Abstract
Sugarcane is one of the essential raw materials for sugar production worldwide. From a present perspective, extending the storage time of sugarcane after harvest is a crucial step toward increasing sugar production. Particularly, sugarcane harvested by sugarcane harvesters is more susceptible to biological damage due to the increased number of wounds created during harvesting. Once harvested, the most effective way to reduce bio-injury is to deliver sugarcane to the factory as soon as possible. In recent years, microwave radiation has been increasingly used in food products to reduce biological contamination. The present study examines the effect of microwave irradiation on the sucrose loss that occurs in post-harvest sugarcane. A microwave was employed for different time intervals, particularly from 5 to 9 s at a 2450 MHz frequency and 5100 W power. The results reveal that microwave radiation can effectively kill microorganisms in sugarcane wounds in a very short time. With a microwave irradiation time of 6 s, a microbial disinfection efficiency of 91.2% was achieved. The maximum temperature in the billets at that time was only 35.1 °C. Microwave radiation reduced the sucrose loss rate due to a significant decrease in the number of microflora during the 48 h period. As a result, the storage time of harvested sugarcane was prolonged, leading to improved economic benefits in sugar extraction.
1. Introduction
Sugarcane is one of the significant sugar crops, accounting for more than 85% of the total global sugar supply [1]. The yield of sugar is greatly affected by the quality of the sugarcane in the production process. The main reason for the serious degradation of sugarcane quality in China after harvest is the delay in transporting sugarcane to the sugar mill [2]. During the stacking process of harvested sugarcane, the sugar content, gravity purity, apparent purity, and unit weight of sugarcane varieties gradually decrease, while the fiber content and reducing sugar content increase with extended stacking time [3,4]. The mechanized production of sugarcane is considered an effective method to reduce the cost of sugarcane planting and improve the economic benefits of planting [5,6]. However, one of the key problems caused by the untimely squeezing of sugarcane after mechanical harvesting is the loss of sucrose. Additionally, mechanical harvesting causes more wounds or cuts, such as cane rupture, resulting in financial losses for sugar mills by hastening the decline in sugarcane quality [7].
Previous studies have identified the main cause of post-harvest sugarcane spoilage as the transformation of sucrose by enzymes and microorganisms, with more than 90% of sucrose loss in the first 14 h of sugarcane juice spoilage caused by microorganisms [8]. Sugarcane contains high concentrations of sucrose, providing a favorable environment for the proliferation of microorganisms, which can invade the harvested sugarcane stalk through wounds or cuts [9]. Studies have shown that more than 400 species of bacteria and fungi may be present in cuts and wounds after sugarcane harvesting [10]. These acid-producing microorganisms cause spoilage, reducing sucrose content, juice purity, and pH, especially under anaerobic conditions, such as bulk-stored and poorly ventilated sugarcane [11,12]. Therefore, it has become urgent to seek a method that can control the number of microbial floras.
In a previous study, a strong recommendation was made regarding the utilization of chemical formulations for safeguarding harvested sugarcane against microbial infestation and curbing sucrose losses. The combination of benzalkonium chloride and sodium metasilicate emerged as particularly effective in achieving these objectives. Notably, the implementation of this chemical formulation resulted in a notable decrease in the levels of microbial infestation, dextran, soluble acid invertase, and reducing sugars in drought-affected sugarcane samples, all of which were observed within a span of 240 h following harvest. This outcome played a pivotal role in mitigating the degradation of sucrose and, in turn, enhancing the overall sugar recovery process at the milling stage [13]. On the other hand, the utilization of a pulsed electric field (PEF) was explored as a means of treating freshly extracted sugarcane juice with the incorporation of lemon and ginger, which could affect the process. Nevertheless, it is essential to acknowledge that the application of PEF treatment may entail substantial costs and necessitate specialized equipment, potentially posing limitations on its applicability across various scenarios [14]. Within the realm of traditional methodologies, pasteurization has been a widely adopted means of sterilizing sugarcane juice. By subjecting the juice to a continuous sterilization process at a temperature of 808 °C, it was possible to maintain the juice’s shelf life for a duration of 60 days. Further enhancing the longevity of sugarcane juice, certain chemicals, such as potassium metabisulphite, have been introduced to prolong its shelf life [15].
Microwave sterilization technology has emerged as a new type of physical sterilization technology and has become a research hotspot in recent years. Compared with traditional chemical and biological control methods, microwave treatment offers advantages, such as short time, rapid heating, high efficiency, energy saving, sensitive response, simple equipment, safety, effectiveness, and easy control. It has been widely used in the food industry to kill pathogenic microorganisms. A particularly intriguing avenue of investigation involves the amalgamation of microwave and ultrasonic technologies. This innovative combination adeptly retains the desirable attributes of sugarcane juice, including color preservation, total phenolic and flavonoid content, levels of ascorbic acid, and the capacity for antioxidant action. Impressively, this collaborative approach not only ensures stability throughout storage, but also effectively curtails the proliferation of microbial populations. By adopting this synergistic microwave–ultrasonic strategy, a remarkable extension of the shelf life of sugarcane juice has been achieved [16].
Microwaves refer to electromagnetic waves with a frequency of 300 MHz to 300 GHz and a wavelength between 1 mm and 1 m [17]. This is an electromagnetic method of heating that is used as an alternative to traditional thermal processing methods and offers a better protection of the nutritional and sensory properties of food. Microwave heating occurs when materials absorb microwave energy and convert it to heat. This is primarily driven by dipolar and ionic mechanisms. Due to the dipolar nature of water, the presence of moisture or water causes dielectric heating. When an oscillating electric field is incident on water molecules, the permanently polarized dipolar molecules attempt to rearrange themselves in the direction of the electric field. With the high frequency of the electric field, this rearrangement occurs at a high rate of one million times per second and causes internal friction within the molecules, which leads to volumetric heating of the material [18]. Microwave treatment swiftly deactivates microorganisms and enzymes compared to traditional thermal processing approaches, thus minimizing nutrient loss [19]. Microwave technology demonstrates the ability to manage microbial load and enzyme activity, enhancing the safety of fruit juices as well [20,21,22,23]. In recent years, researchers have also investigated the use of microwaves to control crop biological diseases. Reports have shown that microwaves can effectively kill endogenous pathogenic bacteria [24] and vector insect eggs and pupae [25] under conditions that ensure plant survival. Furthermore, microwave radiation has been applied to control soil pathogenic microorganisms, pests, and weeds [26,27,28,29].
The advantages of microwave sterilization come from its thermal and non-thermal effects. The thermal effect of microwaves can effectively enhance the sterilization effect of food, thereby reducing the temperature threshold of microbial death, leading to sterilization and sugar preservation [30]. Considering these findings, it is meaningful to research the effect of microwave radiation on the microbial flora of post-harvest sugarcane to evaluate the impact of this technology on sucrose loss.
2. Materials and Methods
2.1. Experimental Sugarcane
From December 2022 to January 2023, the experiment was conducted at the China Agricultural University Professor Workstation, which is located in Fusui County, the Guangxi Zhuang Autonomous Region, China. Since the workstation was located in the sugarcane plantation, it was very convenient to gain access to the test materials. The sugarcane variety used in this experiment was Guitang 43 (Guangxi, China). Firstly, a certain quantity of sugarcane was cut in the field, and then the sugarcane with similar lengths and diameters were selected as the test samples. The abovementioned sample selection could minimize the errors caused plant differences. In order to ensure the universality of the experimental data, the sugarcane samples were evenly and randomly divided into 6 groups, 5 of which were the experimental group and the last group was the control group.
2.2. Experimental Equipment
The Nanning Zhongke Microwave Advanced Manufacturing Industry Technology Research Institute, which is based at the Guangxi Academy of Sciences, provided the equipment for the microwave experimental apparatus. The WaveLane WLKJ-D6 microwave system (WaveLane Co., Ltd., Zhuzhou, Hunan, China) was used in this study for testing purposes. The WLKJ-D6 microwave oven is a stainless-steel chamber composed of an 800 by 800 by 800 mm block (maximum size) and weighed 20 kg (maximum weight). The microwave system operated at 2450 MHz, which was more powerful and allowed for the short treating time of products compared with the frequently used 915 MHz microwave frequency. Heating homogeneity was achieved by the use of microwave coupling from the top and bottom of the product and the rotation of a turntable. The output power of 5100 W maximum was produced by six 1050 W generators (magnetron) and was adjustable from 1000 to 5100 W. The system was wind-cooled. The sugarcane crusher adopted the Midea MJ-PB40E254D juicer (Midea Co., Ltd., Foshan, Guangdong, China) with a rated power of 1200 W and a rated speed of 30,000 RPM. SWEVY SW-32D (SWEVY Technology Co., Ltd., Guangzhou, Guangdong, China) is a sugar content measuring instrument that uses a high-precision refractive sugar content detector with a resolution of 0.1%/Brix, a measurement range of 0~32%/Brix, and an accuracy of ±0.2%. The weight measuring instrument adopted the OHAUS 602ZH (OHAUS Co., Ltd., Parsippany, NJ, USA) model high-precision balance scale with a measurement accuracy of 0.01 g.
2.3. Experiment Preparation
Experimental errors are likely to be caused by the differences in diameter, maturity, and sugar content among different sugarcane varieties. Therefore, it was necessary to pretreat the sugarcane before conducting the experiment to increase the accuracy of the test. Figure 1 shows some of the samples. The sugarcane used for the weight measurement was cut into 20 cm/segment by a cutting knife for a total of 18 segments, and randomly divided into 6 groups, where each group of 3 segments was randomly selected. Those that were used for sugar and microbial measurements were cut into 2 cm/segment, where the sugar content was measured and randomly divided into 31 groups with 5 sections in each group, where 1 group was measured before the experiment. The microorganism test groups were randomly divided into 31 groups with 5 sections in each group, where 1 group was measured before the microwave experiment. Each group samples was repeated 3 times as well. After completing the abovementioned processes, the microwave experiment was conducted. Immediately after the experimental procedure, the measurements of weight, sugar content, and microbial flora quantity were obtained for a total of 6 groups. The remaining post-experimental materials were placed in a conventional indoor environment and assayed every 24 h.

Figure 1.
Experimental sugarcane.
Cane weight was measured after the harvesting of canes of each pile and before the extraction of juice to measure the loss in cane weight. Sucrose percentage was estimated using the lead acetate method, where 4 g of lead acetate was added to approx. 100 mL of juice and was left to precipitate. Then, the juice was filtered through Whatman filter paper and a reading was with a SWEVY polarimeter [31]. The temperature was measured after the microwave irradiation of canes from each pile.
2.4. Microwave Output Power and Time of Irradiation
A total of 2450 MHz was selected for the microwave frequency, with a maximum power of 5100 W used during the whole processing stage. The radiation time of the microwaves for this experiment was set as shown in Table 1.

Table 1.
Time of experiment.
2.5. Experimental Procedure
The experimental procedure was shown in Figure 2. Before the test began, all the test materials were measured for their physicochemical properties, including temperature, microbial content, and weight. Then, the formal test operation process started as follows. Firstly, place the sugarcane onto the microwave platform with a certain number of billets according to the grouping in Table 1. Start the microwave immediately after setting the radiation time. Once the equipment stops running, remove the billets immediately, and first measure the temperature, then the weight, and finally the microbial content of the billets. Then, place the remaining billets in the natural environment. Measure and record their physicochemical properties every 24 h for 4 consecutive days. After four days of measurements, conduct the statistical analysis. The above steps are the complete process of one set of experiments. A total of five sets of experiments were conducted in this study.
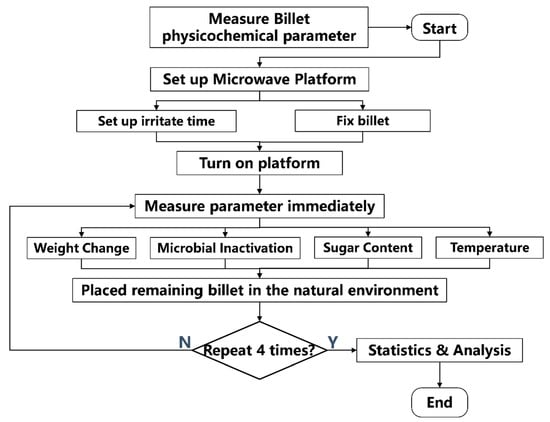
Figure 2.
Experimental procedure. “Measure Billet physicochemical parameter” means measure the billet’s temperature, weight, microbial load, and sucrose percentage before the experiment.
3. Results
3.1. Temperature of Tested Sugarcane
Due to the thermal effect of microwaves, the temperature of the sugarcane sharply increased after being irradiated. It can be observed from Figure 3 that the maximum and average temperatures of the microwaved sugarcane gradually increase during the irradiation process, while the minimum shows a very gradual increased. Notably, after 8 s of microwave irradiation, the overall temperature of the billet increased to over 50 °C, which is the temperature known to kill traditionally soaked sugarcane sprouts in warm water [29].
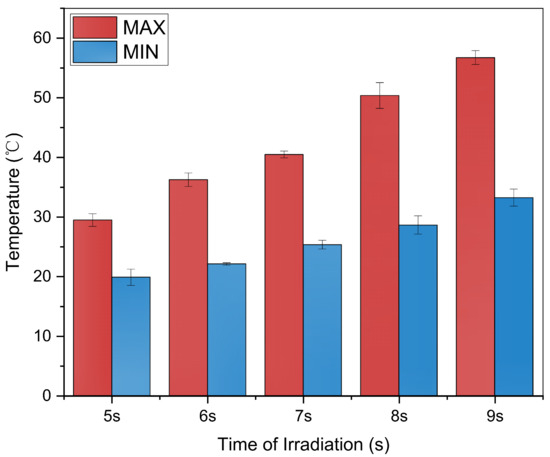
Figure 3.
Temperature record.
Moreover, the temperature at both ends of the microwaved sugarcane, specifically at the incision points, was higher than in the middle of the segment, as shown in Figure 4. However, the temperature gap between the edges and middle segments reduced with the prolongation of the testing time. This particular microwave equipment had its microwave guide port positioned on the side, necessitating the placement of sugarcane on its side during irradiation to minimize any unanticipated incidents during its use. This positioning allows the microwave radiation to accurately target the sugarcane’s incision area, which harbors significantly denser microbial flora than the inside. As a result, the microwave’s automatic processing effectively targeted more microorganisms in a shorter time, thereby increasing th economic benefits.
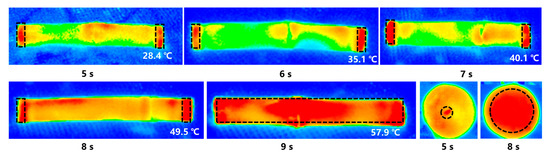
Figure 4.
Infrared temperature map.
3.2. Alterations in Microbial Flora
Bacteria contain a lot of water, and water molecules are dipolar molecules as well. Previous research has shown that water molecules polarize when absorbing microwave energy, allowing them to gain kinetic energy and move in the electric field. The accelerated movement of water molecules significantly increases the likelihood of collisions with other molecules. These continuous collisions generate a lot of heat, which not only denatures proteins due to the heat but also leads to the inactivation and death of the organism [30]. The number of microbial floras was counted on the sugarcanes irradiated by microwaves, and the statistical results are shown in Figure 5. Compared with the control group, the number of microbial floras in the experimental group’s sugarcane was very low during the first 48 h and remained at a relatively low level afterward. The level of microbial flora was reduced to between 52.4% and 91.2% in the irradiation-treated samples after 48 h of harvest, compared to their control. This phenomenon indicates that an overwhelming number of microorganisms are killed when the irradiation interval is 6 s. Additionally, the data suggest that the microorganism population experiences a boom between 48 and 72 h of treatment. Therefore, 6 s is the optimal microwave irradiation time required to effectively eliminate microorganisms under the conditions of this experiment.
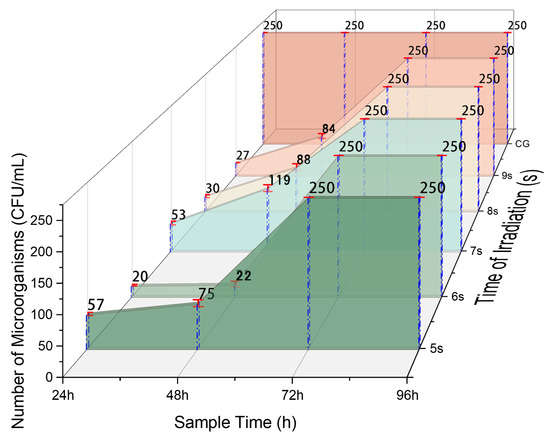
Figure 5.
Alterations in microbial flora. CG is the abbreviation for the control group. A total of 250 was defined as the maximum number of microorganisms throughout the experiment, which meant that a quantity much higher than that would be ignored.
3.3. Weight Loss of Testing Sugarcane
All groups of billets were weighed before being fed into the microwave facility. After microwave irradiation, the weight of each group of sugarcane samples was measured again, as shown in Figure 6. The weight of the experimental sugarcane remained almost the same when the irradiation time was shorter than 7 s. However, if the irradiation time was longer than or equal to 7 s, the weight loss increased with time, and then the average weight decreased by 0.7% per day for each group, as depicted in the figure. Microwave radiation did not cause obvious wounds to the billets. The weight loss of sugarcane when irradiated by microwaves primarily occurred due to the evaporation of water, following the principle of the thermal effect of microwaves [30]. However, this evaporation of water was not aggressive because the microwave radiation exposure was brief, and the temperature increase was far from reaching the evaporation temperature of water caused by the microwave radiation. On the other hand, the sugarcane in the positioned area experienced respiration, leading to the consumption of some water. This was why the weight of the sugarcane decreased every day.
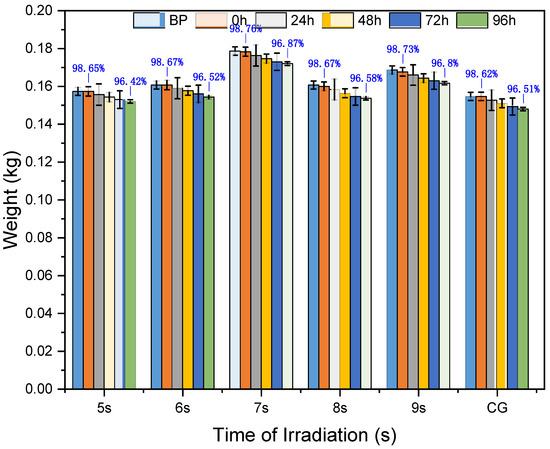
Figure 6.
Weight lost during the process of time. The blue percentage in the graph is the weight loss compared with before the test at 0 and 96 h, respectively. BP = before processing of irradiation.
3.4. Sucrose Percentage
Traditionally, the sugar content is the most commonly used index to describe the amount of sugar in cane, referring to the amount of sugar in cane per unit weight. The change in sugar content of sugarcane after being irradiated by microwaves is shown in Figure 7. As shown, the microwave-irradiated sugarcane contained more sucrose compared to the control group. The thermal effects of the microwaves caused a portion of the sucrose to convert into glucose and fructose, leading to a reduction in water content. However, the total sugar content in the sample remained the same. The sum of sucrose, glucose, and fructose was maintained as well. This reduction in water content resulted in an increase in sugar concentration. However, some of the glucose and fructose gradually began to degrade after 24 h. Consequently, the total sugar content in the sample decreased, leading to a reduction in brix, which is a measurement of sugar content. The observable manifestation of the sugar level depended on the reaction that dominated. If the hydrolysis reaction of sucrose led to excessive water consumption and only the minimal degradation of glucose and fructose occurred, the rate of total sugar reduction was slower than the rate of water evaporation. This led to a macroscopic increase in the sugar concentration. Conversely, if the degradation of glucose and fructose was more pronounced, the sugar level decreased. On the other hand, due to the thermal effect of microwave radiation, the number of microbial floras in the sugarcane was maintained at a low level during the first 48 h; however, it began to rapidly multiply after 48 h. High levels of sucrose and water were consumed during this process, causing the sucrose percentage to drop rapidly after 48 h. After 72 h, the reproduction rate of the microbial flora decreased; however, the percentage of sucrose began to increase due to the evaporation of water, and the brix remained relatively stable over the subsequent time. Therefore, microwave radiation can effectively inhibit the growth of microbial flora within 48 h, which slows down the consumption of sucrose and prolongs the storage time of sugarcane after harvest.
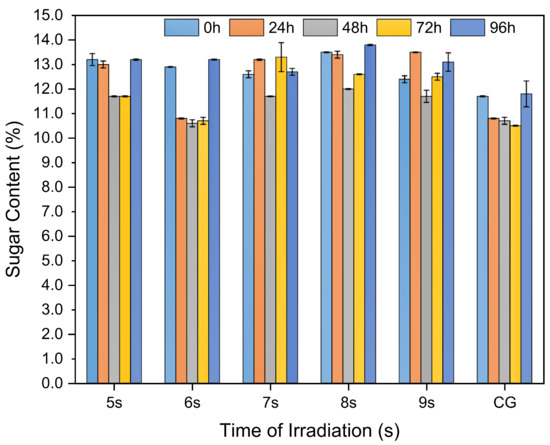
Figure 7.
Sucrose percentage during the elapse of time.
4. Discussion
Since the major application of microwave radiation is in the incision, the microwave treatment of sugarcane is more precisely targeted and also may effectively reduce the quantity of microbial flora at the incision of sugarcane after harvest. When the microwave kills the microorganisms, it is firstly applied to the incision of the sugarcane section. This is due to the fact that there are water molecules directly exposed to the air at the incision of the sugarcane section. Through the thermal effect of the microwave, it can rapidly cause the vigorous vibration of the water molecules at the incision. This phenomenon results in higher temperatures at the ends of the sugarcane section during the initial stage of microwave radiation (radiation time less than 6 s) compared to the inner part of the sugarcane section (Figure 4). The temperature of the whole cane section gradually increases throughout the radiation time. However, the temperature at the cut of the cane section remains higher than that of the interior of the cane section. On the other hand, microorganisms in the natural environment can easily enter the inner part of the sugarcane section through the incision, and the sucrose at the incision becomes one of the important energy sources supporting the reproduction of microorganisms. This makes the incision of the sugarcane section more susceptible to microbial infection. Therefore, the thermal effect of the microwave can rapidly kill the microorganisms at the cuts of sugarcane, thereby prolonging its storage time. In other words, it protects the sucrose content.
Microorganisms can be killed effectively by microwave radiation in sugarcane wounds in a very short time. We concluded that the highest efficiency of killing microorganisms was achieved when the irradiation was set at 6 s. From the experimental results, it can be observed that the microorganisms are inactivated with the highest efficiency when the microwave treatment time is 6 s. Although the microorganisms in the billets with a microwave radiation time of longer than 7 s can be inactivated to the same level, the microwave radiation time is positively correlated with energy consumption, and a microwave radiation time longer than 7 s results in higher energy consumption rates per throughput of sugarcane. Therefore, a shorter microwave radiation time reduces the time needed to treat all the billets and decrease the energy required, thereby improving the efficiency of sugarcane production.
Microwave technology can slow down the loss of sucrose within 48 h after irradiation, prolonging the storage time before significant sucrose loss results in the economic benefits of sugar extraction. After the sugarcane is radiated by microwaves, a high number of harmful microorganisms are killed, and there is a certain negative correlation between the content of microorganisms and sucrose consumption. In other words, when the microorganisms begin to reproduce in large quantities, a significant amount of sucrose is consumed. However, due to the substantial number of microorganisms being extinguished, a certain size of the microbial population cannot be formed in a short period of time (Figure 5). Consequently, the rate of sucrose depletion can be reduced, thus improving the storage time of the sugarcane.
The microwave irradiation of sugarcane not only does not damage the sugarcane, but it also does not produce harmful substances. The only effect on the sugarcane is the evaporation of water. On the other hand, microwave radiation is an indirect treatment of sugarcane and is capable of destroying microorganisms with very high treatment efficiency. Therefore, microwaves are an ideal technology to significantly extend the storage time of sugarcane and protect the sucrose. Additionally, since the time of microwave radiation is short, it is possible to try to improve and upgrade the elevator of the combined chopper sugarcane harvester. In other words, upgrading the existing elevator to one with a microwave-treatment installation allows the billets to be treated immediately while harvesting the sugarcane, reducing the time from harvesting to the sugar mill, and further increasing the production efficiency.
5. Conclusions
In the experimental study of microwave irradiation on the billets, which was observed to have effectively killed microorganisms in a short time, over 91.2% of the microorganisms were inactivated. Billets that were harvested by a combined chopper sugarcane harvester typically became susceptible to a significant microbial infection at the end. Due to the limited penetration capacity of 2450 MHz microwaves, the ends of the billets received the highest concentration of microwave radiation. Consequently, the temperature at the ends of the billets also increased more than the billets’ insides. On the other hand, microwave radiation contributed to an overall temperature increase across the billets. However, higher temperatures accelerate the rate of the hydrolysis reaction of sucrose. Therefore, the irradiation time is essential when using microwave radiation. Among the various factors we considered, a microwave radiation time of 6 s was deemed optimal. The experiment demonstrated that microwave radiation extended the storage life of billets by 24 h.
Most of the previous research primarily revolve around enhancing the shelf life and flavor of sugarcane juice. However, the research aims remain largely consistent, which is to control the microbial flora within sugarcane juice (or billets) to prolong the shelf life. Compared with conventional methods, such as chemical treatments [14], ozone exposure [32], and pasteurization [15], the microwave approach showed several advantages, such as a superior sterilization efficiency, short treatment time (20 min required for the pasteurization method), no by-products, and no chemical additives. With the short irradiation time, the sugarcane harvester can be upgraded to a brand new machine, which will allow the sugarcane harvester to simultaneously harvest and subject the billets to microwave treatment, thereby enhancing the overall production efficiency.
Author Contributions
Author Contributions: conceptualization, L.L. and S.M.; methodology, L.L.; software, L.L.; writing—original draft preparation, L.L.; writing—review and editing, S.M.; visualization, L.L. and S.M.; supervision, S.M.; funding acquisition, S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data that are used are confidential.
Acknowledgments
We acknowledge that the research was supported by the Guangxi Academy of Sciences. Any opinions, findings, and conclusions expressed in this publication are those of the authors and do not necessarily reflect the view of the China Agricultural University. The authors would also like to express their gratitude to the Bio-based Material Innovation Team of the Guangxi Academy of Sciences for providing their help to ensure the correct statistics for the microbial flora.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, Y.; Wu, C. Progress and Development Analysis of Sugarcane Industry in the World. Sugar Crops China 2017, 39, 47–50. [Google Scholar]
- Kong, R.; Luo, L.; Li, D.; Su, J. Storage Quality of Five Sugarcane Varieties after Harvest. Chin. J. Trop. Crops 2018, 39, 2391–2395. [Google Scholar]
- Feng, W.; Jing, Y.; An, R.; Bian, X.; Zhou, Q.M.; Dong, L.H.; Tao, L.A.; Sun, Y.F.; Lang, R.B.; Yu, H.X.; et al. Comparative Test on Storability of Different Sugarcane Cultivar. Sugar Crops China 2016, 38, 27–28. [Google Scholar]
- Huang, F.; Xu, J.; Chen, C. Comparative Study of Storage- Fast in Some Species of Sugar Cane. Guangxi Sugarcane Canesugar 2005, 3, 10–13, 31. [Google Scholar]
- Bai, Z.; Zhang, W.; Lu, B. Analysis of Major Factors Affecting Transformation and Upgrading of Sugar Industry in China. Sugar Crops China 2018, 40, 62–65. [Google Scholar]
- Chen, S.; Liao, X.; Xu, F.; Liu, Q.Q.; Xv, R.Q. Analysis and Discussion on Sugarcane Mechanized Harvest in Guangken Sugar Industry. J. Guangdong AIB Polytech. Coll. 2019, 35, 6–12. [Google Scholar]
- Su, J.; Kong, R.; Luo, L.; Zhang, H.; Chen, Y.Q.; Chen, R.K. Comparative Analysis of the Sugar Content Between Two Sugarcane Cultivars after Mechanized Harvesting. Chin. J. Trop. Crops 2015, 36, 1415–1418. [Google Scholar]
- Eggleston, G. Deterioration of cane juice—Sources and indicators. Food Chem. 2002, 78, 95–103. [Google Scholar] [CrossRef]
- Solomon, S.; Singh, P.; Shrivastava, A.K.; Singh, P.; Chandra, A.; Jain, R. Physico-chemical method of preserving sucrose in harvested sugarcane at high ambient tempera ture in a sub-tropical climate. Sugar Tech. 2011, 13, 60–67. [Google Scholar] [CrossRef]
- Stevenson, J.A.; Rands, R.D. An annotated list of the fungi and bacte ria associated with sugarcane and its products. Hawaii Plant Rec. 1938, 42, 247–313. [Google Scholar]
- Solomon, S. Postharvest deterioration of sugarcane. Sugar Tech. 2009, 11, 109–123. [Google Scholar] [CrossRef]
- Misra, V.; Mall, A.K.; Pathak, A.D.; Solomon, S.; Kishor, R. Microorganisms affecting post harvest sucrose losses in sugarcane. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2554–2566. [Google Scholar] [CrossRef][Green Version]
- Misra, V.; Solomon, S.; Hashem, A.; Abd, E.F.; Al-Arjani, A.F.; Mall, A.K.; Prajapati, C.P.; Ansari, M.I. Minimization of post-harvest sucrose losses in drought affected sugarcane using chemical formulation. Saudi J. Biol. Sci. 2020, 27, 309–317. [Google Scholar] [CrossRef]
- Kayalvizhi, V.; Pushpa AJ, S.; Antony, U. Effect of pulsed electric field (PEF) treatment on sugarcane juice. J. Food Sci. Technol. 2016, 53, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Sankhla, S.; Chaturvedi, A.; Kuna, A.; Dhanlakshmi, K. Preservation of sugarcane juice using hurdle technology. Sugar Tech. 2012, 14, 26–39. [Google Scholar] [CrossRef]
- Zia, S.; Khan, M.R.; Zeng Aadil, R.M. Combined effect of microwave and ultrasonication treatments on the quality and stability of sugarcane juice during cold storage. Int. J. Food Sci. Technol. 2019, 54, 2563–2569. [Google Scholar] [CrossRef]
- Sadeghi, A.; Hakimzadeh, V.; Karimifar, B. Microwave assisted extraction of bioactive compounds from food: A review. Int. J. Food Sci. Nutr. Eng. 2017, 7, 19–27. [Google Scholar]
- Datta, A.K.; Davidson, P.M. Microwave and radio frequency processing. J. Food Sci. 2000, 65, 32–41. [Google Scholar] [CrossRef]
- Siguemoto, E.S.; Gut, J.A.W.; Martinez, A.; Rodrigo, D. Inactivation kinetics of Escherichia coli O157:H7 and Listeria monocytogenes in apple juice by microwave and conventional thermal processing. Innov. Food Sci. Emerg. Technol. 2018, 45, 84–91. [Google Scholar] [CrossRef]
- Aadil, R.M.; Zeng, X.A.; Zhang, Z.H.; Wang, M.-S.; Han, Z.; Jing, H.; Jabbar, S. Thermosonication: A potential technique that influences the quality of grapefruit juice. Int. J. Food Sci. Technol. 2015, 50, 1275–1282. [Google Scholar] [CrossRef]
- Aadil, R.M.; Roobab, U.; Maan, A.A.; Madni, G.M. Effect of heat on food properties. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 70–75. [Google Scholar]
- Chang, J.D.; Zheng, H.; Mantri, N.; Xu, L.; Jiang, Z.; Zhang, J.; Song, Z.; Lu, H. Chemometrics coupled with ultraviolet spectroscopy: A tool for the analysis of variety, adulteration, quality and ageing of apple juices. Int. J. Food Sci. Technol. 2016, 51, 2474–2484. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, S.; Yan, W.; Tang, Y.; Yang, R.; Zhao, W. Inactivation of apple (Malus domestica Borkh) polyphenol oxidases by radio frequency combined with pulsed electric field treatment. Int. J. Food Sci. Technol. 2018, 53, 2054–2063. [Google Scholar] [CrossRef]
- Zhang, R.; An, S.; Shi, C.; Nurdanbek, K. Germicidal Efficacy of Microwave Treatment Against Endophyte and Its Effects on the Germinating Vigor of Achnatherum Inebrians. J. Nucl. Agric. Sci. 2016, 30, 1792–1797. [Google Scholar]
- Su, X.; Yang, Q.; Tan, W.; Li, B.; Hu, X.F.; Wang, W.; Qi, S.Y.; Diao, S.H.; Wang, P.F. Preliminary Study on the Insecticidal Effect of Microwave on Eggs and Pupae of Medical Insects. Chin. J. Hyg. Insectic. Equip. 2017, 23, 106–108. [Google Scholar]
- Brodie, G.; Khan, M.J.; Gupta, D. Microwave Soil Treatment and Plant Growth. In Sustainable Crop Production; IntechOpen: Rijeka, Croatia, 2019; pp. 1–18. [Google Scholar]
- Maynaud, G.; Baudoin, E.; Bourillon, J.; Duponnois, R.; Cleyet-Marel, J.-C.; Brunel, B. Short-term effect of 915-MHz microwave treatments on soil physicochemical and biological properties. Eur. J. Soil Sci. 2019, 70, 443–453. [Google Scholar] [CrossRef]
- Graham, B.; Muhammed, J.K.; Dorin, G.; Foletta, S.; Bootes, N. Microwave Weed and Soil Treatment in Agricultural Systems. AMPERE Newsl. 2017, 93, 9–17. [Google Scholar]
- Lu, W.; Li, W.; Huang, Y.; Wang, M.Q.; Wang, X.Y.; Li, J.; Yang, H.C.; Luo, M.Z. Production Technology and Yield Increase Efficiency of Disease-Free Sugarcane Seedlings with Hot Water Treatment. Sugar Crops China 2010, 3, 52–53, 57. [Google Scholar]
- Fan, W.; Huang, H. Application of Microwave Sterilization in Food Industry. Food Mach. 2007, 23, 143–147. [Google Scholar]
- Misra, V.; Solomon, S.; Ansari, M.I. Impact of drought on post-harvest quality of sugarcane crop. Adv. Life Sci. 2016, 5, 8204–8213. [Google Scholar]
- Panigrahi, C.; Mishra, H.N.; De, S. Ozone treatment of ultrafiltered sugarcane juice: Process optimization using multi-objective genetic algorithm and correlation analysis by multivariate technique. LWT 2022, 154, 112861. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).