Decomposition of Hemp Residues in Soil as Facilitated by Different Nitrogen Sources
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Treatments
2.2. Carbon and Nitrogen Contents
2.3. Statistical and Numerical Analyses
3. Results
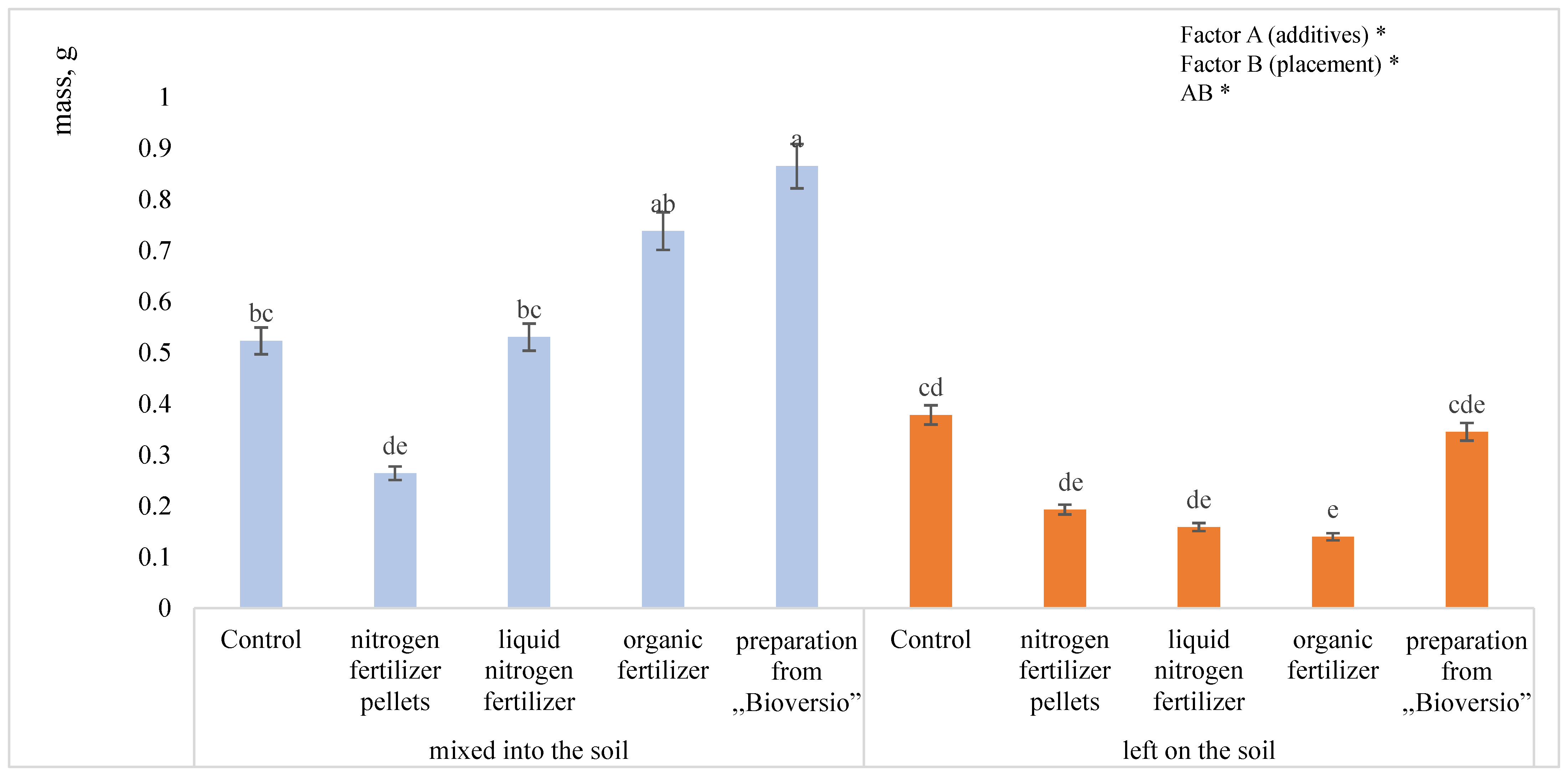
3.1. Mass Weight of Mineralized Hemp Residues
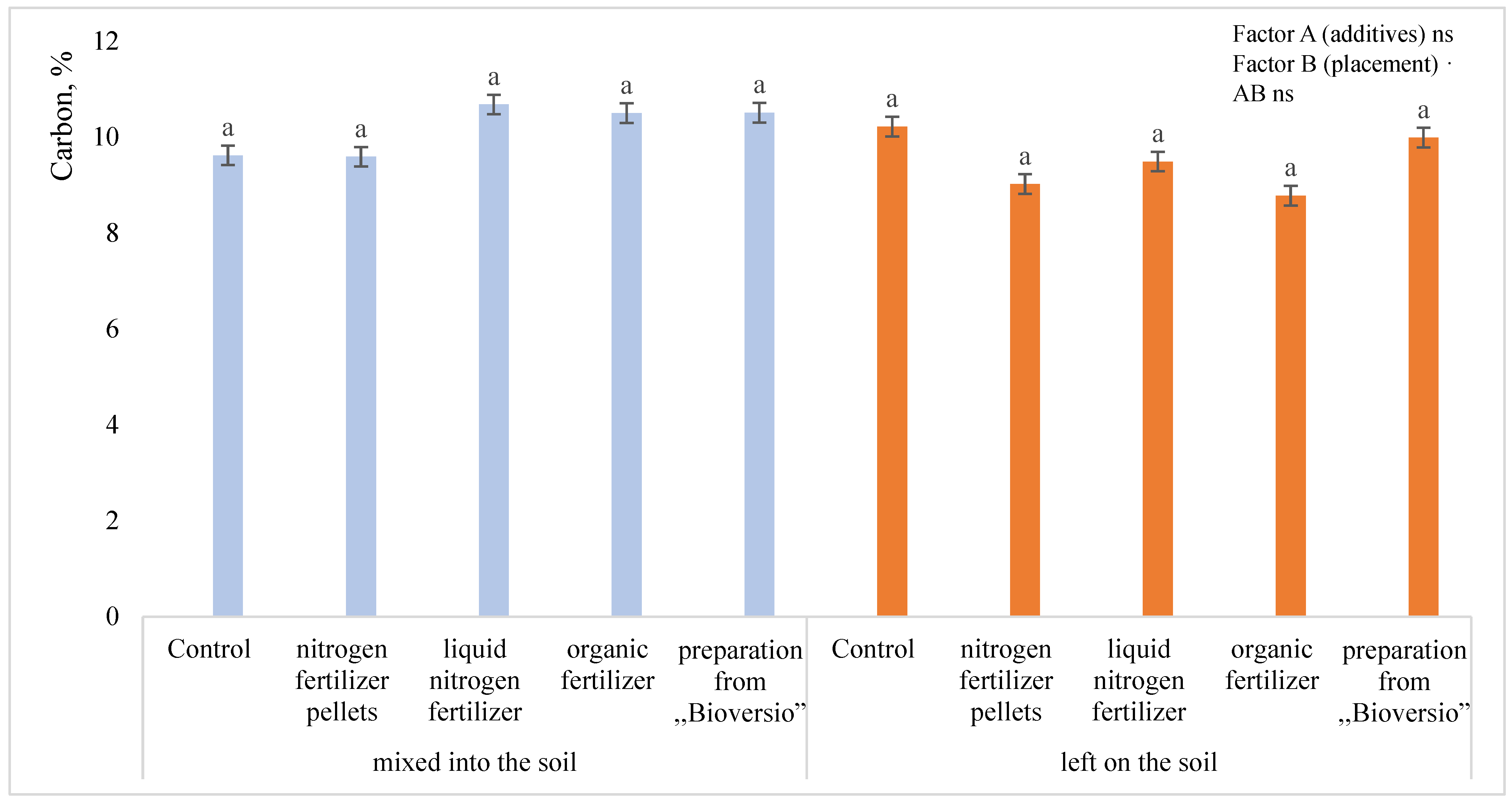
3.2. Carbon Content in Hemp Residues
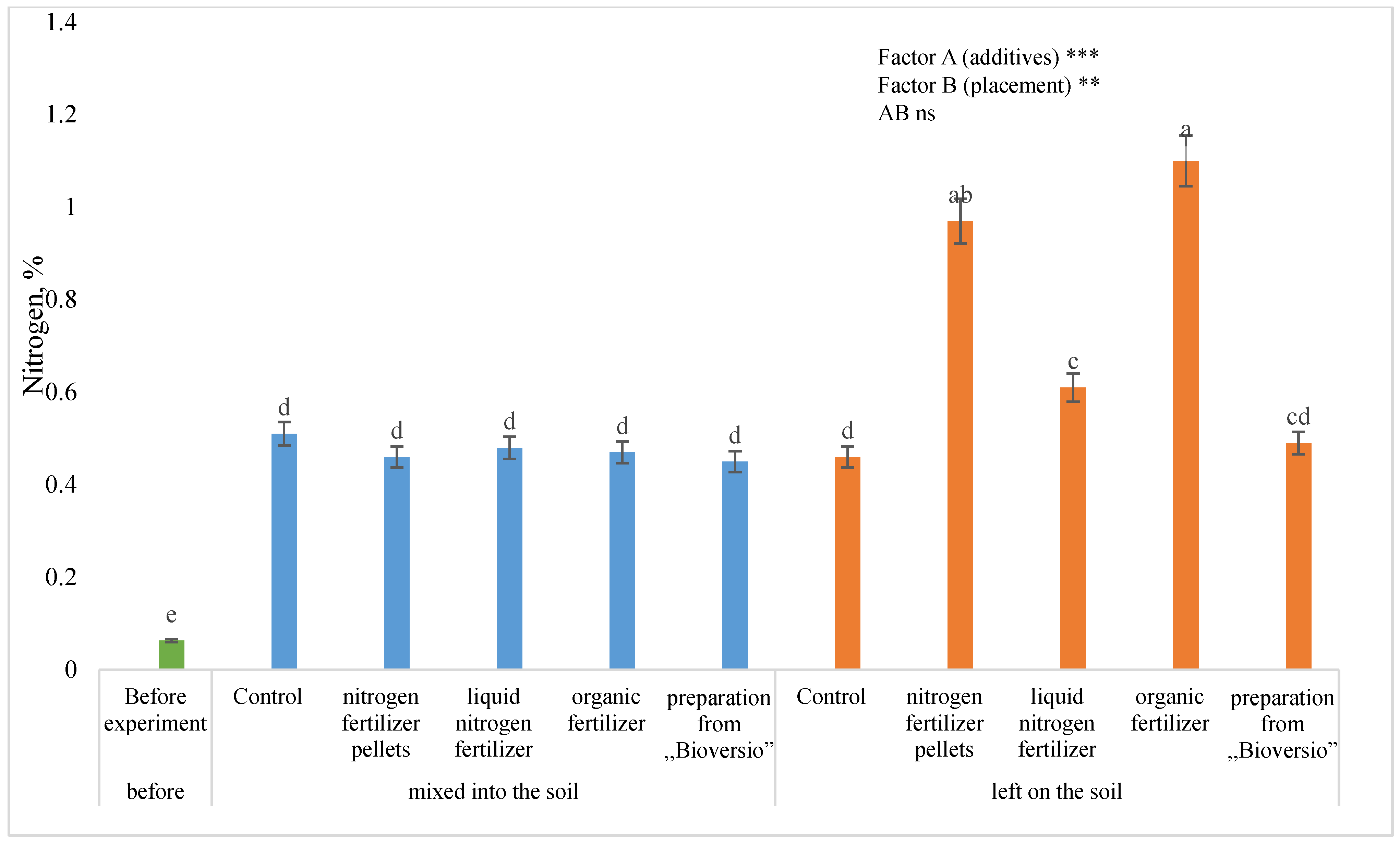
3.3. Nitrogen Content in Hemp Residues
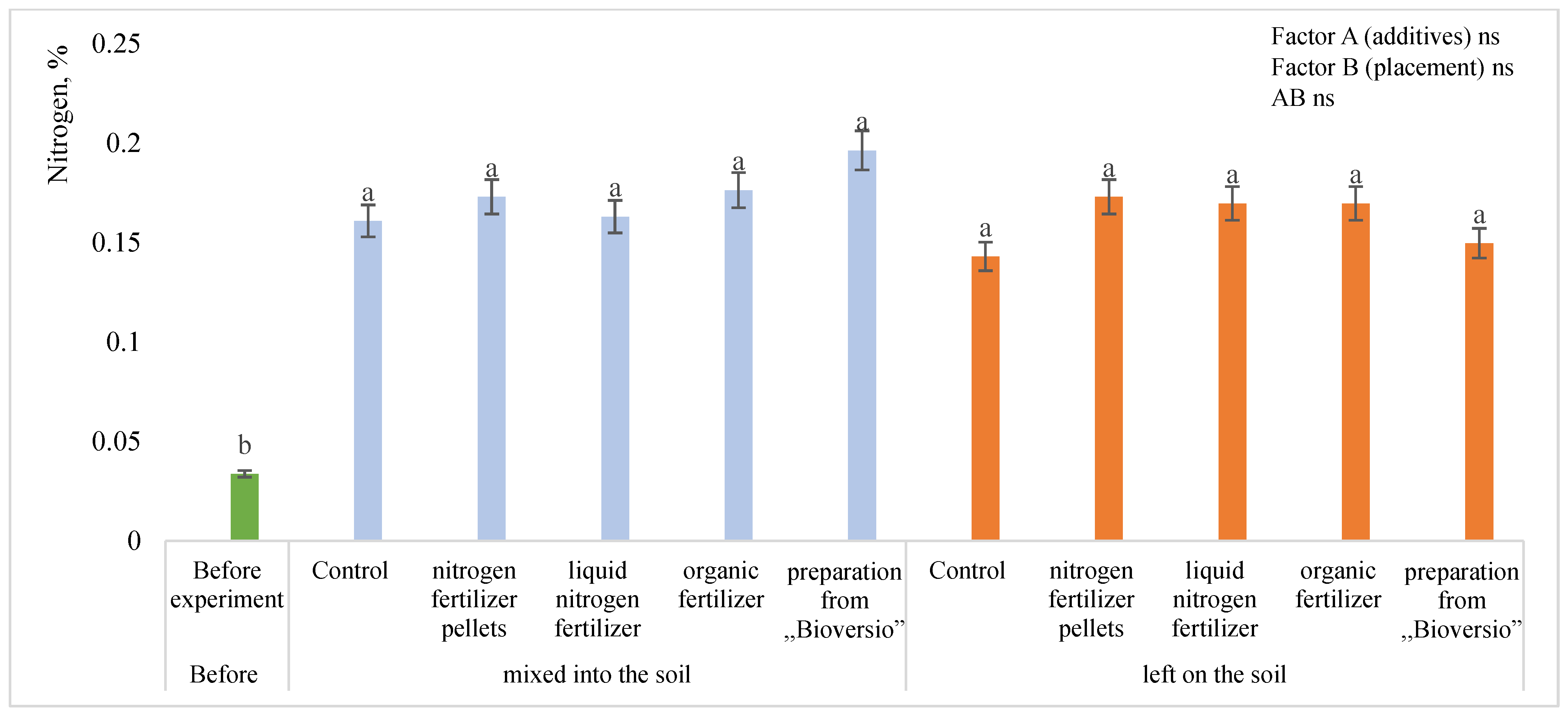
3.4. Nitrogen and Carbon Contents in the Soil
4. Discussion
4.1. Mass Loss in Hemp Residue Litter
4.2. Carbon Content in the Hemp Residues
4.3. Nitrogen Content in the Hemp Residues
4.4. Carbon Content in the Soil
4.5. Nitrogen Content in the Soil
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dessie, W.; Tang, J.; Wang, M.; Luo, X.; Liu, X.; Qin, Z. One-pot conversion of industrial hemp residue into fermentable feedstocks using green catalyst and enzyme cocktails generated by solid-state fermentation. Ind. Crops Prod. 2022, 182, 114885. [Google Scholar] [CrossRef]
- Filazi, A.; Tortuk, S.; Pul, M. Determination of optimum blast furnace slag ash and hemp fiber ratio in cement mortars. Structures 2023, 57, 105024. [Google Scholar] [CrossRef]
- Foley, J.A.; DeFries, R.; Asner, G.P.; Barford, C.; Bonan, G.; Carpenter, S.R.; Chapin, F.S.; Coe, M.T.; Daily, G.C.; Gibbs, H.K.; et al. Global consequences of land use. Science 2005, 309, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Howden, S.M.; Soussana, J.F.; Tubiello, F.N.; Chhetri, N.; Dunlop, M.; Meinke, H. Adapting agriculture to climate change. Proc. Natl. Acad. Sci. USA 2007, 104, 19691–19696. [Google Scholar] [CrossRef] [PubMed]
- Almagro, M.; de Vente, J.; Boix-Fayos, C.; García-Franco, N.; Melgares de Aguilar, J.; González, D.; Solé-Benet, A.; Martínez-Mena, M. Sustainable land management practices as providers of several ecosystem services under rainfed Mediterranean agroecosystems. Mitig. Adapt. Strateg. Glob. Change 2016, 21, 1029–1043. [Google Scholar] [CrossRef]
- Stavi, I.; Bel, G.; Zaady, E. Soil functions and ecosystem services in conventional, conservation, and integrated agricultural systems. A review. Agron. Sustain. Dev. 2016, 36, 32. [Google Scholar] [CrossRef]
- Chenu, C.; Angers, D.A.; Barré, P.; Derrien, D.; Arrouays, D.; Balesdent, J. Increasing organic stocks in agricultural soils: Knowledge gaps and potential innovations. Soil Tillage Res. 2019, 188, 41–52. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Change Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef]
- Almagro, M.; Ruiz-Navarro, A.; Díaz-Pereira, E.; Albaladejo, J.; Martínez-Mena, M. Plant residue chemical quality modulates the soil microbial response related to decomposition and soil organic carbon and nitrogen stabilization in a rainfed Mediterranean agroecosystem. Soil Biol. Biochem. 2021, 156, 108198. [Google Scholar] [CrossRef]
- Fanin, N.; Kardol, P.; Farrell, M.; Nilsson, M.C.; Gundale, M.J.; Wardle, D.A. The ratio of Gram-positive to Gram-negative bacterial PLFA markers as an indicator of carbon availability in organic soils. Soil Biol. Biochem. 2019, 128, 111–114. [Google Scholar] [CrossRef]
- Wu, H.; Wiesmeier, M.; Yu, Q.; Steffens, M.; Han, X.; Kögel-Knabner, I. Labile organic C and N mineralization of soil aggregate size classes in semiarid grasslands as affected by grazing management. Biol. Fertil. Soils 2012, 48, 305–313. [Google Scholar] [CrossRef]
- Bimüller, C.; Kreyling, O.; Kölbl, A.; von Lützow, M.; Kögel-Knabner, I. Carbon and nitrogen mineralization in hierarchically structured aggregates of different size. Soil Tillage Res. 2016, 160, 23–33. [Google Scholar] [CrossRef]
- Kimura, S.D.; Melling, L.; Goh, K.J. Influence of soil aggregate size on greenhouse gas emission and uptake rate from tropical peat soil in forest and different oil palm development years. Geoderma 2012, 185–186, 1–5. [Google Scholar] [CrossRef]
- Sarker, J.R.; Singh, B.P.; Fang, Y.; Cowie, A.L.; Dougherty, W.J.; Collins, D.; Dalal, R.C.; Singh, B.K. Tillage history and crop residue input enhanced native carbon mineralisation and nutrient supply in contrasting soils under long-term farming systems. Soil Tillage Res. 2019, 193, 71–84. [Google Scholar] [CrossRef]
- Saidy, A.R.; Smernik, R.J.; Baldock, J.A.; Kaiser, K.; Sanderman, J. The sorption of organic carbon onto differing clay minerals in the presence and absence of hydrous iron oxide. Geoderma 2013, 209–210, 15–21. [Google Scholar] [CrossRef]
- Frederiksen, L.; Bean, M.; Nance, H. PEST and SWOT analysis of international interlibrary loan. Glob. Resour. Shar. 2012, 35–59. [Google Scholar] [CrossRef]
- Kim, N.; Zabaloy, M.C.; Guan, K.; Villamil, M.B. Do cover crops benefit soil microbiome? A meta-analysis of current research. Soil Biol. Biochem. 2020, 142, 107701. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Ruis, S.J. Cover crop impacts on soil physical properties: A review. Soil Sci. Soc. Am. J. 2020, 84, 1527–1576. [Google Scholar] [CrossRef]
- Poeplau, C.; Don, A. Carbon sequestration in agricultural soils via cultivation of cover crops—A meta-analysis. Agric. Ecosyst. Environ. 2015, 200, 33–41. [Google Scholar] [CrossRef]
- Basche, A.D.; Miguez, F.E.; Kaspar, T.C.; Castellano, M.J. Do cover crops increase or decrease nitrous oxide emissions? A meta-analysis. J. Soil Water Conserv. 2014, 69, 471–482. [Google Scholar] [CrossRef]
- García-Tejero, I.F.; Durán Zuazo, V.H.; Sánchez-Carnenero, C.; Hernández, A.; Ferreiro-Vera, C.; Casano, S. Seeking suitable agronomical practices for industrial hemp (Cannabis sativa L.) cultivation for biomedical applications. Ind. Crops Prod. 2019, 139, 111524. [Google Scholar] [CrossRef]
- Cherney, J.H.; Small, E. Industrial hemp in North America: Production, politics and potential. Agronomy 2016, 6, 58. [Google Scholar] [CrossRef]
- Salentijn, E.M.J.; Zhang, Q.; Amaducci, S.; Yang, M.; Trindade, L.M. New developments in fiber hemp (Cannabis sativa L.) breeding. Ind. Crops Prod. 2015, 68, 32–41. [Google Scholar] [CrossRef]
- Tedeschi, A.; Cerrato, D.; Menenti, M. Is the Potential for Multi-Functional Use of Industrial Hemp Greater than Maize under Saline Conditions? Sustainability 2022, 14, 15646. [Google Scholar] [CrossRef]
- Visković, J.; Zheljazkov, V.D.; Sikora, V.; Noller, J.; Latković, D.; Ocamb, C.M.; Koren, A. Industrial Hemp (Cannabis sativa L.) Agronomy and Utilization: A Review. Agronomy 2023, 13, 931. [Google Scholar] [CrossRef]
- Kaleeem Abbasi, M.; Mahmood Tahir, M.; Sabir, N.; Khurshid, M. Impact of the addition of different plant residues on nitrogen mineralization-immobilization turnover and carbon content of a soil incubated under laboratory conditions. Solid Earth 2015, 6, 197–205. [Google Scholar] [CrossRef]
- Paul, E.A. The nature and dynamics of soil organic matter: Plant inputs, microbial transformations, and organic matter stabilization. Soil Biol. Biochem. 2016, 98, 109–126. [Google Scholar] [CrossRef]
- Zhang, P.; Lin, J.; Hao, J.; Li, C.; Quan, W. Decomposition Characteristics of Lignocellulosic Biomass in Subtropical Rhododendron Litters under Artificial Regulation. Metabolites 2023, 13, 279. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.M.-F.; Barbour, N.W.; Weyers, S.L. Chemical Composition of Crop Biomass Impacts Its Decomposition. Soil Sci. Soc. Am. J. 2007, 71, 155–162. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, H.; Cheng, X.; Liu, G. Nitrogen addition stimulates litter decomposition rate: From the perspective of the combined effect of soil environment and litter quality. Soil Biol. Biochem. 2023, 179, 108992. [Google Scholar] [CrossRef]
- Chen, B.; Liu, E.; Tian, Q.; Yan, C.; Zhang, Y. Soil nitrogen dynamics and crop residues. A review. Agron. Sustain. Dev. 2014, 34, 429–442. [Google Scholar] [CrossRef]
- Abera, G.; Wolde-Meskel, E.; Bakken, L.R. Unexpected high decomposition of legume residues in dry season soils from tropical coffee plantations and crop lands. Agron. Sustain. Dev. 2014, 34, 667–676. [Google Scholar] [CrossRef]
- Grzyb, A.; Wolna-Maruwka, A.; Niewiadomska, A. Environmental factors affecting the mineralization of crop residues. Agronomy 2020, 10, 1951. [Google Scholar] [CrossRef]
- Vahdat, E.; Nourbakhsh, F.; Basiri, M. Lignin content of range plant residues controls N mineralization in soil. Eur. J. Soil Biol. 2011, 47, 243–246. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, K.; Zhang, H.; Zhou, K.; Chang, Y.; Zhan, Y.; Pan, C.; Shi, X.; Zuo, H.; Li, J.; et al. Humic acid and phosphorus fractions transformation regulated by carbon-based materials in composting steered its potential for phosphorus mobilization in soil. J. Environ. Manag. 2023, 325, 116553. [Google Scholar] [CrossRef]
- Kühling, I.; Mikuszies, P.; Helfrich, M.; Flessa, H.; Schlathölter, M.; Sieling, K.; Kage, H. Effects of winter cover crops from different functional groups on soil-plant nitrogen dynamics and silage maize yield. Eur. J. Agron. 2023, 148, 126878. [Google Scholar] [CrossRef]
- Ferdush, J.; Paul, V. A review on the possible factors influencing soil inorganic carbon under elevated CO2. Catena 2021, 204, 105434. [Google Scholar] [CrossRef]
- Yao, Y.; Dai, Q.; Gao, R.; Yi, X.; Wang, Y.; Hu, Z. Characteristics and factors influencing soil organic carbon composition by vegetation type in spoil heaps. Front. Plant Sci. 2023, 14, 1240217. [Google Scholar] [CrossRef] [PubMed]
- Chaves, B.; Redin, M.; Giacomini, S.J.; Schmatz, R.; Léonard, J.; Ferchaud, F.; Recous, S. The combination of residue quality, residue placement and soil mineral N content drives C and N dynamics by modifying N availability to microbial decomposers. Soil Biol. Biochem. 2021, 163, 108434. [Google Scholar] [CrossRef]
- Xu, X.; An, T.; Zhang, J.; Sun, Z.; Schaeffer, S.; Wang, J. Geoderma Transformation and stabilization of straw residue carbon in soil affected by soil types, maize straw addition and fertilized levels of soil. Geoderma 2019, 337, 622–629. [Google Scholar] [CrossRef]
- Kroschewski, B.; Richter, C.; Baumecker, M.; Kautz, T. Effect of crop rotation and straw application in combination with mineral nitrogen fertilization on soil carbon sequestration in the Thyrow long-term experiment Thy_D5. Plant Soil 2023, 488, 121–136. [Google Scholar] [CrossRef]
- Fontaine, D.; Eriksen, J.; Sørensen, P. Cover crop and cereal straw management influence the residual nitrogen effect. Eur. J. Agron. 2020, 118, 126100. [Google Scholar] [CrossRef]

| Fertilizer | N Content, % | Application Dose, g |
|---|---|---|
| Nitrogen pellets | 34 | 1.22 |
| Liquid nitrogen | 36 | 1.15 |
| Organic fertilizer | 84 | 49.46 |
| Bioversio | - | 0.2 |
| N Content, % | C Content, % | |
|---|---|---|
| Soil | 0.03 | 1.89 |
| Hemp | 0.06 | 33.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stulpinaite, U.; Tilvikiene, V.; Doyeni, M.O. Decomposition of Hemp Residues in Soil as Facilitated by Different Nitrogen Sources. Agriculture 2024, 14, 508. https://doi.org/10.3390/agriculture14030508
Stulpinaite U, Tilvikiene V, Doyeni MO. Decomposition of Hemp Residues in Soil as Facilitated by Different Nitrogen Sources. Agriculture. 2024; 14(3):508. https://doi.org/10.3390/agriculture14030508
Chicago/Turabian StyleStulpinaite, Urte, Vita Tilvikiene, and Modupe Olufemi Doyeni. 2024. "Decomposition of Hemp Residues in Soil as Facilitated by Different Nitrogen Sources" Agriculture 14, no. 3: 508. https://doi.org/10.3390/agriculture14030508
APA StyleStulpinaite, U., Tilvikiene, V., & Doyeni, M. O. (2024). Decomposition of Hemp Residues in Soil as Facilitated by Different Nitrogen Sources. Agriculture, 14(3), 508. https://doi.org/10.3390/agriculture14030508







