Assessment of the Chemical Composition and Nutritional Quality of Breast Muscle from Broiler Chickens Receiving Various Levels of Fe Glycine Chelate
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Factor
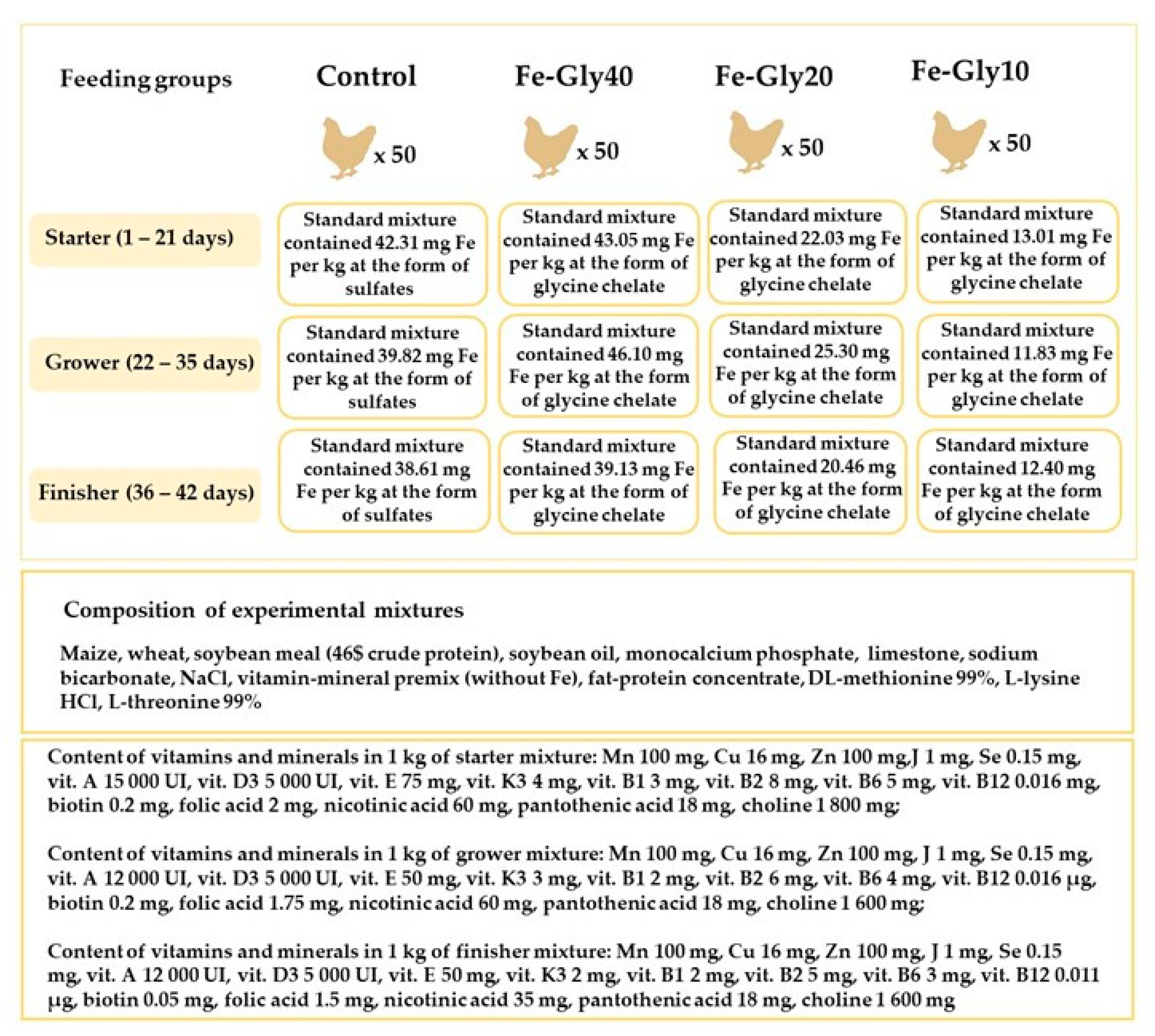
2.2. Chickens and the Design of the Experiment
2.3. Muscle Samples
2.4. Chemical Analyses
2.5. Measurement of Meat pH
2.6. Calculations and Statistical Analysis
3. Results
3.1. Content of Basic Chemical Components, Total Cholesterol Content, and pH
3.2. Fatty Acid Profile in Breast Meat
3.3. Nutritional Value of Meat
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dinh, T.T.N.; Thompson, L.D.; Galyean, M.L.; Brooks, J.C.; Patterson, K.Y.; Boylan, L.M. Cholesterol content and methods for cholesterol determination in meat and poultry. Compr. Rev. Food Sci. Food Saf. 2011, 10, 269–289. [Google Scholar] [CrossRef]
- Goluch, Z.; Słupczyńska, M.; Okruszek, A.; Haraf, G.; Wereńska, M.; Wołoszyn, J. The Energy and Nutritional Value of Meat of Broiler Chickens Fed with Various Addition of Wheat Germ Expeller. Animals 2023, 13, 499. [Google Scholar] [CrossRef]
- Pisulewski, P.M. Nutritional potential for improving meat quality in poultry. Anim. Sci. Pap. Rep. 2005, 23, 303–315. [Google Scholar]
- Long, S.; Liu, S.; Wu, D.; Mahfuz, S.; Piao, X. Effects of Dietary Fatty Acids from Different Sources on Growth Performance, Meat Quality, Muscle Fatty Acid Deposition, and Antioxidant Capacity in Broilers. Animals 2020, 10, 508. [Google Scholar] [CrossRef]
- Jachimowicz, K.; Winiarska-Mieczan, A.; Tomaszewska, E. The Impact of Herbal Additives for Poultry Feed on the Fatty Acid Profile of Meat. Animals 2022, 12, 1054. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A.; Kwiecień, M. The effects of copper-glycine complexes on chemical composition and sensory attributes of raw, cooked and grilled chicken meat. J. Food Sci. Tech. 2015, 52, 4226–4235. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A.; Kwiecień, M.; Kwiatkowska, K.; Baranowska-Wójcik, E.; Szwajgier, D.; Zaricka, E. Fatty acid profile, antioxidative status and dietary value of the breast muscle of broiler chickens receiving glycine-Zn chelates. Anim. Prod. Sci. 2020, 60, 1095–1102. [Google Scholar] [CrossRef]
- Zając, M.; Kiczorowska, B.; Samolińska, W.; Klebaniuk, R.; Andrejko, D.; Kiczorowski, P.; Milewski, S.; Winiarska-Mieczan, A. Supplementation of Broiler Chicken Feed Mixtures with Micronised Oilseeds and the Effects on Nutrient Contents and Mineral Profiles of Meat and Some Organs, Carcass Composition Parameters, and Health Status. Animals 2022, 12, 1623. [Google Scholar] [CrossRef]
- Lin, X.; Gou, Z.; Wang, Y.; Li, L.; Fan, Q.; Ding, F.; Zheng, C.; Jiang, S. Effects of Dietary Iron Level on Growth Performance, Immune Organ Indices and Meat Quality in Chinese Yellow Broilers. Animals 2020, 10, 670. [Google Scholar] [CrossRef]
- Jarosz, Ł.; Kwiecień, M.; Marek, A.; Grądzki, Z.; Winiarska-Mieczan, A.; Kalinowski, M.; Laskowska, E. Effects of feed supplementation with glycine chelate and iron sulfate on selected parameters of cell-mediated immune response in broiler chickens. Res. Vet. Sci. 2016, 107, 68–74. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, Y.; Qian, Z.; Shen, X. The mechanism of Fe2+-initiated lipid peroxidation in liposomes: The dual function of ferrous ions, the roles of the pre-existing lipid peroxides and the lipid peroxyl radical. Biochem. J. 2000, 352, 27–36. [Google Scholar] [CrossRef]
- Kwiecień, M.; Winiarska-Mieczan, A.; Milczarek, A.; Klebaniuk, R. Biological response of broiler chickens to decreasing dietary inclusion levels of zinc glycine chelate. Biol. Trace Elem. Res. 2017, 175, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Rockfield, S.; Chhabra, R.; Robertson, M.; Rehman, N.; Bisht, R.; Nanjundan, M. Links between iron and lipids: Implications in some major human diseases. Pharmaceuticals 2018, 11, 113. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, H.; Ailati, A.; Chen, W.; Chilton, F.H.; Lowther, W.T.; Chen, Y.Q. Substrate specificity and membrane topologies of the iron-containing ω3 and ω6 desaturases from Mortierella alpina. Appl. Microbiol. Biotechnol. 2018, 102, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Kwiecień, M.; Samolińska, W.; Bujanowicz-Haraś, B. Effects of iron-glycine chelate on growth, carcass characteristic, liver mineral concentrations and haematological and biochemical blood parameters in broilers. J. Anim. Physiol. Anim. Nutr. 2015, 99, 1184–1196. [Google Scholar] [CrossRef]
- Kwiatkowska, K.; Winiarska-Mieczan, A.; Kwiecień, M. Effect of application of Fe-glycinate chelate in diet for broiler chickens in an amount covering 50 or 25% of the requirement on physical, morphometric, and strength parameters of tibia bones. Biol. Trace Elem. Res. 2018, 184, 483–490. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A.; Kwiecień, M.; Grela, E.R.; Tomaszewska, E.; Klebaniuk, R. The chemical composition and sensory properties of raw, cooked and grilled thigh meat of broiler chickens fed with Fe-Gly chelate. J. Food Sci. Tech. 2016, 53, 3825–3833. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A.; Kwiecień, M.; Mieczan, T.; Kwiatkowska, K.; Jachimowicz, K. The effect of Cu, Zn and Fe chelates on the antioxidative status of thigh meat of broiler chickens. Animal 2021, 15, 100367. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A.; Jachimowicz, K.; Kwiecień, M.; Kislova, S.; Baranowska-Wójcik, E.; Zasadna, Z.; Yanovych, D.; Kowalczuk-Vasilev, E. The Impact of Zn, Cu and Fe Chelates on the Fatty-Acid Profile and Dietary Value of Broiler-Chicken Thigh Meat. Animals 2021, 11, 3115. [Google Scholar] [CrossRef]
- Aviagen. Ross 308 Parent Stock: Nutrition Specifications. Available online: www.aviagen.com (accessed on 20 October 2019).
- NRC. National Research Council. Nutrient Requirements of Poultry; National Academy Press: Washington, DC, USA, 1994. [Google Scholar]
- AOAC. Official Methods of Analysis, 17th ed.; AOAC International: Gaithersburg, MA, USA, 2000. [Google Scholar]
- Santé, V.; Fernandez, X. The measurement of pH in raw and frozen turkey Pectoralis superficial is muscle. Meat Sci. 2000, 55, 503–506. [Google Scholar] [CrossRef]
- De Sousa, A.B.B.; de Santos Júnior, O.; Visentainer, J.V.; de Almeida, N.M. Total lipid nutritional quality of the adipose tissue from the orbital cavity in Nile tilapia from continental aquaculture. Acta Sci. Anim. Sci. 2017, 39, 335–341. [Google Scholar] [CrossRef]
- Kwiecień, M.; Winiarska-Mieczan, A.; Milczarek, A.; Tomaszewska, E.; Matras, J. Effects of zinc glycine chelate on growth performance, carcass characteristics, bone quality, and mineral content in bone of broiler chicken. Livest. Sci. 2016, 191, 43–50. [Google Scholar] [CrossRef]
- Bu, W.; Liu, R.; Cheung-Lau, J.C.; Dmochowski, I.J.; Loll, P.J.; Eckenhoff, R.G. Ferritin couples iron and fatty acid metabolism. FASEB J. 2012, 26, 2394–2400. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, U.; Latham, P.S.; Oates, P.S. Interactions between hepatic iron and lipid metabolism with possible relevance to steatohepatitis. World J. Gastroenterol. 2012, 18, 4651–4658. [Google Scholar] [CrossRef]
- Żekanowska, E.; Boinska, J.; Giemza-Kucharska, P.; Kwapisz, J. Obesity and iron metabolism. J. Biotech. Comput. Biol. Bionanotech. 2011, 92, 147–152. [Google Scholar] [CrossRef]
- Feldman, A.; Aigner, E.; Weghuber, D.; Paulmichl, K. The Potential Role of Iron and Copper in Pediatric Obesity and Nonalcoholic Fatty Liver Disease. Biomed. Res. Int. 2015, 2015, 287401. [Google Scholar] [CrossRef]
- Zhang, L.; Dai, X.; Wang, L.; Cai, J.; Shen, J.; Shen, Y.; Li, X.; Zhao, Y. Iron overload accelerated lipid metabolism disorder and liver injury in rats with non-alcoholic fatty liver disease. Front Nutr. 2022, 9, 961892. [Google Scholar] [CrossRef]
- Hilton, C.; Sabaratnam, R.; Drakesmith, H.; Karpe, F. Iron, glucose and fat metabolism and obesity: An intertwined relationship. Int. J. Obes. 2023, 47, 554–563. [Google Scholar] [CrossRef]
- Ogłuszka, M.; Lipiński, P.; Starzyński, R.R. Interaction between iron and omega-3 fatty acids metabolisms: Where is the cross-link? Crit. Rev. Food Sci. Nutr. 2022, 62, 3002–3022. [Google Scholar] [CrossRef]
- Konstorum, A.; Lynch, M.L.; Torti, S.V.; Torti, F.M.; Laubenbacher, R.C. A Systems Biology Approach to Understanding the Pathophysiology of High-Grade Serous Ovarian Cancer: Focus on Iron and Fatty Acid Metabolism. Omics A J. Integr. Biol. 2018, 22, 502–513. [Google Scholar] [CrossRef]
- Valenzuela, R.; Rincon-Cervera, M.A.; Echeverria, F.; Barrera, C.; Espinosa, A.; Hernandez-Rodas, M.C.; Ortiz, M.; Valenzuela, A.; Videla, L.A. Iron-induced pro-oxidant and pro-lipogenic responses in relation to impaired synthesis and accretion of long-chain polyunsaturated fatty acids in rat hepatic and extrahepatic tissues. Nutrition 2018, 45, 49–58. [Google Scholar] [CrossRef]
- Mattera, R.; Stone, G.P.; Bahhur, N.; Kuryshev, Y.A. Increased release of arachidonic acid and eicosanoids in iron-overloaded cardiomyocytes. Circulation 2001, 103, 2395–2401. [Google Scholar] [CrossRef] [PubMed]
- Łopacka, J.; Lipińska, A. Oxidative changes during storage of beef under modified atmosphere and thermal treatment and its potential impact on human health. Probl. Hig. Epidemiol. 2015, 96, 719–726. [Google Scholar]
- Mapiye, C.; Chimonyo, M.; Dzama, K.; Hugo, A. Fatty acid composition of beef from Nguni steers supplemented with Acacia karroo leaf-meal. J. Food Compos. Anal. 2011, 24, 523–528. [Google Scholar] [CrossRef]
- Woloszyn, M.R.; Clyde, K.; Corno, D. The relative impact of looks, income, warmth, and intelligence on female online dating preferences. Soc. Sci. Humanit. Open 2020, 2, 100089. [Google Scholar] [CrossRef]
| Nutrient Value of 1 kg of Mixture | Starter (1–21 days) | Grower (22–35 days) | Finisher (36–42 days) |
|---|---|---|---|
| a Metabolizable energy (ME, MJ kg−1) | 12.7 | 13.1 | 13.2 |
| b Crude protein, g·kg–1 | 202 | 182 | 181 |
| b Crude fibre, g·kg–1 | 30.6 | 29.9 | 29.9 |
| b Crude fat, g·kg–1 | 46.6 | 60.8 | 64.3 |
| a Lysine, g·kg–1 | 12.9 | 11.3 | 10.9 |
| a Methionine + cysteine, g·kg–1 | 9.3 | 8.3 | 8.1 |
| a Total Ca, g·kg–1 | 8.8 | 7.8 | 7.5 |
| a Total P, g·kg–1 | 6.6 | 6.5 | 6.3 |
| a Available P, g·kg–1 | 4.2 | 4.1 | 3.9 |
| a Total Ca/available P | 21.2 | 19.0 | 19.2 |
| b Fe, mg | |||
| 40 mg FeSO4 | 113.59 | 109.80 | 106.69 |
| 40 mg Fe-Gly | 110.28 | 107.32 | 104.61 |
| 20 mg Fe-Gly | 90.25 | 89.84 | 85.37 |
| 10 mg Fe-Gly | 83.38 | 79.81 | 76.92 |
| Starter 1–21 days | Grower 22–35 days | Finisher 36–42 days | |
|---|---|---|---|
| Myristic (14:0) | 0.03 | 0.07 | 0.07 |
| Palmitic (16:0) | 1.42 | 1.17 | 1.15 |
| Stearic (18:0) | 0.29 | 0.32 | 0.33 |
| Oleic (18:1n − 9) | 2.25 | 2.24 | 2.19 |
| Linoleic (18:2n − 6) | 4.72 | 4.95 | 4.96 |
| Linolenic (18:3n − 3) | 1.18 | 0.86 | 0.89 |
| Control n = 10 | Fe-Gly40 n = 10 | Fe-Gly20 n = 10 | Fe-Gly10 n = 10 | SEM | p Value | |
|---|---|---|---|---|---|---|
| pH15 | 6.01 ± 0.03 | 6.03 ± 0.02 | 6.03 ± 0.04 | 6.02 ± 0.01 | 0.03 | 0.51 |
| pH45 | 5.72 ± 0.01 | 5.75 ± 0.02 | 5.74 ± 0.02 | 5.73 ± 0.04 | 0.20 | 0.45 |
| Moisture, % | 74.5 ± 0.73 | 74.4 ± 0.65 | 74.3 ± 0.58 | 74.5 ± 0.97 | 0.07 | 0.77 |
| Crude ash, % | 1.25 ± 0.02 a | 1.28 ± 0.02 a | 1.34 ± 0.01 b | 1.39 ± 0.04 c | 0.01 | 0.002 |
| Crude protein, % | 23.2 ± 0.82 | 23.1 ± 0.54 | 23.3 ± 0.92 | 23.1 ± 0.74 | 0.08 | 0.77 |
| Crude fat, % | 1.11 ± 0.03 ab | 1.01 ± 0.02 b | 1.22 ± 0.05 a | 1.22 ± 0.02 a | 0.04 | 0.02 |
| Cholesterol, mg/100 g | 54.2 ± 3.21 | 52.9 ± 4.41 | 53.7 ± 4.12 | 53.7 ± 3.53 | 2.29 | 0.09 |
| Control n = 10 | Fe-Gly40 n = 10 | Fe-Gly20 n = 10 | Fe-Gly10 n = 10 | SEM | p Value | |
|---|---|---|---|---|---|---|
| SFA | ||||||
| Lauric acid (C12:0) | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.01 | 0.64 |
| Myristic acid (C14:0) | 0.49 ± 0.13 b | 0.42 ± 0.09 ab | 0.38 ± 0.11 a | 0.37 ± 0.07 a | 0.02 | 0.02 |
| Pantadecanoic acid (15:0) | 0.10 ± 0.01 | 0.10 ± 0.05 | 0.09 ± 0.06 | 0.09 ± 0.10 | 0.01 | 0.20 |
| Palmitic acid (16:0) | 23.1 ± 0.54 | 21.9 ± 1.01 | 22.2 ± 0.91 | 22.0 ± 1.03 | 0.24 | 0.26 |
| Heptadecanoic acid (17:0) | 0.14 ± 0.11 | 0.12 ± 0.05 | 0.13 ± 0.11 | 0.14 ± 0.07 | 0.01 | 0.31 |
| Stearic acid (18:0) | 7.45 ± 0.21 | 7.50 ± 0.11 | 7.97 ± 0.52 | 8.60 ± 0.34 | 0.21 | 0.19 |
| Arachidic acid (20:0) | 0.10 ± 0.01 | 0.09 ± 0.01 | 0.08 ± 0.01 | 0.10 ± 0.01 | 0.01 | 0.51 |
| MUFA | ||||||
| Palmitoleic acid (16:1) | 4.01 ± 0.21 | 3.94 ± 0.11 | 4.11 ± 0.27 | 3.15 ± 0.31 | 0.18 | 0.22 |
| Margaroleic acid (17:1) | 0.27 ± 0.02 | 0.18 ± 0.01 | 0.31 ± 0.01 | 0.26 ± 0.02 | 0.03 | 0.57 |
| Oleic acid (18:1n − 9) | 31.7 ± 1.11 | 32.0 ± 2.04 | 34.5 ± 2.30 | 34.2 ± 1.21 | 0.52 | 0.11 |
| Vaccenic acid (18:1n − 11) | 2.25 ± 0.14 | 2.37 ± 0.09 | 2.61 ± 0.11 | 2.38 ± 0.21 | 0.06 | 0.13 |
| Eicosenoic acid (20:1n − 11) | 0.32 ± 0.01 ab | 0.28 ± 0.02 a | 0.41 ± 0.03 b | 0.39 ± 0.03 b | 0.02 | 0.01 |
| PUFA | ||||||
| Linoleic acid (18:2n − 6) | 25.0 ± 1.23 | 26.2 ± 2.01 | 22.9 ± 1.17 | 24.2 ± 0.92 | 0.51 | 0.12 |
| Eicosadienoic acid (20:2n − 6) | 0.29 ± 0.02 | 0.32 ± 0.02 | 0.37 ± 0.04 | 0.29 ± 0.02 | 0.02 | 0.27 |
| Arachidonic acid (20:4n − 6) | 1.88 ± 0.11 | 1.78 ± 0.12 | 1.63 ± 0.1 | 1.30 ± 0.11 | 0.19 | 0.78 |
| α-linoleic acid (18:3n − 3) | 2.20 ± 0.21 b | 2.17 ± 0.14 b | 1.83 ± 0.21 a | 1.88 ± 0.15 a | 0.06 | 0.01 |
| Eicosatrenic acid (20:3n − 3) | 0.26 ± 0.02 | 0.27 ± 0.01 | 0.37 ± 0.02 | 0.25 ± 0.02 | 0.02 | 0.06 |
| Σ SFA | 31.4 ± 2.24 | 30.1 ± 1.09 | 30.8 ± 2.77 | 31.3 ± 1.98 | 0.22 | 0.14 |
| Σ MUFA | 38.7 ± 2.01 | 38.9 ± 3.12 | 42.0 ± 2.09 | 40.4 ± 3.36 | 0.58 | 0.15 |
| Σ PUFA | 29.6 ± 1.09 | 30.7 ± 1.96 | 27.1 ± 2.01 | 27.9 ± 1.79 | 0.62 | 0.14 |
| Σ UFA | 68.3 ± 4.55 | 69.7 ± 5.01 | 69.1 ± 5.03 | 68.4 ± 3.46 | 0.23 | 0.09 |
| Σ PUFA n − 6 | 2.18 ± 0.11 | 2.10 ± 0.08 | 1.99 ± 0.11 | 1.59 ± 0.06 | 0.20 | 0.77 |
| Σ PUFA n − 3 | 2.46 ± 0.12 | 2.44 ± 0.09 | 2.20 ± 0.11 | 2.13 ± 0.05 | 0.06 | 0.07 |
| Σ PUFA/Σ SFA | 0.95 ± 0.03 | 1.02 ± 0.02 | 0.88 ± 0.01 | 089 ± 0.03 | 0.02 | 0.11 |
| Σ SFA /Σ UFA | 0.46 ± 0.01 | 0.43 ± 0.01 | 0.45 ± 0.02 | 0.46 ± 0.01 | 0.01 | 0.11 |
| Control n = 10 | Fe-Gly40 n = 10 | Fe-Gly20 n = 10 | Fe-Gly10 n = 10 | SEM | p Value | |
|---|---|---|---|---|---|---|
| S/P | 0.455 | 0.428 | 0.442 | 0.453 | 0.01 | 0.13 |
| n − 6/n − 3 | 0.869 | 0.888 | 0.908 | 0.742 | 0.08 | 0.91 |
| AI | 0.58 b | 0.54 ab | 0.51 a | 0.53 a | 0.01 | 0.01 |
| TI | 1.06 | 1.02 | 1.03 | 1.08 | 0.01 | 0.28 |
| H/H | 2.58 | 2.79 | 2.70 | 2.76 | 0.04 | 0.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winiarska-Mieczan, A.; Kwiecień, M.; Jachimowicz-Rogowska, K.; Kislova, S.; Zasadna, Z.; Yanovych, D. Assessment of the Chemical Composition and Nutritional Quality of Breast Muscle from Broiler Chickens Receiving Various Levels of Fe Glycine Chelate. Agriculture 2023, 13, 1455. https://doi.org/10.3390/agriculture13071455
Winiarska-Mieczan A, Kwiecień M, Jachimowicz-Rogowska K, Kislova S, Zasadna Z, Yanovych D. Assessment of the Chemical Composition and Nutritional Quality of Breast Muscle from Broiler Chickens Receiving Various Levels of Fe Glycine Chelate. Agriculture. 2023; 13(7):1455. https://doi.org/10.3390/agriculture13071455
Chicago/Turabian StyleWiniarska-Mieczan, Anna, Małgorzata Kwiecień, Karolina Jachimowicz-Rogowska, Svitlana Kislova, Zvenyslava Zasadna, and Dmytro Yanovych. 2023. "Assessment of the Chemical Composition and Nutritional Quality of Breast Muscle from Broiler Chickens Receiving Various Levels of Fe Glycine Chelate" Agriculture 13, no. 7: 1455. https://doi.org/10.3390/agriculture13071455
APA StyleWiniarska-Mieczan, A., Kwiecień, M., Jachimowicz-Rogowska, K., Kislova, S., Zasadna, Z., & Yanovych, D. (2023). Assessment of the Chemical Composition and Nutritional Quality of Breast Muscle from Broiler Chickens Receiving Various Levels of Fe Glycine Chelate. Agriculture, 13(7), 1455. https://doi.org/10.3390/agriculture13071455






