Estimation of Carcass Trait Characteristics, Proportions, and Their Correlation with Preslaughter Body Weight in Indigenous Chickens in Southeastern Ethiopia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Study Areas
2.2. Animals and Data Collection
2.3. Statistical Analysis
3. Results
3.1. Preslaughter Body Weight, Dressed Carcass Weight, and Dressing Percentages
3.2. Characteristics of Carcass Components and Edible Giblet Yields
3.3. Effect of District, Age, and the District–Age Interaction on Carcass Components Characteristics
3.4. Carcass Component and Edible Giblet Proportions
3.5. Multivariate Analysis of Carcass Component and Edible Giblets
3.6. Carcass Component and Preslaughter Body Weight Correlations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tegegne, A.; Gebremedhin, B.; Hoekstra, D. Livestock Input Supply and Service Provision in Ethiopia: Challenges and Opportunities for Market-Oriented Development; IPMS Working Paper; ILRI: Nairobi, Kenya, 2010. [Google Scholar]
- Yami, A. Poultry production in Ethiopia. World’s Poult. Sci. J. 1995, 51, 197–201. [Google Scholar] [CrossRef]
- Melesse, A. Significance of scavenging chicken production in the rural community of Africa for enhanced food security. World’s Poult. Sci. J. 2014, 70, 593–606. [Google Scholar] [CrossRef]
- Moges, F.; Mellesse, A.; Dessie, T. Assessment of village chicken production system and evaluation of the productive and reproductive performance of local chicken ecotype in Bure district, North West Ethiopia. Afr. J. Agric. Res. 2010, 5, 1739–1748. [Google Scholar]
- CSA (Central Statistical Authority). Livestock and Livestock Characteristics (Private Peasant Holdings); CSA: Addis Ababa, Ethiopia, 2022; p. 219. [Google Scholar]
- Melesse, A. Comparative Studies on Performance and Physiological Responses of Ethiopian Indigenous Naked Neck (Angete-Melata) Chickens and Their F1 Crosses to Long-Term Heat Exposure; Logos: Berlin, Germany, 2000. [Google Scholar]
- De Marchi, M.; Dalvit, C.; Targhetta, C.; Cassandro, M. Assessing genetic diversity in indigenous Veneto chicken breeds using AFLP markers. Anim. Genet. 2006, 37, 101–105. [Google Scholar] [CrossRef] [PubMed]
- FAO. Phenotypic Characterization of Animal Genetic Resources; Animal Production and Health Guidelines No. 11; Food and Agricultural Organization: Rome, Italy, 2012. [Google Scholar]
- Castellini, C.; Mugnai, C.; Dal Bosco, A. Effect of organic production system on broiler carcass and meat quality. Meat Sci. 2002, 60, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Castellini, C.; Dal Bosco, A.; Mugnai, C.; Pedrazzoli, M. Comparison of two chicken genotypes organically reared: Oxidative stability and other qualitative traits of the meat. Ital. J. Anim. Sci. 2006, 5, 29–42. [Google Scholar] [CrossRef]
- Faridi, A.; Sakomura, N.; Golian, A.; Marcato, S. Predicting body and carcass characteristics of 2 broiler chicken strains using support vector regression and neural network models. Poult. Sci. 2012, 91, 3286–3294. [Google Scholar] [CrossRef]
- da Silva Souza, J.; do Santos Difante, G.; Neto, J.V.E.; Lana, Â.M.Q.; da Silva Roberto, F.F.; Ribeiro, P.H.C. Biometric measurements of Santa Inês meat sheep reared on Brachiaria brizantha pastures in Northeast Brazil. PLoS ONE 2019, 14, e0219343. [Google Scholar] [CrossRef]
- Moreda, E.; Hareppal, S.; Johansson, A.; Sisaye, T.; Sahile, Z. Characteristics of indigenous chicken production system in south west and south part of Ethiopia. Br. J. Poult. Sci. 2013, 2, 25–32. [Google Scholar]
- Kingori, A.; Wachira, A.; Tuitoek, J. Indigenous chicken production in Kenya: A review. Int. J. Poult. Sci. 2010, 9, 309–316. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2010. [Google Scholar]
- Mogesse, H.H. Phenotypic and Genetic Characterization of Indigenous Chicken Populations in Northwest Ethiopia. Ph.D. Thesis, University of the Free State, Bloemfontein, South Africa, 2007. [Google Scholar]
- Alemneh, W.; Berihun, K.; Melesse, A. Comparative study on carcass quality characteristics of indigenous chickens and their F1-crosses with the Sasso chicken breed in Sheka zone, South Western Ethiopia. Int. J. Food Sci. Agric. 2021, 5, 692–697. [Google Scholar] [CrossRef]
- Iqbal, S.; Pampori, Z.; Hasin, D. Carcass and egg characteristics of indigenous chicken of Kashmir (Kashmir favorella). Indian J. Anim. Res. 2009, 43, 194–196. [Google Scholar]
- Motsepe, R.; Mabelebele, M.; Norris, D.; Brown, D.; Ginindza, J.N.M. Carcass and meat quality characteristics of South African indigenous chickens. Indian J. Anim. Res. 2016, 50, 580–587. [Google Scholar] [CrossRef]
- Yousif, I.; Binda, B.; Elamin, K.; Malik, H.; Babiker, M. Evaluation of Carcass Characteristics and Meat Quality of Indigenous Fowl Ecotypes and Exotic Broiler Strains Raised under Hot Climate. Glob. J. Anim. Sci. Res. 2014, 2, 365–371. [Google Scholar]
- Youssao, I.; Alkoiret, I.; Dahouda, M.; Assogba, M.; Idrissou, N.; Kayang, B.; Yapi-Gnaoré, V.; Assogba, H.; Houinsou, A.; Ahounou, S. Comparison of growth performance, carcass characteristics and meat quality of Benin indigenous chickens and Label Rouge (T55× SA51). Afr. J. Biotechnol. 2012, 11, 15569–15579. [Google Scholar]
- Magala, H.; Kugonza, D.R.; Kwizera, H.; Kyarisiima, C. Influence of management system on growth and carcass characteristics of Ugandan local chickens. J. Anim. Sci. Adv. 2012, 2, 558–567. [Google Scholar]
- Melesse, A.; Dotamo, E.; Banerjee, S.; Berihun, K.; Beyan, M. Studies on carcass traits, nutrient retention and utilization of Koekoeck chickens fed diets containing different protein levels with Iso-Caloric ration. J. Anim. Sci. Adv. 2013, 3, 532–543. [Google Scholar]
- Azahan, E.; Zahari, W. Observation on some characteristics of carcass and meat of Malaysian kampong chickens. Mardi Res. Bull. 1983, 11, 225–232. [Google Scholar]
- Jaturasitha, S.; Kayan, A.; Wicke, M. Carcass and meat characteristics of male chickens between Thai indigenous compared with improved layer breeds and their crossbred. Arch. Anim. Breed. 2008, 51, 283–294. [Google Scholar] [CrossRef]
- Melesse, A.; Getye, Y.; Berihun, K.; Banerjee, S. Effect of feeding graded levels of Moringa stenopetala leaf meal on growth performance, carcass traits and some serum biochemical parameters of Koekoek chickens. Livest. Sci. 2013, 157, 498–505. [Google Scholar] [CrossRef]
- Yitbarek, M.B. Carcass characteristics of Rhode Island Red (RIR) grower chicks feed on different levels of dried tomato pomace (DTP). Int. J. Adv. Res. 2013, 1, 17–22. [Google Scholar]
- Alemu, Y.; Tadelle, D. The Status of Poultry Research and Development in Ethiopia; Research Bulletin No. 4, Poultry Commodity Research Program; Debrezeit Agricultural Research Center, Alemaya University of Agriculture: Haramaya, Ethiopia, 1997; p. 62.
- Wang, K.; Shi, S.; Dou, T.; Sun, H. Effect of a free-range raising system on growth performance, carcass yield, and meat quality of slow-growing chicken. Poult. Sci. 2009, 88, 2219–2223. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, F.; Buchtova, H. Comparison of qualitative and quantitative properties of the wings, necks and offal of chicken broilers from organic and conventional production systems. Veterinární Med. 2016, 61, 643–651. [Google Scholar] [CrossRef]
- Coban, O.; Lacin, E.; Aksu, M.; Kara, A.; Sabuncuoglu, N. The impact of slaughter age on performance, carcass traits, properties of cutup pieces of carcasses, and muscle development in broiler chickens. Eur. Poult. Sci. Arch. Geflügelkunde 2014, 78. [Google Scholar] [CrossRef]
- Kim, C.H.; Kang, H.K. Effects of stock density on the growth performance, and meat quality of Korean native chickens. Korean J. Poult. Sci. 2020, 47, 1–7. [Google Scholar] [CrossRef]
- Havenstein, G.; Ferket, P.; Scheideler, S.; Rives, D. Carcass composition and yield of 1991 vs. 1957 broilers when fed “typical” 1957 and 1991 broiler diets. Poult. Sci. 1994, 73, 1795–1804. [Google Scholar] [CrossRef]
- Thiruvenkadan, A.; Prabakaran, R.; Panneerselvam, S. Broiler breeding strategies over the decades: An overview. World’s Poult. Sci. J. 2011, 67, 309–336. [Google Scholar] [CrossRef]
- Venturini, G.; Cruz, V.; Rosa, J.; Baldi, F.; El Faro, L.; Ledur, M.; Peixoto, J.; Munari, D. Genetic and phenotypic parameters of carcass and organ traits of broiler chickens. Genet. Mol. Res. 2014, 13, 10294–10300. [Google Scholar] [CrossRef]
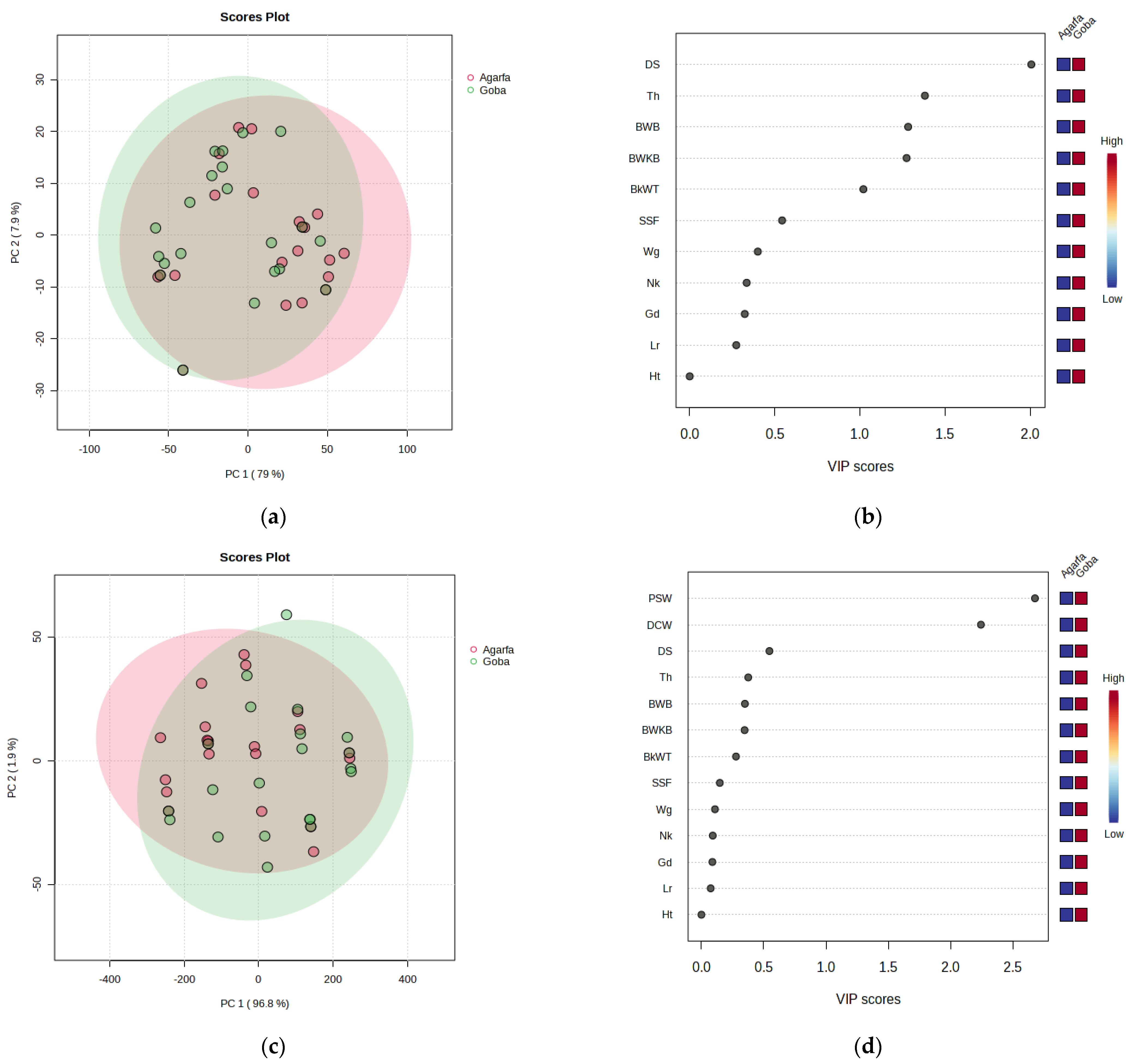
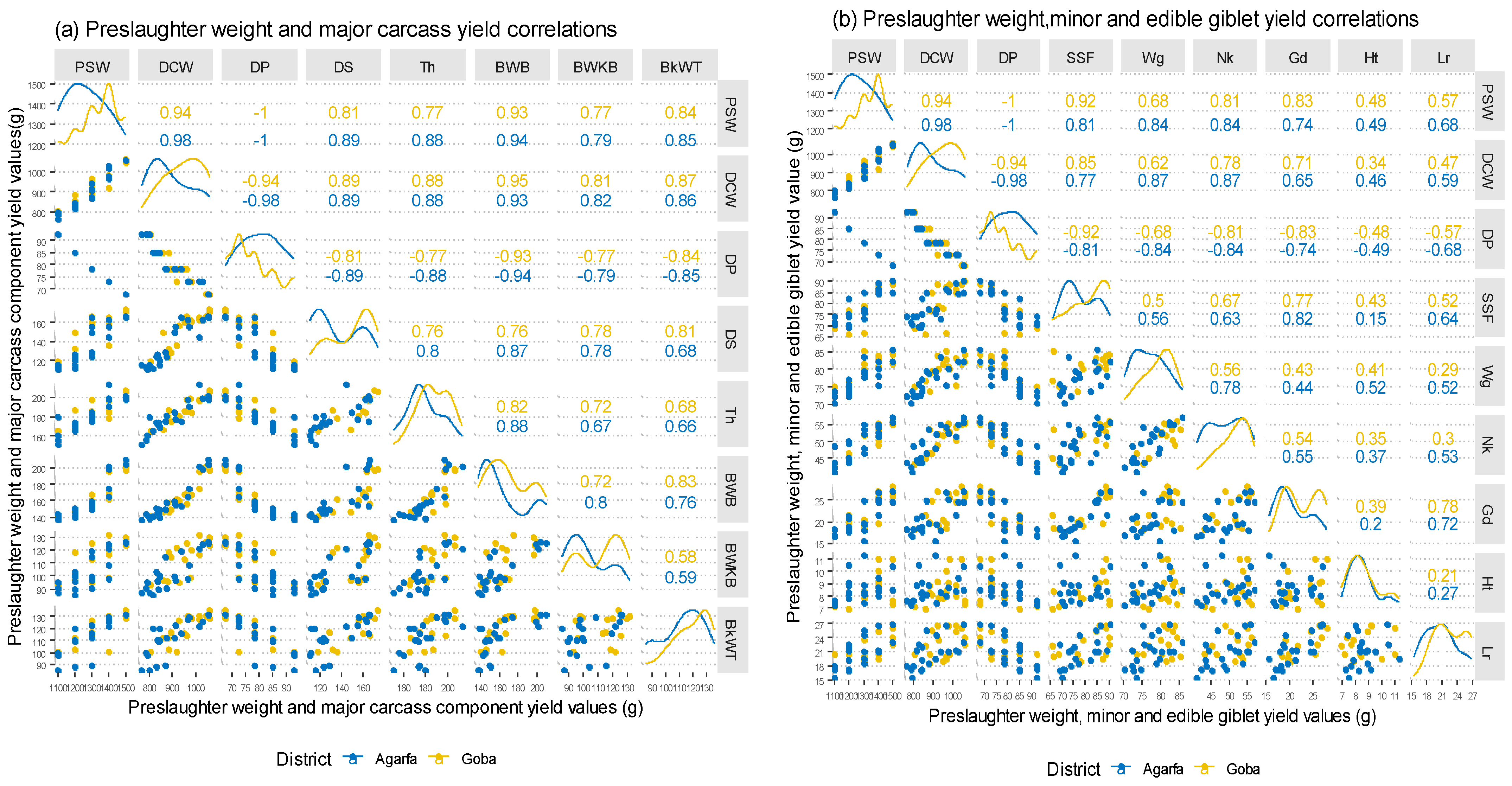
| Traits | Ages (A) | p-Values | District (D) | p-Values | |||
|---|---|---|---|---|---|---|---|
| AG-1 | AG-2 | AG-3 | Goba | Agarfa | |||
| PSW | 1300 ± 57.74 | 1270.59 ± 25.4 | 1338.89 ± 32.5 | 0.341 | 1338.1 ± 27.15 a | 1271.4 ± 27.73 a | 0.094 |
| DCW | 923.69 ± 39.09 | 901.36 ± 19.34 | 939.57 ± 23.15 | 0.542 | 949.39 ± 18.18 a | 893.52 ± 20.19 b | 0.046 |
| DP | 79.38 ± 3.69 | 80.71 ± 1.55 | 76.95 ± 2.01 | 0.434 | 76.83 ± 1.65 a | 80.92 ± 1.74 a | 0.096 |
| DS | 142.33 ± 9.25 | 138.51 ± 4.79 | 146.79 ± 4.99 | 0.597 | 149.49 ± 4.16 a | 135.9 ± 4.59 b | 0.034 |
| Th | 184.91 ± 5.04 | 182.39 ± 3.48 | 184.67 ± 4.09 | 0.907 | 188.47 ± 2.94 a | 179.11 ± 3.45 b | 0.045 |
| BWB | 165.27 ± 8.3 | 158.92 ± 5.09 | 169.27 ± 5.78 | 0.459 | 168.76 ± 4.77 a | 160.07 ± 5.06 a | 0.219 |
| BWKB | 107.3 ± 6.52 | 102.98 ± 3.17 | 110.59 ± 3.57 | 0.381 | 111.28 ± 3.22 a | 102.65 ± 2.99 a | 0.057 |
| BkWT | 114.59 ± 6.74 | 114.98 ± 2.81 | 115.3 ± 3.93 | 0.999 | 118.51 ± 2.9 a | 111.6 ± 3.37 a | 0.128 |
| SSF | 79.97 ± 2.67 | 77.18 ± 1.79 | 82.4 ± 1.8 | 0.159 | 81.72 ± 1.77 a | 78.04 ± 1.5 a | 0.121 |
| Wg | 78.86 ± 1.61 | 77.11 ± 0.87 | 79.41 ± 1.05 | 0.282 | 79.74 ± 0.78 a | 77.03 ± 0.93 b | 0.031 |
| Nk | 50.46 ± 1.82 | 49.29 ± 1.05 | 51.13 ± 1.1 | 0.547 | 51.41 ± 0.81 a | 49.14 ± 1.1 a | 0.104 |
| Gd | 21.63 ± 1.51 | 20.36 ± 0.81 | 22.86 ± 0.87 | 0.161 | 22.74 ± 0.79 a | 20.55 ± 0.76 b | 0.053 |
| Ht | 8.54 ± 0.39 | 8.66 ± 0.27 | 8.51 ± 0.29 | 0.921 | 8.58 ± 0.26 a | 8.58 ± 0.24 a | 0.989 |
| Lr | 20.51 ± 1.55 | 21.82 ± 0.72 | 22.04 ± 0.74 | 0.639 | 22.62 ± 0.65 a | 20.77 ± 0.71 a | 0.060 |
| Traits | Goba | Agarfa | p-Values | ||||
|---|---|---|---|---|---|---|---|
| AG-1 | AG-2 | AG-3 | AG-1 | AG-2 | AG-3 | ||
| PSW | 1266.67 ± 88.19 | 1300 ± 46.29 | 1390 ± 31.45 | 1325.00 ± 85.39 | 1244.44 ± 24.22 | 1275 ± 55.9 | 0.312 |
| DCW | 922.9 ± 63.87 | 933.86 ± 30.42 | 969.76 ± 24.56 | 924.28 ± 57.51 | 872.47 ± 21.71 | 901.84 ± 39.93 | 0.680 |
| DP (%) | 81.28 ± 5.91 | 79.11 ± 2.85 | 73.68 ± 1.74 | 77.95 ± 5.34 | 82.13 ± 1.50 | 81.04 ± 3.60 | 0.309 |
| DS | 147.03 ± 15.68 | 148.13 ± 7.18 | 151.32 ± 5.61 | 138.8 ± 12.91 | 129.96 ± 5.22 | 141.13 ± 8.80 | 0.806 |
| Th | 187.13 ± 5.04 | 187.64 ± 4.71 | 189.54 ± 4.94 | 183.25 ± 8.60 | 177.73 ± 4.75 | 178.59 ± 6.54 | 0.874 |
| BWB | 159.83 ± 10.98 | 164.24 ± 8.27 | 175.06 ± 6.84 | 169.35 ± 12.97 | 154.19 ± 6.22 | 162.04 ± 9.69 | 0.540 |
| BWKB | 110.43 ± 12.93 | 107.55 ± 5.36 | 114.52 ± 4.12 | 104.95 ± 7.80 | 98.92 ± 3.34 | 105.69 ± 5.99 | 0.965 |
| BkWT | 110.50 ± 13.30 | 117.64 ± 4.46 | 121.61 ± 3.43 | 117.65 ± 7.96 | 112.62 ± 3.60 | 107.41 ± 7.04 | 0.265 |
| SSF | 78.83 ± 3.15 | 78.64 ± 3.00 | 85.06 ± 2.45 | 80.83 ± 4.42 | 75.89 ± 2.16 | 79.08 ± 2.30 | 0.483 |
| Wg | 79.00 ± 3.33 | 78.69 ± 1.42 | 80.81 ± 0.79 | 78.75 ± 1.86 | 75.70 ± 0.86 | 77.66 ± 2.06 | 0.699 |
| Nk | 50.13 ± 0.87 | 51.35 ± 1.58 | 51.84 ± 1.19 | 50.70 ± 3.34 | 47.46 ± 1.15 | 50.25 ± 2.03 | 0.522 |
| Gd | 21.03 ± 1.88 | 21.81 ± 1.45 | 24.00 ± 1.00 | 22.08 ± 2.46 | 19.08 ± 0.62 | 21.44 ± 1.40 | 0.458 |
| Ht | 8.10 ± 0.40 | 8.43 ± 0.35 | 8.84 ± 0.46 | 8.88 ± 0.59 | 8.88 ± 0.42 | 8.10 ± 0.25 | 0.208 |
| Lr | 20.70 ± 2.27 | 23.31 ± 1.03 | 22.65 ± 0.89 | 20.38 ± 2.42 | 20.49 ± 0.83 | 21.28 ± 1.25 | 0.649 |
| Classification | Trait Components | Goba (n = 21) | Agarfa (n = 21) | p-Values |
|---|---|---|---|---|
| Major carcass component proportion | Drumstick | 15.71 ± 0.23 | 15.15 ± 0.24 | 0.1045 |
| Thigh | 19.90 ± 0.20 | 20.09 ± 0.19 | 0.4838 | |
| Breast without keel bone | 17.73 ± 0.21 | 17.86 ± 0.21 | 0.6548 | |
| Breast with keel bone | 11.71 ± 0.22 | 11.48 ± 0.18 | 0.4296 | |
| Back with Thorax | 12.47 ± 0.17 | 12.48 ± 0.25 | 0.9760 | |
| Minor carcass component proportion | Skin with subcutaneous fat | 8.62 ± 0.13 | 8.76 ± 0.12 | 0.4087 |
| Wing | 8.44 ± 0.12 | 8.67 ± 0.12 | 0.1910 | |
| Neck | 5.43 ± 0.07 | 5.51 ± 0.07 | 0.4200 | |
| Edible giblet proportion | Gizzard | 1.70 ± 0.04 | 1.61 ± 0.04 | 0.1283 |
| Heart | 0.64 ± 0.02 | 0.68 ± 0.02 | 0.1540 | |
| Liver | 1.69 ± 0.04 | 1.63 ± 0.04 | 0.2782 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mekonnen, K.T.; Lee, D.-H.; Cho, Y.-G.; Son, A.-Y.; Seo, K.-S. Estimation of Carcass Trait Characteristics, Proportions, and Their Correlation with Preslaughter Body Weight in Indigenous Chickens in Southeastern Ethiopia. Agriculture 2024, 14, 50. https://doi.org/10.3390/agriculture14010050
Mekonnen KT, Lee D-H, Cho Y-G, Son A-Y, Seo K-S. Estimation of Carcass Trait Characteristics, Proportions, and Their Correlation with Preslaughter Body Weight in Indigenous Chickens in Southeastern Ethiopia. Agriculture. 2024; 14(1):50. https://doi.org/10.3390/agriculture14010050
Chicago/Turabian StyleMekonnen, Kefala Taye, Dong-Hui Lee, Young-Gyu Cho, Ah-Yeong Son, and Kang-Seok Seo. 2024. "Estimation of Carcass Trait Characteristics, Proportions, and Their Correlation with Preslaughter Body Weight in Indigenous Chickens in Southeastern Ethiopia" Agriculture 14, no. 1: 50. https://doi.org/10.3390/agriculture14010050
APA StyleMekonnen, K. T., Lee, D.-H., Cho, Y.-G., Son, A.-Y., & Seo, K.-S. (2024). Estimation of Carcass Trait Characteristics, Proportions, and Their Correlation with Preslaughter Body Weight in Indigenous Chickens in Southeastern Ethiopia. Agriculture, 14(1), 50. https://doi.org/10.3390/agriculture14010050









