Optimal Preharvest Melatonin Applications to Enhance Endogenous Melatonin Content, Harvest and Postharvest Quality of Japanese Plum
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Physicochemical Parameters
2.3. Determination of Respiration Rate and Ethylene Production Rate
2.4. Bioactive Parameters
2.5. Quantification of Melatonin
2.6. Statistical Analysis
3. Results
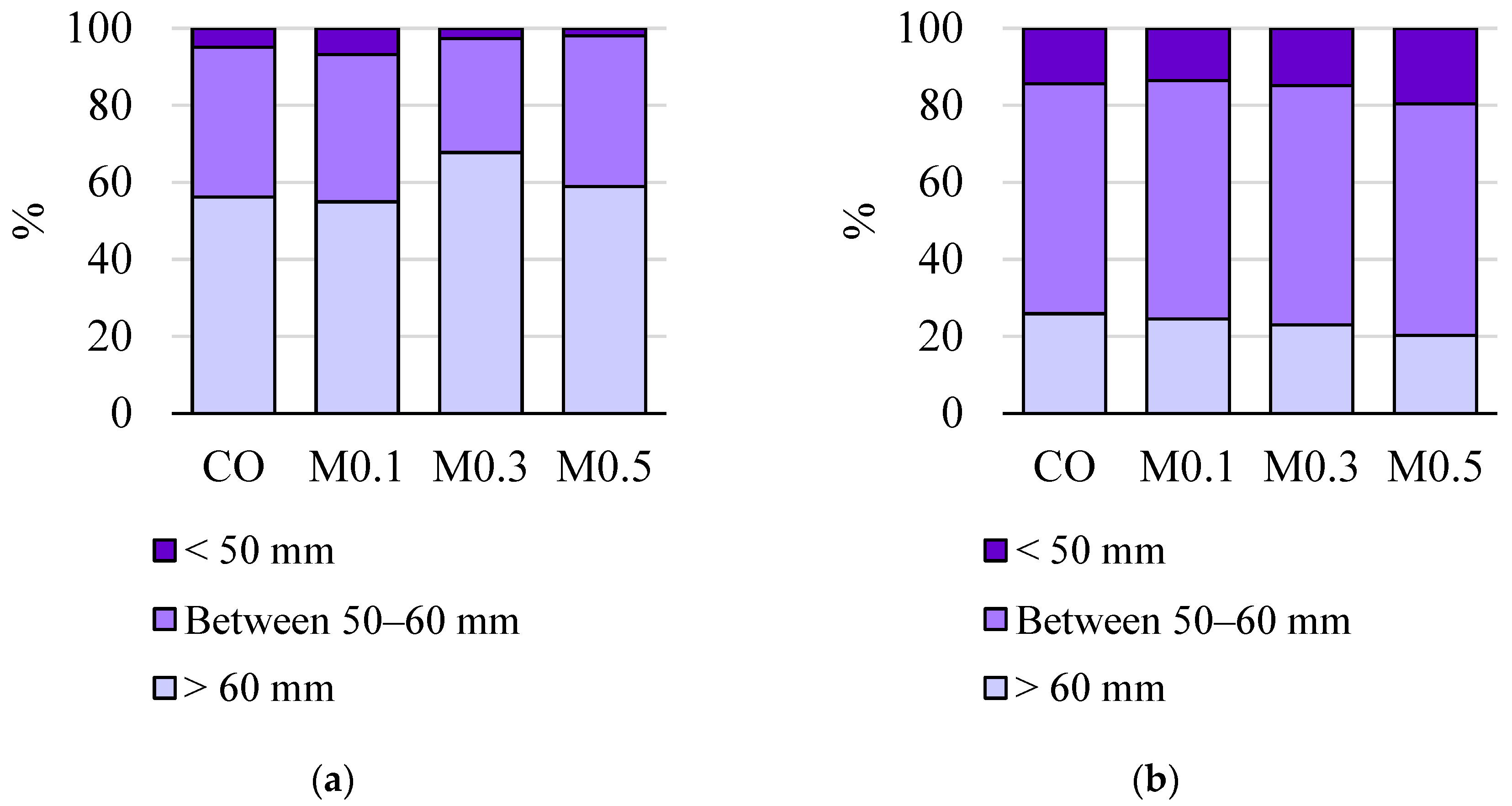
3.1. Quality at the Time of Harvest
3.1.1. Physicochemical Parameters
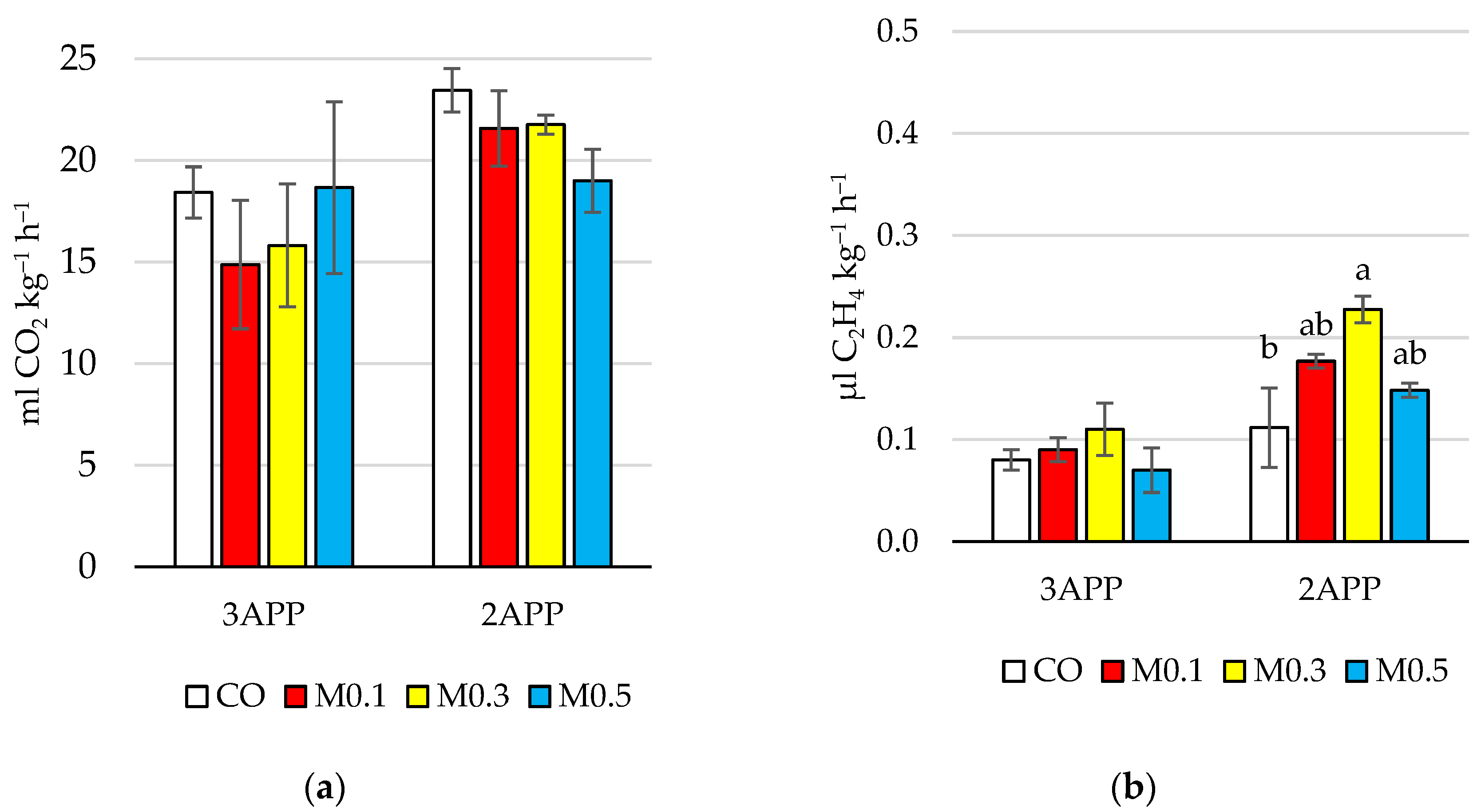
3.1.2. Determination of Respiration Rate and Ethylene Production Rate
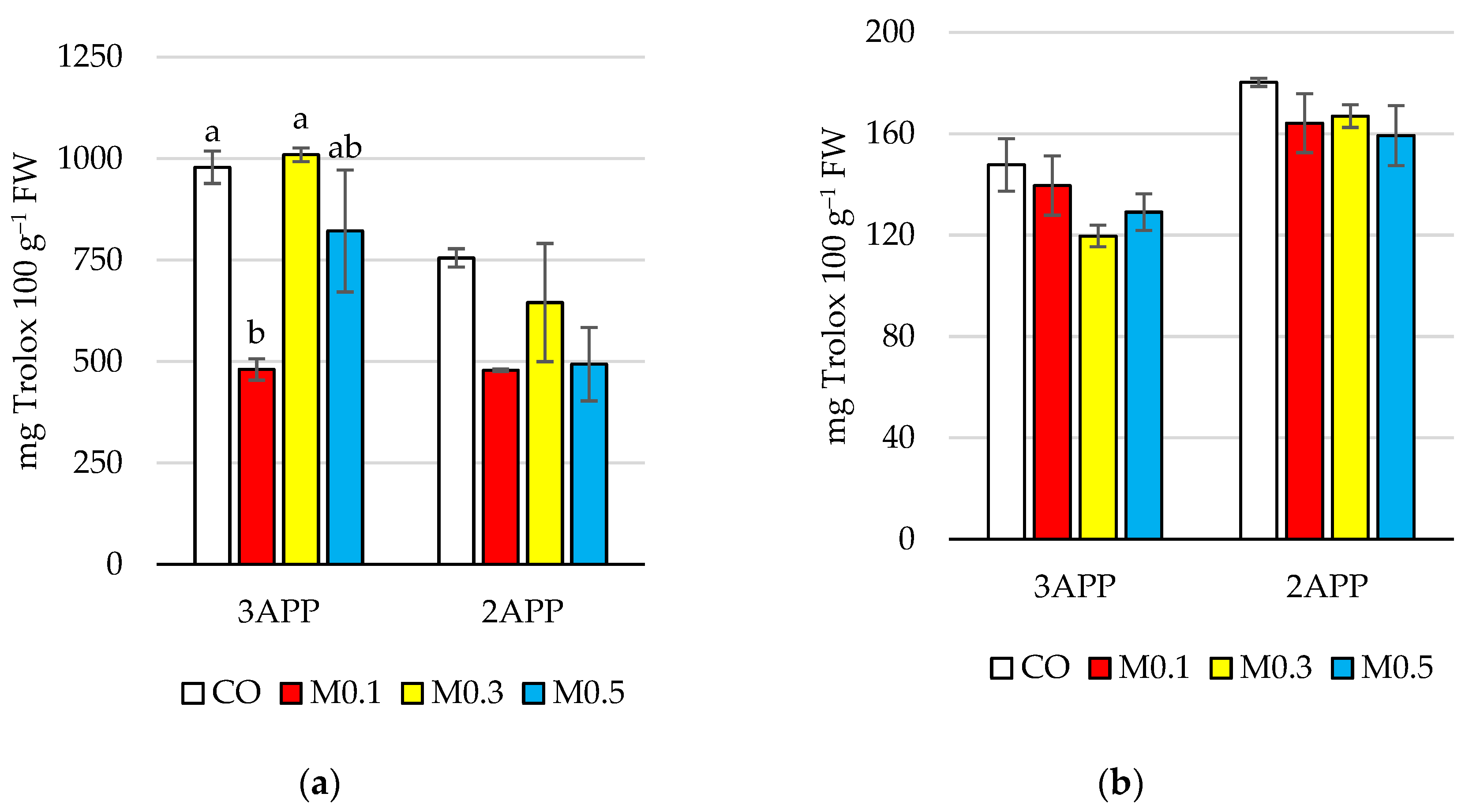
3.1.3. Bioactive Parameters
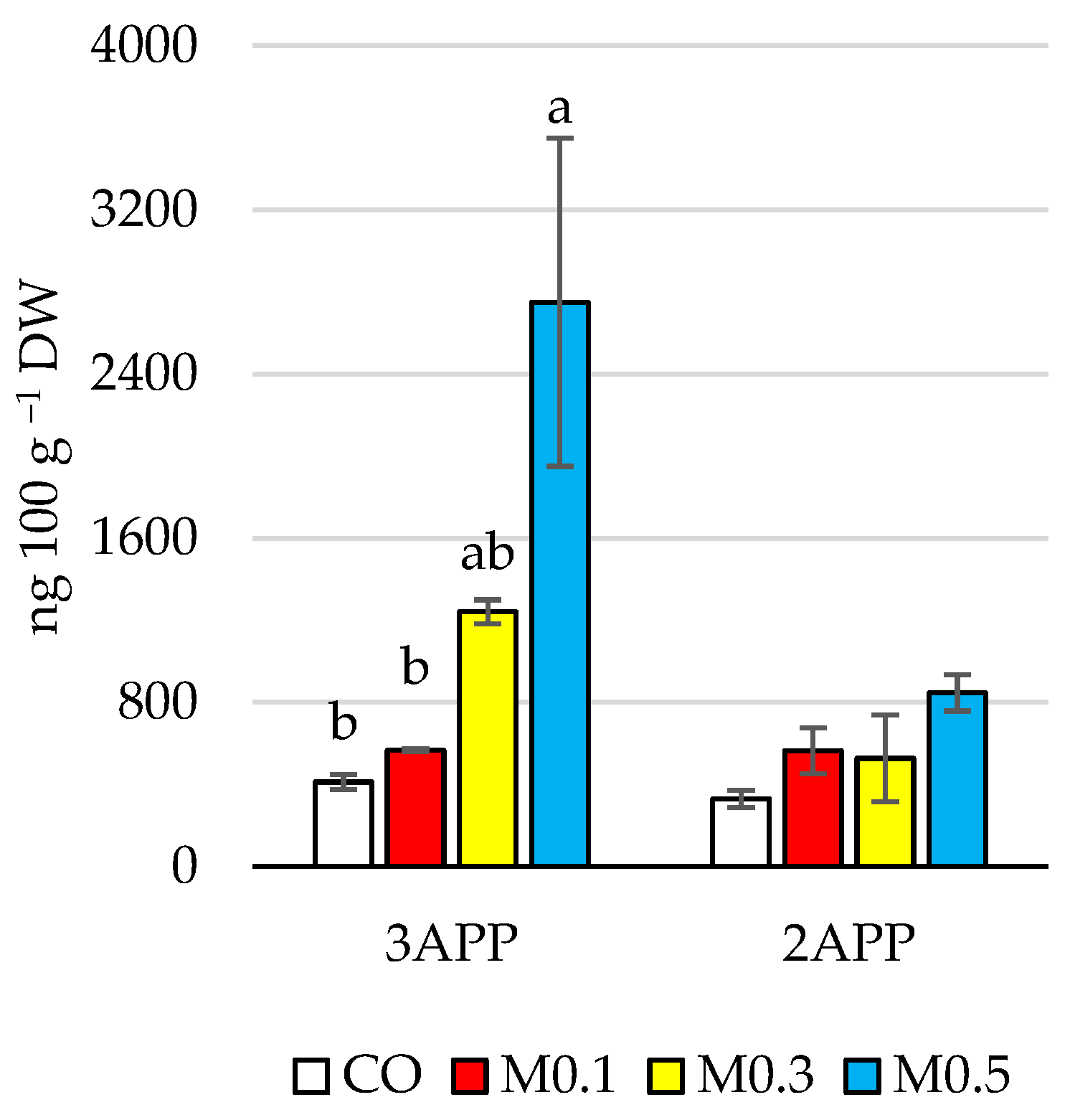
3.1.4. Quantification of Melatonin
3.2. Postharvest Quality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, D.O.; Chun, O.K.; Kim, Y.J.; Moon, H.Y.; Lee, C.Y. Quantification of Polyphenolics and Their Antioxidant Capacity in Fresh Plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Li, W.; You, B.; Yang, S.; Xian, W.; Deng, Y.; Huang, W.; Yang, R. Phenolic Profiles, Bioaccessibility and Antioxidant Activity of Plum (Prunus Salicina Lindl). Food Res. Int. 2021, 143, 110300. [Google Scholar] [CrossRef] [PubMed]
- González-Flores, D.; Velardo, B.; Garrido, M.; González-Gómez, D.; Lozano, M.; Concepción Ayuso, M.; Barriga, C.; Paredes, S.D.; Rodríguez, A.B. Ingestion of Japanese Plums (Prunus Salicina Lindl. Cv. Crimsonglobe) Increases the Urinary 6-Sulfatoxymelatonin and Total Antioxidant Capacity Levels in Young, Middle-Aged and Elderly Humans: Nutritional and Functional Characterization of Their Content. J. Food Nutr. Res. 2011, 50, 229–236. [Google Scholar]
- Valero, D.; Serrano, M. Postharvest Biology and Technology for Preserving Fruit Quality; CRC Press: Boca Raton, FL, USA, 2010; ISBN 9781439802670. [Google Scholar]
- Sabeera, M.; Munazah, S. Chapter 13—Effects of Pre- and Postharvest Processing Technologies on Bioactive Compounds of Plum. In Hand Book of Plum Fruit: Production, Postharvest Science, and Processing Technology; Gull, A., Nayik, G.A., Wani, S.M., Nanda, V., Eds.; CRC Press: Boca Raton, FL, USA, 2022; pp. 249–282. ISBN 9781003205449. [Google Scholar]
- Martínez-Esplá, A.; Serrano, M.; Martínez-Romero, D.; Valero, D.; Zapata, P.J. Oxalic Acid Preharvest Treatment Increases Antioxidant Systems and Improves Plum Quality at Harvest and during Postharvest Storage. J. Sci. Food Agric. 2019, 99, 235–243. [Google Scholar] [CrossRef]
- Arabia, A.; Munné-Bosch, S.; Muñoz, P. Melatonin Triggers Tissue-Specific Changes in Anthocyanin and Hormonal Contents during Postharvest Decay of Angeleno Plums. Plant Sci. 2022, 320, 111287. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: A New Plant Hormone and/or a Plant Master Regulator? Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin in Flowering, Fruit Set and Fruit Ripening. Plant Reprod. 2020, 33, 77–87. [Google Scholar] [CrossRef]
- Back, K. Melatonin Metabolism, Signaling and Possible Roles in Plants. Plant J. 2021, 105, 376–391. [Google Scholar] [CrossRef]
- Carrión-Antolí, A.; Lorente-Mento, J.M.; Valverde, J.M.; Castillo, S.; Valero, D.; Serrano, M. Effects of Melatonin Treatment on Sweet Cherry Tree Yield and Fruit Quality. Agronomy 2021, 12, 3. [Google Scholar] [CrossRef]
- Carrión-Antolí, A.; Martínez-Romero, D.; Guillén, F.; Zapata, P.J.; Serrano, M.; Valero, D. Melatonin Pre-Harvest Treatments Leads to Maintenance of Sweet Cherry Quality during Storage by Increasing Antioxidant Systems. Front. Plant Sci. 2022, 13, 863467. [Google Scholar] [CrossRef]
- Bal, E. Effect of Melatonin Treatments on Biochemical Quality and Postharvest Life of Nectarines. J. Food Meas. Charact. 2021, 15, 288–295. [Google Scholar] [CrossRef]
- Chen, Q.; Arnao, M.B. Phytomelatonin: An Emerging New Hormone in Plants. J. Exp. Bot. 2022, 73, 5773–5778. [Google Scholar] [CrossRef]
- Meng, J.F.; Xu, T.F.; Song, C.Z.; Yu, Y.; Hu, F.; Zhang, L.; Zhang, Z.W.; Xi, Z.M. Melatonin Treatment of Pre-Veraison Grape Berries to Increase Size and Synchronicity of Berries and Modify Wine Aroma Components. Food Chem. 2015, 185, 127–134. [Google Scholar] [CrossRef]
- Tijero, V.; Muñoz, P.; Munné-Bosch, S. Melatonin as an Inhibitor of Sweet Cherries Ripening in Orchard Trees. Plant Physiol. Biochem. 2019, 140, 88–95. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Mukherjee, S.; Flores, F.B.; Arnao, M.B.; Luo, Z.; Corpas, F.J. Functions of Melatonin during Postharvest of Horticultural Crops. Plant Cell Physiol. 2023, 63, 1764–1786. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Wu, M.; Wang, Y.; Yan, Y.; Mao, Q.; Ren, J.; Ma, R.; Liu, A.; Chen, S. Melatonin Alleviates Iron Stress by Improving Iron Homeostasis, Antioxidant Defense and Secondary Metabolism in Cucumber. Sci. Hortic. 2020, 265, 109205. [Google Scholar] [CrossRef]
- Sheikhalipour, M.; Mohammadi, S.A.; Esmaielpour, B.; Zareei, E.; Kulak, M.; Ali, S.; Nouraein, M.; Bahrami, M.K.; Gohari, G.; Fotopoulos, V. Exogenous Melatonin Increases Salt Tolerance in Bitter Melon by Regulating Ionic Balance, Antioxidant System and Secondary Metabolism-Related Genes. BMC Plant Biol. 2022, 22, 380. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, L.; Li, H.; Zhang, S.; Fu, X.; Zhai, X.; Yang, N.; Shen, J.; Li, R.; Li, D. Exogenous Melatonin Promotes the Salt Tolerance by Removing Active Oxygen and Maintaining Ion Balance in Wheat (Triticum aestivum L.). Front. Plant Sci. 2022, 12, 787062. [Google Scholar] [CrossRef]
- Feng, B.-S.; Kang, D.-C.; Sun, J.; Leng, P.; Liu, L.-X.; Wang, L.; Ma, C.; Liu, Y.-G. Research on Melatonin in Fruits and Vegetables and the Mechanism of Exogenous Melatonin on Postharvest Preservation. Food Biosci. 2022, 50, 102196. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, R.; Sun, Y.; Liu, Z.; Jin, W.; Sun, Y. The Beneficial Effects of Exogenous Melatonin on Tomato Fruit Properties. Sci. Hortic. 2016, 207, 14–20. [Google Scholar] [CrossRef]
- Arabia, A.; Muñoz, P.; Pallarés, N.; Munné-Bosch, S. Experimental Approaches in Studying Active Biomolecules Modulating Fruit Ripening: Melatonin as a Case Study. Plant Physiol. 2023, kiad106. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Shen, Y.; Deng, H.; Wang, J.; Lin, L.; Deng, Q.; Lv, X.; Liang, D.; Hu, R.; Wang, Z.; et al. Melatonin Application Improves Berry Coloration, Sucrose Synthesis, and Nutrient Absorption in ‘Summer Black’ Grape. Food Chem. 2021, 356, 129713. [Google Scholar] [CrossRef]
- Medina-Santamarina, J.; Zapata, P.J.; Valverde, J.M.; Valero, D.; Serrano, M.; Guillén, F. Melatonin Treatment of Apricot Trees Leads to Maintenance of Fruit Quality Attributes during Storage at Chilling and Non-Chilling Temperatures. Agronomy 2021, 11, 917. [Google Scholar] [CrossRef]
- Medina-Santamarina, J.; Serrano, M.; Lorente-Mento, J.M.; García-Pastor, M.E.; Zapata, P.J.; Valero, D.; Guillén, F. Melatonin Treatment of Pomegranate Trees Increases Crop Yield and Quality Parameters at Harvest and during Storage. Agronomy 2021, 11, 861. [Google Scholar] [CrossRef]
- Cos, S.; Sánchez-Barceló, E.J. Melatonin, Experimental Basis for a Possible Application in Breast Cancer Prevention and Treatment. Histol. Histopathol. 2000, 15, 637–647. [Google Scholar] [CrossRef]
- Reiter, R.J.; Sharma, R.; Simko, F.; Dominguez-Rodriguez, A.; Tesarik, J.; Neel, R.L.; Slominski, A.T.; Kleszczynski, K.; Martin-Gimenez, V.M.; Manucha, W.; et al. Melatonin: Highlighting Its Use as a Potential Treatment for SARS-CoV-2 Infection. Cell. Mol. Life Sci. 2022, 79, 143. [Google Scholar] [CrossRef]
- Garrido, M.; Rodríguez, A.B.; Terròn, M.P. Chapter 13—Tryptophan and Melatonin-Enriched Foodstuffs to Improve Antioxidant Status in Aging. In Aging; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 129–136. ISBN 9780124059337. [Google Scholar] [CrossRef]
- Garrido, M.; Espino, J.; González-Gómez, D.; Lozano, M.; Cubero, J.; Toribio-Delgado, A.F.; Maynar-Mariño, J.I.; Terrón, M.P.; Muñoz, J.L.; Pariente, J.A.; et al. A Nutraceutical Product Based on Jerte Valley Cherries Improves Sleep and Augments the Antioxidant Status in Humans. e-SPEN Eur. e-J. Clin. Nutr. Metab. 2009, 4, e321–e323. [Google Scholar] [CrossRef]
- Delgado, J.; Terrón, M.P.; Garrido, M.; Pariente, J.A.; Barriga, C.; Rodríguez Moratinos, A.B.; Paredes, S.D. A Cherry Nutraceutical Modulates Melatonin, Serotonin, Corticosterone, and Total Antioxidant Capacity Levels: Effect on Ageing and Chronotype. J. Appl. Biomed. 2012, 10, 109–117. [Google Scholar] [CrossRef]
- Van Tassel, D.L.; Roberts, N.; Lewy, A.; O’Neill, S.D. Melatonin in Plant Organs. J. Pineal Res. 2001, 31, 8–15. [Google Scholar] [CrossRef]
- Usenik, V.; Štampar, F.; Veberič, R. Anthocyanins and Fruit Colour in Plums (Prunus domestica L.) during Ripening. Food Chem. 2009, 114, 529–534. [Google Scholar] [CrossRef]
- Saltveit, M.E. MEASURING RESPIRATION. Available online: https://ucanr.edu/datastoreFiles/234-20.pdf (accessed on 16 February 2023).
- Yang, Q.; Wang, L.; Li, F.; Ma, J.; Zhang, Z. Impact of 1-MCP on Postharvest Quality of Sweet Cherry during Cold Storage. Front. Agric. China 2011, 5, 631–636. [Google Scholar] [CrossRef]
- Manzano Durán, R.; Sánchez, J.E.F.; Velardo-Micharet, B.; Gómez, M.J.R. Multivariate Optimization of Ultrasound-Assisted Extraction for the Determination of Phenolic Compounds in Plums (Prunus salicina L.) by High-Performance Liquid Chromatography (HPLC). Instrum. Sci. Technol. 2020, 48, 113–127. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Arranz, S.; Tabernero, M.; Díaz- Rubio, M.E.; Serrano, J.; Goñi, I.; Saura-Calixto, F. Updated Methodology to Determine Antioxidant Capacity in Plant Foods, Oils and Beverages: Extraction, Measurement and Expression of Results. Food Res. Int. 2008, 41, 274–285. [Google Scholar] [CrossRef]
- González-Gómez, D.; Lozano, M.; Fernández-León, M.F.; Ayuso, M.C.; Bernalte, M.J.; Rodríguez, A.B. Detection and Quantification of Melatonin and Serotonin in Eight Sweet Cherry Cultivars (Prunus avium L.). Eur. Food Res. Technol. 2009, 229, 223–229. [Google Scholar] [CrossRef]
- Usenik, V.; Kastelec, D.; Veberič, R.; Štampar, F. Quality Changes during Ripening of Plums (Prunus domestica L.). Food Chem. 2008, 111, 830–836. [Google Scholar] [CrossRef]
- Wang, Y.; Gun, S.; Li, Y.; Qu, L. Meta-Analysis of Effects of Melatonin Treatment on Plant Drought Stress Alleviation. Agriculture 2022, 12, 1335. [Google Scholar] [CrossRef]
- Okatan, V.; Aşkın, M.A.; Polat, M.; Bulduk, I.; Çolak, A.M.; Güçlü, S.F.; Kahramanoğlu, İ.; Tallarita, A.V.; Caruso, G. Effects of Melatonin Dose on Fruit Yield, Quality, and Antioxidants of Strawberry Cultivars Grown in Different Crop Systems. Agriculture 2023, 13, 71. [Google Scholar] [CrossRef]
- Xia, H.; Shen, Y.; Shen, T.; Wang, X.; Zhang, X.; Hu, P.; Liang, D.; Lin, L.; Deng, H.; Wang, J.; et al. Melatonin Accumulation in Sweet Cherry and Its Influence on Fruit Quality and Antioxidant Properties. Molecules 2020, 25, 753. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Garner, D.; Crisosto, G.M.; Bowerman, E. Increasing “Blackamber” Plum (Prunus salicina Lindell) Consumer Acceptance. Postharvest Biol. Technol. 2004, 34, 237–244. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Crisosto, G.M.; Metheney, P. Consumer Acceptance of ‘Brooks’ and ‘Bing’ Cherries Is Mainly Dependent on Fruit SSC and Visual Skin Color. Postharvest Biol. Technol. 2003, 28, 159–167. [Google Scholar] [CrossRef]
- Bi, Y.; Li, Y.; Wang, Y.; Prusky, D.; Alkan, N.; Gong, D.; Bi, Y.; Li, Y.; Wang, Y.; Prusky, D.; et al. Preharvest Elicitors Spray Improves Antioxidant Activity, Alleviates Chilling Injury, and Maintains Quality in Harvested Fruit. Horticulturae 2022, 8, 1208. [Google Scholar] [CrossRef]
- Yan, R.; Xu, Q.; Dong, J.; Kebbeh, M.; Shen, S.; Huan, C.; Zheng, X. Effects of Exogenous Melatonin on Ripening and Decay Incidence in Plums (Prunus salicina L. Cv. Taoxingli) during Storage at Room Temperature. Sci. Hortic. 2022, 292, 110655. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Yang, Q.; Zhao, Q. Exogenous Melatonin Delays Postharvest Fruit Senescence and Maintains the Quality of Sweet Cherries. Food Chem. 2019, 301, 125311. [Google Scholar] [CrossRef] [PubMed]
- Michailidis, M.; Tanou, G.; Sarrou, E.; Karagiannis, E.; Ganopoulos, I.; Martens, S.; Molassiotis, A. Pre- and Post-Harvest Melatonin Application Boosted Phenolic Compounds Accumulation and Altered Respiratory Characters in Sweet Cherry Fruit. Front. Nutr. 2021, 8, 695061. [Google Scholar] [CrossRef]
- Fan, S.; Xiong, T.; Lei, Q.; Tan, Q.; Cai, J.; Song, Z.; Yang, M.; Chen, W.; Li, X.; Zhu, X. Melatonin Treatment Improves Postharvest Preservation and Resistance of Guava Fruit (Psidium guajava L.). Foods 2022, 11, 262. [Google Scholar] [CrossRef]
- Shochat, T. Impact of Lifestyle and Technology Developments on Sleep. Nat. Sci. Sleep 2012, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Posadzki, P.P.; Bajpai, R.; Kyaw, B.M.; Roberts, N.J.; Brzezinski, A.; Christopoulos, G.I.; Divakar, U.; Bajpai, S.; Soljak, M.; Dunleavy, G.; et al. Melatonin and Health: An Umbrella Review of Health Outcomes and Biological Mechanisms of Action. BMC Med. 2018, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Badria, F.A. Melatonin, Serotonin, and Tryptamine in Some Egyptian Food and Medicinal Plants. J. Med. Food 2002, 5, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Montaña, D.; Josefa Bernalte-García, M.; Velardo-Micharet, B.; Serrano, M.; Joaquín Serradilla, M. Impact of Pre-Storage Melatonin Application on the Standard, Sensory, and Bioactive Quality of Early Sweet Cherry. Foods 2023, 12, 1723. [Google Scholar] [CrossRef]
- Manganaris, G.A.; Vicente, A.R.; Crisosto, C.H. Effect of Pre-Harvest and Post-Harvest Conditions and Treatments on Plum Fruit Quality. CAB Rev. Perspect. Agric. Veter- Sci. Nutr. Nat. Resour. 2008, 3, 1–10. [Google Scholar] [CrossRef]


| CO | M0.1 | M0.3 | M0.5 | ||
|---|---|---|---|---|---|
| TSS | 3APP | 12.77 ± 0.20 a 1 | 10.60 ± 0.21 b | 10.37 ± 0.69 b | 13.20 ± 0.15 a |
| 2APP | 11.07 ± 0.27 | 10.67 ± 0.53 | 10.70 ± 0.23 | 10.73 ± 0.37 | |
| TA | 3APP | 1.35 ± 0.09 | 1.36 ± 0.03 | 1.31 ± 0.04 | 1.41 ± 0.05 |
| 2APP | 1.44 ± 0.04 | 1.36 ± 0.02 | 1.35 ± 0.02 | 1.43 ± 0.02 | |
| RI | 3APP | 9.59 ± 0.73 | 7.79 ± 0.26 | 7.92 ± 0.33 | 9.40 ± 0.38 |
| 2APP | 7.71 ± 0.18 | 7.83 ± 0.30 | 7.90 ± 0.11 | 7.50 ± 0.24 | |
| Firmness | 3APP | 28.46 ± 1.28 ab | 23.79 ± 0.13 b | 25.67 ± 0.72 ab | 30.39 ± 1.70 a |
| 2APP | 23.71 ± 0.87 | 22.39 ± 0.30 | 22.47 ± 0.91 | 21.93 ± 1.17 |
| 3APP | CO | M0.1 | M0.3 | M0.5 |
|---|---|---|---|---|
| CIRG exocarp | 4.49 ± 0.14 a1 | 4.03 ± 0.18 ab | 3.81 ± 0.15 b | 3.70 ± 0.14 b |
| CIRG mesocarp | 2.55 ± 0.07 | 2.52 ± 0.10 | 2.37 ± 0.08 | 2.43 ± 0.11 |
| TSS | 12.33 ± 0.03 a | 10.67 ± 0.09 ab | 11.83 ± 0.98 a | 9.40 ± 0.32 b |
| TA | 1.10 ± 0.06 | 1.14 ± 0.06 | 1.02 ± 0.08 | 1.11 ± 0.08 |
| RI | 11.31 ± 0.52 | 9.46 ± 0.56 | 11.87 ± 1.90 | 8.52 ± 0.33 |
| Firmness | 3.29 ± 0.25 | 3.59 ± 0.29 | 4.12 ± 0.30 | 4.36 ± 0.47 |
| Weight loss | 3.03 ± 0.27 | 3.00 ± 0.25 | 3.57 ± 0.34 | 2.61 ± 0.29 |
| Respiration rate | 29.17 ± 1.56 | 32.22 ± 2.85 | 32.04 ± 2.08 | 33.14 ± 1.23 |
| Ethylene production | 1.34 ± 0.35 | 1.04 ± 0.12 | 0.52 ± 0.04 | 0.93 ± 0.13 |
| C3OG exocarp | 115.49 ± 15.74 ab | 114.72 ± 8.90 ab | 77.96 ± 0.38 b | 147.93 ± 9.01 a |
| C3OG mesocarp | 8.07 ± 2.64 | 8.93 ± 1.02 | 9.24 ± 0.69 | 7.84 ± 0.13 |
| TAA exocarp | 508.25 ± 7.74 b | 504.77 ± 0.41 b | 375.52 ± 7.72 c | 656.67 ± 17.03 a |
| TAA mesocarp | 141.32 ± 5.50 a | 136.71 ± 4.91 a | 99.01 ± 1.27 c | 117.41 ± 0.30 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortés-Montaña, D.; Bernalte-García, M.J.; Serradilla, M.J.; Velardo-Micharet, B. Optimal Preharvest Melatonin Applications to Enhance Endogenous Melatonin Content, Harvest and Postharvest Quality of Japanese Plum. Agriculture 2023, 13, 1318. https://doi.org/10.3390/agriculture13071318
Cortés-Montaña D, Bernalte-García MJ, Serradilla MJ, Velardo-Micharet B. Optimal Preharvest Melatonin Applications to Enhance Endogenous Melatonin Content, Harvest and Postharvest Quality of Japanese Plum. Agriculture. 2023; 13(7):1318. https://doi.org/10.3390/agriculture13071318
Chicago/Turabian StyleCortés-Montaña, Daniel, María Josefa Bernalte-García, Manuel Joaquín Serradilla, and Belén Velardo-Micharet. 2023. "Optimal Preharvest Melatonin Applications to Enhance Endogenous Melatonin Content, Harvest and Postharvest Quality of Japanese Plum" Agriculture 13, no. 7: 1318. https://doi.org/10.3390/agriculture13071318
APA StyleCortés-Montaña, D., Bernalte-García, M. J., Serradilla, M. J., & Velardo-Micharet, B. (2023). Optimal Preharvest Melatonin Applications to Enhance Endogenous Melatonin Content, Harvest and Postharvest Quality of Japanese Plum. Agriculture, 13(7), 1318. https://doi.org/10.3390/agriculture13071318