1. Introduction
Bovine respiratory disease (BRD) continues to be one of the most challenging issues for the cattle industry, despite years of research and development into different vaccination and management strategies. It is the most common feedlot illness, responsible for more than 50% of all deaths and 75% of morbidities in the United States [
1]. Vaccination is the most common preventative measure. Theurer et al. [
2] reported that adopting a standard vaccination protocol for a cow–calf operation can improve health outcomes at the feedlot. However, the control of BRD is difficult due to the complex nature of the disease and the numerous risk factors involved in the triad complex, including bacterial and viral pathogens, environmental conditions, and host immunity [
3,
4]. Host immunity is further affected by other biological animal factors, which include age at vaccination [
5], vaccination protocol utilized [
6], sex [
7], and various other factors that may contribute to the continued high BRD prevalence in the feedlot [
8]. Minimal studies have assessed the effect of individual animal factors, such as the age of the dam or their vaccination history, on the immune response of their progeny to vaccinations.
The specific immune response to BRD vaccination depends on multiple factors, including vaccine type, such as a modified live virus or an inactivated one [
9]. For example, different types of vaccines use different antigens that activate different components of the adaptive immune system. Modified live virus vaccines induce T- and B-cells, resulting in complete cell-mediated and humoral immune responses. Inactivated virus vaccines primarily stimulate B-cells, inducing a strong humoral immune response but a less robust cell-mediated immunity. A booster vaccination is generally administered regardless of vaccine type, but at least two doses of the inactivated vaccine are required to stimulate a more effective immune response [
10].
Another factor that influences the immune response to vaccination is age. Signer et al. [
11] found that an individual’s immune response to vaccination decreases as age increases. This may be partly due to decreased B-cell production necessary to produce antigenic memory; thus, as animals age, their immune system is less productive in producing serum-neutralizing antibodies, and the antigen affinity is reduced [
12]. This, in turn, leads to a longer time to reach peak titers and a faster decline in titers post-vaccination [
12]. This age-related reduction in immunity is termed immunosenescence, which impacts the robustness of the adaptive immune system [
13]. Thus, it is plausible that as the dam ages (or vaccine doses increase), this may affect her humoral immune response, influencing the vaccine responsiveness of her progeny.
Often, cattle, especially those in breeding herds, receive annual vaccinations, with one before breeding and then again while pregnant. The accumulative effect of annual revaccination may lead to vaccine exhaustion, or the ‘Hoskins Effect’ [
14]. It has been shown in humans that repeated vaccination reduces the effectiveness of influenza protection, as evidenced by reduced titer values [
15,
16] and virus-specific antibody affinity maturation [
17]. Some have speculated that a reduced immune response to vaccination may be partly due to the animal’s first exposure to the antigen being the vaccination, followed by subsequent antigenically identical vaccinations. This may result in the antigenic distance hypothesis, in which the virus has mutated from the original vaccine formulation, resulting in new antigen variants and reducing the efficacy of the immune response to the mutation, as it relies on antibodies made for the original form encountered through vaccination [
18]. Moreover, it is plausible that a reduced immune response to vaccines in the dam may affect passive immunity, which may have implications for the immune response of the progeny.
Maternal vaccination against BRD is believed to be beneficial for calf protection [
19,
20]. However, there is some concern that maternal interference may reduce vaccination efficacy in the calf [
21]. It may be plausible that maternal interference is reduced in calves born to older dams or that maternal passive immunity is affected, thus affecting the immune response to vaccination in the progeny. A more comprehensive understanding of the effect of the age of the dam related to repeated vaccination is important to implement a more effective vaccination protocol for cow–calf operations. We previously reported the differential immune phenotypes induced by vaccination type in heifer calves [
6]; therefore, the objective of this preliminary study was to characterize the effect of dam AGE, and thus her vaccination history, on overall calf response to BRD vaccination and to further characterize if dam AGE affected the calves’ immune response to being vaccinated at weaning and again 28 days later with either modified live or inactivated vaccines.
2. Materials and Methods
All procedures were approved by the Oklahoma State University Institutional Animal Care and Use Committee (Protocol No. AG-15-21 and AG-19-8).
2.1. Animals and Experimental Design
At breeding, Angus dams kept at Oklahoma State University Field and Research Service Unit (Valliant, OK, USA) received a modified live vaccine (MLV; Bovishield® Gold FP5 VL5, Zoetis Animal Health, Parsippany-Troy Hills, NJ, USA) containing infectious bovine rhinotracheitis (IBR), bovine viral diarrhea virus type 1 and 2 (BVD1 and BVD2), parainfluenza 3 (PI3), and bovine respiratory syncytial virus (BRSV), and then a booster four weeks later. This standard vaccination protocol was repeated each breeding period that the dam remained in the breeding herd.
All calves were vaccinated at 3–4 months of age with Titanium®5 (Elanco Animal Health, Greenfield, IN, USA), a modified live vaccine containing IBR, BVD1 and 2, PI3, and BRSV, but blood samples were only taken from a subset of dams and calves (n = 21) at this time. Calves were weaned at 6 to 7 months old and then transported to the Oklahoma State University Willard Sparks Beef Research Center (WSBRC; Stillwater, OK, USA). Dams remained with the breeding herd in Valliant, OK.
Calves were bled before transport. Upon arrival at WSBRC in Stillwater, OK, calves were held overnight in holding pens with ad libitum access to water and hay. The next day, calves were bled, weighed, and allocated to a vaccine treatment group where they were revaccinated with either MLV or an inactivated (INA) (inactivated IBR, BVD1 and 2, PI3, and BRSV) vaccine, and 28 days later, they received a booster with the same treatment vaccine. This resulted in two treatment groups: MLV = 3 MLV (n = 30) or INA = 1 MLV and 2 INA (n = 25) vaccinations. Blood samples were obtained from these calves (n = 55) at 14 and 28 days post-revaccination (PRv) and post-booster (PB) with Titanium®5 (MLV) or Virashield®6 (INA; Elanco Animal Health). Animals were also weighed on blood collection days.
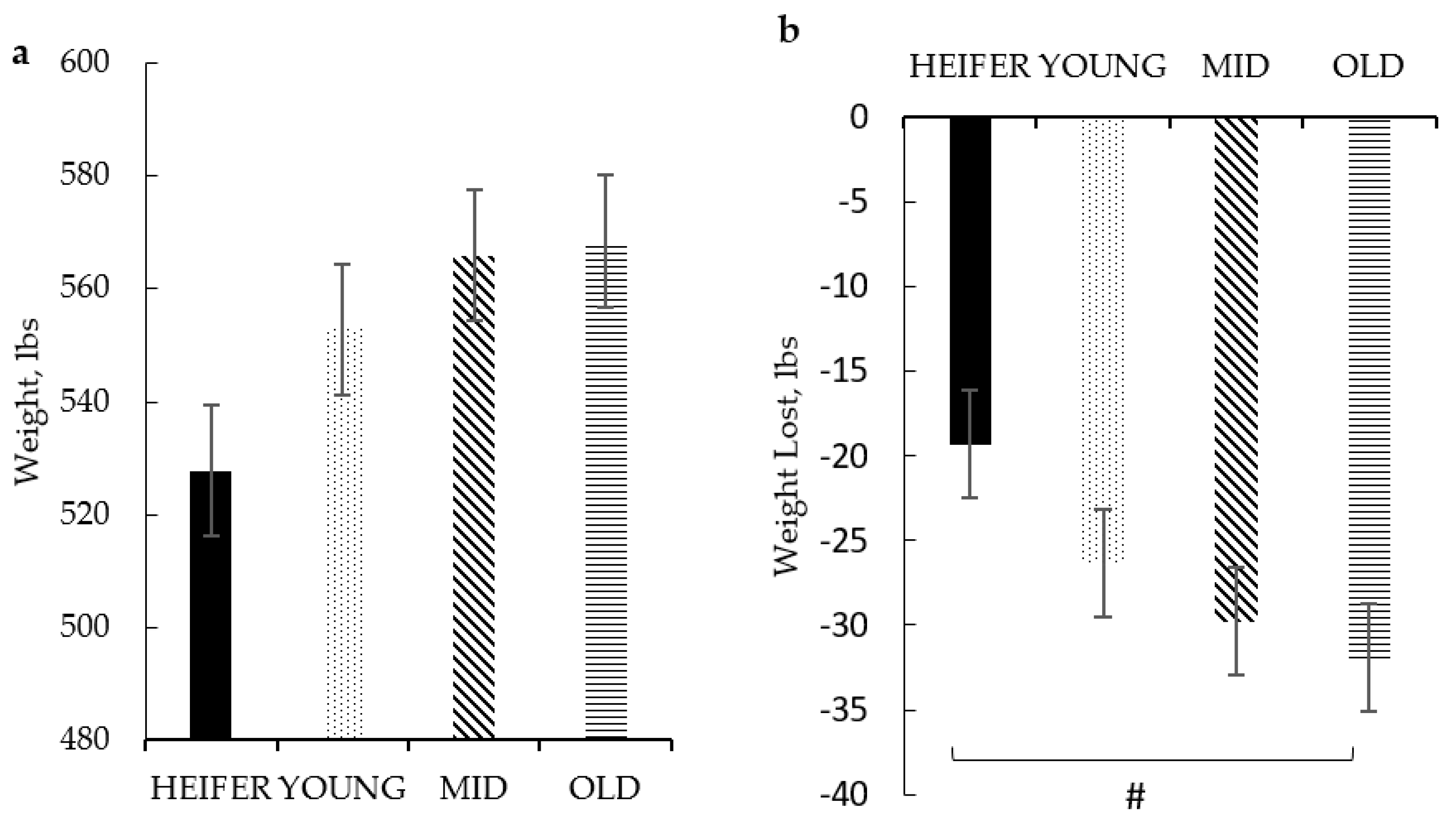
Dam age categories were created based on her age (or parity), indicative of the number of BRD vaccinations she received each pregnancy, which resulted in four age groups: HEIFER (2-year-olds; 2 vaccinations; and n = 9), YOUNG (3- to 4-year-olds; 4–6 vaccinations; and n = 22), MID (5- to 6-year-olds; 8–10 vaccinations; and n = 13), and OLD (≥7-year-olds; ≥12 vaccinations; and n = 11).
2.2. Sample Collection and Blood Processing
Whole blood and serum samples were collected via jugular venipuncture using serum, heparin (plasma), and EDTA (complete cell counts; CBC) vacutainers (BD Vacutainers; Franklin Lakes, NJ, USA). The CBC was determined electronically from whole blood using the Element HT5 Hematology Analyzer (Heska, Loveland, CO, USA). Serum tubes were allowed to clot at room temperature, centrifuged at 3000× g for 20 min, and then aliquoted. Plasma samples were stored at −20 °C and serum at −80 °C until subsequent analysis.
2.3. Cortisol, Cytokines, and Immunoglobulin-G Subsets
Plasma cortisol was measured using a commercially available enzyme-linked immunoassay (ELISA) following the manufacturer’s protocol (Enzo Life Sciences, Farmingdale, NY, USA). Samples were diluted 1:8 in assay buffer and run in duplicate in a 96-well microtiter plate coated with goat anti-mouse IgG. The conjugate (alkaline phosphatase conjugate with cortisol) and antibody (mouse monoclonal antibody to cortisol) were added. Plates were placed on a shaker at 500 rpm for 2 h at room temperature and washed three times, then substrate (P-nitrophenylphosphate) was added, and plates were incubated for 1 h at room temperature. The reaction was stopped with the solution provided. Plates were read using a microplate reader (BioTek Epoch, Winooski, VT, USA) at 405 nm. A standard curve was used to determine the concentration of the unknown samples using the Gen5 Data Analysis Software version 3.0 (Bio Tek). The minimal detectable concentration of the assay was 56.7 pg/mL, and the mean intra- and interassay coefficients of variability were 7.3% and 13.4%, respectively.
Interleukin-4, -6, -10, -17, -21 (Invitrogen Corp., Waltham, MA, USA), and -8 (Biomatik, Wilmington, DE, USA) were measured using commercially available bovine ELISA kits for each cytokine following the manufacturer’s protocols. Briefly, the 96-well microtiter plates were pre-coated for all cytokines except interleukin-6 and -8. Standards and samples were pipetted in duplicate, and plates were incubated at room temperature with gentle shaking. Anti-bovine detection antibodies IL-4 or IL-6 and biotin-conjugated antibodies IL-10, IL-17, or IL-21 were added to the appropriate plate and incubated for 1 h at room temperature. Plates were washed several times, Streptavidin-HRP was added to each well, and plates were incubated with moderate shaking at room temperature for 30 min (IL-6 and IL-4) or 45 min (IL-10, IL-17, and IL-21). Tetramethylbenzidine dihydrochloride (TMB) substrate was added to each well. Plates were incubated for 20 min (IL-6 and IL-4) or 30 min with gentle shaking (IL-10, IL-17, and IL-21); the reaction was terminated with stop solution, and plates were read at 450 nm wavelengths using the BioTek Epoch plate reader. Using the Gen5 Data Analysis Software (BioTek, Winooski, VT, USA), the minimal detectable concentrations of the assays for interleukin-4, -6, -8, -10, -17, and -21 were 15.6 pg/mL, 78.1 pg/mL, 5.9 pg./mL, 0.12 ng/mL, 2 pg./mL, and 0.41 ng/mL, respectively. The mean intra- and interassay coefficients of variability were 10% and 12% for IL-4, -6, -17, and -21; 8% and 10% for IL-8; and 5% and 7.2% for IL-10, respectively.
Immunoglobulin-G (IgG) subset IgG1 and IgG2 concentrations were measured at South Dakota State University (Brookings, SD, USA) using commercially available bovine IgG1 (E11-16) and IgG2 (E11-17) ELISA kits (Bethyl Laboratories Inc., Montgomery, TX, USA), following the manufacturer’s protocol. Briefly, samples were diluted in sample diluting buffer at 1:500,000, and samples and standards were pipetted in duplicate onto 96-well microtiter plates coated with either anti-bovine IgG1 or IgG2 antibody. Plates were incubated at room temperature for 1 h and washed four times. Anti-IgG1 or anti-IgG2 detection antibodies were pipetted into the wells. Plates were incubated at room temperature for 1 h and washed four times. Horseradish peroxidase solution was added to each well, and plates were incubated at room temperature for 30 min and washed. The TMB substrate was added, and plates were incubated in the dark for 30 min at room temperature. The reaction was stopped using the provided solution, and plates were read at 450 nm. The standard curve was plotted using Soft-Max pro software version 7.1.2 (Molecular Devices LLC, San Jose, CA, USA) to estimate concentrations of IgG1 and IgG2. Total IgG was calculated by adding the values of the concentrations of IgG1 and IgG2. The minimal detectable concentration of both assays was 1.0 ng/mL.
2.4. Serum-Neutralizing Antibody Titers
Serum samples were shipped to the Texas Veterinary Medical Diagnostic Laboratory (Canyon, TX, USA) on dry ice to analyze serum-neutralizing (SN) antibody titers for infectious bovine rhinotracheitis (IBR), bovine viral diarrhea virus types 1a, 1b, and 2 (BVDV 1a, 1b, 2), parainfluenza 3 (PI3), and bovine respiratory syncytial virus (BRSV). The reciprocal of the highest dilution of serum that neutralizes the infectivity of the virus was determined as the SN antibody titer.
2.5. Statistical Analysis
Data were analyzed using Pearson’s correlation and mixed procedure in SAS 9.4 (Inst. Inc., Cary, NC, USA) with repeated measures. All traits were tested for departure from a normal distribution through analysis of the residuals, and log transformation was applied to non-normally distributed traits. The model included random effects of dam or calf and fixed effects of dam AGE (HEIFER, YOUNG, MID, or OLD) and vaccination treatment (MLV or INA). Days post-revaccination or booster were used as a repeated measure. The Tukey–Kramer post hoc analysis adjusted the p-value to account for Type I error for multiple comparisons. Significance was set at (p ≤ 0.05), and trends were discussed from (p > 0.05) to (p ≤ 0.10).
4. Discussion
Understanding how biological factors (i.e., age) may alter the vaccination response of the progeny and how these factors may interact with vaccination protocol is important to developing efficient vaccination strategies for cow–calf operations to improve calf health upon receiving at the feedlot. More specifically, the age of the dam and her vaccination history in terms of the number of times she received a modified live bovine respiratory disease vaccination may affect passive immunity, which may affect the immune response of her progeny to vaccinations. It should be noted that these data are a subset of a larger study that characterizes the immune phenotype of calves that received either a modified live or inactivated vaccine, resulting in a smaller sample size when dam age is considered. Regardless of the smaller sample size, we found that immunoglobulin concentrations at 14 days post-revaccination and booster were reduced in calves born to older dams (≥7 years of age; ≥12 vaccinations) compared to those born to younger dams (≤6 years of age; ≥8 vaccinations). Also, serum-neutralizing antibody titers were higher in calves vaccinated with one MLV at 3 to 4 months old and then revaccinated with INA vaccines at weaning, and 28 days later, they were higher in calves born to the youngest (2 years of age; 2 vaccinations) and the oldest (≥7 years of age; ≥2 vaccinations) dams than calves born to middle-aged dams (3 to 6 years of age; 4–8 vaccinations) for the majority of tested antigens. These data may begin to indicate that dam AGE may affect passive immunity, and her vaccination history may also differentially affect her calves’ immune response to vaccine type (e.g., modified-live or killed). Old dams seem to have a suppressive effect on the immune response of their progeny, which is indicative of influencing both a cell-mediated (Th-1; IgG2) and humoral (Th-2; IgG1) immune response at the time of vaccination, regardless of vaccination protocol. A robust Th-1 and Th-2 response may need to be initiated for protection and viral clearance of BRD [
22,
23].
In the current study, calves born to heifers or young dams began to exhibit a pattern of having the highest IgG1 concentrations at weaning and upon revaccination 28 days later. This may begin to indicate, at least in the calves of young-aged dams, higher levels of IgG1 being passed from the dam, as this is the predominant subset in milk [
24], or a more robust response. Maternal antibodies may still be detectable in the calf at 6 months old [
21]. However, these results are interesting, as it is generally believed that heifers produce lower IgGs in the milk [
25,
26]. There are multiple speculations about why heifers normally produce reduced IgGs in the colostrum and milk; one theory is that less exposure to antigens due to younger age results in fewer antibodies transferred to the calf [
27]. Another speculation is that in heifers, mammary development is not yet complete and may reduce the transfer of IgGs into mammary secretions [
28]. Higher IgG production in the calves of these heifers may be due to naivety from reduced maternal antibodies, thus increasing the need of the calf to produce their antibodies when exposed and having fewer specific antibodies to clear pathogens faster and with fewer antibodies produced [
29].
Conversely, in calves from older dams, IgG1 and IgG2 levels only peaked at 14 d post-booster vaccination, regardless of vaccination protocol, possibly indicating that calves from older dams may require more exposures to these antigens than those from younger dams in order to mount an efficient immune response, especially since the magnitude of the concentrations at 14 d post-booster was less than their conspecifics from younger dams. However, it should be conceded that there was a lack of statistical significance found in these differences, possibly due to the sample size of the current study. This possible decrease in IgGs may be due to a reduction in passive immunity in older dams, as B cells have been found to decrease as the dam ages [
11]. Moreover, the immunoglobulins’ antigen affinity may decrease as individuals age [
12], thus indicating a reduction in the quantity or quality of maternal passive antibodies the offspring receives, which may, in the short term, be indicative of reduced adaptive immunity in response to vaccination and a booster partly due to reduced passive immunity. A reduction in antibody quality may also explain why we did not see a similar IgG response to vaccination in calves from old-aged dams as we did from calves from heifer dams. However, both are speculated to receive reduced passive immunity from their dams. However, we did not assess the dam’s adaptive immunity or maternal antibody quantity and quality in the colostrum and milk.
Reduced passive immunity may be partly due to an altered specific immune response to antigens in the BRD vaccine administered to calves born to heifers and old dams. This is more evident in those calves that received one MLV and two INA vaccines since naivety toward these antigens may have led to more robust titer responses in these calves than their counterparts from young or mid-aged dams. Previous research has shown that titer differences due to vaccination type may be due to lower exposure of heifers to antigens via vaccination or natural exposure, thus resulting in a more robust titer response [
27]. On the other hand, in humans, it has been reported that as individuals age, their titer response to vaccination results in lower titer levels declining rapidly [
12]. It is also plausible that the older dams reached a point of vaccine exhaustion from repetitive vaccinations, similar to what occurs in humans. Individuals subjected to repetitive vaccinations, especially those using antigenically similar vaccine formulations, have a reduced immune response to subsequent vaccinations [
14,
15,
16,
17,
18]. Moreover, we speculate that this may be occurring in cattle as well. Lee et al. [
30] found that dairy cows under 5 years old showed higher titers when vaccinated yearly for BHV, a causative agent for IBR, than cows older than 5 years. For both calves from heifers and old dams, the possible reduction in the dam’s ability to produce specific antibodies may have resulted in less specific BRD antibodies being passed to the offspring. Thus, decreased maternal antibodies may have increased progeny antibody titer values, as maternal antibodies for viruses within the BRD complex may interfere with calf antibody production [
13,
21,
31]. However, these higher titer responses do not necessarily indicate a higher level of protection [
32].
Calves of young and mid-aged dams appear to have less variability in IgG and serum-neutralizing antibody titer responses to vaccination. Serum-neutralizing antibody titer responses were fairly similar between calves of young dams who received either vaccination protocol or between calves of mid dams who received either vaccination protocol. While these calves of young and mid-aged dams did not exhibit the highest titer values overall in response to vaccination, excluding PI3 responses, this could indicate they had a more efficient immune response, as some have reported low to medium titer values are protective against immune challenges [
32]. Previous research shows that young to mid-age may increase the passive immune factors produced by the dam and the level of these factors detectable in the offspring. Parity 2 to 5 cows, which are 3 to 6 years old, have been found to have increased milk yield [
33], which may aid in increased passing of maternal immunity but also increased IgG concentration in the milk and colostrum [
24,
25,
26,
27,
34]. In other species, such as swine, as parity increases, passive immunity increases in the progeny [
35,
36], which may indicate an optimal dam age range for maternal passage of humoral immunity. While the current study has characterized the immune responses to BRD vaccination of offspring born to dams of various ages, further research should be conducted to determine if dam age and vaccine history hinder vaccine efficacy. Moreover, as the current study acts as a conduit for preliminary data, more focused studies should be conducted to investigate this possible phenomenon further.