Abstract
Camelina sativa is an annual oilseed crop that requires low inputs. Recently, interest in camelina oil for both human use and biofuel production has increased. Camelina oil extraction is performed through two main methods, namely, mechanical expulsion and solvent extraction. The resulting meals from the oil extraction process show promise as an animal feed due to their high crude protein content. Solvent extraction removes more oil from the seed, which results in a meal that is lower in fat and higher in crude protein concentration than expelled meal (3.52 vs. 13.69% and 41.04 vs. 34.65%, respectively). Solvent-extracted camelina meal has a similar chemical composition to canola meal but less crude protein and more fiber than soybean meal. Camelina meal is also limited by its anti-nutritional factors, mainly glucosinolates. Camelina meal contains 23.10 to 44.90 mmol/kg of glucosinolates, but processing methods may be able to decrease the total glucosinolates. Heat-treating the camelina meal can decrease glucosinolates and remove residual solvent in the solvent-extracted meal. The fungal fermentation of canola meal has also decreased glucosinolates, which could be used in camelina meal as well. The selective breeding of camelina varieties to decrease glucosinolates in the plant is also a solution to the high glucosinolates found in camelina meal. Current feed regulations in the US and Canada limit camelina meal to 10% inclusion in broiler chicken, laying hen, and cattle diets.
1. Introduction
Camelina (Camelina sativa) is a member of the Brassicaceae family and is an annual oilseed crop. Camelina is also known as false flax or gold of pleasure. Grown as either a summer or winter crop, camelina shows potential as a low-input cover crop in the semiarid climate of the great plains [1]. Camelina has a short lifecycle, making it useful in double cropping systems with soybeans and corn, and its cold and drought tolerance make it useful in a wheat-fallow system [2]. Camelina has both winter and summer varieties, making it suitable for winter annual production in the Northern Great Plains of the US [3]. The winter camelina yielded 743 kg/ha in the US [3]. The production of camelina has decreased in the US from 2009 to 2017 [1], with 937 acres planted in 2017 [4]. The decrease in camelina production is due to decreasing camelina seed prices, partially from lower crude oil prices [1]. Despite the decrease in US camelina acreage, interest in camelina has been increasing, especially as biofuel production increases [5]. The seed contains 38 to 43% fat and is primarily used for its oil in human use and biofuel [2]. Camelina oil contains high levels of the essential fatty acids linoleic and alpha-linolenic acid, making it useful as an edible oil [6]. Recently, camelina oil has been investigated for use in biofuel production, especially its use in jet biofuel [1]. Once the oil has been removed from the seed, camelina meal becomes the leftover as a byproduct. The oil is primarily extracted from the seed in one of two ways: mechanical expulsion or solvent extraction. Mechanical expulsion involves physically pressing the oil from the seed, while solvent extraction then uses a solvent, often hexane, to further remove oil from the seed. This results in two different camelina meals of differing chemical compositions being produced.
An increase in camelina and biofuel production will result in an increase in byproducts, which may provide an additional opportunity for camelina meal as a feed ingredient in animal production. Animal agriculture already utilizes a wide variety of byproducts, including byproducts from the ethanol industry (distillers’ grains) and byproducts of oilseeds (soybean and canola meals). Camelina meal has a high concentration of crude protein (CP) and the essential fatty acid, alpha-linolenic acid (omega-3), making it attractive as a feed ingredient; however, anti-nutritional factors in camelina may limit the inclusion in diets. This paper discusses the efficacy of utilizing camelina meal as a feed ingredient for the cattle, poultry, and swine industry.
2. Production of Camelina Meal
2.1. Mechanical Extraction of Camelina Oil
The mechanical extraction of oilseeds uses pressure to extract the oil from the seed. The mechanical extraction of oil is one of the oldest methods of oil extraction and is commonly used because of its relative ease and low costs [7]. The two main methods of mechanical extraction are a screw press and a hydraulic press. Although mechanical extraction is less expensive than other methods, oil yields are lower than solvent extraction (29.9% vs. 35.9% oil recovered from seeds, respectively; [8]). A screw press has slightly improved yields compared to hydraulic pressing and can also be adapted to continuous extraction which is beneficial when processing large quantities of oilseeds [7]. The lower oil yield from mechanical extraction means that the resulting meal is lower in CP content because it is diluted by the greater fat content. This greater fat content can be beneficial, as it can supply extra energy to the diet; however, in ruminant diets, care must be taken not to over supplement fat, as it can negatively impact the rumen microbes.
2.2. Solvent Extraction of Camelina Oil
Solvent extraction has higher capital costs but results in a greater recovery of oil [2]. Solvent extraction generally begins with pressing the seeds to remove most of the oil or pretreating the seeds via grinding and heating to reduce the viscosity of the oil, and then running a solvent, most commonly hexane, through the press cake to remove the residual oil. Solvent extraction results in oil yields of 95–97% [2]. Once the oil has been extracted, the solvent must be removed from both the oil and the meal. This solvent can be reused to help reduce costs. The greater oil yield from solvent extraction results in a meal that has less fat, and CP is more concentrated because of the lower fat content. The meal is toasted after the solvent is removed at 105 C for 30–40 min; then, it is ground or pelleted once the meal has been cooled [2]. Toasting the meal after oil extraction using steam helps remove residual solvent and reduce glucosinolates [9]. Although research on camelina meal is limited, when canola meal was toasted at 100 C, the concentration of glucosinolates decreased [10]. The solvent-extracted camelina meal contains about 3.7% fat, 41.6% CP, and 33.0% neutral detergent fiber (NDF) on a dry matter (DM) basis (Table 1).

Table 1.
Chemical composition of solvent-extracted and expelled camelina meals, solvent-extracted canola meal, solvent-extracted soybean meal, and caranita meal.
3. Nutrient Composition of Camelina Meal
Oilseed meals are a common feed ingredient in the livestock industry due to their high concentrations of CP. Canola and soybean meals are both readily acceptable ingredients in ruminant and monogastric diets; however, less research has been conducted on camelina meal and its use as a feed ingredient. Camelina seeds have high levels of fat; however, post oil extraction, the meal contains mostly CP and fiber [11]. Camelina meal has a similar nutrient composition to canola meal, although this can vary greatly depending on the oil extraction process used [26]. Using a solvent results in a meal that has less fat and greater CP concentrations.
3.1. Feeding Value of Crude Protein of Camelina Meal
The primary role of camelina meal is most likely as a protein supplement due to its high levels of CP, which are similar to canola meal but less than soybean meal, both of which are commonly fed as a protein source. Comparing the ruminal protein degradation rate and rumen unavailable protein (RUP) among 10 camelina varieties resulted in a range from 0.123 to 0.191 h−1 and 255 to 332 g/kg of CP, respectively [11]. Colombini et al. [11] also evaluated a canola meal that had a ruminal protein degradation rate of 0.156 h−1 and RUP content of 275 g/kg of CP. Three varieties of camelina meal had greater RUP contents than the canola meal with an average of 356 g/kg of CP between the three of them, which is similar to the recommendations of 350 g/kg of CP in [23]. It is important to note that although the camelina and canola meals were solvent-extracted, the extraction was performed using a Soxhlet apparatus, and no heat treatment was performed, which may increase the RUP. When Camelina meal is fed to pigs, the ileal standardized digestibility of crude protein was greater in the solvent-extracted meal than the expelled meal (0.67 vs. 0.58; p = 0.007) despite the solvent-extracted camelina meal being heat-treated, which generally decreases CP and amino acid digestibility [15]. The increase in CP and amino acid digestibility in pigs fed solvent compared to expelled camelina meal was likely due to the solvent-extracted camelina having lower levels of glucosinolates and soluble fiber [15]. Overall, Cerisuelo et al. [15] concluded that the digestibility of specific amino acids in camelina meal was similar to canola and soybean meals.
Camelina meal has a similar amino acid profile to both canola and soybean meals (Figure 1). Glutamate, aspartate, arginine, and leucine are the four most prevalent amino acids in both camelina meal and soybean meal (Table 2), although both canola and soybean meals have greater proportions of lysine. The amino acid profile of camelina meal that was produced via solvent extraction and expulsion did not differ in amino acid profile, which is consistent with Cerisuelo et al. [15] who compared the same source of camelina either expelled or solvent-extracted.
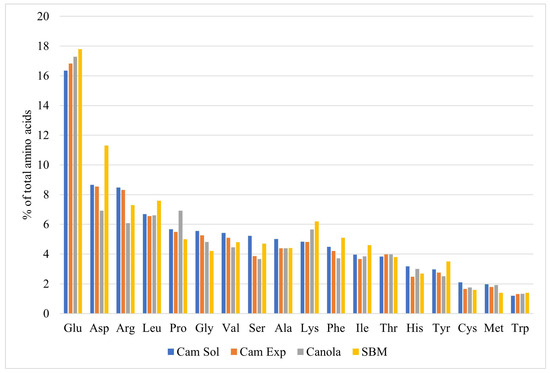
Figure 1.
Amino acid profile of solvent-extracted (Cam Sol) and expelled (Cam Exp) camelina meals, solvent-extracted canola meal (Canola), and solvent-extracted soybean meal (SBM) adapted from Table 2.

Table 2.
Amino acid profile of solvent-extracted and expelled camelina meals, solvent-extracted canola meal, and solvent-extracted soybean meal.
3.2. Neutral Detergent Fiber and Energy Value of Camelina Meal
Most of the energy that is supplied by camelina meal, and oilseed meals in general, comes from residual fat, NDF, and protein. The energy value of a feed can be expressed in a variety of ways; in this paper, gross energy (GE) is used to compare feeds. Gross energy is the total energy released when a feedstuff is completely oxidized through combustion, which is associated more with the chemical composition of the feed than the nutritional quality [23]. Although GE does not consider the energy available to the animal, because of the limited reports on more appropriate energy measures in the literature, and the fact that this paper is covering a wide range of animals, GE can provide a general idea of the energy content of the feeds. Expelled camelina meal has a greater GE content than the solvent-extracted meal, as expected due to the greater fat content in the expelled meal (Table 1). Both canola meal and soybean meal have lower GE concentrations than the solvent-extracted camelina meal, likely due to the greater NDF concentration in the camelina meal. When comparing the digestible energy (DE) of solvent-extracted camelina meal to canola and soybean meals, Cerisuelo et al. [15] found that the camelina meal had a lower DE than both when fed to pigs, highlighting the need to be cautious when using just GE. The lower DE of camelina meal is likely due to the high NDF concentration.
Another major component of camelina meal is the NDF and ADF contents. Solvent-extracted camelina meal generally has greater NDF and ADF concentrations, likely due to less dilution from fat when compared to the expelled meal. Canola meal and solvent-extracted camelina meal have similar NDF concentrations, with camelina meal having a wider range and greater mean than canola. Soybean meal has significantly less NDF than camelina meal. The significant amounts of NDF in camelina meal may limit its use in animal feeds as NDF has limited digestion in non-ruminants; however, ruminants are still able to utilize the NDF, and glucosinolates are still the main limiting factor for camelina meal inclusion.
The mineral composition of solvent-extracted camelina meal is poorly represented in the literature with no studies found that lists the magnesium, potassium, chloride, sodium, or sulfur content. Expelled camelina meal has a similar mineral profile to canola meal, and it is reasonable to assume that the solvent-extracted meal would be similar to the expelled meal with slightly elevated values because of less dilution from fat.
4. Anti-Nutritional Factors Present in Camelina Meal
The greatest factors limiting the use of camelina meal as a feed ingredient for animals are the anti-nutritional factors that are present [29]. The anti-nutritional factors in camelina meal are glucosinolates, sinapines, erucic acid, trypsin inhibitors, and tannins, with glucosinolates being the main concern. Glucosinolates are hydrolyzed into isothiocyanates, thiocyanates, nitriles, and epithionitriles that are highly toxic to animals [29]. Sinapines are present in plants for the biosynthesis of lignin and flavonoids but have a bitter flavor, which can decrease DMI and cause a fishy taste in eggs [29]. Camelina meal contains lower concentrations of sinapine (0.1–0.5%) than canola, and, thus, sinapine does not induce any unpleasant effects [29]. Erucic acid is a fatty acid that reduces the palatability of feed and in monogastrics may induce myocardial lipidosis [15]. Canola was developed from rapeseed primarily to reduce its erucic acid content and must now contain <2% erucic acid in its fatty acid profile [9]. Erucic acid content in camelina meal is slightly higher than canola meal at 3.1% in solvent-extracted camelina meal [15]. Concentrations of trypsin inhibitors and tannins are both low in camelina meal. Trypsin inhibitors reduce amino acid digestion and intake at a concentration of 3 trypsin inhibitor units (THI)/mg in the feed, and solvent-extracted camelina meal contains 0.00663 TIU/mg [15]. Overall, glucosinolates present the biggest concerns for anti-nutritional factors in camelina meal and are the main component limiting its use as an animal feed ingredient.
Glucosinolates are present in all members of the brassica plant species. The metabolites of glucosinolates cause reductions in feed intake and decreased growth and production [30]. Pigs are more sensitive to glucosinolates with tolerance levels for pigs, ruminants, and poultry of 0.78, 1.5 to 4.22, and 5.4 mmol/kg, respectively [30]. The glucosinolate content of expelled and solvent-extracted camelina meals ranges from 39.12 to 44.90 and 23.10 to 37.63 mmol/kg, respectively (Table 1). It is important to note that only two papers were available on the glucosinolate levels in solvent-extracted camelina meal. In [13], the authors performed solvent extraction with hexane but did not state the temperature used and whether the meal was toasted or not. Cerisuelo et al. [15] also performed solvent extraction, but they used a Soxhlet apparatus and did not state whether the resulting meal was toasted or not. Although speculative, the similar glucosinolate content to the expelled camelina meal suggests that these meals were either not toasted or heated at a low temperature. Although there is limited research on glucosinolates in solvent-extracted camelina meal, similar glucosinolate problems and solutions have been researched in canola meal.
Alternatives to Reduce Glucosinolates in Camelina Meal
A solution for decreasing the glucosinolate load in camelina meal is through the heat treatment of the meal. Cerisuelo et al. [15] compared expelled and solvent-extracted camelina meals fed to pigs. Although they did not measure glucosinolates, they did measure allyl-isothyocyanate, which is a metabolite of glucosinolates. Solvent-extracted camelina meal had significantly less allyl-isothyocyanate than the expelled meal (180 and 500 mg/kg, respectively), which the authors attributed to the toasting that the solvent-extracted camelina meal went through after extraction to remove residual solvent. Heating canola meal has also been shown to reduce glucosinolates. Toasting canola meal at 100 C for 15, 30, 60, and 120 min decreased the glucosinolates by 24, 46, 70, and 90%, respectively [10]. A concern with heating the meal is a decrease in CP digestibility and lysine content. Heating the canola meal at 100 C for 0, 15, 30, 60, and 120 min also resulted in a linear decrease in true CP digestibility in rats from 77.0, 73.9, 72.1, 72.9, and 71.2%, respectively [10]. Jensen et al. [10] found that toasting up to 30 min at 100 C resulted in a protein solubility of ~60% and was the optimal processing time in canola meal. Although this was conducted in canola meal, due to similar chemical compositions, it is likely that camelina meal would be similar. Heat treatment is likely to be an effective method of reducing glucosinolates, and at the same time recovering residual solvent; however, care must be taken to not depress protein and amino acid digestibility. A primary detriment to using heat treatment to reduce glucosinolates includes cost and the negative environmental impact this could have if fossil fuels were used as the primary fuel source. There is potential in an economy based upon carbon credits and carbon offsets that one might be able to recoup costs and lessen the environmental impact of this process since the meal results from the production of an oilseed that is used as a source of renewable oil in place of fossil fuels.
The fungal fermentation of camelina meal may also be a novel method of reducing glucosinolates. Aureobasidium pullulans and Trichoderma reesei are fungi species that may have the potential to decrease the glucosinolate concentrations in camelina meal [31,32]. The fungi are grown using a solid-state fermentation process which helps limit bacterial contamination from decreased water activity and reduces costs compared to submerged bioprocessing. When-solvent extracted canola meal was incubated for 168 h at 30 C, T. reesei and A. pullulans reduced glucosinolates by up to 99 and 94%, respectively [31]. When T. reesei and A. pullulans were used in a coculture, glucosinolates were reduced by 81.3% [32]. These fungi also increased the protein content of the canola meal in both studies [31,32]. Although the utilization of fungal fermentation to reduce glucosinolates in canola meal has proven effective, information on the costs associated with it and the ability to scale this to commercial production of camelina meal is uncertain.
Another method to decrease glucosinolates in camelina meal begins in the field with the selective breeding of camelina to reduce glucosinolate content. Canola has successfully been bred to reduce erucic acid and glucosinolates from the original rapeseed [9]. Over 4000 lines of rapeseed were surveyed to identify low-erucic lines for use in breeding, and canola must now contain <2% erucic acid in its fatty acid profile and <30 mmol/kg of glucosinolates in its meal [9]. Glucosinolate concentrations across 47 accensions of camelina were analyzed to identify varieties for selective breeding of low glucosinolate strains [29]. The total glucosinolates ranged from 19.6 to 40.3 mmol/kg, and the authors found that individual glucosinolates also varied between camelina accensions [29]. Due to the high variability in glucosinolate concentrations, glucosinolate levels could be reduced through conventional breeding. In addition, no correlation was found between the individual glucosinolates GSL1 and GSL3, which could be used to cross varieties that lower both individual glucosinolates [29]. Although not a short-term solution, much like canola which has been selectively bred to be low in erucic acid and glucosinolates, the selective breeding of camelina meal can help improve the value of camelina meal in the future. This, however, would come at the expense of other agronomic benefits that camelina provides in regard to drought, pest, and disease tolerance. Ultimately, treatment costs are dependent upon infrastructure and location. There is potential that this might be re-couped if sustainable fuel from the oil that was extracted from camelina is allowed to be used as carbon offsets to improve the quality of the by-product produced.
5. Beneficial Nutritional Factors in Camelina Meal
Camelina meal contains high levels of omega-3 (α-linolenic acid) and omega-6 (linoleic acid) fatty acids (Table 3). Omega-3 fatty acids have been of special importance due to their health-promoting effects in humans [33]. The inclusion of omega-3 fatty acids in poultry diets helps increase the omega-3 content in both the meat and eggs because of their high capacity for lipid biosynthesis. This makes camelina meal a potential source of omega-3 for enriched poultry products.

Table 3.
Fatty acid profile of camelina meal [33].
Feeding broiler chickens a diet containing 10% camelina meal led to a 2- to 2.5-fold increase in omega-3 fatty acids in the white and dark meats compared to a control diet based on corn and soybeans [33]. When laying hens were fed a diet containing 10% camelina meal, even greater increases in omega-3 fatty acids in eggs were observed. Compared to those on a corn–soybean control diet, the camelina-fed hens had an eight-fold increase in omega-3 fatty acids in their eggs [33]. Consuming two large eggs from the camelina-fed hens could provide over 300 mg of omega-3 fatty acids to a human diet. In a soybean-meal-based diet fed to brown egg layers that had a 10% inclusion of either camelina meal or flax meal, the camelina-fed group had a greater yolk content of omega-3 and total n-3 poly-unsaturated fatty acids as well [34]. Thus, feeding camelina meal to chickens has the potential to increase the nutritional quality of the final product, especially in laying hens. However, in solvent-extracted camelina meal, the effect of omega-3 fatty acids may not be as pronounced because of the lower fat content in these meals.
Camelina meal can also improve the fatty acid composition of milk when fed to dairy cattle [35]. When sunflower meal was replaced with camelina meal, milk yield, fat content, and protein content were not affected, but milk fat content was numerically decreased in the camelina-fed groups [36]. The saturated fatty acid content of the camelina-fed groups was decreased, and the poly unsaturated fatty acid content was increased. This is consistent with other studies that have found that feeding camelina meal decreases milk fat content and modifies the fatty acid composition with greater poly unsaturated and less saturated fatty acids [35,37].
6. Current Feeding Regulations of Camelina Meal
Camelina meal is currently approved in both the US and Canada as a feed ingredient for broiler chickens at up to 10% inclusion in the total ration. In the US, camelina meal is also approved for use in laying hens and cattle fed in confinement for slaughter at up to 10% inclusion. The inclusion levels are based on the glucosinolate concentrations in camelina meal.
Animal feed regulations in the US are enforced by the United States Food and Drug Administration (FDA) under the Federal Food, Drug, and Cosmetic Act. The FDA works with the Association of American Feed Control Officials (AAFCO), which is a voluntary-membership organization responsible for the execution of laws and regulations pertaining to the production, labeling, distribution, use, or sale of animal feed and feed ingredients. The AAFCO is responsible for maintaining the definitions of feed ingredients and requests for new or modifications to existing feed ingredient definitions. The AAFCO publishes an annual publication with up-to-date approved documents.
In Canada, animal feed is regulated by the Canadian Food Inspection Agency (CFIA) under the authority of the Feeds Act and Regulations and the Health of Animals Regulations. The CFIA approves feed ingredients and lists them in Schedule IV and V of the feed regulations. Use of ingredients not listed in Schedule IV and V requires registration through the Ottawa Feed Division.
Information in this section may be revised as regulations and feed ingredients change. The AAFCO publication for the US and Schedules IV and V for Canada should contain the most up-to-date approved feeds. The approval and modification of feed ingredients in the US and Canada should be directed toward the FDA and CFIA, respectively.
7. Conclusions
Camelina meal can result in decreased DM intake; it has greater NDF and ADF contents, as well as greater antinutritional factors than protein meal produced from some of the more commonly cultivated oilseeds. It is, however, still a viable feed ingredient in animal diets as a protein source. The nutrient composition of camelina is similar to canola meal, with high levels of protein and fiber, but is not as good of a source as soybean meal because soybean meal has more protein and less fiber. The oil extraction method produces similar meals, but expelled camelina meal has much greater fat and lower protein, while the solvent-extracted meal has lower fat and greater protein. In addition, the solvent-extracted camelina meal may be produced under greater temperatures, especially if the meal is toasted to remove residual solvent, which can reduce the glucosinolate content of the meal. This is especially important as the primary barrier to the utilization of camelina meal in animal feeds, especially pigs, is the high levels of glucosinolates. Decreasing the glucosinolates either through heat treatment, fungal fermentation, or selective breeding to produce low-glucosinolate varieties could improve the feeding value of camelina meal. Currently, camelina meal is safe to feed at 10% inclusion in poultry and cattle diets, and the utilization of camelina meal in animal feeds will be dependent on the cost competitiveness with other oilseed meals. Camelina meal is currently an acceptable protein source for animal feeds but could be improved similarly to canola, where future research should be focused on reducing anti-nutritional factors through breeding and production methods.
If camelina is to increase in production, research gaps in utilizing camelina meal should be filled. One of the largest research needs is to determine effective and economic methods for reducing glucosinolates in camelina, either through plant breeding or the treatment of the meal. Especially important is determining the costs of various methods of treating the meal and the ability to perform these at larger scales. More information is also required on the energy value of camelina meal, as, currently, there is limited data available on the net energy value of camelina meal.
Author Contributions
Conceptualization, J.J.D. and Z.K.S.; methodology, J.J.D. and Z.K.S.; formal analysis, J.J.D.; writing—original draft preparation, J.J.D.; writing—review and editing, Z.K.S.; project administration, Z.K.S.; funding acquisition, Z.K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Beef Nutrition Program and funds appropriated to South Dakota State University by USDA-NIFA (HATCH-SD00H690-19) and Vision Bioenergy Oilseeds.
Institutional Review Board Statement
Institutional Animal Care and Use Committee approval was not obtained for this study because the data were obtained from the published and industry literature.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the corresponding author on reasonable request.
Conflicts of Interest
The author declares no conflicts of interest, except that Vision Bioenergy Oilseeds provided a stipend to support the corresponding author during the writing of this review.
Abbreviations
| ADF | Acid detergent fiber |
| AFFCO | Association of American Feed Control Officials |
| CFIA | Canadian Food Inspection Agency |
| CP | Crude protein |
| DE | Digestible energy |
| DM | Dry matter |
| FDA | United States Food and Drug Administration |
| GE | Gross energy |
| GLSN | Glucosinolates |
| NDF | Neutral detergent fiber |
| RUP | Rumen unavailable protein |
| THI | Trypsin inhibitor units |
References
- Obour, A.K.; Sintim, H.Y.; Obeng, E.; Jeliazkov, D.V. Oilseed Camelina (Camelina sativa L. Crantz): Production Systems, Prospects and Challenges in the USA Great Plains. Adv. Plants Agric. Res 2015, 2, 00043. [Google Scholar] [CrossRef]
- Veljković, V.B.; Kostić, M.D.; Stamenković, O.S. Camelina Seed Harvesting, Storing, Pretreating, and Processing to Recover Oil: A Review. Ind. Crops Prod. 2022, 178, 114539. [Google Scholar] [CrossRef]
- Zanetti, F.; Gesch, R.W.; Walia, M.K.; Johnson, J.M.F.; Monti, A. Winter Camelina Root Characteristics and Yield Performance under Contrasting Environmental Conditions. Field Crops Res. 2020, 252, 107794. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture National Agricultural Statistics Service (NASS). 2017 Census of Agriculture; U.S. Department of Agriculture National Agricultural Statistics Service (NASS): Washington, DC, USA, 2019.
- Sydor, M.; Kurasiak-Popowska, D.; Stuper-Szablewska, K.; Rogoziński, T. Camelina Sativa. Status Quo and Future Perspectives. Ind. Crops Prod. 2022, 187, 115531. [Google Scholar] [CrossRef]
- Moloney, A.P.; Woods, V.B.; Crowley, J.G. A Note on the Nutritive Value of Camelina Meal for Beef Cattle. Ir. J. Agric. Food Res. 1998, 37, 243–247. [Google Scholar]
- Nde, D.B.; Foncha, A.C. Optimization Methods for the Extraction of Vegetable Oils: A Review. Processes 2020, 8, 209. [Google Scholar] [CrossRef]
- Berti, M.; Gesch, R.; Eynck, C.; Anderson, J.; Cermak, S. Camelina Uses, Genetics, Genomics, Production, and Management. Ind. Crops Prod. 2016, 94, 690–710. [Google Scholar] [CrossRef]
- Mejicanos, G.; Sanjayan, N.; Kim, I.H.; Nyachoti, C.M. Recent Advances in Canola Meal Utilization in Swine Nutrition. J. Anim. Sci. Technol. 2016, 58, 7. [Google Scholar] [CrossRef]
- Jensen, S.K.; Liu, Y.-G.; Eggum, B.O. The Effect of Heat Treatment on Glucosinolates and Nutritional Value of Rapeseed Meal in Rats. Anim. Feed. Sci. Technol. 1995, 53, 17–28. [Google Scholar] [CrossRef]
- Colombini, S.; Broderick, G.A.; Galasso, I.; Martinelli, T.; Rapetti, L.; Russo, R.; Reggiani, R. Evaluation of Camelina sativa (L.) Crantz Meal as an Alternative Protein Source in Ruminant Rations. J. Sci. Food Agric. 2014, 94, 736–743. [Google Scholar] [CrossRef]
- Ye, C.L.; Anderson, D.M.; Lall, S.P. The Effects of Camelina Oil and Solvent Extracted Camelina Meal on the Growth, Carcass Composition and Hindgut Histology of Atlantic Salmon (Salmo Salar) Parr in Freshwater. Aquaculture 2016, 450, 397–404. [Google Scholar] [CrossRef]
- Brandao, V.L.N.; Silva, L.G.; Paula, E.M.; Monteiro, H.F.; Dai, X.; Lelis, A.L.J.; Faccenda, A.; Poulson, S.R.; Faciola, A.P. Effects of Replacing Canola Meal with Solvent-Extracted Camelina Meal on Microbial Fermentation in a Dual-Flow Continuous Culture System. J. Dairy Sci. 2018, 101, 9028–9040. [Google Scholar] [CrossRef]
- Salas, H.; Castillejos, L.; López-Suárez, M.; Ferret, A. In Vitro Digestibility, In Situ Degradability, Rumen Fermentation and N Metabolism of Camelina Co-Products for Beef Cattle Studied with a Dual Flow Continuous Culture System of Camelina Co-Products for Beef Cattle. Animals 2019, 9, 1079. [Google Scholar] [CrossRef] [PubMed]
- Cerisuelo, A.; Ferrer, P.; Gómez, E.Á.; Woyengo, T.A.; Stein, H.H.; Martínez, M.; Cano, J.L.; Piquer, O. Nutritional Value of Spanish Camelina Sativa Co-Products for Pigs. Anim. Feed. Sci. Technol. 2023, 301, 115665. [Google Scholar] [CrossRef]
- Moriel, P.; Nayigihugu, V.; Cappellozza, B.I.; Gonçalves, E.P.; Krall, J.M.; Foulke, T.; Cammack, K.M.; Hess, B.W. Camelina Meal and Crude Glycerin as Feed Supplements for Developing Replacement Beef Heifers1. J. Anim. Sci. 2011, 89, 4314–4324. [Google Scholar] [CrossRef] [PubMed]
- Kahindi, R.K.; Woyengo, T.A.; Thacker, P.A.; Nyachoti, C.M. Energy and Amino Acid Digestibility of Camelina Cake Fed to Growing Pigs. Anim. Feed. Sci. Technol. 2014, 193, 93–101. [Google Scholar] [CrossRef]
- Pekel, A.Y.; Kim, J.I.; Chapple, C.; Adeola, O. Nutritional Characteristics of Camelina Meal for 3-Week-Old Broiler Chickens. Poult. Sci. 2015, 94, 371–378. [Google Scholar] [CrossRef]
- Lawrence, R.D.; Anderson, J.L.; Clapper, J.A. Evaluation of Camelina Meal as a Feedstuff for Growing Dairy Heifers. J. Dairy Sci. 2016, 99, 6215–6228. [Google Scholar] [CrossRef]
- Smit, M.N.; Beltranena, E. Effects of Feeding Camelina Cake to Weaned Pigs on Safety, Growth Performance, and Fatty Acid Composition of Pork1. J. Anim. Sci. 2017, 95, 2496–2508. [Google Scholar] [CrossRef]
- Lawrence, R.D.; Anderson, J.L. Ruminal Degradation and Intestinal Digestibility of Camelina Meal and Carinata Meal Compared with Other Protein Sources. Prof. Anim. Sci. 2018, 34, 10–18. [Google Scholar] [CrossRef]
- Interactive Feed Composition Libraries|Dairy One. Available online: https://apps.dairyone.com/feedcomposition/ (accessed on 14 November 2023).
- National Academy of Science, Engineering, and Medicine (NASEM). Nutrient Requirements of Beef Cattle, 8th ed.; National Academies Press: Washington, DC, USA, 2016; ISBN 978-0-309-27335-0. [Google Scholar]
- Liu, Y.; Jaworski, N.W.; Rojas, O.J.; Stein, H.H. Energy Concentration and Amino Acid Digestibility in High Protein Canola Meal, Conventional Canola Meal, and in Soybean Meal Fed to Growing Pigs. Anim. Feed. Sci. Technol. 2016, 212, 52–62. [Google Scholar] [CrossRef]
- Schulmeister, T.M.; Ruiz-Moreno, M.; Silva, G.M.; Garcia-Ascolani, M.; Ciriaco, F.M.; Henry, D.D.; Lamb, G.C.; Dubeux, J.C.B.; DiLorenzo, N. Characterization of Dietary Protein in Brassica carinata Meal When Used as a Protein Supplement for Beef Cattle Consuming a Forage-Based Diet. J. Anim. Sci. 2021, 99, skaa383. [Google Scholar] [CrossRef]
- Paula, E.M.; da Silva, L.G.; Brandao, V.L.N.; Dai, X.; Faciola, A.P. Feeding Canola, Camelina, and Carinata Meals to Ruminants. Animals 2019, 9, 704. [Google Scholar] [CrossRef]
- Canola Meal Nutrient Composition. Available online: https://www.canolacouncil.org/canolamazing/feed-guide/nutrient-composition/ (accessed on 17 October 2023).
- Soybean Meal, Type 48 and Similar|Feedipedia. Available online: https://www.feedipedia.org/node/26068 (accessed on 17 October 2023).
- Russo, R.; Reggiani, R. Glucosinolates and Sinapine in Camelina Meal. Food Nutr. Sci. 2017, 8, 1063–1073. [Google Scholar] [CrossRef]
- Tripathi, M.K.; Mishra, A.S. Glucosinolates in Animal Nutrition: A Review. Anim. Feed. Sci. Technol. 2007, 132, 1–27. [Google Scholar] [CrossRef]
- Croat, J.R.; Berhow, M.; Karki, B.; Muthukumarappan, K.; Gibbons, W.R. Conversion of Canola Meal into a High-Protein Feed Additive via Solid-State Fungal Incubation Process. J. Am. Oil Chem. Soc. 2016, 93, 499–507. [Google Scholar] [CrossRef]
- Alhomodi, A.F.; Zavadil, A.; Berhow, M.; Gibbons, W.R.; Karki, B. Application of Cocultures of Fungal Mycelium during Solid-State Fermentation of Canola Meal for Potential Feed Application. J. Am. Oil Chem. Soc. 2021, 98, 509–517. [Google Scholar] [CrossRef]
- Cherian, G. Camelina Sativa in Poultry Diets: Opportunities and Challenges. In Biofuel Co-Products as Livestock feed: Opportunities and Challenges; FAO: Rome, Italy, 2012; pp. 303–310. [Google Scholar]
- Aziza, A.E.; Panda, A.K.; Quezada, N.; Cherian, G. Nutrient Digestibility, Egg Quality, and Fatty Acid Composition of Brown Laying Hens Fed Camelina or Flaxseed Meal. J. Appl. Poult. Res. 2013, 22, 832–841. [Google Scholar] [CrossRef]
- Riaz, R.; Ahmed, I.; Sizmaz, O.; Ahsan, U. Use of Camelina Sativa and By-Products in Diets for Dairy Cows: A Review. Animals 2022, 12, 1082. [Google Scholar] [CrossRef]
- Toma, S.; Dragomir, C.; Habeanu, M.; Ropota, M.; Cismileanu, A.; Grosu, H. Effects of Partial or Total Replacement of Sunflower Meal with Camelina Meal on Dairy Cows’ Milk Fatty Acids Profile. Arch. Zootech. 2015, 18, 85–94. [Google Scholar]
- Hurtaud, C.; Peyraud, J.L. Effects of Feeding Camelina (Seeds or Meal) on Milk Fatty Acid Composition and Butter Spreadability. J. Dairy Sci. 2007, 90, 5134–5145. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).