Abstract
The gastrointestinal tract of broilers is susceptible to oxidative stress induced by heat stress (HS) and diet, which can be mitigated by the supply of exogenous vitamins and antioxidants. The aim of this study was to compare the extent of the effects of cyclic HS, and high levels of polyunsaturated fatty acids (PUFA) (HP) on gut health. It also aimed to investigate whether additional supplementation with vitamins E, C and selenium (HA) is required to support gut health under these conditions. In the present study, 192 one-day-old male Ross 308 broilers were randomly divided into eight experimental groups. Exposure to HS and HP significantly (p < 0.001) decreased villus height and villus-to-crypt ratio in the duodenum, while no differences were observed in the jejunum and ileum. In addition, oxidative stress in the liver, indicated by increased malondialdehyde (MDA) levels, was increased in the HP groups (p < 0.0001), while the HA groups had a positive effect on lowering MDA levels. The results confirm that cyclic HS and HP induce oxidative stress that damages the structure of intestinal morphology and that supplementation with HA could be a potential approach to mitigate the negative effects of these stressors.
1. Introduction
Keeping the gastrointestinal tract (GIT) of chickens in good condition is important for animal health and, thus, for achieving good performance results. The GIT is the most important organ responsible for digestion and nutrient absorption [1]. The intestinal mucosa is responsible for the absorption of nutrients, and the antioxidant system maintains a diverse microbiota in the luminal epithelia. If the physiological level is exceeded, the production of reactive oxygen species (ROS)/RNS (reactive nitrogen species) leads to intestinal inflammation and impairs absorption capacity [2].
In the poultry industry, chickens are exposed to various stressors on a daily basis, especially due to genetic selection. On the other hand, heat stress (HS) is a major problem in poultry production that affects chicken performance [3], especially in tropical and subtropical regions [4]. Poultry may be exposed to HS when there is an imbalance between the body’s heat production and its ability to dissipate that heat [5]. Poultry are exposed to thermal stress because they typically reduce their heat production by decreasing feed intake, which can negatively affect their performance, productivity, and welfare [6]. Elevated ambient temperatures can negatively affect morphology, behavior, growth performance, and overall productivity, as documented in references [7,8]. Poultry are comfortable and perform their life functions optimally in a temperature range of approximately 23.9 °C to 26.7 °C [9]. Numerous studies have reported the negative effects of HS on poultry production, such as decreased body antioxidant capacity, gut health and gut immunity, and impaired gut morphology [10,11,12,13].
The inclusion of fats and oils in the diet is crucial for enhancing productivity, as they contribute to a high energy content, which is important to increase the productivity of animals by including various fats and oils in the diet. With the aim of creating functional foods, the commonly used vegetable oil in broiler chickens is replaced by n-3 polyunsaturated fatty acid (PUFA)-rich oil sources [14]. However, excessive consumption of PUFAs can potentially promote oxidative stress and inflammation [15,16].
The negative effects of oxidative stress in chickens can be mitigated by various technological and nutritional strategies, like feed supplementation with exogenous vitamins, antioxidants, and plants (e.g., berries, microalgae) or plant extracts [2,17]. Commonly used nutritional strategies include supplementation with various vitamins (e.g., vitamins A, C, and E) and minerals (e.g., Fe, Zn, Cr, Cu, Mn, and Se) can mitigate the adverse effects of oxidative stress, while supplementation with vitamins E, C, and selenium (Se) has shown promising results in attenuating the adverse effects of HS and high intake of linseed oil, which is rich in n-3 PUFA [15,16,18,19]. In addition, vitamins E, C, and Se are known to play an important role in the antioxidant defense system by acting synergistically and thus could contribute to better antioxidant activity and attenuate oxidative stress by reducing free radical production when supplemented in combination [20]. Current practical Aviagen [21] dietary guidelines for broilers already consider the need for additional supplementation with vitamin E and Se in the feed regarding the exposure of birds to different stressors, with the recommendations for vitamin E in the range of 55 to 80 IU/kg feed, compared with NRC [22] nutrient requirements, which consider 10 IU vitamin E/kg feed. Furthermore, the recommendations for Se are set at 0.30 mg Se/kg feed [21] and 0.15 mg Se/kg feed [22], respectively. Additional amounts of antioxidants are recommended for animals exposed to oxidative stress [15,23,24].
The intestinal tract exhibits increased sensitivity to various stressors, including HS [25]. Optimal functioning of the intestinal tract is very important in the context of poultry production, as it has profound effects on overall animal health and performance [26]. Numerous studies have documented morphometric and histopathological changes in the intestinal tract of poultry exposed to HS conditions. The predominant trend in most of these studies consistently shows a notable decrease in villus height and an increase in crypt depth, resulting in a lower villus-to-crypt ratio [27,28,29]. However, Santos et al. [30] found an increase in villus base width and a decrease in epithelial cell area in the duodenal, jejunal, and ileal mucosa of broiler chickens exposed to HS conditions. Strategies to cope with HS conditions have mainly focused on nutritional manipulations (i.e., diet composition according to the metabolic state of the animals) and addition of feed additives (e.g., antioxidants, vitamins, minerals, probiotics, prebiotics, phytogens) and water supplementation with electrolytes [31,32,33]. A study shows that broiler chickens receiving oxidized oils/fats in their diets have an imbalance of antioxidants and immune responses in their intestinal mucosa [34]. The intestinal mucosa serves as the primary protection against oxidative stress and has an extensive arsenal of antioxidants, including enzymes, such as catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx), as well as nonenzymatic radical scavengers, such as glutathione, transition ions (e.g., Fe2+, Cu2+), and flavonoids [35].
The objective of this study was to investigate the effects of cyclic HS and dietary oxidative stress induced by high dietary PUFA levels (HP) on intestinal morphology, gut fermentation activity and histological parameters of different intestinal segments in broiler chickens. Furthermore, we aim to determine whether dietary supplementation with a mixture of vitamins E, C and Se above the Aviagen recommendations compared to the low NRC recommendations for antioxidants is essential for maintaining the gut health of broilers when exposed to various external stressors.
2. Materials and Methods
The nutritional study was conducted at the research facility of the Department of Animal Science, Biotechnical Faculty, University of Ljubljana, Slovenia. In this study, all experimental procedures were in accordance with the applicable Slovenian animal experimentation regulations, which are consistent with the European Union standards for research involving experimental animals. The protocol was approved by the Animal Ethics Committee of the Veterinary Administration of the Republic of Slovenia under project license number U34401-5/2021/4.
The 192 one-day-old broiler chickens were housed in two separate experimental rooms with different environmental conditions (thermoneutral (TN) and heat stress (HS)) within the same experimental facility in 24 pens (2 × 2 × 2 factorial design in randomized complete blocks, with 2 × 2 diet treatments and 2 environmental conditions). Each experimental group consisted of three replicate pens measuring 0.95 by 1.26 m for a total area of 1.20 square meters. These pens were equipped with wood shavings as bedding, a plastic feeder, and nipple drinkers. Diet and water were provided ad libitum. Starter diet was fed for the first 10 days, grower diet from day 11 to day 24, and finisher diet for the remainder of the experimental period until day 42. The experimental diets, which the animals received from the first day, used the main fat source, consisting of a mixture of animal fats and plant oils (low PUFA; LP), or 5% cold-pressed linseed oil create experimental diets enriched in PUFA (high PUFA; HP). In addition, diets were formulated according to NRC [22] (low antioxidants; LA) or Aviagen recommendations for Ross 308 broilers [21] and supplemented with 200 IU vitamin E, 250 mg vitamin C, and 0.15 mg Se/kg diet (high antioxidants; HA). Therefore, 4 diet treatments were used in the experiment: LP/LA: basal diet according to NRC, no supplementation; HP/LA: +5% linseed oil and NRC; LP/HA: basal diet according to Aviagen + 200 IU dl-α-tocopheryl acetate + 250 mg vitamin C + 0.15 mg Se/kg diet; HP/HA: +5% linseed oil and Aviagen + 200 IU dl-α-tocopheryl acetate + 250 mg vitamin C + 0.15 mg Se/kg diet. Table 1 provides a detailed listing of the components and calculated energy and nutrient contents for the starter, grower, and finisher diet formulations. In addition, proximate and mineral content, α- and γ-tocopherol composition, antioxidant capacity of water-soluble (ACW) antioxidants, MDA, and fatty acid composition (FA) were measured in the finisher diets (Table 2).

Table 1.
Composition and calculated nutrient content of the experimental diets fed to broiler chickens during the starter (1–10 d), grower (11–24 d) and finisher (25–42 d) period.
Animals were reared according to a standard lighting schedule that included 23 h of light and 1 h of darkness during the first week. From day 8 until the end of the experiment, they were reared under an 18 h light and 6 h dark cycle program. Chickens in the TN groups were raised under controlled environmental conditions for temperature and humidity throughout the experiment, following Aviagen [36] guidelines for Ross 308 broiler chickens. The chickens in the HS room were kept under the same conditions as in the TN room until the 22nd day of rearing. To induce cyclic HS, a modified temperature regime was used from day 22 to the end of the experiment. During this period, chickens experienced the following temperature phases daily: 12 h at 24 ± 0.5 °C, a 2 h warm-up period from 24 ± 0.5 °C to 34 ± 1 °C, 7 h at 34 ± 1 °C (HS), and 3 h to cool down from 34 ± 1 °C to 24 ± 0.5 °C. Relative humidity was monitored at a specific time to ensure that it fluctuated but never dropped below 45%. To evaluate the effects of these thermal conditions on broiler thermoregulatory status and overall thermal comfort, especially during heat treatment, the daily temperature–humidity index (THI) for chicken was calculated.

Table 2.
Proximate composition, concentration of some minerals, content of α-, and γ-tocopherol, MDA, antioxidant capacity of water-soluble compounds (ACW) and the FA composition of the finisher experimental diets.
Table 2.
Proximate composition, concentration of some minerals, content of α-, and γ-tocopherol, MDA, antioxidant capacity of water-soluble compounds (ACW) and the FA composition of the finisher experimental diets.
| Component | Dietary Treatments 1 | |||
|---|---|---|---|---|
| LP/LA | HP/LA | LP/HA | HP/HA | |
| Dry matter, g/kg | 899.3 | 898.5 | 897.6 | 897.5 |
| Crude protein, g/kg | 215.0 | 213.7 | 214.1 | 212.6 |
| Ether extract, g/kg | 84.39 | 86.70 | 83.82 | 84.07 |
| Crude ash, g/kg | 55.04 | 56.65 | 58.93 | 54.83 |
| Crude fiber, g/kg | 68.40 | 71.19 | 68.81 | 75.14 |
| Cu, mg/kg | 15.47 | 19.75 | 21.64 | 22.97 |
| Se, mg/kg | 0.175 | 0.147 | 0.387 | 0.351 |
| Tocopherol isomers | ||||
| α-tocopherol, mg/kg | 12.15 | 8.87 | 250.2 | 232.2 |
| γ-tocopherol, mg/kg | 26.76 | 43.37 | 27.23 | 45.09 |
| MDA and ACW 3 | ||||
| MDA, nmol/g | 3.81 | 7.53 | 3.35 | 4.40 |
| ACW, µmol/g | 3.37 | 2.62 | 2.95 | 2.51 |
| Fatty acid composition 2, g of fatty acids/100 g of fatty acids 3 | ||||
| C16:0 | 22.36 | 8.99 | 22.09 | 8.95 |
| C18:0 | 10.97 | 3.66 | 10.66 | 3.61 |
| C18:2 n-6 | 26.53 | 28.36 | 27.53 | 28.52 |
| C18:3 n-3 | 2.08 | 37.84 | 2.09 | 37.77 |
| ∑ SFA | 37.02 | 13.40 | 36.34 | 13.30 |
| ∑ MUFA | 33.99 | 20.41 | 33.65 | 20.40 |
| ∑ PUFA | 28.97 | 66.20 | 29.98 | 66.29 |
| ∑ n-6 PUFA | 26.75 | 28.36 | 27.74 | 28.52 |
| ∑ n-3 PUFA | 2.14 | 37.84 | 2.16 | 37.77 |
| n-6/n-3 PUFA | 12.53 | 0.75 | 12.87 | 0.76 |
Proximate composition [37] of feed mixtures, concentration of minerals determined by the standard methods. Concentrations of MDA by Fukunaga et al. [38] and vitamin E in diet samples were measured according to the methodologies of Rupérez et al. [39] using Agilent 1260 Infinity HPLC. The ACW in feed samples was analyzed using PhotoChem commercial kits (Analytik Jena, Jena, Germany). Methyl esters of fatty acids were prepared according to Park and Goins’ procedure [40]. 1 LP/LA: basal diet according to NRC, no supplementation; HP/LA: +5% linseed oil and NRC; LP/HA: basal diet according to Aviagen + 200 IU dl-α-tocopheryl acetate + 250 mg vitamin C + 0.15 mg Se/kg diet; HP/HA: +5% linseed oil and Aviagen + 200 IU dl-α-tocopheryl acetate + 250 mg vitamin C + 0.15 mg Se/kg diet. 2 All values are means of two analyses per measured sample. Only prevalent and important dietary fatty acids are listed. 3 Abbreviations: MDA, malondialdehyde; ACW, antioxidant capacity of water-soluble compounds; ∑, sum of isomers; SFA, saturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid.
Each animal was individually labeled on the first day. Throughout the experiment, body weight (BW) was documented on a weekly basis, as well as on the day of slaughter. Also, body weight gain (BWG), average daily feed intake (ADFI) per pen, and feed conversion ratio (FCR) (per pen) were recorded to determine broiler growth performance. The production parameters were monitored until day 40. The chickens were slaughtered on day 42.
At the end of the experiment, 12 chickens per group were randomly selected and slaughtered. The animals were slaughtered in an experimental slaughterhouse at our faculty. The birds were treated gently and calmly, the cervical dislocation was used and the large blood vessels in the neck were severed to cause rapid bleeding and death. Organs (heart, liver, pancreas, proventriculus, gizzard, and intestine) and samples of different parts of intestine contents from each chicken were collected. Tissue samples of individual parts of the gastrointestinal tract (small intestine: duodenum, jejunum and ileum and caecum) were taken for histological measurements as described by Pirman et al. [41].
Liver was weighed, stored at −80 °C and homogenized before analysis. Levels of MDA were measured by High-Performance Liquid Chromatography (HPLC) according to the method of Wong et al. [42] with some modifications described by Voljč et al. [15]. Concentrations of tocopherols were determined by HPLC according to the protocol described by Leskovec et al. [18]. The ACW were analyzed using PhotoChem commercial kits (Analytik Jena, Jena, Germany). The content of selenium in liver samples was determined according to the European Standard 14627 (EN 14627, Determination of trace elements–Determination of total arsenic and selenium by hydride generation atomic absorption spectrometry (HGAAS) after pressure digestion. CEN: Brussels, Belgium, 200) and the method described by García et al. [43]. Fatty acids were analyzed using gas chromatography according to the method described by Leskovec [44].
The pH of the contents of different parts of the intestine (stick pH meter Mettler Toledo, Mettler-Toledo GmbH, Greifensee, Switzerland) and the viscosity of small intestine contents of broilers were measured. Samples of small intestine contents were centrifuged at 9500× g for 10 min, and intestinal viscosity analysis was carried out according to Bedford and Classen [45].
The concentrations of Short Chain Fatty Acids (SCFA) in the contents of the small intestine and caecum were determined by gas chromatography using the Agilent 6890A GC system equipped with FID detector (Agilent, Santa Clara, CA, USA) and DB-FASTWAX UI capillary column (30 m × 0.25 mm × 0.25 μm) (Agilent) using the method reported in Holdeman and Moore [46], with some modifications, described by Pirman et al. [47].
For the histological measurements of the small intestine (duodenum, jejunum and ileum) and the caecum, a section of each was taken, fixed in 5% buffered formalin solution and embedded in paraffin according to a standard procedure. Subsequently, an evenly spaced series of histologic sections (50 μm section interval) were then cut at 5 μm intervals and stained with hematoxylin and eosin (H&E). Histomorphometric analysis was performed on the H&E-stained tissue sections using a Nikon Ni/U light microscope equipped with a DS-Fi1 camera and analyzed with NIS-Elements imaging software, NIS-Elements Basic Research 4.60 (Nikon Instruments Europe B.V., Badhoevedorp, The Netherlands). Villus height was measured from the tip to the crypt–villus junction, and crypt depth was measured from the crypt–villus junction to the crypt base (Figure 1).
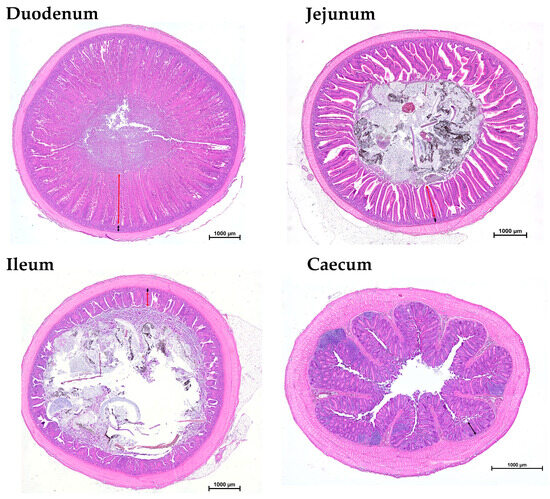
Figure 1.
A representative histological cross section of duodenum, jejunum, ileum and caecum. * Villus height (double arrow line in red); crypt depth (double arrow line in black).
Statistical analysis of the data was performed using the mixed procedure of SAS software Ver. 9.4 (SAS Institute Inc., Cary, NC, USA). In the statistical model, environment (E), dietary fat treatments (P), antioxidants supplementations (A) and their interactions were determined as main effects, and replication pen was determined as a random effect, except for FCR. When significant interactions emerged among the primary effects, comparisons were made within each experimental variable. The least squares mean (LSM) was estimated by applying the LSMEANS statement in the model. Differences between LSMs were determined by a Tukey–Kramer multiple comparison test. Dispersion was expressed as the standard error of the mean (SEM). Statistical significance was assumed at p < 0.05.
3. Results
Throughout the trial, the birds acclimated efficiently to the experimental conditions and remained in good health without any problems.
3.1. Performance and Relative Organ Weight
The broilers in the HP/LA group had a lower BW than those in the LP/LA group, regardless of the housing system HS (p < 0.0001) (Table 3). At the end of the experiment, after heating at day 22, broilers from TN environment had generally higher BW than broilers from HS. However, the environment statistically poured on BW on day 40 (p = 0.001). The addition of antioxidants to the diet had a significant effect on the increase in BW of broiler chickens (p < 0.0001) on the 21st and 40th day of the experiment compared with chickens that did not receive diets containing higher levels of antioxidants (LP/LA and HP/LA groups) depending on the rearing conditions. However, significant differences in feed conversion ratio (FCR) from day 21 to day 40 were found as a result of fat (p = 0.009) and antioxidants (p = 0.003), but no influence of the rearing environment was found (p = 0.626). However, higher FCR was measured in both groups of chickens fed higher dietary PUFA levels (HP) than in the other experimental groups in both rearing conditions.

Table 3.
Body weight and feed conversion ratio (FCR), relative organ weight and markers of oxidative stress and antioxidant levels measured in the liver of broiler chickens reared under different environmental conditions and fed different experimental diets.
Environmental conditions had a negative significant effect on the relative weight of the heart, pancreas, and intestine (Table 3). The addition of PUFA increased the relative organ weight. Antioxidant supplements in most decreased the relative organ weight in both rearing conditions. However, the interaction effect of diet and environment was not detected.
3.2. Markers of Oxidative Stress (MDA), Antioxidant Levels and Fatty Acids Profile of the Liver
Nutritional supplementation and also environmental conditions negatively influenced liver mass (Table 3). Heat stress conditions negatively influenced relative liver weight. PUFA supplementation in HS decreased relative liver weight. Higher dietary PUFA levels in diets had a significant effect on liver MDA content, which was measured as a marker to determine the extent of lipid oxidation. Liver MDA concentrations were higher in the HP/LA groups than in the other experimental groups (p < 0.0001) under both conditions TN and HS. Antioxidant supplementation (HA) decreased MDA content in the liver in all supplemented groups in both rearing conditions (p < 0.0001). A significant interaction between dietary treatments and environmental conditions was observed for liver MDA concentrations (p = 0.002).
As expected, vitamins and Se supplementation increased levels of α-tocopherol compared with the LP/LA and HP/LA groups under TN and HS conditions in the liver. Supplementation with antioxidants decreased γ-tocopherol concentration in both HA groups reared under TN and HS conditions. However, Se concentration and α-tocopherol level were higher in supplemented groups. The environmental conditions affected the ACW content in the liver (p < 0.0001), which was lower in all experimental groups in HS, while the influence of dietary PUFA levels and antioxidant supplementation was not observed (Table 3).
The groups with linseed oil had higher liver PUFA content, as expected (p < 0.0001). The total n-6 PUFA content in chicken liver was higher in the LP groups than in the HP (p < 0.0001). The impact of the environment was also measured (p = 0.01). On the other hand, in HS, antioxidant supplement increased n-3 PUFA (HP/LA vs. HP/HA) in comparison to TN rearing conditions. Antioxidant supplement increased n-3 PUFA in HP/HA group in HS in comparison to TN condition. The ratio of n-6 to n-3 PUFA was narrower in the liver of chickens fed linseed oil compared to other groups (p < 0.0001) (Table 4).

Table 4.
Fatty acid profile of the liver (mg of fatty acids/100 g of liver).
3.3. Relative Weight of Individual Parts of Intestine, pH and Viscosity Measurements in Intestinal Content
Heat stress decreased (p < 0.0001) relative weight (% per body weight weight) of the small intestine (Table 5) as compared to TN. The dietary PUFA levels (p = 0.011) and antioxidant supplementation (p = 0.001) also had a statistical influence. On the other hand, the different feed supplements had no effect on the differences in the proportions of caecum and colon. Differences were observed only in the environment; in general, chickens housed in HS had lower small intestine proportions and higher large intestine proportions than chickens housed in TN. Interactions between diet and housing conditions were not observed.

Table 5.
Relative weight of individual parts of intestine and pH of the contents of different parts of intestine, and viscosity of small intestine contents of broilers exposed to different stressors and antioxidant levels.
The content of small intestine pH of chickens was lower in HA than in LA group and HP/HA group (p < 0.0001). No influence of environment was observed. Chickens raised in HS had a more alkaline pH of colonic contents than chickens from TN (p < 0.0001). No interaction between the stressors was found.
The higher dietary PUFA levels in the groups fed without antioxidant supplementation (HP/LA) had a significant effect on the higher viscosity rate of small intestinal contents compared to the HP/HA and LP/HA experimental groups (p = 0.039). The addition of antioxidants had a significant effect on small intestinal viscosity (p < 0.0001); beyond this, environment (p = 0.456) and interaction (p = 0.1301) had no effect.
3.4. Concentration of Short Chain Fatty Acid (SCFA) in the Content of Small Intestine and Caecum
Different antioxidant supplementation influenced the content of ethanoic acid in the small intestine, whereby the content was lower in the LP/HA and HP/HA groups under HS conditions (p = 0.001). Different diets and different rearing environments did not affect the concentration of propanoic acid and butanoic acid in the content of the small intestine. There was a significant interaction between the feed additives and the environment in the concentration of propanoic acid in the content of the small intestine (p = 0.041). In the groups with antioxidant supplement LP/HA and HP/HA, the sum of SCFAs in HS was significantly lower (p = 0.001) (Table 6).

Table 6.
Concentration of short-chain fatty acid (SCFA) in content of small intestine and caecum of broiler chickens fed different experimental diets and reared under different environmental conditions.
Significant differences in the content of butanoic acid in the content of caecum were measured among chickens receiving higher amounts of antioxidants and different PUFA levels (group LP/HA and HP/HA) in TN environment, but HS decreased butanoic acid in caecum (p = 0.002). The influence of antioxidants was also measured (p = 0.034).
The proportion of ethanoic acid in caecum was significantly higher in HS (p = 0.016). On the other hand, a greater proportion of butanoic acid was measured in the caecum of chickens raised at TN compared to chickens raised at HS (p = 0.0001). The influence of antioxidants was also measured (p = 0.027).
3.5. Villus and Crypt Measurements in the Small Intestine and Caecum
Heat stress decreased duodenum villus height (p < 0.0001). The results show that PUFA supplements also decreased villus height in the duodenum (p = 0.002) under both environmental conditions. Environmental conditions also affected crypt depth (p = 0.001), with statistically lower depth in the LP/HA group at HS compared with LP/HA at TN. Rearing conditions had an effect on the villus:crypt ratio in the duodenum (p = 0.007). Antioxidants supplementation decreased ileum villus height (p = 0.010) and crypt depth (p = 0.001) (Table 7).

Table 7.
Villus height, crypt depth and villus:crypt ratio in the small intestine and caecum of broiler chickens fed different experimental diets and reared under different environmental conditions.
4. Discussion
It is known that cyclic HS (environmental) [20,27,28] and PUFA (dietary) [15,24,44] supplementation can independently trigger oxidative stress. However, to our knowledge, the results of the effects of the interaction of both stressors on the intestinal health of broilers have not yet been published. On the other hand, research has shown that the addition of antioxidants to the diet is necessary for the mitigation of lipid peroxidation of broiler chickens under stress conditions. Supplementation with an additional combination of vitamins E, C, and Se increased plasma α-tocopherol and vitamin C concentrations, serum antioxidant capacity of lipid-soluble levels (ACL), whole blood GPx activity, and lower serum corticosterone and alkaline phosphatase levels (results not yet published).
Oxidative stress in animals and also in GIT refers to an imbalance between the production of harmful ROS and the body’s ability to counteract or neutralize their harmful effects through antioxidant defenses. It can be caused by a variety of factors, both external (exogenous, e.g., HS) and internal (endogenous, e.g., diet). In addition, antioxidants play a crucial role in mitigating oxidative stress by neutralizing harmful ROS and protecting against cellular damage. Vitamin E, C and Se supplementation showed promising results in attenuating the adverse effects of HS [19] and high dietary n-3 PUFA levels [15,16,18,19]. In addition, by acting synergistically, vitamins E, C, and Se can make an important contribution to better antioxidant activity and attenuate oxidative stress by reducing free radical production. Studies have shown the synergistic effect of two vitamins E and C [19,48] and vitamin E and Se [49,50,51], but our unpublished results show the impact of all three.
Rearing birds under HS conditions has been reported to have negative effects on their growth performance (BW, BWG, ADFI and FCR) [52]. Moreover, the combination of both stressors resulted in a greater negative impact in our study, which was also supported by other results [52]. It is known that PUFA-rich vegetable oils can increase oxidative deterioration in chickens [15,44], leading to accelerated lipid oxidation and even the formation of toxic compounds that can negatively affect broiler performance parameters [53]. Our results also confirm that the HP/LA groups had the lowest BW of chickens at the end of the experiment under both environmental conditions. In contrast, previous studies on broiler chickens with similar dietary PUFA supplements did not find negative effects on performance [18,41]. Studies confirm the positive effect of antioxidant supplementation on growth performance [16,50], which was also confirmed by our results. In addition, vitamins E, C, and Se play a significant role in the antioxidant defense system [16,48,49,50,51]. Vitamin C is important for the restoration of vitamin E [54,55]. Selenium plays an important role in the antioxidant defense system as a cofactor for enzymes such as GPx, SOD, and thioredoxin reductase [55]. However, previous research by Calik et al. [19] and Jang et al. [56] indicated that supplementation with a combination of vitamins E and Se or a combination of vitamins E and C had no effect on the growth performance of broilers exposed to HS.
In the present study, environmental conditions affected the relative weight of heart, pancreas, and intestine. Also, different dietary supplements significantly affected the relative weights of heart, pancreas, proventriculus, gizzard, and intestine (Table 3). In contrast, Hosseini-Vashan et al. [57] reported that HS did not change the relative weight of lymphoid organs. However, studies [58,59] suggest a decrease in the relative weight of lymphoid organs and antibody production under HS conditions, but there are limited data on the effects of supplementation with antioxidants on the immune response of heat-stressed birds.
In the study, MDA concentrations in the liver were used as a marker to determine the extent of lipid oxidation (Table 3). MDA concentrations in the liver were higher in the HP/LA group than in the other experimental groups (p < 0.0001). In addition, the broilers in the LA groups had higher liver MDA concentrations than those in the HA groups (p < 0.001). Moreover, a significant negative interaction was found between cyclic HS, high dietary PUFA intake and antioxidant supplementation. Studies reported that exposure of broilers to acute HS induced higher production of ROS, resulting in lipid peroxidation in the liver and serum [60]. According to data from the literature, antioxidant supplementation was effective in reducing MDA levels in the liver of broilers exposed to nutritional or HS [15,51,61,62], which was also supported by the results of our previous studies, where the combination of added antioxidants was even more effective [18,54].
The content of additional vitamin E in the liver was higher in the HA groups than in the LA groups (p < 0.0001). The effectiveness of the addition of antioxidants is confirmed also in other studies [15,19,44,51]. Supplementation with exogenous vitamins and antioxidants with antioxidant properties scavenges ROS and has a positive effect on attenuating oxidative stress in GIT [2]. Vitamin E supplementation has been shown to be effective in poultry production as it provides antioxidant protection and balances the immune system, especially under stressful conditions [55]. It is known as an essential nutrient for growth, strengthening the immune system, and promoting animal health. Consequently, the inclusion of PUFA in broiler chicken diets is critical for creating functional foods for human health through the consumption of healthier poultry products [63]. However, antioxidants such as vitamin E and Se also play an important role in protecting cells from ROS by reducing free radicals and preventing lipid peroxidation [49]. Due to their important role in the cellular antioxidant defense system, a deficiency of them strongly affects growth, meat products, and immunological functions [64]. Although poultry can produce vitamin C endogenously, research has shown that supplementing chicken diets with vitamin C during periods of HS can mitigate oxidative stress [23]. In the present study, liver ACW levels decreased at HS, confirming the importance of vitamin C supplementation at HS. Research suggests that endogenous synthesis of vitamin C may not be adequate under all stress conditions. In addition, vitamins E, C and Se play an important role in the antioxidant defense network, and they function most effectively when they work together. Vitamin E counteracts lipid peroxidation caused by free radicals but is quickly depleted if not replenished by vitamin C. Selenium-containing enzymes restore the antioxidant effect of vitamin C [65].
Dietary supplementation with PUFA affects fat content and fatty acid composition in the liver. The present results show that the percentage of n-3 PUFA in the liver was higher in the groups to which linseed oil was added (HP) than in the chicken groups that did not receive linseed oil (LP) (p < 0.0001). Lu et al. [66] reported that prolonged heat-induced stress affects aerobic metabolism, glycolysis, and deposition of triglycerides in the liver, which may also affect meat quality. However, the authors suggested that a combination of early acclimation and linseed dietary inclusion in the diet to enrich PUFAs could be an efficient way to improve thermoresistance in broiler chickens, which could be a strategy to mitigate HS [67]. As highlighted in the present results, environmental conditions had no major effect on n-3 PUFA content in the liver. Furthermore, the addition of antioxidants in HS had no effect on PUFA concentration in the liver.
It has been reported that various stressors in broiler chickens lead to physiological and immunological impairments that increase the susceptibility of the animals to diseases in broilers [68]. In chickens, a healthy gut is important to ensure efficient digestion and absorption of nutrients, a stable gut microbial population, gut barrier structure and function, and effective immune system function, which plays an important role in intestinal physiology, good production, and animal welfare. There are several stressors that can negatively affect the delicate balance between poultry components GIT, which, in turn, affects the health status and productivity of poultry [2]. Therefore, it is important to develop a strategy to mitigate oxidative stress. Supplementation with vitamins C and E improves antioxidant capacity and immune performance [69]. However, some studies have proposed that early posthatch nutrition significantly influences the development of the intestines [70,71] and gut health [72] of broilers.
A few studies are available in the literature with respect to the effect of HS conditions and PUFA addition in connection to antioxidant supplementation. In their study, Wang et al. [73] reported that supplementation with vitamin E and n-3 PUFA independently and in combination showed a more beneficial effect on improving intestinal morphology during the grower phase than the starter phase. In the present study, the relative weight of the small intestine to the total body weight of the chickens was the highest in TN (p < 0.0001) and in the HP/LA experimental group (p = 0.011). On the other hand, the different feed supplements had no effect on the differences in the proportions of caecum and colon. The strain generated by HS may also trigger changes in intestinal development by reducing the small intestine weight, the number of villi, and the proliferation rate of enterocytes [74] and directly affects the bird’s ability to digest and assimilate nutrients necessary for maintenance and production [68,75].
The relationship between the viscosity of the digestive tract and decreased fat digestibility in chickens has long been recognized [76]. Increased viscosity in the small intestine slows the digestion of nutrients by endogenous enzymes, resulting in a greater supply of substrate for the microbiota. Environmental factors that can cause changes in mucin dynamics have the potential to affect the viscosity and integrity of the mucus layer and nutrient transport [77]. Therefore, disruption of intestinal homeostasis leads to shifts in the mucus barrier that protects the intestinal mucosa. Increased permeability of the intestinal mucosa can lead to inflammatory processes and injury to mucosal cells [78]. The above research results also confirmed our findings that the higher content of n-3 PUFA in the chicken feed without antioxidant additives (HP/LA group) had a significant effect on the higher viscosity of the small intestinal contents compared to the HP/HA and LP/HA experimental groups (p = 0.039). In addition, supplementation with antioxidants also had a significant effect on reducing the viscosity of the small intestine contents. However, HS and interactions had no effect on the viscosity of the small intestinal contents.
The GIT plays a critical role in the digestion and absorption of dietary nutrients. SCFAs are products of bacterial fermentation of dietary fiber. SCFAs are defined as groups of fatty acids with fewer than six carbons, mainly acetate, propionate, and butyrate [79]. The three predominant fatty acids, which account for more than 95% of total SCFAs with a ratio of 60:20:20, exhibit variability depending on factors such as dietary components, microbiota composition, and site of fermentation [80]. The present results show that the ratio of ethanoic, propanoic and butanoic acid in the caecum is, on average, 72:7:14, indicating the dominance of ethanoic acid. Studies have shown that the ratio of acetate, propionate, and butyrate in the caecum can be influenced by dietary factors [81]. Studies also have highlighted the essential role of SCFAs in maintaining gut health in poultry [82]. SCFAs perform various functions in the gastrointestinal tract, including regulating the intestinal microflora, promoting the growth and specialization of intestinal epithelial cells, and thus increasing the absorption area of the gastrointestinal tract. In addition, acetate and propionate serve as energy sources for various tissues [47,81]. In the current study, ethanoic acid content in the small intestinal contents was higher in the LA groups than in the HA group (p = 0.001) under both rearing conditions. Ethanoic acid is known to be the major product of fermentation by heterofermentative bacteria in the intestine [83]. The content of the other SCFAs studied in the small intestine was minimal. Nevertheless, there is growing evidence that HS is a relevant environmental factor that can significantly affect gut viability and functioning and compromise animal welfare [84]. However, the main nutritional strategies investigated to date to mitigate the effects of HS include fortification with antioxidants in the diet [85,86]. Higher total SCFAs in LA compared to HA groups in the small intestine (p = 0.001) were observed.
The caecum Is an important part of the gastrointestinal tract where no absorbed fermentable materials (mainly carbohydrates) transported from the upper digestive tract are converted into SCFA and gasses [87]. In the present study, none of the stressors had an effect on the sum of SCFA. On the other hand, a greater amount of butanoic acid was measured in the caecum content of chickens raised on TN than in chickens raised on HS (p = 0.0001).
The primary locations for lipid digestion and absorption in broiler chickens are the duodenum and upper jejunum [88], and the intestinal epithelium is highly sensitive to oxidative damage. Lipids have been observed to be the nutrients most susceptible to oxidative reactions, leading to increased production of free radicals [65]. PUFA diets in the duodenum reduced villus height and crypt depth (HP/LA and HP/HA groups) in TN and HS. The results disagree with Leskovec et al. [18], who showed that a high concentration of n-3 fatty acids does not significantly affect nutrient utilization. The HS environment may further increase exposure to oxidative stress. The morphology of duodenum and jejunum was slightly affected by environmental conditions in the present study, which agrees with the findings of Santos et al. [30]. They also found that the duodenum and jejunum were more damaged than the ileum. Their results showed that HS-induced changes compared to normal villi were also seen in villus height (reduced), villus base width (increased), and epithelial cell area (reduced). However, the results of other studies also confirmed this result. The HS birds showed reduced crypt depth, mucosal area and villus height of the duodenum, as well as reduced intestinal length at 42 days of age [89]. The results of the present study also showed that PUFA supplementation decreased villus height (p = 0.002). The addition of antioxidants had no effect on reducing the decreased depth of the crypt duodenum (p = 0.136). In contrast, the addition of vitamin C alone had no effect on villus height, crypt depth or villus-to-crypt ratio in broilers under HS (42 days) [90]. No studies were found in the literature that investigated the addition of a combination of supplemental vitamins in relation to gut health. The current findings indicate that supplementary PUFAs have a negative impact on villus height in the duodenum, and HS had an additional negative impact. To fully understand the potential benefits of supranational supplementation of broiler diets with PUFA diet or/and under HS conditions on gut health, further studies are recommended.
5. Conclusions
The results of the present study indicate that the gut health of broilers was negatively affected by heat- and diet-induced oxidative stress. Cyclic HS resulted in lower final BW, lower relative weight of the heart, liver, pancreas, intestine and ACW content in the liver, and caused damage to duodenal villi height and crypt depth, which may negatively affect nutrient absorption in the small intestine. High dietary n-3 PUFA levels also affected the fatty acid profile of the liver and resulted in lower FCR during the experiment, higher MDA levels in the liver and higher viscosity of the small intestinal contents. Furthermore, we found a correlation between stressors and antioxidants affecting lipid oxidation in the liver, as evidenced by increased MDA levels. In addition, antioxidant supplementation with vitamins E, C and Se improved final body weight, relative organ weights, antioxidant protection in the liver and viscosity of the small intestine and had a potentially positive effect on ileal villus height and crypt depth. Nevertheless, further studies should be conducted to confirm the negative effects of various stressors on gut health and mucosal morphology and to determine the amounts of antioxidants that should be used in broilers reared under stressful conditions.
Author Contributions
Conceptualization: J.L., J.S. and V.R.; formulation of experimental diets: J.L., J.S. and V.R.; methodology, investigation: M.P.P., J.L., A.L. (Alenka Levart), J.S., T.P., M.V. and V.R.; data curation: M.P.P. and J.L.; writing-original draft preparation: V.R. and T.P.; supervision, T.P. and A.L. (Andrej Lavrenčič). All authors have read and agreed to the published version of the manuscript.
Funding
The present research study was financially supported by The Slovenian Research and Innovation Agency (Ljubljana, Slovenia), grant number: P4-0097 and P4-0053.
Data Availability Statement
All procedures with animals were performed according to current legislation on animal experimentation in Slovenia, which comply with the EU regulations regarding research on experimental animals. The animal study protocol was approved by the Animal Ethics Committee of the Veterinary Administration of the Republic of Slovenia (project license number U34401-9/2019/2). The experiment was performed in the research facility of the Department of Animal Science, Biotechnical Faculty, University of Ljubljana, Slovenia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tellez-Isaias, G.; Eisenreich, W.; Petrone-Garcia, V.M.; Hernandez-Velasco, X.; Castellanos-Huerta, I.; Tellez, G., Jr.; Latorre, J.D.; Bottje, W.G.; Señas-Cuesta, R.; Coles, M.E.; et al. Effects of chronic stress and intestinal inflammation on commercial poultry health and performance: A review. Ger. J. Vet. Res. 2023, 3, 38–57. [Google Scholar] [CrossRef]
- Mishra, B.; Jha, R. Oxidative stress in the poultry gut: Potential challenges and interventions. Front. Vet. Sci. 2019, 6, 60. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ren, M.; Ren, K.; Jin, Y.; Yan, M. Heat stress impacts on broiler performance: A systematic review and meta-analysis. Poult. Sci. 2020, 99, 6205–6211. [Google Scholar] [CrossRef] [PubMed]
- Gregory, N.G. How climatic changes could affect meat quality. Food Res. Int. 2010, 3, 1866–1873. [Google Scholar] [CrossRef]
- Abbas, A.; Khan, M.J.; Naeem, M.; Ayaz, M.; Sufyan, A.; Somro, M.H. Cation anion balance in avian diet: A review. Agric. Sci. Res. J. 2012, 2, 302–307. [Google Scholar]
- Vandana, G.D.; Sejian, V.; Lees, A.M.; Pragna, P.; Silpa, M.V.; Maloney, S.K. Heat stress and poultry production: Impact and amelioration. Int. J. Biometeorol. 2021, 65, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Mashaly, M.; Hendricks, G.; Kalama, M.; Gehad, A.; Abbas, A.; Patterson, P. Effect of heat stress on production parameters and immune responses of commercial laying hens. Poult. Sci. 2004, 83, 889–894. [Google Scholar] [CrossRef]
- El-Kholy, M.S.; El-Hindawy, M.M.; Alagawany, M.; El-Hack, A.; Ezzat, M.; El-Sayed, S.A.E.-G.A.E. Dietary supplementation of chromium can alleviate negative impacts of heat stress on performance, carcass yield, and some blood hematology and chemistry indices of growing Japanese quail. Biol. Trace Elem. Res. 2017, 179, 148–157. [Google Scholar] [CrossRef]
- Bell, D.D.; Weaver, W.D.; North, M.O. Commercial Chicken Meat and Egg Production, 5th ed.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2002; p. 1416. [Google Scholar] [CrossRef]
- Sahin, N.; Hayirli, A.; Orhan, C.; Tuzcu, M.; Akdemir, F.; Komorowski, J.R.; Sahin, K. Effects of the supplemental chromium form on performance and oxidative stress in broilers exposed to heat stress. Poult. Sci. 2017, 96, 4317–4324. [Google Scholar] [CrossRef]
- He, X.; Lu, Z.; Ma, B.; Zhang, L.; Li, J.; Jiang, Y.; Zhou, G.; Gao, F. Effects of chronic heat exposure on growth performance, intestinal epithelial histology, appetite-related hormones and genes expression in broilers. J. Sci. Food Agric. 2018, 98, 4471–4478. [Google Scholar] [CrossRef]
- Song, Z.H.; Cheng, K.; Zheng, X.C.; Ahmad, H.; Zhang, L.L.; Wang, T. Effects of dietary supplementation with enzymatically treated Artemisia annua on growth performance, intestinal morphology, digestive enzyme activities, immunity, and antioxidant capacity of heat-stressed broilers. Poult. Sci. 2018, 97, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Yu, Y.H.; Hsiao, F.S.H.; Su, C.H.; Liu, H.C.; Tobin, I.; Zhang, G.; Cheng, Y.-H. Influence of heat stress on poultry growth performance, intestinal inflammation, and immune function and potential mitigation by probiotics. Animals 2022, 12, 2297. [Google Scholar] [CrossRef] [PubMed]
- Kalakuntla, S.; Nagireddy, N.K.; Panda, A.K.; Jatoth, N.; Thirunahari, R.; Vangoor, R.R. Effect of dietary incorporation of n-3 polyunsaturated fatty acids rich oil sources on fatty acid profile, keeping quality and sensory attributes of broiler chicken meat. Anim. Nutr. 2017, 3, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Voljč, M.; Levart, A.; Žgur, S.; Salobir, J. The effect of α-tocopherol, sweet chestnut wood extract and their combination on oxidative stress in vivo and the oxidative stability of meat in broilers. Br. Poult. Sci. 2013, 54, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Attia, Y.A.; Al-Harthi, M.A.; El-Shafey, A.S.; Rehab, Y.A.; Kim, W.K. Enhancing tolerance of broiler chickens to heat stress by supplementation with Vitamin E, Vitamin C and/or probiotics. Ann. Anim. Sci. 2017, 17, 1155–1169. [Google Scholar] [CrossRef]
- Wang, N.; Pei, H.; Xiang, W.; Li, T.; Lin, S.; Wu, J.; Chen, Z.; Wu, H.; Li, C.; Wu, H. Rapid screening of microalgae as potential sources of natural antioxidants. Foods 2023, 12, 2652. [Google Scholar] [CrossRef] [PubMed]
- Leskovec, J.; Levart, A.; Nemec, S.; Perić, L.; Đukić Stojčić, M.; Žikić, D.; Salobir, J.; Rezar, V. Effects of supplementation with α-tocpherol, ascorbic acid, Se, or their combination in linseed oil-enriched diets on the oxidative status in broilers. Poult. Sci. 2018, 97, 1641–1650. [Google Scholar] [CrossRef] [PubMed]
- Calik, A.; Emami, N.K.; Schyns, G.; White, M.B.; Walsh, M.C.; Romero, L.F.; Dalloul, R.A. Influence of dietary vitamin E and selenium supplementation on broilers subjected to heat stress, Part II: Oxidative stress, immune response, gut integrity, and intestinal microbiota. Poult. Sci. 2022, 101, 101858. [Google Scholar] [CrossRef]
- Shakeri, M.; Oskoueian, E.; Le, H.H.; Shakeri, M. Strategies to combat heat stress in broiler chickens: Unveiling the roles of selenium, vitamin E and vitamin C. Vet. Sci. 2020, 7, 71. [Google Scholar] [CrossRef]
- Aviagen. Ross 308 Broiler: Nutrition Specifications. 2019. Available online: https://eu.aviagen.com/assets/Tech_Center/Ross_Broiler/RossBroilerNutritionSpecs2019-EN.pdf (accessed on 18 March 2021).
- NRC. Nutrient Requirements of Poultry; National Academy Press: Washington, DC, USA, 1994; p. 176. [Google Scholar] [CrossRef]
- Leeson, S.; Summers, J.D. Scott’s Nutrition of the Chicken, 4th ed.; University Books: Guelph, ON, Canada, 2001; p. 602. [Google Scholar]
- Barroeta, A. Nutritive value of poultry meat: Relationship between vitamin E and PUFA. World’s Poult. 2007, 63, 277–284. [Google Scholar] [CrossRef]
- Slawinska, A.; Mendes, S.; Dunislawska, A.; Siwek, M.; Zampiga, M.; Sirri, F.; Meluzzi, A.; Tavaniello, S.; Maiorano, G. Avian model to mitigate gut-derived immune response and oxidative stress during heat. Biosystems 2019, 178, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Kadykalo, S.; Roberts, T.; Thompson, M.; Wilson, J.; Lang, M.; Espeisse, O. The value of anticoccidials for sustainable global poultry production. Int. J. Antimicrob. Agents 2018, 51, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Dong, X.F.; Tong, J.M.; Zhang, Q. The probiotic Bacillus licheniformis ameliorates heat stress-induced impairment of egg production, gut morphology, and intestinal mucosal immunity in laying hens. Poult. Sci. 2012, 91, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Xiao, K.; Ke, Y.L.; Jiao, L.F.; Hu, C.H.; Diao, Q.Y.; Shi, B.; Zou, X.T. Effect of a probiotic mixture on intestinal microflora, morphology, and barrier integrity of broilers subjected to heat stress. Poult. Sci. 2014, 93, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.J.; Liu, N.; Wu, X.H.; Wang, G.Y.; Lin, L. Glutamine alleviates heat stress-induced impairment of intestinal morphology, intestinal inflammatory response, and barrier integrity in broilers. Poult. Sci. 2018, 97, 2675–2683. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.R.; Awati, A.; Roubos-van den Hil, P.J.; Tersteeg-Zijderveld, M.H.; Koolmees, P.A.; Fink-Gremmels, J. Quantitative histo-morphometric analysis of heat-stress-related damage in the small intestines of broiler chickens. Avian. Pathol. 2015, 44, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Rostagno, M.H. Effects of heat stress on the gut health of poultry. J. Anim. Sci. 2020, 98, skaa090. [Google Scholar] [CrossRef] [PubMed]
- Oke, O.E.; Alo, E.T.; Oke, F.O.; Oyebamiji, Y.A.; Ijaiya, M.A.; Odefemi, M.A.; Kazeem, R.Y.; Soyode, A.A.; Aruwajoye, O.M.; Ojo, R.T.; et al. Early age thermal manipulation on the performance and physiological response of broiler chickens under hot humid tropical climate. J. Therm. Biol. 2020, 88, 102517. [Google Scholar] [CrossRef]
- Onagbesan, O.M.; Uyanga, V.A.; Oso, O.; Tona, K.; Oke, O.E. Alleviating heat stress effects in poultry: Updates on methods and mechanisms of actions. Front. Vet. Sci. 2023, 10, 1255520. [Google Scholar] [CrossRef]
- Liang, F.; Jiang, S.; Mo, Y.; Zhou, G.; Yang, L. Consumption of oxidized soybean oil increased intestinal oxidative stress and affected intestinal immune variables in yellow-feathered broilers. Asian-Austr. J. Anim. Sci. 2015, 28, 1194–1201. [Google Scholar] [CrossRef]
- Circu, M.L.; Aw, T.Y. Intestinal redox biology and oxidative stress. Semin. Cell Dev. Biol. 2012, 23, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Aviagen. Ross Broiler Management Handbook. 2018. Available online: https://en.aviagen.com/assets/Tech_Center/Ross_Broiler/Ross-BroilerHandbook2018-EN.pdf (accessed on 12 April 2021).
- AOAC. Official Methods of Analysis of AOAC International. In AOAC International; Horwith, W., Ed.; AOAC: Gaithersburg, MD, USA, 2000; p. 2200. [Google Scholar]
- Fukunaga, K.; Takama, K.; Suzuki, T. High-performance liquid chromatographic determination of plasma malondialdehyde level without a solvent extraction procedure. Anal. Biochem. 1995, 230, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Rupérez, F.J.; Martín, D.; Herrera, E.; Barbas, C. Chromatographic analysis of alpha-tocopherol and related compounds in various matrices. J. Chromatogr. A 2001, 935, 45–69. [Google Scholar] [CrossRef] [PubMed]
- Park, P.W.; Goins, R.E. In situ preparation of fatty acid methyl esters for analysis of fatty acid composition in foods. J. Food Sci. 1994, 59, 1262–1266. [Google Scholar] [CrossRef]
- Pirman, T.; Rezar, V.; Vrecl, M.; Salobir, J.; Levart, A. Effect of olive leaves or marigold petal extract on oxidative stress, gut fermentative activity, and mucosa morphology in broiler chickens fed a diet rich in n-3 polyunsaturated fats. J. Poult. Sci. 2021, 58, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.H.; Knight, J.A.; Hopfer, S.M.; Zaharia, O.; Leach, C.N.; Sunderman, F.W. Lipoperoxides in plasma as measured by liquidchromatographic separation of malondialdehyde-thiobarbituric acid adduct. Clin. Chem. 1987, 33, 214–220. [Google Scholar] [CrossRef] [PubMed]
- García, J.B.; Krachler, M.; Chen, B.; Shotyk, W. Improved determination of selenium in plant and peat samples using hydride generation-atomic fluorescence spectrometry (HG-AFS). Anal. Chim. Acta 2005, 534, 255–261. [Google Scholar] [CrossRef]
- Leskovec, J.; Rezar, V.; Nemec Svete, A.; Salobir, J.; Levart, A. Antioxidative effects of olive polyphenols compared to vitamin E in piglets fed a diet rich in n-3 PUFA. Animals 2019, 9, 161. [Google Scholar] [CrossRef]
- Bedford, M.R.; Classen, H.L. Reduction in intestinal viscosity through manipulation of dietary rye and pentosanase concentration is effected through changes in the carbohydrate: Composition in the intestinal aqueous phase and results in improved growth rate and food conversion efficiency of broiler chicks. J. Nutr. 1992, 122, 560–569. [Google Scholar] [CrossRef]
- Holdeman, L.V.; Moore, W.E.C. Anaerobe Laboratory Manual, 3rd ed.; Virginia Polytechnic Institute and State University; University of Michigan: Ann Arbor, MI, USA, 1975; p. 132. [Google Scholar]
- Pirman, T.; Ribeyre, M.C.; Mosoni, L.; Remond, D.; Vrecl, M.; Salobir, J.; Patureau Mirand, P. Dietary pectin stimulates protein metabolism in the digestive tract. Nutrition 2007, 23, 69–75. [Google Scholar] [CrossRef]
- Sahin, K.; Sahin, N.; Yaralioglu, S. Effects of vitamin C and vitamin E on lipid peroxidation, blood serum metabolites, and mineral concentrations of laying hens reared at high ambient temperature. Biol. Trace Elem. Res. 2002, 85, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Ghazi Harsini, S.; Habibiyan, M.; Moeini, M.M.; Abdolmohammadi, A.R. Effects of dietary selenium, vitamin E, and their combination on growth, serum metabolites, and antioxidant defense system in skeletal muscle of broilers under heat stress. Biol. Trace Elem. Res. 2012, 148, 322–330. [Google Scholar] [CrossRef]
- Habibian, M.; Ghazi, S.; Moeini, M.M. Effects of dietary selenium and vitamin E on growth performance, meat yield, and selenium content and lipid oxidation of breast meat of broilers reared under heat Stress. Biol. Trace Elem. Res. 2016, 169, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Kumbhar, S.; Khan, A.Z.; Parveen, F.; Nizamani, Z.A.; Siyal, F.A.; El-Hack, M.E.A.; Gan, F.; Liu, Y.; Hamid, M.; Nido, S.A.; et al. Impacts of selenium and vitamin E supplementation on mRNA of heat shock proteins, selenoproteins and antioxidants in broilers exposed to high temperature. AMB Express 2018, 8, 112. [Google Scholar] [CrossRef] [PubMed]
- Goo, D.; Kim, J.H.; Park, G.H.; Delos Reyes, J.B.; Kil, D.Y. Effect of heat stress and stocking density on growth performance, breast meat quality, and intestinal barrier function in broiler chickens. Animals 2019, 9, 107. [Google Scholar] [CrossRef] [PubMed]
- Delles, R.M.; Xiong, Y.L.; True, A.D.; Ao, T.; Dawson, K.A. Dietary antioxidant supplementation enhances lipid and protein oxidative stability of chicken broiler meat through promotion of antioxidant enzyme activity. Poult. Sci. 2014, 93, 1561–1570. [Google Scholar] [CrossRef] [PubMed]
- Pečjak, M.; Leskovec, J.; Levart, A.; Salobir, J.; Rezar, V. Effects of Dietary Vitamin E, Vitamin C, Selenium and Their Combination on Carcass Characteristics, Oxidative Stability and Breast Meat Quality of Broiler Chickens Exposed to Cyclic Heat Stress. Animals 2022, 12, 1789. [Google Scholar] [CrossRef] [PubMed]
- Surai, P.F. Effect of selenium and vitamin E content of the maternal diet on the antioxidant system of the yolk and the developing chick. Br. Poult. Sci. 2002, 41, 235–243. [Google Scholar] [CrossRef]
- Jang, I.S.; Ko, Y.H.; Moon, Y.S.; Sohn, S.H. Effects of vitamin C or E on the pro-inflammatory cytokines, heat shock protein 70 and antioxidant status in broiler chicks under summer conditions. Asian-Australas. J. Anim. Sci. 2014, 27, 749–756. [Google Scholar] [CrossRef]
- Hosseini-Vashan, S.J.; Golian, A.; Yaghobfar, A. Growth, immune, antioxidant, and bone responses of heat stress-exposed broilers fed diets supplemented with tomato pomace. Int. J. Biometeorol. 2016, 60, 1183–1192. [Google Scholar] [CrossRef]
- Lara, L.J.; Rostagno, M.H. Impact of heat stress on poultry production. Animals 2013, 3, 356–369. [Google Scholar] [CrossRef]
- Habibian, M.; Ghazi, S.; Moeini, M.M.; Abdolmohammadi, A. Effect s of dietary selenium and vitamin E on immune response and biological blood parameters of broilers reared under thermoneutral or heat stress conditions. Int. J. Biometeorol. 2014, 58, 741–752. [Google Scholar] [CrossRef]
- Yang, L.; Tan, G.Y.; Fu, Y.Q.; Feng, J.H.; Zhang, M.H. Effects of acute heat stress and subsequent stress removal on function of hepatic mitochondrial respiration, ROS production and lipid peroxidation in broiler chickens. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2010, 151, 204–208. [Google Scholar] [CrossRef]
- Khan, R.; Naz, S.; Nikousefat, Z.; Tufarelli, V.; Javdani, M.; Rana, N.; Laudadio, V. Effect of vitamin E in heat-stressed poultry. World’s Poult. Sci. J. 2011, 67, 469–478. [Google Scholar] [CrossRef]
- Jena, B.; Panda, N.; Patra, R.; Mishra, P.; Behura, N.; Panigrahi, B. Supplementation of vitamin E and C reduces oxidative stress in broiler breeder hens during summer. Food Nut. Sci. 2013, 4, 33–37. [Google Scholar] [CrossRef]
- Galli, F.; Azzi, A.; Birringer, M.; Cook Mills, J.M.; Eggersdorfer, M.; Frank, J.; Cruciani, G.; Lorkowski, S.; Özer, N.K. Vitamin E: Emerging aspects and new directions. Free Radic. Biol. Med. 2017, 102, 16–36. [Google Scholar] [CrossRef]
- Niu, Z.Y.; Liu, F.Z.; Yan, Q.L.; Li, L. Effects of different levels of selenium on growth performance and immunocompetence of broilers under heat stress. Arch. Anim. Nutr. 2009, 63, 56–65. [Google Scholar] [CrossRef]
- Lykkesfeldt, J.; Svendsen, O. Oxidants and antioxidants in disease: Oxidative stress in farm animals. Vet. J. 2007, 173, 502–511. [Google Scholar] [CrossRef]
- Lu, Z.; He, X.; Ma, B.; Zhang, L.; Li, J.; Jiang, Y.; Zhou, G.; Gao, F. Chronic heat stress impairs the quality of breast-muscle meat in broilers by affecting redox status and energy-substance metabolism. J. Agric. Food Chem. 2017, 65, 11251–11258. [Google Scholar] [CrossRef]
- Zineb, B.; Said, D.; Djilali, B. Impact of both early-age acclimation and linseed dietary inclusion on fat deposition and fatty acids’ meat traits in heat-stressed broiler chickens. J. Adv. Vet. Anim. Res. 2021, 19, 237–245. [Google Scholar] [CrossRef]
- Quintero-Filho, W.M.; Ribeiro, A.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Sakai, M.; Sá, L.R.M.; Ferreira, A.J.P.; Palermo-Neto, J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 2010, 89, 1905–1914. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.N.; Niu, Z.Y.; Sun, T.T.; Wang, Z.P.; Jiao, P.X.; Zi, B.B.; Chen, P.P.; Tian, D.L.; Liu, F.F. Vitamin E and vitamin C supplementation improves antioxidant status and immune function in oxidative-stressed breeder roosters by up-regulating expression of GSH-Px gene. Poult. Sci. 2018, 97, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Noy, Y.; Geyra, A.; Sklan, D. The effect of early feeding on growth and small intestinal development in the posthatch poult. Poult. Sci. 2001, 80, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Batal, A.B.; Parsons, C.M. Effect of fasting versus feeding Oasis after hatching on nutrient utilization in chicks. Poult. Sci. 2002, 81, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Ao, Z.; Kocher, A.; Choct, M. Effects of dietary additives and early feeding on performance, gut development and immune status of broiler chickens challenged with Clostridium perfringens. Asian-Australas. J. Anim. Sci. 2012, 25, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Clark, D.L.; Jacobi, S.K.; Velleman, S.G. Supplementation of vitamin E and omega-3 fatty acids during the early posthatch period on intestinal morphology and gene expression differentiation in broilers. Poult. Sci. 2021, 100, 100954. [Google Scholar] [CrossRef] [PubMed]
- Uni, Z.; Gal-Garber, O.; Geyra, A.; Sklan, D.; Yahav, S. Changes in growth and function of chick small intestine epithelium due to early thermal conditioning. Poult. Sci. 2001, 80, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Marchini, C.F.; Café, M.B.; Araújo, E.G.; Nascimento, M.R. Physiology, cell dynamics of small intestinal mucosa, and performance of broiler chickens under heat stress: A review. Rev. Colomb. Cienc. Pecu. 2016, 29, 159–168. [Google Scholar] [CrossRef]
- Smits, C.H.M.; Annison, G. Non-starch plant polysaccharides in broiler nutrition—Towards a physiologically valid approach to their determination. Worlds Poult. Sci. J. 1996, 52, 203–221. [Google Scholar] [CrossRef]
- Horn, N.L.; Donkin, S.S.; Applegate, T.J.; Adeola, O. Intestinal mucin dynamics: Response of broiler chicks and White Pekin ducklings to dietary threonine. Poult. Sci. 2009, 88, 1906–1914. [Google Scholar] [CrossRef]
- Dharmani, P.; Srivastava, V.; Kissoon-Singh, V.; Chadee, C. Role of intestinal mucins in innate host defense mechanisms against pathogens. J. Innate Immun. 2009, 1, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Abelardo, M.; Gueimonde, M.; de los Reyes-Gavilán, C.G.; Salazar, N. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Fredstrom, S.B.; Lampe, J.W.; Jung, H.J.; Slavin, J.L. Apparent fiber digestibility and fecal short-chain fatty acid concentrations with ingestion of two types of dietary fiber. J. Parenter. Enter. Nutr. 1994, 18, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Jha, R. Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J Anim. Sci. Biotechnol. 2019, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, Q.; Yang, Y.; Guo, A. Biological function of short-chain fatty acids and its regulation on intestinal health of poultry. Front. Vet. Sci. 2021, 18, 736739. [Google Scholar] [CrossRef]
- Immersell, V.F.; de Buck, J.; Pasmans, F.; Veelg, P.; Bottreau, E.; Fievez, V.; Haesebrouck, F.; Ducatelle, R. Invasion of Salmonella enteritidis in avian intestinal epithelia l cells in vitro is influenced by short-chain fatty acids. Int. J. Food Microbiol. 2003, 85, 237–248. [Google Scholar] [CrossRef]
- Kikusato, M.; Toyomizu, M. Mechanisms underlying the effects of heat stress on intestinal integrity, inflammation, and microbiota in chickens. J. Poult. Sci. 2023, 9, 2023021. [Google Scholar] [CrossRef]
- Kikusato, M.; Toyomizu, M. Differential effects of heat stress on oxidative status of skeletal muscle with different muscle fibre compositions in broiler chicken. J. Anim. Feed Sci. 2019, 28, 78–82. [Google Scholar] [CrossRef]
- Sumanu, V.O.; Naidoo, V.; Oosthuizen, M.C.; Chamunorwa, J.P. Adverse effects of heat stress during summer on broiler chickens production and antioxidant mitigating effects. Int. J. Biometeorol. 2022, 66, 2379–2393. [Google Scholar] [CrossRef]
- Jamroz, D.; Jakobsen, K.; Knudsen, K.E.B.; Wiliczkjewicz, A.; Orda, J. Digestibility and energy value of non-starch polysaccharides in young chickens, ducks and geese, fed diets containing high amounts of barley. Comp. Biochem. Physiol. Part A 2002, 131, 357–668. [Google Scholar] [CrossRef]
- Tancharoenrat, P.; Ravindran, V.; Zaefarian, F.; Ravindran, G. Digestion of fat and fatty acids along the gastrointestinal tract of broiler chickens. Poult. Sci. 2014, 93, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Marchini, C.F.P.; Silva, P.L.; Nascimento, M.R.B.M.; Beletti, M.E.; Silva, N.M.; Guimarães, E.C. Body weight, intestinal morphometry and cell proliferation of broiler chickens submitted to cyclic heat stress. Int. J. Poult. Sci. 2011, 10, 455–460. [Google Scholar] [CrossRef]
- Gungor, E.; Altop, A.; Erener, G. Effect of raw and fermented grape seed on growth performance, antioxidant capacity, and cecal microflora in broiler chickens. Animals 2021, 15, 100194. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).