A Study on the Effect Mechanism of Pectin Modification on the Carrot Cell Wall’s Texture Formation under Ultrasonic and Infrared Drying
Abstract
1. Introduction
2. Materials and Methods
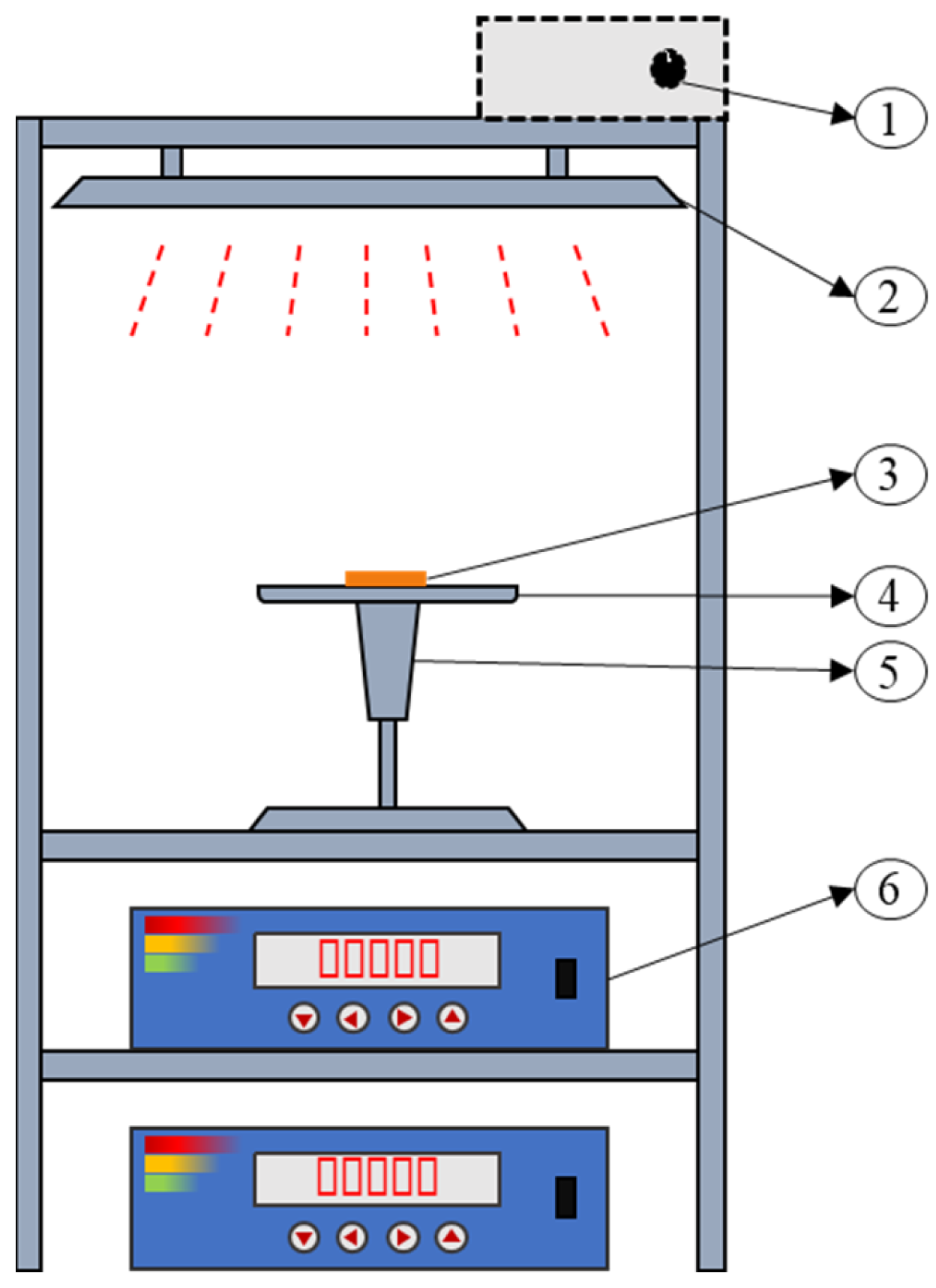
2.1. Sample Preparation and Equipment
2.2. Extraction of Carrot Cell Wall Alcohol-Insoluble Residues
2.3. The Separation and Extraction of Carrot Pectin Fractions
2.4. Fourier-Transform Infrared Spectrometry
2.5. The Thermodynamic Characterisation of Pectin
2.6. The X-ray Diffraction of the Carrot Cell Wall-Insoluble Residues
2.7. The Determination of Pectin Esterification
2.8. The Determination of the Calcium Ion Content
2.9. Molecular Weight Determination of Pectin
2.10. Analysis of the Pectin’s Monosaccharide Composition
2.11. Sugar Ratio Calculation
2.12. Microscopic Morphology of Pectin
2.13. Statistical Analysis
3. Results and Discussion
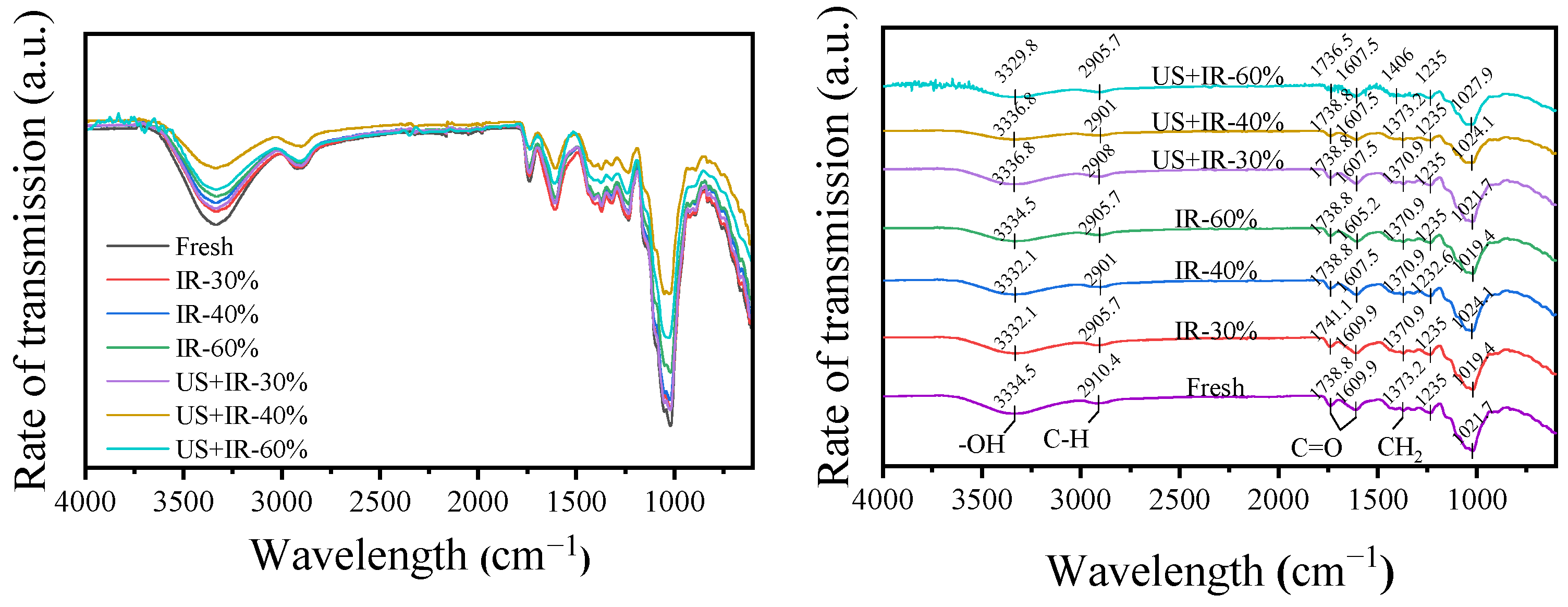
3.1. The Effect of Ultrasonic Co-Operative Infrared Drying on the FTIR of Carrot AIR
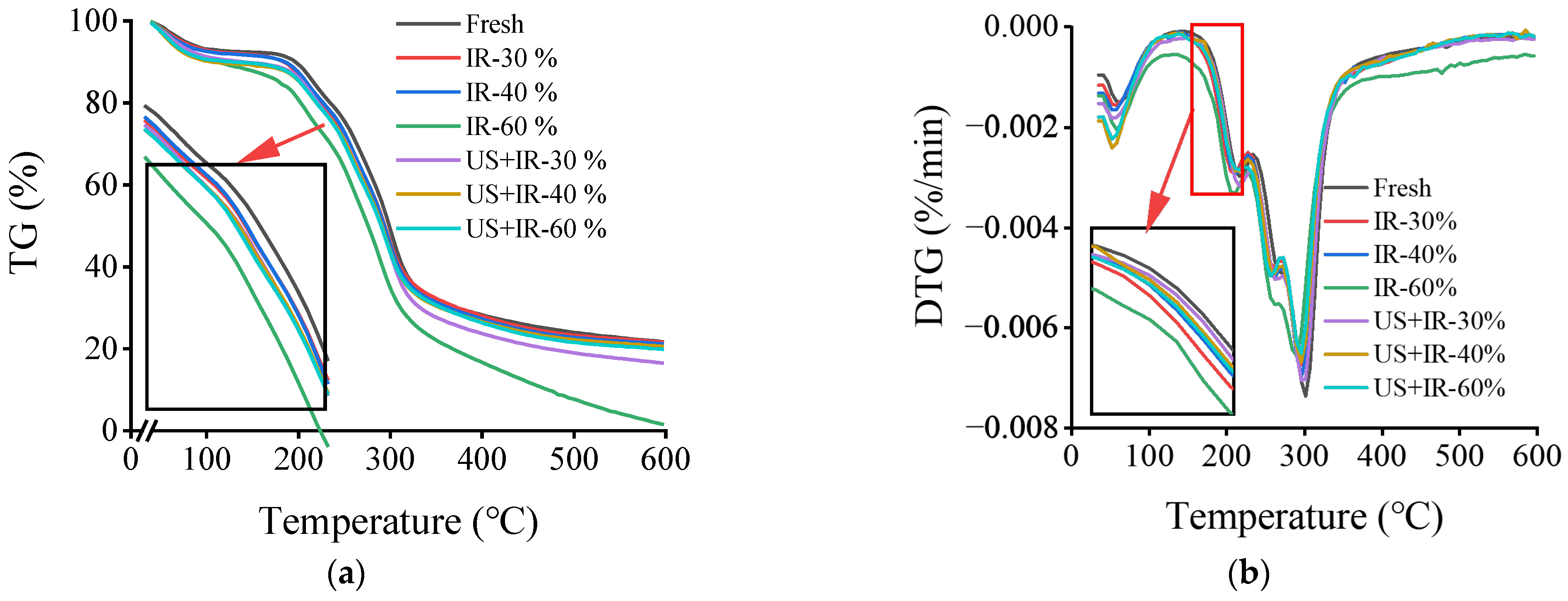
3.2. The Effect of Ultrasonic Co-Operative Infrared Drying on the Thermal Stability of Carrot AIR
3.3. The Effect of Ultrasonic Co-Operative Infrared Drying on Carrot AIR X-ray Diffraction
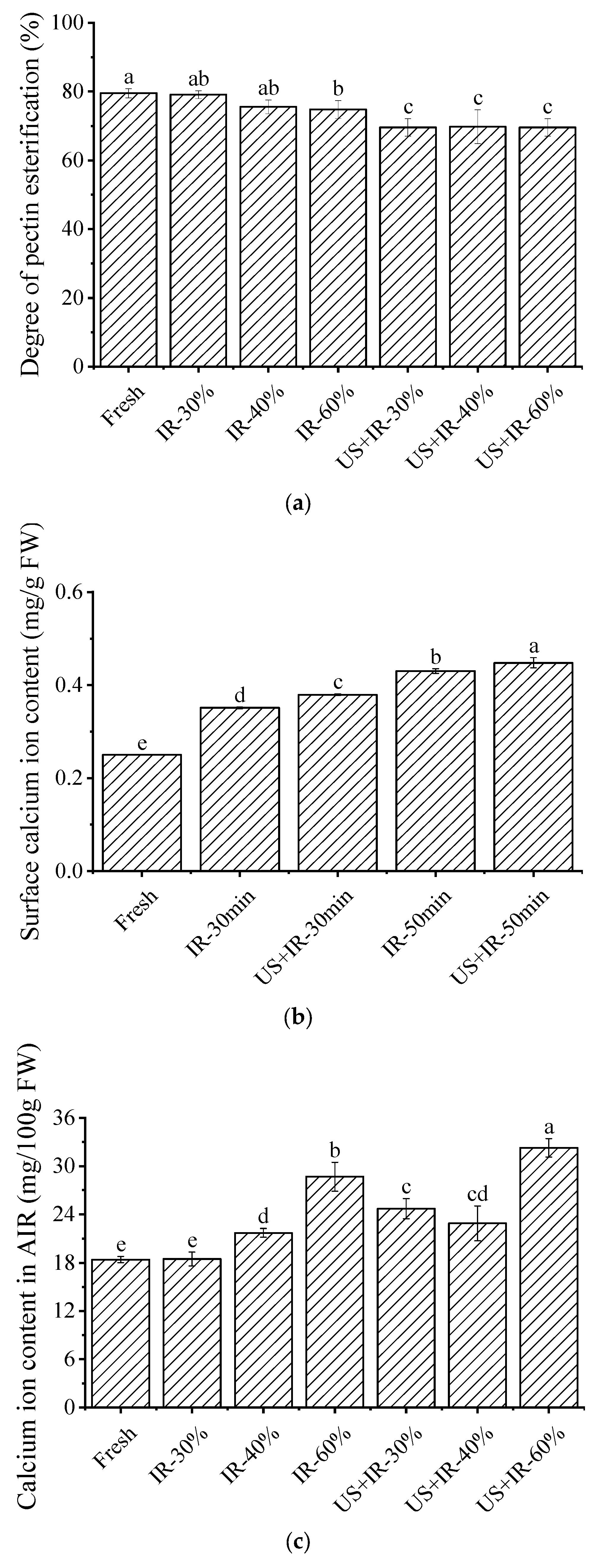
3.4. The Effect of Ultrasonic Co-Operative Infrared Drying on the Esterification Degree of Carrot Pectin
3.5. The Effect of Ultrasonic Co-Operative Infrared Drying on the Calcium Ion Content in the Surface Layer and the AIR of the Carrot Samples
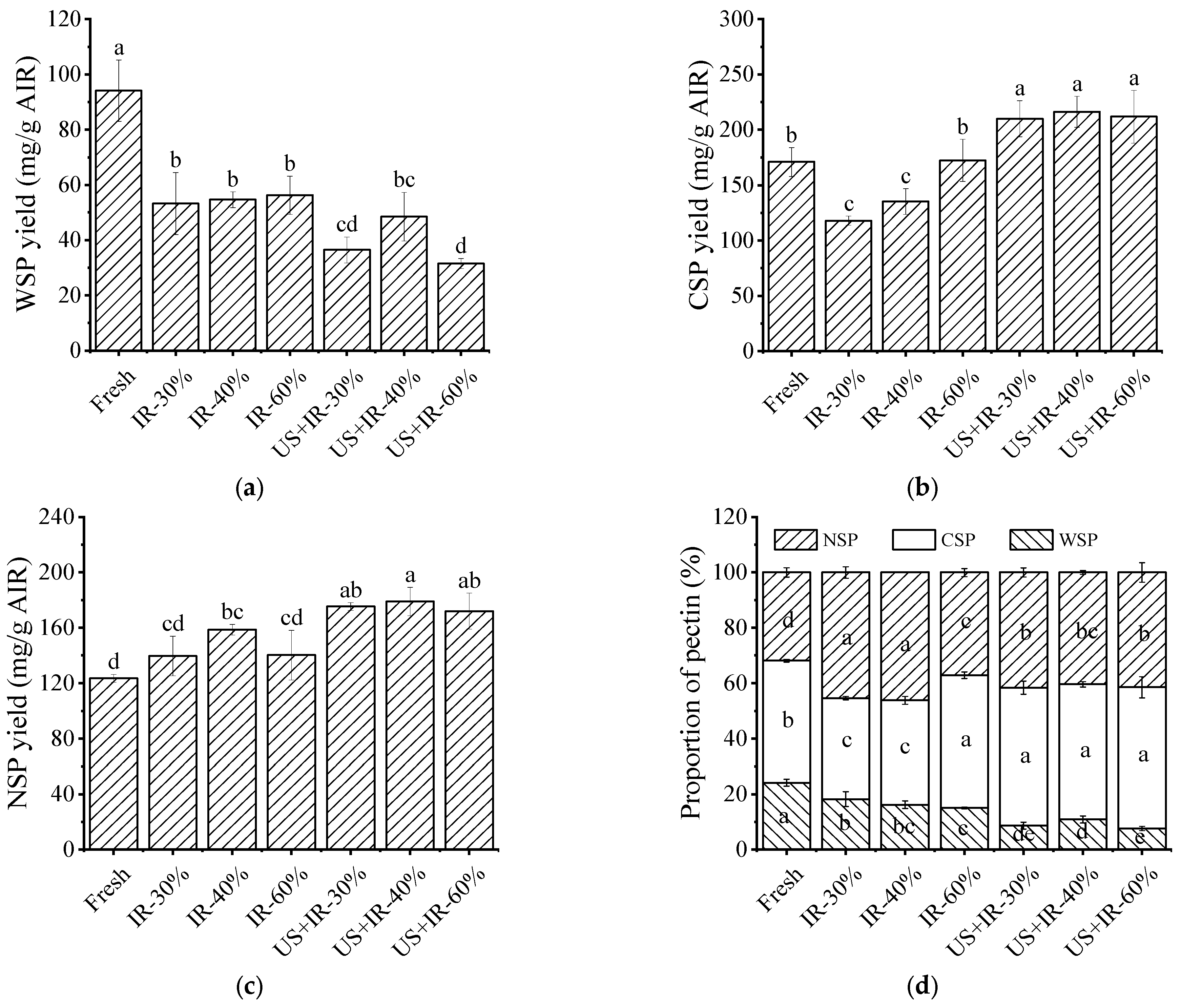
3.6. The Effect of Ultrasonic Co-Operative Infrared Drying on the Change in the Carrot Pectin Fraction Content
3.7. The Effect of Ultrasonic Co-Operative Infrared Drying on the Monosaccharide Composition and the Sugar Ratio of Carrot Pectin
3.8. The Effect of Ultrasonic Co-Operative Infrared Drying on the Molecular Weight of Carrot Pectin
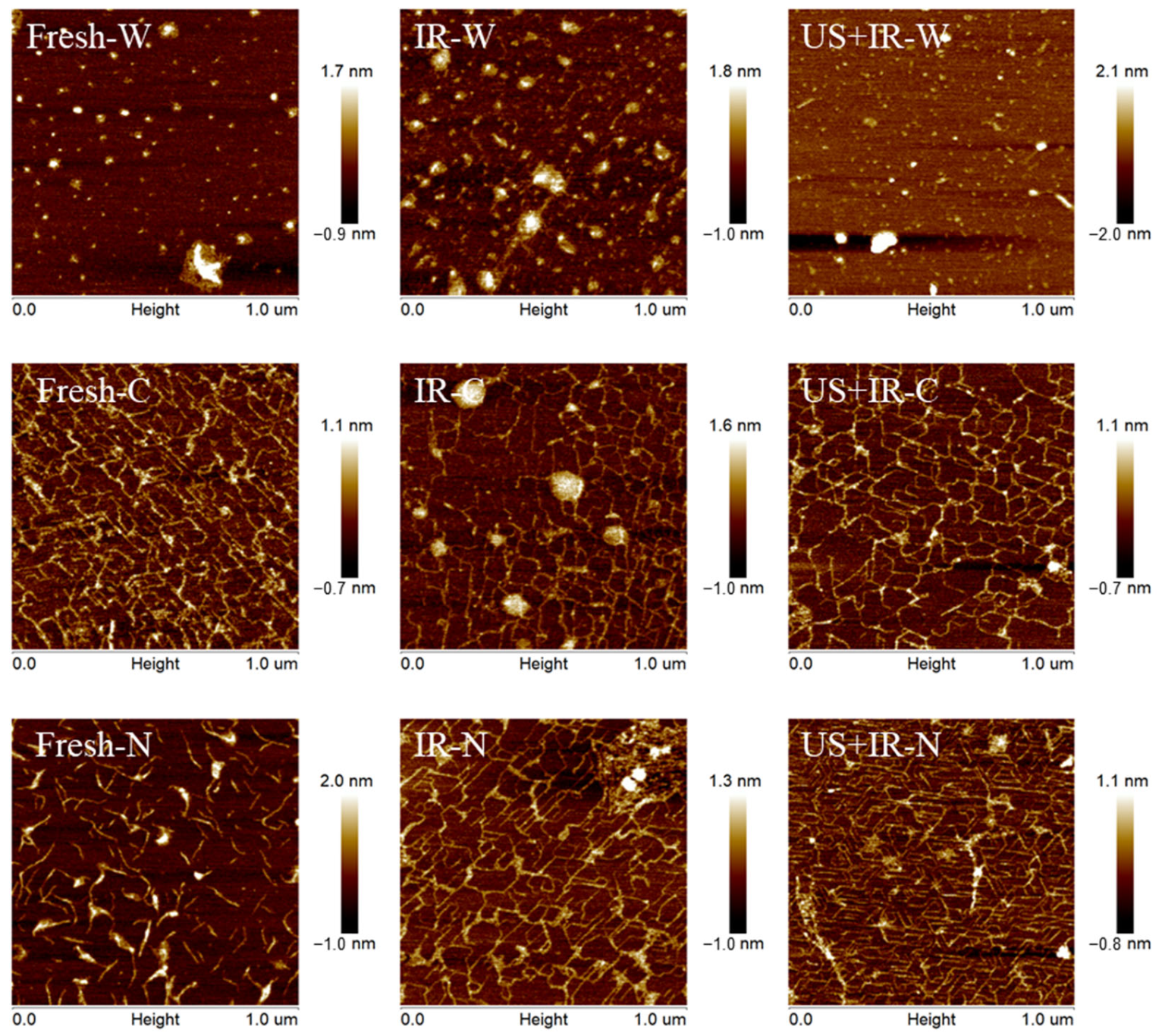
3.9. The Effect of Ultrasonic Co-Operative Infrared Drying on the Micro-Morphological Changes in Carrot Pectin
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, Y.; Wu, B.; Guo, X.; Ding, F.; Pan, Z.; Ma, H. Effects of Power Ultrasound Enhancement on Infrared Drying of Carrot Slices: Moisture Migration and Quality Characterizations. LWT-Food Sci. Technol. 2020, 126, 109312. [Google Scholar] [CrossRef]
- Wu, B.; Ma, H.; Qu, W.; Wang, B.; Zhang, X.; Wang, P.; Wang, J.; Atungulu, G.; Pan, Z. Catalytic Infrared and Hot Air Dehydration of Carrot Slices. J. Food Process Eng. 2014, 37, 111–121. [Google Scholar] [CrossRef]
- Bi, J.; Chen, Q.; Zhou, Y.; Liu, X.; Wu, X.; Chen, R. Optimization of Short- and Medium-Wave Infrared Drying and Quality Evaluation of Jujube Powder. Food Bioprocess Technol. 2014, 7, 2375–2387. [Google Scholar] [CrossRef]
- Khampakool, A.; Soisungwan, S.; Park, S.H. Potential Application of Infrared Assisted Freeze Drying (Irafd) for Banana Snacks: Drying Kinetics, Energy Consumption, and Texture. LWT-Food Sci. Technol. 2019, 99, 355–363. [Google Scholar] [CrossRef]
- Khan MI, H.; Karim, M.A. Cellular Water Distribution, Transport, and Its Investigation Methods for Plant-Based Food Material. Food Res. Int. 2017, 99, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Prothon, F.; Ahrné, L.M.; Sjöholm, I. Mechanisms and Prevention of Plant Tissue Collapse During Dehydration: A Critical Review. Crit. Rev. Food Sci. Nutr. 2003, 43, 447–479. [Google Scholar] [CrossRef]
- Wu, B.; Guo, X.; Guo, Y.; Ma, H.; Zhou, C. Enhancing Jackfruit Infrared Drying by Combining Ultrasound Treatments: Effect on Drying Characteristics, Quality Properties and Microstructure. Food Chem. 2021, 358, 129845. [Google Scholar] [CrossRef] [PubMed]
- Xi, H.; Liu, Y.; Guo, L.; Hu, R. Effect of Ultrasonic Power on Drying Process and Quality Properties of Far-Infrared Radiation Drying on Potato Slices. Food Sci. Biotechnol. 2020, 29, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wan, F.; Zang, Z.; Jiang, C.; Xu, Y.; Huang, X. Effect of Ultrasonic Far-Infrared Synergistic Drying on the Characteristics and Qualities of Wolfberry (Lycium barbarum L.). Ultrason. Sonochem. 2022, 89, 106134. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, M.; Guo, L.; Shao, R.; Wang, X.; Liu, F. Effect of Direct-Contact Ultrasonic and Far Infrared Combined Drying on the Drying Characteristics and Quality of Ginger. Processes 2024, 12, 98. [Google Scholar] [CrossRef]
- Keegstra, K. Plant Cell Walls. Plant Physiol. 2010, 154, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Sila, D.N.; Duvetter, T.; De Roeck, A.; Verlent, I.; Smout, C.; Moates, G.K.; Hills, B.P.; Waldron, K.K.; Hendrickx, M.; Van Loey, A. Texture Changes of Processed Fruits and Vegetables: Potential Use of High-Pressure Processing. Trends Food Sci. Technol. 2008, 19, 309–319. [Google Scholar] [CrossRef]
- Kyomugasho, C.; Christiaens, S.; Shpigelman, A.; Van Loey, A.M.; Hendrickx, M.E. Ft-Ir Spectroscopy, a Reliable Method for Routine Analysis of the Degree of Methylesterification of Pectin in Different Fruit- and Vegetable-Based Matrices. Food Chem. 2015, 176, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Sila, D.N.; Doungla, E.; Smout, C.; Van Loey, A.; Hendrickx, M. Pectin Fraction Interconversions: Insight into Understanding Texture Evolution of Thermally Processed Carrots. J. Agric. Food Chem. 2006, 54, 8471–8479. [Google Scholar] [CrossRef]
- Liu, J.; Bi, J.; Mcclements, D.J.; Liu, X.; Yi, J.; Lyu, J.; Zhou, M.; Verkerk, R.; Dekker, M.; Wu, X.; et al. Impacts of Thermal and Non-Thermal Processing on Structure and Functionality of Pectin in Fruit- and Vegetable- Based Products: A Review. Carbohydr. Polym. 2020, 250, 116890. [Google Scholar] [CrossRef]
- Huang, L.-L.; Zhang, M.; Wang, L.-P.; Mujumdar, A.S.; Sun, D.-F. Influence of Combination Drying Methods on Composition, Texture, Aroma and Microstructure of Apple Slices. LWT-Food Sci. Technol. 2012, 47, 183–188. [Google Scholar] [CrossRef]
- De Roeck, A.; Sila, D.N.; Duvetter, T.; Van Loey, A.; Hendrickx, M. Effect of High Pressure/High Temperature Processing on Cell Wall Pectic Substances in Relation to Firmness of Carrot Tissue. Food Chem. 2008, 107, 1225–1235. [Google Scholar] [CrossRef]
- Rose, J.K.; Hadfield, K.A.; Labavitch, J.M.; Bennett, A.B. Temporal Sequence of Cell Wall Disassembly in Rapidly Ripening Melon Fruit. Plant Physiol. 1998, 117, 345–361. [Google Scholar] [CrossRef]
- Sila, D.N.; Smout, C.; Elliot, F.; Van Loey, A.; Hendrickx, M. Non-Enzymatic Depolymerization of Carrot Pectin: Toward a Better Understanding of Carrot Texture During Thermal Processing. J. Food Sci. 2006, 71, E1–E9. [Google Scholar] [CrossRef]
- Shaha, R.K.; Punichelvana, Y.; Afandi, A. Optimized Extraction Condition and Characterization of Pectin from Kaffir Lime (Citrus Hystrix). Res. J. Agric. For. Sci. 2013, 2320, 6063. [Google Scholar]
- Chen, T.; Zhang, Z.; Wang, Z.; Chen, Z.-L.; Ma, H.; Yan, J.-K. Effects of Ultrasound Modification at Different Frequency Modes on Physicochemical, Structural, Functional, and Biological Properties of Citrus Pectin. Food Hydrocoll. 2021, 113, 106484. [Google Scholar] [CrossRef]
- Houben, K.; Jolie, R.P.; Fraeye, I.; Van Loey, A.M.; Hendrickx, M.E. Comparative Study of the Cell Wall Composition of Broccoli, Carrot, and Tomato: Structural Characterization of the Extractable Pectins and Hemicelluloses. Carbohydr. Res. 2011, 346, 1105–1111. [Google Scholar] [CrossRef]
- Zhang, M.; Qin, H.; An, R.; Zhang, W.; Liu, J.; Yu, Q.; Liu, W.; Huang, X. Isolation, Purification, Structural Characterization and Antitumor Activities of a Polysaccharide from Lilium Davidii Var. Unicolor Cotton. J. Mol. Struct. 2022, 1261, 132941. [Google Scholar] [CrossRef]
- Szymanska-Chargot, M.; Zdunek, A. Use of Ft-Ir Spectra and Pca to the Bulk Characterization of Cell Wall Residues of Fruits and Vegetables Along a Fraction Process. Food Biophys. 2013, 8, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, K.; Yadav, S.K. Ultrasonication Assisted Salt-Spices Impregnation in Black Carrots to Attain Anthocyanins Stability, Quality Retention and Antimicrobial Efficacy on Hot-Air Convective Drying. Ultrason. Sonochem. 2019, 58, 104661. [Google Scholar]
- Liu, N.; Yang, W.; Li, X.; Zhao, P.; Liu, Y.; Guo, L.; Huang, L.; Gao, W. Comparison of Characterization and Antioxidant Activity of Different Citrus Peel Pectins. Food Chem. 2022, 386, 132683. [Google Scholar] [CrossRef]
- Hastuti, B.; Totiana, F.; Winiasih, R. The Role of Pectin in Pb Binding by Carrot Peel Biosorbents: Isoterm Adsorption Study. In Proceedings of the 12th Joint Conference on Chemistry, Semarang, Indonesia, 19–20 September 2017. [Google Scholar] [CrossRef]
- Koziol, A.; Sroda-Pomianek, K.; Gorniak, A.; Wikiera, A.; Cyprych, K.; Malik, M. Structural Determination of Pectins by Spectroscopy Methods. Coatings 2022, 12, 546. [Google Scholar] [CrossRef]
- Wang, W.; Ma, X.; Jiang, P.; Hu, L.; Zhi, Z.; Chen, J.; Ding, T.; Ye, X.; Liu, D. Characterization of Pectin from Grapefruit Peel: A Comparison of Ultrasound-Assisted and Conventional Heating Extractions. Food Hydrocoll. 2016, 61, 730–739. [Google Scholar] [CrossRef]
- Sucheta Misra, N.N.; Yadav, S.K. Extraction of Pectin from Black Carrot Pomace Using Intermittent Microwave, Ultrasound and Conventional Heating: Kinetics, Characterization and Process Economics. Food Hydrocoll. 2020, 102, 105592. [Google Scholar] [CrossRef]
- Feng, Y.; Tan, C.P.; Zhou, C.; Yagoub, A.E.A.; Xu, B.; Sun, Y.; Ma, H.; Xu, X.; Yu, X. Effect of Freeze-Thaw Cycles Pretreatment on the Vacuum Freeze-Drying Process and Physicochemical Properties of the Dried Garlic Slices. Food Chem. 2020, 324, 126883. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, L.; Yagoub, A.A.E.; Yu, X.; Ma, H.; Zhou, C. Effects of Ultrasound, Freeze-Thaw Pretreatments and Drying Methods on Structure and Functional Properties of Pectin During the Processing of Okra. Food Hydrocoll. 2021, 120, 106965. [Google Scholar] [CrossRef]
- Celus, M.; Kyomugasho, C.; Van Loey, A.M.; Grauwet, T.; Hendrickx, M.E. Influence of Pectin Structural Properties on Interactions with Divalent Cations and Its Associated Functionalities. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1576–1594. [Google Scholar] [CrossRef] [PubMed]
- Sajjaanantakul, T.; Van Buren, J.P.; Downing, D. Effect of Methyl Ester Content on Heat Degradation of Chelator-Soluble Carrot Pectin. J. Food Sci. 1989, 54, 1272–1277. [Google Scholar] [CrossRef]
- Cui, J.; Zhao, C.; Feng, L.; Han, Y.; Du, H.; Xiao, H.; Zheng, J. Pectins from Fruits: Relationships between Extraction Methods, Structural Characteristics, and Functional Properties. Trends Food Sci. Technol. 2021, 110, 39–54. [Google Scholar] [CrossRef]
- De Roeck, A.; Mols, J.; Duvetter, T.; Van Loey, A.; Hendrickx, M. Carrot Texture Degradation Kinetics and Pectin Changes During Thermal versus High-Pressure/High-Temperature Processing: A Comparative Study. Food Chem. 2010, 120, 1104–1112. [Google Scholar] [CrossRef]
- Christiaens, S.; Van Buggenhout, S.; Vandevenne, E.; Jolie, R.; Van Loey, A.M.; Hendrickx, M.E. Towards a Better Understanding of the Pectin Structure-Function Relationship in Broccoli During Processing: Part II—Analyses with Anti-Pectin Antibodies. Food Res. Int. 2011, 44, 2896–2906. [Google Scholar] [CrossRef]
- Feng, W.; Kita, D.; Peaucelle, A.; Cartwright, H.N.; Doan, V.; Duan, Q.; Liu, M.-C.; Maman, J.; Steinhorst, L.; Schmitz-Thom, I.; et al. The Feronia Receptor Kinase Maintains Cell-Wall Integrity during Salt Stress through Ca2+ Signaling. Curr. Biol. 2018, 28, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Moens, L.G.; De Laet, E.; Van Wambeke, J.; Van Loey, A.M.; Hendrickx, M.E.G. Pulsed Electric Field and Mild Thermal Processing Affect the Cooking Behaviour of Carrot Tissues (Daucus Carota) and the Degree of Methylesterification of Carrot Pectin. Innov. Food Sci. Emerg. Technol. 2020, 66, 102483. [Google Scholar] [CrossRef]
- Christiaens, S.; Van Buggenhout, S.; Houben, K.; Fraeye, I.; Van Loey, A.M.; Hendrickx, M.E. Towards a Better Understanding of the Pectin Structure-Function Relationship in Broccoli during Processing: Part I-Macroscopic and Molecular Analyses. Food Res. Int. 2011, 44, 1604–1612. [Google Scholar] [CrossRef]
- Moens, L.G.; Huang, W.; Van Loey, A.M.; Hendrickx, M.E.G. Effect of Pulsed Electric Field and Mild Thermal Processing on Texture-Related Pectin Properties to Better Understand Carrot (Daucus Carota) Texture Changes during Subsequent Cooking. Innov. Food Sci. Emerg. Technol. 2021, 70, 102700. [Google Scholar] [CrossRef]
- Xiao, M.; Yi, J.; Bi, J.; Lv, J.; Zhou, L.; Zhou, M. Influence of Different Dehydration Processes on the Texture and Pectin Characteristics of Apple Chips. Mod. Food Sci. Technol. 2017, 33, 157–162+117. [Google Scholar]
- Sun, Y.; Kang, X.; Liang, D.; Chen, F.; Hu, X. Study on Effect and Mechanism of High Pressure Processing on Hardness of Fresh-Cut Carrot. Sci. Technol. Food Ind. 2017, 38, 200–204+208. [Google Scholar]
- Yi, J.; Bi, J.; Liu, X.; Lv, J.; Zhou, M.; Wu, X.; Zhao, Y.; Du, Q. A Review: Domain Fine Structure of Pectic Polysaccharides. Food Sci. 2020, 41, 292–299. [Google Scholar]
- Nagano, T.; Hirotsuka, M.; Mori, H.; Kohyama, K.; Nishinari, K. Dynamic Viscoelastic Study on the Gelation of 7 S Globulin from Soybeans. J. Agric. Food Chem. 1992, 40, 941–944. [Google Scholar] [CrossRef]
- Yi, J.; Lv, J.; Bi, J.; Zhou, L.; Wu, X.; Zhou, M. Research Process of Structure and Function Relationship of Pectin in Processed Fruits and Vegetables. J. Chin. Inst. Food Sci. Technol. 2017, 17, 175–181. [Google Scholar]
- Zhang, L.; Ye, X.; Ding, T.; Sun, X.; Xu, Y.; Liu, D. Ultrasound Effects on the Degradation Kinetics, Structure and Rheological Properties of Apple Pectin. Ultrason. Sonochem. 2013, 20, 222–231. [Google Scholar] [CrossRef]
- Binnig, G.; Quate, C.F.; Gerber, C. Atomic Force Microscope. Phys. Rev. Lett. 1986, 56, 930. [Google Scholar] [CrossRef]
- Cybulska, J.; Zdunek, A.; Kozio, A. The Self-Assembled Network and Physiological Degradation of Pectins in Carrot Cell Walls. Food Hydrocoll. 2015, 43, 41–50. [Google Scholar] [CrossRef]
- Zhang, H.; Nie, S.; Guo, Q.; Wang, Q.; Cui, S.W.; Xie, M. Conformational Properties of a Bioactive Polysaccharide from Ganoderma Atrum by Light Scattering and Molecular Modeling. Food Hydrocoll. 2018, 84, 16–25. [Google Scholar] [CrossRef]

| Standard Product | Time of Peak (min) | Standard Curve | R2 |
|---|---|---|---|
| Mannose | 21.053 | 0.998 | |
| Rhamnose | 26.172 | 0.998 | |
| Glucuronic acid | 27.309 | 0.997 | |
| Galacturonic acid | 31.529 | 0.996 | |
| Glucose | 33.458 | 0.990 | |
| Galactose | 36.069 | 0.990 | |
| Xylose | 36.752 | 0.990 | |
| Arabinose | 38.036 | 0.990 | |
| Fucose | 41.237 | 0.991 |
| Pectin Types | Conditions | The Contents of Neutral Sugars and Uronic Acids (mg/g AIR) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Man | Rha | Glu | Man | Xyl | Ara | Man | GluA | GalA | ||
| WSP | Fresh | 2.36 ± 0.2 e | 3.42 ± 0.4 h | 2.81 ± 1.0 bc | 14.07 ± 3.1 l | 8.66 ± 1.0 k | 6.40 ± 0.1 b | 0.78 ± 0.1 fg | 2.10 ± 0.2 i | 38.79 ± 2.7 klm |
| IR-30% | 2.63 ± 0.0 d | 3.26 ± 1.2 h | 264.74 ± 34.0 a | 20.03 ± 0.3 k | 8.24 ± 0.2 k | 5.45 ± 0.1 c | 5.03 ± 0.1 a | 2.39 ± 0.0 h | 47.22 ± 1.2 hi | |
| IR-40% | 2.60 ± 0.1 d | 3.45 ± 0.2 h | 5.45 ± 0.1 bc | 23.61 ± 0.9 ij | 9.92 ± 0.1 j | 4.32 ± 0.2 d | 0.57 ± 0.2 gh | 2.01 ± 0.1 i | 36.67 ± 0.8 lm | |
| IR-60% | 5.30 ± 0.1 a | 5.25 ± 0.0 g | 265.38 ± 0.8 a | 26.46 ± 0.3 gh | 10.72 ± 0.4 j | 0.71 ± 0.0 n | 0.54 ± 0.3 gh | 2.83 ± 0.0 g | 48.24 ± 1.0 h | |
| US + IR-30% | 3.05 ± 0.1 b | 1.48 ± 0.1 i | 2.54 ± 0.4 c | 15.47 ± 0.8 l | 5.58 ± 0.4 l | 3.37 ± 0.1 f | 0.10 ± 0.0 ij | 1.08 ± 0.1 k | 21.11 ± 1.1 n | |
| US + IR-40% | 3.13 ± 0.0 b | 0.96 ± 0.0 ij | 3.19 ± 0.1 bc | 11.91 ± 0.1 m | 5.51 ± 0.0 l | 2.28 ± 0.0 i | 0.05 ± 0.0 j | 1.12 ± 0.0 k | 18.70 ± 0.0 n | |
| US + IR-60% | 2.76 ± 0.0 c | 0.59 ± 0.0 j | 2.78 ± 0.0 bc | 8.46 ± 0.0 n | 3.74 ± 0.0 m | 0.87 ± 0.0 m | 0.06 ± 0.0 ij | 0.79 ± 0.0 l | 5.14 ± 0.0 o | |
| CSP | Fresh | 1.94 ± 0.1 g | 9.02 ± 0.2 d | 1.56 ± 0.1 c | 27.79 ± 0.0 g | 17.17 ± 0.0 g | 2.16 ± 0.0 i | 1.09 ± 0.1 def | 3.07 ± 0.1 f | 108.19 ± 2.7 b |
| IR-30% | 1.41 ± 0.0 k | 2.69 ± 0.4 h | 2.85 ± 0.4 bc | 6.78 ± 1.3 n | 7.95 ± 0.8 k | 7.38 ± 0.2 a | 0.46 ± 0.2 ghi | 1.73 ± 0.0 j | 40.23 ± 2.8 jkl | |
| IR-40% | 1.61 ± 0.0 ij | 5.21 ± 0.2 g | 0.90 ± 0.0 c | 19.31 ± 0.2 k | 15.56 ± 0.2 h | 1.71 ± 0.0 kl | 0.61 ± 0.3 g | 2.42 ± 0.1 h | 82.85 ± 3.5 d | |
| IR-60% | 2.39 ± 0.0 e | 5.45 ± 0.1 fg | ND | 14.32 ± 1.7 l | 13.00 ± 0.9 i | 3.04 ± 0.0 g | 1.12 ± 0.1 defg | 3.50 ± 0.0 c | 74.85 ± 9.4 e | |
| US + IR-30% | 2.82 ± 0.0 c | 6.18 ± 0.0 ef | ND | 22.79 ± 0.1 ij | 16.86 ± 0.4 g | 2.86 ± 0.0 h | 1.48 ± 0.4 cd | 3.97 ± 0.0 a | 114.24 ± 3.4 b | |
| US + IR-40% | 2.64 ± 0.0 d | 6.11 ± 0.1 ef | ND | 22.31 ± 0.1 j | 19.22 ± 0.2 f | 2.73 ± 0.0 h | 1.33 ± 0.14 cde | 3.68 ± 0.0 b | 90.90 ± 0.9 c | |
| US + IR-60% | 2.39 ± 0.0 e | 4.87 ± 0.0 g | ND | 18.93 ± 0.3 k | 15.10 ± 0.3 h | 2.18 ± 0.0 i | 0.79 ± 0.2 f | 3.22 ± 0.0 e | 138.21 ± 0.6 a | |
| NSP | Fresh | 1.52 ± 0.0 j | 18.53 ± 0.2 a | 16.51 ± 0.0 b | 92.49 ± 0.2 b | 37.93 ± 0.0 c | 1.58 ± 0.0 l | 1.39 ± 0.6 cde | 2.81 ± 0.1 g | 47.60 ± 2.1 hi |
| IR-30% | 1.66 ± 0.0 hi | 18.79 ± 0.3 a | ND | 107.70 ± 0.1 a | 60.62 ± 0.2 a | 1.77 ± 0.0 k | 2.00 ± 0.1 b | 2.93 ± 0.1 g | 45.34 ± 2.1 hij | |
| IR-40% | 1.75 ± 0.0 h | 15.64 ± 0.2 b | ND | 79.14 ± 0.1 c | 42.63 ± 0.2 b | 2.01 ± 0.0 j | 1.55 ± 0.1 c | 3.31 ± 0.1 e | 54.16 ± 3.6 g | |
| IR-60% | 1.74 ± 0.0 h | 12.55 ± 0.4 c | ND | 66.43 ± 0.3 d | 38.69 ± 0.2 c | 1.77 ± 0.0 k | 1.44 ± 0.0 cde | 2.87 ± 0.1 g | 43.19 ± 2.9 ijk | |
| US + IR-30% | 1.95 ± 0.1 g | 6.78 ± 1.3 e | 0.04 ± 0.0 c | 24.82 ± 3.5 hi | 17.38 ± 1.2 g | 3.70 ± 0.1 e | 0.19 ± 0.0 hij | 2.83 ± 0.0 g | 36.19 ± 0.7 m | |
| US + IR-40% | 2.10 ± 0.0 f | 12.34 ± 0.1 c | ND | 58.39 ± 0.1 e | 34.44 ± 0.0 d | 2.26 ± 0.0 i | 1.07 ± 0.0 ef | 3.46 ± 0.0 cd | 59.62 ± 0.0 f | |
| US + IR-60% | 2.15 ± 0.0 f | 9.72 ± 0.0 d | ND | 37.33 ± 0.2 f | 26.94 ± 0.0 e | 2.17 ± 0.0 i | 1.29 ± 0.1 cde | 3.34 ± 0.0 de | 55.79 ± 0.2 fg | |
| Pectin Types | Conditions | Ratio of Sugars | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| WSP | Fresh | 1.164 | 0.088 | 5.981 |
| IR-30% | 1.124 | 0.069 | 7.804 | |
| IR-40% | 0.876 | 0.094 | 8.088 | |
| IR-60% | 1.104 | 0.109 | 5.178 | |
| US + IR-30% | 0.812 | 0.070 | 12.762 | |
| US + IR-40% | 0.902 | 0.051 | 14.786 | |
| US + IR-60% | 0.375 | 0.114 | 15.851 | |
| CSP | Fresh | 1.890 | 0.083 | 3.320 |
| IR-30% | 1.593 | 0.067 | 5.269 | |
| IR-40% | 1.954 | 0.063 | 4.039 | |
| IR-60% | 2.491 | 0.059 | 3.185 | |
| US + IR-30% | 2.277 | 0.054 | 4.152 | |
| US + IR-40% | 1.758 | 0.067 | 4.101 | |
| US + IR-60% | 2.685 | 0.043 | 4.331 | |
| NSP | Fresh | 0.313 | 0.389 | 5.076 |
| IR-30% | 0.238 | 0.414 | 5.824 | |
| IR-40% | 0.384 | 0.289 | 5.189 | |
| IR-60% | 0.357 | 0.291 | 5.433 | |
| US + IR-30% | 0.685 | 0.187 | 4.209 | |
| US + IR-40% | 0.549 | 0.207 | 4.914 | |
| US + IR-60% | 0.720 | 0.174 | 4.065 | |
| Pectin Types | Mw (×105 Da) | Mn (×105 Da) | Mw/Mn | Rg(nm) | |
|---|---|---|---|---|---|
| WSP | Fresh | 1.815 (±0.72%) | 1.152 (±1.56%) | 1.575 (±1.72%) | 42.5 (±3.7%) |
| IR-60% | 1.709 (±1.08%) | 1.045 (±1.00%) | 1.636 (±1.47%) | 52.1 (±3.7%) | |
| US + IR-60% | 3.550 (±0.64%) | 1.707 (±0.77%) | 2.080 (±1.00%) | 50.9 (±2.3%) | |
| CSP | Fresh | 3.029 (±0.71%) | 1.746 (±1.17%) | 1.735 (±1.37%) | 64.8 (±1.7%) |
| IR-60% | 3.303 (±0.73%) | 2.068 (±1.18%) | 1.597 (±1.39%) | 73.1 (±1.4%) | |
| US + IR-60% | 3.495 (±1.10%) | 2.643 (±1.22%) | 1.322 (±1.65%) | 85.8 (±1.6%) | |
| NSP | Fresh | 1.216 (±0.90%) | 5.463 (±2.21%) | 2.227 (±2.39%) | 45.4 (±4.0%) |
| IR-60% | 5.679 (±0.42%) | 1.962 (±1.23%) | 2.895 (±1.30%) | 55.4 (±1.3%) | |
| US + IR-60% | 2.858 (±0.90%) | 1.212 (±2.39%) | 2.358 (±2.55%) | 55.8 (±2.7%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, K.; Liu, B.; Wu, B.; Guo, Y.; Song, C.; Nan, S.; Dai, J.; Shen, Y.; Ma, H. A Study on the Effect Mechanism of Pectin Modification on the Carrot Cell Wall’s Texture Formation under Ultrasonic and Infrared Drying. Agriculture 2024, 14, 803. https://doi.org/10.3390/agriculture14060803
Gao K, Liu B, Wu B, Guo Y, Song C, Nan S, Dai J, Shen Y, Ma H. A Study on the Effect Mechanism of Pectin Modification on the Carrot Cell Wall’s Texture Formation under Ultrasonic and Infrared Drying. Agriculture. 2024; 14(6):803. https://doi.org/10.3390/agriculture14060803
Chicago/Turabian StyleGao, Kun, Bin Liu, Bengang Wu, Yiting Guo, Chenyu Song, Shenao Nan, Junjun Dai, Yan Shen, and Haile Ma. 2024. "A Study on the Effect Mechanism of Pectin Modification on the Carrot Cell Wall’s Texture Formation under Ultrasonic and Infrared Drying" Agriculture 14, no. 6: 803. https://doi.org/10.3390/agriculture14060803
APA StyleGao, K., Liu, B., Wu, B., Guo, Y., Song, C., Nan, S., Dai, J., Shen, Y., & Ma, H. (2024). A Study on the Effect Mechanism of Pectin Modification on the Carrot Cell Wall’s Texture Formation under Ultrasonic and Infrared Drying. Agriculture, 14(6), 803. https://doi.org/10.3390/agriculture14060803









