Abstract
Although the cherry already has an excellent bioactive profile, the implementation of breeding programs is a valuable tool to enhance it further. Thus, a breeding program was conducted on ‘Picota’ type cherries (the ‘Ambrunés’ cultivar, in particular) by crossing them with the ‘Hudson’ cultivar, with the primary aim of obtaining new cherry cultivars that enhance the overall quality traits of the parental cultivars, but above all that increase the concentration of phenolic compounds, which are of significant relevance to human health, providing greater added value for the consumer. The fruits obtained from new cultivars were tested for their physicochemical profiles, phenolic compounds profile, and antioxidant activity. The obtained data were statistically analyzed by applying ANOVA and hierarchical cluster analysis. After screening these data, three new cultivars were found that maintain the physicochemical profile of ‘Ambrunés’ but enhance their phenolic compound content and could, therefore, be interesting cultivars to be used as parents in new cross-breeding.
1. Introduction
The sweet cherry (Prunus avium L.), belonging to the Rosaceae family, is a crucial stone fruit crop for fresh consumption worldwide. The Jerte Valley (located in the north of Extremadura, Spain) is well known for producing ‘Picota’ sweet cherries, which are characterized by being naturally harvested without the stem and are marketed under the protected designation of origin (PDO) ‘Cereza del Jerte’ []. According to its specifications [], the PDO includes four ‘Picota’-type cultivars, specifically ‘Ambrunés’, ‘Pico Limón Negro’, ‘Pico Negro’, and ‘Pico Colorado’, with ‘Ambrunés’ the most important cultivar in terms of production volume []. Consumers highly appreciate these ‘Picota’-type cherries due to their high organoleptic quality, with a taste that combines a high sugar content with slight acidity [].
Nowadays, consumers are well aware of the health benefits of various fruits, especially berries. Berries are a natural source of antioxidant compounds, mainly anthocyanins and other phenolic compounds, which contribute to a healthy diet []. Their intake has been reported to alleviate arthritic pain and gout, reduce the incidence of cancer, and reduce the risk of various degenerative and chronic diseases related to oxidative stress [,,,,].
As ‘Ambrunés’ cherries are characterized by mahogany skin and orange flesh, they are not typically associated with a high antioxidant composition [,]. Therefore, enhancing the concentration of antioxidant compounds in ‘Ambrunés’ sweet cherries could provide additional commercial value for consumers.
For this reason, a breeding program was established in 2006 in the Jerte Valley by the Scientific and Technological Research Centre of Extremadura (CICYTEX) to obtain new Picota-type cultivars with a higher antioxidant composition from the cross ‘Ambrunés’ × ‘Hudson’ by controlled cross-breeding []. The ‘Ambrunés’ parental cultivar is a late, medium-sized cherry with the above physicochemical fruit characteristics []. The ‘Hudson’ parental cultivar is also a very late cherry, with large-sized fruit, dark burgundy skin, dark red flesh, and presumably high in phenolic compounds.
This study aimed to investigate whether the new cultivars obtained from the cross of ‘Ambrunés’ × ‘Hudson’ enhanced the concentration of phenolic compounds and improved the antioxidant capacity compared with the parental cultivars.
2. Materials and Methods
2.1. Plant Material
Samples of thirteen new cherry cultivars (Prunus avium L.) obtained from the controlled cross of ‘Ambrunés’ × ‘Hudson’ parental cultivars, using the methodology described by Lopez-Corrales et al. [], were harvested at commercial maturity from an experimental orchard of CICYTEX, located in the Jerte Valley (latitude 40°07′43.5″ N, longitude 5°54′02.3″ W, Cáceres, Spain), during two consecutive seasons (2022–2023). To analyze the total antioxidant activity, anthocyanins and polyphenols, samples were vacuum-frozen using a vacuum (VirTis Genesis 25L; Hucoa Erlöss SA, Madrid, Spain), packed in plastic bags, and stored at −80 °C in triplicate. Physicochemical analyses were carried out on fresh fruit, also in triplicate.
2.2. Chemicals
ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt), Trolox ((±)-6-hydroxy-2,5,7,8-tetramethyl-chromane-2-carboxylic acid), peroxidase from horseradish, and anthocyanidins and polyphenols HPLC standards were purchased from Sigma-Aldrich (Madrid, Spain).
Acetonitrile (ACN) HPLC-grade, formic acid (CH2O2), sodium hydroxide (NaOH), hydrochloric acid (HCl), potassium dihydrogen phosphate (KH2PO4), potassium hydrogen phosphate (K2HPO4), ethyl acetate (C4H8O2), ethanol (EtOH; 96%), oxygen peroxide (H2O2; 30%), and glass wool were purchased from Panreac (Barcelona, Spain).
Methanol (MeOH; analytical grade) was obtained from Fisher (Madrid, Spain); and ultrapure Milli-Q water and 0.45 µm nylon filters were obtained from Millipore (Madrid, Spain).
2.3. Weight, Size and Firmness
Weight and size were measured employing an AE-166 balance (Mettler, Madrid, Spain) and a DL-10 digital micrometer (Mitutoyo, Kawasaki, Japan), respectively, in 50 randomly selected fruits of each cultivar (n = 3).
The firmness of fifty randomly selected fruits (n = 3) was measured employing a TA.XT2i Texture Analyser (Stable Micro Systems, Godalming, UK) connected to a computer and equipped with a 25 mm flat base probe that moved at 0.2 mm s−1 during the test. The results (expressed in N mm−1) show the ratio between the force needed to produce a 2% deformation and the fruit size.
2.4. Total Soluble Solids, Titratable Acidity and Maturation Index
Fifty randomly selected fruits of each cultivar (n = 3) were homogenized using an Omni Mixer homogenizer (Omni International, Marietta, GA, USA). Each homogenate was measured in an automatic temperature-compensated DR101 digital refractometer (Optic Ivymen System, Barcelona, Spain) to obtain the total soluble solids (TSS) expressed as °Brix.
Then, 5 g of each homogenate was diluted in 50 mL of ultrapure water. Samples were titrated with NaOH (0.1 M) up to pH 8.1, and results were given in g malic acid 100 g−1 fresh weight (FW; 716 DMS Tritino automatic titrator, Metrohm, Herisau, Switzerland). The mature index was obtained as the ratio is between total soluble solids and titratable acidity (TA).
2.5. Color
The skin and flesh color of fifty randomly selected fruits of each cultivar (n = 3) were measured in a CR-400 tristimulus colorimeter (Minolta, Tokyo, Japan), employing a viewing area of 8 mm diameter and the illuminant D65. Chromatic analyses were conducted in accordance with the CIE (Commission Internacionale de l’Eclairage) system of 1976. Each datum represents the mean of four measures at equidistant points of the equatorial region.
2.6. Total Phenolic and Anthocyanins Content
The total phenolic content was determined following the method described by Lima et al. []. Briefly, 5 g of the homogenate obtained from the 50 fruits of each cultivar was extracted successively with an 80% aqueous ethanol extractant solution with 1% hydrochloric acid (HCl) in the absence of light and at room temperature (25 °C) and then filtered and evaporated. The total phenolic content was measured spectrophotometrically at 760 nm using the Folin–Ciocalteu reagent. The results are expressed as mg gallic acid 100 g−1 FW.
The anthocyanin content was obtained by employing the double-pH method []. Each batch was homogenized, and 10 g was extracted for 24 h at −20 °C using acidified methanol (0.1% HCl). The resulting extracts were filtrated, and the absorbance was measured at 510 and 700 nm in buffers at two different pH values: 1.0 (potassium chloride, 0.025 M) and 4.5 (sodium acetate, 0.4 M), employing a UV-2401PC spectrophotometer (Shimadzu Scientific Instruments, Kyoto, Japan). The results are expressed as mg cyanidin-3-O-rutinoside 100 g−1 FW.
2.7. Hydrophilic and Lipophilic Antioxidant Activities
The antioxidant compounds were extracted by mixing 5 g of a homogenate of each sample with 5 mL of a dihydrogen phosphate/hydrogen phosphate buffer (50 mM; pH 7.5) and 3 mL of ethyl acetate in a centrifuge tube. Then, the tubes were centrifuged (Sorvall Legend XTR, Thermo Scientific, Waltham, MA, USA) for 10 min (10,000 rpm; 4 °C). Finally, the upper phase contained lipophilic compounds, while the lower phase contained hydrophilic compounds.
The hydrophilic total antioxidant activity (H-TAA) was determined spectrophotometrically (UV-2401PC spectrophotometer, Shimadzu Scientific Instruments, Kyoto, Japan) using a slightly modified version of the method proposed by Pereira et al. []. Briefly, 1 mL of the radical cation ABTS (dissolved in dihydrogen phosphate/hydrogen phosphate buffer) was placed in a spectrophotometer cuvette. After 60 s, the absorbance at 730 nm was measured. Then, 20 µL of the hydrophilic extract was added, and the absorbance was measured after 20 min of reaction to compare the results. The results are expressed as mg Trolox 100 g−1 FW.
The lipophilic total antioxidant activity (L-TAA) was determined similarly, but the radical cation ABTS was dissolved in acidified ethanol.
2.8. HPLC Analysis of Anthocyanins and Polyphenols
Samples were homogenized using an Omni Mixer homogenizer (Omni International, Marietta, GA, USA). 10 g of each homogenate per cultivar (n = 3) was extracted for 24 h at −20 °C, employing 50 mL of acidified methanol (0.1% HCl). The resulting extracts were filtered through glass wool and 0.45 µm nylon filters and were transferred to topaz vials.
The resulting filtrates were directly injected into the high-performance liquid chromatography (HPLC) equipment without evaporating the solvent, as they were completely soluble in the mobile phase used. The instrument employed was an Agilent Model 1100 LC instrument (Agilent Technologies, Palo Alto, CA, USA) equipped with an on-line degasser, a quaternary pump, an automatic injection valve, a diode array (DAD), and a fluorescence (FLD) detector. The chromatographic conditions in terms of the stationary and mobile phases, as well as the running regime, were those established by Manzano-Durán et al. []. Briefly, a Gemini-NX C18 column (150 × 4.6 mm; 5 µm) thermostated at 40 °C was employed as the stationary phase, while the mobile phase was composed of a gradient mix of phase A (water) and phase B (ACN), both acidified with 0.1% of formic acid. The total time of analysis was 50 min.
DAD quantified 515.8, 320.8, and 350.8 nm for cyanidins, hydroxycinnamic acids, and flavonols families. The family of flavan-3-ols was determined using FLD (275/315 nm). Compounds were identified by comparing the retention time and spectra and spiking samples with known amounts of pure standards. The results are expressed as mg 100 g−1 FW.
2.9. Statistical Analysis
Statistical data were analyzed using SPSS 21.0 (SPSS Inc., Chicago, IL, USA). Data are expressed as mean values, and the error associated with each measure is listed as the relative standard deviation (RSD). The physicochemical characteristics and bioactive compounds composition were studied by an analysis of variance (ANOVA), which allows the comparison of the mean differences between groups that have been split into two dependent between-subject factors: ‘year’ and ‘cultivar’. For the comparison of mean values, Tukey’s Honest Significant Difference (HSD) test (p < 0.05) was used.
On the other hand, categorizing the new cultivars based on their characteristics and their similarity with the different parentals was performed using hierarchical cluster analysis, expressly, by using XLSTAT software, version 2014.5.03 (Addinsoft, Paris, France). Ward’s method was selected, an agglomerative method that groups the individual samples based on their similarity, looking for the minimum residual variance within each cluster and obtaining homogeneous groups of similar size.
3. Results
3.1. Physicochemical Analysis
The sweet cherry weight (Figure 1) varied between 2.95 and 11.25 g, with the parental ‘Hudson’ being the heaviest. None of the new sweet cherry cultivars presented a weight comparable to that of ‘Hudson’. However, three new cultivars improved the weight of the ‘Ambrunés’ parental cultivar. The size (Figure 1) ranged between 17.25 and 29.00 mm, being the ‘Hudson’ parental cultivar, again, the bigger fruit. Six different cultivars equaled or improved the size of the parental ‘Ambrunés’, but none of them could surpass ‘Hudson’ in size. Concerning the firmness (Figure 1), it varied between 0.84 and 2.14 N mm−1, but none of the new cultivars improved the firmness of the parental cultivars. However, four new cultivars equaled their value.
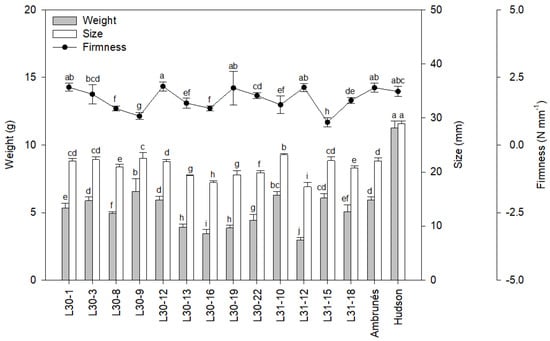
Figure 1.
Average weight (g), size (mm), and firmness (N mm−1) of two years of the new and the parental cultivars. Values are shown as the mean of the two years, together with the standard deviation (SD). Different letters in each plot indicate significant differences.
On the other hand, as seen in Figure 2, the total soluble solids (TSS) and titratable acidity (TA) were generally higher in the new cultivars than the parental cultivars. The TSS values varied between 17.38 and 22.13 °Brix, while the TA values ranged between 0.52 and 1.06 g malic acid 100 g−1 FW. Regarding TSS, all cultivars except one (L31-18) overcame ‘Ambrunés’, and five also overcame ‘Hudson’. Speaking of TA, all cultivars except for L30-12, L30-22, and L30-9 exceeded both parental cultivars. Also, L30-9 was the only cultivar with a lower TA than ‘Ambrunés’. Finally, the mature index (MI; Figure 2) ranged from 19.80 to 42.65. Four new cultivars enhanced the mature index of the ‘Hudson’ parental cultivar, and two also overtook the ‘Ambrunés’ parental cultivar.
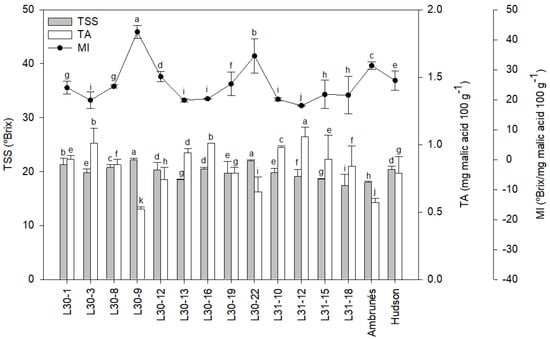
Figure 2.
Average total soluble solids (TSS, °Brix), titratable acidity (TA, mg malic acid 100 g−1), and mature index (MI, °Brix/ mg malic acid 100 g−1) of two years of the new and parental cultivars. Values are shown as the mean of the two years, together with the standard deviation (SD). Different letters in each plot indicate significant differences.
3.2. Color
Table 1 shows the color of new and parental cultivars, represented by L* (luminosity or brightness), h (hue angle) and C* (chroma or chromaticity) parameters and the conversion into RGB color for both for the skin and flesh color. As gathered, about skin color, most of the new cultivars obtained higher L* and h values, although to different extents depending on the cultivar. Regarding L* parameter, values varied between 24.44 and 41.64. Most of the new cultivars presented values more comparable with the ‘Ambrunés’ parent (28.29) than to ‘Hudson’ (24.44). Concerning the h value, values oscillated between 10.88 and 28.63. Maximum differences were found in L30-13 (28.63), which presented a more yellow skin color, while the rest of the new cultivars showed a red-purple skin. C* values were quite heterogeneous, ranging between 9.64 and 48.04.

Table 1.
Mean values and standard deviation (SD) for two years of L*, h and C*, for skin and flesh color, of new and parental cultivars and conversion into RGB color.
On the other hand, flesh color presented L* and h values generally between those of parental cultivars (values of 59.44 and 70.83 and of 28.68 and 20.19, for ‘Ambrunés’ and ‘Hudson’, respectively), except, again, for L30-13, which showed a flesh color practically identical to the ‘Ambrunés’ parental cultivar. C* was, in general, higher than ‘Hudson’ (29.93) in all new cultivars. Thus, it can be said that new sweet cherry cultivars showed, in general, dark red skin and yellow-red pulp.
3.3. Total Phenolic and Anthocyanins Content
The total phenolic content ranged between 76.9 and 288.83 mg gallic acid 100 g−1 FW (Figure 3). Eleven out of the thirteen cultivars improved the content of phenols with respect to ‘Ambrunés’ (94.65 mg gallic acid 100 g−1 FW), although L30-16 (277.38 mg gallic acid 100 g−1 FW) was the only cultivar with a total phenolic concentration significantly similar to that of ‘Hudson’ (288.83 mg gallic acid 100 g−1 FW).
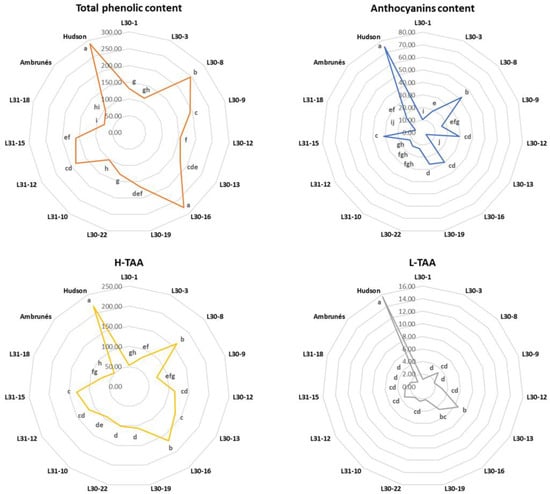
Figure 3.
Radar chart of the average concentration of total phenolic content (orange), anthocyanins content (blue), hydrophilic antioxidant activity (yellow), and lipophilic antioxidant activity (grey) of the new and parental cultivars. Values are shown as the mean of the two years. Different lowercase letters in each plot indicate significant differences.
The content of anthocyanins followed the same trend. In this case, the increase in anthocyanin content was lower, and five cultivars did not surpass that of ‘Ambrunés’ (16.82 mg cyanidin 3-O-rutinoside 100 g−1 FW), and none of the cultivars were statically comparable to ‘Hudson’ (74.79 mg cyanidin 3-O-rutinoside 100 g−1 FW). The values, in this case, varied between 2.94 and 74.79 mg cyanidin 3-O-rutinoside 100 g−1 FW.
3.4. Hydrophilic and Lipophilic Antioxidant Activities
The mean values of TAA (both hydrophilic and lipophilic) are also shown in Figure 3. For all of the new and parental cultivars, H-TAA (50.60–218.60 mg Trolox 100 g−1 FW) was higher than L-TAA (1.10–15.67 mg Trolox 100 g−1 FW). The TAA for ‘Hudson’ (218.60 and 15.67 mg Trolox 100 g−1 FW, for H-TAA and L-TAA, respectively) was not surpassed by any of the new cultivars. However, all cultivars equaled or improved the TAA of ‘Ambrunés’, especially in H-TAA, where most of them doubled the value of ‘Ambrunés’ (50.60 mg Trolox 100 g−1 FW). Concerning L-TAA, all of the new cultivars equaled or improved on the ‘Ambrunés’ (1.10 mg Trolox 100 g−1 FW) values, but none of them were comparable to ‘Hudson’ (i.e., L30-13, which was the cultivar with the highest L-TAA with a concentration of 6.52 mg Trolox 100 g−1 FW, did not even reach half of the L-TAA value of ‘Hudson’).
3.5. HPLC Analysis Polyphenols
The composition and concentrations of the significant phenolics of the new cultivars studied are shown in Table 2, in units of mg 100 g−1 FW. A prototype chromatogram of one of the cultivars analyzed is shown in Figure S1 of Supplementary Materials. The content of cyanidins ranged between 11.88 and 75.72 mg 100 g−1 FW, with cyanidin 3-O-rutinoside (C3R) being the main one and representing almost 90% of this family. Five new cultivars showed a lower content of cyanidins than ‘Ambrunés’ (20.52 mg 100 g−1 FW), while the rest improved their content. No new cultivars exceeded the ‘Hudson’ concentration (75.72 mg 100 g−1 FW), but three doubled or even tripled the concentration of the ‘Ambrunés’ parental cultivar (L31-15, L30-16, and L30-8, with concentrations of 50.14, 54.65, and 60.85 mg 100 g−1 FW, respectively).

Table 2.
The concentration of the different phenolic compounds determined by HPLC-DAD/FLD for each new and parental cultivar, grouped by families. Concentrations were determined over two years. Each value is the mean value of three replicates, in units of mg 100 g−1, and it is presented together with the standard deviation (SD).
Regarding hydroxycinnamic acids, all of the new sweet cherry cultivars enhanced the content of the ‘Ambrunés’ parental cultivar (22.82 mg 100 g−1 FW), and two (L30-13 and L30-16, with concentrations of 78.13 and 76.43 mg 100 g−1 FW, respectively) even exceeded the content of the ‘Hudson’ parental cultivar (72.33 mg 100 g−1 FW). The concentration of this family of compounds ranged between 22.82 and 78.13 mg 100 g−1 FW. The main compound within this family was p-coumarylquinic acid (pCQA), accounting for approximately 75% of the total. However, the most significant changes in concentration occurred in the neochlorogenic acid (NCGA) concentration, where all new cultivars exceeded the concentration of both parental cultivars, being 4.98 and 8.33 mg 100 g−1 FW, for ‘Ambrunés’ and ‘Hudson’ cultivars, respectively.
Contrary to the tendency presented by the other families of compounds, in the case of flavonols, the ‘Ambrunés’ parental cultivar presented the highest concentration (6.14 mg 100 g−1 FW), and only two new cultivars surpassed it (L30-22 and L30-8, with concentrations of 6.38 and 7.71 mg 100 g−1 FW, respectively). Moreover, eight cultivars showed a lower concentration of these compounds than the ‘Hudson’ parental cultivar (4.90 mg 100 g−1 FW). The total concentration of this family ranged between 2.07 and 7.71 mg 100 g−1 FW. The main compound within this family was quercetin 3-O-rutinoside (Q3R), which represented three-quarters of the total concentration of this family.
Finally, bearing in mind the flavan-3-ols family, all of the new cultivars surpassed the concentration of the ‘Ambrunés’ parental cultivar (2.24 mg 100 g−1 FW), but none managed to reach even half the concentration of the ‘Hudson’ parent cultivar (40.09 mg 100 g−1 FW). The concentration of these compounds ranged between 2.24 and 40.09 mg 100 g−1 FW, with epicatechin (ECA) being the main one and representing half the total concentration of flavan-3-ols.
3.6. Hierarchical Cluster Analysis
The new sweet cherry cultivars were grouped based on their characteristics, so a hierarchical cluster analysis (HCA) was applied to the data obtained. This algorithm employed data from physicochemical analysis and the total phenolic and anthocyanin content, and hydrophilic and lipophilic antioxidant activities were employed. Also, L*, C*, and h values of the skin and flesh color and the total concentration of each polyphenolic family were used.
The results of HCA showed that the thirteen new and two parental cultivars were classified into three clusters (C1, C2, and C3). As shown in Figure 4, C1 contained the ‘Hudson’ parental cultivar, the most distinct cluster. C2 and C3 start from the same branch of the dendrogram, implying more similarities between them and more differences with C1. C3 contains the ‘Ambrunés’ parental cultivar, while C2 is only formed by new sweet cherry cultivars.
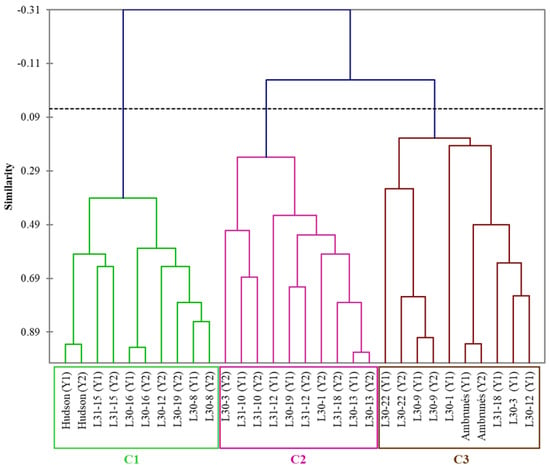
Figure 4.
Dendrogram obtained after the application of hierarchical cluster analysis. Y1 and Y2 represent year 1 and year 2; C1, C2, and C3 indicate clusters 1, 2, and 3, respectively.
Consequently, the new cultivars were divided into two groups: those that achieved the breeding program’s objective and improved their bioactive characteristics (C1), and those that did not manage to achieve this objective. Within this second group, there is a subgroup practically equal to the ‘Ambrunés’ parental cultivar (C3), and a subgroup with characteristics different from both parental cultivars (C2).
Within the group C1, it is important to note that although there are five new cultivars (apart from ‘Hudson’), only three of them are present in both years, so it would be these (L31-15, L30-16, and L30-8) that could be said to be similar to the ‘Hudson’ parental cultivar.
4. Discussion
Many studies have reported that cherries are an excellent source of natural antioxidants, but their concentration depends on the cultivar []. For this reason, this breeding program aimed to obtain new cultivars of ‘Picota’-type cherries, which would maintain the highly appreciated organoleptic traits of ‘Ambrunés’ and improve the bioactive profile, employing a cultivar characterized by large and dark-colored fruits (‘Hudson’).
The physicochemical characteristics of the new sweet cherry cultivars were hardly modified by the cross with the ‘Hudson’ parental cultivar, obtaining fruit with a weight, size, and firmness in order of the ‘Ambrunés’ parental cultivar. Regarding TSS and TA, these new fruits are slightly sweeter and sourer than the ‘Ambrunés’ parental cultivar. Sweetness and flavor intensity are the most valuable parameters for the flavor/texture appreciation of sweet cherries [,,] and are mainly linked to the maturation index []. These traits appear to be a key factor influencing preference and acceptability by consumers [,], so their improvement could translate into a better consumer acceptance of these new cultivars.
It can also be verified that the variations obtained in these parameters depended significantly according to the year and the cultivar, with the p-value being less than 0.001, except for the year in the attributes weight and firmness, where it was insignificant. Furthermore, the interaction of both parameters was highly significant (p < 0.001) in all cases. These results are by Gonçalves et al. [], who, in their study, analyzed how different cherry quality traits were affected by cultivar, rootstock, and the combination of both, concluding that the combination of the traits was a critical parameter to consider in cherry planting strategies.
On the other hand, the cross of ‘Ambrunés’ × ‘Hudson’ increased the total antioxidant activity of all new cultivars. This increase was mainly due to the contribution of H-TAA, which, in general, became two or three times greater than ‘Ambrunés’. For its part, the L-TAA was affected to a much lesser extent, although practically all new cultivars have doubled it at least. These results concord with the results of Tomás-Barberán et al. [], which showed that the major contributors to antioxidant activity were hydrophilic compounds, such as polyphenols and anthocyanins. Antioxidant vitamins, such as tocopherols and carotenoids, are lipophilic compounds that might contribute to L-TAA [].
Although these traits were hardly affected, the color of the new sweet cherry cultivars, as well as the concentration and profile of phenolic compounds, were the characteristics that varied the most in the different cultivars studied. Both traits are essential and are related between them, skin color being the most important indicator of the quality and maturity of fresh sweet cherries and one of the most valuable parameters for visual appreciation of sweet cherries. It depends on the anthocyanidin content [,], as the color becomes darker by increasing the amount []. Among the cyanidins found in sweet cherries, C3R is the dominant cyanidin—accounting for approximately 90% of the total cyanidin content [,,,] as observed in this study. However, the composition of the individual cyanidins depends on the cherry cultivar []. It is also important to note that cyanidins are involved in color and in other traits like fruit flavor, bitterness, or astringency [].
Considering this, sweet cherries that showed low values of L* (luminosity), h (tone angle), and C* (chroma) were dark cherries, with a high content of bioactive compounds and good acceptability by consumers.
Regarding the color of the new cultivars and considering the classification applied by the HCA, different patterns can be distinguished depending on the cluster to which the new cultivar belongs. In the case of C1 (which contains the ‘Hudson’ parental cultivar), redder and darker cherries than the ‘Ambrunés’ parental cultivar were obtained. The skin color of these cherries was more similar to ‘Hudson’s’, but not as dark. The flesh color was significantly modified, going from a yellowish tone to a reddish one. In the case of C3, which includes the ‘Ambrunés’ parental cultivar, skin color was a little more heterogeneous, but in general, they were more similar to ‘Ambrunés’ than to ‘Hudson’. Moreover, the flesh of these new cultivars was yellowish (as in ‘Ambrunés’), although with C* values more like ‘Hudson’. Finally, C2 cherries presented higher values of L*, C*, and h than ‘Ambrunés’ and ‘Hudson’ for skin and flesh color. This originated light red fruit with yellow flesh.
Related to those color changes, different bioactive compound profiles were found in the different fruit of each cluster. C1 sweet cherries, which are redder and darker than ‘Ambrunés’, showed a significant increase in the concentration of bioactive compounds, doubling and even tripling the phenolic and anthocyanin values, respectively. C3 fruit on the contrary, barely showed any color change compared to the ‘Ambrunés’ parental cultivar, and the concentration of bioactive compounds (total phenols and anthocyanins) was quite similar to that of the parental cultivar. Finally, C2 sweet cherries showed an average total phenol content slightly higher than that of the ‘Ambrunés’ parental cultivar, although some new cultivars did not even reach this value. However, anthocyanins greatly reduced their concentration, with some new cultivars not reaching a third of the concentration of ‘Ambrunés’.
Focusing on the determination by families, all new cultivars improved the hydroxycinnamic acid content of the ‘Ambrunés’ parental cultivar, some surpassing even the ‘Hudson’ parental cultivar. These compounds were exceptionally high in those cultivars which were more reddish and less dark. These compounds have been associated with the color, sensory, and nutritional qualities of fruit and are also related to general fruit maturation and prevention of enzymatic browning [,,]. Also, they are related to health benefits through their potent antioxidant action, such as the prevention of cardiovascular diseases, gout, obesity, and diabetes, chemopreventive properties, and the ability to inhibit low-density lipoprotein oxidation [,]. Therefore, it can be concluded that an enhancement in the bioactive profile of the new fruit has been achieved.
Concerning cyanidins, they are essential because they have presented health-promoting effects in various in vitro and in vivo studies and have been linked with preventing cancers and cardiovascular diseases through a direct and indirect antioxidant effect (via activation of different enzymatic systems). In addition, cyanidins have also presented anti-proliferative and apoptosis-inducing activity [,]. Furthermore, their presence is closely related to the visual quality of the fruits, as they contribute to the tissues’ red, blue, and purple coloring []. This group of compounds presented approximately three times the ‘Ambrunés’ concentration in C1 cherries, while C3 cherries sometimes even presented less than ‘Ambrunés’ itself. C2 cherries did not achieve any increase by the crossing with the ‘Hudson’ parental cultivar.
Flavonoids are a class of naturally occurring polyphenolic compounds in fruit, vegetables, nuts, and plant-derived beverages. Most of them present a three-ring structure, with two of them being aromatic and the third one heterocyclic. The variation in this third ring produces the division of flavonoids into various subclasses, such as flavonols and flavan-3-ols []. Flavonoids are absorbed in the large intestine, and the possibility that they may affect microflora and gut health by inhibiting cancer cell growth and promoting probiotic bacteria growth is being studied []. Flavonols present a potent antioxidant activity, and the quercetin’s capacity to act as a free radical scavenger suggests that it could be beneficial in reducing reactive oxygen species (ROS) associated with chronic diseases such as cardiovascular diseases or cancer [,]. Also, quercetin has presented other healthy effects in different in vitro studies, such as anti-inflammatory, anti-hypertensive, or anti-bacterial effects []. These new sweet cherry cultivars presented concentrations similar to the ‘Ambrunés’ parental cultivar in C1 fruits, while C2 and C3 cherries slightly lowered this value.
As regards the flavan-3-ols identified in the sweet cherry, these include catechin and epicatechin, the second being higher, and the procyanidins B1 and B2. The ratio between the two first compounds is characteristic of each cultivar []. C1 cherries again present a concentration similar to ‘Ambrunés’. However, the cherries from the other two clusters showed a slightly higher concentration of this family of compounds. In general, it could be noted that new cultivars with higher concentrations of hydroxycinnamic acids also showed the highest concentrations of flavan-3-ols.
Therefore, of the thirteen new sweet cherry cultivars, the ones that would be of the most interest for further study because they fulfilled the objective of improving the profile of bioactive compounds are those appearing in C1. However, it should be taken into account that data from two consecutive seasons have been analyzed so those cultivars that appeared in both seasons in this group are of more interest. Thus, the new sweet cherry cultivars with the highest bioactive potential would be L31-15, L30-16, and L30-8.
5. Conclusions
A breeding program was conducted with the ‘Ambrunés’ cultivar, the most important in terms of yield and organoleptic characteristics within the group of ‘Picota’ produced in the PDO ‘Cereza del Jerte’, through its crossing with the ‘Hudson’ cultivar, to increase the content of bioactive compounds in these cherries.
After the physicochemical and bioactive analysis of sweet cherries obtained from thirteen new sweet cherry cultivars for two seasons, a group of new cultivars has been found which, while maintaining the physicochemical characteristics of ‘Ambrunés’, have managed to increase the phenolic profile, bringing it closer to ‘Hudson’. Of these new potential cultivars, L31-15, L30-16, and L30-8 have enhanced this during both seasons. Therefore, these new cultivars have the potential to be further studied and could be used as parental cultivars in future cross-breeding.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture14111938/s1, Figure S1: Prototype chromatograms of one of the new cherry cultivars tested. Abbreviations: C3G: cyanidin 3-O-glucoside; C3R: cyanidin 3-O-rutinoside; P3G: peonidin 3-O-glucoside; P3R: peonidin 3-O-rutinoside; NCGA: neochlorogenic acid; CGA: chlorogenic acid; pCQA: p-coumarylquinic acid; Q3R: quercetin 3-O-rutinoside; Q3G: quercetin 3-O-glucoside; KMP: kaempferol; IHM: isorhamnetin; PB1: procyanidin B1; CA: catechin; PB2: procyanidin B2; ECA: epicatechin.
Author Contributions
Conceptualization, M.J.S.; formal analysis, M.P.-V.; investigation, M.P.-V.; resources, P.B.; writing—original draft preparation, M.P.-V.; writing—review and editing, M.L.-C., M.J.B., A.M. and M.J.S.; project administration, M.J.S.; funding acquisition, M.J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Regional Research Plan (Junta de Extremadura) and FEDER funds, projects IB13111 and ADAPFRUIT. M.P.V. is grateful for the obtention of a JDC2022-049532-I contract, funded by MICIU/AEI /10.13039/501100011033 and Unión Europea NextGenerationEU/PRTR.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Dataset available on request from the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Serradilla, M.J.; Lozano, M.; Bernalte, M.J.; Ayuso, M.C.; López-Corrales, M.; González-Gómez, D. Physicochemical and bioactive properties evolution during ripening of “Ambrunés” sweet cherry cultivar. LWT 2011, 44, 199–205. [Google Scholar] [CrossRef]
- Comisión Europea. Pliego De Condiciones DOP Cereza Del Jerte. 2014. Available online: https://cerezadeljerte.org/wp-content/uploads/2021/10/PUBLICACION-PLIEGO-DE-CONDICIONES-MODIFICADO-DOP-CEREZA-DEL-JERTE.pdf (accessed on 6 June 2023).
- Serradilla, M.J.; Martín, A.; Ruiz-Moyano, S.; Hernández, A.; López-Corrales, M.; Córdoba, M.D.G. Physicochemical and sensorial characterisation of four sweet cherry cultivars grown in Jerte Valley (Spain). Food Chem. 2012, 133, 1551–1559. [Google Scholar] [CrossRef]
- Rahaman, M.M.; Hossain, R.; Herrera-Bravo, J.; Islam, M.T.; Atolani, O.; Adeyemi, O.S.; Owolodun, O.A.; Kambizi, L.; Daştan, S.D.; Calina, D.; et al. Natural antioxidants from some fruits, seeds, foods, natural products, and associated health benefits: An update. Food Sci. Nutr. 2023, 11, 1657–1670. [Google Scholar] [CrossRef] [PubMed]
- Lara, I.; Camats, J.A.; Comabella, E.; Ortiz, A. Eating quality and health-promoting properties of two sweet cherry (Prunus avium L.) cultivars stored in passive modified atmosphere. Food Sci. Technol. Int. 2013, 21, 133–144. [Google Scholar] [CrossRef]
- Afonso, S.; Oliveira, I.; Ribeiro, C.; Vilela, A.; Meyer, A.S.; Gonçalves, B. Innovative edible coatings for postharvest storage of sweet cherries. Sci. Hortic. 2023, 310, 111738. [Google Scholar] [CrossRef]
- Faienza, M.F.; Corbo, F.; Carocci, A.; Catalano, A.; Clodoveo, M.L.; Grano, M.; Wang, D.Q.H.; D’Amato, G.; Muraglia, M.; Franchini, C.; et al. Novel insights in health-promoting properties of sweet cherries. J. Funct. Foods 2020, 69, 103945. [Google Scholar] [CrossRef]
- Chaovanalikit, A.; Wrolstad, R.E. Total anthocyanins and total phenolics of fresh and processed cherries and their antioxidant properties. J. Food Sci. 2004, 69, FCT67–FCT72. [Google Scholar] [CrossRef]
- Serra, A.T.; Duarte, R.O.; Bronze, M.R.; Duarte, C.M.M. Identification of bioactive response in traditional cherries from Portugal. Food Chem. 2011, 125, 318–325. [Google Scholar] [CrossRef]
- Calle, A.; Balas, F.; Cai, L.; Iezzoni, A.; López-Corrales, M.; Serradilla, M.J.; Wünsch, A. Fruit size and firmness QTL alleles of breeding interest identified in a sweet cherry ‘Ambrunés’ × ‘Sweetheart’ population. Mol. Breed. 2020, 40, 86. [Google Scholar] [CrossRef]
- López-Corrales, M.; Mateos, J.R.; Alarcón, M.V.; Bañuls, P.; Pérez, F.; Serradilla, M.J.; Manzano, M.A. Sweet cherry (Prunus avium L.) breeding program in southern Spain. Acta Hortic. 2014, 1020, 53–56. [Google Scholar] [CrossRef]
- Lima, V.L.A.G.; Mélo, E.A.; Maciel, M.I.S.; Prazeres, F.G.; Musser, R.S.; Lima, D.E.S. Total phenolic and carotenoid contents in acerola genotypes harvested at three ripening stages. Food Chem. 2005, 90, 565–568. [Google Scholar] [CrossRef]
- Giusti, M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV-Visible spectroscopy. Curr. Prot. Food Anal. Chem. 2001, 1, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Pereira, C.; López-Corrales, M.; Serradilla, M.J.; Villalobos, M.C.; Ruiz-Moyano, S.; Martín, A. Influence of ripening stage on bioactive compounds and antioxidant activity in nine fig (Ficus carica L.) varieties grown in Extremadura, Spain. J. Food Compos. Anal. 2017, 64, 203–212. [Google Scholar] [CrossRef]
- Manzano Durán, R.; Sánchez, J.E.F.; Velardo-Micharet, B.; Gómez, M.J.R. Multivariate optimization of ultrasound-assisted extraction for the determination of phenolic compounds in plums (Prunus salicina Lindl.) by high-performance liquid chromatography (HPLC). Instrum. Sci. Technol. 2019, 48, 113–127. [Google Scholar] [CrossRef]
- Serradilla, M.J.; Hernández, A.; López-Corrales, M.; Ruiz-Moyano, S.; de Guía Córdoba, M.; Martín, A. Composition of the cherry (Prunus avium L. and Prunus cerasus L.; Rosaceae). In Nutritional Composition of Fruit, Cultivars; Simmonds, M.S.J., Preedy, V.R., Eds.; Academic Press: Waltham, MA, USA, 2016; pp. 127–147. [Google Scholar] [CrossRef]
- Romano, G.S.; Cittadini, E.D.; Schouten, R. Sweet cherry quality in the horticultural production chain. Stewart Postharvest Rev. 2006, 2, 1–9. [Google Scholar] [CrossRef]
- López, L.; Larrigaudière, C.; Giné-Bordonaba, J.; Echeverria, G. Defining key parameters and predictive markers of ‘Early Bigi’ cherry consumer satisfaction by means of differential storage scenarios. Postharv Biol. Technol. 2023, 195, 112117. [Google Scholar] [CrossRef]
- Serradilla, M.J.; Akšic, M.F.; Manganaris, G.A.; Ercisli, S.; González-Gómez, D.; Valero, D. Fruit chemistry, nutritional benefits and social aspects of cherries. In Cherries: Botany, Production and Uses; Quero-García, J., Lezzoni, A., Pulawska, J., Lang, G., Eds.; CAB International: Oxfordshire, UK, 2017; pp. 420–441. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Crisosto, G.M.; Metheney, P. Consumer acceptance of “Brooks” and “Bing” cherries is mainly dependent on fruit SSC and visual skin color. Postharvest Biol. Technol. 2003, 28, 159–167. [Google Scholar] [CrossRef]
- Ballistreri, G.; Continella, A.; Gentile, A.; Amenta, M.; Fabroni, S.; Rapisarda, P. Fruit quality and bioactive compounds relevant to human health of sweet cherry (Prunus avium L.) cultivars grown in Italy. Food Chem. 2013, 140, 630–638. [Google Scholar] [CrossRef]
- Serrano, M.; Diaz-Mula, H.M.; Zapata, P.J.; Castillo, S.; Guillén, F.; Martínez-Romero, D.; Valverde, J.M.; Valero, D. Maturity stage at harvest determines the fruit quality and antioxidant potential after storage of sweet cherry cultivars. J. Agric. Food Chem. 2009, 57, 3240–3246. [Google Scholar] [CrossRef]
- Gonçalves, B.; Moutinho-Pereira, J.; Santos, A.; Silva, A.P.; Bacelar, E.; Correia, C.; Rosa, E. Scion-rootstock interaction affects the physiology and fruit quality of sweet cherry. Tree Physiol. 2006, 26, 93–104. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Ruiz, D.; Valero, D.; Rivera, D.; Obón, C.; Sánchez-Roca, C.; Gil, M.I. Health benefits from pomegranates and stone fruit, including plums, peaches, apricots and cherries. Bioact. Fruit 2013, 125–167. [Google Scholar] [CrossRef]
- Esti, M.; Cinquanta, L.; Sinesio, F.; Moneta, E.; Di Matteo, M. Physicochemical and sensory fruit characteristics of two sweet cherry cultivars after cool storage. Food Chem. 2002, 76, 399–405. [Google Scholar] [CrossRef]
- Cortés-Montaña, D.; Bernalte-García, M.J.; Palomino-Vasco, M.; Serradilla, M.J.; Velardo-Micharet, B. Effect of preharvest melatonin applications at dusk on quality and bioactive compounds content of early sweet cherries. J. Sci. Food Agric. 2024, 104, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Usenik, V.; Fabčič, J.; Štampar, F. Sugars, organic acids, phenolic composition and antioxidant activity of sweet cherry (Prunus avium L.). Food Chem. 2008, 107, 185–192. [Google Scholar] [CrossRef]
- Kim, D.O.; Ho, J.H.; Young, J.K.; Hyun, S.Y.; Lee, C.Y. Sweet and sour cherry phenolics and their protective effects on neuronal cells. J. Agric. Food Chem. 2005, 53, 9921–9927. [Google Scholar] [CrossRef]
- Kelebek, H.; Selli, S. Evaluation of chemical constituents and antioxidant activity of sweet cherry (Prunus avium L.) cultivars. Int. J. Food Sci. Technol. 2011, 46, 2530–2537. [Google Scholar] [CrossRef]
- Calle, A.; Serradilla, M.J.; Wünsch, A. QTL mapping of phenolic compounds and fruit color in sweet cherry using a 6+9K SNP array genetic map. Sci. Hortic. 2021, 280, 109900. [Google Scholar] [CrossRef]
- Chockchaisawasdee, S.; Golding, J.B.; Vuong, Q.V.; Papoutsis, K.; Stathopoulos, C.E. Sweet cherry: Composition, postharvest preservation, processing and trends for its future use. Trends Food Sci. Technol. 2016, 55, 72–83. [Google Scholar] [CrossRef]
- Hayaloglu, A.A.; Demir, N. Phenolic compounds, volatiles, and sensory characteristics of twelve sweet cherry (Prunus avium L.) cultivars grown in Turkey. J. Food Sci. 2016, 81, C7–C18. [Google Scholar] [CrossRef]
- Cortés-Montaña, D.; Bernalte-García, M.J.; Velardo-Micharet, B.; Serrano, M.; Serradilla, M.J. Impact of pre-storage melatonin application on the standard, sensory, and bioactive quality of early sweet cherry. Foods 2023, 12, 1723. [Google Scholar] [CrossRef]
- Del Rio, D.; Costa, L.G.; Lean, M.E.J.; Crozier, A. Polyphenols and health: What compounds are involved? Nutr. Metab. Cardiovasc. Dis. 2010, 20, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Barberán, F.A.; Andrés-Lacueva, C. Polyphenols and health: Current state and progress. J. Agric. Food Chem. 2012, 60, 8773–8775. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Giusti, M. Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Stoner, G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008, 269, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Haenen, G.R.M.M.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef]
- Wilms, L.C.; Hollman, P.C.H.; Boots, A.W.; Kleinjans, J.C.S. Protection by quercetin and quercetin-rich fruit juice against induction of oxidative DNA damage and formation of BPDE-DNA adducts in human lymphocytes. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2005, 582, 155–162. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).