Genetic Improvement of Drought Tolerance in a Mega-Rice Variety Improved White Ponni through Marker-Assisted Backcross Breeding
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Development of IWP BILs Harboring Mega-Effect Drought-Tolerant QTLs from Apo through MABB
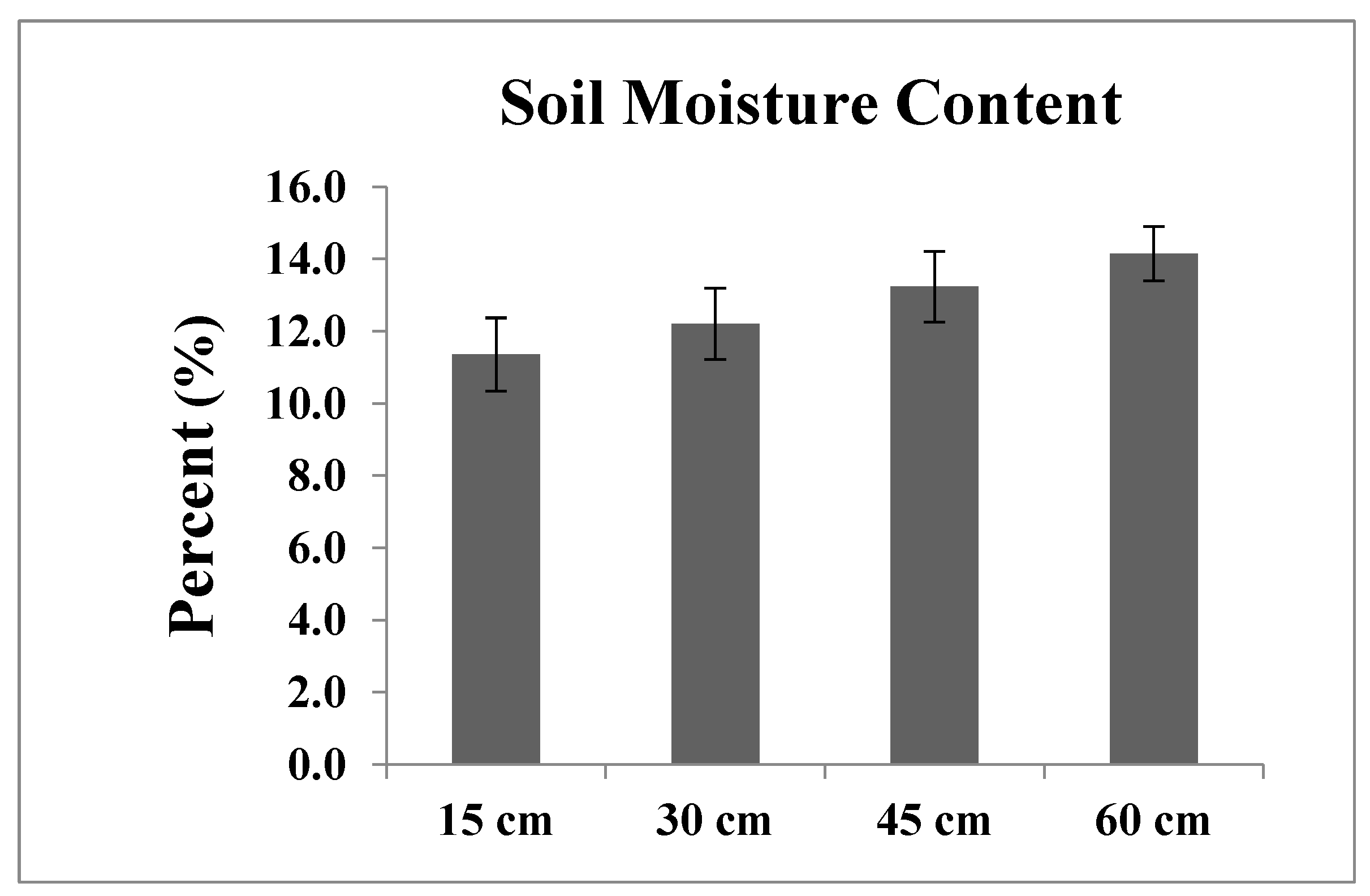
2.3. Phenotypic Evaluation of IWP BILs under Water Deficit Conditions
3. Results
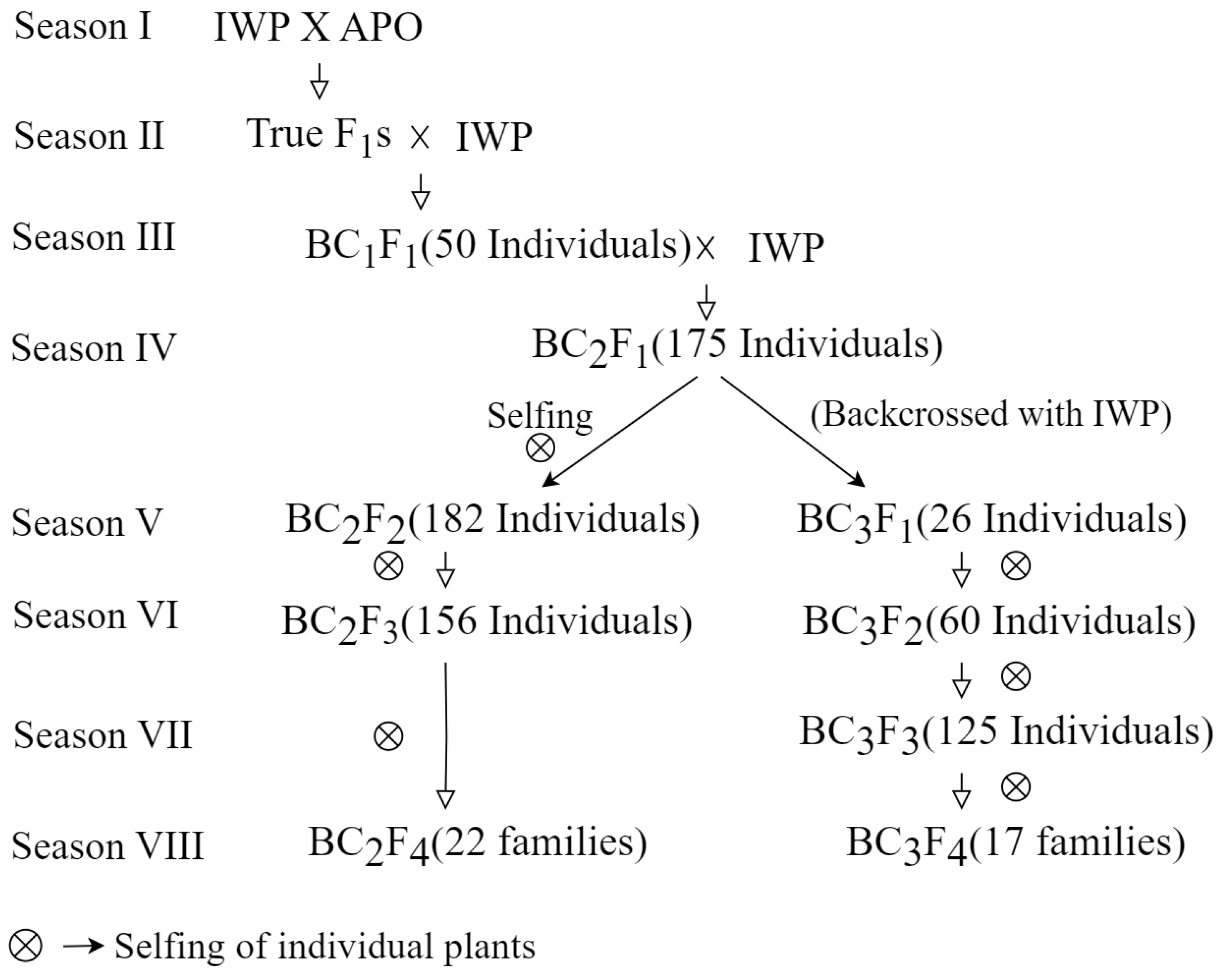
3.1. Development of Backcross Inbred Lines (BILs) of IWP Pyramided with Drought-Tolerant QTLs
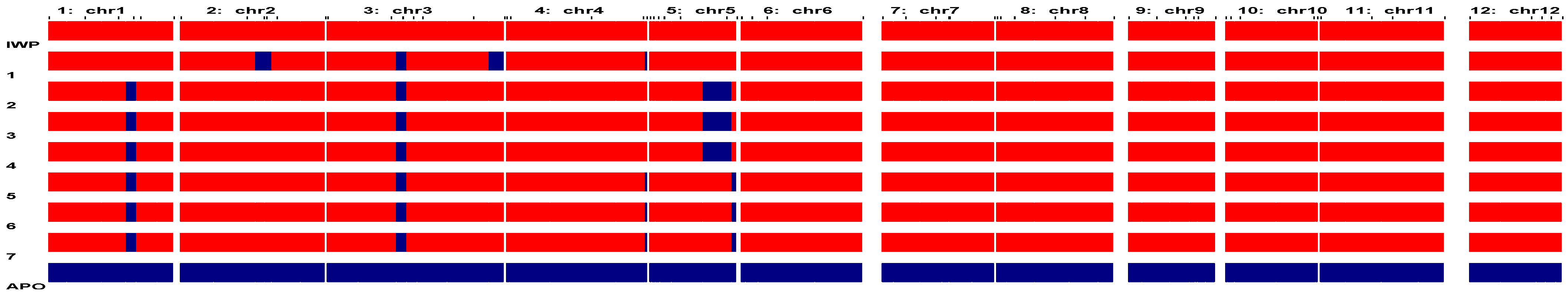
3.2. Identification of Elite Inbred Lines of IWP BILs Harboring Drought-Tolerant QTLs
3.3. Measuring Responses of BILs for Drought Tolerance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dar, M.H.; Waza, S.A.; Shukla, S.; Zaidi, N.W.; Nayak, S.; Hossain, M.; Kumar, A.; Ismail, A.M.; Singh, U.S. Drought Tolerant Rice for Ensuring Food Security in Eastern India. Sustainability 2020, 12, 2214. [Google Scholar] [CrossRef]
- Oladosu, Y.; Rafii, M.Y.; Samuel, C.; Fatai, A.; Magaji, U.; Kareem, I.; Kamarudin, Z.S.; Muhammad, I.; Kolapo, K. Drought Resistance in Rice from Conventional to Molecular Breeding: A Review. Int. J. Mol. Sci. 2019, 20, 3519. [Google Scholar] [CrossRef] [PubMed]
- Barik, S.R.; Pandit, E.; Pradhan, S.K.; Mohanty, S.P.; Mohapatra, T. Genetic Mapping of Morpho-Physiological Traits Involved during Reproductive Stage Drought Tolerance in Rice. PLoS ONE 2019, 14, e0214979. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.R.; Palakolanu, S.R.; Chopra, P.; Rajurkar, A.B.; Gupta, R.; Iqbal, N.; Maheshwari, C. Improving Drought Tolerance in Rice: Ensuring Food Security through Multi-dimensional Approaches. Physiol. Plant. 2021, 172, 645–668. [Google Scholar] [CrossRef] [PubMed]
- Caine, R.S.; Yin, X.; Sloan, J.; Harrison, E.L.; Mohammed, U.; Fulton, T.; Biswal, A.K.; Dionora, J.; Chater, C.C.; Coe, R.A.; et al. Rice with Reduced Stomatal Density Conserves Water and Has Improved Drought Tolerance under Future Climate Conditions. New Phytol. 2019, 221, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Bertolino, L.T.; Caine, R.S.; Gray, J.E. Impact of Stomatal Density and Morphology on Water-Use Efficiency in a Changing World. Front. Plant Sci. 2019, 10, 225. [Google Scholar] [CrossRef]
- Liao, S.; Qin, X.; Luo, L.; Han, Y.; Wang, X.; Usman, B.; Nawaz, G.; Zhao, N.; Liu, Y.; Li, R. CRISPR/Cas9-Induced Mutagenesis of Semi-Rolled Leaf1, 2 Confers Curled Leaf Phenotype and Drought Tolerance by Influencing Protein Expression Patterns and ROS Scavenging in Rice (Oryza sativa L.). Agronomy 2019, 9, 728. [Google Scholar] [CrossRef]
- Salsinha, Y.C.F.; Maryani; Indradewa, D.; Purwestri, Y.A.; Rachmawati, D. Leaf Physiological and Anatomical Characters Contribute to Drought Tolerance of Nusa Tenggara Timur Local Rice Cultivars. J. Crop Sci. Biotechnol. 2021, 24, 337–348. [Google Scholar] [CrossRef]
- Tarun, J.A.; Mauleon, R.; Arbelaez, J.D.; Catausan, S.; Dixit, S.; Kumar, A.; Brown, P.; Kohli, A.; Kretzschmar, T. Comparative Transcriptomics and Co-Expression Networks Reveal Tissue-and Genotype-Specific Responses of qDTYs to Reproductive-Stage Drought Stress in Rice (Oryza sativa L.). Genes 2020, 11, 1124. [Google Scholar] [CrossRef]
- Xu, P.; Ali, A.; Han, B.; Wu, X. Current Advances in Molecular Basis and Mechanisms Regulating Leaf Morphology in Rice. Front. Plant Sci. 2018, 9, 1528. [Google Scholar] [CrossRef] [PubMed]
- Henry, A.; Swamy, B.M.; Dixit, S.; Torres, R.D.; Batoto, T.C.; Manalili, M.; Anantha, M.S.; Mandal, N.P.; Kumar, A. Physiological Mechanisms Contributing to the QTL-Combination Effects on Improved Performance of IR64 Rice NILs under Drought. J. Exp. Bot. 2015, 66, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Vikram, P.; Swamy, B.M.; Dixit, S.; Ahmed, H.U.; Teresa Sta Cruz, M.; Singh, A.K.; Kumar, A. qDTY1.1, a Major QTL for Rice Grain Yield under Reproductive-Stage Drought Stress with a Consistent Effect in Multiple Elite Genetic Backgrounds. BMC Genet 2011, 12, 89. [Google Scholar] [CrossRef] [PubMed]
- Venuprasad, R.; Dalid, C.O.; Del Valle, M.; Zhao, D.; Espiritu, M.; Sta Cruz, M.T.; Amante, M.; Kumar, A.; Atlin, G.N. Identification and Characterization of Large-Effect Quantitative Trait Loci for Grain Yield under Lowland Drought Stress in Rice Using Bulk-Segregant Analysis. Theor. Appl. Genet 2009, 120, 177–190. [Google Scholar] [CrossRef]
- Varshney, R.K.; Tuberosa, R. Translational Genomics in Crop Breeding for Biotic Stress Resistance: An Introduction. In Translational Genomics for Crop Breeding; Varshney, R.K., Tuberosa, R., Eds.; Wiley: Hoboken, NJ, USA, 2013; pp. 1–9. ISBN 978-0-470-96290-9. [Google Scholar]
- Bhukya, J.N.; Bollineni, S.N.; Kadambari, G.; Bommisetty, R.; Gudikati, E.R.; Darsha, W.M.; Issa, K.; Akkareddy, S.; Eslavath, S.N.; Dokuparthi, A.K.; et al. Marker-assisted Introgression of QTLs for Yield under Moisture Stress into Elite Varieties of Rice (Oryza sativa). Plant Breed. 2020, 139, 1076–1089. [Google Scholar] [CrossRef]
- Dhawan, G.; Kumar, A.; Dwivedi, P.; Gopala Krishnan, S.; Pal, M.; Vinod, K.K.; Nagarajan, M.; Bhowmick, P.K.; Bollinedi, H.; Ellur, R.K. Introgression of qDTY1.1 Governing Reproductive Stage Drought Tolerance into an Elite Basmati Rice Variety “Pusa Basmati 1” through Marker Assisted Backcross Breeding. Agronomy 2021, 11, 202. [Google Scholar] [CrossRef]
- Dwivedi, S.K.; Arora, A.; Singh, V.P.; Singh, G.P. Induction of Water Deficit Tolerance in Wheat Due to Exogenous Application of Plant Growth Regulators: Membrane Stability, Water Relations and Photosynthesis. Photosynthetica 2018, 56, 478–486. [Google Scholar] [CrossRef]
- Muthu, V.; Abbai, R.; Nallathambi, J.; Rahman, H.; Ramasamy, S.; Kambale, R.; Thulasinathan, T.; Ayyenar, B.; Muthurajan, R. Pyramiding QTLs Controlling Tolerance against Drought, Salinity, and Submergence in Rice through Marker Assisted Breeding. PLoS ONE 2020, 15, e0227421. [Google Scholar] [CrossRef]
- Oo, K.S.; Krishnan, S.G.; Vinod, K.K.; Dhawan, G.; Dwivedi, P.; Kumar, P.; Bhowmick, P.K.; Pal, M.; Chinnuswamy, V.; Nagarajan, M. Molecular Breeding for Improving Productivity of Oryza sativa L. Cv. Pusa 44 under Reproductive Stage Drought Stress through Introgression of a Major QTL, qDTY12.1. Genes 2021, 12, 967. [Google Scholar] [CrossRef]
- Priyadarshini, S.K.; Raveendran, M.; Manonmani, S.; Robin, S. Effect of QTLs Controlling Grain Yield under Drought Stress in the Genetic Background of ADT45 Rice Variety. Indian J. Genet. Plant Breed. 2014, 74, 374–377. [Google Scholar] [CrossRef]
- Dixit, S.; Singh, A.; Kumar, A. Rice Breeding for High Grain Yield under Drought: A Strategic Solution to a Complex Problem. Int. J. Agron. 2014, 2014, 863683. [Google Scholar] [CrossRef]
- Shaik, N.M.; Arun Kumar, S.; Praveen, R.; Waris, A.; Voleti, S.R. Promising Technologies to Bridge the Rice Yield Gaps across the Country: Experiences from Frontline Demonstrations Program. J. Rice Res. 2018, 11, 73–80. [Google Scholar]
- ICAR-Indian Institute of Rice Research. Production Oriented Survey 2022. In All India Coordinated Research Project on Rice; ICAR-Indian Institute of Rice Research: Hyderabad, India, 2023. [Google Scholar]
- Venuprasad, R.; Bool, M.E.; Quiatchon, L.; Sta Cruz, M.T.; Amante, M.; Atlin, G.N. A Large-Effect QTL for Rice Grain Yield under Upland Drought Stress on Chromosome 1. Mol. Breed. 2012, 30, 535–547. [Google Scholar] [CrossRef]
- Ausubel, F.M.; Brent, R.; Kingston, R.E.; Moore, D.D.; Seidman, J.G.; Smith, J.A.; Struhl, K. Current Protocols in Molecular Biology; John Wiley & Sons. Inc.: Brooklyn, NY, USA, 2003; Volume 1, pp. 1994–2005. [Google Scholar]
- Cagampang, G.B.; Perez, C.M.; Juliano, B.O. A Gel Consistency Test for Eating Quality of Rice. J. Sci. Food Agric. 1973, 24, 1589–1594. [Google Scholar] [CrossRef]
- Little, R.R.; Hilder, G.B.; Dawson, E.H. Differential Effect of Dilute Alkali on 25 Varieties of Milled White Rice. Cereal Chem. 1958, 35, 111–126. [Google Scholar]
- Kumar, A.; Dixit, S.; Ram, T.; Yadaw, R.B.; Mishra, K.K.; Mandal, N.P. Breeding High-Yielding Drought-Tolerant Rice: Genetic Variations and Conventional and Molecular Approaches. J. Exp. Bot. 2014, 65, 6265–6278. [Google Scholar] [CrossRef] [PubMed]
- Brumlop, S.; Finckh, M.R. Applications and Potentials of Marker Assisted Selection (MAS) in Plant Breeding: Final Report of the F+ E Project" Applications and Potentials of Smart Breeding"(FKZ 350 889 0020)-on Behalf of the Federal Agency for Nature Conservation; Federal Agency for Nature Conservation (BfN): Bonn, Germany, 2011; ISBN 978-3-89624-033-0. [Google Scholar]
- Hasan, M.M.; Rafii, M.Y.; Ismail, M.R.; Mahmood, M.; Rahim, H.A.; Alam, M.A.; Ashkani, S.; Malek, M.A.; Latif, M.A. Marker-Assisted Backcrossing: A Useful Method for Rice Improvement. Biotechnol. Biotechnol. Equip. 2015, 29, 237–254. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.A.; Rafii, M.Y.; Yusoff, M.M.; Ali, N.S.; Yusuff, O.; Datta, D.R.; Anisuzzaman, M.; Ikbal, M.F. Recent Advances in Rice Varietal Development for Durable Resistance to Biotic and Abiotic Stresses through Marker-Assisted Gene Pyramiding. Sustainability 2021, 13, 10806. [Google Scholar] [CrossRef]
- Dixit, S.; Singh, A.; Sandhu, N.; Bhandari, A.; Vikram, P.; Kumar, A. Combining Drought and Submergence Tolerance in Rice: Marker-Assisted Breeding and QTL Combination Effects. Mol. Breed. 2017, 37, 143. [Google Scholar] [CrossRef] [PubMed]
- Vikram, P.; Swamy, B.M.; Dixit, S.; Singh, R.; Singh, B.P.; Miro, B.; Kohli, A.; Henry, A.; Singh, N.K.; Kumar, A. Drought Susceptibility of Modern Rice Varieties: An Effect of Linkage of Drought Tolerance with Undesirable Traits. Sci. Rep. 2015, 5, 14799. [Google Scholar] [CrossRef] [PubMed]
- Swain, P.; Raman, A.; Singh, S.P.; Kumar, A. Breeding Drought Tolerant Rice for Shallow Rainfed Ecosystem of Eastern India. Field Crops Res. 2017, 209, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Venuprasad, R.; Lafitte, H.R.; Atlin, G.N. Response to Direct Selection for Grain Yield under Drought Stress in Rice. Crops Sci. 2007, 47, 285–293. [Google Scholar] [CrossRef]
- Selamat, N.; Nadarajah, K.K. Meta-Analysis of Quantitative Traits Loci (QTL) Identified in Drought Response in Rice (Oryza sativa L.). Plants 2021, 10, 716. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Lin, H.; Sasaki, T.; Yano, M. Identification of Heading Date Quantitative Trait Locus Hd6 and Characterization of Its Epistatic Interactions with Hd2 in Rice Using Advanced Backcross Progeny. Genetics 2000, 154, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Shamini, K.; Sudhagar, R.; Raveendran, M.; Joel, A.J. qDTY3.1, a Major Drought Tolerant Locus of APO Promotes Early Flowering in the Genetic Back Ground of a Local Cultivar Improved White Ponni. Electron. J. Plant Breed. 2019, 10, 155–159. [Google Scholar] [CrossRef]
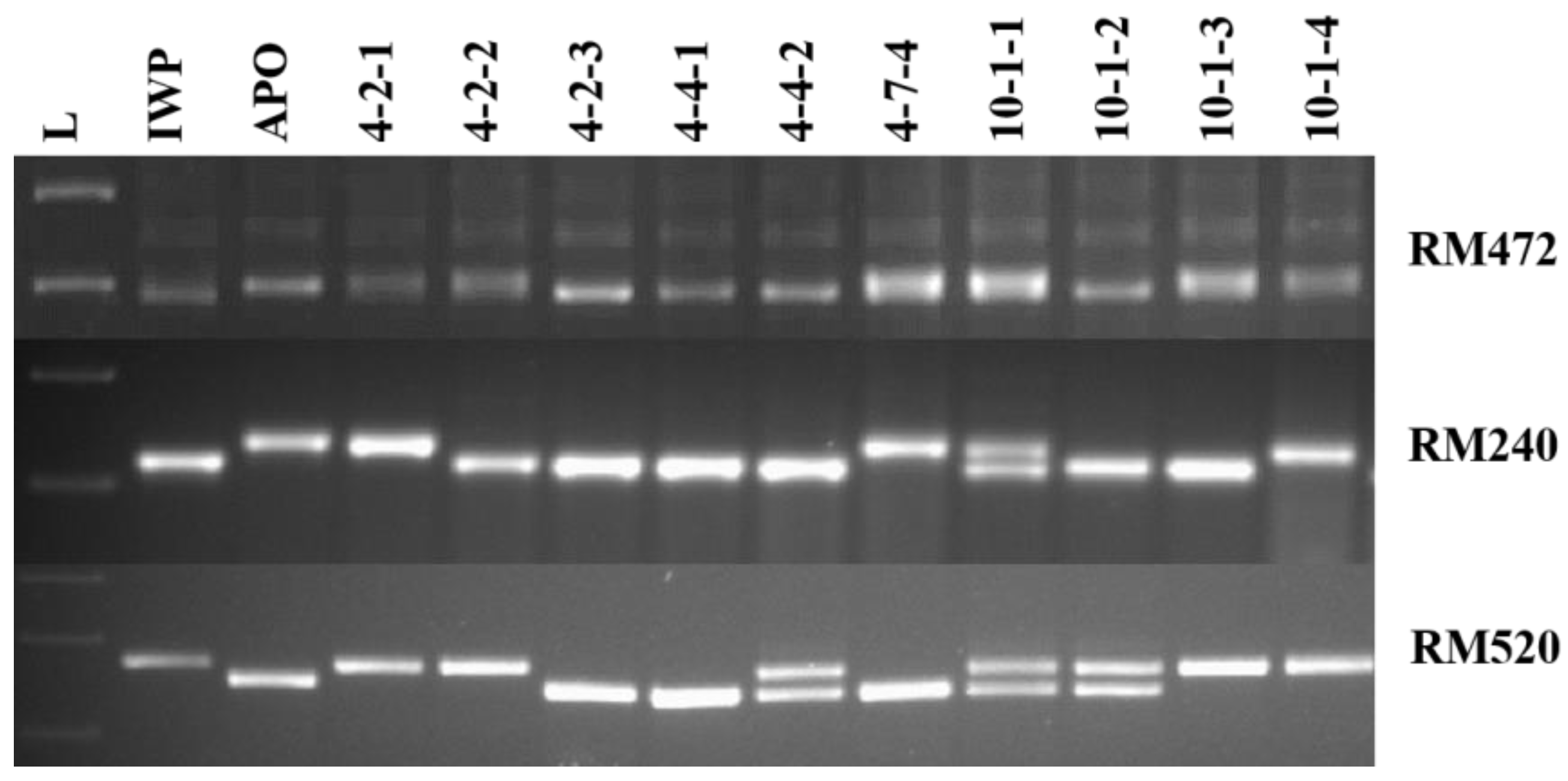
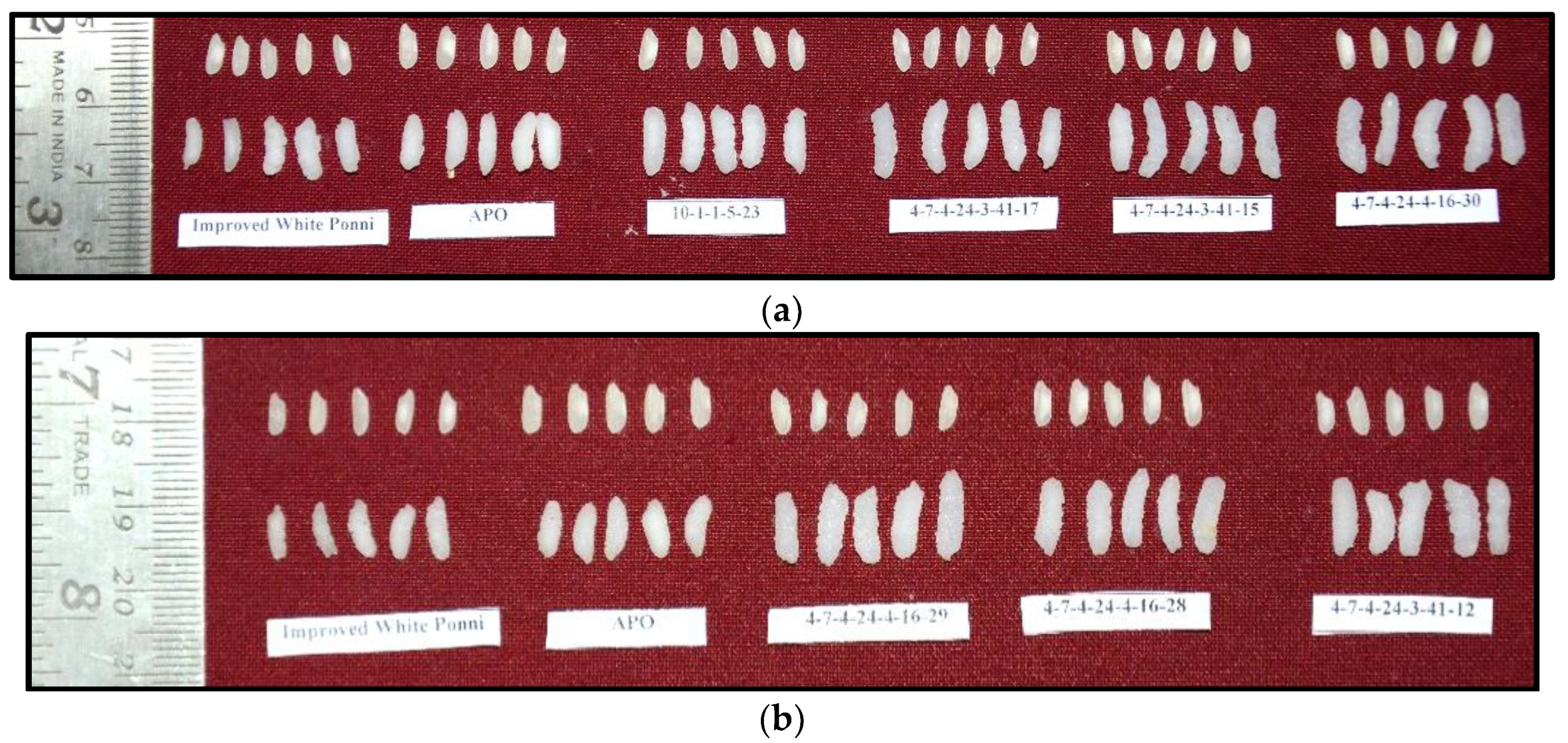

| QTL | Donor | Chromosome | Marker Interval | Linked Marker | Position (Mb) | R2 (%) |
|---|---|---|---|---|---|---|
| qDTY1.1 | APO | 1 | RM486-RM472 | RM472 | 37.89 | 58 |
| qDTY2.1 | APO | 2 | RM327-RM262 | RM240 | 31.5 | 13–16 |
| qDTY3.1 | APO | 3 | RM520-RM16030 | RM520 | 30.91 | 31 |
| Markers | Forward Primer | Reverse Primer |
|---|---|---|
| RM472 | CATTGACGTGGCACTTTGTTCC | AGAGAGCACGCAATGGAGTATGC |
| RM240 | CCTTAATGGGTAGTGTGCAC | TGTAACCATTCCTTCCATCC |
| RM520 | ACGATAACGCCGACATCACTGG | GCTAAGCATCCACGGTTTCTCTCC |
| Generation | Total Number of Plants Evaluated | Number of Positive Plants for Two or More QTLs | Number of Markers Used for Background Selection | Recurrent Parent Genome Recovery (%) |
|---|---|---|---|---|
| F1 | ||||
| BC1F1 | 50 | 14 | 55 | 64–68 |
| BC2F1 | 175 | 34 | 72 | 78.3–87.05 |
| BC2F2 | 182 | 43 | - | - |
| BC2F3 | 156 | 52 | - | - |
| BC2F4 | 22 | 22 | 69 | 80.9–92 |
| BC3F1 | 26 | 3 | 72 | 89.7–90.8 |
| BC3F2 | 60 | 21 | - | - |
| BC3F3 | 125 | 28 | 72 | 94.2–95.6 |
| BC3F4 | 17 | 17 | - | - |
| Line | 100 Seed Weight (g) | L/B Ratio | Grain Type | Gelatinization Temperature | Gel Consistency | |||
|---|---|---|---|---|---|---|---|---|
| Mean | Range | Mean | Range | mm | Category | |||
| 10-1-1-5-23 | 1.80 | 17.0–19.0 | 2.73 | 2.62–2.90 | Medium slender | Intermediate | 80 | Soft |
| 4-7-4-24-3-41-17 | 1.89 | 18.3–20.0 | 2.64 | 2.52–2.80 | Medium slender | Intermediate | 72 | Soft |
| 4-7-4-24-3-41-15 | 1.90 | 18.9–20.0 | 2.66 | 2.55–2.80 | Medium slender | Intermediate | 70 | Soft |
| 4-7-4-24-4-16-30 | 1.92 | 18.8–20.2 | 2.57 | 2.52–2.62 | Medium slender | Intermediate | 73 | Soft |
| 4-7-4-24-4-16-28 | 1.94 | 19.0–21.2 | 2.57 | 2.59–2.67 | Medium slender | Intermediate | 69 | Soft |
| 4-7-4-24-4-16-29 | 1.91 | 18.4–20.7 | 2.65 | 2.60–2.71 | Medium slender | Intermediate | 76 | Soft |
| 4-7-4-24-3-41-12 | 1.93 | 19.0–20.4 | 2.53 | 2.50–2.57 | Medium slender | Intermediate | 68 | Soft |
| IWP | 1.66 | 16.3–17.0 | 2.79 | 2.68–2.89 | Medium slender | Intermediate | 82 | Soft |
| Mean | 1.90 | 17.0–21.2 | 2.62 | 2.52–2.90 | 73 | |||
| Season/Year | Stress | Duration | No. of IWP BILs | DTF | NT | GY (g) | |||
|---|---|---|---|---|---|---|---|---|---|
| Mean IWP | Mean IWP BILs | Mean IWP | Mean IWP BILs | Mean IWP | Mean IWP BILs | ||||
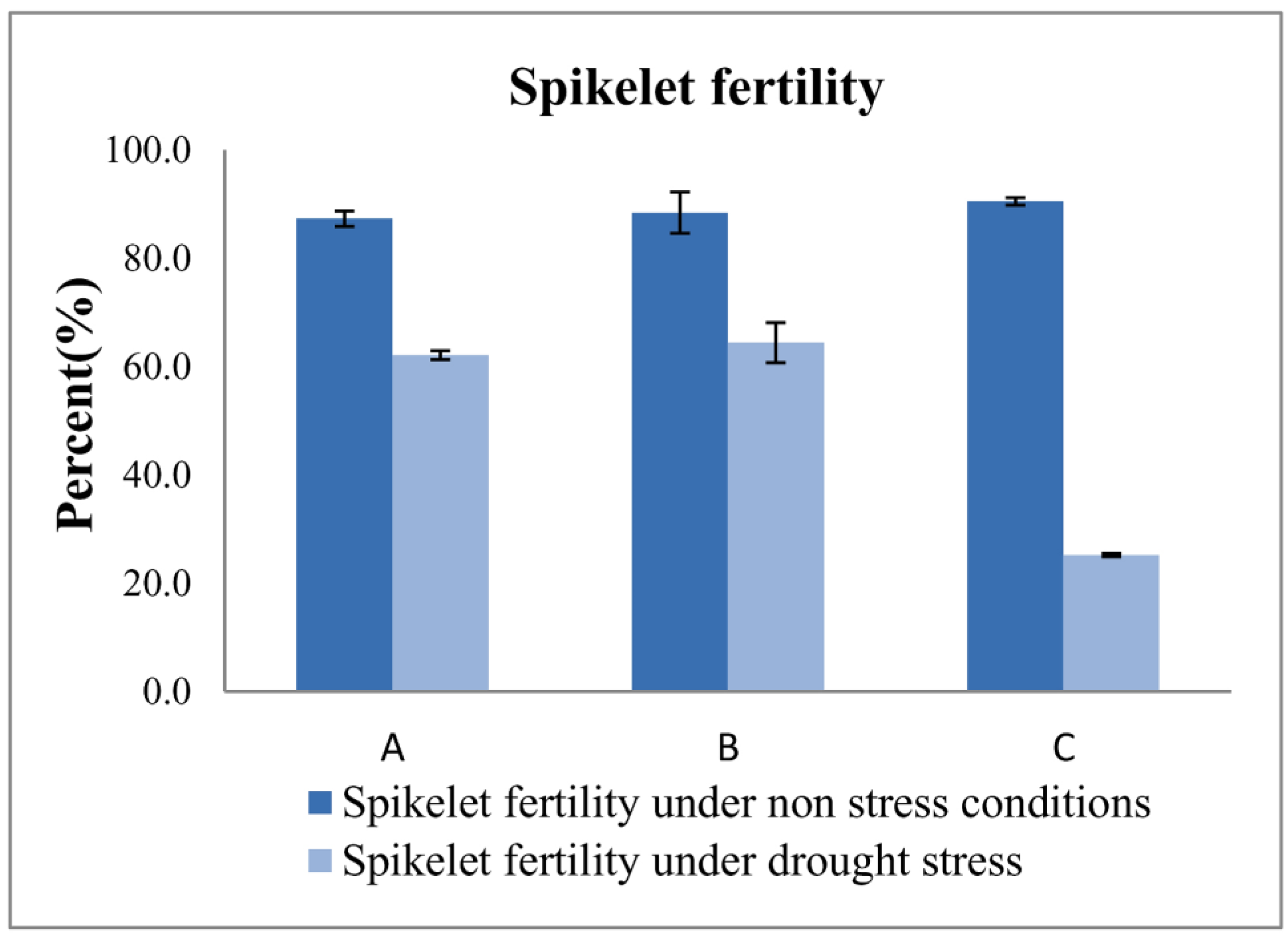
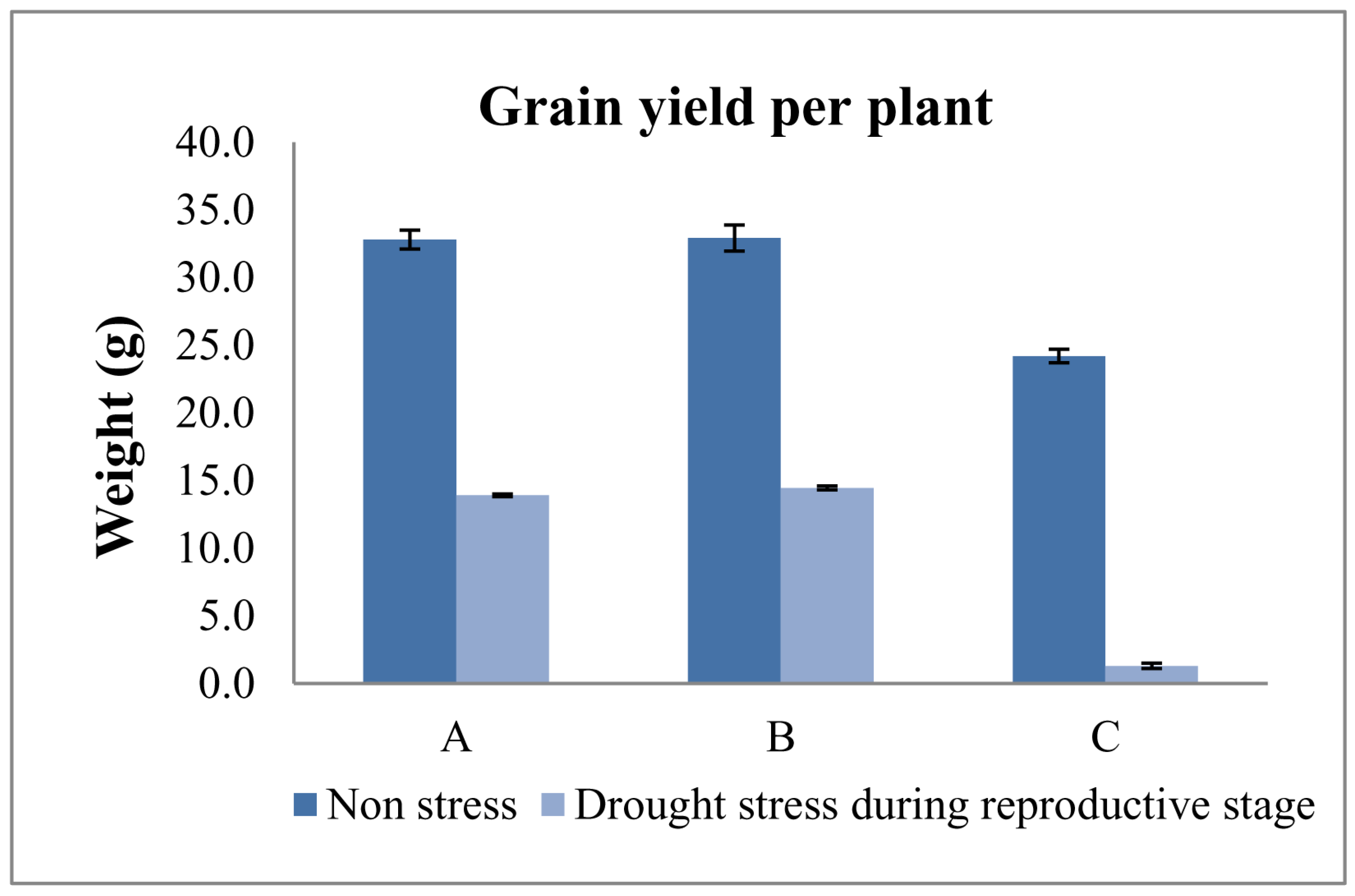
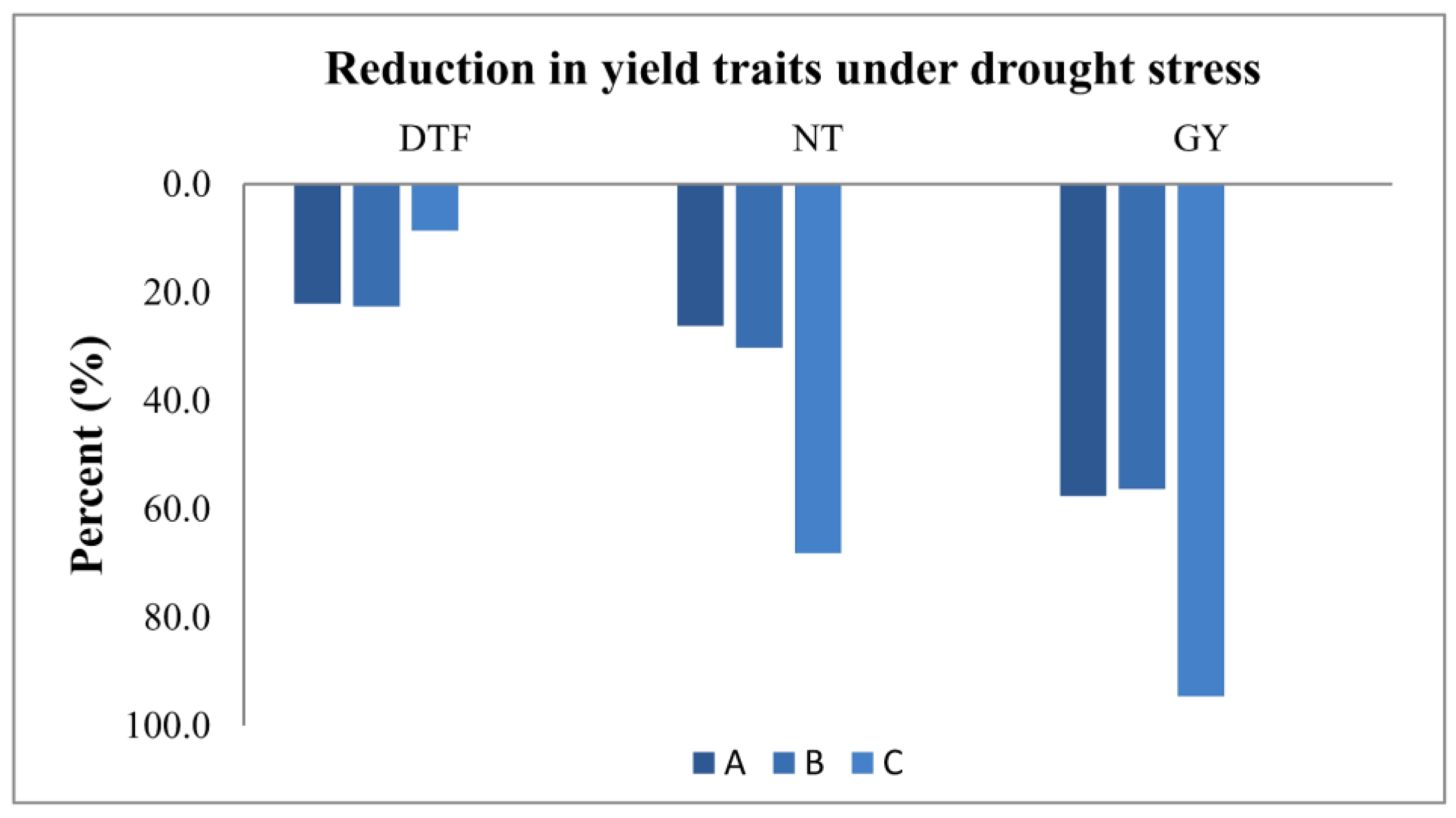
| 2020 | Control | Medium | 7 | 117 ± 1.2 | 106.3 ± 0.8 | 22 ± 1.0 | 24.4 ± 0.5 | 24.2 ± 0.5 | 32.9 ± 0.5 |
| 2020 | Drought stress | Medium | 7 | 107 ± 1.2 | 81.9 ± 0.6 | 7 ± 0.6 | 17.2 ± 0.7 | 1.3 ± 0.2 | 14.4 ± 0.8 |
| Parents/BILs | qDTY 1.1 | qDTY 2.1 | qDTY 3.1 | Days to Fifty Percent Flowering | Number of Productive Tillers | Grain Yield (g) | |||
|---|---|---|---|---|---|---|---|---|---|
| Control | Stress | Control | Stress | Control | Stress | ||||
| IWP | − | − | − | 117.2 ± 1.2 | 107.2 ± 1.2 | 22.2 ± 1.0 | 7.2± 0.6 | 24.2 ± 0.5 | 1.3 ± 0.2 |
| APO | + | + | + | 98.1 ± 0.6 | 80.2 ± 1.5 | 12.3 ± 0.1 | 11.3 ± 0.1 | 20.0 ± 0.6 | 15.2 ± 0.6 * |
| 10-1-1-5-23 | + | − | + | 104.3 ± 1.5 | 81.1 ± 1.5 | 26.2 ± 1.5 * | 19.2 ± 0.2 * | 32.8 ± 0.7 * | 13.9 ± 0.1 * |
| 4-7-4-24-3-41-17 | − | + | + | 108.6 ± 2.2 | 82.1 ± 1.5 | 25.8 ± 0.2 * | 18.7 ± 0.2 * | 33.8 ± 0.6 * | 16.5 ± 0.1 * |
| 4-7-4-24-3-41-15 | − | + | + | 107.1 ± 1.2 | 84.1± 1.2 | 24.5 ± 0.1 * | 18.6 ± 0.1 * | 33.2 ± 0.2 * | 14.2 ± 0.1 * |
| 4-7-4-24-4-16-30 | − | + | + | 105.0 ± 1.2 | 84.3 ± 2.0 | 25.1 ± 0.2 * | 17.4 ± 0.1 * | 34.6 ± 0.1 * | 17.1 ± 0.2 * |
| 4-7-4-24-4-16-28 | − | + | + | 104.2 ± 1.1 | 80.5 ± 1.2 | 23.8 ± 0.1 | 16.8 ± 0.1 * | 33.5 ± 0.1 * | 15.4 ± 0.1 * |
| 4-7-4-24-4-16-29 | − | + | + | 109.1 ± 0.6 | 82.1 ± 1.2 | 23.2 ± 1.2 | 15.2 ± 0.2 * | 31.9 ± 0.4 * | 12.1 ± 0.1 * |
| 4-7-4-24-3-41-12 | − | + | + | 107.8 ± 1.2 | 80.3 ± 1.2 | 22.9 ± 0.3 | 14.8 ± 0.1 * | 30.5 ± 0.4 * | 11.4 ± 0.1 * |
| C.D. (5%) | 4.488 | 4.612 | 2.365 | 0.426 | 1.314 | 0.416 | |||
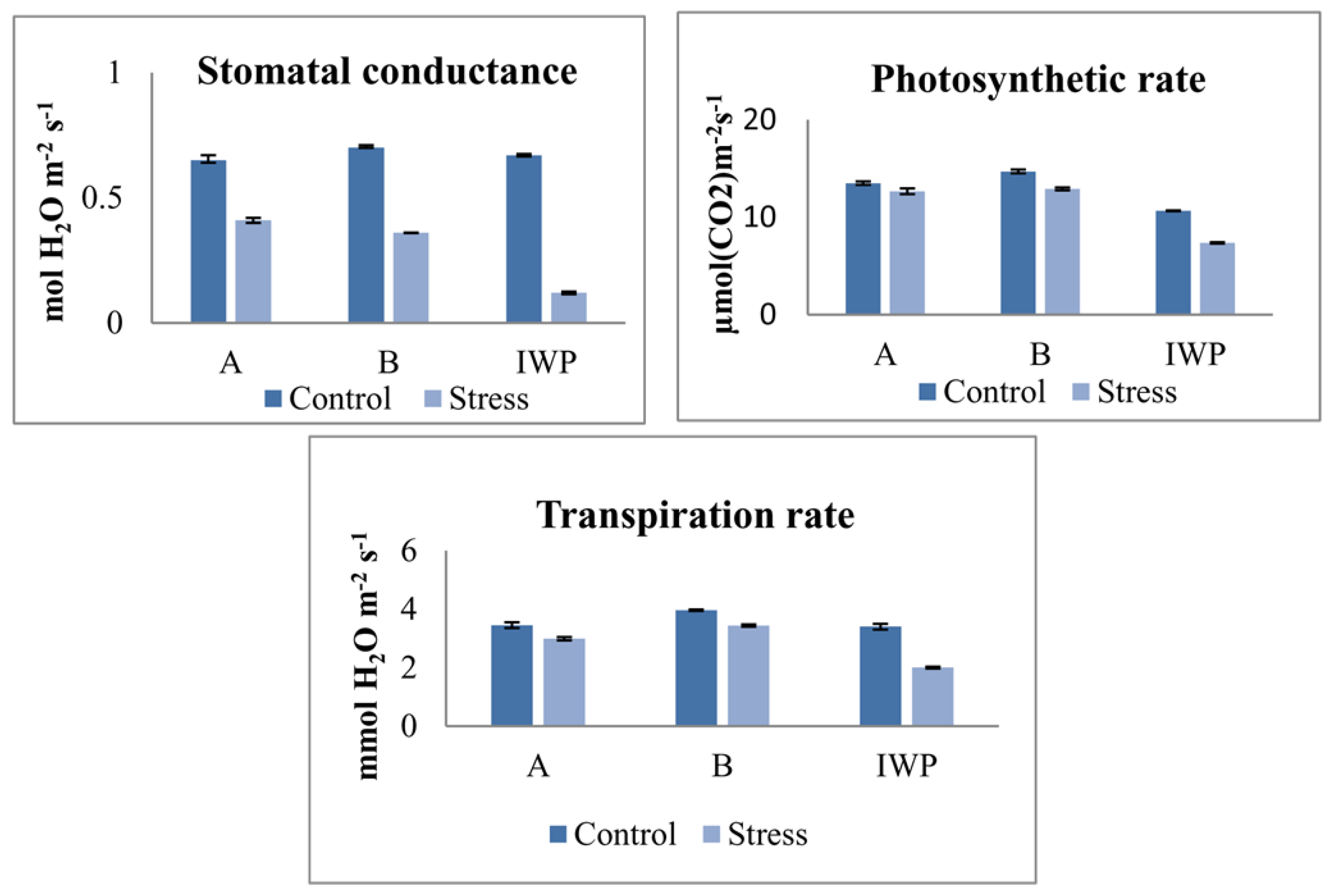
| Parents/BLs | Transpiration Rate | Stomatal Conductance | Photosynthetic Rate | |||
|---|---|---|---|---|---|---|
| Control | Stress | Control | Stress | Control | Stress | |
| IWP | 3.40 ± 0.10 | 2.26 ± 0.04 | 0.67 ± 0.01 | 0.12 ± 0.01 | 10.66 ± 0.04 | 7.37 ± 0.06 |
| APO | 3.43 ± 0.04 | 3.18 ± 0.02 | 0.70 ± 0.03 | 0.30 ± 0.02 | 12.58 ± 0.06 | 11.73 ± 0.04 |
| 10-1-1-5-23 | 3.45 ± 0.10 | 2.99 ± 0.06 * | 0.56 ± 0.02 | 0.28 ± 0.01 * | 13.50 ± 0.18 * | 12.67 ± 0.30 * |
| 4-7-4-24-3-41-17 | 3.67 ± 0.09 * | 2.95 ± 0.03 * | 0.61 ± 0.04 | 0.32 ± 0.02 * | 14.51 ± 0.11 * | 13.34 ± 0.05 * |
| 4-7-4-24-3-41-15 | 4.24 ± 0.08 * | 3.34 ± 0.12 * | 0.65 ± 0.03 | 0.36 ± 0.01 * | 16.39 ± 0.07 * | 15.26 ± 0.11 * |
| 4-7-4-24-4-16-30 | 4.01 ± 0.02 * | 3.93 ± 0.07 * | 0.76 ± 0.06 | 0.30 ± 0.02 * | 14.23 ± 0.16 * | 11.40 ± 0.17 * |
| 4-7-4-24-4-16-28 | 3.31 ± 0.04 | 2.82 ± 0.06 * | 0.75 ± 0.04 | 0.41 ± 0.01 * | 13.34 ± 0.11 * | 13.01 ± 0.03 * |
| 4-7-4-24-4-16-29 | 4.34 ± 0.05 * | 4.25 ± 0.09 * | 0.71 ± 0.02 | 0.38 ± 0.02 * | 14.85 ± 0.09 * | 13.16 ± 0.04 * |
| 4-7-4-24-3-41-12 | 4.21 ± 0.04 * | 3.34 ± 0.03 * | 0.72 ± 0.03 | 0.39 ± 0.01 * | 14.90 ± 0.10 * | 11.28 ± 0.01 * |
| C.D. (5%) | 0.222 | 0.234 | 0.105 | 0.041 | 0.351 | 1.891 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seeli, F.D.P.; Manoharan, M.; Ayyenar, B.; Kambale, R.; Mohanavel, V.; Rajagopalan, V.R.; Manickam, S.; Muthurajan, R.; Swaminathan, M. Genetic Improvement of Drought Tolerance in a Mega-Rice Variety Improved White Ponni through Marker-Assisted Backcross Breeding. Agriculture 2024, 14, 431. https://doi.org/10.3390/agriculture14030431
Seeli FDP, Manoharan M, Ayyenar B, Kambale R, Mohanavel V, Rajagopalan VR, Manickam S, Muthurajan R, Swaminathan M. Genetic Improvement of Drought Tolerance in a Mega-Rice Variety Improved White Ponni through Marker-Assisted Backcross Breeding. Agriculture. 2024; 14(3):431. https://doi.org/10.3390/agriculture14030431
Chicago/Turabian StyleSeeli, F. D. Prisca, Muthukumar Manoharan, Bharathi Ayyenar, Rohit Kambale, Vignesh Mohanavel, Veera Ranjani Rajagopalan, Sudha Manickam, Raveendran Muthurajan, and Manonmani Swaminathan. 2024. "Genetic Improvement of Drought Tolerance in a Mega-Rice Variety Improved White Ponni through Marker-Assisted Backcross Breeding" Agriculture 14, no. 3: 431. https://doi.org/10.3390/agriculture14030431
APA StyleSeeli, F. D. P., Manoharan, M., Ayyenar, B., Kambale, R., Mohanavel, V., Rajagopalan, V. R., Manickam, S., Muthurajan, R., & Swaminathan, M. (2024). Genetic Improvement of Drought Tolerance in a Mega-Rice Variety Improved White Ponni through Marker-Assisted Backcross Breeding. Agriculture, 14(3), 431. https://doi.org/10.3390/agriculture14030431







