Effects of Seed Quality and Hybrid Type on Maize Germination and Yield in Hungary
Abstract
1. Introduction
2. Materials and Methods
2.1. Laboratory Experiment
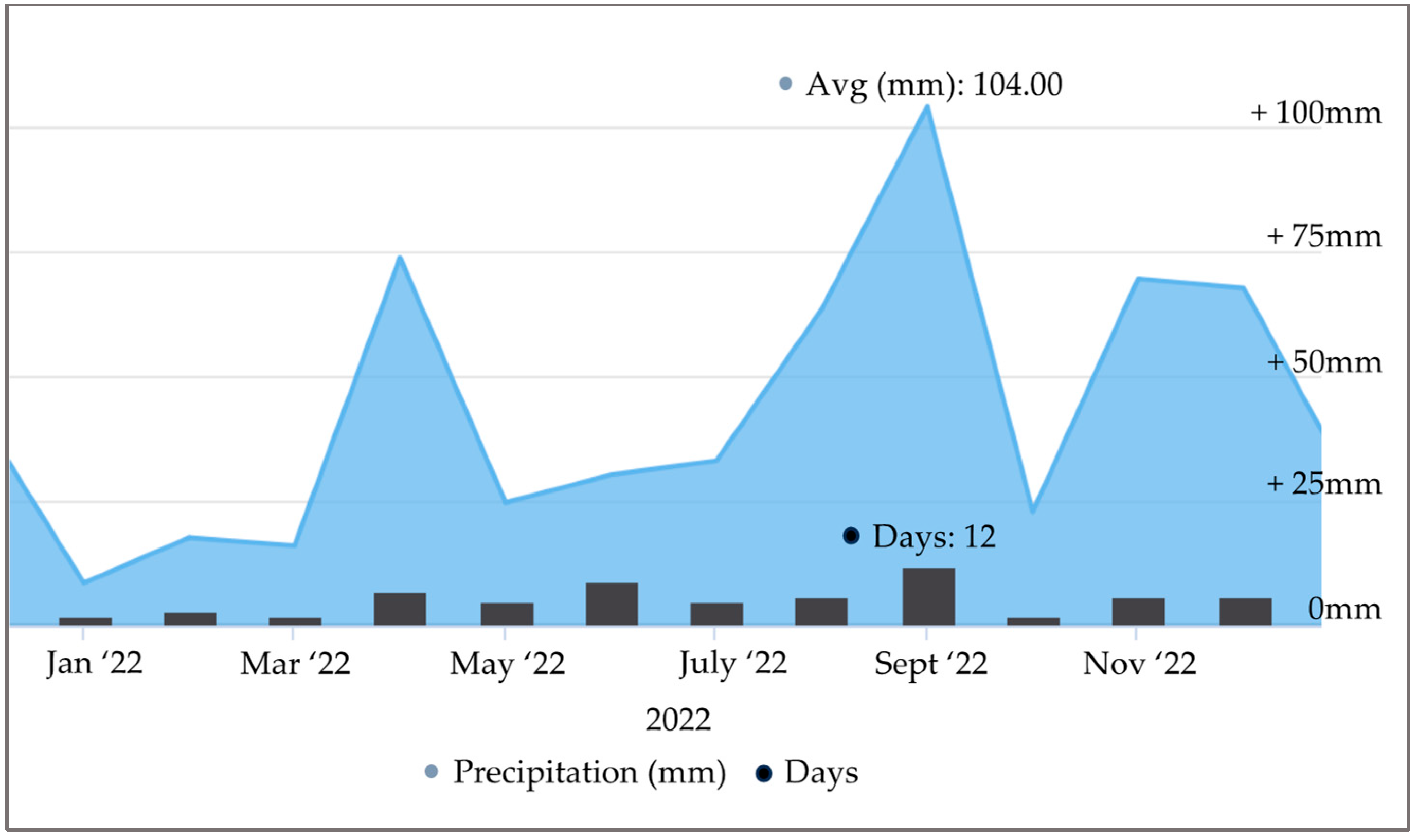
2.2. Open Field Experiment
2.3. Experimental Design
2.4. Data Collection
2.5. Statistical Analysis
3. Results
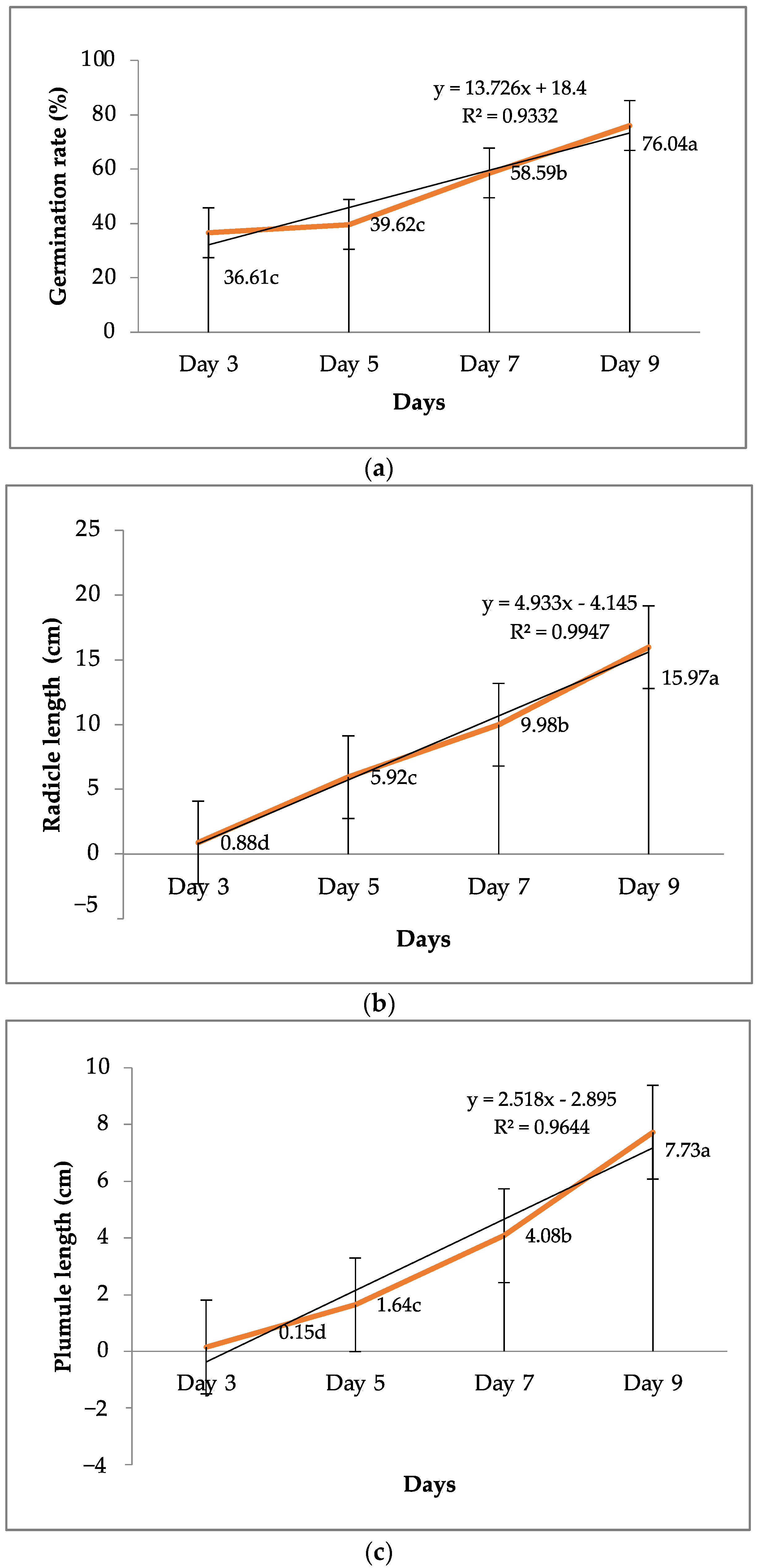
3.1. Seed Viability and Vigor
3.2. Yield Performance
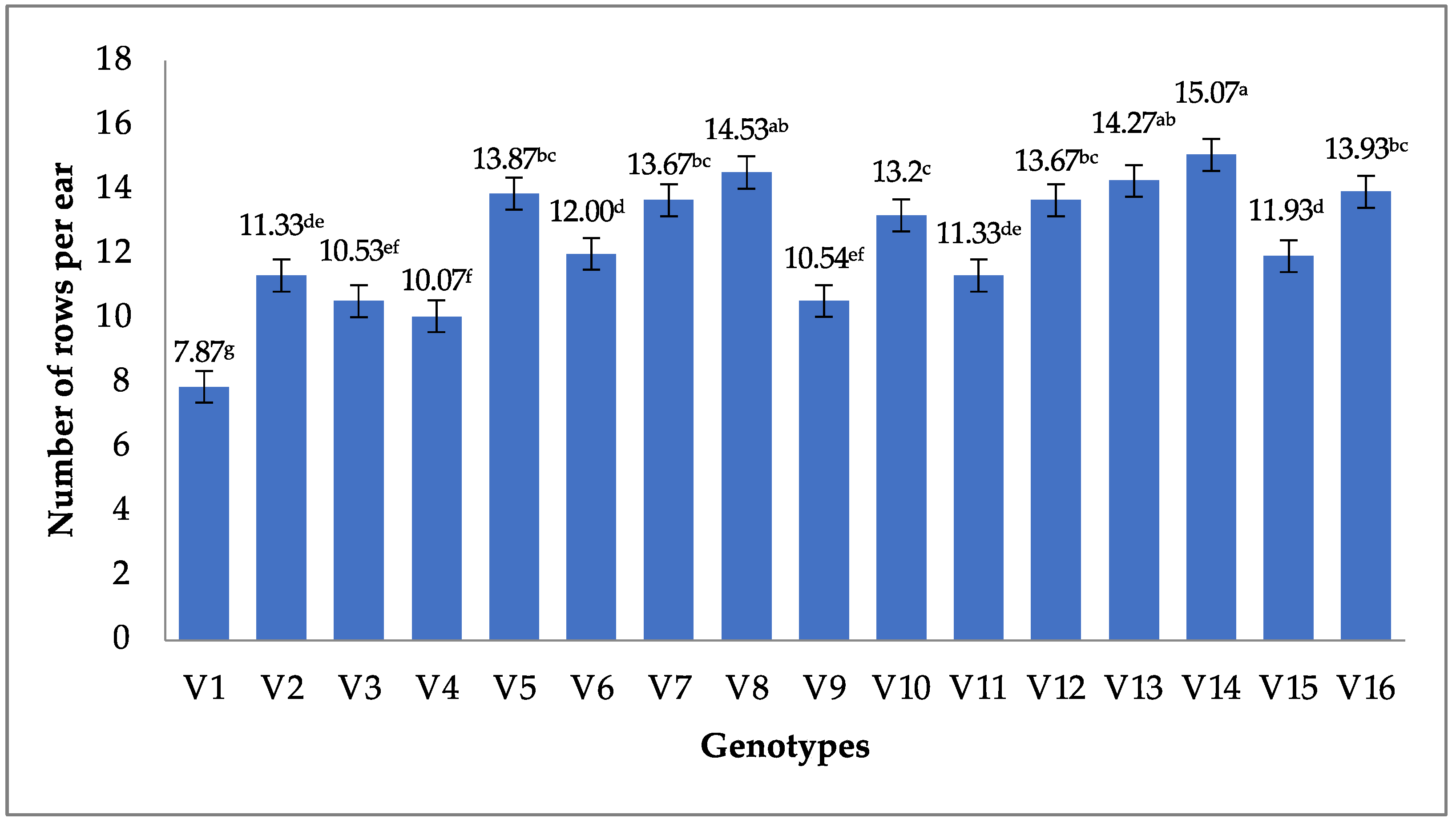
3.2.1. Number of Rows Per Ear
3.2.2. Number of Kernels Per Ear
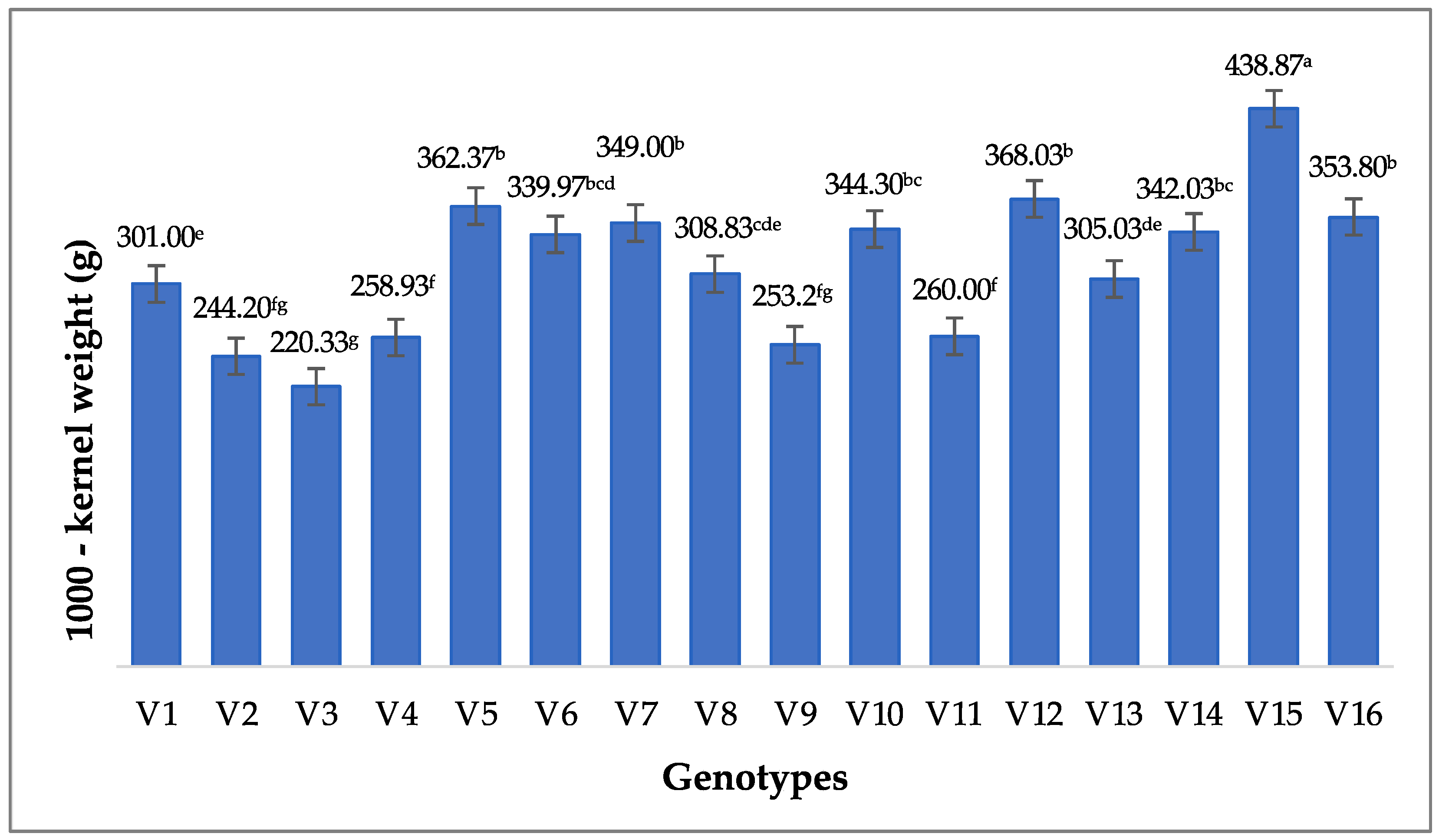
3.2.3. 1000-Kernel Weight (g)
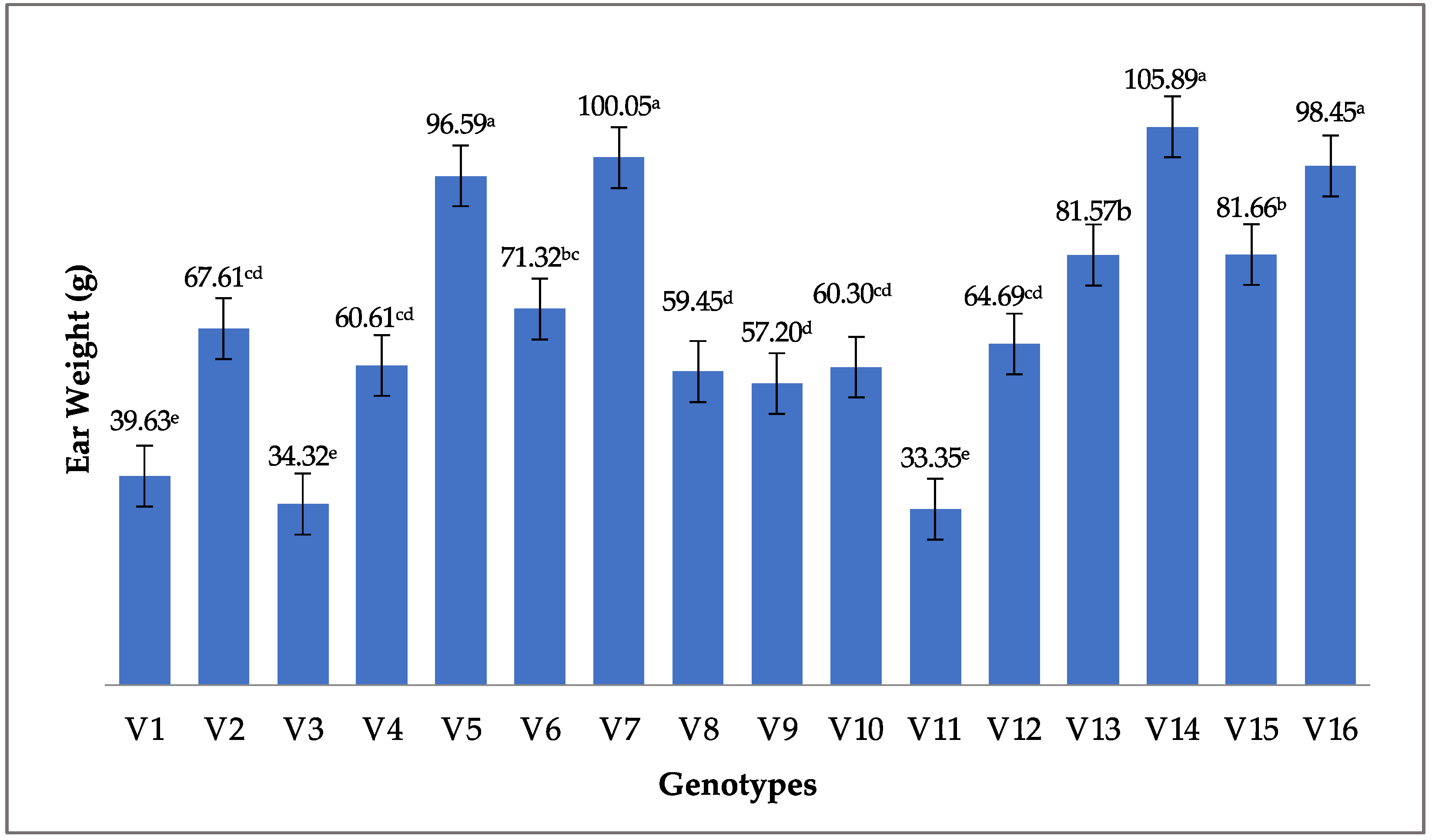
3.2.4. Ear Weight (g)
3.3. Relationship between Seed Viability, Vigor, and Yield Traits
4. Discussion
4.1. Seed Viability and Vigor
4.2. Yield Performance
4.3. Relationship between Seed Viability, Vigor, and Yield Traits
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Golob, P.; Kutukwa, N.; Devereau, A.; Bartosik, R.E.; Rodriguez, J.C. Chapter two: In Maize. In Crop Post-Harvest: Science and Technology; Hodges, R., Farrell, G., Eds.; Blackwell Publishing Ltd.: Ames, Iowa, 2004; Volume 2. [Google Scholar]
- Suleiman, R.; Rosentrater, K.A.; Bern, C. Effects of Deterioration Parameters on Storage of Maize: A Review. Wuhan Univ. J. Nat. Sci. 2003, 3, 147–165. [Google Scholar]
- Breadley, P.R. British Herbal Compendium; British Herbal Medicine Association: Bournemouth, UK, 1992; Volume 2. [Google Scholar]
- Ranum, P.; Peña-Rosas, J.P.; García-Casal, M.N. Global maize production, utilization, and consumption. Ann. N. Y. Acad. Sci. 2014, 1312, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Jhariya, A.N. Nutritional, Medicinal and Economical Importance of Corn: A Mini Review. Res. J. Pharm. Sci. 2013, 2, 7–8. Available online: http://www.isca.in/IJPS/Archive/v2/i7/2.ISCA-RJPcS-2013-029.pdf (accessed on 23 June 2023).
- Pruitt, J.D. A Brief History of Corn: Looking Back to Move Forward. Doctoral Document. 2016, pp. 5–6. Available online: http://digitalcommons.unl.edu/planthealthdoc/7 (accessed on 23 June 2023).
- Hake, S.; Ross-Ibarra, J. Genetic, evolutionary and plant breeding insights from the domestication of maize. eLife 2015, 4, e05861. [Google Scholar] [CrossRef] [PubMed]
- States, T.U.; Maize, S.A.; Uses, O. Maize Cultivation: Advanced Maize Production Technologies Introduction: Importance: Land Preparation: Ecological Requirement. 2010. Available online: https://kvk.icar.gov.in/API/Content/PPupload/k0376_3.pdf (accessed on 23 June 2023).
- Hungarian Central Statistical Office Home Page. Harvest Results of Main Crops. 2020. Available online: https://www.ksh.hu/docs/eng/xftp/stattukor/fobbnoveny/2020/index.html#furtherdataandinformation (accessed on 30 June 2023).
- Hungarian Central Statistical Office Home Page. 19.1.2.5. Production of Maize by Country and Region. 2023. Available online: https://www.ksh.hu/stadat_files/mez/en/mez0072.html (accessed on 30 June 2023).
- Harvey, F. Falls in Europe’s Crop Yields Due to Heatwaves Could Worsen Price Rises. 2022. Available online: https://www.theguardian.com/environment/2022/jul/27/big-falls-in-crop-yields-across-europe-feared-due-to-heatwaves (accessed on 27 June 2023).
- Xiao, Y.; Wang, M.; Song, Y. Abiotic and biotic stress cascades in the era of climate change pose a challenge to genetic improvements in plants. Forests 2022, 13, 780. [Google Scholar] [CrossRef]
- Li, Y.; Guan, K.; Schnitkey, G.; DeLucia, E.H.; Peng, B. Excessive rainfall leads to maize yield loss of a comparable magnitude to extreme drought in the United States. Glob. Change Biol. 2022, 25, 2325–2337. [Google Scholar] [CrossRef]
- Roger, E. Influence of Soil Temperature on Corn Germination and Growth. Integrated Crop Management 2012. Available online: https://crops.extension.iastate.edu/cropnews/2012/03/influence-soil-temperature-corn-germination-and-growth (accessed on 27 June 2023).
- Denis, T.; Gales, D.; Chiriac, G.; Răus, L.; Jităreanu, G. Impact of plowing on some soil physical properties under hybrid seed corn production. ProEnvironment/ProMediu 2013, 6, 183–186. [Google Scholar]
- Venkatesh, T.V.; Breeze, M.L.; Liu, K.; Harrigan, G.G.; Culler, A.H. Compositional analysis of grain and forage from MON 87427, an inducible male sterile and tissue selective glyphosate-tolerant maize product for hybrid seed production. J. Agric. Food Chem. 2014, 62, 1964–1973. [Google Scholar] [CrossRef]
- Arisnabarreta, S.; Solari, F. Hybrid maize seed production yield associations with inbred line performance in multi-environment trials. Crop Sci. 2017, 57, 3203–3216. [Google Scholar] [CrossRef]
- Marton, L.C. Hybrid maize in Hungary is 60 years old. In 60 Years of Hungarian Hybrid Maize; Pannonian Plant Biotechnology Association: Tolna County, Hungary, 2013; pp. 10–16. Available online: https://www.pannonbiotech.hu/_upload/editor/Hibridkukorica-beliv-angol-screen.pdf (accessed on 27 June 2023).
- Omar, S.; Tarnawa, Á.; Kende, Z.; Abd Ghani, R.; Kassai, M.K.; Jolánkai, M. Germination characteristics of different maize inbred hybrids and their parental lines. Cereal Res. Commun. 2022, 50, 1229–1236. [Google Scholar] [CrossRef]
- MacRobert, J.F.; Setimela, P.S.; Gethi, J.; Worku, M. Maize Hybrid Seed Production Manual. 2014. Mexico, D.F. CIMMYT. Available online: https//repositorycimmyt.org (accessed on 27 June 2023).
- Nagy, J. Maize Production: Food, Energy Biomass; Akadémiai Kiadó: Budapest, Hungary, 2008; ISBN 9789630586368. [Google Scholar]
- Berzy, T.; Záborszky, S.; Marton, L.C.; Spitkó, T.; Pintér, J.; Tóthné Zsubori, Z.; Szőke, C. Effect of seed deterioration, seed dressing agents and seed vigours parameters in maize (Zea mays L.) hybrids. Int. J. Agric. Environ. Biores. 2020, 5, 72–81. [Google Scholar] [CrossRef]
- Rajesh, S.; Ram, L.; Srivastava, R.P. A Journey of Hybrids in Maize: An Overview. India Res. J. Ext. Educ. 2012, 1, 340–344. [Google Scholar]
- El-Abady, M.I. Influence of maize seed size/shape, planted at different depths and temperature on seed emergence and seedling vigor. Res. J. Seed Sci. 2015, 8, 1–11. [Google Scholar] [CrossRef][Green Version]
- Goggi, A.S.; Caragea, P.; Pollak, L.; McAndrews, G.; DeVries, M.; Montgomery, K. Seed quality assurance in maize breeding program: Test to explain variations in maize inbreds and populations. Agron. J. 2002, 100, 337–343. [Google Scholar] [CrossRef]
- Shibata, M.; Coelho, C.M.M.; Araldy, C.G.; Adan, N.; Peroni, N. Physiological and physical quality of local Araucaria angustifolia seed variety. Acta Sci. Agron. 2016, 38, 249–256. [Google Scholar] [CrossRef]
- Munamava, M.R.; Goggi, A.S.; Pollak, L. Seed quality of maize inbred lines with different composition and genetic backgrounds. Crop Sci. Soc. Am. 2004, 44, 542–548. [Google Scholar] [CrossRef]
- Illipronti, J.; Langerak, L.A.; Lommen, W.J.M. Variation in and relation between physical and physiological seed attributes within soyben seed lot. Seed Sci. Technol. 1997, 5, 215–231. [Google Scholar]
- Tuan, P.A.; Sun, M.; Nguyen, T.-N.; Park, S.; Ayele, B.T. Molecular mechanisms of seed germination. Sprouted Grains 2019, 1–24. [Google Scholar] [CrossRef]
- Rizzardi, M.A.; Luiz, A.R.; Roman, E.S.; Vargas, L. Temperatura cardeal e potencial hídrico na germinação de sementes de corda-de-viola (Ipomoea triloba). Planta Daninha 2009, 27, 13–21. [Google Scholar] [CrossRef]
- Aderounmu, A.F.; Nkemnkeng, F.J.; Anjah, G.M. Effects of seed provenance and growth media on the growth performance of Vitellaria paradoxa C.F. Gaertn. Int. J. Biol. Chem. Sci. 2020, 14, 2659–2669. [Google Scholar] [CrossRef]
- Prerna, D.I.; Govindaraju, K.; Tamilselvan, S.; Kannan, M.; Vasantharaja, R.; Chaturvedi, S.; Shkolnik, D. Influence of nanoscale micro-nutrient α-Fe2O3 on seed germination, seedling growth, translocation, physiological effects, and yield of rice (Oryza sativa) and maize (Zea mays). Plant Physiol. Biochem. 2021, 162, 564–580. [Google Scholar] [CrossRef] [PubMed]
- Masubelele, N.H.; Dewitte, W.; Menges, M.; Maughan, S.; Collins, C.; Huntley, R.; Nieuwland, J.; Scofield, S.; Murray, J.A.H. D-type cyclins activate division in the root apex to promote seed germination in Arabidopsis. Proc. Natl. Acad. Sci. USA 2005, 102, 15694–15699. [Google Scholar] [CrossRef]
- Ltroutwar, P.D.; Kasivelu, G.; Raguraman, V.; Malaichamy, K.; Sevathapandian, S.K. Effects of biogenic zinc oxide nanoparticles on seed germination and seedling vigor of maize (Zea mays). Biocatal. Agric. Biotechnol. 2020, 29, 101778. [Google Scholar]
- Vishal, B.; Kumar, P.P. Regulation of seed germination and abiotic stresses by gibberellins and abscisic acid. Front. Plant Sci. 2018, 9, 838. [Google Scholar] [CrossRef] [PubMed]
- Clor, M.A.; Al-Ani, T.A.; Charchafchy, F. Germinability and seedling vigor of Haloxylon salicornicumi as affected by storage and seed size. J. Range Manag. 1976, 29, 60–62. [Google Scholar] [CrossRef]
- Krishnan, P.; Rao, A.V.S. Effects of genotypes and environment on seed yield and quality of rice. J. Agric. Sci. 2005, 143, 283–292. [Google Scholar] [CrossRef]
- Lafta, A.K.; Chilab, Y.K. Evaluation of the vigor and viability of maize (Zea mays L.) seeds which resultant from planting date and plant density on yield character. Plant Arch. 2019, 19, 1663–1671. [Google Scholar] [CrossRef]
- Hamza, J.H. The Effect of Seed Size and Which Produced from Planting Dates on Seed Vigor and Grain Yield of (Sorghum bicolor L. Monech). Ph.D. Thesis, Field Crops Department, Agriculture College, Baghdad University, Baghdad, Iraq, 2006; p. 131. [Google Scholar]
- Shirin, M.; Enayatgholizadeh, M.R.; Siadat, E.; Fathi, G. Comparison of suitable seed vigour of hybrid Zea mays (CV. SC.704) in the field condition of Ahvaz. In Proceedings of the 10th Congress of Agronomy and Plant Breeding of Iran, Karaj, Iran, 18–20 August 2008; p. 330. [Google Scholar]
- Marcos, J. Seed vigor testing: An overview of the past, present, and future perspective. Sci. Agric. 2015, 72, 363–374. [Google Scholar] [CrossRef]
- ISTA. International Rules for Seed Testing; International Seed Testing Association: Bassersdorf, Switzerland, 2014. [Google Scholar]
- World Weather Online. Available online: https://www.worldweatheronline.com/ (accessed on 7 September 2023).
- Hoffmann, R. A Kukorica Trágyázása. In Agroforum.hu. 8 October 2018; Hungary. Available online: https://agroforum.hu/szakcikkek/tapanyag-utanpotlas/a-kukorica-tragyazasa/ (accessed on 7 September 2023).
- Soyelu, L.O.; Ajayi, S.A.; Aluko, O.B.; Fakorede, M.A.B. Varietal differences for development of maize (Zea mays L.) seedlings on compacted soils. J. Agron. Crop Sci. 2001, 186, 157–166. [Google Scholar] [CrossRef]
- Oluwaranti, A.; Badmus, O.T.; Awoniyi, S.O.; Akintola, A.M.; Bankole, O.O.; Awosanmi, F.E. Comparative analysis of physiological seed quality and field performance of single-, three-way and double-cross hybrids of tropical maize germplasm. Life J. Agric. 2018, 30, 83–98. [Google Scholar]
- Bewley, J.D.; Lack, M. Physiology and Biochemistry of Seeds in Relation to Germination, 2nd ed.; Springer Press: New York, NY, USA, 1982; p. 365. [Google Scholar] [CrossRef]
- Leishman, M.R.; Westoby, M.; Jurado, E. Correlates of seed size variation: A comparison among five temperate floras. J. Ecol. 1995, 83, 517–529. [Google Scholar] [CrossRef]
- Silvertown, J.; Bullock, J.M. Do Seedlings in Gaps Interact? A Field Test of Assumptions in ESS Seed Size Models. Oikos 2003, 101, 499–504. [Google Scholar] [CrossRef]
- Andersson, S.; Mansby, E.; Prentice, H.C. Paternal effects on seed germination: A barrier to the genetic assimilation of an endemic plant taxon? J. Evol. Biol. 2008, 21, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Kadafi, M.; Anne, N.; Agus, W.; Anita Lestari, S. The Relationship Between Seed Size and Seed Quality in UNPAD New Seed Collection of Sweet Corn Lines After Storage. In Proceedings of the International Conference on Food, Agriculture and Natural Resources (FANRes 2018), Bantul, Indonesia, 12–14 September 2018. [Google Scholar] [CrossRef]
- Jallow, R.A.J.; Fissah, A.T.; Al-Beiruty, R.Z.; Shakir, S.H. Effect of seed maize and depth of planting on field germination percentage and it’s relation to maize grain yield and components of maize. Iraqi J. Agric. 2009, 14, 9–20. [Google Scholar]
- Ambika, S.; Manonmani, V.; Somasundaram, G. Review on effect of seed size on seedling vigour and seed yield. Res. J. Seed Sci. 2014, 7, 31–38. [Google Scholar] [CrossRef]
- Al-Karaki, G.N. Seed size and water potential effects on water uptake, germination, and growth of lentil. J. Agron. Crop Sci. 1998, 181, 237–242. [Google Scholar] [CrossRef]
- Shi, R.; Tong, L.; Du, T.; Shukla, M.K. Response and Modeling of Hybrid Maize Seed Vigor to Water Deficit at Different Growth Stages. Water 2020, 12, 3289. [Google Scholar] [CrossRef]
- Meena, R.K.; Pullaiahgari, D.; Gudipalli, P. Proteomic analysis of heterotic seed germination in maize using F1 hybrid DHM 117 and its parental inbreds. Turk. J. Biol. Turk Biyol. Derg. 2018, 42, 345–363. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Dalil, B. Effects of seed vigor on growth and grain yield of maize. Plant Breed. Seed Sci. 2014, 70, 81–90. [Google Scholar] [CrossRef]
- Ashakina, A.; Hasanuzzaman, M.; Arifuzzaman, M.; Rahman, M.; Kabir, M. Performance of single, double, and three-way cross hybrids in tomato (Lycopersicon esculentum Mill.). J. Food Agric. Environ. 2016, 1414, 71–77. [Google Scholar]
- Yıldırım, M.; Cakmak, M. Evaluation of hybrid seeds of three-way and single cross for grain number and weight in bread and durum wheat. Turk. J. Agric. -Food Sci. Technol. 2018, 6, 22. [Google Scholar] [CrossRef][Green Version]
- Li, D.; Zhou, Z.; Lu, X.; Jiang, Y.; Li, G.; Li, J.; Liu, W. Genetic dissection of hybrid performance and heterosis for yield-related traits in maize. Front. Plant Sci. 2021, 12, 774478. [Google Scholar] [CrossRef]
- Joshi, P.; Gautam, D. Genetic insights on single cross maize hybrid and its importance on maize self-sufficiency in Nepal. Arch. Agric. Environ. Sci. 2021, 6, 218–226. [Google Scholar] [CrossRef]
- Tahir, M.; Tanveer, A.; Ali, A.; Abbas, M.; Wasaya, A. Comparative Yield Performance of Different Maize (Zea mays L.) Hybrids under Local Conditions of Faisalabad-Pakistan. Pak. J. Life Soc. Sci. 2008, 6, 118–120. Available online: https://www.researchgate.net/publication/236330855 (accessed on 28 June 2023).
- Ladan, K.M.; Hassan, A.H. Yield performance of three maize (Zea mays L.) varieties as influenced by time of nitrogen fertilizer topdressing in savannah zone of Nigeria. J. Agric. Environ. 2020, 16, 43–52. [Google Scholar]
- Ali, Z. Studies on Comparative Economic Returns of Different Maize Genotypes. Master’s Thesis, Department Agronomy, University Agriculture, Faisalabad, Pakistan, 1994. [Google Scholar]
- EnayatGholizadeh, M.; Bakhshandeh, A.; Shoar, M.D.; Ghaineh, M.; Saeid, K.A.; Sharafizadeh, M. Effect of Source and Seed Size on Yield Component of Corn S.C704 in Khuzestan. Afr. J. Biotechnol. 2012, 11, 2938–2944. Available online: https://www.ajol.info/index.php/ajb/article/view/100681 (accessed on 28 June 2023). [CrossRef]
- Geletata, L. Comparative performance and heterosis in single, three-way, and double cross pepper hybrids. J. Agric. Sci. 2004, 142, 659–663. [Google Scholar] [CrossRef]
- Stephen, D.S. Corn Grain Yield in Relation to Stress during Ear Development. Crop Insights 2016, 26, 1–5. Available online: https://www.pioneer.com/CMRoot/Pioneer/US/Non_Searchable/programs_services/earn-the-right/Corn-Yield-Stress-Ear-Development.pdf (accessed on 28 June 2023).
- Harder, H.J.; Carlson, R.E.; Shaw, R.H. Yield, yield components, and nutrient content of corn grain as influenced by post-silking moisture stress1. Agron. J. 1982, 74, 275–278. [Google Scholar] [CrossRef]
- Herrero, M.P.; Johnson, R.R. High temperature stress and pollen viability of maize1. Crop Sci. 1980, 20, 796–800. [Google Scholar] [CrossRef]
- Jing, Q.; Bingwv, W.; Yong, M. A study on comprehensive evaluation of maize hybrids. J. Jilin-Agric. Univ. 2003, 25, 139–142. [Google Scholar]
- McCutcheon, J.; Siegrist, H.; Rzenwncki, P. Fair Field, Licking, and Perry Counties- Commercial Corn Hybrid Side by Side Performance Trials. Spec. Circ. Ohio Agric. Res. Dev. Cent. 2001, 179, 54–56. Available online: https://agcrops.osu.edu/research-report/1999/fairfield-licking-and-perry-counties-commercial-corn-hybrid-side-side (accessed on 1 July 2023).
- Zeng, L.; Wu, J.; Bechere, E. Comparative genetic analysis of lint yield and fibre quality among single, three-way, and double crosses in upland cotton. Crop Sci. 2017, 57, 192–201. [Google Scholar] [CrossRef]
- Zemach, S.; Wassu, M.; Dagne, W.; Amsal, T. Performances of three-way cross hybrids over their respective single crosses and related heterosis of maize (Zea mays L.) hybrids evaluated in Ethiopia. Heliyon 2023, 9, 1–12. [Google Scholar] [CrossRef]
- Owen, E.; Jones, F. Statistics 3rd; Renold Publishing Company: New York, NY, USA, 1977; 30p. [Google Scholar]
- Yagdi, K.O.K.S.A.L.; Sozen, E. Heritability, variance components and correlations of yield and quality traits in durum wheat (Triticum durum Desf.). Pak. J. Bot 2009, 41, 753–759. [Google Scholar]
- Finch-Savage, W.E.; Bassel, G.W. Seed vigour and crop establishment: Extending performance beyond adaptation. J. Exp. Bot. 2016, 67, 567–591. [Google Scholar] [CrossRef]
- TeKrony, D.M.; Egli, D.B. Relationship of seed vigor to crop yield: A review. Crop Sci. 1991, 31, 816–822. [Google Scholar] [CrossRef]


| Source | Entry | Genotypes | Description |
|---|---|---|---|
| Martonvásár | V1 | B1026/17 | Parent |
| V2 | MCS901/19 | Parent | |
| V3 | TK/15/DV | Parent | |
| V4 | TK1083/18 | Parent | |
| V5 | TK623/18 | SC Hybrid 1 | |
| V6 | TK256/17 | DC Hybrid 2 | |
| V7 | TK222/17 | TC Hybrid 3 | |
| Szeged University | V8 | GK131 | Parent |
| V9 | GK144 | Parent | |
| V10 | GK150 | Parent | |
| V11 | GK154 | Parent | |
| V12 | GK155 | Parent | |
| V13 | GK144X150 | SC Hybrid 1 | |
| V14 | GK154X155 | SC Hybrid 1 | |
| V15 | Szegedi 521 | SC Hybrid 1 | |
| Commercial | V16 | MV277 | Control |
| Source | Dependent Variables | Sum Square | Df | Mean Square | F |
|---|---|---|---|---|---|
| Days | Germination rate (%) | 388.953 | 3 | 129.651 | 142.246 ** |
| Radicle length (cm) | 7824.545 | 3 | 2608.182 | 1681.805 ** | |
| Plumule length (cm) | 2110.986 | 3 | 703.662 | 1419.352 ** | |
| Genotypes | Germination rate (%) | 158.109 | 15 | 10.541 | 11.565 ** |
| Radicle length (cm) | 182.861 | 15 | 12.191 | 7.861 ** | |
| Plumule length (cm) | 51.976 | 15 | 3.465 | 6.989 ** | |
| Days × genotypes | Germination rate (%) | 62.422 | 45 | 1.387 | 1.522 * |
| Radicle length (cm) | 247.334 | 45 | 5.496 | 3.544 ** | |
| Plumule length (cm) | 33.788 | 45 | 0.751 | 1.515 * | |
| Error | Germination rate (%) | 175.000 | 192 | 0.911 | |
| Radicle length (cm) | 297.758 | 192 | 1.551 | ||
| Plumule length (cm) | 95.186 | 192 | 0.496 | ||
| Total | Germination rate (%) | 3714.000 | 256 | ||
| Radicle length (cm) | 25,731.023 | 256 | |||
| Plumule length (cm) | 5249.053 | 256 |
| 1 Genotypes | 2 Germination Rate (%) | 3 Radicle Length (cm) | 4 Plumule Length (cm) |
|---|---|---|---|
| V1 (parent) | 83.33 a–d | 13.42 fg | 8.23 b |
| V2 (parent) | 95.83 ab | 18.95 a | 7.41 b |
| V3 (parent) | 100 a | 18.45 ab | 7.54 b |
| V4 (parent) | 87.50 abc | 14.60 ef | 7.45 b |
| V5 (SC hybrid) | 79.17 bcd | 16.92 a–e | 7.76 b |
| V6 (DC hybrid) | 100 a | 16.37 b–e | 7.22 b |
| V7 (TC hybrid) | 66.67 de | 15.86 c–f | 5.79 c |
| V8 (parent) | 54.17 e | 16.78 a–e | 7.91 b |
| V9 (parent) | 83.33 a–d | 11.41 g | 7.42 b |
| V10 (parent) | 91.65 abc | 17.70 a–d | 8.30 b |
| V11 (parent) | 87.49 abc | 13.9 f | 7.65 b |
| V12 (parent) | 50.00 ef | 18.09 abc | 7.75 b |
| V13 (SC hybrid) | 95.83 ab | 16.91 a–e | 8.04 b |
| V14 (SC hybrid) | 33.33 f | 13.68 fg | 9.88 a |
| V15 (SC hybrid) | 75.00 cd | 15.17 def | 7.65 b |
| V16 (control) | 91.67 abc | 17.30 a–d | 7.78 b |
| Grand mean | 79.69 | 15.97 | 7.73 |
| SEM (±) | 2.71 | 0.31 | 0.14 |
| Source | Sum of Square | df | Mean Square | F | Sig. | |
|---|---|---|---|---|---|---|
| Rows ear−1 | Between groups | 893.133 | 15 | 59.542 | 40.482 | 0.00 |
| Within groups | 329.467 | 224 | 1.471 | |||
| Total | 1222.600 | 239 | ||||
| Source | No. | Mean | Minimum | Maximum | Std. Deviation |
|---|---|---|---|---|---|
| Rows ear−1 | 240 | 12.3500 | 7.00 | 18.00 | 2.26174 |
| Valid N (listwise) | 240 |
| Source | Sum of Square | df | Mean Square | F | Sig. | |
|---|---|---|---|---|---|---|
| No. of kernels per ear−1 | Between groups | 2,064,762.863 | 15 | 137,650.858 | 118.243 | 0.000 |
| Within groups | 260,766.993 | 224 | 1164.138 | |||
| Total | 2,325,529.796 | 239 | ||||
| Source | No. | Mean | Minimum | Maximum | Std. Deviation |
|---|---|---|---|---|---|
| No. of kernels per ear−1 | 240 | 214.80 | 64.00 | 538.00 | 98.64203 |
| Valid N (listwise) | 240 |
| Source | Sum of Square | df | Mean Square | F | Sig. | |
|---|---|---|---|---|---|---|
| 1000-kernel weight (g) | Between groups | 141,061.910 | 15 | 9404.127 | 9.561 | 0.000 |
| Within groups | 31,476.453 | 32 | 983.639 | |||
| Total | 172,538.363 | 47 | ||||
| Source | No. | Mean | Minimum | Maximum | Std. Deviation |
|---|---|---|---|---|---|
| 1000-kernel weight (g) | 48 | 318.23 | 209.30 | 472.20 | 60.58902 |
| Valid N (listwise) | 48 |
| Source | Sum of Square | df | Mean Square | F | Sig. | |
|---|---|---|---|---|---|---|
| Ear weight (g) | Between groups | 120,460.654 | 15 | 8030.710 | 41.815 | 0.000 |
| Within groups | 45,822.069 | 224 | 192.054 | |||
| Total | 166,282.723 | 239 | ||||
| Source | No. | Mean | Minimum | Maximum | Std. Deviation |
|---|---|---|---|---|---|
| Ear weight | 240 | 318.23 | 22.20 | 140.40 | 26.15377 |
| Valid N (listwise) | 240 |
| 1RPE | 2NKPE | 31000 KWT | 4EW | 5GR % | 6RL | 7PL | |
| 1RPE | 1 | ||||||
| 2NKPE | 0.81 ** | 1 | |||||
| 31000 KWT | 0.38 ** | 0.41 ** | 1 | ||||
| 4EW | 0.65 ** | 0.77 ** | 0.49 ** | 1 | |||
| 5GR % | −0.04 ns | 0.12 ns | −0.004 ns | −0.06 ns | 1 | ||
| 6RL | 0.15 ns | 0.07 ns | 0.03 ns | 0.11 ns | 0.18 * | 1 | |
| 7PL | −0.19 ns | −0.17 * | −0.05 ns | −0.08 ns | −0.20 ** | 0.09 ns | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omar, S.; Abd Ghani, R.; Khalid, N.; Jolánkai, M.; Tarnawa, Á.; Percze, A.; Mikó, P.P.; Kende, Z. Effects of Seed Quality and Hybrid Type on Maize Germination and Yield in Hungary. Agriculture 2023, 13, 1836. https://doi.org/10.3390/agriculture13091836
Omar S, Abd Ghani R, Khalid N, Jolánkai M, Tarnawa Á, Percze A, Mikó PP, Kende Z. Effects of Seed Quality and Hybrid Type on Maize Germination and Yield in Hungary. Agriculture. 2023; 13(9):1836. https://doi.org/10.3390/agriculture13091836
Chicago/Turabian StyleOmar, Suhana, Rosnani Abd Ghani, Noriza Khalid, Márton Jolánkai, Ákos Tarnawa, Attila Percze, Péter Pál Mikó, and Zoltán Kende. 2023. "Effects of Seed Quality and Hybrid Type on Maize Germination and Yield in Hungary" Agriculture 13, no. 9: 1836. https://doi.org/10.3390/agriculture13091836
APA StyleOmar, S., Abd Ghani, R., Khalid, N., Jolánkai, M., Tarnawa, Á., Percze, A., Mikó, P. P., & Kende, Z. (2023). Effects of Seed Quality and Hybrid Type on Maize Germination and Yield in Hungary. Agriculture, 13(9), 1836. https://doi.org/10.3390/agriculture13091836








