after Weaning. Toxins 2019, 11, 25. [Google Scholar] [CrossRef]
- Choy, Y.Y.; Quifer-Rada, P.; Holstege, D.M.; Frese, S.A.; Calvert, C.C.; Mills, D.A.; Lamuela-Raventos, R.M.; Waterhouse, A.L. Phenolic Metabolites and Substantial Microbiome Changes in Pig Feces by Ingesting Grape Seed Proanthocyanidins. Food Funct. 2014, 5, 2298–2308. [Google Scholar] [CrossRef]
- Hughes, R.; Brooker, J.; Smyl, C. Growth Rate of Broiler Chickens given Condensed Tannins Extracted from Grape Seed. In Proceedings of the 17th Australian Poultry Science Symposium, Sydney, Australia, 7–9 February 2005. [Google Scholar]
- Blander, J.M.; Longman, R.S.; Iliev, I.D.; Sonnenberg, G.F.; Artis, D. Regulation of Inflammation by Microbiota Interactions with the Host. Nat. Immunol. 2017, 18, 851–860. [Google Scholar] [CrossRef]
- Peterson, L.W.; Artis, D. Intestinal Epithelial Cells: Regulators of Barrier Function and Immune Homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef]
- Winer, D.A.; Luck, H.; Tsai, S.; Winer, S. The Intestinal Immune System in Obesity and Insulin Resistance. Cell Metab. 2016, 23, 413–426. [Google Scholar] [CrossRef]
- Mosele, J.I.; Macià, A.; Motilva, M.-J. Metabolic and Microbial Modulation of the Large Intestine Ecosystem by Non-Absorbed Diet Phenolic Compounds: A Review. Molecules 2015, 20, 17429–17468. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary Phenolics: Chemistry, Bioavailability and Effects on Health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef]
- Bustos, I.; Garcia-Cayuela, T.; Hernandez-Ledesma, B.; Pelaez, C.; Requena, T.; Martínez-Cuesta, M.C. Effect of Flavan-3-Ols on the Adhesion of Potential Probiotic Lactobacilli to Intestinal Cells. J. Agric. Food Chem. 2012, 60, 9082–9088. [Google Scholar] [CrossRef]
- Tzounis, X.; Vulevic, J.; Kuhnle, G.G.; George, T.; Leonczak, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P. Flavanol Monomer-Induced Changes to the Human Faecal Microflora. Br. J. Nutr. 2008, 99, 782–792. [Google Scholar] [CrossRef] [PubMed]
- González-Quilen, C.; Rodríguez-Gallego, E.; Beltrán-Debón, R.; Pinent, M.; Ardévol, A.; Blay, M.T.; Terra, X. Health-Promoting Properties of Proanthocyanidins for Intestinal Dysfunction. Nutrients 2020, 12, 130. [Google Scholar] [CrossRef]
- Gonthier, M.-P.; Donovan, J.L.; Texier, O.; Felgines, C.; Remesy, C.; Scalbert, A. Metabolism of Dietary Procyanidins in Rats. Free Radic. Biol. Med. 2003, 35, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A. Antimicrobial Properties of Tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Anhê, F.F.; Pilon, G.; Roy, D.; Desjardins, Y.; Levy, E.; Marette, A. Triggering Akkermansia with Dietary Polyphenols: A New Weapon to Combat the Metabolic Syndrome? Gut Microbes 2016, 7, 146–153. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, N.; Song, P.; He, T.; Levesque, C.; Bai, Y.; Zhang, A.; Ma, X. Grape Seed Proanthocyanidin Affects Lipid Metabolism via Changing Gut Microflora and Enhancing Propionate Production in Weaned Pigs. J. Nutr. 2019, 149, 1523–1532. [Google Scholar] [CrossRef]
- KruegerChristian, G.; MeudtJennifer, J.; ReedJess, D. Effect of Sweetened Dried Cranberry Consumption on Urinary Proteome and Fecal Microbiome in Healthy Human Subjects. Omics A J. Integr. Biol. 2018, 22, 145–153. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, Z.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Surface Components and Metabolites of Probiotics for Regulation of Intestinal Epithelial Barrier. Microb. Cell Fact. 2020, 19, 23. [Google Scholar] [CrossRef]
- Williams, A.R.; Krych, L.; Fauzan Ahmad, H.; Nejsum, P.; Skovgaard, K.; Nielsen, D.S.; Thamsborg, S.M. A Polyphenol-Enriched Diet and Ascaris suum Infection Modulate Mucosal Immune Responses and Gut Microbiota Composition in Pigs. PLoS ONE 2017, 12, e0186546. [Google Scholar] [CrossRef] [PubMed]
- Pineiro, M.; Asp, N.-G.; Reid, G.; Macfarlane, S.; Morelli, L.; Brunser, O.; Tuohy, K. FAO Technical Meeting on Prebiotics. J. Clin. Gastroenterol. 2008, 42, S156–S159. [Google Scholar] [CrossRef] [PubMed]
- Christaki, E.; Giannenas, I.; Bonos, E.; Florou-Paneri, P. Chapter 2—Innovative Uses of Aromatic Plants as Natural Supplements in Nutrition. In Feed Additives; Florou-Paneri, P., Christaki, E., Giannenas, I., Eds.; Academic Press: New York, NY, USA, 2020; pp. 19–34. ISBN 978-0-12-814700-9. [Google Scholar] [CrossRef]
- Mohammadi Gheisar, M.; Kim, I.H. Phytobiotics in Poultry and Swine Nutrition—A Review. Ital. J. Anim. Sci. 2018, 17, 92–99. [Google Scholar] [CrossRef]
- Yang, C.; Chowdhury, M.K.; Hou, Y.; Gong, J. Phytogenic Compounds as Alternatives to In-Feed Antibiotics: Potentials and Challenges in Application. Pathogens 2015, 4, 137–156. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Bok, S.-H.; Jeon, S.-M.; Park, Y.B.; Lee, S.-J.; Jeong, T.-S.; Choi, M.-S. Effect of Rutin and Tannic Acid Supplements on Cholesterol Metabolism in Rats. Nutr. Res. 2002, 22, 283–295. [Google Scholar] [CrossRef]
- Guinane, C.M.; Cotter, P.D. Role of the Gut Microbiota in Health and Chronic Gastrointestinal Disease: Understanding a Hidden Metabolic Organ. Therap. Adv. Gastroenterol. 2013, 6, 295–308. [Google Scholar] [CrossRef]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human Nutrition, the Gut Microbiome and the Immune System. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef]
- Krajmalnik-Brown, R.; Ilhan, Z.; Kang, D.; DiBaise, J.K. Effects of Gut Microbes on Nutrient Absorption and Energy Regulation. Nutr. Clin. Pract. 2012, 27, 201–214. [Google Scholar] [CrossRef]
- Everard, A.; Cani, P.D. Diabetes, Obesity and Gut Microbiota. Best Pract. Res. Clin. Gastroenterol. 2013, 27, 73–83. [Google Scholar] [CrossRef]
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut Microbiota and IBD: Causation or Correlation? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 573–584. [Google Scholar] [CrossRef]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R. Gut Microbiota from Twins Discordant for Obesity Modulate Metabolism in Mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Galluzzi, L.; Viaud, S.; Vétizou, M.; Daillère, R.; Merad, M.; Kroemer, G. Cancer and the Gut Microbiota: An Unexpected Link. Sci. Transl. Med. 2015, 7, 271ps1. [Google Scholar] [CrossRef] [PubMed]
- Burdulis, D.; Sarkinas, A.; Jasutiene, I.; Stackevicené, E.; Nikolajevas, L.; Janulis, V. Comparative Study of Anthocyanin Composition, Antimicrobial and Antioxidant Activity in Bilberry (Vaccinium myrtillus L.) and Blueberry (Vaccinium corymbosum L.) Fruits. Acta Pol. Pharm. 2009, 66, 399–408. [Google Scholar]
- Cushnie, T.T.; Lamb, A.J. Recent Advances in Understanding the Antibacterial Properties of Flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Perumalla, A.; Hettiarachchy, N.S. Green Tea and Grape Seed Extracts—Potential Applications in Food Safety and Quality. Food Res. Int. 2011, 44, 827–839. [Google Scholar] [CrossRef]
- Jayaprakasha, G.; Selvi, T.; Sakariah, K. Antibacterial and Antioxidant Activities of Grape (Vitis vinifera) Seed Extracts. Food Res. Int. 2003, 36, 117–122. [Google Scholar] [CrossRef]
- Rhodes, P.; Mitchell, J.; Wilson, M.; Melton, L. Antilisterial Activity of Grape Juice and Grape Extracts Derived from Vitis vinifera Variety Ribier. Int. J. Food Microbiol. 2006, 107, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadi, M.; Chorianopoulos, N.G.; Nychas, G.-J.E.; Haroutounian, S.A. Antilisterial Activities of Polyphenol-Rich Extracts of Grapes and Vinification Byproducts. J. Agric. Food Chem. 2009, 57, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Gañan, M.; Martínez-Rodríguez, A.J.; Carrascosa, A.V. Antimicrobial Activity of Phenolic Compounds of Wine against Campylobacter Jejuni. Food Control 2009, 20, 739–742. [Google Scholar] [CrossRef]
- Papadopoulou, C.; Soulti, K.; Roussis, I.G. Potential Antimicrobial Activity of Red and White Wine Phenolic Extracts against Strains of Staphylococcus Aureus, Escherichia Coli and Candida Albicans. Food Technol. Biotechnol. 2005, 43, 41–46. [Google Scholar]
- Özkan, G.; Sagdiç, O.; Göktürk Baydar, N.; Kurumahmutoglu, Z. Antibacterial Activities and Total Phenolic Contents of Grape Pomace Extracts. J. Sci. Food Agric. 2004, 84, 1807–1811. [Google Scholar] [CrossRef]
- Silván, J.M.; Mingo, E.; Hidalgo, M.; de Pascual-Teresa, S.; Carrascosa, A.V.; Martinez-Rodriguez, A.J. Antibacterial Activity of a Grape Seed Extract and Its Fractions against Campylobacter spp. Food Control 2013, 29, 25–31. [Google Scholar] [CrossRef]
- Daroch, F.; Hoeneisen, M.; González, C.L.; Kawaguchi, F.; Salgado, F.; Solar, H.; García, A. In Vitro Antibacterial Activity of Chilean Red Wines against Helicobacter Pylori. Microbios 2001, 104, 79–85. [Google Scholar] [PubMed]
- Just, J.; Daeschel, M. Antimicrobial Effects of Wine on Escherichia coli O157: H7 and Salmonella typhimurium in a Model Stomach System. J. Food Sci. 2003, 68, 285–290. [Google Scholar] [CrossRef]
- Zhang, J.; Hou, X.; Ahmad, H.; Zhang, H.; Zhang, L.; Wang, T. Assessment of Free Radicals Scavenging Activity of Seven Natural Pigments and Protective Effects in AAPH-Challenged Chicken Erythrocytes. Food Chem. 2014, 145, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Hervert-Hernández, D.; Pintado, C.; Rotger, R.; Goñi, I. Stimulatory Role of Grape Pomace Polyphenols on Lactobacillus Acidophilus Growth. Int. J. Food Microbiol. 2009, 136, 119–122. [Google Scholar] [CrossRef]
- Morris, G.; Berk, M.; Carvalho, A.; Caso, J.R.; Sanz, Y.; Walder, K.; Maes, M. The Role of the Microbial Metabolites Including Tryptophan Catabolites and Short Chain Fatty Acids in the Pathophysiology of Immune-Inflammatory and Neuroimmune Disease. Mol. Neurobiol. 2017, 54, 4432–4451. [Google Scholar] [CrossRef]
- Leser, T.D.; Amenuvor, J.Z.; Jensen, T.K.; Lindecrona, R.H.; Boye, M.; Møller, K. Culture-Independent Analysis of Gut Bacteria: The Pig Gastrointestinal Tract Microbiota Revisited. Appl. Environ. Microbiol. 2002, 68, 673–690. [Google Scholar] [CrossRef]
- Kim, H.B.; Isaacson, R.E. The Pig Gut Microbial Diversity: Understanding the Pig Gut Microbial Ecology through the next Generation High Throughput Sequencing. Vet. Microbiol. 2015, 177, 242–251. [Google Scholar] [CrossRef]
- Yutin, N.; Galperin, M.Y. A Genomic Update on Clostridial Phylogeny: G Ram-negative Spore Formers and Other Misplaced Clostridia. Environ. Microbiol. 2013, 15, 2631–2641. [Google Scholar] [CrossRef]
- Shah, H.N.; Collins, D.M. Prevotella, a New Genus to Include Bacteroides Melaninogenicus and Related Species Formerly Classified in the Genus Bacteroides. Int. J. Syst. Evol. Micr. 1990, 40, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Hara, H.; Orita, N.; Hatano, S.; Ichikawa, H.; Hara, Y.; Matsumoto, N.; Kimura, Y.; Terada, A.; Mitsuoka, T. Effect of Tea Polyphenols on Fecal Flora and Fecal Metabolic Products of Pigs. J. Vet. Med. Sci. 1995, 57, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, N.; Chu, D.-C.; Akachi, S.; Juneja, L. Improvement of Intestinal Microflora Balance and Prevention of Digestive and Respiratory Organ Diseases in Calves by Green Tea Extracts. Livest. Prod. Sci. 2001, 68, 217–229. [Google Scholar] [CrossRef]
- Rastall, R.A.; Gibson, G.R.; Gill, H.S.; Guarner, F.; Klaenhammer, T.R.; Pot, B.; Reid, G.; Rowland, I.R.; Sanders, M.E. Modulation of the Microbial Ecology of the Human Colon by Probiotics, Prebiotics and Synbiotics to Enhance Human Health: An Overview of Enabling Science and Potential Applications. FEMS Microbiol. Ecol. 2005, 52, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Lopetuso, L.R.; Scaldaferri, F.; Petito, V.; Gasbarrini, A. Commensal Clostridia: Leading Players in the Maintenance of Gut Homeostasis. Gut Pathog. 2013, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Wu, X.; Chen, D.; Yu, B.; He, J. Adaptation of Gut Microbiome to Different Dietary Nonstarch Polysaccharide Fractions in a Porcine Model. Mol. Nutr. Food Res. 2017, 61, 1700012. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, Y.; Liu, S.; Huang, J.; Zhai, Z.; He, C.; Ding, J.; Wang, J.; Wang, H.; Fan, W. The Dynamic Distribution of Porcine Microbiota across Different Ages and Gastrointestinal Tract Segments. PLoS ONE 2015, 10, e0117441. [Google Scholar] [CrossRef]
- Castrica, M.; Rebucci, R.; Giromini, C.; Tretola, M.; Cattaneo, D.; Baldi, A. Total Phenolic Content and Antioxidant Capacity of Agri-Food Waste and by-Products. Ital. J. Anim. Sci. 2019, 18, 336–341. [Google Scholar] [CrossRef]
- Luehring, M.; Blank, R.; Wolffram, S. Vitamin E-Sparing and Vitamin E-Independent Antioxidative Effects of the Flavonol Quercetin in Growing Pigs. Anim. Feed Sci. Tech. 2011, 169, 199–207. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, J.; Ahmad, H.; Zhang, H.; Xu, Z.; Wang, T. Evaluation of Antioxidant Activities of Ampelopsin and Its Protective Effect in Lipopolysaccharide-Induced Oxidative Stress Piglets. PLoS ONE 2014, 9, e108314. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, N.; Peng, Y.; Qi, D. Interaction of Zearalenone and Soybean Isoflavone in Diets on the Growth Performance, Organ Development and Serum Parameters in Prepubertal Gilts. J. Anim. Physiol. Anim. Nutr. 2012, 96, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Starčević, K.; Krstulović, L.; Brozić, D.; Maurić, M.; Stojević, Z.; Mikulec, Ž.; Bajić, M.; Mašek, T. Production Performance, Meat Composition and Oxidative Susceptibility in Broiler Chicken Fed with Different Phenolic Compounds. J. Sci. Food Agric. 2015, 95, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-J.; Jung, U.J.; Park, H.-J.; Kim, H.-J.; Park, Y.B.; Kim, S.R.; Choi, M.-S. Combined Ethanol Extract of Grape Pomace and Omija Fruit Ameliorates Adipogenesis, Hepatic Steatosis, and Inflammation in Diet-Induced Obese Mice. Evid. Based Complement. Altern. Med. 2013, 2013, 212139. [Google Scholar] [CrossRef] [PubMed]
- Han, H.J.; Jung, U.J.; Kim, H.-J.; Moon, B.S.; Cho, S.-J.; Park, Y.B.; Lee, D.G.; Choi, M.-S. Dual Effects of a Mixture of Grape Pomace (Campbell Early) and Omija Fruit Ethanol Extracts on Lipid Metabolism and the Antioxidant Defense System in Diet-Induced Obese Mice. Nutr. Res. Pract. 2015, 9, 227. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Mei, X.; Xu, D.; Xu, S.; Lv, J. Protective effects of polysacchride of Spirulina platensis and Sargassum thunbeergii on vascular of alloxan induced diabetic rats. Zhongguo Zhong Yao Za Zhi 2005, 30, 211–215. [Google Scholar] [PubMed]
- Evans, M.; Wilson, D.; Guthrie, N. A Randomized, Double-Blind, Placebo-Controlled, Pilot Study to Evaluate the Effect of Whole Grape Extract on Antioxidant Status and Lipid Profile. J. Funct. Foods 2014, 7, 680–691. [Google Scholar] [CrossRef]
- Ngamukote, S.; Mäkynen, K.; Thilawech, T.; Adisakwattana, S. Cholesterol-Lowering Activity of the Major Polyphenols in Grape Seed. Molecules 2011, 16, 5054–5061. [Google Scholar] [CrossRef] [PubMed]
- Baselga-Escudero, L.; Blade, C.; Ribas-Latre, A.; Casanova, E.; Suarez, M.; Torres, J.L.; Salvado, M.J.; Arola, L.; Arola-Arnal, A. Resveratrol and EGCG Bind Directly and Distinctively to MiR-33a and MiR-122 and Modulate Divergently Their Levels in Hepatic Cells. Nucleic Acids Res. 2013, 42, 882–892. [Google Scholar] [CrossRef]
- Catoni, C.; Peters, A.; Schaefer, H.M. Life History Trade-Offs Are Influenced by the Diversity, Availability and Interactions of Dietary Antioxidants. Anim. Behav. 2008, 76, 1107–1119. [Google Scholar] [CrossRef]
- Kannan, G.; Heath, J.; Wabeck, C.; Mench, J. Shackling of Broilers: Effects on Stress Responses and Breast Meat Quality. Brit. Poult. Sci. 1997, 38, 323–332. [Google Scholar] [CrossRef]
- Xing, T.; Gao, F.; Tume, R.K.; Zhou, G.; Xu, X. Stress Effects on Meat Quality: A Mechanistic Perspective. Compr. Rev. Food Sci. Food Saf. 2019, 18, 380–401. [Google Scholar] [CrossRef] [PubMed]
- Lipiński, K.; Antoszkiewicz, Z.; Mazur-Kuśnirek, M.; Korniewicz, D.; Kotlarczyk, S. The Effect of Polyphenols on the Performance and Antioxidant Status of Sows and Piglets. Ital. J. Anim. Sci. 2019, 18, 174–181. [Google Scholar] [CrossRef]
| Typ | Name | Chemical Structure | Radicals | Compound | |||
|---|---|---|---|---|---|---|---|
| Flavonoid | Flavanol |  | R1 | R2 | R3 | R4 | |
| H | OH | H | OH | Catechin | |||
| H | OH | OH | H | Epicatechin | |||
| H | OH | G | H | Epicatechingallate | |||
| PH | OH | OH | H | Epigallocatechina | |||
| OH | OH | H | OH | Gallocatechin | |||
| Anthocyanin |  | ||||||
| Proanthocyanidin B-type link | 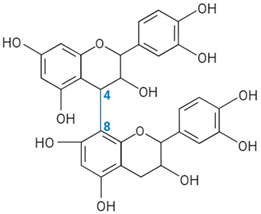 | ||||||
| Proanthocyanidin B-type link | 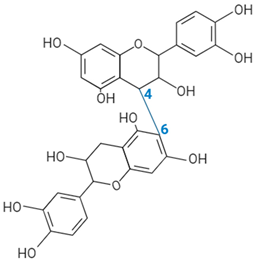 | ||||||
| Proanthocyanidin A-type link | 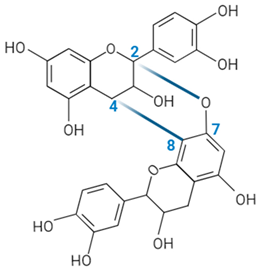 | ||||||
| Flavonol | 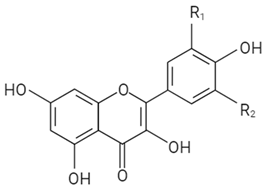 | R1 | R2 | ||||
| OH | OH | Myricetin | |||||
| OH | H | Quercetin | |||||
| H | H | Kaempferol | |||||
| OCH3 | H | Isorhamnetin | |||||
| Non-flavonoid | Hydroxybenzoic acid | 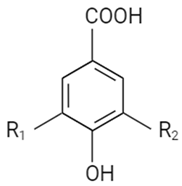 | R1 | R2 | |||
| OH | OH | Gallic acid | |||||
| OH | H | Protocatechuic acid | |||||
| OCH3 | OCH3 | Syringic acid | |||||
| H | H | P-Hydroxybenzoic acid | |||||
| Hydroxycinnamic acid | 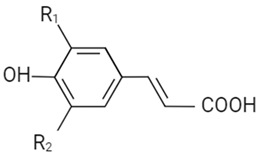 | R1 | R2 | ||||
| OH | H | Cafeic acid | |||||
| OCH3 | H | Ferulic acid | |||||
| OCH3 | OCH3 | Sinaptic acid | |||||
| H | H | p-Coumaric acid | |||||
| Stilbene | 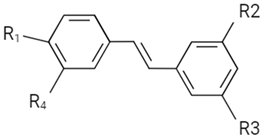 | R1 | R2 | R3 | R4 | ||
| OH | OH | OH | H | Resveratrol | |||
| OH | OH | OH | OH | Piceatannol | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Proca, A.C.; Horodincu, L.; Solcan, C.; Solcan, G. The Potential of Grape Polyphenols Additive in Pig Nutrition: Chemical Structure, Bioavailability and Their Effect on Intestinal Health of Pigs. Agriculture 2024, 14, 1142. https://doi.org/10.3390/agriculture14071142
Proca AC, Horodincu L, Solcan C, Solcan G. The Potential of Grape Polyphenols Additive in Pig Nutrition: Chemical Structure, Bioavailability and Their Effect on Intestinal Health of Pigs. Agriculture. 2024; 14(7):1142. https://doi.org/10.3390/agriculture14071142
Chicago/Turabian StyleProca, Andrei Claudiu, Loredana Horodincu, Carmen Solcan, and Gheorghe Solcan. 2024. "The Potential of Grape Polyphenols Additive in Pig Nutrition: Chemical Structure, Bioavailability and Their Effect on Intestinal Health of Pigs" Agriculture 14, no. 7: 1142. https://doi.org/10.3390/agriculture14071142
APA StyleProca, A. C., Horodincu, L., Solcan, C., & Solcan, G. (2024). The Potential of Grape Polyphenols Additive in Pig Nutrition: Chemical Structure, Bioavailability and Their Effect on Intestinal Health of Pigs. Agriculture, 14(7), 1142. https://doi.org/10.3390/agriculture14071142






