Increasing Productivity and Fruit Quality of ‘Mutsu’ Apple Orchard by Dwarfing Treatments
Abstract
1. Introduction
2. Materials and Methods
- Control (no growth-limiting treatments)—C;
- Unilateral root pruning with an oblique knife—RP;
- Regalis Plus 10 WG applied from the balloon stage (BBCH 60–69)—RB;
- Regalis Plus 10 WG applied at standard times, according to the label (BBCH 71–73)—RS;
- Flordimex 480 SL applied 4 times, from the end of flowering, at 2-week intervals, at doses of 250, 200, 150, and 100 mL·ha−1—Ethephon;
- Unilateral root pruning with an angled knife + Regalis Plus 10 WG applied from the balloon stage (BBCH 60–69)—RP + RB;
- One-sided root pruning with an angled knife + Regalis Plus 10 WG applied at standard dates, according to the label (BBCH 71–73)—RP + RS;
- Unilateral root pruning with an angled knife + spraying the trees 4 times with Flordimex 480 SL from the end of flowering, at 2-week intervals, at doses of 250, 200, 150, and 100 mL·ha−1—RP + Ethephon.
2.1. Growth-Restricting Treatments
2.2. Fruit Storage Conditions
2.3. Indicators Determined in the Orchard
2.4. Physiological Status of Fruit at Harvest Directly After Storage and After 7 Days of Shelf-Life
2.5. Statistical Analysis
3. Results
3.1. Indicators Determined in the Orchard
3.2. Physiological State of Apples Directly After Harvest
3.3. Quality of Apples Directly After Storage and After Shelf-Life
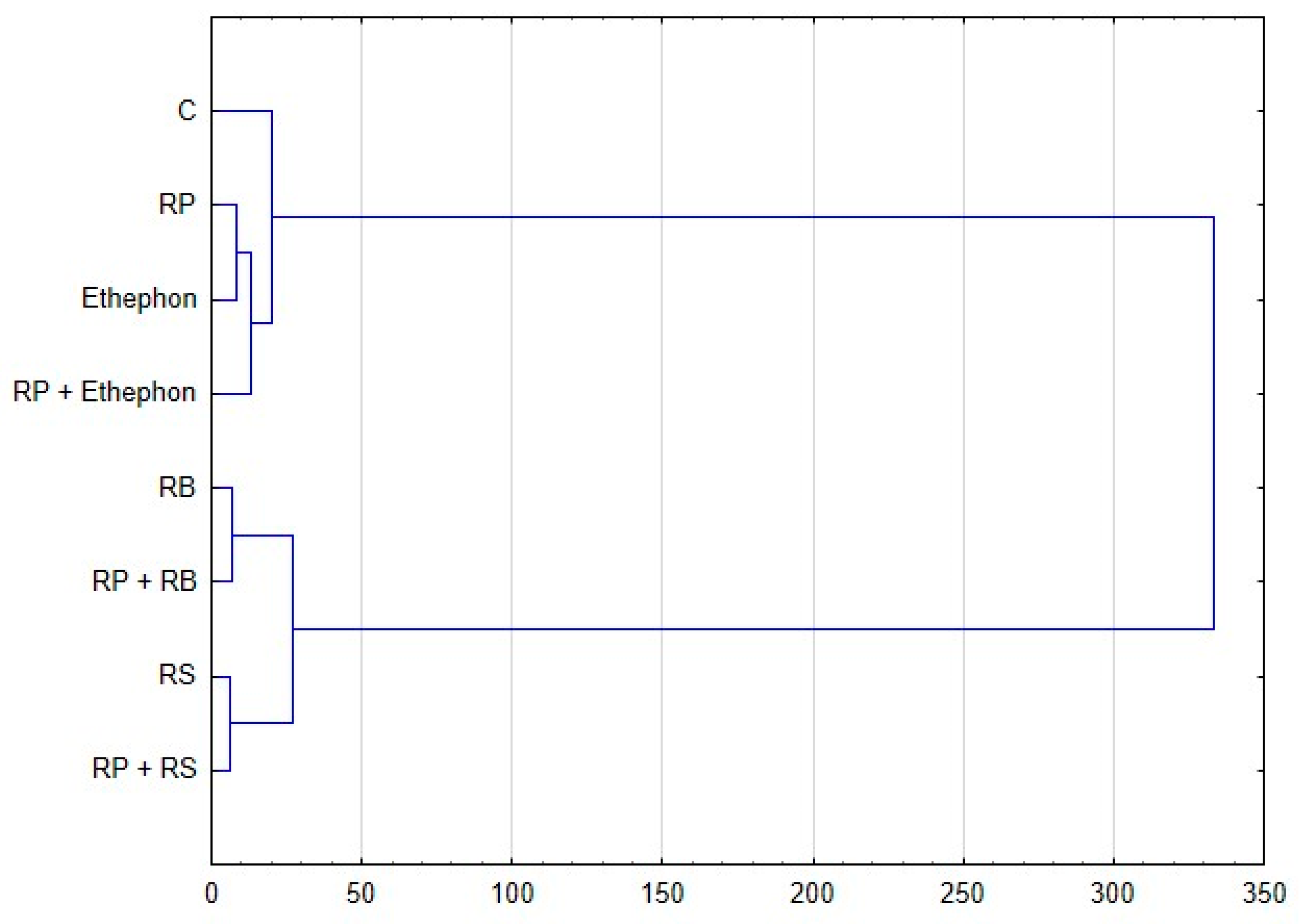
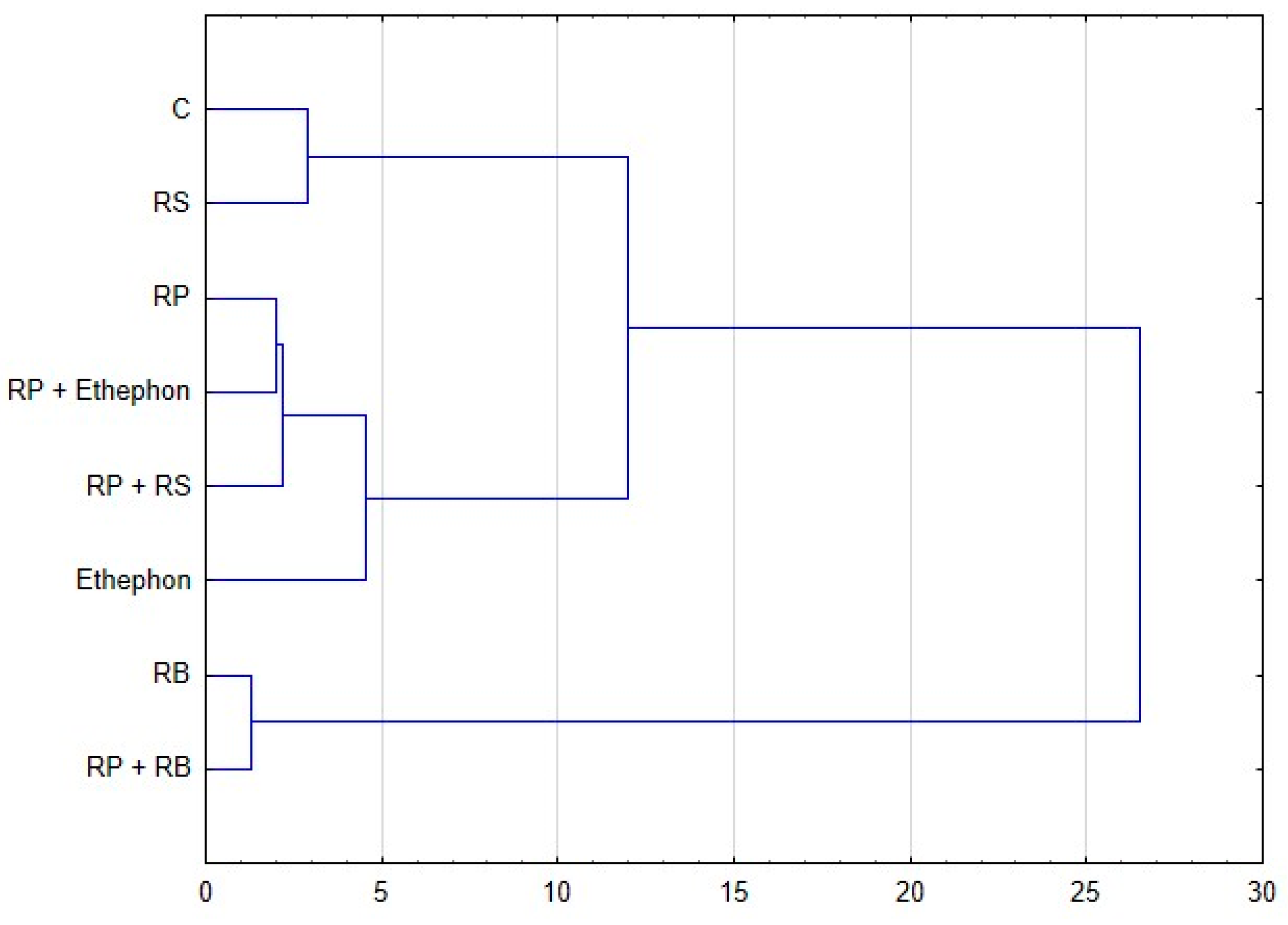
3.4. Values of Correlation Coefficients Between Selected Parameters
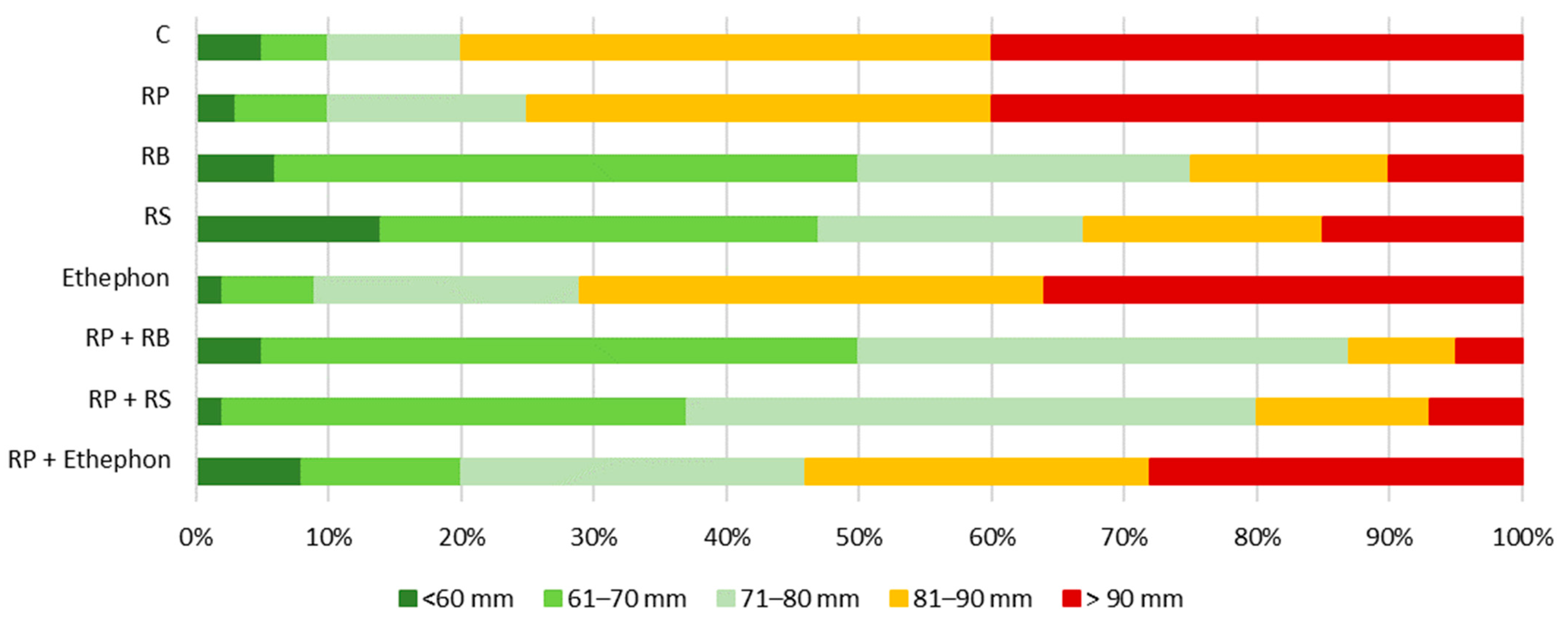
3.5. Size Structure of Apple Yield
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Daugaard, H.; Callesen, O. The effect of rootstock on yield and quality of ‘Mutsu’apples. J. Hortic. Sci. Biotechnol. 2002, 77, 248–251. [Google Scholar] [CrossRef]
- Peşteanu, A. Influence of Regalis Plus product on the growth and fruiting of apple trees. Ann. Univ. Craiova 2022, XXVII, 291–296. [Google Scholar] [CrossRef]
- Łysiak, G.P.; Kurlus, R.; Michalska, A. Increasing the frost resistance of ‘Golden Delicious’, ‘Gala’ and ‘Šampion’ apple cultivars. Folia Hortic. 2016, 28, 25–135. [Google Scholar] [CrossRef][Green Version]
- Bakos, J.L.; Ladányi, M.; Szalay, L. Frost hardiness of flower buds of 16 apricot cultivars during dormancy. Folia Hortic. 2024, 36, 81–93. [Google Scholar] [CrossRef]
- Kurlus, R.; Świerczyński, S.; Rutkowski, K.; Ratajkiewicz, H.; Malinowska, A.; Wyrwał, A. Exogenus ‘Ga3’ and ‘Ga4+7’ effects on phenological indices, frost hardiness and quality properties of ‘English morello’ sour cherry (Prunus cerasus L.). Acta Sci. Polonorum. Hortorum Cultus 2017, 16, 99–109. [Google Scholar] [CrossRef]
- The Apple Market in the EU: Vol. 1: Production, Areas and Yields. 2022. Available online: https://agriculture.ec.europa.eu/system/files/2022-10/apples-production_en.pdf (accessed on 27 July 2024).
- Kacprzak, T.; Himstedt, K. The Influence of the Russian Embargo on the Economic Situation of Apple Producers in the Eastern Part of the Masovia Province. Zesz. Nauk. SGGW Warsz. Probl. Roln. Świat. 2019, 19, 54–64. (In Polish) [Google Scholar] [CrossRef]
- Elias, T.S. The Complete Trees of North America. Field Guide and Natural History; Van Nostrand Reinhold Company & Times Mirror Magazines. Inc.: New York, NY, USA, 1980. [Google Scholar]
- Schupp, J.; Ferree, D. Influence of time of root pruning on growth, mineral nutrition, net photosynthesis and transpiration of young apple trees. Sci. Hortic. 1990, 42, 299–306. [Google Scholar] [CrossRef]
- Maggs, D. Growth-rates in relation to assimilate supply and demand: I. Leaves and roots as limiting regions. J. Exp. Bot. 1964, 15, 574–583. [Google Scholar] [CrossRef]
- Geisler, D.; Ferree, D. The influence of root pruning on water relations, net photosynthesis, and growth of young ‘Golden Delicious’ apple trees. J. Am. Soc. Hortic. Sci. 1984, 109, 827–831. [Google Scholar] [CrossRef]
- Fang, Y.; Xu, B.; Turner, N.; Li, F. Grain yield, dry matter accumulation and remobilization, and root respiration in winter wheat as affected by seeding rate and root pruning. Eur. J. Agron. 2010, 33, 257–266. [Google Scholar] [CrossRef]
- Ma, S.; Xu, B.; Li, F.; Liu, W.; Huang, Z. Effects of root pruning on competitive ability and water use efficiency in winter wheat. Field Crops Res. 2010, 105, 56–63. [Google Scholar] [CrossRef]
- Cline, J.A.; Embree, C.G.; Hebb, J.; Nichols, D.S. Performance of prohexadione-calcium on shoot growth and fruit quality of apple—Effect of spray surfactants. Can. J. Plant Sci. 2008, 88, 165–174. [Google Scholar] [CrossRef]
- Miller, S.S. Prohexadione-calcium controls vegetative shoot growth in apple. J. Tree Fruit Prod. 2002, 3, 11–28. [Google Scholar] [CrossRef]
- Milyaev, A.; Kofler, J.; Moya, Y.; Lempe, J.; Stefanelli, D.; Hanke, M.; Flachowsky, H.; Wirén, N.; Wünsche, J. Profiling of phytohormones in apple fruit and buds regarding their role as potential regulators of flower bud formation. Tree Physiol. 2022, 42, 2319–2335. [Google Scholar] [CrossRef] [PubMed]
- Beauvieux, R.; Wenden, B.; Dirlewanger, E. Bud Dormancy in Perennial Fruit Tree Species: A Pivotal Role for Oxidative Cues. Front. Plant Sci. 2018, 9, 657. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Kim, J.; Hamayun, M.; Hwang, I.; Khan, A.; Kim, Y.; Lee, J.; Lee, I. Influence of prohexadione-calcium on growth and gibberellins content of Chinese cabbage grown in Alpine region of South Korea. Sci. Hortic. 2010, 125, 88–92. [Google Scholar] [CrossRef]
- Kim, Y.; Khan, A.; Hamayun, M.; Kim, J.; Lee, J.; Hwang, I.; Yoon, C.; Lee, I. Effects of Prohexadione Calcium on growth and gibberellins contents of Chrysanthemum morifolium R. cv Monalisa White. Sci. Hortic. 2010, 123, 423–427. [Google Scholar] [CrossRef]
- Amarante, C.; Silveira, J.; Steffens, C.; Freitas, S.; Mitcham, E.; Miqueloto, A. Post-bloom and preharvest treatment of ‘Braeburn’ apple trees with prohexadione-calcium and GA4+7 affects vegetative growth and postharvest incidence of calcium-related physiological disorders and decay in the fruit. Sci. Hortic. 2020, 261, 108919. [Google Scholar] [CrossRef]
- Thorp, T.; Aspinall, D.; Sedgley, M. Influence of shoot age on floral development and early fruit set in avocado (Persea americana Mill.) cv. Hass. J. Hortic. Sci. 1993, 68, 645–651. [Google Scholar] [CrossRef]
- Samach, A.; Smith, H. Constraints to obtaining consistent annual yields in perennials. II: Environment and fruit load affect induction of flowering. Plant Sci. 2013, 207, 168–176. [Google Scholar] [CrossRef]
- Martín-Fontecha, E.; Tarancón, C.; Cubas, P. To grow or not to grow, a power-saving program induced in dormant buds. Curr. Opin. Plant Biol. 2018, 41, 102–109. [Google Scholar] [CrossRef]
- Jain, R.; Rathod, S.; Banjare, M.; Nidhi, R.; Sood, N.; Sharma, A. Physiological Aspects of Flowering, Fruit Setting, Fruit Development and Fruit Drop, Regulation and their Manipulation: A Review. Int. J. Environ. Clim. Change 2023, 13, 205–224. [Google Scholar] [CrossRef]
- Ramírez, H.; Alonso, S.; Benavides, A.; Antonio, A. Prohexadione-Ca Modifies Growth and Endogenous Hormones in the Shoot Apex in Apple Trees. Acta Hortic. 2006, 727, 117–124. [Google Scholar] [CrossRef]
- Ramírez, H.; Sanchez-Canseco, J.; Zamora-Villa, V.; Rancaño-Arrioja, J. Effect of Prohexadione calcium, 6-benzyl amino purine and 6-furfuryladenine on vegetative growth and fruit quality in apple. Phyton 2017, 86, 282–289. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, D.; An, N.; Fan, S.; Zuo, X.; Zhang, X.; Zhang, L.; Gao, C.; Han, M.; Xing, L. Transcriptomic analysis reveals the regulatory module of apple (Malus × domestica) floral transition in response to 6-BA. BMC Plant Biol. 2019, 19, 93 . [Google Scholar] [CrossRef]
- Schwarz, I.; Scheirlinck, M.; Otto, E.; Bartrina, I.; Schmidt, R.; Schmülling, T. Cytokinin regulates the activity of the inflorescence meristem and components of seed yield in oilseed rape. J. Exp. Bot. 2020, 71, 7146–7159. [Google Scholar] [CrossRef]
- Vaughan-Hirsch, J.; Li, D.; Martinez, A.; Roden, S.; Pattyn, J.; Taira, S.; Shikano, H.; Miyama, Y.; Okano, Y.; Voet, A.; et al. A 1-aminocyclopropane-1-carboxylic-acid (ACC) dipeptide elicits ethylene responses through ACC-oxidase mediated substrate promiscuity. Front. Plant Sci. 2022, 13, 995073. [Google Scholar] [CrossRef]
- Houben, M.; Poel, B. 1-Aminocyclopropane-1-Carboxylic Acid Oxidase (ACO): The Enzyme That Makes the Plant Hormone Ethylene. Front. Plant Sci. 2019, 10, 695. [Google Scholar] [CrossRef]
- Gamalero, E.; Glick, B. Bacterial Modulation of Plant Ethylene Levels. Plant Physiol. 2015, 169, 13–22. [Google Scholar] [CrossRef]
- Rademacher, W. Plant growth regulators: Backgrounds and uses in plant production. J. Plant Growth Regul. 2015, 34, 845–872. [Google Scholar] [CrossRef]
- Sosna, I.; Spiak, Z.; Sosna, I.; Gudarowska, E. Effect of rootstock on leaf nutrient concentration and productive value of ‘Mutsu’ apple trees. J. Elem. 2020, 25, 1581–1593. [Google Scholar] [CrossRef]
- Jackson, J.E.; Palmer, J.W. Light distribution within hedgerow orchards. J. Appl. Ecol. 1972, 9, 341–357. [Google Scholar] [CrossRef]
- Swarbrick, T. The Seasonal elongation growth of apple varieties on some vegetative rootstocks and its possible relation to fruit bud formation. J. Pomol. Hortic. Sci. 1928, 7, 100–129. [Google Scholar] [CrossRef]
- Hoying, S.A.; Robinson, T.L. Effects of chain saw girdling and root pruning of apple trees. Acta Hortic. 1992, 322, 167–172. [Google Scholar] [CrossRef]
- Marandici, S. Increasing productivity of apple orchard by using the growth regulator based on prohexadion-calcium. An. Univ. Craiova Ser. Biol. Hortic. Tehnol. Preluc. Prod. Agric. Ing. Med. 2016, 21, 141–144. [Google Scholar]
- Medjdoub, R.; Val, J.; Blanco, A. Prohexadione–Ca inhibits vegetative growth of ‘Smoothee Golden Delicious’ apple trees. Sci. Hortic. 2004, 101, 243–253. [Google Scholar] [CrossRef]
- Skic, A.; Szymańska-Chargot, M.; Kruk, B.; Chylińska, M.; Pieczywek, P.M.; Kurenda, A.; Zdunek, A.; Rutkowski, K.P. Determination of the Optimum Harvest Window for Apples Using the Non-Destructive Biospeckle Method. Sensors 2016, 16, 661. [Google Scholar] [CrossRef]
- Tomala, K. Orchard Factors Affecting Fruit Storage Quality And Prediction Of Harvest Date Of Apples. Acta Hortic. 1999, 485, 373–382. [Google Scholar] [CrossRef]
- Łysiak, G. The determination of harvest index of ‘Sampion’ apples intended for long storage. Acta Sci. Pol. Hortorum Cultus 2011, 10, 273–282. [Google Scholar]
- Bulens, I.; Van de Poel, B.; Hertog, M.P.; De Proft, M.P.; Geeraerd, A.H.; Nicolai, B.M. Influence of harvest time and 1-MCP application on postharvest ripening and ethylene biosynthesis of ‘Jonagold’ apple. Postharvest Biol. Technol. 2012, 72, 11–19. [Google Scholar] [CrossRef]
- Lafer, G. Optimaler Erntetermin—Optimale Ergebnisse. Besseres Obst 1998, 43, 15–20. [Google Scholar]
- Małachowska, M.; Tomala, K. Apple Quality during Shelf-Life after Long-Term Storage and Simulated Transport. Agriculture 2023, 13, 2045. [Google Scholar] [CrossRef]
- Tomala, K.; Grzęda, M.; Guzek, D.; Głąbska, D.; Gutkowska, K. Analysis of Possibility to Apply Preharvest 1-Methylcyclopropene (1-MCP) Treatment to Delay Harvesting of Red Jonaprince Apples. Sustainability 2020, 12, 4575. [Google Scholar] [CrossRef]
- Tomala, K.; Guzek, D.; Głąbska, D.; Małachowska, M.; Widłak, Ł.; Krupa, T.; Gutkowska, K. Assessment of the quality of ‘Red Jonaprince’ apples during storage after delayed harvesting and 1-methylcyclopropene (1-MCP) preharvest and postharvest treatment. Agronomy 2023, 13, 1730. [Google Scholar] [CrossRef]
- Vermeulen, P. Crown depth as a result of evolutionary games: Decreasing solar angle should lead to shallower, not deeper crowns. New Phytol. 2014, 202, 1249–1256. [Google Scholar] [CrossRef]
- Dayatilake, G.A.; Wunschke, J.N.; Wood, P.; McArtney, S.; Manktelow, D.; Gurnsey, S.; Tustin, D.S. The use of prohexadione-ca for improved crop management. Acta Hortic. 2005, 694, 315–319. [Google Scholar] [CrossRef]
- Jackson, J.; Palmer, J. Effects of Shade on the Growth and Cropping of Apple Trees. II. Effects on Components of Yield. J. Hortic. Sci. 1977, 52, 253–266. [Google Scholar] [CrossRef]
- Pitchers, B.; Do, F.; Pradal, C.; Dufour, L.; Lauri, P. Apple tree adaptation to shade in agroforestry: An architectural approach. Am. J. Bot. 2021, 108, 732–743. [Google Scholar] [CrossRef]
- Solomakhin, A.; Blanke, M. Coloured hailnets alter light transmission, spectra and phytochrome, as well as vegetative growth, leaf chlorophyll and photosynthesis and reduce flower induction of apple. Plant Growth Regul. 2008, 56, 211–218. [Google Scholar] [CrossRef]
- Łysiak, G. The base colour of fruit as an indicator of optimum harvest date for two apple cultivars (Malus domestica Borkh.). Folia Hort. 2012, 24, 81–89. [Google Scholar] [CrossRef]
- Ferree, D.C.; Knee, M. Influence of root pruning and rootstocks on growth and performance of ‘Golden Delicious’ apple. HortScience 1997, 32, 645–648. [Google Scholar] [CrossRef]
- Wajja-Musukwe, T.; Wilson, J.; Sprent, J.; Ong, C.; Deans, J.; Okorio, J. Tree growth and management in Ugandan agroforestry systems: Effects of root pruning on tree growth and crop yield. Tree Physiol. 2008, 28, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Schupp, J.; Ferree, D. Effect of Root Pruning at Different Growth Stages on Growth and Fruiting of Apple Trees. HortScience 1987, 22, 387–390. [Google Scholar] [CrossRef]
- Schupp, J.R. Root pruning of apple trees. Fruit Notes 1990, 55, 2–3. [Google Scholar]
- Kim, H.; Lee, I.; Hamayun, M.; Kim, J.; Won, J.; Hwang, I.; Kim, K. Effect of Prohexadione Calcium on Growth Components and Endogenous Gibberellins Contents of Rice (Oryza sativa L.). J. Agron. Crop Sci. 2007, 193, 445–451. [Google Scholar] [CrossRef]
- Grossmann, K.; Konig-Kranz, S.; Kwiatkowski, J. Phytohormonal changes in intact shoots of wheat and oilseed rape treated with the acylcyclohexanedione growth retardant prohexadione calcium. Physiol. Plant. 1994, 90, 139–143. [Google Scholar] [CrossRef]
- Greene, D.W. The effect of prohexadione-calcium on fruit set and chemical thinning of apple trees. HortScience 2007, 42, 1361–1365. [Google Scholar] [CrossRef]
- Zadravec, P.; Čmelik, Z.; Tojnko, S.; Unuk, T.; Schlauer, B. Vegetative Growth, Yield and Fruit Quality of ‘Gala’ Apple Treated with Regalis (Prohexadione-Ca). Acta Hortic. 2008, 774, 287–290. [Google Scholar] [CrossRef]
- Musacchi, S.; Sheick, R.; Mia, M.J.; Serra, S. Studies on physiological and productive effects of multi-leader training systems and Prohexadione-Ca applications on apple cultivar ‘WA 38’. Sci. Hortic. 2023, 312, 111850. [Google Scholar] [CrossRef]
- Carrà, B.; Fachinello, J.; Abreu, E.; Pasa, M.; Spagnol, D.; Giovanaz, M.; Silva, C. Control of the vegetative growth of ‘Shinseiki’ pear trees by prohexadione calcium and root pruning. Pesq. Agropec. Bras. 2017, 52, 177–185. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, X.; Liang, H.; Ji, Y.; Liu, M. Effects of Comparative Metabolism on Tomato Fruit Quality under Different Levels of Root Restriction. HortScience 2023, 58, 885–892. [Google Scholar] [CrossRef]
- Shehaj, M.; Hodaj, B.; Rama, P.; Lepaja, K. Effect of prohexadione-calcium (Regalis) on shoot growth and fruit set in pear var. ‘Passe Crassane’. J. Int. Sci. Publ. Agric. Food 2014, 2, 298–304. [Google Scholar]
- Smith, S.; Johnson, D.; Mori, J. The effect of the growth regulator Ethephon on the quality of discovery apples during simulated marketing. J. Hortic. Sci. 1985, 60, 305–310. [Google Scholar] [CrossRef]
- McArtney, S.; Unrath, D.; Obermiller, J.; Green, A. Naphthaleneacetic Acid, Ethephon, and Gibberellin A4 + A7 Have Variable Effects on Flesh Firmness and Return Bloom of Apple. Horttechnology 2007, 17, 32–38. [Google Scholar] [CrossRef]
- Whale, S.; Singh, Z.; Behboudian, M.; Janes, J.; Dhaliwal, S. Fruit quality in ‘Cripp’s Pink’ apple, especially colour, as affected by preharvest sprays of aminoethoxyvinylglycine and Ethephon. Sci. Hortic. 2008, 115, 342–351. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, Z.; Jiang, Y.; Zhang, X.; Li, X.; Li, F. Effects of preharvest regulation of ethylene on carbohydrate metabolism of apple (Malus domestica Borkh cv. Starkrimson) fruit at harvest and during storage. Sci. Hortic. 2021, 276, 109748. [Google Scholar] [CrossRef]
- Jędrszczyk, E.; Skowera, B.; Kędzior, R.; Gawęda, M. The influence of Ethephon application to processing tomato plants on yield structure in relation to weather conditions during the growing period. Folia Hortic. 2017, 29, 75–81. [Google Scholar] [CrossRef][Green Version]
- Zhang, Z. Prohexadione calcium regulates wheat tolerance to drought stress by maintaining water balance and promoting antioxidant metabolism and photosynthesis. Plant Soil Environ. 2024, 70, 673–681. [Google Scholar] [CrossRef]
- Jones, K.M.; Koen, T.B.; Oakford, M.J. Thinning ‘Red Fuji’ apples using Ethephon at two timings. J. Hortic. Sci. 2015, 65, 381–384. [Google Scholar] [CrossRef]
- Koen, T.B.; Jones, K.M.; Longley, S.B. Spray thinning strategies for ‘Red Delicious’ apple using naphthalene acetic acid and Ethephon. J. Hortic. Sci. 1988, 63, 31–35. [Google Scholar] [CrossRef]

| Treatment | Number of Fruitlets per 100 Inflorescences | Shoot Length [cm] | Yield [kg·tree−1] | Fruit Weight [g] | |
|---|---|---|---|---|---|
| Top of the Tree | Bottom of the Tree | ||||
| C | 47.8 b 1 | 69.9 h | 56.3 g | 18.3 a | 393 b |
| RP | 44.0 ab | 59.7 g | 52.6 f | 21.5 b | 367 b |
| RB | 126.3 e | 18.0 b | 15.0 a | 37.9 h | 296 a |
| RS | 118.1 cd | 26.1 d | 23.9 c | 32.1 f | 286 a |
| Ethephon | 44.8 ab | 58.1 f | 49.0 e | 25.5 d | 378 b |
| RP + RB | 120.5 d | 15.6 a | 14.6 a | 34.0 g | 284 a |
| RP + RS | 115.0 c | 22.7 c | 19.9 b | 27.9 e | 284 a |
| RP + Ethephon | 42.4 a | 53.2 e | 42.7 d | 23.3 c | 365 b |
| Treatment | IEC [µL·L−1] | SI [-] | Background Color | FF [N] | SSC [°Bx] | TA [%] | ||
|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | ||||||
| C | 0.081 a 1 | 6.6 a | 66.3 ab | −15.1 a | 35.1 a | 76.9 a | 11.5 a | 0.544 b |
| RP | 0.041 a | 8.5 c | 68.6 b | −15.5 a | 36.2 a | 77.9 ab | 12.3 b | 0.504 ab |
| RB | 0.067 a | 7.6 b | 65.4 ab | −15.1 a | 35.2 a | 87.4 c | 13.0 c | 0.479 a |
| RS | 0.054 a | 7.4 b | 67.4 b | −15.2 a | 35.3 a | 81.8 b | 12.9 c | 0.494 a |
| Ethephon | 0.049 a | 9.4 d | 66.9 ab | −15.3 a | 34.7 a | 82.3 b | 12.8 bc | 0.475 a |
| RP + RB | 0.036 a | 7.4 b | 65.2 a | −15.0 a | 34.8 a | 88.7 c | 12.9 c | 0.487 a |
| RP + RS | 0.064 a | 7.3 b | 68.3 b | −15.2 a | 35.4 a | 81.7 b | 13.2 c | 0.491 a |
| RP + Ethephon | 0.058 a | 7.4 b | 68.2 b | −15.5 a | 35.4 a | 88.7 c | 13.1 c | 0.488 a |
| Treatments | Background Color | FF [N] | SSC [°Bx] | TA [%] | ||
|---|---|---|---|---|---|---|
| L* | a* | b* | ||||
| Directly after storage | ||||||
| C | 66.6 a 1 | −13.3 a | 35.7 a | 72.8 b | 14.8 ab | 0.403 a |
| RP | 69.3 a | −13.6 a | 36.4 a | 66.4 a | 14.9 ab | 0.417 ab |
| RB | 64.4 a | −13.8 a | 35.6 a | 69.1 ab | 14.3 a | 0.445 b |
| RS | 66.1 a | −13.1 a | 34.6 a | 66.1 a | 14.6 ab | 0.406 a |
| Ethephon | 65.5 a | −13.0 a | 34.4 a | 67.4 a | 14.5 ab | 0.415 ab |
| RP + RB | 66.2 a | −14.3 a | 36.8 a | 75.1 b | 14.4 a | 0.443 b |
| RP + RS | 67.0 a | −13.3 a | 35.3 a | 68.6 ab | 14.7 ab | 0.425 ab |
| RP + Ethephon | 66.1 a | −13.4 a | 37.4 a | 66.8 a | 15.4 b | 0.398 a |
| After 7 days of shelf life | ||||||
| C | 71.9 ab | −10.6 a | 41.3 b | 56.3 b | 15.0 a | 0.375 a |
| RP | 72.9 b | −10.7 a | 41.3 b | 47.8 a | 14.5 a | 0.366 a |
| RB | 71.5 ab | −11.0 a | 41.2 b | 61.7 c | 14.8 a | 0.357 a |
| RS | 72.2 ab | −10.6 a | 41.3 b | 53.4 b | 15.0 a | 0.375 a |
| Ethephon | 69.3 a | −10.1 a | 39.1 a | 49.5 a | 14.7 a | 0.379 a |
| RP + RB | 70.4 ab | −10.8 a | 40.7 ab | 62.0 c | 15.0 a | 0.373 a |
| RP + RS | 72.4 ab | −10.6 a | 41.4 b | 54.8 b | 15.1 a | 0.376 a |
| RP + Ethephon | 71.4 ab | −10.4 a | 40.2 ab | 48.4 a | 14.9 a | 0.374 a |
| A | B | C | D | E | F | G | H | I | J | K | L | M | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 1.00 | ||||||||||||
| B | −0.96 ** | 1.00 | |||||||||||
| C | −0.99 ** | 0.98 ** | 1.00 | ||||||||||
| D | 0.88 ** | −0.92 ** | −0.89 ** | 1.00 | |||||||||
| E | −0.96 ** | 0.98 ** | 0.95 ** | −0.87 ** | 1.00 | ||||||||
| F | −0.05 | 0.13 | 0.07 | −0.11 | 0.14 | 1.00 | |||||||
| G | −0.35 * | 0.24 | 0.35 * | −0.04 | 0.17 | −0.37 * | 1.00 | ||||||
| H | −0.36 * | 0.31 | 0.37 * | −0.46 * | 0.22 | −0.01 | 0.18 | 1.00 | |||||
| I | 0.28 | −0.23 | −0.26 | 0.23 | −0.20 | 0.07 | −0.17 | −0.57 ** | 1.00 | ||||
| J | −0.15 | 0.12 | 0.11 | −0.18 | 0.12 | −0.06 | 0.03 | 0.60 ** | −0.62 ** | 1.00 | |||
| K | 0.38 * | −0.55 ** | −0.46 * | 0.61 ** | −0.43 * | −0.09 | −0.08 | −0.40 * | 0.25 | −0.36 * | 1.00 | ||
| L | 0.50 ** | −0.67 ** | −0.53 ** | 0.63 ** | −0.68 ** | −0.11 | 0.18 | 0.13 | 0.05 | −0.01 | 0.60 ** | 1.00 | |
| M | −0.29 | 0.44 * | 0.31 | −0.50 ** | 0.43 * | 0.34 * | −0.39 * | 0.02 | 0.13 | 0.05 | −0.49 ** | −0.58 ** | 1.00 |
| A | B | C | D | E | F | G | H | I | J | K | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| F | −0.25 | 0.24 | 0.21 | −0.35 * | 0.20 | 1.00 | |||||
| G | −0.24 | 0.27 | 0.29 | −0.25 | 0.20 | −0.15 | 1.00 | ||||
| H | −0.11 | 0.03 | 0.02 | −0.07 | 0.11 | 0.46 * | −0.67 ** | 1.00 | |||
| I | −0.08 | 0.09 | 0.05 | 0.03 | 0.15 | −0.01 | −0.09 | 0.14 | 1.00 | ||
| J | −0.44 * | 0.36 * | 0.40 * | −0.46 * | 0.38 * | 0.36 * | −0.12 | 0.61 ** | −0.02 | 1.00 | |
| K | 0.53 ** | −0.56 ** | −0.58 ** | 0.58 ** | −0.50 ** | −0.13 | −0.31 | −0.07 | 0.10 | −0.59 ** | 1.00 |
| A | B | C | D | E | F | G | H | I | J | K | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| F | 0.05 | 0.01 | −0.06 | −0.14 | −0.01 | 1.00 | |||||
| G | −0.26 | 0.26 | 0.31 | −0.23 | 0.21 | −0.09 | 1.00 | ||||
| H | 0.33 * | −0.21 | −0.31 | 0.09 | −0.21 | 0.56 ** | −0.22 | 1.00 | |||
| I | 0.61 ** | −0.54 ** | −0.63 ** | 0.63 ** | −0.42 * | −0.17 | −0.32 | 0.11 | 1.00 | ||
| J | 0.22 | −0.19 | −0.21 | 0.05 | −0.18 | −0.01 | −0.06 | −0.13 | 0.19 | 1.00 | |
| K | −0.09 | 0.08 | 0.09 | −0.10 | 0.05 | 0.06 | −0.08 | −0.10 | −0.16 | 0.07 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Małachowska, M.; Majak, T.; Krupa, T.; Tomala, K. Increasing Productivity and Fruit Quality of ‘Mutsu’ Apple Orchard by Dwarfing Treatments. Agriculture 2024, 14, 1838. https://doi.org/10.3390/agriculture14101838
Małachowska M, Majak T, Krupa T, Tomala K. Increasing Productivity and Fruit Quality of ‘Mutsu’ Apple Orchard by Dwarfing Treatments. Agriculture. 2024; 14(10):1838. https://doi.org/10.3390/agriculture14101838
Chicago/Turabian StyleMałachowska, Maria, Tomasz Majak, Tomasz Krupa, and Kazimierz Tomala. 2024. "Increasing Productivity and Fruit Quality of ‘Mutsu’ Apple Orchard by Dwarfing Treatments" Agriculture 14, no. 10: 1838. https://doi.org/10.3390/agriculture14101838
APA StyleMałachowska, M., Majak, T., Krupa, T., & Tomala, K. (2024). Increasing Productivity and Fruit Quality of ‘Mutsu’ Apple Orchard by Dwarfing Treatments. Agriculture, 14(10), 1838. https://doi.org/10.3390/agriculture14101838







