In-Vivo and In-Vitro Investigation of Germination Rate of Buried Sclerotia, and Variability in Carpogenic Germination Among Sclerotinia sclerotiorum Isolates
Abstract
1. Introduction
2. Materials and Methods
2.1. S. sclerotiorum Isolates and Production of Sclerotia
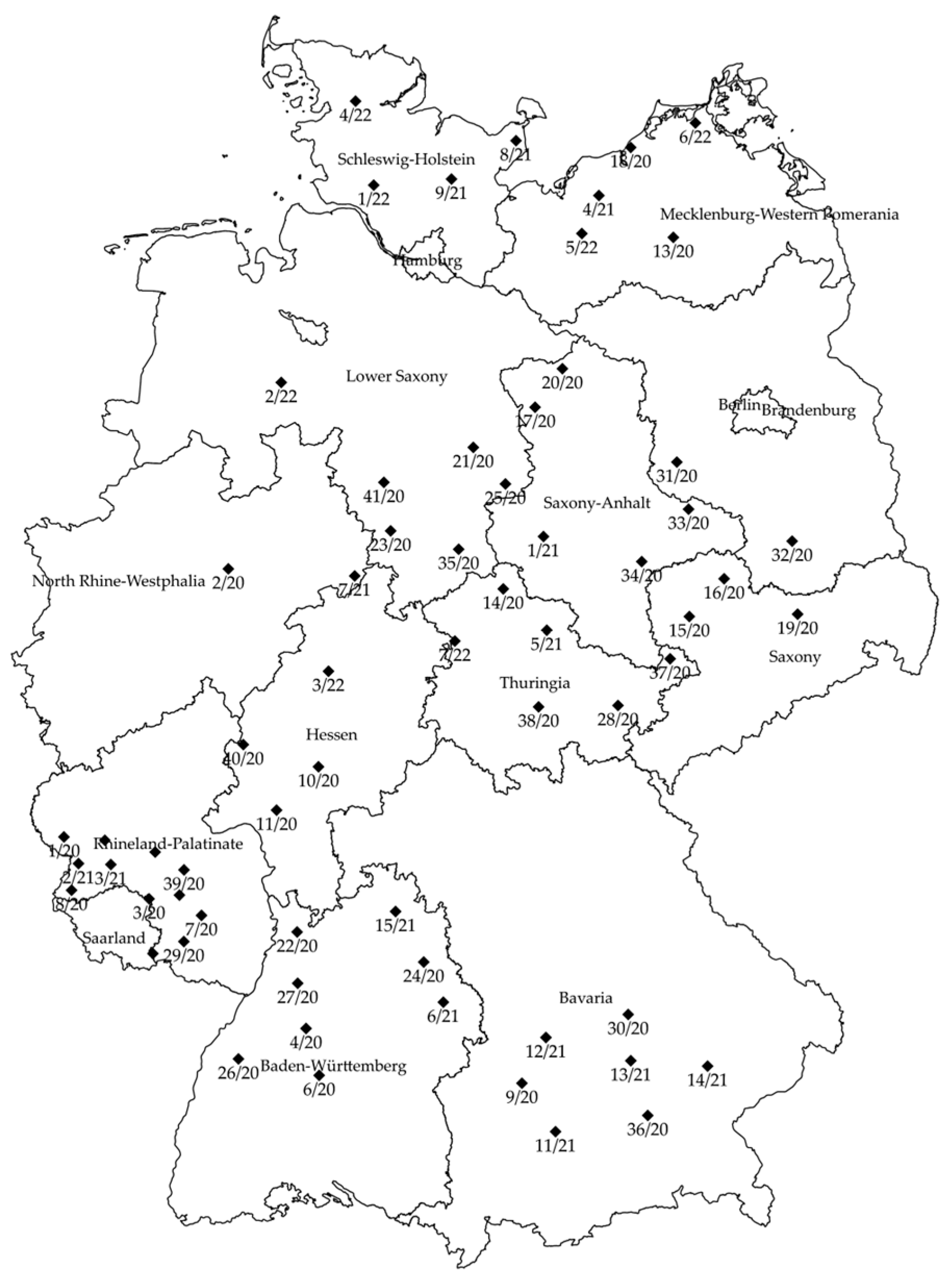
2.2. Establishing Sclerotia Depots and Apothecial Emergence Observation
2.3. Controlled Environment Studies on Carpogenic Germination of Sclerotinia sclerotiorum
2.3.1. Assessment of Temperature and Soil Moisture on Sclerotial Germination
2.3.2. Examining the Impact of Ultraviolet (UV) Light on Apothecia Development
2.3.3. Carpogenic Germination and Apothecia Development Among S. sclerotiorum Isolates
2.4. Statistical Analysis
3. Results
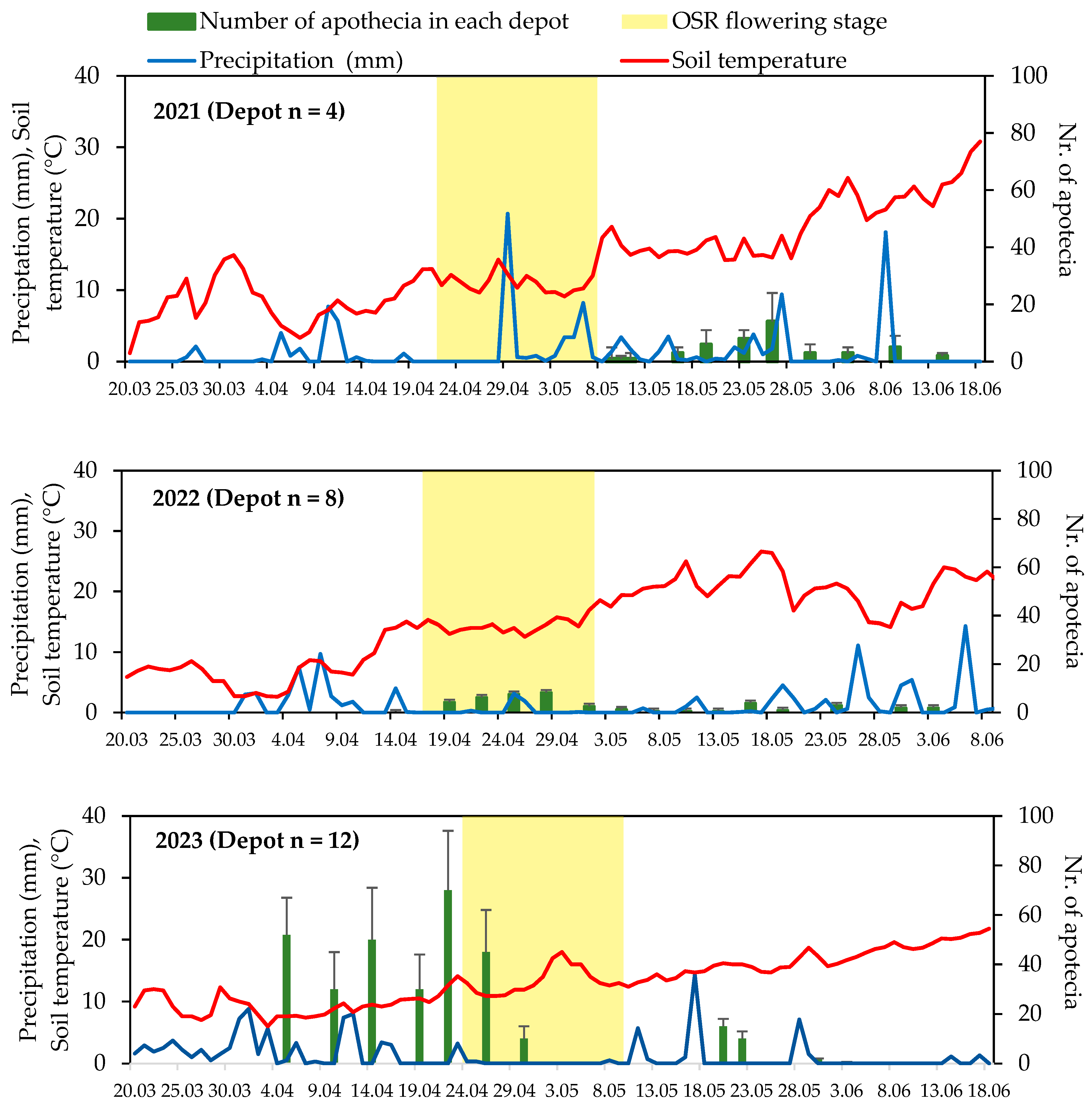
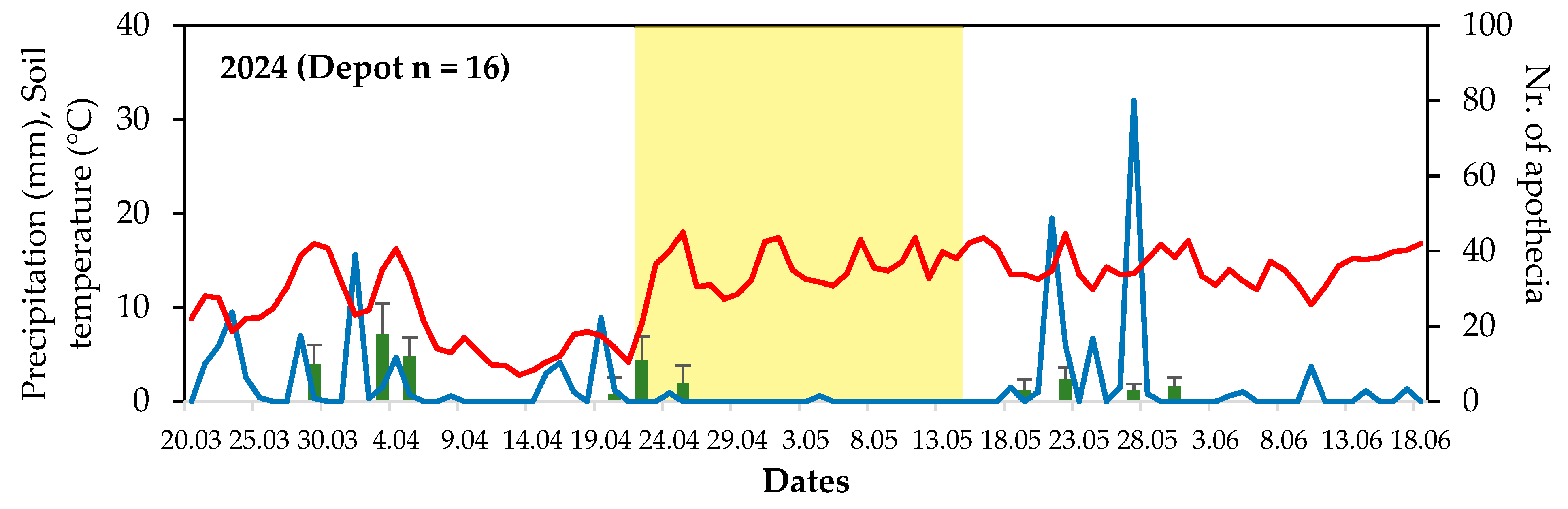
3.1. Sclerotia Depots and Monitoring Apothecial Emergence
3.2. Carpogenic Germination of S. sclerotiorum Under Controlled Conditions
3.2.1. Effect of Temperature and Soil Moisture on Sclerotial Germination
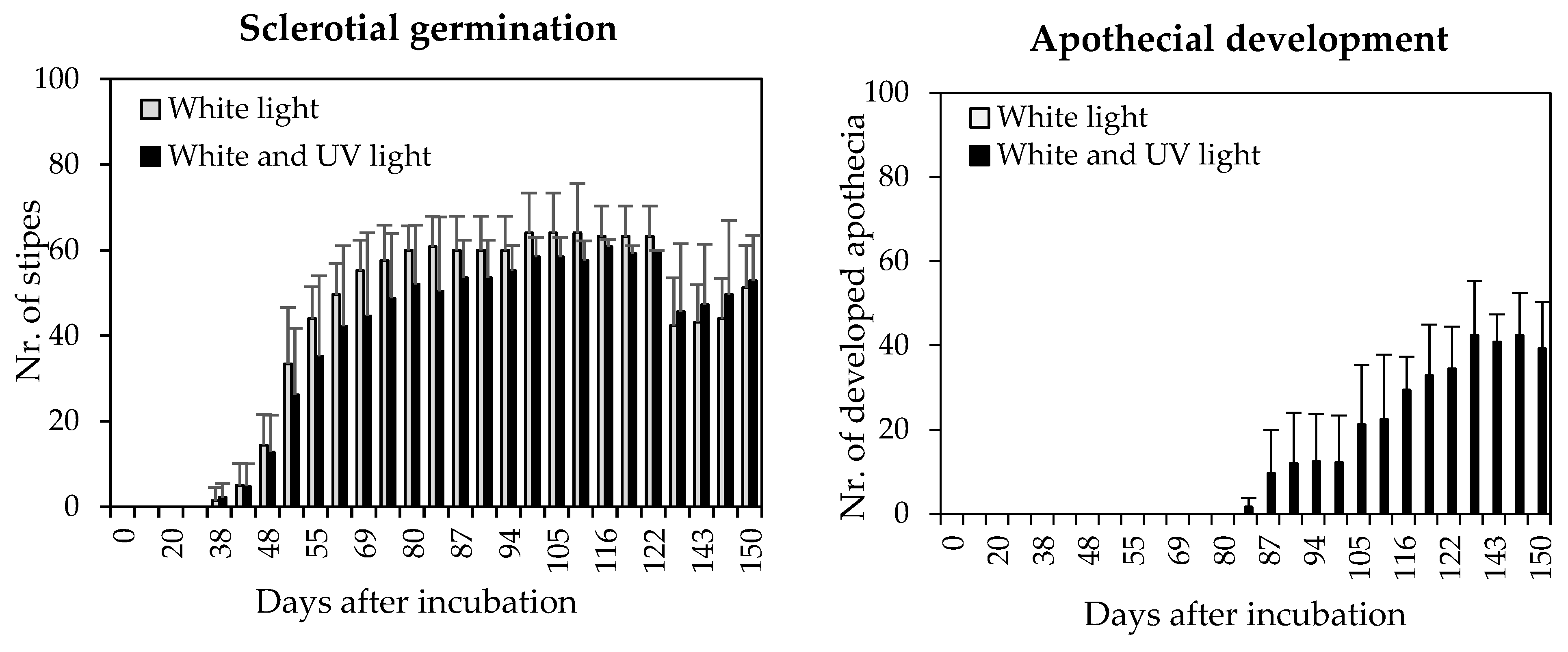
3.2.2. Effect of UV Light on Development of Apothecia
3.2.3. Carpogenic Germination and Apothecia Development Among S. sclerotiorum Isolates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smolinska, U.; Kowalska, B. Biological control of the soil-borne fungal pathogen Sclerotinia sclerotiorum—A review. J. Plant Pathol. 2018, 100, 1–12. [Google Scholar] [CrossRef]
- Antwi-Boasiako, A.; Wang, Y.; Dapaah, H.K.; Zhao, T. Mitigating against Sclerotinia Diseases in Legume Crops: A Comprehensive Review. Agronomy 2022, 12, 3140. [Google Scholar] [CrossRef]
- Reich, J.; Chatterton, S. Predicting field diseases caused by Sclerotinia sclerotiorum: A review. Plant Pathol. 2023, 72, 3–18. [Google Scholar] [CrossRef]
- Zamani-Noor, N.; Brand, S.; Noshin, F.; Söchting, H.-P. Variation in pathogenicity and subsequent production of sclerotia of Sclerotinia sclerotiorum isolates in different cover crops, flower strips and weeds. Plant Dis. 2024, 108, 1688–1694. [Google Scholar] [CrossRef]
- Chaudhary, S.; Lal, M.; Sagar, S.; Tyagi, H.; Kumar, M.; Sharma, S.; Chakrabarti, S. Genetic diversity studies based on morpho-pathological and molecular variability of the Sclerotinia sclerotiorum population infecting potato (Solanum tuberosum L.). World J. Microbiol. Biotechnol. 2020, 36, 177. [Google Scholar] [CrossRef]
- Faruk, M.I.; Rahman, M. Collection, isolation and characterization of Sclerotinia sclerotiorum, an emerging fungal pathogen causing white mold disease. J. Plant Sci. Phytopathol. 2022, 6, 043–051. [Google Scholar]
- Zamani-Noor, N.; Brand, S.; Wüsthoff, N.; Klocke, B.; Papenbrock, J. Diversity in morphological traits, cultural characteristics, and virulence of Sclerotinia sclerotiorum isolates in oilseed rape in Germany. J. Crop Health 2024, in press. [Google Scholar]
- Derbyshire, M.C.; Denton-Giles, M. The control of Sclerotinia stem rot on oilseed rape (Brassica napus): Current practices and future opportunities. Plant Pathol. 2016, 65, 859–877. [Google Scholar] [CrossRef]
- Ben-Yephet, Y.; Genizi, A.; Siti, E. Sclerotial Survival and Apothecial Production by Sclerotinia sclerotiorum Following Outbreaks of Lettuce Drop. Phytopathology 1993, 83, 509–513. [Google Scholar] [CrossRef]
- Wu, B.M.; Subbarao, K.V.; Liu, Y.-B. Comparative survival of sclerotia of Sclerotinia minor and S. sclerotiorum. Phytopathology 2008, 98, 659–665. [Google Scholar] [CrossRef]
- Wu, B.M.; Subbarao, K.V. Effects of soil temperature, moisture, and burial depths on carpogenic germination of Sclerotinia sclerotiorum and S. minor. Phytopathology 2008, 98, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, J.P.; Phelps, K.; Whipps, J.M.; Young, C.S.; Smith, J.A.; Watling, M. Forecasting Sclerotinia disease on lettuce: A predictive model for carpogenic germination of Sclerotinia sclerotiorum sclerotia. Phytopathology 2007, 97, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Mila, A.L.; Yang, X.B. Effects of fluctuating soil temperature and water potential on sclerotia germination and apothecial production of Sclerotinia sclerotiorum. Plant Dis. 2008, 92, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Nordin, R.; Sigvald, R.; Svensson, C. Forecasting the incidence of Sclerotinia stem rot on spring-sown rapeseed. J. Plant Dis. Prot. 1992, 99, 245–255. [Google Scholar]
- Dillard, H.R.; Ludwig, J.W.; Hunter, J.E. Conditioning sclerotia of Sclerotinia sclerotiorum for carpogenic germination. Plant Dis. 1995, 79, 411–415. [Google Scholar] [CrossRef]
- Huang, H.; Kozub, G. Temperature requirements for carpogenic germination of sclerotia of Sclerotinia sclerotiorum isolates of different geographic origin. Bot. Bull. Acad. Sin. 1991, 32, 279–286. [Google Scholar]
- Sun, P.; Yang, X. Light, temperature, and moisture effects on apothecium production of Sclerotinia sclerotiorum. Plant Dis. 2000, 84, 1287–1293. [Google Scholar] [CrossRef]
- Thaning, C.; Nilsson, H.E. A narrow range of wavelengths active in regulating apothecial development in Sclerotinia sclerotiorum. J. Phytopathol. 2000, 148, 627–631. [Google Scholar] [CrossRef]
- Zhou, T.; Boland, G.J. Suppression of dollar spot by hypovirulent isolates of Sclerotinia homoeocarpa. Phytopathology 1998, 88, 788–794. [Google Scholar] [CrossRef]
- Michael, P.J.; Lui, K.Y.; Thomson, L.L.; Lamichhane, A.R.; Bennett, S.J. Impact of Preconditioning Temperature and Duration Period on Carpogenic Germination of Diverse Sclerotinia sclerotiorum Populations in Southwestern Australia. Plant Dis. 2021, 105, 1798–1805. [Google Scholar] [CrossRef]
- Euteneuer, P.; Wagentristl, H.; Pauer, S.; Keimerl, M.; Schachinger, C.; Bodner, G.; Piepho, H.-P.; Steinkellner, S. Field investigation of topsoil moisture and temperature as drivers for decomposition or germination of sclerotia (Sclerotinia sclerotiorum) under winter-killed cover crops. Acta Agric. Scand. 2022, 72, 527–537. [Google Scholar] [CrossRef]
- Paula Júnior, T.J.D.; Vieira, R.F.; Rocha, P.R.R.; Bernardes, A.; Costa, É.L.; Carneiro, J.E.S.; Vole, F.X.R.; Zambolim, L. White mold intensity on common bean in response to plant density, irrigation frequency, grass mulching, Trichoderma spp., and fungicide. Summa Phytopathol. 2009, 35, 44–48. [Google Scholar] [CrossRef]
- Zamani-Noor, N. Baseline Sensitivity and Control Efficacy of Various Group of Fungicides against Sclerotinia sclerotiorum in Oilseed rape Cultivation. Agronomy 2021, 11, 1758. [Google Scholar] [CrossRef]
- Bolton, M.D.; Thomma, B.P.H.J.; Nelson, B.D. Sclerotinia sclerotiorum (Lib.) de Bary: Biology and molecular traits of a cosmopolitan pathogen. Mol. Plant Pathol. 2006, 7, 1–16. [Google Scholar] [CrossRef]
- Ferraz, L.L.; Cafe, A.C.; Nasser, L.B.; Azevedo, J. Effects of soil moisture, organic matter and grass mulching on the carpogenic germination of sclerotia and infection of bean by Sclerotinia sclerotiorum. Plant Pathol. 1999, 48, 77–82. [Google Scholar] [CrossRef]
- Twengström, E.; Köpmans, E.; Sigvald, R.; Svensson, C. Influence of different irrigation regimes on carpogenic germination of sclerotia of Sclerotinia sclerotiorum. J. Phytopath. 1998, 146, 487–493. [Google Scholar] [CrossRef]
- Bom, M.; Boland, G.J. Evaluation of disease forecasting variables for Sclerotinia stem rot (Sclerotinia sclerotiorum) of canola. Can. J. Plant Sci. 2000, 80, 889–898. [Google Scholar] [CrossRef]
- Foster, A.J.; Kora, C.; McDonald, M.R.; Boland, G.J. Development and validation of a disease forecast model for Sclerotinia rot of carrot. Can. J. Plant Pathol. 2011, 33, 187–201. [Google Scholar] [CrossRef]
- Willbur, J.F.; Fall, M.L.; Byrne, A.M.; Chapman, S.A.; McCaghey, M.M.; Mueller, B.D.; Schmidt, R.; Chilvers, M.I.; Muller, D.S.; Kabbage, M.; et al. Validating Sclerotinia sclerotiorum apothecial models to predict Sclerotinia stem rot in soybean (Glycine max) fields. Plant Dis. 2018, 102, 2592–2601. [Google Scholar] [CrossRef]
- Hao, J.; Subbarao, K.; Duniway, J. Germination of Sclerotinia minor and S. sclerotiorum sclerotia under various soil moisture and temperature combinations. Phytopathology 2003, 93, 443–450. [Google Scholar] [CrossRef]
- Fall, M.L.; Willbur, J.F.; Smith, D.L.; Byrne, A.M.; Chilvers, M.I. Spatiotemporal distribution pattern of Sclerotinia sclerotiorum apothecia is modulated by canopy closure and soil temperature in an irrigated soybean field. Plant Dis. 2018, 102, 1794–1802. [Google Scholar] [CrossRef] [PubMed]
- Cook, G.E.; Steadman, J.R.; Boosalis, M.G. Survival of Whetzelinia sclerotiorum and initial infection of dry edible beans. Phytopathology 1975, 65, 250–255. [Google Scholar] [CrossRef]
- Cosic, J.; Jurkowic, D.; Vrandecic, K.; Kaucic, D. Survival of buried Sclerotinia sclerotiorum sclerotia in undisturbed soil. Helia 2012, 35, 73–78. [Google Scholar] [CrossRef]
- Adams, P.B.; Ayers, W.A. Ecology of Sclerotinia species. Phytopathology 1979, 69, 896–899. [Google Scholar] [CrossRef]
- Matheron, M.E.; Porchas, M. Influence of soil temperature and moisture on eruptive germination and viability of sclerotia of Sclerotinia minor and S. sclerotiorum. Plant Dis. 2005, 89, 50–54. [Google Scholar] [CrossRef]
- Duncan, R.W.; Dilantha Fernando, W.G.; Rashid, K.Y. Time and burial depth influencing the viability and bacterial colonization of sclerotia of Sclerotinia sclerotiorum. Soil Biol. Biochem. 2006, 38, 275–284. [Google Scholar] [CrossRef]
- Clarkson, J.P.; Phelps, K.; Whipps, J.M.; Young, C.S.; Smith, J.A.; Watling, M. Forecasting Sclerotinia Disease on Lettuce: Toward Developing a Prediction Model for Carpogenic Germination of Sclerotia. Phytopathology 2004, 94, 268–279. [Google Scholar] [CrossRef]
- Nepal, A.; del Río Mendoza, L.E. Effect of Sclerotial Water Content on Carpogenic Germination of Sclerotinia sclerotiorum. Plant Dis. 2012, 96, 1315–1322. [Google Scholar] [CrossRef]
- Sansford, C.E.; Coley-Smith, J.R. Production and germination of sclerotia of Sclerotinia sclerotiorum. Plant Pathol. 1992, 41, 154–156. [Google Scholar] [CrossRef]
- Veluchamy, S.; Rollins, J.A. A CRY-DASH-type photolyase/cryptochrome from Sclerotinia sclerotiorum mediates minor UV-A-specific effects on development. Fungal Genet. Biol. 2008, 45, 1265–1276. [Google Scholar] [CrossRef]
- Mylchreest, S.J.; Wheeler, B.E.J. A method for inducing apothecia from sclerotia of Sclerotinia sclerotiorum. Plant Pathol. 1987, 36, 16–20. [Google Scholar] [CrossRef]
- Abreu, M.J.; Souza, E.A. Investigation of Sclerotinia sclerotiorum strains variability in Brazil. Genet. Mol. Res. 2015, 14, 6879–6896. [Google Scholar] [CrossRef]



| Independent Variables | d.f. | F | p |
|---|---|---|---|
| Soil moisture | 3 | 58.29 | <0.05 * |
| Temperature | 2 | 19.82 | <0.05 * |
| Soil moisture × Temperature | 6 | 8.83 | <0.05 * |
| Isolate Number | Year of Collection | Geographical Origin (Federal State) | Days Until the Appearance of the First Stipe | Days Until the Appearance of the First Apothecium | ||||
|---|---|---|---|---|---|---|---|---|
| Scl 030/20 | 2020 | Baden-Württemberg | 78 | ±6 | c | 140 | ±13 | cd |
| Scl 036/20 | 2020 | Baden-Württemberg | 93 | ±16 | cd | 132 | ±8 | cd |
| Scl 010/21 | 2021 | Baden-Württemberg | 60 | ±17 | bc | 121 | ±25 | c |
| Scl 011/21 | 2021 | Baden-Württemberg | 50 | ±22 | b | 102 | ±22 | b |
| Scl 012/21 | 2021 | Baden-Württemberg | 69 | ±14 | bc | 0 | ±0 | a |
| Scl 013/21 | 2021 | Baden-Württemberg | 69 | ±21 | bc | 144 | ±7 | d |
| Scl 014/21 | 2021 | Baden-Württemberg | 47 | ±18 | b | 95 | ±14 | b |
| Scl 015/21 | 2021 | Baden-Württemberg | 62 | ±17 | bc | 117 | ±15 | bc |
| Scl 013/20 | 2020 | Bavaria | 55 | ±12 | b | 132 | ±13 | cd |
| Scl 014/20 | 2020 | Bavaria | 107 | ±12 | d | 126 | ±8 | c |
| Scl 003/21 | 2021 | Bavaria | 68 | ±15 | bc | 120 | ±13 | bc |
| Scl 004/21 | 2021 | Bavaria | 64 | ±17 | bc | 143 | ±5 | cd |
| Scl 005/21 | 2021 | Bavaria | 54 | ±6 | b | 124 | ±10 | c |
| Scl 006/22 | 2022 | Bavaria | 63 | ±7 | bc | 113 | ±12 | bc |
| Scl 007/22 | 2022 | Bavaria | 50 | ±14 | b | 102 | ±16 | b |
| Scl 022/20 | 2020 | Hesse | 75 | ±22 | bc | 101 | ±6 | b |
| Scl 023/20 | 2020 | Hesse | 75 | ±6 | bc | 101 | ±10 | b |
| Scl 024/20 | 2020 | Hesse | 62 | ±21 | bc | 136 | ±4 | cd |
| Scl 041/20 | 2020 | Hesse | 46 | ±2 | b | 99 | ±14 | b |
| Scl 017/20 | 2020 | Lower Saxony | 71 | ±6 | bc | 120 | ±12 | bc |
| Scl 020/20 | 2020 | Lower Saxony | 51 | ±13 | b | 108 | ±5 | bc |
| Scl 021/20 | 2020 | Lower Saxony | 79 | ±3 | c | 132 | ±5 | cd |
| Scl 025/20 | 2020 | Lower Saxony | 38 | ±11 | b | 125 | ±19 | c |
| Scl 040/20 | 2020 | Lower Saxony | 90 | ±23 | cd | 120 | ±6 | bc |
| Scl 008/22 | 2022 | Lower Saxony | 71 | ±13 | bc | 121 | ±13 | c |
| Scl 018/20 | 2020 | Mecklenburg Pomerania | 72 | ±12 | bc | 118 | ±6 | bc |
| Scl 008/21 | 2021 | Mecklenburg Pomerania | 88 | ±23 | cd | 144 | ±7 | cd |
| Scl 009/21 | 2021 | Mecklenburg Pomerania | 57 | ±10 | b | 126 | ±17 | c |
| Scl 004/22 | 2022 | Mecklenburg Pomerania | 47 | ±28 | b | 99 | ±11 | b |
| Scl 005/22 | 2022 | Mecklenburg Pomerania | 64 | ±22 | bc | 101 | ±15 | b |
| Scl 035/20 | 2020 | Northrhine-Westphalia | 76 | ±15 | bc | 138 | ±17 | cd |
| Scl 006/21 | 2021 | Northrhine-Westphalia | 75 | ±13 | bc | 146 | ±4 | d |
| Scl 001/20 | 2020 | Rhineland Palatinate | 84 | ±30 | c | 144 | ±3 | d |
| Scl 002/20 | 2020 | Rhineland Palatinate | 72 | ±9 | bc | 127 | ±5 | c |
| Scl 003/20 | 2020 | Rhineland Palatinate | 65 | ±19 | bc | 123 | ±13 | c |
| Scl 004/20 | 2020 | Rhineland Palatinate | 0 | ±0 | a | 0 | ±0 | a |
| Scl 005/20 | 2020 | Rhineland Palatinate | 87 | ±7 | cd | 153 | ±10 | d |
| Scl 006/20 | 2020 | Rhineland Palatinate | 80 | ±12 | c | 133 | ±10 | cd |
| Scl 007/20 | 2020 | Rhineland Palatinate | 72 | ±12 | bc | 127 | ±8 | c |
| Scl 008/20 | 2020 | Rhineland Palatinate | 70 | ±16 | bc | 124 | ±8 | c |
| Scl 009/20 | 2020 | Rhineland Palatinate | 63 | ±8 | bc | 133 | ±14 | cd |
| Scl 010/20 | 2020 | Rhineland Palatinate | 47 | ±7 | b | 107 | ±12 | bc |
| Scl 011/20 | 2020 | Rhineland Palatinate | 58 | ±3 | b | 120 | ±16 | bc |
| Scl 012/20 | 2020 | Rhineland Palatinate | 70 | ±6 | bc | 121 | ±27 | c |
| Scl 015/20 | 2020 | Saxony | 59 | ±3 | b | 88 | ±6 | b |
| Scl 016/20 | 2020 | Saxony | 79 | ±2 | c | 134 | ±13 | cd |
| Scl 019/20 | 2020 | Saxony | 83 | ±8 | c | 141 | ±5 | cd |
| Scl 033/20 | 2020 | Saxony-Anhalt | 74 | ±7 | bc | 125 | ±4 | c |
| Scl 034/20 | 2020 | Saxony-Anhalt | 74 | ±8 | bc | 126 | ±11 | c |
| Scl 039/20 | 2020 | Saxony-Anhalt | 79 | ±18 | c | 131 | ±8 | cd |
| Scl 001/21 | 2021 | Saxony-Anhalt | 59 | ±14 | b | 139 | ±11 | cd |
| Scl 002/21 | 2021 | Saxony-Anhalt | 70 | ±21 | bc | 145 | ±3 | d |
| Scl 007/21 | 2021 | Schleswig Holstein | 75 | ±34 | bc | 135 | ±23 | cd |
| Scl 001/22 | 2022 | Schleswig Holstein | 55 | ±12 | b | 98 | ±10 | b |
| Scl002/22 | 2022 | Schleswig Holstein | 72 | ±21 | bc | 107 | ±13 | bc |
| Scl 003/22 | 2022 | Schleswig Holstein | 66 | ±10 | bc | 95 | ±14 | b |
| Scl 026/20 | 2020 | Thuringia | 93 | ±23 | cd | 125 | ±15 | c |
| Scl 027/20 | 2020 | Thuringia | 84 | ±13 | c | 110 | ±11 | bc |
| Scl 028/20 | 2020 | Thuringia | 102 | ±38 | d | 125 | ±3 | c |
| Scl 029/20 | 2020 | Thuringia | 110 | ±24 | d | 145 | ±12 | d |
| Scl 037/20 | 2020 | Thuringia | 92 | ±17 | cd | 137 | ±28 | cd |
| Scl 038/20 | 2020 | Thuringia | 65 | ±10 | bc | 120 | ±6 | bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamani-Noor, N.; Klocke, B.; Dominic, A.R.; Brand, S.; Wüsthoff, N.; Papenbrock, J. In-Vivo and In-Vitro Investigation of Germination Rate of Buried Sclerotia, and Variability in Carpogenic Germination Among Sclerotinia sclerotiorum Isolates. Agriculture 2024, 14, 1939. https://doi.org/10.3390/agriculture14111939
Zamani-Noor N, Klocke B, Dominic AR, Brand S, Wüsthoff N, Papenbrock J. In-Vivo and In-Vitro Investigation of Germination Rate of Buried Sclerotia, and Variability in Carpogenic Germination Among Sclerotinia sclerotiorum Isolates. Agriculture. 2024; 14(11):1939. https://doi.org/10.3390/agriculture14111939
Chicago/Turabian StyleZamani-Noor, Nazanin, Bettina Klocke, Anto Raja Dominic, Sinja Brand, Niklas Wüsthoff, and Jutta Papenbrock. 2024. "In-Vivo and In-Vitro Investigation of Germination Rate of Buried Sclerotia, and Variability in Carpogenic Germination Among Sclerotinia sclerotiorum Isolates" Agriculture 14, no. 11: 1939. https://doi.org/10.3390/agriculture14111939
APA StyleZamani-Noor, N., Klocke, B., Dominic, A. R., Brand, S., Wüsthoff, N., & Papenbrock, J. (2024). In-Vivo and In-Vitro Investigation of Germination Rate of Buried Sclerotia, and Variability in Carpogenic Germination Among Sclerotinia sclerotiorum Isolates. Agriculture, 14(11), 1939. https://doi.org/10.3390/agriculture14111939








