Cooperative Fermentation Using Multiple Microorganisms and Enzymes Potentially Enhances the Nutritional Value of Spent Mushroom Substrate
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Single Cellulase, Xylanase, and β-Galactosidase Enzyme Assay
2.3. Multi-Enzyme Assays
2.4. Box–Behnken Design Test
3. Results
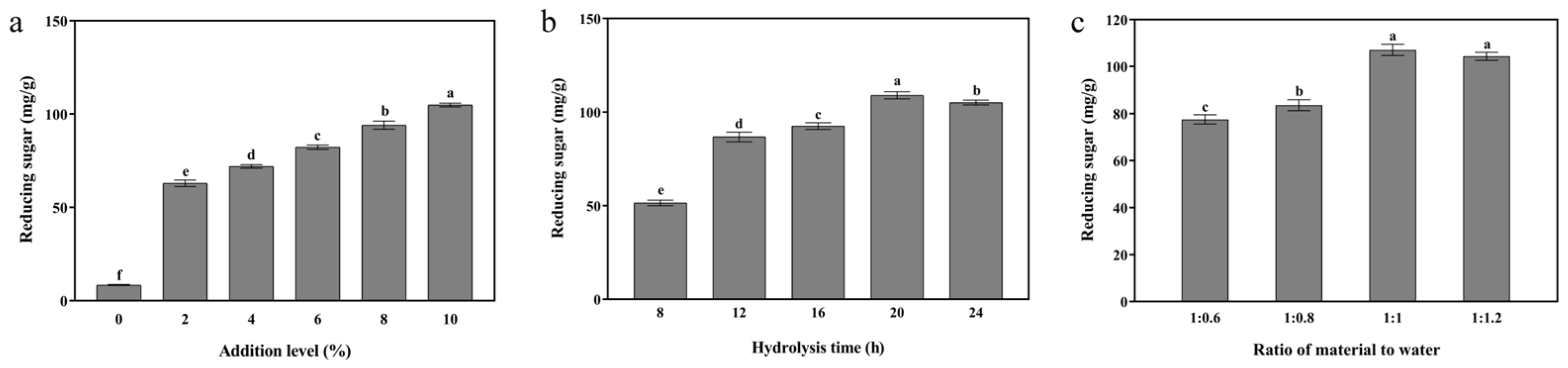
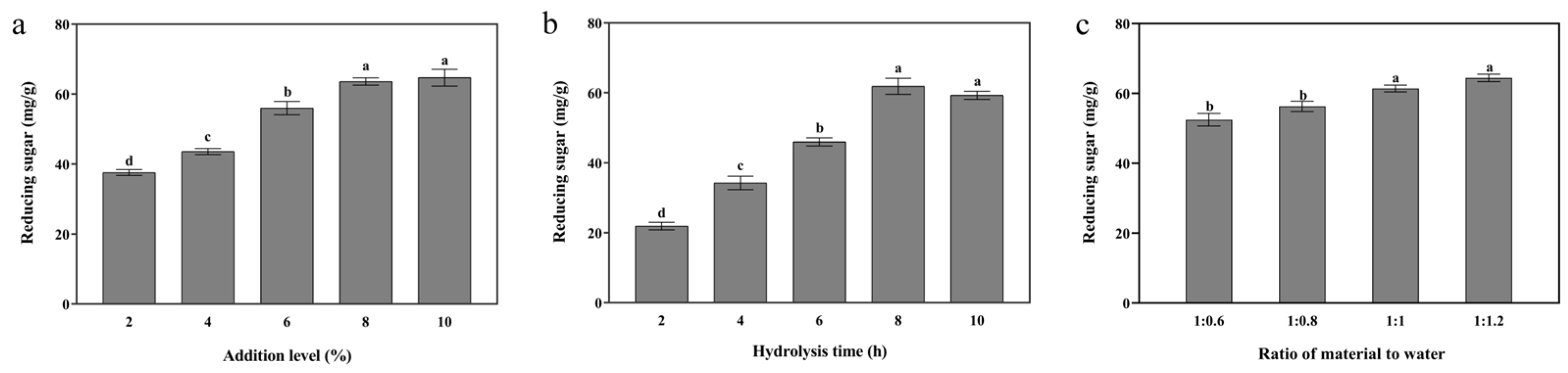
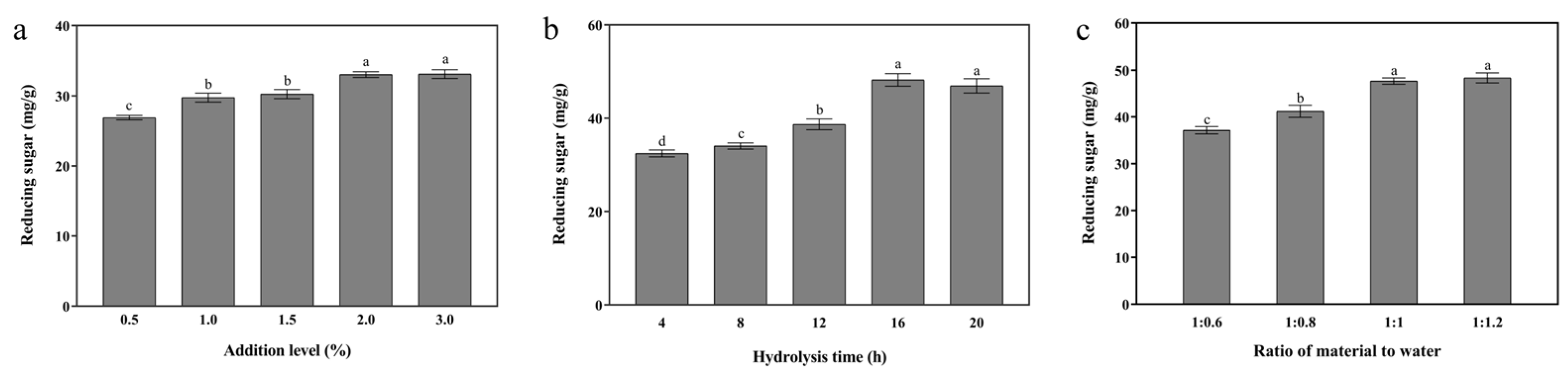
3.1. Single-Enzymatic Hydrolysis of Cellulase, Xylanase, and β-Galactosidase
3.1.1. Cellulase
3.1.2. Xylanase
3.1.3. β-Galactosidase
3.2. Multiple Ratios of Complex Enzymes
3.3. Plackett–Burman Design (PBD) Screening Test
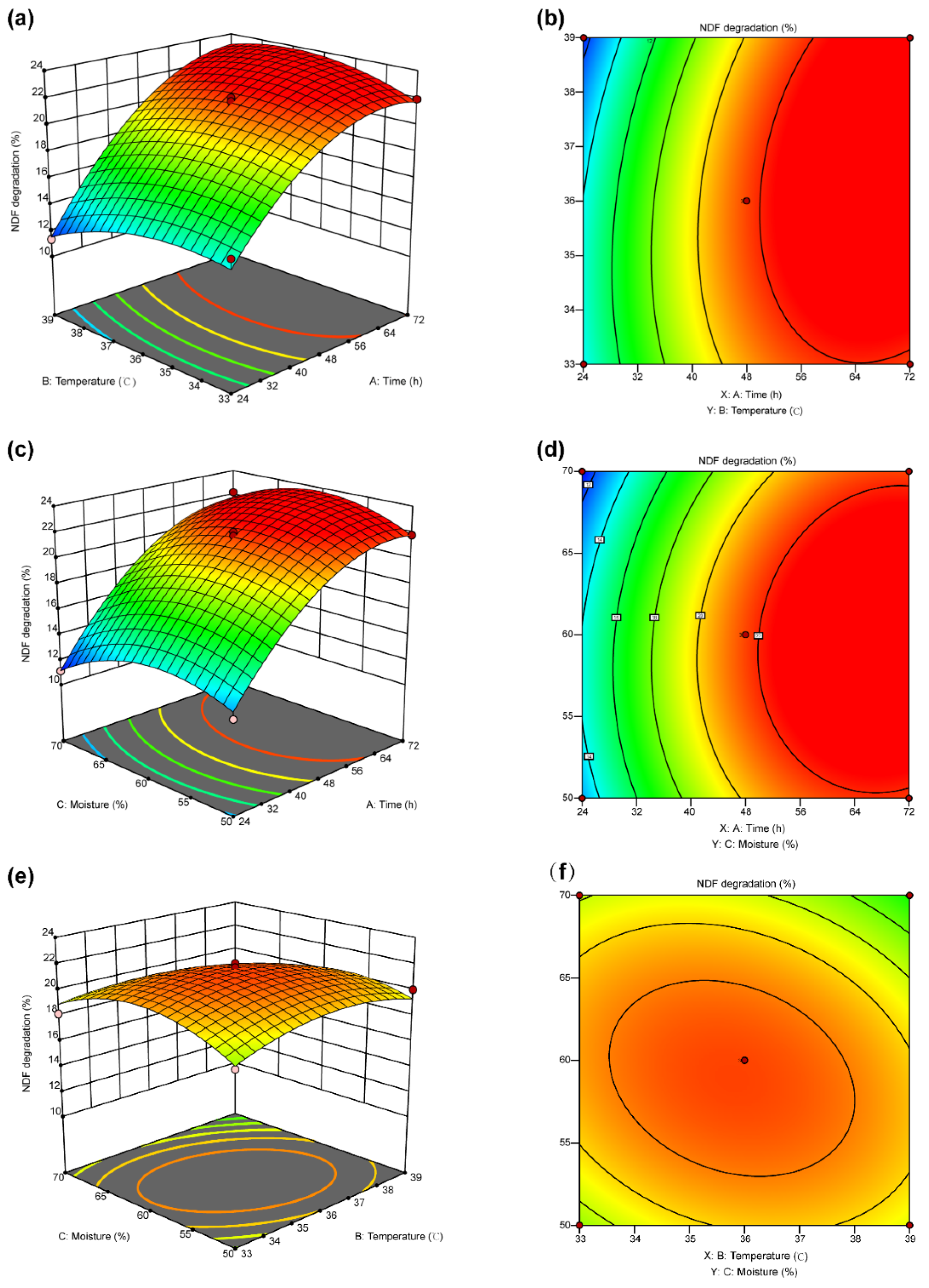
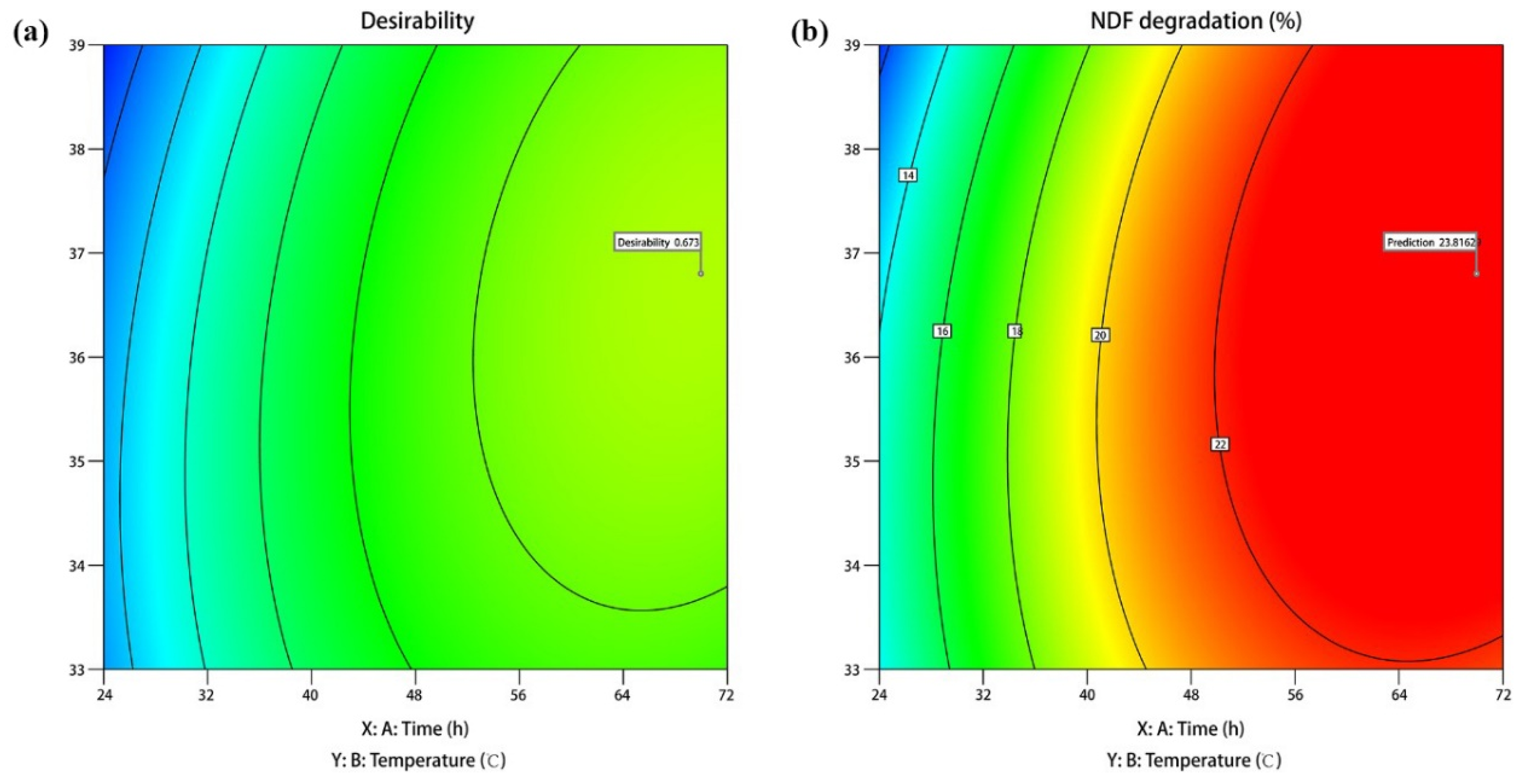
3.4. Response Surface Optimization
3.5. Optimization and Verification
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Atallah, E.; Zeaiter, J.; Ahmad, M.N.; Leahy, J.J.; Kwapinski, W. Hydrothermal carbonization of spent mushroom compost waste compared against torrefaction and pyrolysis. Fuel Process. Technol. 2021, 216, 106795. [Google Scholar] [CrossRef]
- Valverde, M.E.; Hernández-Pérez, T.; Paredes-López, O. Edible mushrooms: Improving human health and promoting quality life. Int. J. Microbiol. 2015, 2015, 376387. [Google Scholar] [CrossRef] [PubMed]
- Mahari, W.A.W.; Peng, W.; Nam, W.L.; Yang, H.; Lee, X.Y.; Lee, Y.K.; Liew, R.K.; Ma, N.L.; Mohammad, A.; Sonne, C.; et al. A review on valorization of oyster mushroom and waste generated in the mushroom cultivation industry. J. Hazard. Mater. 2020, 400, 123156. [Google Scholar] [CrossRef] [PubMed]
- Najafi, B.; Ardabili, S.F.; Shamshirband, S.; Chau, K.-W. Spent mushroom compost (SMC) as a source for biogas production in Iran. Eng. Appl. Comput. Fluid Mech. 2019, 13, 967–982. [Google Scholar] [CrossRef]
- Gao, X.; Tang, X.; Zhao, K.; Balan, V.; Zhu, Q. Biogas Production from Anaerobic Co-Digestion of Spent Mushroom Substrate with Different Livestock Manure. Energies 2021, 14, 570. [Google Scholar] [CrossRef]
- Li, T.-H.; Che, P.-F.; Zhang, C.-R.; Zhang, B.; Ali, A.; Zang, L.-S. Recycling of spent mushroom substrate: Utilization as feed material for the larvae of the yellow mealworm Tenebrio molitor (Coleoptera: Tenebrionidae). PLoS ONE 2020, 15, e0237259. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Li, S.; Carson, M.A.; Chang, S.X.; Wu, Q.; Wang, L.; An, Z.; Sun, X. Spent mushroom substrate and cattle manure amendments enhance the transformation of garden waste into vermicomposts using the earthworm Eisenia fetida. J. Environ. Manag. 2019, 248, 109263. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, M.; Chen, J.; Li, S.; Shao, Y.; Yang, J.; Li, J. Characteristics of bio-oil produced by the pyrolysis of mixed oil shale semi-coke and spent mushroom substrate. Fuel 2017, 200, 218–224. [Google Scholar] [CrossRef]
- Lam, S.S.; Lee, X.Y.; Nam, W.L.; Phang, X.Y.; Liew, R.K.; NY Yek, P.; Ho, Y.L.; Ma, N.L.; Rosli, M.H. Microwave vacuum pyrolysis conversion of waste mushroom substrate into biochar for use as growth medium in mushroom cultivation. J. Chem. Technol. Biotechnol. 2019, 94, 1406–1415. [Google Scholar] [CrossRef]
- Liu, D.; Ding, W.; Yuan, J.; Xiang, J.; Lin, Y. Substrate and/or substrate-driven changes in the abundance of methanogenic archaea cause seasonal variation of methane production potential in species-specific freshwater wetlands. Appl. Microbiol. Biotechnol. 2014, 98, 4711–4721. [Google Scholar] [CrossRef]
- Waqas, M.; Nizami, A.; Aburiazaiza, A.; Barakat, M.; Ismail, I.; Rashid, M. Optimization of food waste compost with the use of biochar. J. Environ. Manag. 2018, 216, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, J.; Chang, K.; Buyukada, M.; Evrendilek, F. (Co-)pyrolytic performances and by-products of textile dyeing sludge and spent mushroom substrate. J. Clean. Prod. 2020, 261, 121195. [Google Scholar] [CrossRef]
- Qiao, J.-J.; Zhang, Y.-F.; Sun, L.-F.; Liu, W.-W.; Zhu, H.-J.; Zhang, Z. Production of spent mushroom substrate hydrolysates useful for cultivation of Lactococcus lactis by dilute sulfuric acid, cellulase and xylanase treatment. Bioresour. Technol. 2011, 102, 8046–8051. [Google Scholar] [CrossRef]
- Economou, C.N.; Philippoussis, A.N.; Diamantopoulou, P.A. Spent mushroom substrate for a second cultivation cycle of Pleurotus mushrooms and dephenolization of agro-industrial wastewaters. FEMS Microbiol. Lett. 2020, 367, fnaa060. [Google Scholar] [CrossRef]
- Matute, R.G.; Figlas, D.; Mockel, G.; Curvetto, N. Degradation of Metsulfuron Methyl by Agaricus blazei Murrill Spent Compost Enzymes. Bioremediat. J. 2012, 16, 31–37. [Google Scholar] [CrossRef]
- Pérez-Chávez, A.M.; Mayer, L.; Albertó, E. Mushroom cultivation and biogas production: A sustainable reuse of organic resources. Energy Sustain. Dev. 2019, 50, 50–60. [Google Scholar] [CrossRef]
- Ribas, L.C.; de Mendonca, M.M.; Camelini, C.M.; Soares, C.H. Use of spent mushroom substrates from Agaricus subrufescens (syn. A. blazei, A. brasiliensis) and Lentinula edodes productions in the enrichment of a soil-based potting media for lettuce (Lactuca sativa) cultivation: Growth promotion and soil bioremediation. Bioresour. Technol. 2009, 100, 4750–4757. [Google Scholar] [PubMed]
- Ramirez-Bribiesca, J.E.; Soto-Sanchez, A.; Hernandez-Calva, L.M.; Salinas-Chavira, J.; Galaviz-Rodriguez, J.R.; Cruz-Monterrosa, R.G.; Vargas-Lopez, S. Influence of Pleurotus ostreatus spent corn straw on performance and carcass characteristics of feedlot Pelibuey lambs. Indian J. Anim. Sci. 2010, 80, 754–757. [Google Scholar]
- Baek, Y.C.; Kim, M.S.; Reddy, K.E.; Oh, Y.K.; Jung, Y.H.; Yeo, J.M.; Choi, H. Rumen fermentation and digestibility of spent mushroom (Pleurotus ostreatus) substrate inoculated with Lactobacillus brevis for Hanwoo steers. Rev. Colomb. De Cienc. Pecu. 2017, 30, 267–277. [Google Scholar] [CrossRef]
- Mahfuz, S.; He, T.; Liu, S.; Wu, D.; Long, S.; Piao, X. Dietary Inclusion of Mushroom (Flammulina velutipes) Stem Waste on Growth Performance, Antibody Response, Immune Status, and Serum Cholesterol in Broiler Chickens. Animals 2019, 9, 692. [Google Scholar] [CrossRef]
- Chuang, W.Y.; Liu, C.L.; Tsai, C.F.; Lin, W.C.; Chang, S.C.; Der Shih, H.; Shy, Y.M.; Lee, T.-T. Evaluation of Waste Mushroom Compost as a Feed Supplement and Its Effects on the Fat Metabolism and Antioxidant Capacity of Broilers. Animals 2020, 10, 445. [Google Scholar] [CrossRef]
- Lee, T.-T.; Ciou, J.-Y.; Chiang, C.-J.; Chao, Y.-P.; Yu, B. Effect of Pleurotus eryngii Stalk Residue on the Oxidative Status and Meat Quality of Broiler Chickens. J. Agric. Food Chem. 2012, 60, 11157–11163. [Google Scholar] [CrossRef]
- Chang, S.-C.; Lin, M.-J.; Chao, Y.-P.; Chiang, C.-J.; Jea, Y.-S.; Lee, T.-T. Effects of spent mushroom compost meal on growth performance and meat characteristics of grower geese. Rev. Bras. Zootec. 2016, 45, 281–287. [Google Scholar] [CrossRef]
- Rangubhet, K.T.; Mangwe, M.C.; Mlambo, V.; Fan, Y.K.; Chiang, H.I. Enteric methane emissions and protozoa populations in Holstein steers fed spent mushroom (Flammulina velutipes) substrate silage-based diets. Anim. Feed Sci. Technol. 2017, 234, 78–87. [Google Scholar] [CrossRef]
- Dai, C.; Ma, H.; He, R.; Huang, L.; Zhu, S.; Ding, Q.; Luo, L. Improvement of nutritional value and bioactivity of soybean meal by solid-state fermentation with Bacillus subtilis. LWT 2017, 86, 1–7. [Google Scholar] [CrossRef]
- Shi, H.; Yang, E.; Li, Y.; Chen, X.; Zhang, J. Effect of Solid-State Fermentation on Nutritional Quality of Leaf Flour of the Drumstick Tree (Moringa oleifera Lam.). Front. Bioeng. Biotechnol. 2021, 9, 626628. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cao, F.; Su, E.; Zhao, L.; Qin, W. Improvement of Animal Feed Additives of Ginkgo Leaves through Solid-state Fermentation using Aspergillus niger. Int. J. Biol. Sci. 2018, 14, 736–747. [Google Scholar] [CrossRef]
- Wu, S.; Lan, Y.; Wu, Z.; Peng, Y.; Chen, S.; Huang, Z.; Xu, L.; Gelbič, I.; Guan, X.; Zhang, L.; et al. Pretreatment of spent mushroom substrate for enhancing the conversion of fermentable sugar. Bioresour. Technol. 2013, 148, 596–600. [Google Scholar] [CrossRef]
- Si, M.; Yan, X.; Liu, M.; Shi, M.; Wang, Z.; Wang, S.; Zhang, J.; Gao, C.; Chai, L.; Shi, Y. In situ lignin bioconversion promotes complete carbohydrate conversion of rice straw by Cupriavidus basilensis B-8. ACS Sustain. Chem. Eng. 2018, 6, 7969–7978. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Y.H.; Chu, J.; Luo, L.Z.; Zhuang, Y.P.; Zhang, S.L. Optimization of cellulase mixture for efficient hydrolysis of steam-exploded corn stover by statistically designed experiments. Bioresour. Technol. 2009, 100, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Soltanian, S.; Aghbashlo, M.; Almasi, F.; Hosseinzadeh-Bandbafha, H.; Nizami, A.-S.; Ok, Y.S.; Lam, S.S.; Tabatabaei, M. A critical review of the effects of pretreatment methods on the exergetic aspects of lignocellulosic biofuels. Energy Convers. Manag. 2020, 212, 112792. [Google Scholar] [CrossRef]
- Brandon, A.G.; Scheller, H.V. Engineering of Bioenergy Crops: Dominant Genetic Approaches to Improve Polysaccharide Properties and Composition in Biomass. Front. Plant Sci. 2020, 11, 282. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Awasthi, M.K.; Li, P.; Meng, X.; Wang, Z. Comparative analysis of prediction models for methane potential based on spent edible fungus substrate. Bioresour. Technol. 2020, 317, 124052. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Yuan, X.; Wang, S.; Sun, F.; Hou, Z.; Hu, Q.; Zhai, L.; Cui, Z.; Zou, Y. Methane production and characteristics of the microbial community in the co-digestion of spent mushroom substrate with dairy manure. Bioresour. Technol. 2018, 250, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Lin, M.; Fan, J.; Chen, Y.; Zhao, C.; Liu, B. Anaerobic digestion of spent mushroom substrate under thermophilic conditions: Performance and microbial community analysis. Appl. Microbiol. Biotechnol. 2018, 102, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Mago, G.; Balan, V.; Wyman, C.E. Physical and chemical characterizations of corn stover and poplar solids resulting from leading pretreatment technologies. Bioresour. Technol. 2009, 100, 3948–3962. [Google Scholar] [CrossRef] [PubMed]
- Shibata, N.; Suetsugu, M.; Kakeshita, H.; Igarashi, K.; Hagihara, H.; Takimura, Y. A novel GH10 xylanase from Penicillium sp. accelerates saccharification of alkaline-pretreated bagasse by an enzyme from recombinant Trichoderma reesei expressing Aspergillus β-glucosidase. Biotechnol. Biofuels 2017, 10, 278. [Google Scholar] [CrossRef]
- Zhang, J.; Tuomainen, P.; Siika-Aho, M.; Viikari, L. Comparison of the synergistic action of two thermostable xylanases from GH families 10 and 11 with thermostable cellulases in lignocellulose hydrolysis. Bioresour. Technol. 2011, 102, 9090–9095. [Google Scholar] [CrossRef]
- Liao, H.; Xu, Y.; Zhang, J. Efficient production of xylooligosaccharides and fermentable sugars from corncob by propionic acid and enzymatic hydrolysis. Bioresour. Technol. 2021, 342, 125680. [Google Scholar] [CrossRef]
- Zhou, Y.; Kajiyama, S.; Itoh, K.; Tanino, T.; Fukuda, N.; Tanaka, T.; Kondo, A.; Fukui, K. Development of an enzyme activity screening system for beta-glucosidase-displaying yeasts using calcium alginate micro-beads and flow sorting. Appl. Microbiol. Biotechnol. 2009, 84, 375–382. [Google Scholar] [CrossRef]
- Preeti, V.E.; Sandhya, S.V.; Kuttiraja, M.; Sindhu, R.; Vani, S.; Kumar, S.R.; Pandey, A.; Binod, P. An evaluation of chemical pretreatment methods for improving enzymatic saccharification of chili postharvest residue. Appl. Biochem. Biotechnol. 2012, 167, 1489–1500. [Google Scholar] [CrossRef] [PubMed]
- Vergara-Barberán, M.; Lerma-García, M.; Herrero-Martínez, J.; Simó-Alfonso, E. Use of an enzyme-assisted method to improve protein extraction from olive leaves. Food Chem. 2015, 169, 28–33. [Google Scholar] [CrossRef] [PubMed]
- de Figueiredo, V.R.G.; Yamashita, F.; Vanzela, A.L.L.; Ida, E.I.; Kurozawa, L.E. Action of multi-enzyme complex on protein extraction to obtain a protein concentrate from okara. J. Food Sci. Technol. 2018, 55, 1508–1517. [Google Scholar] [CrossRef]
- Berlin, A.; Maximenko, V.; Gilkes, N.; Saddler, J. Optimization of enzyme complexes for lignocellulose hydrolysis. Biotechnol. Bioeng. 2007, 97, 287–296. [Google Scholar] [CrossRef]
- Woodward, J.; Hayes, M.K.; Lee, N.E. Hydrolysis of cellulose by saturating and non-saturating concentrations of cellulase-implications for synergism. Bio-Technology 1988, 6, 301–304. [Google Scholar] [CrossRef]
- Asha, P.C.; Prema, P. Production of cellulase-free endoxylanase from novel alkalophilic thermotolerent Bacillus pumilus by solid-state fermentation and its application in wastepaper recycling. Bioresour. Technol. 2007, 98, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Lee, Y.Y.; Elander, R.T. Conversion of aqueous ammonia-treated corn stover to lactic acid by simultaneous saccharification and cofermentation. Appl. Biochem. Biotech. 2007, 137, 721–738. [Google Scholar]
- Al Loman, A.; Ju, L. Towards complete hydrolysis of soy flour carbohydrates by enzyme mixtures for protein enrichment: A modeling approach. Enzym. Microb. Technol. 2016, 86, 25–33. [Google Scholar] [CrossRef]
| Level | Factors | ||
|---|---|---|---|
| Cellulase/% | Xylanase/% | β-Galactosidase/% | |
| −1 | 6 | 6 | 1 |
| 0 | 8 | 8 | 2 |
| +1 | 10 | 10 | 3 |
| Number | Factors | Results | ||||
|---|---|---|---|---|---|---|
| Cellulase | Xylanase | β-Galactosidase | RS, mg/g | NDF Degradation Rate, % | Enzymatic Hydrolysis Effect | |
| 1 | −1 | −1 | −1 | 185.36 | 11.12 | 49.45 |
| 2 | −1 | 0 | 0 | 196.65 | 15.64 | 55.46 |
| 3 | −1 | +1 | +1 | 198.79 | 16.60 | 56.68 |
| 4 | 0 | −1 | 0 | 202.14 | 15.32 | 56.42 |
| 5 | 0 | 0 | +1 | 206.97 | 17.50 | 59.18 |
| 6 | 0 | +1 | −1 | 201.07 | 16.72 | 57.28 |
| 7 | +1 | −1 | +1 | 207.37 | 13.51 | 56.16 |
| 8 | +1 | 0 | −1 | 202.81 | 16.51 | 57.50 |
| 9 | +1 | +1 | 0 | 210.19 | 18.05 | 60.32 |
| k1 | 53.87 | 54.01 | 54.74 | |||
| k2 | 57.63 | 57.38 | 57.40 | |||
| k3 | 57.99 | 58.09 | 57.34 | |||
| Range | 4.13 | 4.08 | 2.66 | |||
| Best level | +1 | +1 | 0 | |||
| Sequence | cellulase > xylanase > β-galactosidase | |||||
| Level | Bacillus subtilis Inoculum/% F1 | Pediococcus acidilactici Inoculum/% F2 | Saccharomyces cerevisiae Inoculum/% F3 | Time/h F4 | Temperature/°C F5 | Moisture/% F6 |
|---|---|---|---|---|---|---|
| −1 | 2 | 2 | 2 | 48 | 30 | 50 |
| +1 | 10 | 10 | 10 | 96 | 36 | 60 |
| Experimental Number | F1 | F2 | F3 | F4 | F5 | F6 | NDF Degradation Rate/% |
|---|---|---|---|---|---|---|---|
| 1 | −1 | +1 | +1 | −1 | +1 | −1 | 19.78 |
| 2 | +1 | −1 | +1 | −1 | −1 | −1 | 15.79 |
| 3 | +1 | +1 | −1 | +1 | −1 | −1 | 9.35 |
| 4 | +1 | −1 | −1 | −1 | +1 | +1 | 21.33 |
| 5 | −1 | −1 | +1 | +1 | +1 | −1 | 19.37 |
| 6 | +1 | +1 | −1 | +1 | +1 | −1 | 15.87 |
| 7 | −1 | −1 | −1 | −1 | −1 | −1 | 18.22 |
| 8 | +1 | +1 | +1 | −1 | +1 | +1 | 22.87 |
| 9 | −1 | +1 | −1 | −1 | −1 | +1 | 19.71 |
| 10 | −1 | +1 | +1 | +1 | −1 | +1 | 16.35 |
| 11 | +1 | −1 | +1 | +1 | −1 | +1 | 14.27 |
| 12 | −1 | −1 | −1 | +1 | +1 | +1 | 17.64 |
| Sources | Degree of Freedom | Sum of Squares | Mean Square | F-Value | p Value |
|---|---|---|---|---|---|
| Model | 6 | 127.16 | 21.1933 | 7.46 | 0.022 |
| Linear | 6 | 127.16 | 21.1933 | 7.46 | 0.022 |
| F1 | 1 | 11.194 | 11.194 | 3.94 | 0.104 |
| F2 | 1 | 0.603 | 0.603 | 0.21 | 0.664 |
| F3 | 1 | 3.318 | 3.318 | 1.17 | 0.329 |
| F4 | 1 | 51.46 | 51.4602 | 18.12 | 0.008 |
| F5 | 1 | 44.737 | 44.7374 | 15.76 | 0.011 |
| F6 | 1 | 15.847 | 15.847 | 5.58 | 0.065 |
| Error | 5 | 14.197 | 2.8394 | ||
| Total | 11 | 141.357 |
| Level | Time/h | Temperature/°C | Moisture/% |
|---|---|---|---|
| A | B | C | |
| −1 | 24 | 33 | 50 |
| 0 | 48 | 36 | 60 |
| +1 | 72 | 39 | 70 |
| Experimental Number | Time/h A | Temperature/°C B | Moisture/% C | NDF Degradation Rate/% Response |
|---|---|---|---|---|
| 1 | 0 | −1 | +1 | 18.17 |
| 2 | −1 | 0 | +1 | 11.11 |
| 3 | 0 | 0 | 0 | 21.10 |
| 4 | −1 | −1 | 0 | 14.78 |
| 5 | +1 | 0 | −1 | 21.79 |
| 6 | −1 | +1 | 0 | 11.34 |
| 7 | 0 | +1 | −1 | 20.03 |
| 8 | +1 | +1 | 0 | 22.39 |
| 9 | −1 | 0 | −1 | 12.46 |
| 10 | +1 | −1 | 0 | 21.91 |
| 11 | 0 | 0 | 0 | 22.04 |
| 12 | +1 | 0 | +1 | 22.18 |
| 13 | 0 | −1 | −1 | 18.20 |
| 14 | 0 | 0 | 0 | 21.74 |
| 15 | 0 | +1 | +1 | 17.13 |
| Source | Sum of Squares | Degrees of Freedom | Mean Square | F-Value | p Value | |
|---|---|---|---|---|---|---|
| Model | 238.16 | 9 | 26.46 | 39.86 | 0.0004 | ** |
| A: Time | 186.10 | 1 | 186.10 | 280.29 | <0.0001 | ** |
| B: Temperature | 0.58 | 1 | 0.58 | 0.88 | 0.3917 | |
| C: Moisture | 1.90 | 1 | 1.90 | 2.86 | 0.1518 | |
| AB | 3.83 | 1 | 3.83 | 5.77 | 0.0614 | |
| AC | 0.76 | 1 | 0.76 | 1.14 | 0.3345 | |
| BC | 2.05 | 1 | 2.05 | 3.09 | 0.1391 | |
| A2 | 28.12 | 1 | 28.12 | 42.35 | 0.0013 | ** |
| B2 | 5.89 | 1 | 5.89 | 8.88 | 0.0308 | * |
| C2 | 14.51 | 1 | 14.51 | 21.85 | 0.0055 | ** |
| Residual | 3.32 | 5 | 0.66 | |||
| Lack of Fit | 2.86 | 3 | 0.95 | 4.13 | 0.2009 | |
| Pure Error | 0.46 | 2 | 0.23 | |||
| Cor Total | 241.48 | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, A.; He, W.; Han, Y.; Zheng, A.; Chen, Z.; Meng, K.; Yang, P.; Liu, G. Cooperative Fermentation Using Multiple Microorganisms and Enzymes Potentially Enhances the Nutritional Value of Spent Mushroom Substrate. Agriculture 2024, 14, 629. https://doi.org/10.3390/agriculture14040629
Zhang A, He W, Han Y, Zheng A, Chen Z, Meng K, Yang P, Liu G. Cooperative Fermentation Using Multiple Microorganisms and Enzymes Potentially Enhances the Nutritional Value of Spent Mushroom Substrate. Agriculture. 2024; 14(4):629. https://doi.org/10.3390/agriculture14040629
Chicago/Turabian StyleZhang, Anrong, Weizhao He, Yunsheng Han, Aijuan Zheng, Zhimin Chen, Kun Meng, Peilong Yang, and Guohua Liu. 2024. "Cooperative Fermentation Using Multiple Microorganisms and Enzymes Potentially Enhances the Nutritional Value of Spent Mushroom Substrate" Agriculture 14, no. 4: 629. https://doi.org/10.3390/agriculture14040629
APA StyleZhang, A., He, W., Han, Y., Zheng, A., Chen, Z., Meng, K., Yang, P., & Liu, G. (2024). Cooperative Fermentation Using Multiple Microorganisms and Enzymes Potentially Enhances the Nutritional Value of Spent Mushroom Substrate. Agriculture, 14(4), 629. https://doi.org/10.3390/agriculture14040629









