Effects of Water–Nitrogen Interaction on Sandy Soil, Physiology, and Morphology of Tall Fescue (Festuca arundinacea Schreb) Turf
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Design
2.2. Sampling and Measurements
2.2.1. Collection of Samples
2.2.2. Measurement Methods
2.3. Data Analysis
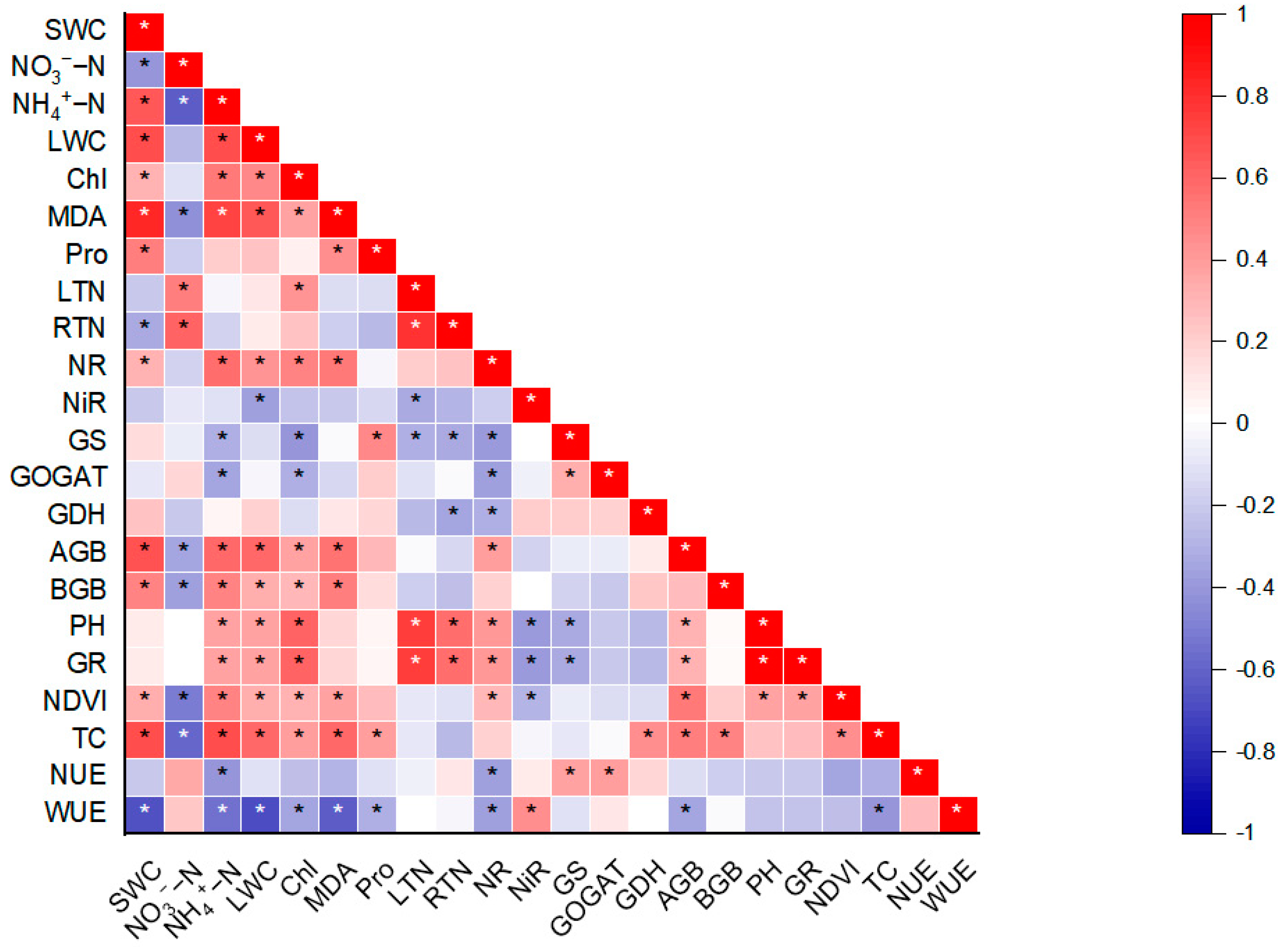
3. Results
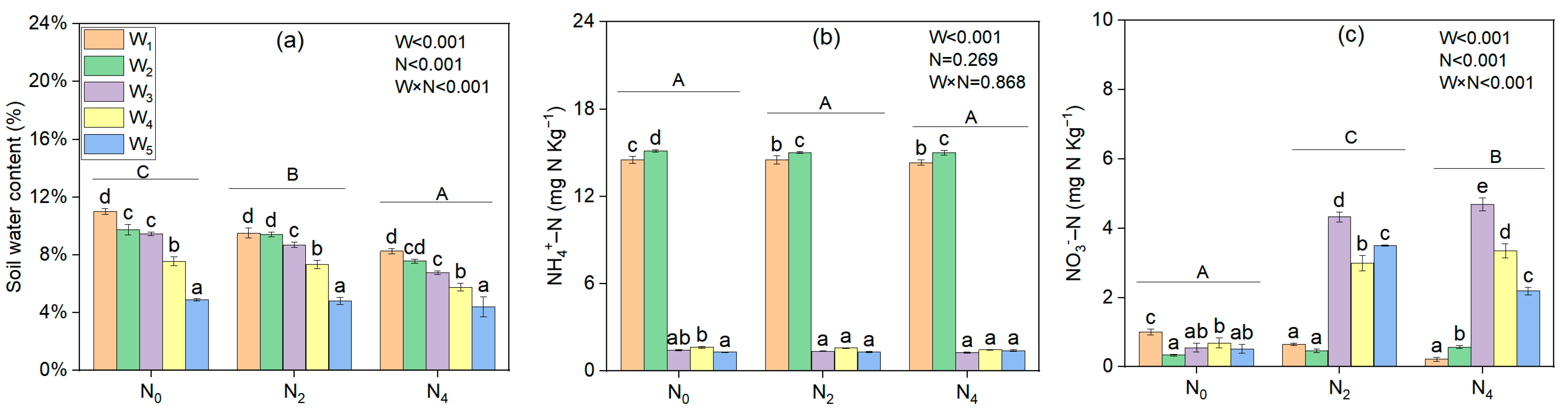
3.1. SWC, NO3−–N, and NH4+–N Levels
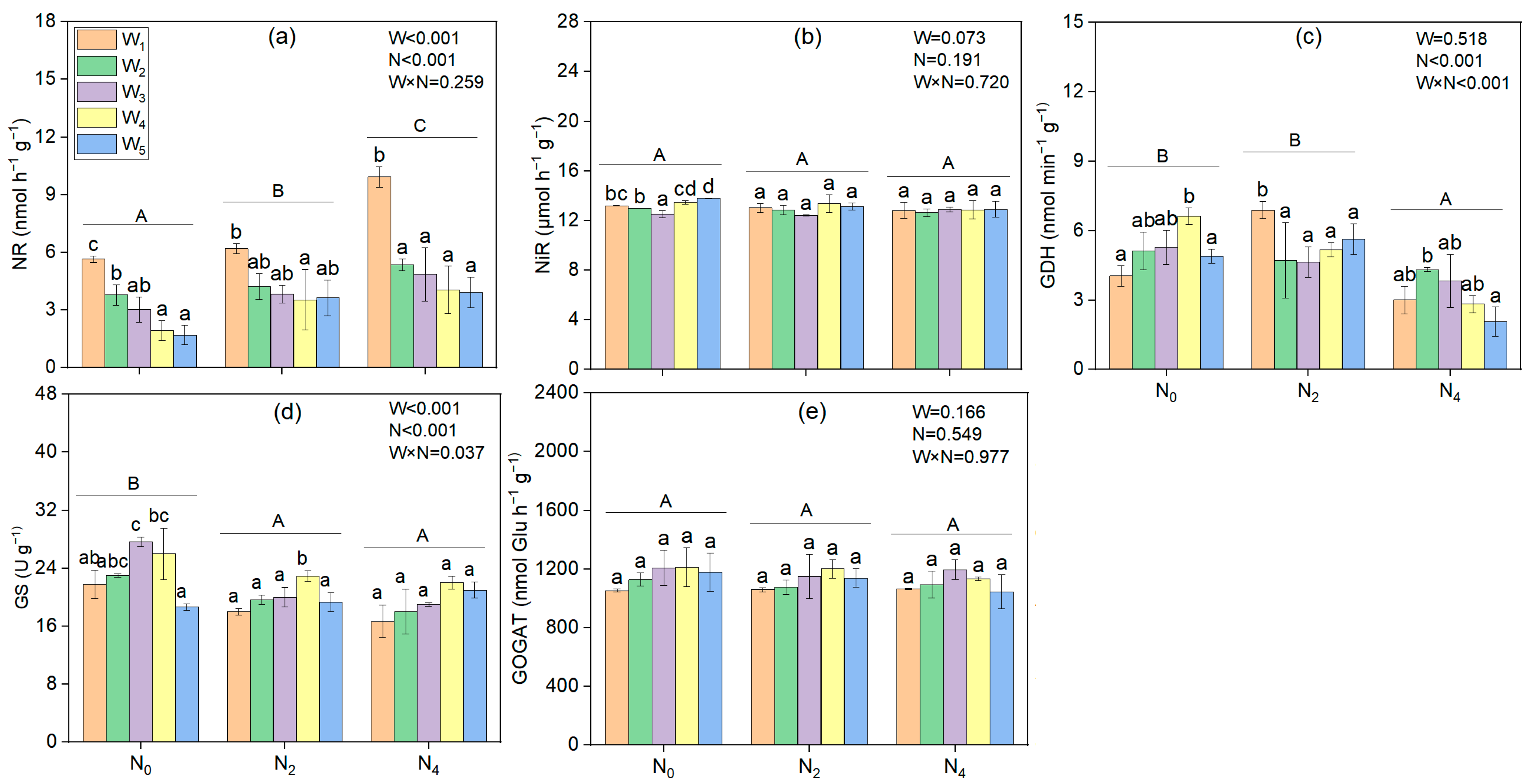
3.2. Physiological Indexes
3.3. Morphological Parameters
3.4. WUE and NUE
4. Discussion
4.1. Effects of Irrigation Treatment
4.2. Effects of N Fertilizer
4.3. Synergistic Effects of Irrigation and N Fertilizer Application
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bachman, M.; Inamdar, S.; Barton, S.; Duke, J.M.; Tallamy, D.; Bruck, J. A Comparative Assessment of Runoff Nitrogen from Turf, Forest, Meadow, And Mixed Landuse Watersheds. J. Am. Water Resour. Assoc. 2016, 2, 397–408. [Google Scholar] [CrossRef]
- Hanks, J.D.; Waldron, B.L.; Johnson, P.G.; Jensen, K.B.; Asay, K.H. Breeding CWG-R Crested Wheatgrass for Reduced-Maintenance Turf. Crop Sci. 2005, 2, 524–528. [Google Scholar] [CrossRef][Green Version]
- Li, D.; Liu, J.; Guo, H.; Zong, J.; Li, J.; Wang, J.; Li, L.; Chen, J. Effects of Low Nitrogen Supply on Nitrogen Uptake, Assimilation and Remobilization in Wild Bermudagrass. Plant Physiol. Biochem. 2022, 191, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Li, J.; Sun, L.; Gao, Y.; Cao, M.; Luo, J. Impacts of Water Deficit and Post-Drought Irrigation on Transpiration Rate, Root Activity, and Biomass Yield of Festuca arundinacea During Phytoextraction. Chemosphere 2022, 294, 133842. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.; Rossi, S.; Yuan, B.; Huang, B. Differential Regulation of Amino Acids and Nitrogen for Drought Tolerance and Poststress Recovery in Creeping Bentgrass. J. Am. Soc. Hortic. Sci. 2022, 4, 208–215. [Google Scholar] [CrossRef]
- DaCosta, M.; Huang, B. Deficit Irrigation Effects on Water Use Characteristics of Bentgrass Species. Crop Sci. 2006, 4, 1779–1786. [Google Scholar] [CrossRef]
- King, K.W.; Balogh, J.C.; Hughes, K.L.; Harmel, R.D. Nutrient Load Generated by Storm Event Runoff from A Golf Course Watershed. J. Environ. Qual. 2007, 4, 1021–1030. [Google Scholar] [CrossRef]
- Ulen, B.; Johansson, G.; Simonsson, M. Changes in Nutrient Leaching and Groundwater Quality During Long-Term Studies of an Arable Field on The Swedish South-West Coast. Hydrol. Res. 2008, 1, 63–77. [Google Scholar] [CrossRef][Green Version]
- Chen, L.; Yue, S.; Sun, L.; Gao, M.; Wang, R. Study on the Effects of Irrigation Quotas and Amendments on Salinized Soil and Maize Growth. Water 2024, 16, 2194. [Google Scholar] [CrossRef]
- Bayat, H.; Nemati, H.; Tehranifar, A.; Gazanchian, A. Screening Different Crested Wheatgrass (Agropyron cristatum (L.) Gaertner.) Accessions for Drought Stress Tolerance. Arch. Agron. Soil Sci. 2016, 6, 769–780. [Google Scholar] [CrossRef]
- Gazanchian, A.; Hajheidari, M.; Sima, N.K.; Salekdeh, G.H. Proteome Response of Elymus elongatum to Severe Water Stress and Recovery. J. Exp. Bot. 2007, 2, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Saud, S.; Li, X.; Chen, Y.; Zhang, L.; Fahad, S.; Hussain, S.; Sadiq, A.; Chen, Y. Silicon Application Increases Drought Tolerance of Kentucky Bluegrass by Improving Plant Water Relations and Morphophysiological Functions. Sci. World J. 2014, 2014, 368694. [Google Scholar] [CrossRef] [PubMed]
- Széles, A.V.; Megyes, A.; Nagy, J. Irrigation and Nitrogen Effects on The Leaf Chlorophyll Content and Grain Yield of Maize in Different Crop Years. Agric. Water Manag. 2012, 107, 133–144. [Google Scholar] [CrossRef]
- Zhang, X.; Taylor, Z.; Goatley, M.; Wang, K.; Brown, I.; Kosiarski, K. Photosynthetic Rate and Root Growth Responses to ascophyllum nodosum Extract-Based Biostimulant in Creeping Bentgrass Under Heat and Drought Stress. Hortscience 2023, 8, 917–921. [Google Scholar] [CrossRef]
- Zhang, C. Nitrate Uptake of Kentucky Bluegrass as a Determinant of Nitrogen Use Efficiency; North Carolina State University: Raleigh, NC, USA, 2012. [Google Scholar]
- Bowman, D.C.; Cramer, G.R.; Devitt, D.A. Effect of Salinity and Nitrogen Status on Nitrogen Uptake by Tall Fescue Turf. J. Plant Nutr. 2006, 8, 1481–1490. [Google Scholar] [CrossRef]
- Li, D.; Chen, J.; Zong, J.; Wang, Y.; Guo, H.; Zhang, B.; Liu, J. Variation in Growth Response and Nitrogen Accumulation and Partitioning of Bermudagrass Under Low Nitrogen Levels. Fresenius Environ. Bull. 2017, 1A, 654–660. [Google Scholar]
- Zere, S.; Bilgili, U. Effects of Different Nitrogen Sources on Turf Quality and Plants Growth of Some Warm-Season Turfgrasses. Turk. J. Field Crops 2022, 1, 167–174. [Google Scholar] [CrossRef]
- Giagnoni, L.; Pastorelli, R.; Mocali, S.; Arenella, M.; Nannipieri, P.; Renella, G. Availability of Different Nitrogen Forms Changes the Microbial Communities and Enzyme Activities in the Rhizosphere of Maize Lines with Different Nitrogen Use Efficiency. Appl. Soil Ecol. 2016, 98, 30–38. [Google Scholar] [CrossRef]
- Sun, X.; Zheng, Q.; Xiong, L.; Xie, F.; Li, X.; Li, Y.; Zhang, L.; Saud, S.; Guo, Z.; Yan, Y.; et al. Nitrogen Assimilation and Gene Regulation of Two Kentucky Bluegrass Cultivars Differing in Response to Nitrate Supply. Sci. Hortic. 2021, 288, 110315. [Google Scholar] [CrossRef]
- Li, G.; Guo, X.; Sun, W.; Hou, L.; Wang, G.; Tian, R.; Wang, X.; Qu, C.; Zhao, C. Nitrogen Application in Pod Zone Improves Yield and Quality of Two Peanut Cultivars by Modulating Nitrogen Accumulation and Metabolism. Bmc Plant Biol. 2024, 1, 1471–2229. [Google Scholar] [CrossRef]
- Cui, J.; Lamade, E.; Fourel, F.; Tcherkez, G. δ15N values in Plants are Determined by Both Nitrate Assimilation and Circulation. New Phytol. 2020, 6, 1696–1707. [Google Scholar] [CrossRef]
- Jiang, Z.; Xu, C.; Huang, B. Enzymatic Metabolism of Nitrogen in Leaves and Roots of Creeping Bentgrass Under Nitrogen Deficiency Conditions. J. Am. Soc. Hortic. Sci. 2011, 5, 320–328. [Google Scholar] [CrossRef]
- Ghaffari, H.; Tadayon, M.R.; Bahador, M.; Razmjoo, J. Investigation of the Proline Role in Controlling Traits Related to Sugar and Root Yield of Sugar Beet Under Water Deficit Conditions. Agric. Water Manag. 2021, 243, 106448. [Google Scholar] [CrossRef]
- Li, Y.H.; Tian, P.; Li, C.Z.; Yu, X.Z. Elucidating Comportment of the Glutamate and Ornithine Pathway on Proline Accumulation in Rice Under Different Nitrogenous Nutrition. Int. J. Environ. Sci. Technol. 2022, 4, 2993–3000. [Google Scholar] [CrossRef]
- Candogan, B.N.; Bilgili, U.; Yazgan, S.; Acikgoz, E. Irrigation Level and Nitrogen Rate Affect Evapotranspiration and Quality of Perennial Ryegrass (Lolium perenne). Int. J. Agric. Biol. 2015, 3, 431–439. [Google Scholar] [CrossRef]
- Latiri-Souki, K.; Nortcliff, S.; Lawlor, D.W. Nitrogen Fertilizer Can Increase Dry Matter, Grain Production and Radiation and Water Use Efficiencies for Durum Wheat Under Semi-Arid Conditions. Eur. J. Agron. 1998, 1, 21–34. [Google Scholar] [CrossRef]
- Candogan, B.N.; Bilgili, U.; Yazgan, S.; Acikgoz, E. Growth and Quality Responses of Tall Fescue (festuca arundinacea schreb.) to Different Irrigation Levels and Nitrogen Rates. Turk. J. Field Crops 2014, 1, 142–152. [Google Scholar]
- Wright, I.J.; Reich, P.B.; Westoby, M. Strategy Shifts in Leaf Physiology, Structure and Nutrient Content Between Species of High- and Low-Rainfall and High- and Low-Nutrient Habitats. Funct. Ecol. 2001, 15, 423–434. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M. Least—Cost Input Mixtures of Water and Nitrogen for Photosynthesis. Am. Nat. 2003, 161, 98–111. [Google Scholar] [CrossRef]
- Wang, H.; Prentice, I.C.; Keenan, T.F.; Davis, T.W.; Wright, I.J.; Cornwell, W.K.; Evans, B.J.; Peng, C. Towards a Universal Model for Carbon Dioxide Uptake by Plants. Nat. Plants 2017, 9, 734–741. [Google Scholar] [CrossRef]
- Prentice, I.C.; Dong, N.; Gleason, S.M.; Maire, V.; Wright, I.J. Balancing the Costs of Carbon Gain and Water Transport: Testing a New Theoretical Framework for Plant Functional Ecology. Ecol. Lett. 2014, 17, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.A.; Devitt, D.A.; Morris, R.L. Water Use and Physiological Response of Tall Fescue Turf Water Deficit Irrigation in an Arid Environment. J. Exp. Bot. 2004, 39, 388–393. [Google Scholar] [CrossRef]
- Kunrath, T.R.; Lemaire, G.; Sadras, V.O.; Gastal, F. Water Use Efficiency in Perennial Forage Species: Interactions Between Nitrogen Nutrition and Water Deficit. Field Crop. Res. 2018, 222, 1–11. [Google Scholar] [CrossRef]
- Baird, J.; Schwenke, G.; Macdonald, B.; Nachimuthu, G.; McPherson, A.; Mercer, C. Efficiency Over Excess: Maximising Cotton Lint Yields with Optimum Irrigation and Nitrogen Fertiliser Application. Field Crop. Res. 2024, 315, 109484. [Google Scholar] [CrossRef]
- Riaz, A.; Younis, A.; Hameed, M.; Kiran, S. Morphological and Biochemical Responses of Turf Grasses to Water Deficit Conditions. Pak. J. Bot. 2010, 5, 3441–3448. [Google Scholar]
- Hunt, K.L. Morphology and Associated Turfgrass Quality of Tall Fescue Cultivars in Response to Management Regimes. Doctoral Dissertation, University of Missouri, Columbia, MO, USA, 1988. [Google Scholar]
- Bilgili, U.; Acikgoz, E. Effect of Nitrogen Fertilization on Quality Characteristics of Four Turf Mixtures Under Different Wear Treatments. J. Plant Nutr. 2007, 30, 1139–1152. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, S.; Wang, R.; Chen, Y.; Siddique, K.H.M.; Xia, G.; Chi, D. Ameliorative roles of Biochar-Based Fertilizer on Morpho-Physiological Traits, Nutrient Uptake and Yield in Peanut (Arachis hypogaea L.) Under Water Stress. Agric. Water Manag. 2021, 257, 107129. [Google Scholar] [CrossRef]
- Ebrahimian, E.; Seyyedi, S.M.; Bybordi, A.; Damalas, C.A. Seed Yield and Oil Quality of sunflower, Safflower, and Sesame Under Different Levels of Irrigation Water Availability. Agric. Water Manag. 2019, 218, 149–157. [Google Scholar] [CrossRef]
- Li, Z.W.; Wang, G.Y.; Khan, K.; Yang, L.; Chi, Y.X.; Wang, Y.; Zhou, X.B. Irrigation Combines with Nitrogen Application to Optimize Soil Carbon and Nitrogen, Increase Maize Yield, and Nitrogen Use Efficiency. Plant Soil 2024, 1–2, 605–620. [Google Scholar] [CrossRef]
- Ding, Y.; Luo, W.; Xu, G. Characterisation of Magnesium Nutrition and Interaction of Magnesium and Potassium in Rice. Ann. Appl. Biol. 2006, 2, 111–123. [Google Scholar] [CrossRef]
- Orta, A.H.; Kuyumcu, S. Evapotranspiration and the Response of Cool-Season and Warm-Season Turfgrass Species to Deficit Irrigation Under a Sprinkler Irrigation Method. Irrig. Sci. 2023, 41, 81–91. [Google Scholar] [CrossRef]
- Qin, X.; Huang, T.; Lu, C.; Dang, P.; Zhang, M.; Guan, X.; Wen, P.; Wang, T.; Chen, Y.; Siddique, K.H.M. Benefits and Limitations of Straw Mulching and Incorporation on Maize Yield, Water Use Efficiency, and Nitrogen Use Efficiency. Agric. Water Manag. 2021, 256, 107128. [Google Scholar] [CrossRef]
- Yu, J.; Fan, N.; Hao, T.; Bian, Y.; Zhuang, L.; Li, Q.; Yang, Z. Ethionine-Mitigation of Drought Stress Associated with Changes in Root Viability, Antioxidant Defense, Osmotic Adjustment, and Endogenous Hormones in Tall Fescue. Plant Growth Regul. 2023, 1, 119–132. [Google Scholar] [CrossRef]
- J, G.P. Plant Roots: Growth, Activity and Interactions with the Soil; John Wiley&Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Geza, M.; Deb, S.K.; Leinauer, B.; Stanek, S.; Sevostianova, E.; Serena, M. Modeling NO3-N Leaching During Establishment of Turfgrasses Irrigated with Tailored Reclaimed Water. Vadose Zone J. 2021, 20, e201123. [Google Scholar] [CrossRef]
- Sainju, U.M.; Stevens, W.B.; Caesar-TonThat, T.; Montagne, C. Nitrogen Dynamics Affected by Management Practices in Croplands Transitioning from Conservation Reserve Program. Agron. J. 2014, 5, 1677–1689. [Google Scholar] [CrossRef]
- Ma, H.; Li, L.; Liu, S.; Shi, W.; Wang, C.; Zhao, Q.; Cui, N.; Wang, Y. Physiological Response, Phytohormone Signaling, Biomass Production and Water Use Efficiency of the CAM Plant Ananas comosus Under Different Water and Nitrogen Regimes. Agric. Water Manag. 2022, 266, 107563. [Google Scholar] [CrossRef]
- Kattge, J.; Bonisch, G.; Diaz, S.; Lavorel, S. TRY Plant Trait Database—Enhanced Coverage and Open Access. Glob. Chang. Biol. 2020, 1, 119–188. [Google Scholar] [CrossRef]
- Chen, L.; Khan, S.; Long, X.; Shao, F.; Huang, J.; Yin, L. Effects of The Ammonium Stress on Photosynthesis and Ammonium Assimilation in Submerged Leaves of Ottelia Cordata—An Endangered Aquatic Plant. Aquat. Toxicol. 2023, 261, 106606. [Google Scholar] [CrossRef]
- Jafarinasab, A.; Azari, A.; Siddique, K.H.M.; Madahhosseini, S. Variation of Yield and Physiological Characteristics of Lathyrus Sativus L. Populations Under Terminal Drought. Agric. Water Manag. 2022, 273, 107886. [Google Scholar] [CrossRef]
- Saud, S.; Fahad, S.; Cui, G.; Chen, Y.; Anwar, S. Determining Nitrogen Isotopes Discrimination Under Drought Stress on Enzymatic Activities, Nitrogen Isotope Abundance and Water Contents of Kentucky Bluegrass. Sci. Rep. 2020, 10, 64151. [Google Scholar] [CrossRef]
- Li, S.; Zhou, L.; Addo-Danso, S.D.; Ding, G.; Sun, M.; Wu, S.; Lin, S. Nitrogen Supply Enhances the Physiological Resistance of Chinese Fir Plantlets Under Polyethylene Glycol (PEG)-Induced Drought Stress. Sci. Rep. 2020, 460, 117905. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Deng, M.; Zhang, N.; Li, Y.; Jia, L.; Niu, D. NADK-Mediated Proline Synthesis Enhances High-Salinity Tolerance in the razor Clam. Comp. Biochem. 2024, 291, 111610. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liang, H.; Gao, D.; Wang, Y.; Jin, K.; Liu, J.; Xue, D.; Chen, Y.; Li, Y.; Gao, T.; et al. Comparative Study on the Effects of Soil Quality Improvement Between Urban Spontaneous Groundcover and Lawn. Ecol. Indic. 2023, 148, 110056. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, H.; Cline, V. Correlations of Leaf Relative Water Content, Canopy Temperature, and Spectral Reflectance in Perennial Ryegrass Under Water Deficit Conditions. Hortscience 2009, 2, 459–462. [Google Scholar] [CrossRef]
- Jiang, Y.H.B. Drought and Heat Stress Injury to Two Cool-Season Turf Grass in Relation to Antioxidant Metabolism and Lipid Peroxidation. Crop Sci. 2001, 41, 436–442. [Google Scholar] [CrossRef]
- Sanchez-Blanco, M.J.; Alvarez, S.; Navarro, A.; Banon, S. Changes in Leaf Water Relations, Gas Exchange, Growth and Flowering Quality in Potted Geranium Plants Irrigated with Different Water Regimes. J. Plant Physiol. 2009, 5, 467–476. [Google Scholar] [CrossRef]
- Shi, J.; Yasuor, H.; Yermiyahu, U.; Zuo, Q.; Ben-Gal, A. Dynamic Responses of Wheat to Drought and Nitrogen Stresses During Re-Watering Cycles. Agric. Water Manag. 2014, 146, 163–172. [Google Scholar] [CrossRef]
- Roseli, A.N.M.; Ying, T.F.; Ramlan, M.F. Morphological and Physiological Response of Syzygium Myrtifolium (Roxb.) Walp. To Paclobutrazol. Sains Malays. 2012, 10, 1187–1192. [Google Scholar]
- Zhang, C.; Liu, Y.; Li, D.; Jiang, J. Influence of Soil Moisture Content on Pullout Properties of Hippophae rhamnoides Linn. Roots. J. Mt. Sci. 2020, 11, 2816–2826. [Google Scholar] [CrossRef]
- Kim, E.; Yejin, L.; Son, H.; Yu, Y.B.; Bae, C. Growth and Ingredient Contents of Platycodon Grandiflorum Roots Under Sensor-Based Soil Moisture Contents of Farmland Conditions. Korean J. Plant Reources 2022, 6, 762–769. [Google Scholar]
- Márquez, A.J.; Betti, M.; García-Calderón, M.; Pal’Ove-Balang, P.; Díaz, P.; Monza, J. Nitrate assimilation in Lotus Japonicus. J. Exp. Bot. 2005, 417, 1741–1749. [Google Scholar] [CrossRef] [PubMed]
- Britto, D.T.; Kronzucker, H.J. NH4+ Toxicity in Higher Plants: A Critical Review. J. Plant Physiol. 2002, 6, 567–584. [Google Scholar] [CrossRef]
- Ireland, R.J.L.P. Plant Amino Acids; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Qi, Z.; Ling, F.; Jia, D.; Cui, J.; Zhang, Z.; Xu, C.; Yu, L.; Guan, C.; Wang, Y.; Zhang, M.; et al. Effects of Low Nitrogen on Seedling Growth, Photosynthetic Characteristics and Antioxidant System of Rice Varieties with Different Nitrogen Efficiencies. Sci. Rep. 2023, 13, 197801. [Google Scholar] [CrossRef] [PubMed]
- Vijayalakshmi, P.; Vishnukiran, T.; Ramana Kumari, B.; Srikanth, B.; Subhakar Rao, I.; Swamy, K.N.; Surekha, K.; Sailaja, N.; Subbarao, L.V.; Raghuveer Rao, P.; et al. Biochemical and Physiological Characterization for Nitrogen Use Efficiency in Aromatic Rice Genotypes. Field Crop. Res. 2015, 179, 132–143. [Google Scholar] [CrossRef]
- Xiang, Z.; Huisen, Z.; Yang, G.; Deying, L. Salinity Tolerance of Turf-Type Tall Fescue as Affected by Nitrogen Sources. Hortscience 2018, 11, 1695–1699. [Google Scholar] [CrossRef]
- Colom, M.R.; Vazzana, C. Photosynthesis and PSII functionality of drought-resistant and Drought-Sensitive Weeping Lovegrass Plants. Environ. Exp. Bot. 2003, 2, 135–144. [Google Scholar] [CrossRef]
- Lopes, M.S.; Reynolds, M.P. Stay-Green in spring wheat can be determined by Spectral Reflectance Measurements (Normalized Difference Vegetation Index) Independently from Phenology. J. Exp. Bot. 2012, 10, 3789–3798. [Google Scholar] [CrossRef]
- Upadhyay, R.G.; Singh, A. Effect of Nitrogen and Zinc on Nodulation, Growth and Yield of Cowpea (Vigna unguiculata). Legume Res. 2016, 1, 149–151. [Google Scholar] [CrossRef]
- Mu, X.; Chen, Q.; Wu, X.; Chen, F.; Yuan, L.; Mi, G. Gibberellins Synthesis is Involved in the Reduction of Cell Flux and Elemental Growth Rate in Maize Leaf Under Low Nitrogen Supply. Environ. Exp. Bot. 2018, 150, 198–208. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, W.; Yang, Q. Reducing Nitrate Content in Lettuce by Pre-Harvest Continuous Light Delivered by Red and Blue Light-Emitting Diodes. J. Plant Nutr. 2013, 3, 481–490. [Google Scholar]


| Aperture (mm) | >3.4 | 2–3.4 | 1–2 | 0.5–1 | 0.25–0.5 | 0.15–0.25 | 0.05–0.15 | <0.05 |
| Percentage (%) | — | — | 0.33 | 16.4 | 73.9 | 8.70 | 0.33 | 0.33 |
| Soil pH | Electrical Conductivity (ms cm–1) | Organic Matter (g kg–1) | Total Nitrogen (g kg–1) | Available Phosphorus (mg kg–1) | Available Potassium (mg kg–1) |
|---|---|---|---|---|---|
| 6.60 | 0.14 | 0.92 | 0.10 | 1.34 | 18.9 |
| Treatment | Above-Ground Biomass (g) | Below-Ground Biomass (g) | Plant Height (cm) | Growth Rate (cm d–1) | NDVI | Turf Color | |
|---|---|---|---|---|---|---|---|
| Nitrogen | Water | ||||||
| N0 | W1 | 0.15 ± 0.03 b | 0.10 ± 0.01 c | 5.24 ± 0.21 a | 0.32 ± 0.03 a | 0.65 ± 0.05 a | 6.40 ± 0.07 ab |
| W2 | 0.13 ± 0.01 b | 0.09 ± 0.00 bc | 6.51 ± 0.12 c | 0.50 ± 0.02 c | 0.74 ± 0.03 b | 6.53 ± 0.04 b | |
| W3 | 0.11 ± 0.02 ab | 0.07 ± 0.01 ab | 5.83 ± 0.21 b | 0.40 ± 0.03 b | 0.71 ± 0.03 ab | 6.47 ± 0.04 ab | |
| W4 | 0.08 ± 0.02 a | 0.07 ± 0.01 a | 5.79 ± 0.15 b | 0.40 ± 0.02 b | 0.66 ± 0.01 a | 6.43 ± 0.04 ab | |
| W5 | 0.07 ± 0.03 a | 0.06 ± 0.01 a | 5.31 ± 0.23 a | 0.33 ± 0.03 a | 0.70 ± 0.01 ab | 6.33 ± 0.09 a | |
| N2 | W1 | 0.16 ± 0.02 b | 0.06 ± 0.01 b | 6.17 ± 0.15 ab | 0.45 ± 0.02 ab | 0.67 ± 0.06 ab | 6.67 ± 0.04 b |
| W2 | 0.15 ± 0.02 b | 0.06 ± 0.01 b | 7.13 ± 0.40 c | 0.59 ± 0.06 c | 0.74 ± 0.042 b | 6.70 ± 0.07 b | |
| W3 | 0.13 ± 0.02 b | 0.06 ± 0.00 ab | 6.41 ± 0.23 bc | 0.49 ± 0.03 bc | 0.65 ± 0.03 ab | 6.33 ± 0.09 a | |
| W4 | 0.08 ± 0.02 a | 0.04 ± 0.01 a | 6.31 ± 0.11 ab | 0.47 ± 0.02 ab | 0.61 ± 0.06 a | 6.20 ± 0.07 a | |
| W5 | 0.07 ± 0.02 a | 0.04 ± 0.00 a | 5.65 ± 0.40 a | 0.38 ± 0.06 a | 0.59 ± 0.01 a | 6.17 ± 0.04 a | |
| N4 | W1 | 0.18 ± 0.03 c | 0.07 ± 0.01 b | 7.07 ± 0.07 bc | 0.58 ± 0.01 bc | 0.75 ± 0.01 b | 6.47 ± 0.09 c |
| W2 | 0.14 ± 0.01 bc | 0.05 ± 0.00 ab | 7.40 ± 0.40 c | 0.63 ± 0.06 c | 0.73 ± 0.01 b | 6.63 ± 0.04 c | |
| W3 | 0.10 ± 0.01 b | 0.05 ± 0.02 ab | 6.77 ± 0.10 ab | 0.54 ± 0.01 ab | 0.68 ± 0.00 ab | 6.27 ± 0.09 b | |
| W4 | 0.06 ± 0.02 a | 0.04 ± 0.01 a | 6.44 ± 0.04 a | 0.49 ± 0.01 a | 0.65 ± 0.06 a | 6.17 ± 0.09 b | |
| W5 | 0.06 ± 0.01 a | 0.04 ± 0.01 a | 6.32 ± 0.16 a | 0.47 ± 0.02 a | 0.64 ± 0.01 a | 5.90 ± 0.07 a | |
| p-value | |||||||
| Water | <0.001 | 0.006 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Nitrogen | 0.683 | 0.183 | <0.001 | <0.001 | 0.037 | <0.001 | |
| Water × Nitrogen | 0.186 | 0.247 | 0.112 | 0.112 | 0.166 | <0.001 | |
| Treatment | WUE (%) | NUE (%) | |
|---|---|---|---|
| Nitrogen | Water | ||
| N0 | W1 | 0.036 ± 0.006 a | —— |
| W2 | 0.039 ± 0.002 ab | —— | |
| W3 | 0.051 ± 0.002 bc | —— | |
| W4 | 0.058 ± 0.004 c | —— | |
| W5 | 0.063 ± 0.013 c | —— | |
| N2 | W1 | 0.034 ± 0.001 a | 31.30 ± 0.41 a |
| W2 | 0.037 ± 0.004 a | 35.31 ± 5.22 a | |
| W3 | 0.050 ± 0.003 b | 36.62 ± 3.09 a | |
| W4 | 0.049 ± 0.009 b | 75.13 ± 5.94 b | |
| W5 | 0.054 ± 0.006 b | 50.61 ± 2.31 ab | |
| N4 | W1 | 0.032 ± 0.003 a | 29.55 ± 1.97 a |
| W2 | 0.033 ± 0.002 a | 32.78 ± 2.25 a | |
| W3 | 0.042 ± 0.001 a | 35.21 ± 0.95 a | |
| W4 | 0.045 ± 0.010 a | 55.59 ± 4.84 b | |
| W5 | 0.045 ± 0.007 a | 25.87 ± 3.89 a | |
| p-value | |||
| Water | <0.001 | <0.001 | |
| Nitrogen | 0.716 | 0.017 | |
| Water × N | <0.001 | 0.187 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, W.; Zhang, W.; Han, L. Effects of Water–Nitrogen Interaction on Sandy Soil, Physiology, and Morphology of Tall Fescue (Festuca arundinacea Schreb) Turf. Agriculture 2024, 14, 1948. https://doi.org/10.3390/agriculture14111948
Guo W, Zhang W, Han L. Effects of Water–Nitrogen Interaction on Sandy Soil, Physiology, and Morphology of Tall Fescue (Festuca arundinacea Schreb) Turf. Agriculture. 2024; 14(11):1948. https://doi.org/10.3390/agriculture14111948
Chicago/Turabian StyleGuo, Wenfei, Wenchao Zhang, and Liebao Han. 2024. "Effects of Water–Nitrogen Interaction on Sandy Soil, Physiology, and Morphology of Tall Fescue (Festuca arundinacea Schreb) Turf" Agriculture 14, no. 11: 1948. https://doi.org/10.3390/agriculture14111948
APA StyleGuo, W., Zhang, W., & Han, L. (2024). Effects of Water–Nitrogen Interaction on Sandy Soil, Physiology, and Morphology of Tall Fescue (Festuca arundinacea Schreb) Turf. Agriculture, 14(11), 1948. https://doi.org/10.3390/agriculture14111948







