Unveiling the Antioxidant Arsenal of Colored Sorghum: A Path to Functional Food Development
Abstract
1. Introduction
2. Material and Methods
2.1. Collection of Sorghum Lines and Seed Characteristics
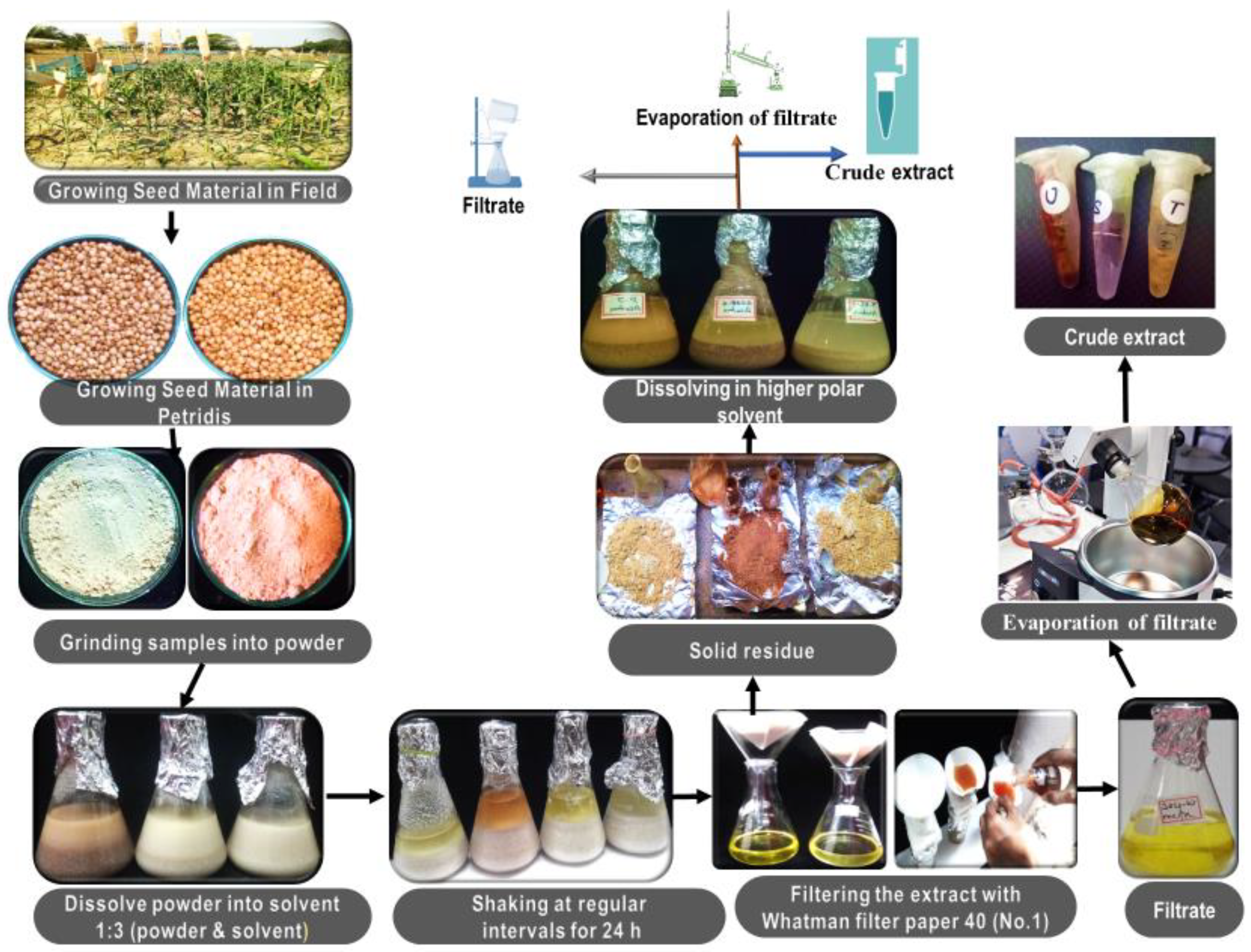
2.2. Extraction of Plant Material
Preliminary Phytochemical Screening
2.3. Quantification of TPC and TFC in Sorghum Lines
2.3.1. Total Phenolic Content Determination (TPC)
2.3.2. Total Flavonoids Content (TFC)
2.4. Antioxidant Activity (Free Radical-Scavenging Activity)
2.4.1. DPPH Assay
2.4.2. ABTS (2,2′-Azino-Bis (3-Ethylbenzothiazoline-6-Sulfonic Acid) Assay
2.4.3. Nitrous Oxide-Scavenging Activity
2.5. In Vitro Antioxidant Potential
Ferrous-Reducing Antioxidant Potential (FRAP)
2.6. Statistical Analysis
3. Results
3.1. Solvent Extract Characteristics
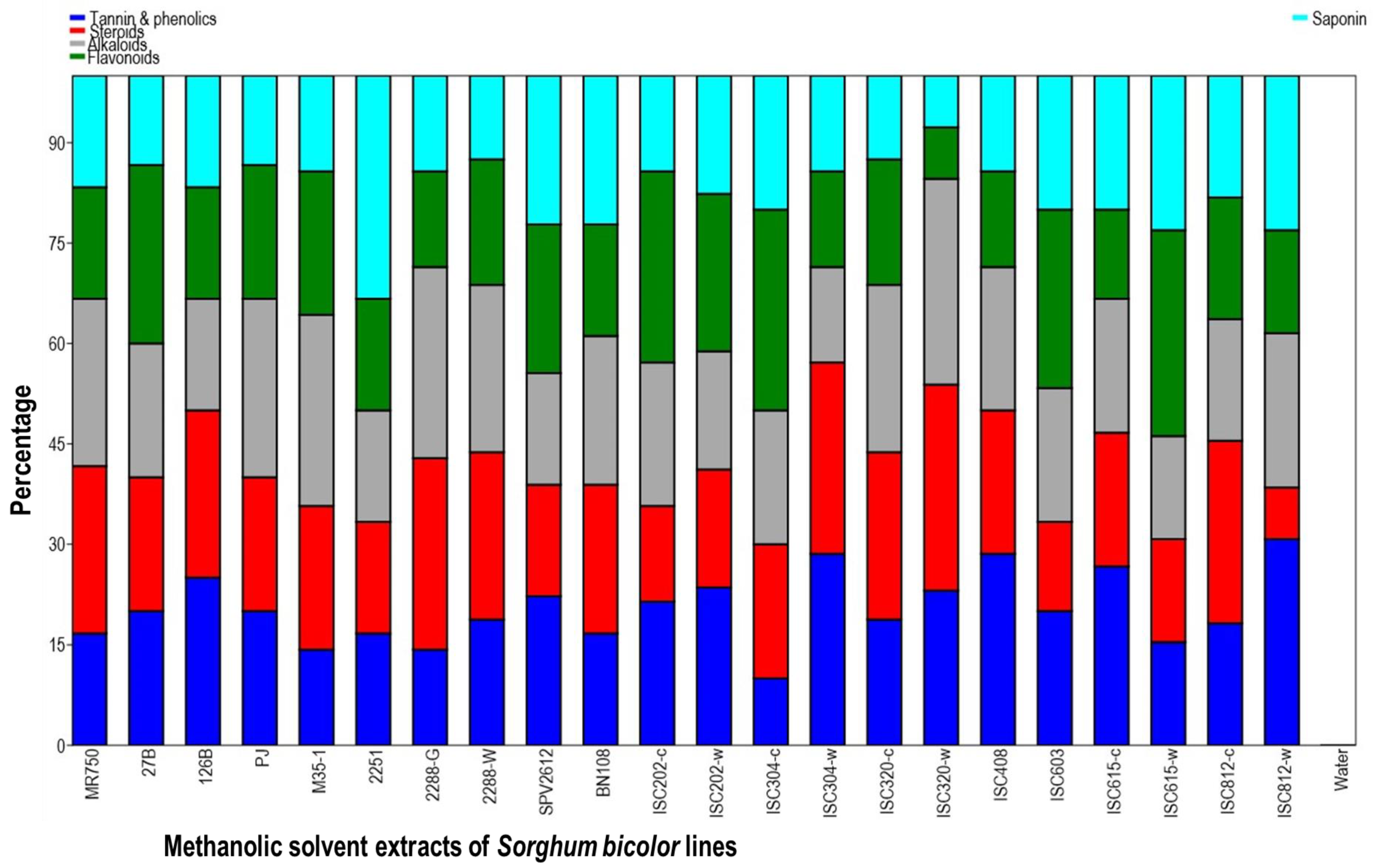
3.2. Qualitative Phytochemical Profiling
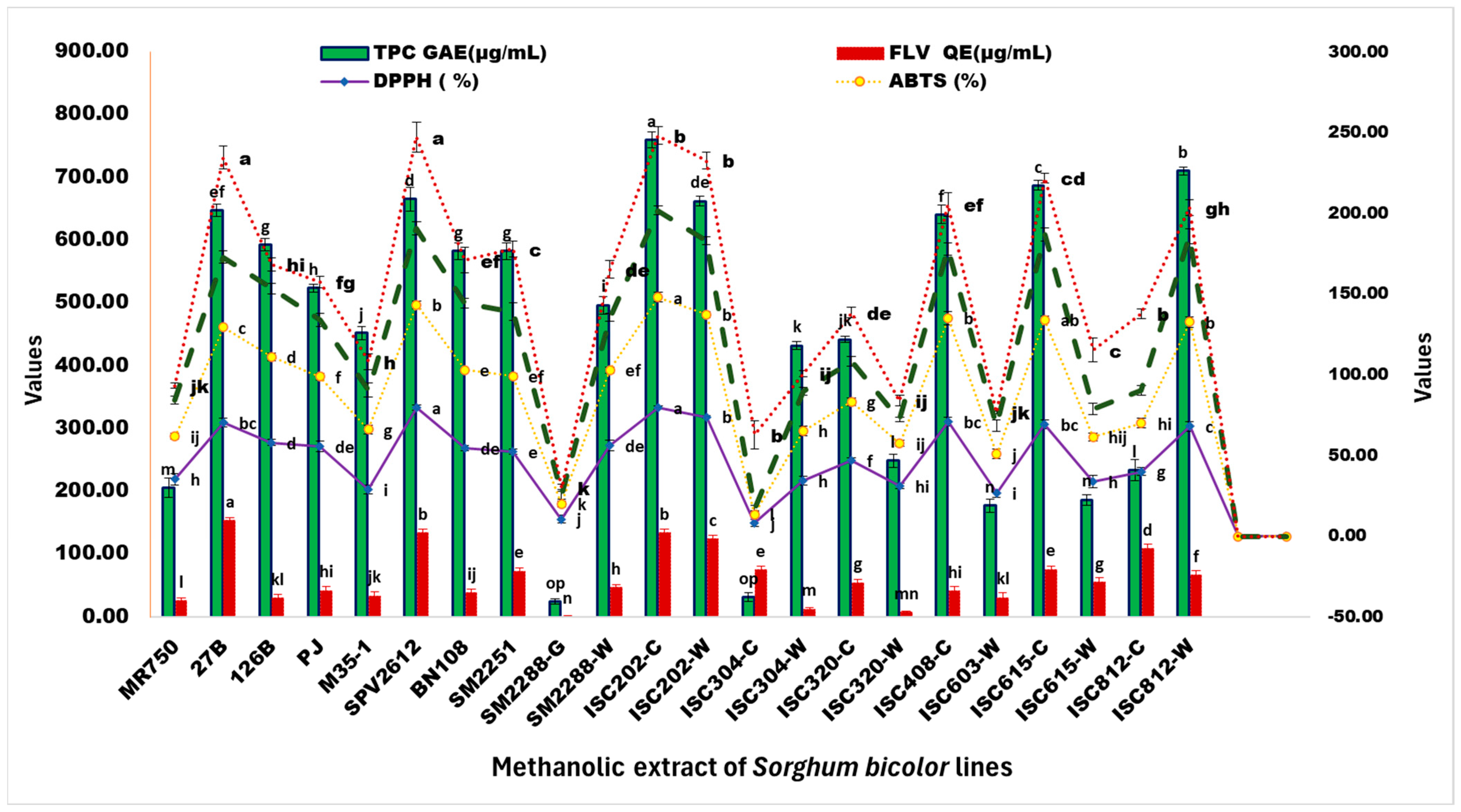
3.2.1. Quantification of Total Flavonoid Content (TFC) of Selected Sorghum Lines
3.2.2. Quantification of Total Phenolic Content (TPC) of Selected Sorghum Lines
3.3. Assessment of Antioxidant Potential
3.3.1. Ferrous-Reducing Antioxidant Potential (FRAP) Assay
3.3.2. Assessment of Free Radical-Scavenging Activity
DPPH Free Radical-Scavenging Assay (%)
Nitrous Oxide-Scavenging Activity (%)
2,2′-and-Bis (3-Ethylbenzothiazoline-6-Sulfonic Acid)-Scavenging Assay (ABTS%)
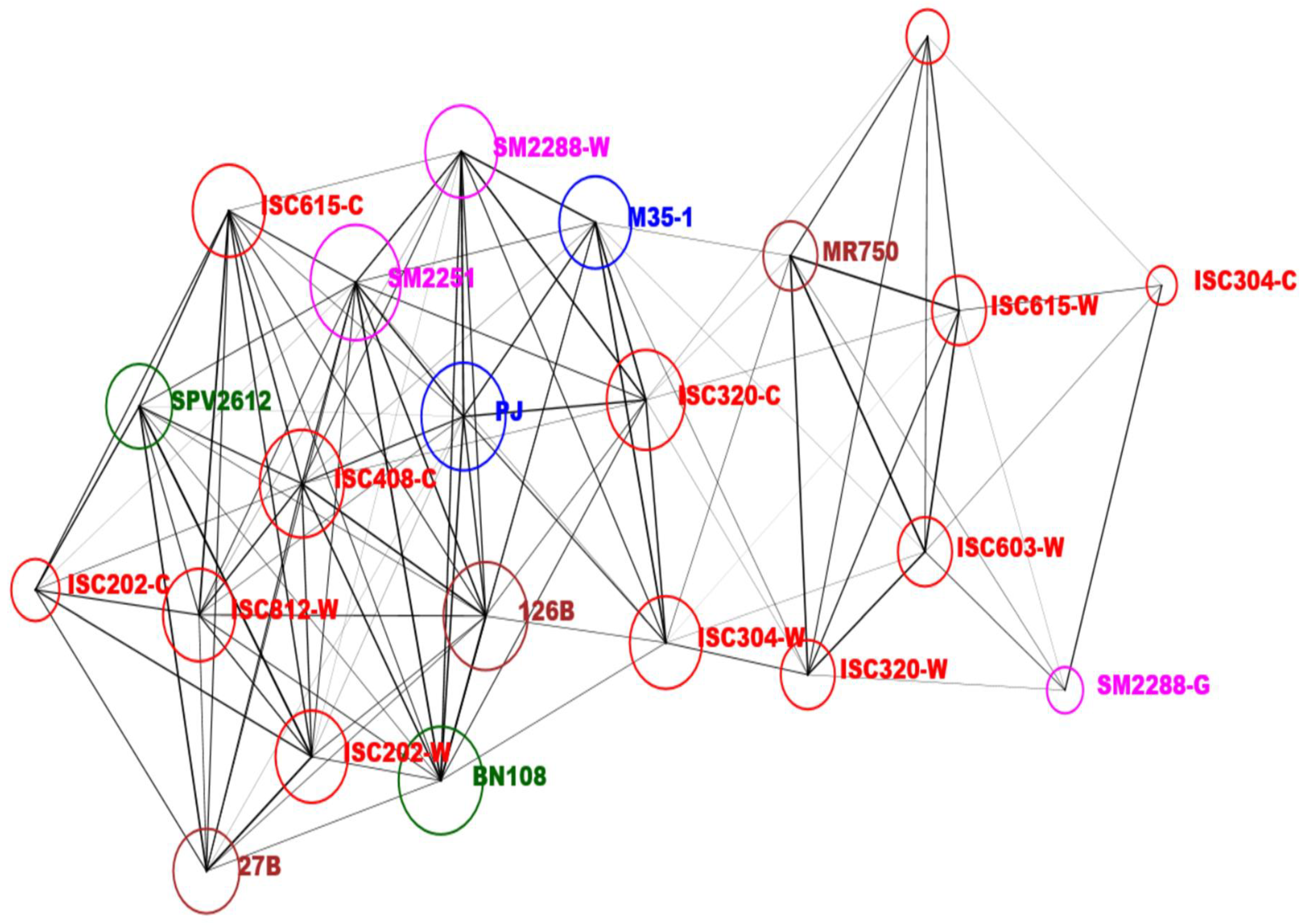
3.4. Networking and PCA Biplot for Solvent Extracts of Sorghum Lines
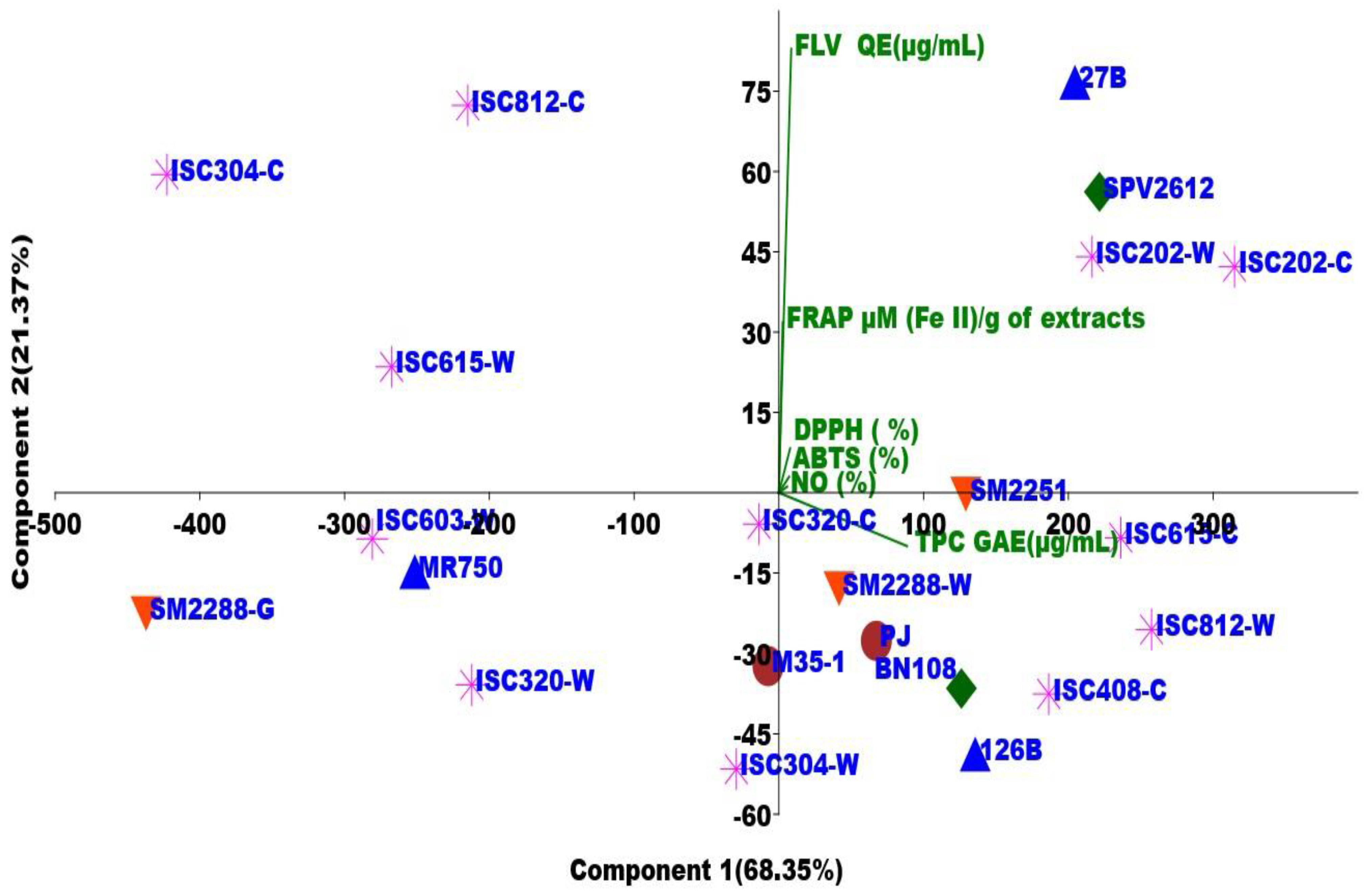
3.5. Principal Component Analysis (PCA)
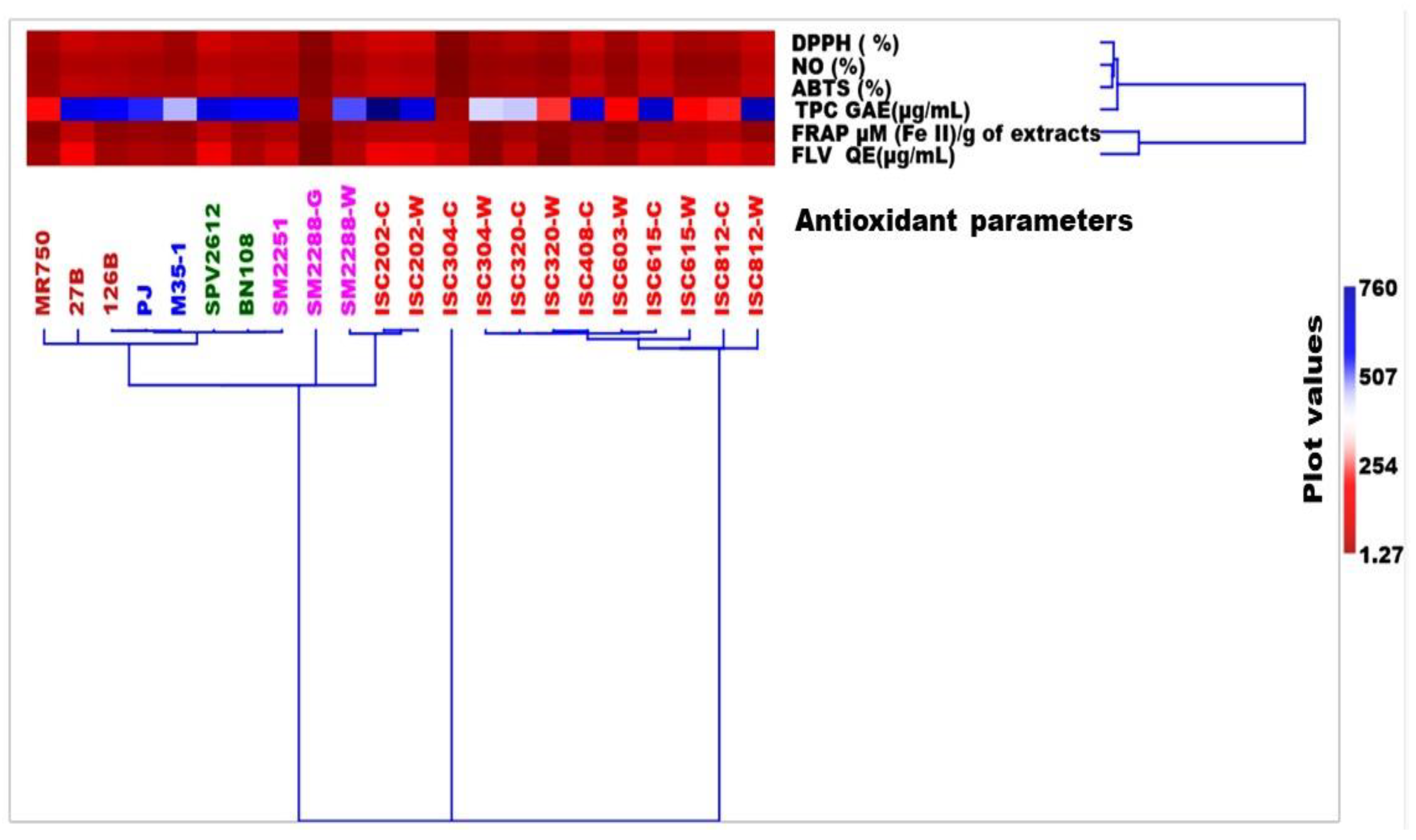
3.6. UPGMA Cluster Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Punia, H.; Tokas, J.; Malik, A.; Sangwan, S. Characterization of phenolic compounds and antioxidant activity in sorghum [Sorghum bicolor (L.) Moench] grains. Cereal Res. Comm. 2021, 49, 343–353. [Google Scholar] [CrossRef]
- International Production Assessment Division. Available online: https://ipad.fas.usda.gov/cropexplorer/cropview/commodityView.aspx?cropid=0459200 (accessed on 5 January 2024).
- Llopart, E.E.; Drago, S.R. Physicochemical properties of sorghum and technological aptitude for popping nutritional changes after popping. LWT Food Sci. Technol. 2016, 71, 316–322. [Google Scholar] [CrossRef]
- Chavez, D.W.H.; Ascheri, J.L.R.; Carvalho, C.W.P.; Godoy, R.L.O.; Pacheco, S. Sorghum and roasted coffee blends as a novel extruded product: Bioactive compounds and antioxidant capacity. J. Funct. Foods 2017, 29, 93–103. [Google Scholar] [CrossRef]
- Przybylska-Balcerek, A.; Frankowski, J.; Sieracka, D.; Sázavská, T.; Wacławek, S.; Raczak, B.K.; Szwajkowska-Michałek, L.; Bu’sko, M.; Graczyk, M.; Niedziela, G.; et al. The Content of Antioxidant Compounds and VOCs in Sorghum Grain Grown in Central and Eastern Europe. Agronomy 2024, 14, 217. [Google Scholar] [CrossRef]
- Pontieri, P.; Mamone, G.; De Caro, S.; Tuinstra, M.R.; Roemer, E.; Okot, J. Sorghum, a healthy and gluten-free food for celiac patients as demonstrated by genome, biochemical, and immunochemical analyses. J. Agric. Food Chem. 2013, 61, 2565–2571. [Google Scholar] [CrossRef]
- de Oliveira, L.L.; Guilherme, T.O.; Ernandes, R.A.; Queiroz, V.A.P.; Figueired, L.F.D. Physical, chemical, and antioxidant analysis of sorghum grain and flour from five hybrids to determine the drivers of liking of gluten-free sorghum breads. LWT 2022, 153, 112407. [Google Scholar] [CrossRef]
- Garzón, A.G.; Albarracín, M.; Drago, S.R. Bioactive Properties of Sorghum-based Beverages from Whole or Refined Grains. Recent Prog. Nutr. 2023, 3, 13. [Google Scholar] [CrossRef]
- Khoddami, A.; Truong, H.H.; Liu, S.Y.; Thomas, H.; Roberts Selle, P.H. Concentrations of specific phenolic compounds in six red sorghums influence nutrient utilisation in broiler chickens. Anim. Feed Sci. Technol. 2015, 210, 190–199. [Google Scholar] [CrossRef]
- Dykes, L.; Rooney, L.; Waniska, R.; Rooney, W. Phenolic Compounds and Antioxidant Activity of Sorghum Grains of Varying Genotypes. J. Agric. Food Chem. 2005, 53, 6813–6818. [Google Scholar] [CrossRef]
- Stefoska-Needham, A.; Beck, E.J.; Johnson, S.K.; Tapsell, L.C. Sorghum: An underutilized cereal whole grain with the potential to assist in the prevention of chronic disease. Food Rev. Int. 2015, 31, 401–437. [Google Scholar] [CrossRef]
- Wu, G.; Johnson, S.K.; Bornman, J.F.; Bennett, S.J.; Fang, Z. Changes in whole grain polyphenols and antioxidant activity of six sorghum genotypes under different irrigation treatments. Food Chem. 2017, 214, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Susan, G.; Prior, R.L. Concentration of proanthocyanidins in common foods and estimations of normal consumptions. J. Nutr. 2004, 134, 613–617. [Google Scholar]
- Kortei, N.K.; Odamtten, G.T.; Appiah, V.; Obodai, M.; Adu-Gyamfi, A.; Wiafe-Kwagyan, M. Comparative occurrence of resident fungi on gamma irradiated and steam sterilized sorghum grains (Sorghum bicolor L.) for spawn production in Ghana. Br. Biotechnol. 2015, 7, 21–32. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Liu, Z.; Wang, J. Extraction, Identification and Antioxidant Activity of 3-Deoxyanthocyanidins from Sorghum bicolor L. Moench Cultivated in China. Antioxidants 2023, 12, 468. [Google Scholar] [CrossRef] [PubMed]
- Awika, J.M.; Rooney, L.W. Sorghum phytochemical and their potential impact on human health. Phytochemistry 2004, 65, 1199–1221. [Google Scholar] [CrossRef] [PubMed]
- Arts, I.C.W.; Hollman, P.C.H. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005, 81, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B.; Williams, C.A. Advances in flavonoids research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef] [PubMed]
- Alfadda, A.A.; Sallam, R.M. Reactive oxygen species in health and disease. J. Biomed. Biotechnol. 2012, 2012, 936486. [Google Scholar] [CrossRef] [PubMed]
- Squarcina, A.; Santoro, A.; Hickey, N.; Zorzi, R.D.; Carraro, M.; Geremia, S.; Bortolus, M.; Valentin, M.D.; Bonchio, M.M. Neutralization of Reactive Oxygen Species at Dinuclear Cu(II)-Cores: Tuning the Antioxidant Manifold in Water by Ligand Design. ACS Catal. 2020, 10, 7295–7306. [Google Scholar] [CrossRef]
- Arbex, P.M.; de Moreira, M.E.C.; Toledo, R.C.L.; de Morais Cardo, L.; Pinheiro-Sant’ana, H.M.; dos Benjamin, L.A.; de Oliveria, L.L.; Carvalho, C.W.P.; Queiroz, V.A.V.; Martino, H. Extruded Sorghum Flour (Sorghum bicolor L.) Modulate Adiposity and Inflammation in High Fat Diet-Induced Obese Rats. J. Funct. Foods 2018, 42, 346–355. [Google Scholar] [CrossRef]
- Chen, X.; Shen, J.; Xu, J.; Herald, T.; Smolensky, D.; Perumal, R.; Wang, W. Sorghum phenolic compounds are associated with cell growth inhibition through cell cycle arrest and apoptosis in human hepatocarcinoma and colorectal adenocarcinoma cells. Foods 2021, 10, 993. [Google Scholar] [CrossRef] [PubMed]
- de Morais, L.C.; Pinheiro, S.S.S.; Martino, H.S.D.S.; Pinheiro-Sant’Ana, H.M.M. Sorghum (Sorghum bicolor L.): Nutrients, bioactive compounds, and potential impact on human health. Crit. Rev. Food Sci. Nutr. 2017, 57, 372–390. [Google Scholar] [CrossRef]
- Visarada, K.B.R.S.; Aruna, C. Sorghum: A Bundle of Opportunities in the 21st Century. In Breeding Sorghum for Diverse End Uses; Aruna, C., Visarada, K.B.R.S., Bhat, B.V., Tonapi, V.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–14. [Google Scholar]
- Lee, S.H.; Lee, J.; Herald, T.; Cox, S.; Noronha, L.; Perumal, R.; Lee, H.-S.; Smolensky, D. Anticancer Activity of a Novel High Phenolic Sorghum Bran in Human Colon Cancer Cells. Oxid. Med. Cell. Longev. 2020, 2020, 2890536. [Google Scholar] [CrossRef]
- Lee, H.; Jeong, W.T.; So, Y.S.; Lim, H.B.; Lee, J. Taxifolin and Sorghum Ethanol Extract Protect against Hepatic Insulin Resistance via the miR-195/IRS1/PI3K/AKT and AMPK Signalling Pathways. Antioxidants 2021, 10, 1331. [Google Scholar] [CrossRef] [PubMed]
- Ducksbury, C.; Neale, E.P.; Stefoska-Needham, A. The effect of sorghum consumption on markers of chronic disease: A systematic review. Crit. Rev. Food Sci. Nutr. 2021, 63, 159–177. [Google Scholar] [CrossRef]
- Hassan, S.M. Nutritional, Functional and Bioactive Properties of Sorghum (Sorghum Bicolor I. Moench) with its Future Outlooks: A Review. Open J. Nutr. Food Sci. 2023, 5, 1030. [Google Scholar]
- Vita, J.A. Polyphenols and cardiovascular disease: Effects on endothelial and platelet function. Am. J. Clin. Nutr. 2005, 81, 292S–297S. [Google Scholar] [CrossRef]
- Wu, L.T.; Chu, C.C.; Chung, J.G.; Chen, C.H.; Hsu, L.S.; Liu, J.K.; Chen, S.C. Effects of tannic acid and its related compounds on food mutagens or hydrogen peroxide-induced DNA strands breaks in human lymphocytes. Mut. Res. 2004, 556, 75. [Google Scholar] [CrossRef] [PubMed]
- Biparva, P.; Ehsani, M.; Hadjmohammadi, M.R. Dispersive liquid–liquid microextraction using extraction solvents lighter than water combined with high phigh-performanced chromatography for determination of synthetic antioxidants in fruit juice samples. J. Food Compos. Anal. 2012, 27, 87–94. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Novel antioxidants in food preservation and health promotion. Eur. J. Lipid Sci. Technol. 2010, 112, 930–940. [Google Scholar] [CrossRef]
- Visarada, K.B.R.S. Variations are introduced in sorghum through pollination with maize. Curr. Sci. 2019, 119, 1540–1548. [Google Scholar] [CrossRef]
- Visarada, K.B.R.S. Overcoming interspecific barriers in Sorghum and molecular characterization of the derivatives. Plant Breed. 2020, 139, 892–905. [Google Scholar] [CrossRef]
- Sofowora, E.A. Medicinal Plants and Traditional Medicine in Africa; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 1982; pp. 64–79. [Google Scholar]
- Trease, G.E.; Evans, W.C. Pharmacognosy, 11th ed.; Bailliere Tindall: London, UK, 1989; pp. 45–50. [Google Scholar]
- Singleton, V.; Rossi, J. Colorimetry of Total Phenolic Compounds with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Harborne, J.B. Textbook of Phytochemical Methods. In A Guide to Modern Techniques of Plant Analysis, 5th ed.; Chapman and Hall Ltd.: London, UK, 1998; pp. 21–72. [Google Scholar]
- Bozin, B.; Mimica-Dukic, N.; Simin, N.; Anackov, G. Characterization of the volatile composition of essential oils of some lamiaceae spices and the antimicrobial and antioxidant activities of the entire oils. J. Agric. Food Chem. 2006, 54, 1822–1828. [Google Scholar] [CrossRef] [PubMed]
- Ilyasov, I.; Beloborodov, V.; Antonov, D.; Dubrovskaya, A.; Terekhov, R.; Zhevlakova, A.; Saydasheva, A.; Evteev, V.; Selivanova, I. Flavonoids with Glutathione Antioxidant Synergy: Influence of Free Radicals Inflow. Antioxidants 2020, 9, 695. [Google Scholar] [CrossRef] [PubMed]
- Garrat, D.C. The Quantitative Analysis of Drugs Japan, 3rd ed.; Chapman and Hall: Tokyo, Japan, 1964; pp. 456–458. [Google Scholar]
- Benzie, I.; Strain, J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power: The FRAP Assay”. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Al-Raqmi, K.A.; AL-Mijizy, Z.H.; Weli, A.M.; Al-Riyami, Q. Study of total phenol, flavonoids contents and phytochemical screening of various leaves crude extracts of locally grown Thymus vulgaris. Asian Pac. J. Trop. Biomed. 2013, 3, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Ali, K.S.; Elsheikh, A.M.; Ahmed, H.M.; Hassan, H.A.; Hamza, N.B.; Osman, M.G.; Daffalla, H.M.; Osman, H.M.H. Phytochemical screening and antibacterial activities of Sorghum bicolor leaves derived from in vitro culture. GSC Biol. Pharm. Sci. 2020, 10, 65–72. [Google Scholar]
- Meena, K. Modified Mechanical and Methodological Attributes Interplay for Isolating Antioxidant Concomitant in Herbal Materials: An Innovative Concept. Res. Biot. 2021, 3, 158–163. [Google Scholar] [CrossRef]
- Tyagi, V.; Saravanan, C.; Wang, Y.; Bhattacharya, B. Solvent Dependency of Sorghum Bran Phytochemicals Acting as Potential Antioxidants and Antibacterial Agents. Food Technol. Biotechnol. 2021, 59, 31–43. [Google Scholar] [CrossRef]
- Fahey, J.W. Reference Module in Food Science. In Encyclopedia of Food and Health, 1st ed.; Elsevier Ltd.: Oxford, UK, 2016; p. 469. [Google Scholar]
- Choi, S.; Beuchat, L.R.; Kim, H.; Ryu, J.H. Viability of sprout seeds as affected by treatment with aqueous chlorine dioxide and dry heat, and reduction of Escherichia coli O157:H7 and Salmonella enterica on pak choi seeds by sequential treatment with chlorine dioxide, drying and dry heat. Food Microbiol. 2016, 54, 127–132. [Google Scholar] [CrossRef]
- Ramakrishna, W.; Anuradha, K.; Rahman, N.; Mandave, P. Anticancer Activities of Plant Secondary Metabolites: Rice Callus Suspension Culture as a New Paradigm. Rice Sci. 2021, 28, 13–30. [Google Scholar] [CrossRef]
- Afrin, S.; Sidhu, S. In vitro study to evaluate anti-inflammatory properties of sorghum extract supplemented bread. Future Foods. 2021, 4, 100039. [Google Scholar] [CrossRef]
- Kim, J.; Park, Y. Anti-diabetic effect of sorghum extract on hepatic gluconeogenesis of streptozotocin-induced diabetic rats. Nutr. Metab. 2012, 9, 106. [Google Scholar] [CrossRef] [PubMed]
- Schnur, S.E.; Amachawadi, R.G.; Baca, G.; Sexton-Bowser, S.; Rhodes, D.H.; Smolensky, D.; Herald, T.J.; Perumal, R.; Thomson, D.U.; Nagaraja, T.G. Antimicrobial Activity of Sorghum Phenolic Extract on Bovine Foodborne and Mastitis-Causing Pathogens. Antibiotics 2021, 10, 594. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Hyun, T.K.; Kim, M. Anti-oxidative activities of sorghum, foxtail millet and proso millet extracts. Afr. J. Biotechnol. 2010, 9, 2683–2690. [Google Scholar]
- Luo, M.; Hou, F.; Dong, L.; Huang, F.; Zhang, R.; Su, D. Comparison of microwave and high-pressure processing on bound phenolic composition and antioxidant activities of sorghum hull. Int. J. Food Sci. Technol. 2020, 55, 3190–3202. [Google Scholar] [CrossRef]
- Suganyadevi, P.; Saravanakumar, M.; Sundhar, M. The antiproliferative activity of 3-deoxyanthocyanins extracted from red sorghum (Sorghum bicolor) bran through P53-dependent and Bcl-2 gene expression in breast cancer cell line. Life Sci. 2013, 92, 379–382. [Google Scholar] [CrossRef]
- Alfieri, M.; Balconi, C.; Cabassi, G.; Habyarimana, E.; Redaelli, R. Antioxidant activity in a set of sorghum landraces and breeding lines. Maydica 2017, 62, 3–32. [Google Scholar]
- Mawouma, S.; Condurache, N.N.; Turturica, M.; Constantin, O.E.; Croitoru, C.; Rapeanu, G. Chemical Composition and Antioxidant Profile of Sorghum (Sorghum bicolor (L.) Moench) and Pearl Millet (Pennisetum glaucum (L.) R.Br.) Grains Cultivated in the Far-North Region of Cameroon. Foods 2022, 11, 2026. [Google Scholar] [CrossRef]
- Jugran, A.K.; Bahukhandi, A.; Dhyani, P.; Bhatt, I.D.; Rawal, R.S.; Nandi, S.K. Impact of altitudes and habitats on valerenic acid, total phenolics, flavonoids, tannins, and antioxidant activity of Valeriana jatamansi. Appl. Biochem. Biotechnol. 2016, 179, 911–926. [Google Scholar] [CrossRef] [PubMed]
- Meena, D.K.; Sahoo, A.K.; Chowdhury, H.; Swain, H.S.; Sahu, N.P.; Behera, B.K. Effects of extraction methods and solvent systems on extract yield, proximate composition and mineral profiling of Terminalia arjuna (Arjuna) dry powders and solvent extracts. J. Innov. Pharm. Biol. Sci. 2019, 7, 22–31. [Google Scholar]
- Meena, D.K.; Sahoo, A.K.; Srivastava, P.P.; Sahu, N.P.; Jadhav, M.; Gandhi, M. On valorization of solvent extracts of Terminalia arjuna (arjuna) upon DNA scission and free radical scavenging improves coupling responses and cognitive functions under in vitro conditions. Sci. Rep. 2021, 11, 10656. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, B.K.; Seo, J.W.; Yu, C.Y.; Kim, S.H.; Chung, I.M. Comparative Study on Seed Characteristics, Antioxidant Activity, and Total Phenolic and Flavonoid Contents in Accessions of Sorghum bicolor (L.) Moench. Molecules 2021, 26, 3964. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M. Catechins as antioxidants from buckwheat (Fagopyrum esculentum Moench) groats. J. Agric. Food Chem. 1998, 46, 839–845. [Google Scholar] [CrossRef]
- Dia, V.P.; Pangloli, P.; Jones, L.; McClure, A.; Patel, A. Phytochemical concentrations and biological activities of Sorghum bicolor alcoholic extracts. Food Funct. 2016, 7, 3410–3420. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Santhakumar, A.B.; Chinkwo, K.A.; Gangcheng, W.; Johnson, S.K.; Blanchard, C.L. Characterization of phenolic compounds and antioxidant activity in sorghum grains. J. Cereal Sci. 2018, 84, 103–111. [Google Scholar] [CrossRef]
- Kamath, V.G.; Chandrashekar, A.; Rajini, P.S. Antiradical properties of sorghum (Sorghum bicolor L. Moench) flour extracts. J. Cereal Sci. 2004, 40, 283–288. [Google Scholar] [CrossRef]
- Shen, S.; Huang, R.; Li, C.; Wu, W.; Chen, H.; Shi, J.; Chen, S.; Ye, X. Phenolic Compositions and Antioxidant Activities Differ Significantly among Sorghum Grains with Different Applications. Molecules 2018, 23, 1203. [Google Scholar] [CrossRef]
- Bouargalne, Y.; Ben Mrid, R.; Bouchmaa, N.; Zouaoui, N.; Benmrid, B.; Kchikich, A.; El Omari, R.; Kabach, I.; Mohamed, N. Genetic diversity for agromorphological traits, phytochemical profile, and antioxidant activity in Moroccan sorghum ecotypes. Sci. Rep. 2022, 12, 5895. [Google Scholar] [CrossRef]
- Awika, J.M.; Mc, D.; Cassandra, M.; Rooney, L.W. Decorticating sorghum to concentrate healthy phytochemicals. J. Agric. Food Chem. 2005, 53, 6230–6234. [Google Scholar] [CrossRef] [PubMed]
- Lalhminghlui, K.; Jagetia, G.C. Evaluation of the free-radical scavenging and antioxidant activities of Chilauni, Schima wallichii Korth in vitro. Future Sci. OA 2018, 4, FSO272. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Teixeira, T.; Zhang, P.; Warner, R.; Shen, S.; Fang, Z. Cellular antioxidant activities of phenolic extracts from five sorghum grain genotypes. Food Biosci. 2021, 41, 101068. [Google Scholar] [CrossRef]
- Baumann, J.; Wurn, G.; Bruchlausen, F.V. Prostaglandin synthetase inhibiting O2 radical scavenging properties of some flavonoids and related phenolic compounds. Deutsche Pharmakologische Gesellschaft abstracts of the 20th spring meeting, Naunyn-Schmiedebergs abstract no: R27 cited. Arch. Pharmacol. 1979, 307, R1–R77. [Google Scholar]
- Huang, D.; Ou, B.; Prior, R.I. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xu, T.; Zheng, W.; Gao, B.; Zhu, H.; Xu, R.; Deng, H.; Wang, B.; Wu, Y.; Sun, X.; et al. Triacylglycerols compositions, soluble and bound phenolics of red sorghums, and their radical scavenging and anti-inflammatory activities. Food Chem. 2021, 340, 128123. [Google Scholar] [CrossRef] [PubMed]
- Ofosu, F.K.; Elahi, F.; Daliri, E.B.M.; Tyagi, A.; Chen, X.Q.; Chelliah, R.; Joong-Hark, K.; Sang-Ik, H.; Deog-Hwan, O. UHPLC-ESI-QTOF-MS/MS Characterization, Antioxidant and Antidiabetic Properties of Sorghum Grains. Food Chem. 2021, 337, 127788. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Int. 2014, 224, 164–175. [Google Scholar] [CrossRef]
- Marković, J.M.D.; Boris, P.; Dejan, M.; Dragan, A.; Nebojša, B.; Miloš, M. Antiradical activity of delphinidin, pelargonidin and malvin towards hydroxyl and nitric oxide radicals: The energy requirements calculations as a prediction of the possible antiradical mechanisms. Food Chem. 2017, 218, 440–446. [Google Scholar] [CrossRef]
- Hong, S.; Pangloli, P.; Perumal, R.; Cox, S.; Noronha, L.E.; Dia, V.P.; Smolensky, D.A. Comparative Study on Phenolic Content, Antioxidant Activity and Anti-Inflammatory Capacity of Aqueous and Ethanolic Extracts of Sorghum in Lipopolysaccharide-Induced RAW 264.7 Macrophages. Antioxidants 2020, 9, 1297. [Google Scholar] [CrossRef]
- Miafo, A.P.; Benoît, K.; Germain, K.; Muralikrishna, G. Antioxidant properties of free and bound phenolic acids from bran, spent grain, and sorghum seeds. Cereal Chem. 2020, 97, 1236–1243. [Google Scholar] [CrossRef]

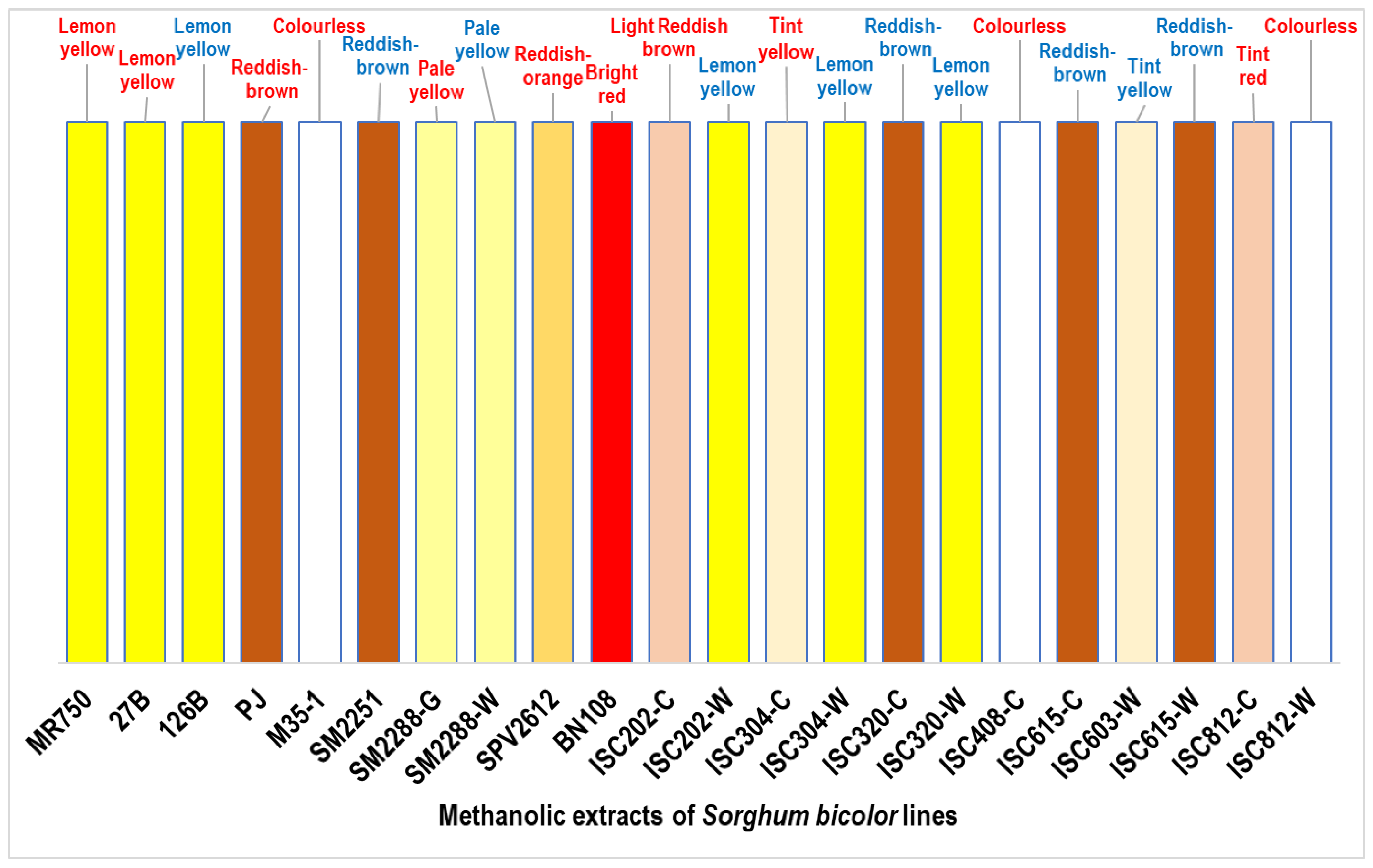
| S. No. | Sample | Description | Pedigree | Pericarp |
|---|---|---|---|---|
| 1 | MR750 | Parental line | - | White |
| 2 | 27B | Parental line | - | White |
| 3 | 126B | Parental line | - | White |
| 4 | Pacha Jonna | Cultivated | - | Yellow |
| 5 | M35-1 | Cultivated | - | White |
| 6 | SM2288-G | Intergeneric | MR750 X CM218 | Grey |
| 7 | SM2288-W | Intergeneric | MR750 X CM218 | White |
| 8 | SM2251 | Intergeneric | 126B X CM108 | Reddish-brown |
| 9 | SPV2612 | Improved breeding line | - | Red |
| 10 | BN108 | Improved breeding line | - | Bright red |
| 11 | ISC202-C | Interspecific | 27B X S. halepens | Red |
| 12 | ISC202-W | Interspecific | 27B X S. halepens | White |
| 13 | ISC304-C | Interspecific | 27B X S. versicolor | Pinkish |
| 14 | ISC304-W | Interspecific | 27B X S. versicolor | White |
| 15 | ISC320-C | Interspecific | 126B X S. versicolor | Reddish-brown |
| 16 | ISC320-W | Interspecific | 126B X S. versicolor | White |
| 17 | ISC408-C | Interspecific | AKMS X S. purpuresericeum | Orangish red |
| 18 | ISC603-W | Interspecific | SSV84 X S. australians | White |
| 19 | ISC615-C | Interspecific | SSV84 X S. australians | Bronze |
| 20 | ISC615-W | Interspecific | SSV84 X S. australians | White |
| 21 | ISC812-C | Interspecific | 27B X S. usumberanse | Whitish-brown |
| 22 | ISC812-W | Interspecific | 27B X S. usumberanse | White |
| Extracts | DPPH (%) | ABTS (%) | NO (%) | TPC GAE (μg/mL) | TFC QE (μg/mL) | FRAP µM (Fe II)/g of Extracts |
|---|---|---|---|---|---|---|
| MR750 | 35.27 ± 2.58 h | 26.53 ± 1.79 ij | 22.7 ± 2.44 g | 206.08 ± 15.46 m | 26.49 ± 4.43 l | 8.59 ± 1.65 jk |
| 27B | 70.19 ± 2.77 bc | 59.28 ± 1.11 c | 43.27 ± 3.8 bcd | 647.75 ± 10.42 ef | 153.26 ± 4.64 a | 61.78 ± 7.12 a |
| 126B | 57.97 ± 1.93 d | 53.11 ± 2.42 d | 42.39 ± 3.36 bcd | 592.75 ± 10.08 g | 30.26 ± 5.46 kl | 14.81 ± 4.04 hi |
| Pacha Jonna | 55.56 ± 3.22 de | 43.34 ± 2.19 f | 35.01 ± 4.03 ef | 523.58 ± 6.36 h | 42.15 ± 6.36 hi | 23.45 ± 3.33 fg |
| M35-1 | 28.99 ± 2.9 i | 37.08 ± 2.95 g | 24.38 ± 4.41 g | 452.19 ± 10.24 j | 33.71 ± 6.81 jk | 17.67 ± 4.79 h |
| SPV2612 | 79.66 ± 1.42 a | 63.66 ± 2.34 b | 47.36 ± 4.35 b | 665.53 ± 18.87 d | 134.1 ± 5.2 b | 56.5 ± 9.22 a |
| BN108 | 54.69 ± 1.51 de | 48.22 ± 1.11 e | 41.59 ± 3.05 cd | 582.75 ± 12.97 g | 38.32 ± 5.62 ij | 26.45 ± 8.01 ef |
| ISC202-C | 79.71 ± 0.97 a | 68.27 ± 2.91 a | 53.73 ± 2.79 a | 759.69 ± 12.5 a | 133.82 ± 6.36 b | 46.53 ± 5.46 b |
| ISC202-W | 73.91 ± 0.97 b | 63.35 ± 1.72 b | 45.93 ± 2.52 bc | 662.19 ± 7.41 de | 123.82 ± 6.36 c | 49.56 ± 5.29 b |
| ISC304-C | 8.21 ± 2.25 j | 5.31 ± 2.02 l | 3.85 ± 1.78 j | 31.92 ± 3.94 op | 75.49 ± 5.55 e | 45.39 ± 8.55 b |
| ISC304-W | 34.3 ± 2.58 h | 30.87 ± 3.07 h | 24.52 ± 2.46 g | 432.19 ± 3.82 k | 12.71 ± 2.1 m | 11.09 ± 1.84 ij |
| ISC320-C | 46.96 ± 1.45 f | 36.34 ± 2.29 g | 25.01 ± 2.96 g | 441.92 ± 4.74 jk | 54.65 ± 5.42 g | 30.11 ± 3.31 de |
| ISC320-W | 30.92 ± 1.61 hi | 26.53 ± 1.79 ij | 14.73 ± 1.4 i | 248.58 ± 10.84 l | 8.01 ± 1.18 mn | 11.78 ± 3.33 ij |
| ISC408-C | 71.01 ± 2.9 bc | 63.97 ± 3.9 b | 43.02 ± 3.82 bcd | 640.8 ± 14.68 f | 41.51 ± 7.19 hi | 27.67 ± 7.38 ef |
| ISC603-W | 26.57 ± 2.58 i | 24.27 ± 2.96 j | 17.52 ± 3.45 hi | 177.19 ± 10 n | 30.76 ± 8.78 kl | 8.34 ± 1.05 jk |
| ISC615-C | 69.18 ± 2.64 bc | 64.99 ± 1.99 ab | 52.71 ± 3.94 a | 687.19 ± 7.95 c | 75.9 ± 4.57 e | 34.95 ± 3.15 cd |
| ISC615-W | 33.82 ± 1.61 h | 27.75 ± 2.37 hij | 17.24 ± 3.43 hi | 186.64 ± 8.39 n | 55.04 ± 7.38 g | 36.5 ± 7.52 c |
| ISC812-C | 40.1 ± 1.61 g | 29.89 ± 2.99 hi | 20.15 ± 2.99 gh | 233.58 ± 16.78 l | 108.54 ± 7.56 d | 47.7 ± 3 b |
| ISC812-W | 68.12 ± 0.97 c | 64.48 ± 3.53 b | 53.03 ± 4.6 a | 710.25 ± 5.91 b | 66.76 ± 6.59 f | 17.89 ± 4.77 gh |
| SM2251 | 52.42 ± 1.58 e | 46.74 ± 1.82 ef | 39.98 ± 5.23 de | 582.47 ± 13.57 g | 73.12 ± 5.63 e | 38.81 ± 4.92 c |
| SM2288-G | 10.63 ± 1.61 j | 9.34 ± 1.79 k | 6.02 ± 3.1 j | 24.97 ± 3.47 op | 1.27 ± 1.27 n | 4 ± 2.1 k |
| SM2288-W | 56.04 ± 2.25 de | 46.85 ± 1.79 ef | 32.74 ± 2.32 f | 496.64 ± 13.8 i | 46.9 ± 4.98 h | 29.73 ± 5.26 de |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meena, K.; Meena, D.K.; Jacob, J.; Aruna, C.; Visarada, K.B.R.S. Unveiling the Antioxidant Arsenal of Colored Sorghum: A Path to Functional Food Development. Agriculture 2024, 14, 566. https://doi.org/10.3390/agriculture14040566
Meena K, Meena DK, Jacob J, Aruna C, Visarada KBRS. Unveiling the Antioxidant Arsenal of Colored Sorghum: A Path to Functional Food Development. Agriculture. 2024; 14(4):566. https://doi.org/10.3390/agriculture14040566
Chicago/Turabian StyleMeena, Kanti, Dharmendra K. Meena, Jinu Jacob, Chandrasekhar Aruna, and Kurella Bala Rama Saraswati Visarada. 2024. "Unveiling the Antioxidant Arsenal of Colored Sorghum: A Path to Functional Food Development" Agriculture 14, no. 4: 566. https://doi.org/10.3390/agriculture14040566
APA StyleMeena, K., Meena, D. K., Jacob, J., Aruna, C., & Visarada, K. B. R. S. (2024). Unveiling the Antioxidant Arsenal of Colored Sorghum: A Path to Functional Food Development. Agriculture, 14(4), 566. https://doi.org/10.3390/agriculture14040566






