Developing Fermentation Liquid of Bacillus amyloliquefaciens PMB04 to Control Bacterial Leaf Spot of Sweet Pepper
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth Conditions for Plants and Bacteria
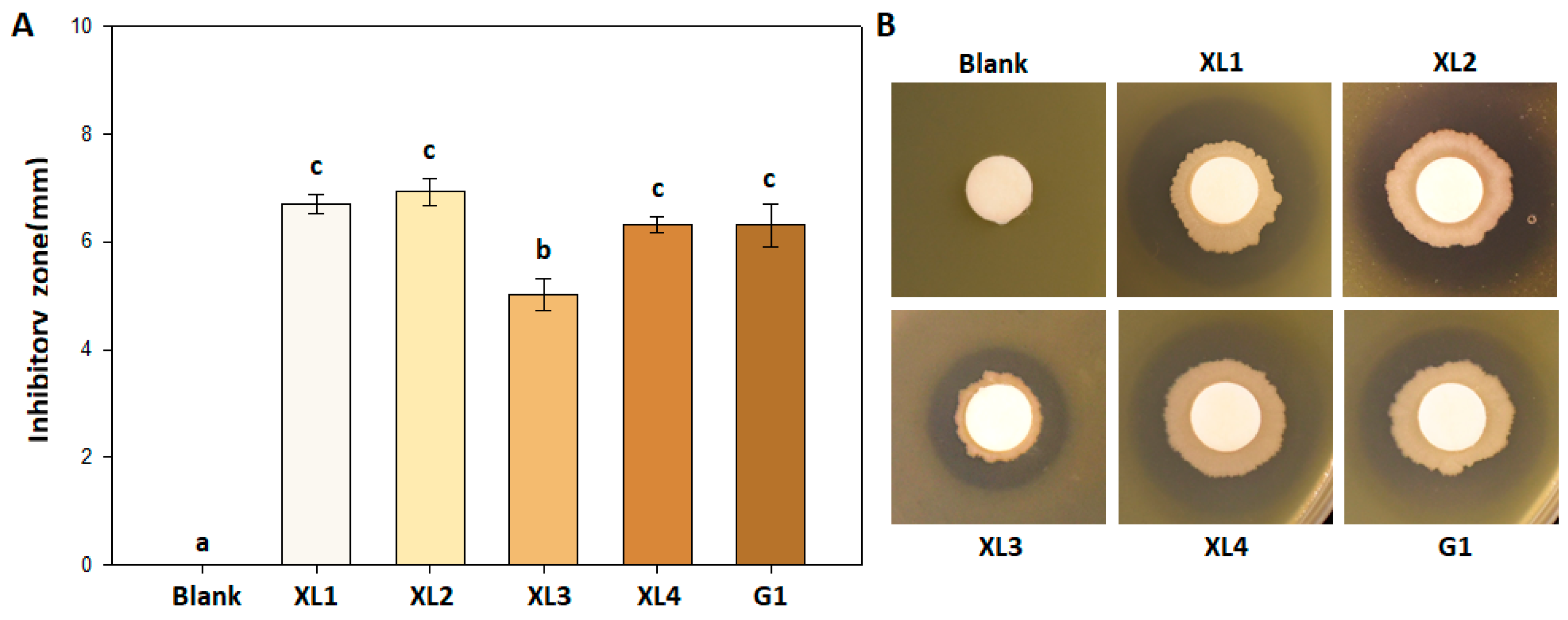
2.2. Inhibitory Assay of B. amyloliquefaciens PMB04 against X. perforans Strains
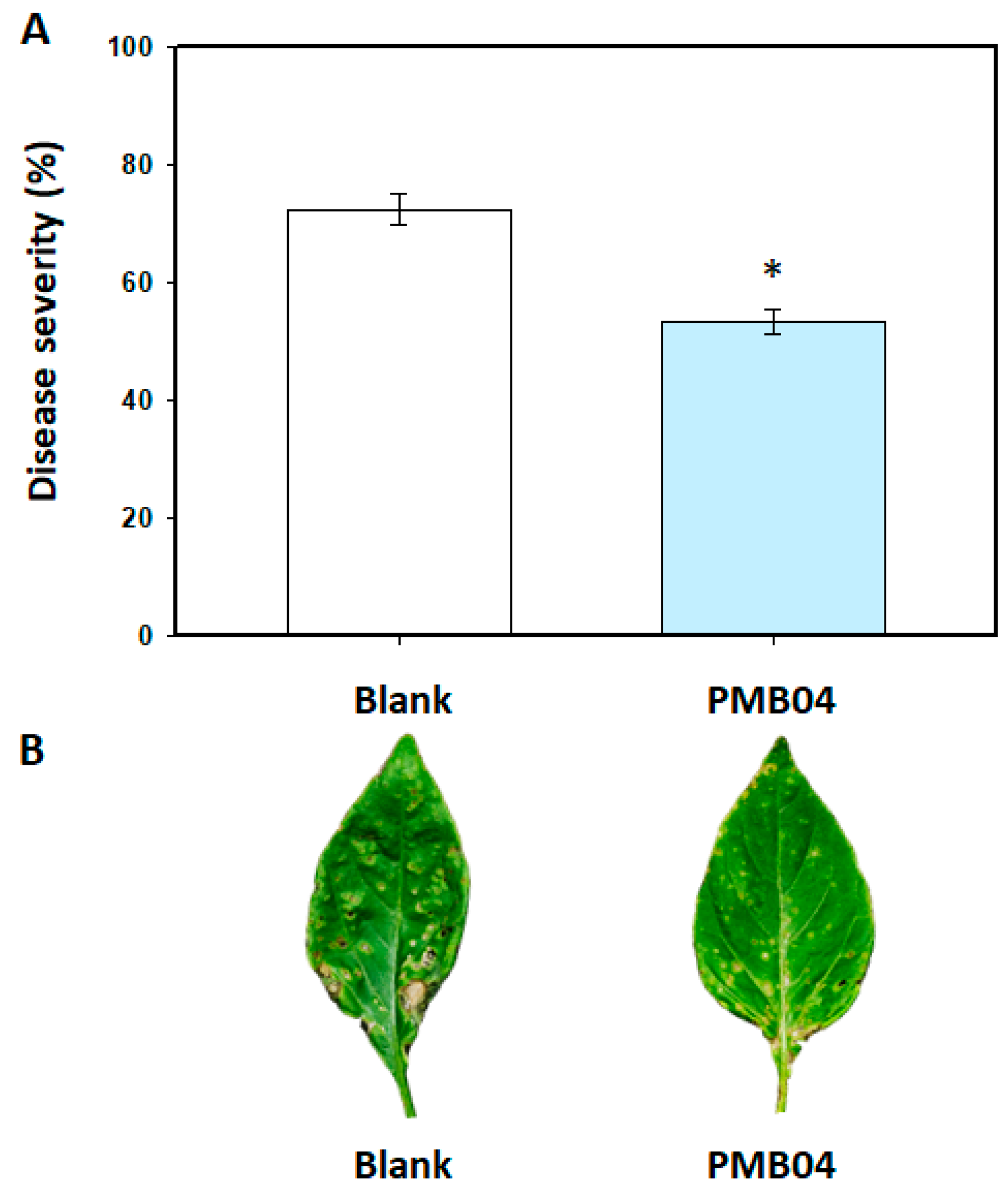
2.3. Effect of B. amyloliquefaciens PMB04 on the Control of Bacterial Leaf Spot Disease
2.4. Effects of Different Fermentation Formulas on Bacterial Population and Sporulation of 30 L Harvested Fermentation Liquid
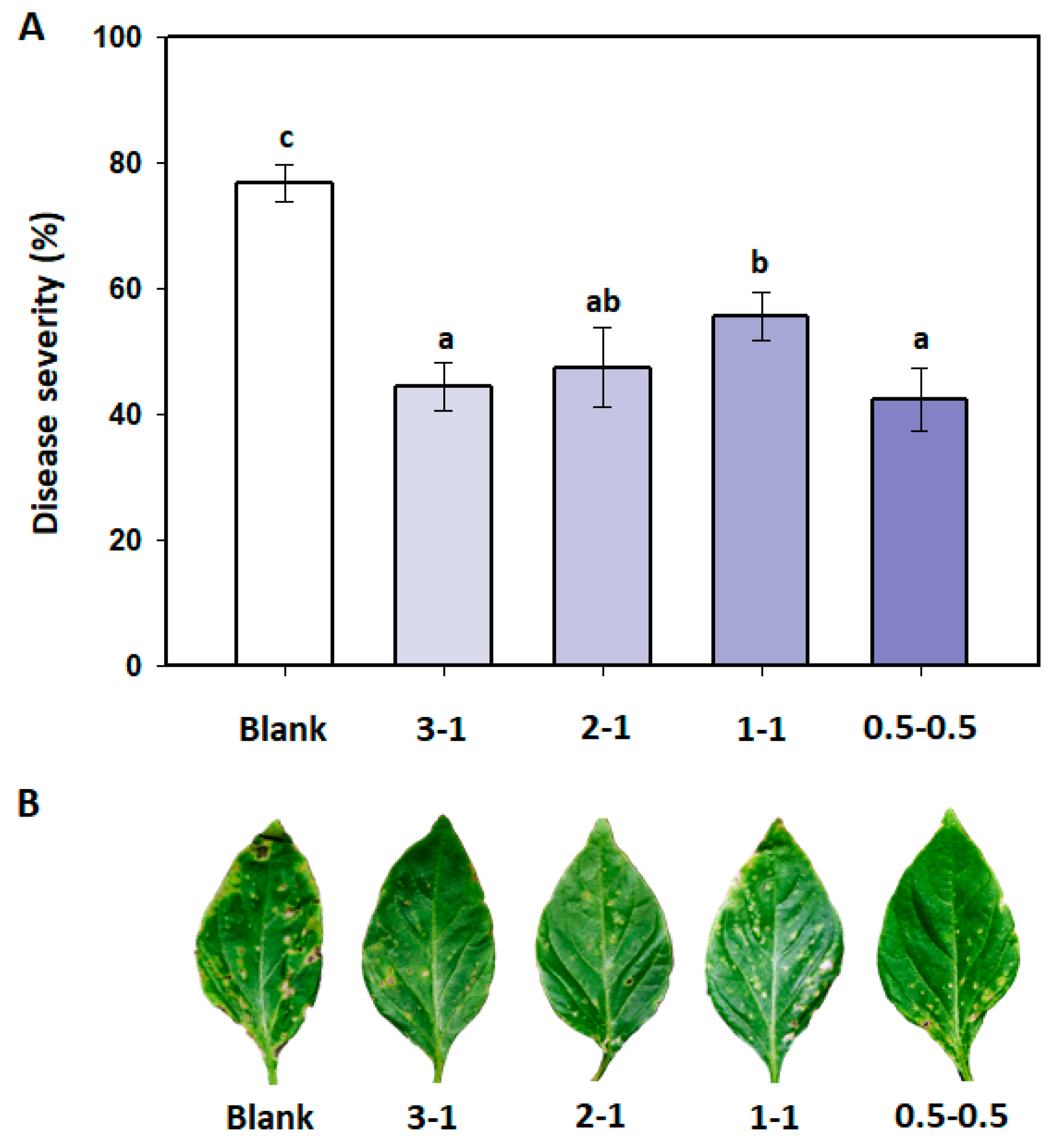
2.5. Control Effect of B. amyloliquefaciens PMB04 Fermentation Liquids on Bacterial Spot of Sweet Pepper
2.6. Sensitivity of B. amyloliquefaciens PMB04 and X. perforans XL1 to Copper-Containing Fungicides
2.7. Effect of B. amyloliquefaciens PMB04 Fermentation Liquid Filtrate against X. perforans
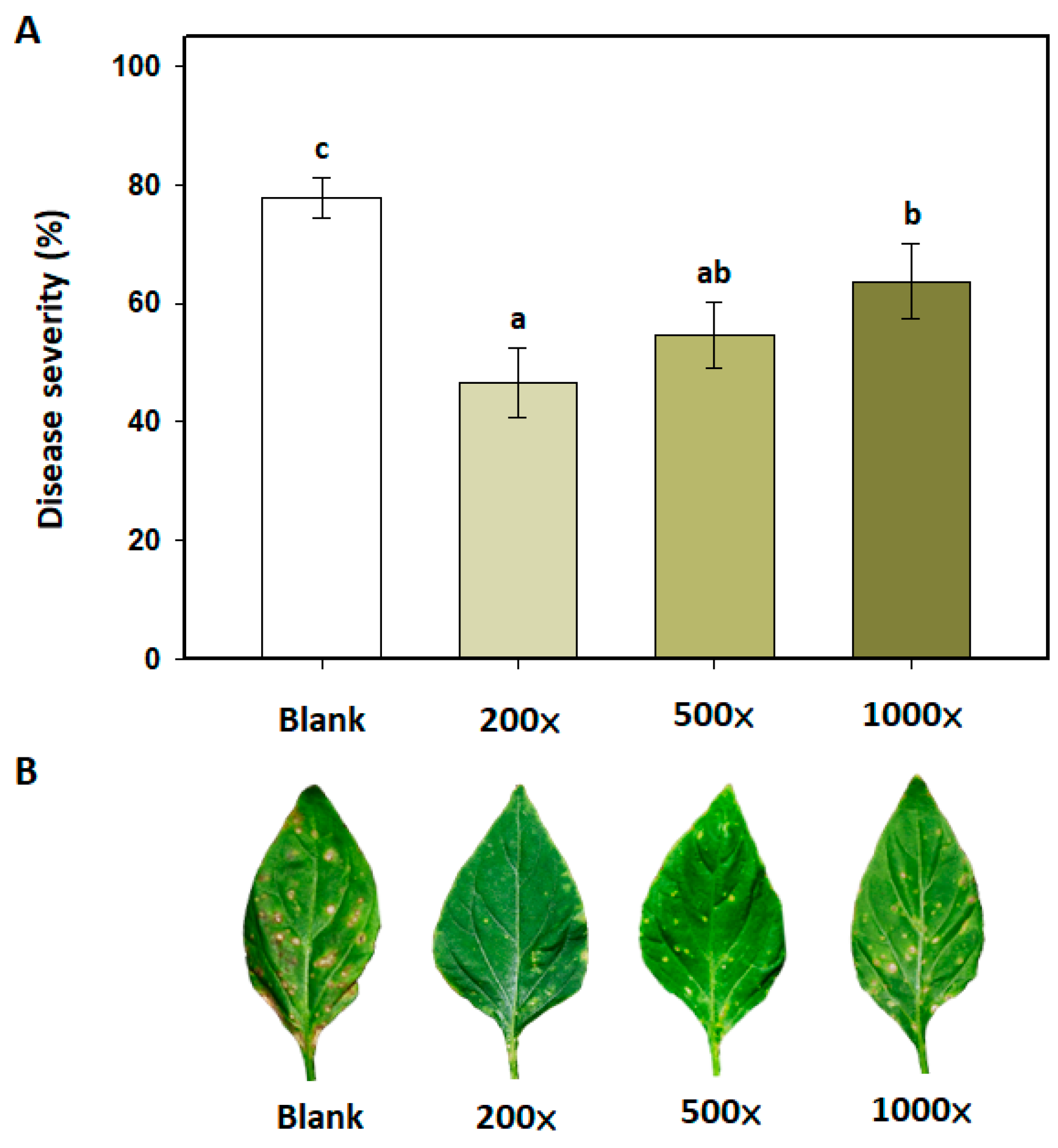
2.8. Effect of Copper Hydroxide on B. amyloliquefaciens PMB04 Fermentation Liquid in the Control of Bacterial Leaf Spot of Sweet Pepper
2.9. Data Analysis
3. Results
3.1. Inhibitory Effect of Bacillus amyloliquefaciens PMB04 against Xanthomonas perforans Strains
3.2. Control Efficacy of Bacillus amyloliquefaciens PMB04 Bacterial Suspension on Bacterial Leaf Spot Disease in Sweet Pepper
3.3. Effects of Formulated Differences on the Population and Sporulation of Bacillus amyloliquefaciens PMB04 Fermentation Liquids from a 30 L Fermenter
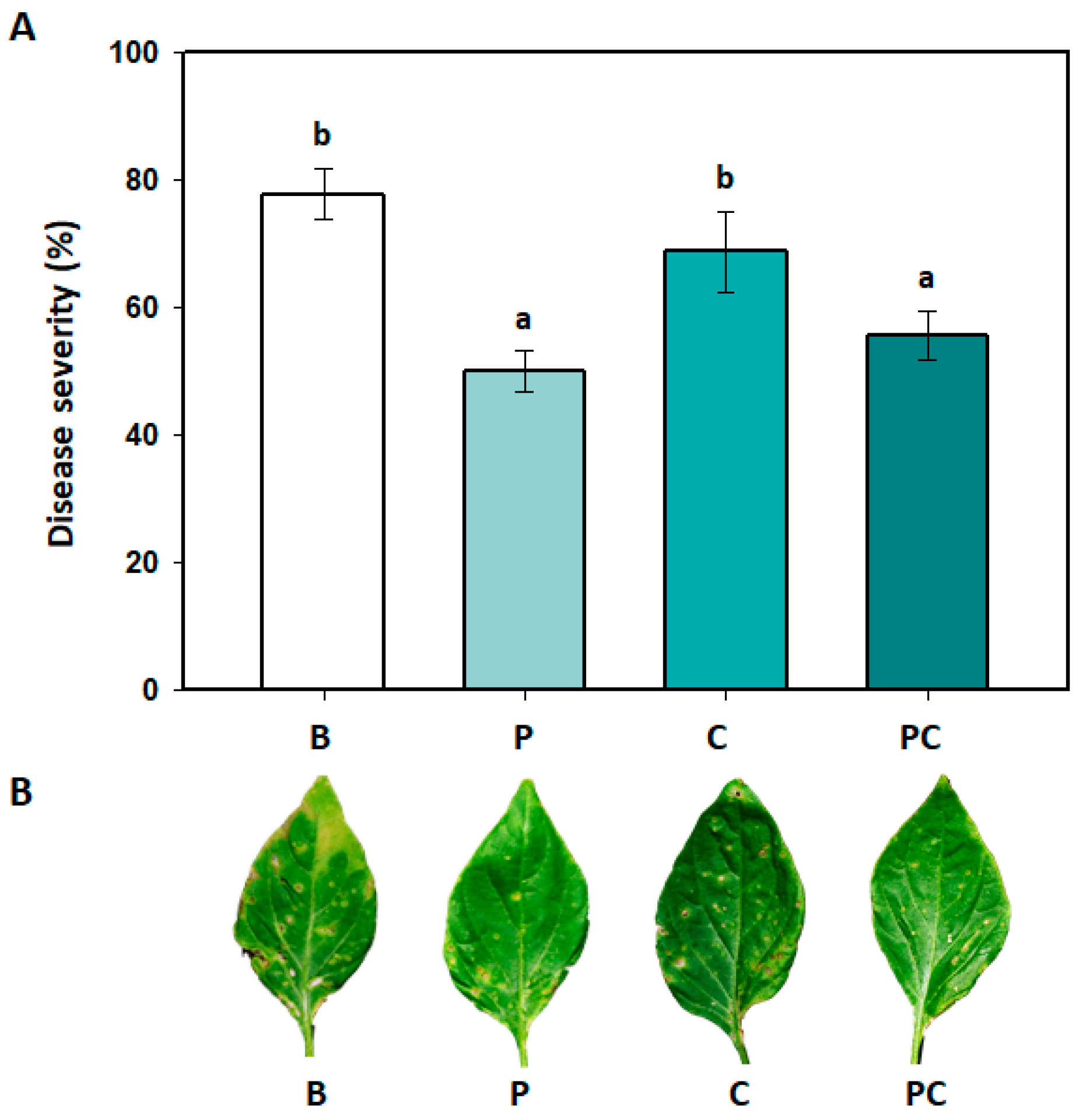
3.4. Effects of B. amyloliquefaciens PMB04 Fermentation Liquids on the Control of Bacterial Spot
3.5. Effect of B. amyloliquefaciens PMB04 Fermentation Filtrate on the Survival of X. perforans Cells
3.6. Inhibitory Effects of Copper-Containing Fungicides against B. amyloliquefaciens PMB04 and X. perforans XL1
3.7. Effects of Copper Hydroxide on PMB4FL in the Control of Bacterial Leaf Spot in Sweet Pepper
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Potnis, N.; Timilsina, S.; Strayer, A.; Shantharaj, D.; Barak, J.D.; Paret, M.L.; Vallad, G.E.; Jones, J.B. Bacterial spot of tomato and pepper: Diverse Xanthomonas species with a wide variety of virulence factors posing a worldwide challenge. Mol. Plant Pathol. 2015, 16, 907–920. [Google Scholar] [CrossRef] [PubMed]
- Roach, R.; Mann, R.; Gambley, C.G.; Shivas, R.G.; Rodoni, B. Identification of Xanthomonas species associated with bacterial leaf spot of tomato, capsicum and chilli crops in eastern Australia. Eur. J. Plant Pathol. 2017, 150, 595–608. [Google Scholar] [CrossRef]
- Sanogo, S.; Clary, M. Bacterial Leaf Spot of Chile Pepper: A Short Guide for Growers; New Mexico State University, College of Agriculture and Home Economics: Las Cruces, NM, USA, 2008. [Google Scholar]
- Hsiao, Y.-M.; Liu, Y.-F.; Lee, P.-Y.; Hsu, P.-C.; Tseng, S.-Y.; Pan, Y.-C. Functional characterization of copA gene encoding multicopper oxidase in Xanthomonas campestris pv. campestris. J. Agric. Food Chem. 2011, 59, 9290–9302. [Google Scholar] [CrossRef]
- Voloudakis, A.E.; Reignier, T.M.; Cooksey, D.A. Regulation of Resistance to Copper in Xanthomonas axonopodis pv. vesicatoria. Appl. Environ. Microbiol. 2005, 71, 782–789. [Google Scholar] [CrossRef]
- Almoneafy, A.A.; Kakar, K.U.; Nawaz, Z.; Li, B.; Saand, M.A.; Chun-lan, Y.; Xie, G.-L. Tomato plant growth promotion and antibacterial related-mechanisms of four rhizobacterial Bacillus strains against Ralstonia solanacearum. Symbiosis 2014, 63, 59–70. [Google Scholar] [CrossRef]
- Cao, Y.; Pi, H.; Chandrangsu, P.; Li, Y.; Wang, Y.; Zhou, H.; Xiong, H.; Helmann, J.D.; Cai, Y. Antagonism of two plant-growth promoting Bacillus velezensis isolates against Ralstonia solanacearum and Fusarium oxysporum. Sci. Rep. 2018, 8, 4360. [Google Scholar] [CrossRef]
- Olishevska, S.; Nickzad, A.; Déziel, E. Bacillus and Paenibacillus secreted polyketides and peptides involved in controlling human and plant pathogens. Appl. Microbiol. Biotechnol. 2019, 103, 1189–1215. [Google Scholar] [CrossRef]
- Chang, J.-J.; Wu, P.-Y.; Lin, Y.-N.; Deng, W.-L.; Lin, Y.-H. Intensification of PAMP-triggered immunity in watermelon by Bacillus spp. strains as a strategy for controlling bacterial fruit blotch disease. J. Plant Med. 2019, 61, 39–48. [Google Scholar]
- Li’aini, A.S.; Lin, Y.-H.; Huang, T.-C.; Sulistyowati, L. Application of Bacillus amyloliquefaciens to control black rot disease on cabbage caused by Xanthomonas campestris pv. campestris. J. Plant Med. 2017, 59, 39–44. [Google Scholar] [CrossRef]
- Wu, Y.-M.; Chen, X.; Wang, F.; Hsiao, C.-Y.; Yang, C.-Y.; Lin, S.-T.; Wu, L.-H.; Chen, Y.-K.; Liang, Y.-S.; Lin, Y.-H. Bacillus amyloliquefaciens strains control strawberry anthracnose through antagonistic activity and plant immune response intensification. Biol. Control 2021, 157, 104592. [Google Scholar] [CrossRef]
- Ahsan, T.; Zang, C.; Yu, S.; Pei, X.; Xie, J.; Lin, Y.; Liu, X.; Liang, C. Screening, and Optimization of Fermentation Medium to Produce Secondary Metabolites from Bacillus amyloliquefaciens, for the Biocontrol of Early Leaf Spot Disease, and Growth Promoting Effects on Peanut (Arachis hypogaea L.). J. Fungi 2022, 8, 1223. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Cao, X.; Cai, Z.; Zhang, L.; Li, D.; Zhang, H.; Li, S.; Sun, X. Improving suppressive activity of compost on phytopathogenic microbes by inoculation of antagonistic microorganisms for secondary fermentation. Bioresour. Technol. 2023, 367, 128288. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.-W.; Liang, Y.-S.; Hsiao, C.-Y.; Wang, F.; Huang, T.-P.; Lin, Y.-H. Application of fermentation broth of Bacillus amyloliquefaciens PMB05 to control bacterial canker disease on lemon. J. Plant Med. 2021, 63, 17–26. [Google Scholar]
- Rathsack, K.; Böllmann, J.; Martienssen, M. Comparative Study of Different Methods for Analyzing Denitrifying Bacteria in Fresh Water Ecosystems. J. Water Resour. Prot. 2014, 6, 609–617. [Google Scholar] [CrossRef]
- Hernández-Huerta, J.; Tamez-Guerra, P.; Gomez-Flores, R.; Delgado-Gardea, M.C.E.; García-Madrid, M.S.; Robles-Hernández, L.; Infante-Ramirez, R. Prevalence of Xanthomonas euvesicatoria (formally X. perforans) associated with bacterial spot severity in Capsicum annuum crops in South Central Chihuahua, Mexico. Peer J. 2021, 15, e10913. [Google Scholar]
- Tsai, Y.-L.; Chen, M.-J.; Hsu, S.-T.; Tzeng, D.D.S.; Tzeng, K.-C. Control Potential of Foliar Pseudomonas putida YLFP14 Against Bacterial Spot of Sweet Pepper. Plant Pathol. Bull. 2004, 13, 191–200. [Google Scholar]
- Chuang, C.-Y.; Lin, S.-T.; Li, A.-T.; Li, S.-H.; Hsiao, C.-Y.; Lin, Y.-H. Bacillus amyloliquefaciens PMB05 Increases Resistance to Bacterial Wilt by Activating Mitogen-Activated Protein Kinase and Reactive Oxygen Species Pathway Crosstalk in Arabidopsis thaliana. Phytopathology 2022, 112, 2495–2502. [Google Scholar] [CrossRef]
- Liang, Y.-S.; Fu, J.-Y.; Chao, S.-H.; Tzean, Y.; Hsiao, C.-Y.; Yang, Y.-Y.; Chen, Y.-K.; Lin, Y.-H. Postharvest Application of Bacillus amyloliquefaciens PMB04 Fermentation Broth Reduces Anthracnose Occurrence in Mango Fruit. Agriculture 2022, 12, 1646. [Google Scholar] [CrossRef]
- Gould, G.; Wrighton, C. Limitations of the initiation of germination of bacterial spores as a spore control procedure. J. Appl. Bacteriol. 1968, 31, 357–366. [Google Scholar] [CrossRef]
- Nicholson, W.L.; Munakata, N.; Horneck, G.; Melosh, H.J.; Setlow, P. Resistance of Bacillus Endospores to Extreme Terrestrial and Extraterrestrial Environments. Microbiol. Mol. Biol. Rev. 2000, 64, 548–572. [Google Scholar] [CrossRef]
- Wu, L.; Wu, H.; Chen, L.; Yu, X.; Borriss, R.; Gao, X. Difficidin and bacilysin from Bacillus amyloliquefaciens FZB42 have antibacterial activity against Xanthomonas oryzae rice pathogens. Sci. Rep. 2015, 5, 12975. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Huang, Y.; Li, Y.; Dong, J.; Liu, X.; Li, C. Biocontrol of Rhizoctonia solani via Induction of the Defense Mechanism and Antimicrobial Compounds Produced by Bacillus subtilis SL-44 on Pepper (Capsicum annuum L.). Front. Microbiol. 2019, 10, 2676. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.-S.; Ahn, Y.-R.; Song, G.C.; Ghim, S.-Y.; Lee, S.; Lee, G.; Ryu, C.-M. Impact of a Bacterial Volatile 2,3-Butanediol on Bacillus subtilis Rhizosphere Robustness. Front. Microbiol. 2016, 7, 993. [Google Scholar] [CrossRef]
- Chen, D.; Liu, X.; Li, C.; Tian, W.; Shen, Q.; Shen, B. Isolation of Bacillus amyloliquefaciens S20 and its application in control of eggplant bacterial wilt. J. Environ. Manag. 2014, 137, 120–127. [Google Scholar] [CrossRef]
- Es-Soufi, R.; Tahiri, H.; Azaroual, L.; El Oualkadi, A.; Martin, P.; Badoc, A.; Lamarti, A. Biocontrol potential of Bacillus amyloliquefaciens Bc2 and Trichoderma harzianum TR against strawberry anthracnose under laboratory and field conditions. Agric. Sci. 2020, 11, 260–277. [Google Scholar]
- Fan, B.; Chen, X.H.; Budiharjo, A.; Bleiss, W.; Vater, J.; Borriss, R. Efficient colonization of plant roots by the plant growth promoting bacterium Bacillus amyloliquefaciens FZB42, engineered to express green fluorescent protein. J. Biotechnol. 2011, 151, 303–311. [Google Scholar] [CrossRef]
- Gao, T.; Wang, X.; Qin, Y.; Ren, Z.; Zhao, X. Watermelon Root Exudates Enhance Root Colonization of Bacillus amyloliquefaciens TR2. Curr. Microbiol. 2023, 80, 110. [Google Scholar] [CrossRef]
- Lu, X.; Liu, S.-F.; Yue, L.; Zhao, X.; Zhang, Y.-B.; Xie, Z.-K.; Wang, R.-Y. Epsc Involved in the Encoding of Exopolysaccharides Produced by Bacillus amyloliquefaciens FZB42 Act to Boost the Drought Tolerance of Arabidopsis thaliana. Int. J. Mol. Sci. 2018, 19, 3795. [Google Scholar] [CrossRef]
- Constesini, F.J.; de Melo, R.R.; Sato, H.H. An overview of Bacillus proteases: From production to application. Crit. Rev. Biotechnol. 2018, 38, 321–334. [Google Scholar] [CrossRef]
- Tian, Y.; Fan, Y.; Liu, J.; Zhao, X.; Chen, W. Effect of nitrogen, carbon sources and agitation speed on acetoin production of Bacillus subtilis SF4-3. Electron. J. Biotechnol. 2016, 19, 41–49. [Google Scholar] [CrossRef]
- Zhu, L.; Yang, X.; Xue, C.; Chen, Y.; Qu, L.; Lu, W. Enhanced rhamnolipids production by Pseudomonas aeruginosa based on a pH stage-controlled fed-batch fermentation process. Bioresour. Technol. 2012, 117, 208–213. [Google Scholar] [CrossRef]
- Chou, H.-P.; Huang, Y.-C.; Lin, Y.-H.; Deng, W.-L. Selection, Formulation, and Field Evaluation of Bacillus amyloliquefaciens PMB01 for Its Application to Manage Tomato Bacterial Wilt Disease. Agriculture 2022, 12, 1714. [Google Scholar] [CrossRef]
- Medeot, D.B.; Fernandez, M.; Morales, G.M.; Jofré, E. Fengycins From Bacillus amyloliquefaciens MEP218 Exhibit Antibacterial Activity by Producing Alterations on the Cell Surface of the Pathogens Xanthomonas axonopodis pv. vesicatoria and Pseudomonas aeruginosa PA01. Front. Microbiol. 2020, 10, 03107. [Google Scholar] [CrossRef]
- Bibi, S.; Weis, K.; Kaur, A.; Bhandari, R.; Goss, E.M.; Jones, J.B.; Potnis, N. A Brief Evaluation of Copper Resistance Mobile Genetic Island in the Bacterial Leaf Spot Pathogen, Xanthomonas euvesicatoria pv. perforans. Phytopathology 2023. [Google Scholar] [CrossRef] [PubMed]
- FRAC. FRAC Code List ©*2022: Fungal Control Agents Sorted by Cross Resistance Pattern and Mode of Action (Including Coding for FRAC Groups on Product Labels). 2022, p. 15. Available online: https://www.frac.info/docs/default-source/publications/frac-code-list/frac-code-list-2022--final.pdf?sfvrsn=b6024e9a_2 (accessed on 29 June 2023).
- Obradovic, A.; Jones, J.B.; Momol, M.T.; Olson, S.M.; Jackson, L.E.; Balogh, B.; Guven, K.; Iriarte, F.B. Integration of Biological Control Agents and Systemic Acquired Resistance Inducers Against Bacterial Spot on Tomato. Plant Dis. 2005, 89, 712–716. [Google Scholar] [CrossRef]
- Korsten, L.; De Villiers, E.E.; Wehner, F.C.; Kotzé, J.M. Field Sprays of Bacillus subtilis and Fungicides for Control of Preharvest Fruit Diseases of Avocado in South Africa. Plant Dis. 1997, 81, 427–452. [Google Scholar] [CrossRef]

| Formulation | Cells (Log CFU/mL) | Endospores (Log CFU/mL) | Sporulation (%) |
|---|---|---|---|
| 3-1 1 | 9.00 ± 0.41 a | 9.29 ± 0.41 a | 100.00 |
| 2-1 | 8.91 ± 0.21 ab | 9.09 ± 0.33 ab | 100.00 |
| 1-1 | 8.54 ± 0.06 b | 8.47 ± 0.08 b | 99.14 |
| 0.5-0.5 | 8.68 ± 0.01 b | 8.75 ± 0.08 b | 100.00 |
| Strain | Fungicide | OD600 | |
|---|---|---|---|
| 12 h | 24 h | ||
| B. amyloliquefaciens PMB04 | |||
| Blank | 0.180 a | 0.367 a | |
| Tribasic copper sulfate | 0.161 a | 0.395 ab | |
| Copper hydroxide | 0.175 a | 0.420 b | |
| X. perforans XL1 | |||
| Blank | 0.123 a | 0.257 a | |
| Tribasic copper sulfate | 0.043 b | 0.162 c | |
| Copper hydroxide | 0.041 b | 0.193 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Chao, S.-H.; Tsai, C.-H.; Blanco, S.D.; Yang, Y.-Y.; Lin, Y.-H. Developing Fermentation Liquid of Bacillus amyloliquefaciens PMB04 to Control Bacterial Leaf Spot of Sweet Pepper. Agriculture 2023, 13, 1456. https://doi.org/10.3390/agriculture13071456
Wang F, Chao S-H, Tsai C-H, Blanco SD, Yang Y-Y, Lin Y-H. Developing Fermentation Liquid of Bacillus amyloliquefaciens PMB04 to Control Bacterial Leaf Spot of Sweet Pepper. Agriculture. 2023; 13(7):1456. https://doi.org/10.3390/agriculture13071456
Chicago/Turabian StyleWang, Fei, Szu-Han Chao, Chen-Hsuan Tsai, Sabrina Diana Blanco, Yung-Yu Yang, and Yi-Hsien Lin. 2023. "Developing Fermentation Liquid of Bacillus amyloliquefaciens PMB04 to Control Bacterial Leaf Spot of Sweet Pepper" Agriculture 13, no. 7: 1456. https://doi.org/10.3390/agriculture13071456
APA StyleWang, F., Chao, S.-H., Tsai, C.-H., Blanco, S. D., Yang, Y.-Y., & Lin, Y.-H. (2023). Developing Fermentation Liquid of Bacillus amyloliquefaciens PMB04 to Control Bacterial Leaf Spot of Sweet Pepper. Agriculture, 13(7), 1456. https://doi.org/10.3390/agriculture13071456







