Sweet Cherry (Prunus avium L.) Cracking during Development on the Tree and at Harvest: The Impact of Methyl Jasmonate on Four Different Growing Seasons
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Sweet Cherry Cracking Evaluation
2.3. Fruit Quality Parameters at Harvest
2.4. Statistical Analysis
3. Results and Discussion
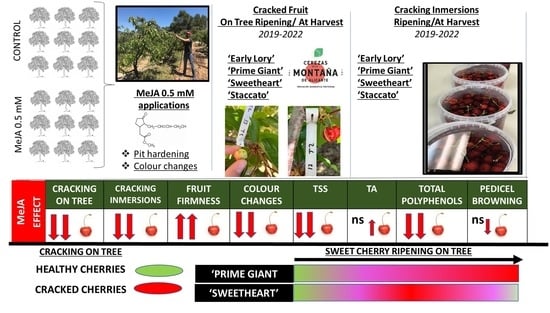
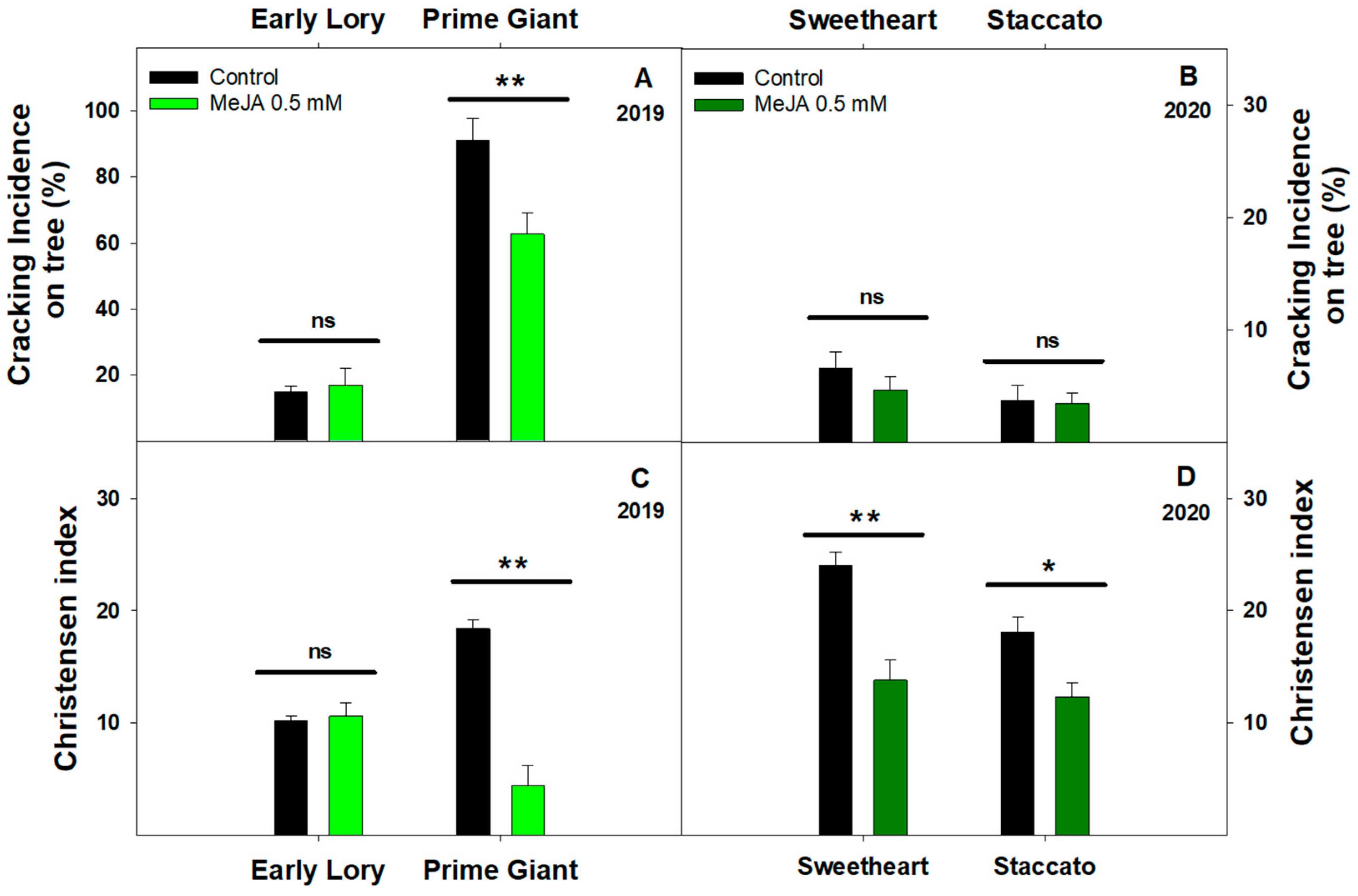
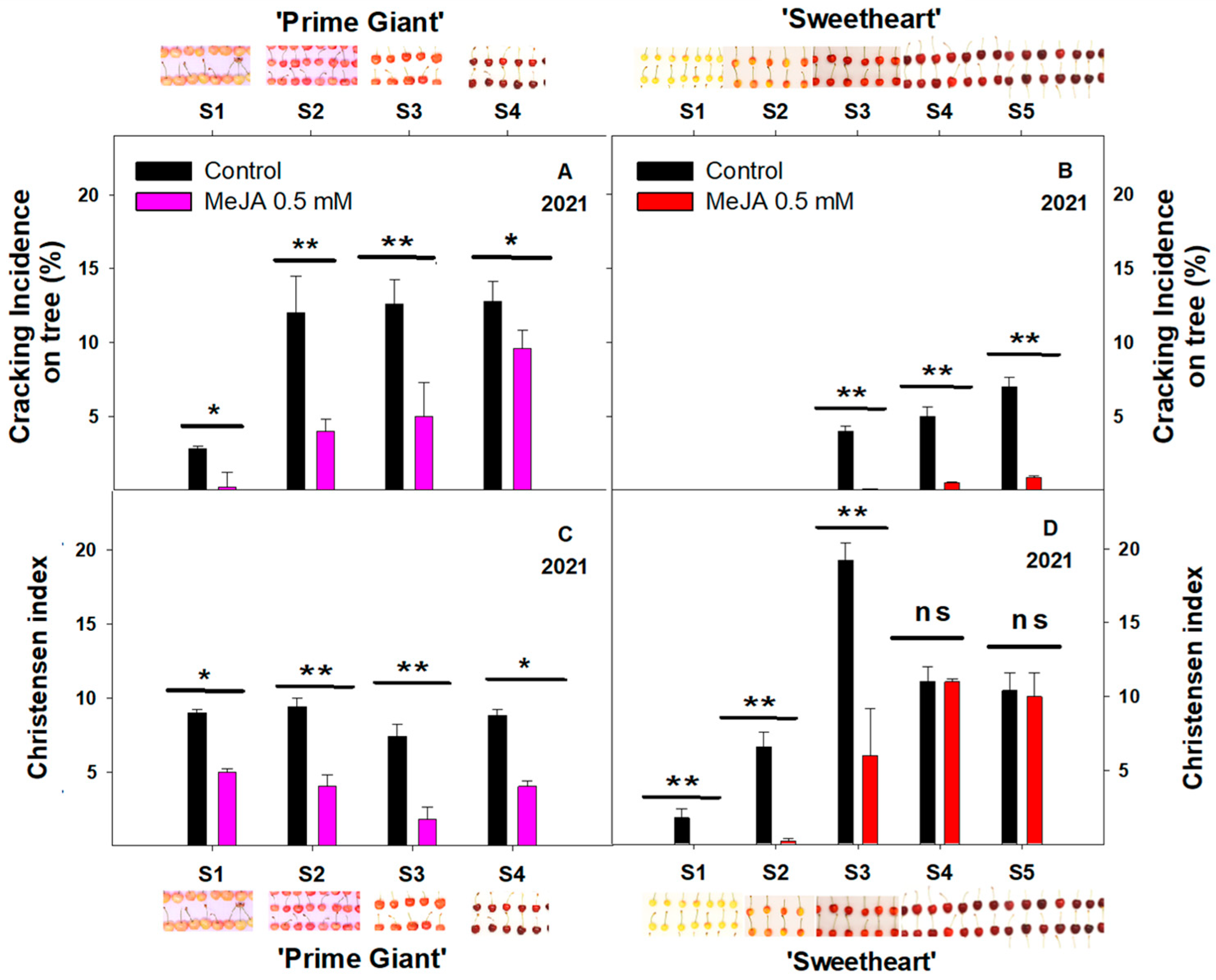
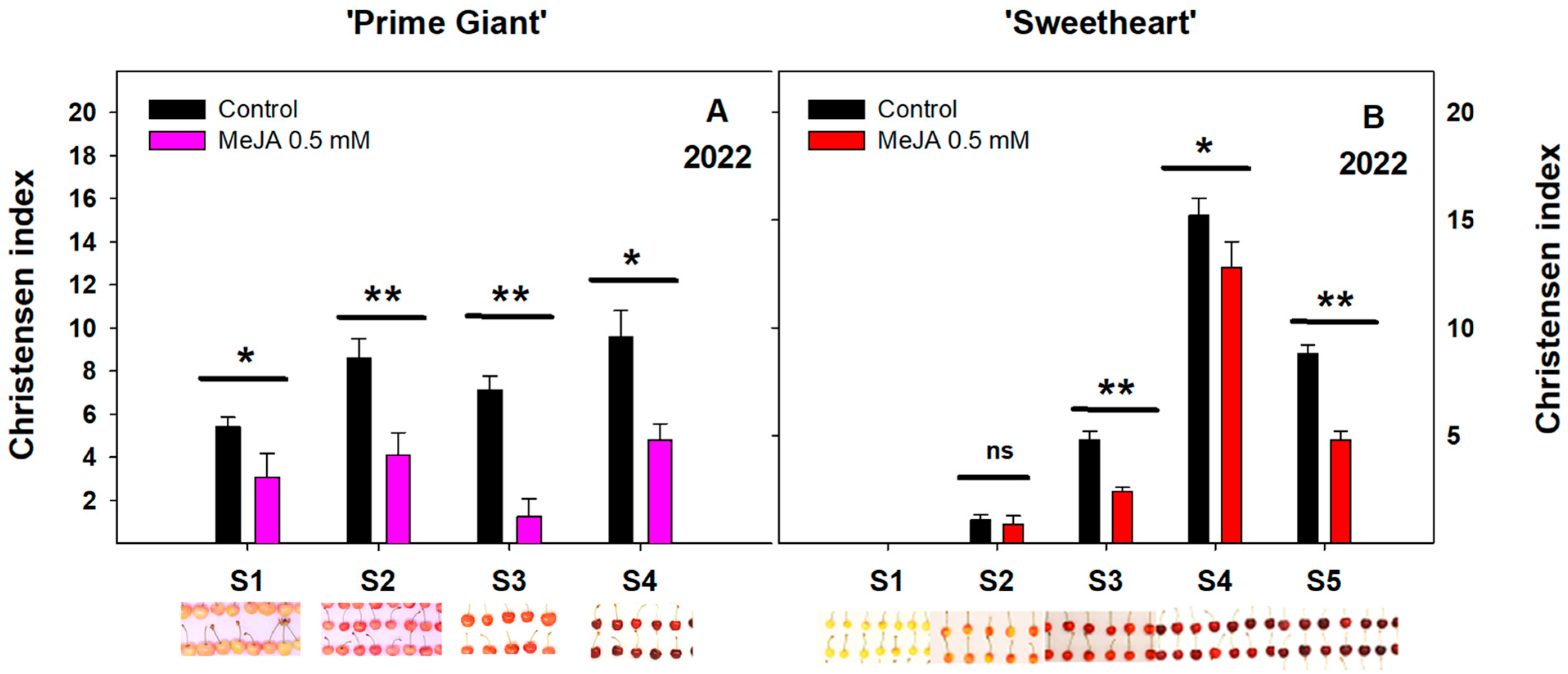
3.1. Effect of Exogenous MeJA on Sweet Cherry Cracking at Harvest
3.2. Effect of Exogenous MeJA on Sweet Cherry Cracking during Ripening on Tree
3.3. Effect of Exogenous MeJA Preharvest Treatments on Quality Parameters at Harvest
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, W.; Gao, Z.; Wang, L.; Zhang, Y.; Fu, X. Advances in Research on Cherry Fruit Cracking: Causes, Prevention and Control. Front. Plant Sci. 2019, 10, 986. [Google Scholar]
- Moing, A.; Renaud, C.; Christmann, H.; Fouilhaux, L.; Tauzin, Y.; Zanetto, A. Is there a relation between changes in osmolarity of cherry fruit flesh or skin and fruit cracking susceptibility? J. Am. Soc. Hortic. Sci. 2004, 129, 635–641. [Google Scholar] [CrossRef]
- Pantelidis, G.; Mavromatis, T.; Drogoudi, P. Consecutive wet days may impede fruit quality of peach and nectarine and cause fruit drop. Sci. Hortic. 2021, 282, 110011. [Google Scholar] [CrossRef]
- Correia, S.; Schouten, R.; Silva, A.P.; Gonçalves, B. Sweet cherry fruit cracking mechanisms and prevention strategies: A review. Sci. Hortic. 2018, 240, 369–377. [Google Scholar] [CrossRef]
- Measham, P.F.; Bound, S.A.; Gracie, A.J.; Wilson, S.J. Crop load manipulation and fruit cracking in sweet cherry (Prunus avium L.). Adv. Hortic. Sci. 2002, 26, 25–31. [Google Scholar]
- Blanco, V.; Blaya-Ros, P.J.; Torres-Sánchez, R.; Domingo, R. Irrigation and Crop Load Management Lessen Rain-Induced Cherry Cracking. Plants 2022, 11, 3249. [Google Scholar] [CrossRef]
- Pereira, S.; Silva, V.; Bacelar, E.; Guedes, F.; Silva, A.P.; Ribeiro, C.; Gonçalves, B. Cracking in sweet cherry cultivars Early Bigi and Lapins: Correlation with quality attributes. Plants 2020, 9, 1557. [Google Scholar] [CrossRef]
- Giné-Bordonaba, J.; Echeverria, G.; Ubach, D.; Aguiló-Aguayo, I.; López, M.L.; Larrigaudière, C. Biochemical and physiological changes during fruit development and ripening of two sweet cherry varieties with different levels of cracking tolerance. Plant Physiol. Biochem. 2017, 111, 216–225. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Sato, I.; Ishiguro, M. Influences of epidermal cell sizes and flesh firmness on cracking susceptibility in sweet cherry (Prunus avium L.) cultivars and selections. J. Jpn. Soc. Hortic. Sci. 2002, 71, 738–746. [Google Scholar]
- Knoche, M.; Peschel, S. Water on the surface aggravates microscopic cracking of the sweet cherry fruit cuticle. J. Am. Soc. Hortic. Sci. 2006, 131, 192–200. [Google Scholar] [CrossRef]
- Pino, S.; Palma, M.; Sepúlveda, Á.; Sánchez-Contreras, J.; Moya, M.; Yuri, J.A. Effect of Rain Cover on Tree Physiology and Fruit Condition and Quality of ‘Rainier’, ‘Bing’ and ‘Sweetheart’ Sweet Cherry Trees. Horticulturae 2023, 9, 109. [Google Scholar] [CrossRef]
- Blanco, V.; Zoffoli, J.P.; Ayala, M. High tunnel cultivation of sweet cherry (Prunus avium L.): Physiological and production variables. Sci. Hortic. 2019, 251, 108–117. [Google Scholar] [CrossRef]
- Matteo, M.; Zoffoli, J.P.; Ayala, M. Calcium Sprays and Crop Load Reduction Increase Fruit Quality and Postharvest Storage in Sweet Cherry (Prunus avium L.). Agronomy 2022, 12, 829. [Google Scholar] [CrossRef]
- Correia, S.; Oliveira, I.; Queirós, F.; Ribeiro, C.; Ferreira, L.; Luzio, A.; Silva, A.P.; Gonçalves, B. Preharvest application of seaweed based biostimulant reduced cherry (Prunus avium L.) cracking. Procedia Environ. Sci. 2015, 29, 251–252. [Google Scholar] [CrossRef]
- Usenik, V.; Kastelec, D.; Štampar, F. Physicochemical changes of sweet cherry fruits related to application of gibberellic acid. Food Chem. 2005, 90, 663–671. [Google Scholar] [CrossRef]
- Correia, S.; Queirós, F.; Ferreira, H.; Morais, M.C.; Afonso, S.; Silva, A.P.; Gonçalves, B. Foliar application of calcium and growth regulators modulate sweet cherry (Prunus avium L.) tree performance. Plants 2020, 9, 410. [Google Scholar] [CrossRef]
- Balbontín, C.; Gutiérrez, C.; Wolff, M.; Figueroa, C.R. Effect of abscisic acid and methyl jasmonate preharvest applications on fruit quality and cracking tolerance of sweet cherry. Chil. J. Agric. Res. 2018, 78, 438–446. [Google Scholar] [CrossRef]
- Yao, H.; Tian, S. Effects of pre-and post-harvest application of salicylic acid or methyl jasmonate on inducing disease resistance of sweet cherry fruit in storage. Postharvest Biol. Technol. 2005, 35, 253–262. [Google Scholar] [CrossRef]
- Faizy, A.H.; Ozturk, B.; Aglar, E.; Yıldız, K. Role of methyl jasmonate application regime on fruit quality and bioactive compounds of sweet cherry at harvest and during cold storage. J. Food Process. 2021, 45, e15882. [Google Scholar] [CrossRef]
- Christensen, J.V. Rain-induced cracking of sweet cherries: Its causes and prevention. In Cherries: Crop Physiology, Production and Uses; Webster, A.D., Looney, N.E., Eds.; CAB International: Wallingford, UK, 2006; pp. 297–327. [Google Scholar]
- Kader, A.A. Methods of gas mixing, sampling and analysis. In Postharvest Technology of Horticultural Crops; Kader, A.A., Ed.; University of California, Division of Agriculture and Natural Resources: Oakland, CA, USA, 1992; pp. 93–95. [Google Scholar]
- Carrión-Antolí, A.; Martínez-Romero, D.; Guillén, F.; Zapata, P.J.; Serrano, M.; Valero, D. Melatonin pre-harvest treatments leads to maintenance of sweet cherry quality during storage by increasing antioxidant systems. Front. Plant Sci. 2022, 13, 984. [Google Scholar]
- Lezoul, N.E.H.; Belkadi, M.; Habibi, F.; Guillén, F. Extraction processes withseveral solvents on total bioactive compounds in different organs of three medicinal plants. Molecules 2020, 25, 4672. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.A.; Singh, P.; Gul, K.; Wani, M.H.; Langowski, H.C. Sweet cherry (Prunus avium): Critical factors affecting the composition and shelf life. Food Packag. Shelf Life 2014, 1, 86–99. [Google Scholar] [CrossRef]
- Blažek, J.; Zelený, L.; Suran, P. Sweet cherry research world overview 2015–2017. Hort. Sci. 2022, 49, 121–146. [Google Scholar] [CrossRef]
- Quero-García, J.; Letourmy, P.; Campoy, J.A.; Branchereau, C.; Malchev, S.; Barreneche, T.; Dirlewanger, E. Multi-year analyses on three populations reveal the first stable QTLs for tolerance to rain-induced fruit cracking in sweet cherry (Prunus avium L.). Hort. Res. 2021, 8, 136. [Google Scholar] [CrossRef]
- Serrano, M.; Guillén, F.; Martínez-Romero, D.; Castillo, S.; Valero, D. Chemical constituents and antioxidant activity of sweet cherry at different ripening stages. J. Agric. Food Chem. 2005, 53, 2741–2745. [Google Scholar] [CrossRef] [PubMed]
- Demirsoy, L.; Bilgener, S. The effects of preharvest calcium hydroxide applications on cracking in ‘0900 Ziraat’, ‘Lambert’ and ‘Van’ sweet cherries. Acta Hortic. 1998, 468, 657–662. [Google Scholar] [CrossRef]
- Rohwer, C.L.; Erwin, J.E. Horticultural applications of jasmonates: A review. J. Hort. Sci. Biotech. 2008, 83, 283–304. [Google Scholar] [CrossRef]
- Demirsoy, L.; Demirsoy, H. The epidermal characteristics of fruit skin of some sweet cherry cultivars in relation to fruit cracking. Pak. J. Bot. 2004, 34, 725–731. [Google Scholar]
- Winkler, A.; Blumenberg, I.; Schürmann, L.; Knoche, M. Rain cracking in sweet cherries is caused by surface wetness, not by water uptake. Sci. Hortic. 2020, 269, 10940023. [Google Scholar] [CrossRef]
- Etiopi, C.; Monari, W. Effectiveness of new rainprotection systems on cracking, ripening date and fruit quality of sweet cherry cultivars. Acta Hortic. 2017, 1161, 213–220. [Google Scholar]
- Bustamante, M.; Muñoz, A.; Romero, I.; Osorio, P.; Mánquez, S.; Arriola, R.; Reyes-Díaz, M.; Ribera-Fonseca, A. Impact of potassium pre-harvest applications on fruit quality and condition of sweet cherry (Prunus avium L.) cultivated under plastic covers in southern chile orchards. Plants 2021, 10, 2778. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, B.; Silva, V.; Bacelar, E.; Guedes, F.; Ribeiro, C.; Silva, A.P.; Pereira, S. Orchard net covers improve resistance to cherry cracking disorder. Foods 2023, 12, 543. [Google Scholar] [CrossRef] [PubMed]
- Brüggenwirth, M.; Knoche, M. Mechanical properties of skins of sweet cherry fruit of differing susceptibilities to cracking. J. Am. Soc. Hortic. Sci. 2016, 141, 162–168. [Google Scholar] [CrossRef]
- Valero, D.; Serrano, M. Postharvest Biology and Technology for Preserving Fruit Quality; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2010. [Google Scholar]
- Saracoglu, O.; Ozturk, B.; Yildiz, K.; Kucuker, E. Pre-harvest methyl jasmonate treatments delayed ripening and improved quality of sweet cherry fruits. Sci. Hortic. 2017, 226, 19–23. [Google Scholar] [CrossRef]
- Erogul, D. Effect of preharvest calcium treatments on sweet cherry fruit quality. Not. Bot. Horti Agrobot. Cluj Napoca 2014, 42, 150–153. [Google Scholar] [CrossRef]
- Yildirim, A.N.; Koyuncu, F. The effect of gibberellic acid applications on the cracking rate and fruit quality in the ‘0900 Ziraat’sweet cherry cultivar. Afr. J. Biotechnol. 2010, 9, 6307–6311. [Google Scholar]
- Díaz-Mula, H.M.; Serrano, M.; Valero, D. Alginate coatings preserve fruit quality and bioactive compounds during storage of sweet cherry fruit. Food Bioproc. Tech. 2012, 5, 2990–2997. [Google Scholar] [CrossRef]
- Serrano, M.; Díaz-Mula, H.M.; Zapata, P.J.; Castillo, S.; Guillén, F.; Martínez-Romero, D.; Valverde, J.M.; Valero, D. Maturity stage at harvest determines the fruit quality and antioxidant potential after storage of sweet cherry cultivars. J. Agric. Food Chem. 2009, 57, 3240–3246. [Google Scholar] [CrossRef]
- Knoche, M.; Grimm, E.; Schlegel, H.J. Mature sweet cherries have low turgor. J. Am. Soc. Hortic. Sci. 2014, 139, 3–12. [Google Scholar] [CrossRef]
- Ruiz, K.B.; Trainotti, L.; Bonghi, C.; Ziosi, V.; Costa, G.; Torrigiani, P. Early methyl jasmonate application to peach delays fruit/seed development by altering the expression of multiple hormone-related genes. J. Plant Growth Regul. 2013, 32, 852–864. [Google Scholar] [CrossRef]
- Shafiq, M.; Singh, Z.; Ahmad, S.K. Pre-harvest spray application of methyl jasmonate improves red blush and flavonoid content in ‘Cripps Pink’ apple. J. Hortic. Sci. Biotechnol. 2011, 86, 422–430. [Google Scholar] [CrossRef]
- Meng, X.; Han, J.; Wang, Q.; Tian, S. Changes in physiology and quality of peach fruits treated by methyl jasmonate under low temperature stress. Food Chem. 2009, 114, 1028–1035. [Google Scholar] [CrossRef]
- Wang, S.Y.; Bowman, L.; Ding, M. Methyl jasmonate enhances antioxidant activity and flavonoid content in blackberries (Rubus sp.) and promotes antiproliferation of human cancer cells. Food Chem. 2008, 107, 1261–1269. [Google Scholar] [CrossRef]
- Wang, S.Y.; Zheng, W. Preharvest application of methyl jasmonate increases fruit quality and antioxidant capacity in raspberries. Int. J. Food Sci. Technol. 2005, 40, 187–195. [Google Scholar] [CrossRef]
- Gonçalves, B.; Silva, A.P.; Moutinho-Pereira, J.; Bacelar, E.; Rosa, E.; Meyer, A.S. Effect of ripeness and postharvest storage on the evolution of colour and anthocyanins in cherries (Prunus avium L.). Food Chem. 2007, 103, 976–984. [Google Scholar] [CrossRef]
- Kondo, S. Roles of jasmonates in fruit ripening and environmental stress. In Proceedings of the XI International Symposium on Plant Bioregulators in Fruit Production. Acta Hortic. 2009, 884, 711–716. [Google Scholar]
- Kucuker, E.; Ozturk, B. The effects of aminoethoxyvinylglycine and methyl jasmonate on bioactive compounds and fruit quality of ‘North Wonder’ sweet cherry. Afr. J. Tradit. Complement. Altern. Med. 2015, 12, 114–119. [Google Scholar] [CrossRef]
- Concha, C.M.; Figueroa, N.E.; Poblete, L.A.; Oñate, F.A.; Schwab, W.; Figueroa, C.R. Methyl jasmonate treatment induces changes in fruit ripening by modifying the expression of several ripening genes in Fragaria chiloensis fruit. Plant Physiol. Biochem. 2013, 70, 433–444. [Google Scholar] [CrossRef]
- Öztürk, B.; Özkan, Y.; Yildiz, K. Methyl jasmonate treatments influence bioactive compounds and red peel color development of Braeburn apple. Turk. J. Agric. For. 2014, 38, 688–699. [Google Scholar] [CrossRef]
- Khan, A.S.; Singh, Z. Methyl jasmonate promotes fruit ripening and improves fruit quality in Japanese plum. J. Hortic. Sci. Biotechnol. 2007, 82, 695–706. [Google Scholar] [CrossRef]
- Kucuker, E.; Ozturk, B.; Celik, S.M.; Aksit, H. Pre-harvest spray application of methyl jasmonate plays an important role in fruit ripening, fruit quality and bioactive compounds of Japanese plums. Sci. Hortic. 2014, 176, 162–169. [Google Scholar] [CrossRef]
- Sudheeran, P.K.; Love, C.; Feygenberg, O.; Maurer, D.; Ovadia, R.; Oren-Shamir, M.; Alkan, N. Induction of red skin and improvement of fruit quality in ‘Kent’, ‘Shelly’ and ‘Maya’ mangoes by preharvest spraying of prohydrojasmon at the orchard. Postharvest Biol. Technol. 2019, 149, 18–26. [Google Scholar] [CrossRef]
- García-Pastor, M.E.; Serrano, M.; Guillén, F.; Zapata, P.J.; Valero, D. Preharvest or a combination of preharvest and postharvest treatments with methyl jasmonate reduced chilling injury, by maintaining higher unsaturated fatty acids, and increased aril colour and phenolics content in pomegranate. Postharvest Biol. Technol. 2020, 167, 111226. [Google Scholar] [CrossRef]
- García-Pastor, M.E.; Serrano, M.; Guillén, F.; Castillo, S.; Martínez-Romero, D.; Valero, D.; Zapata, P.J. Methyl jasmonate effects on table grape ripening, vine yield, berry quality and bioactive compounds depend on applied concentration. Sci. Hortic. 2019, 247, 380–389. [Google Scholar] [CrossRef]
- Blanusa, T.; Else, M.A.; Atkinson, C.J.; Davies, W.J. The regulation of sweet cherry fruit abscission by polar auxin transport. J. Plant Growth Regul. 2005, 45, 189–198. [Google Scholar] [CrossRef]
- Martínez-Romero, D.; Alburquerque, N.; Valverde, J.M.; Guillén, F.; Castillo, S.; Valero, D.; Serrano, M. Postharvest sweet cherry quality and safety maintenance by Aloe vera treatment: A new edible coating. Postharvest Biol. Technol. 2006, 39, 93–100. [Google Scholar] [CrossRef]
- Hewedy, O.A.; Elsheery, N.I.; Karkour, A.M.; Elhamouly, N.; Arafa, R.A.; Mahmoud, G.A.E.; Dawood, M.F.-A.; Hussein, W.E.; Mansour, A.; Amin, D.H.; et al. Jasmonic acid regulates plant development and orchestrates stress response during tough times. Environ. Exp. Bot. 2023, 208, 105260. [Google Scholar] [CrossRef]
- Swain, R.; Sahoo, S.; Behera, M.; Rout, G.R. Instigating prevalent abiotic stress resilience in crop by exogenous application of phytohormones and nutrient. Front. Plant Sci. 2023, 14, 1104874. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Sierra, A.; Thomas, M.; Arul, J. Ethanol and Methyl Jasmonate Fumigation Impact on Quality, Antioxidant Capacity, and Phytochemical Content of Broccoli Florets during Storage. Horticulturae 2023, 9, 465. [Google Scholar] [CrossRef]
| Fruit Firmness (N mm−1) | |||
| Cultivar | Year | Control | MeJA 0.5 mM |
| ‘Early Lory’ | 2019 | 1.10 ± 0.04 | 1.25 ± 0.04 * |
| ‘Prime Giant’ | 2019 | 1.77 ± 0.07 | 1.91 ± 0.08 * |
| ‘Prime Giant’ | 2021 | 1.31 ± 0.04 | 1.51 ± 0.07 * |
| ‘Prime Giant’ | 2022 | 1.19 ± 0.05 | 1.21 ± 0.05 ns |
| ‘Staccato’ | 2020 | 2.84 ± 0.11 | 2.88 ± 0.12 ns |
| ‘Sweetheart’ | 2020 | 1.94 ± 0.07 | 1.99 ± 0.07 ns |
| ‘Sweetheart’ | 2021 | 1.78 ± 0.09 | 2.08 ± 0.08 * |
| ‘Sweetheart’ | 2022 | 1.73 ± 0.08 | 2.17 ± 0.09 ** |
| Fruit Colour (CIE h°) | |||
| Cultivar | Year | Control | MeJA 0.5 mM |
| ‘Early Lory’ | 2019 | 22.50 ± 0.40 | 21.93 ± 0.8 ns |
| ‘Prime Giant’ | 2019 | 15.65 ± 0.65 | 18.61 ± 0.49 * |
| ‘Prime Giant’ | 2021 | 13.37 ± 0.31 | 14.32 ± 0.43 ** |
| ‘Prime Giant’ | 2022 | 21.50 ± 0.52 | 23.92 ± 0.59 * |
| ‘Staccato’ | 2020 | 16.24 ± 0.55 | 18.73 ± 0.72 * |
| ‘Sweetheart’ | 2020 | 16.58 ± 0.58 | 18.19 ± 0.70 * |
| ‘Sweetheart’ | 2021 | 20.64 ± 0.20 | 22.48 ± 0.32 * |
| ‘Sweetheart’ | 2022 | 17.49 ± 0.34 | 19.67 ± 0.25 ** |
| Total Soluble Solids (g 100 g−1) | |||
| Cultivar | Year | Control | MeJA 0.5 mM |
| ‘Early Lory’ | 2019 | 13.05 ± 0.19 | 11.88 ± 0.04 * |
| ‘Prime Giant’ | 2019 | 22.96 ± 0.59 | 18.50 ± 0.45 ** |
| ‘Prime Giant’ | 2021 | 17.56 ± 0.18 | 17.83 ± 0.11 ns |
| ‘Prime Giant’ | 2022 | 22.75 ± 0.41 | 22.06 ± 0.45 ns |
| ‘Staccato’ | 2020 | 21.05 ± 0.05 | 20.17 ± 0.26 * |
| ‘Sweetheart’ | 2020 | 22.42 ± 0.07 | 22.37 ± 0.10 ns |
| ‘Sweetheart’ | 2021 | 19.61 ± 0.35 | 18.90 ± 0.16 * |
| ‘Sweetheart’ | 2022 | 19.03 ± 0.18 | 18.21 ± 0.18 * |
| Titratable Acidity (g 100 g−1) | |||
| Cultivar | Year | Control | MeJA 0.5 mM |
| ‘Early Lory’ | 2019 | 1.12 ± 0.01 | 1.07 ± 0.01 ns |
| ‘Prime Giant’ | 2019 | 1.20 ± 0.05 | 1.31 ± 0.03 ns |
| ‘Prime Giant’ | 2021 | 1.09 ± 0.01 | 1.11 ± 0.01 ns |
| ‘Prime Giant’ | 2022 | 1.52 ± 0.01 | 1.53 ± 0.02 ns |
| ‘Staccato’ | 2020 | 1.21 ± 0.01 | 1.28 ± 0.01 * |
| ‘Sweetheart’ | 2020 | 1.23 ± 0.02 | 1.24 ± 0.01 ns |
| ‘Sweetheart’ | 2021 | 1.33 ± 0.02 | 1.24 ± 0.03 * |
| ‘Sweetheart’ | 2022 | 1.44 ± 0.03 | 1.48 ± 6.12 × 10−3 ns |
| Total Polyphenols (mg 100 g−1) | |||
| Cultivar | Year | Control | Meja 0.5 mM |
| ‘Early Lory’ | 2019 | 99.48 ± 3.91 | 90.75 ± 2.77 * |
| ‘Prime Giant’ | 2019 | 87.15 ± 4.16 | 78.42 ± 7.65 * |
| ‘Prime Giant’ | 2021 | 98.39 ± 6.10 | 102.57 ± 2.55 ns |
| ‘Prime Giant’ | 2022 | 91.45 ± 5.67 | 84.56 ± 3.45 ns |
| ‘Staccato’ | 2020 | 81.66 ± 2.98 | 73.46 ± 1.44 * |
| ‘Sweetheart’ | 2020 | 75.07 ± 2.98 | 73.60 ± 3.44 ns |
| ‘Sweetheart’ | 2021 | 109.73 ± 9.74 | 100.64 ± 9.77 ns |
| ‘Sweetheart’ | 2022 | 85.97 ± 3.21 | 83.56 ± 2.97 ns |
| Pedicel Browning (Scale 0–4) | |||
| Cultivar | Year | Control | Meja 0.5 mM |
| ‘Early Lory’ | 2019 | 0.75 ± 0.20 | 0.72 ± 0.12 ns |
| ‘Prime Giant’ | 2019 | 0.81 ± 0.17 | 0.39 ± 0.16 ** |
| ‘Prime Giant’ | 2021 | 0.83 ± 0.09 | 0.70 ± 0.10 ns |
| ‘Prime Giant’ | 2022 | 0.07 ± 0.02 | 0.06 ± 0.02 ns |
| ‘Staccato’ | 2020 | 0.20 ± 0.03 | 0.16 ± 0.02 ns |
| ‘Sweetheart’ | 2020 | 0.18 ± 0.01 | 0.19 ± 0.01 ns |
| ‘Sweetheart’ | 2021 | 0.62 ± 0.09 | 0.66 ± 0.07 ns |
| ‘Sweetheart’ | 2022 | 0.06 ± 0.02 | 0.05 ± 0.02 ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Aracil, M.C.; Valverde, J.M.; Lorente-Mento, J.M.; Carrión-Antolí, A.; Castillo, S.; Martínez-Romero, D.; Guillén, F. Sweet Cherry (Prunus avium L.) Cracking during Development on the Tree and at Harvest: The Impact of Methyl Jasmonate on Four Different Growing Seasons. Agriculture 2023, 13, 1244. https://doi.org/10.3390/agriculture13061244
Ruiz-Aracil MC, Valverde JM, Lorente-Mento JM, Carrión-Antolí A, Castillo S, Martínez-Romero D, Guillén F. Sweet Cherry (Prunus avium L.) Cracking during Development on the Tree and at Harvest: The Impact of Methyl Jasmonate on Four Different Growing Seasons. Agriculture. 2023; 13(6):1244. https://doi.org/10.3390/agriculture13061244
Chicago/Turabian StyleRuiz-Aracil, María Celeste, Juan Miguel Valverde, Jose Manuel Lorente-Mento, Alberto Carrión-Antolí, Salvador Castillo, Domingo Martínez-Romero, and Fabián Guillén. 2023. "Sweet Cherry (Prunus avium L.) Cracking during Development on the Tree and at Harvest: The Impact of Methyl Jasmonate on Four Different Growing Seasons" Agriculture 13, no. 6: 1244. https://doi.org/10.3390/agriculture13061244
APA StyleRuiz-Aracil, M. C., Valverde, J. M., Lorente-Mento, J. M., Carrión-Antolí, A., Castillo, S., Martínez-Romero, D., & Guillén, F. (2023). Sweet Cherry (Prunus avium L.) Cracking during Development on the Tree and at Harvest: The Impact of Methyl Jasmonate on Four Different Growing Seasons. Agriculture, 13(6), 1244. https://doi.org/10.3390/agriculture13061244