Comparative Effect of Melatonin and 1-Methylcyclopropene Postharvest Applications for Extending ‘Hayward’ Kiwifruit Storage Life
Abstract
1. Introduction
2. Materials and Methods
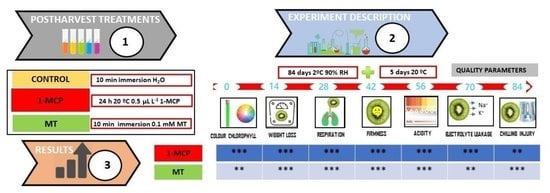
2.1. Plant Material and Experimental Design
2.2. Postharvest Quality Parameters
2.3. Statistical Analysis
3. Results and Discussions
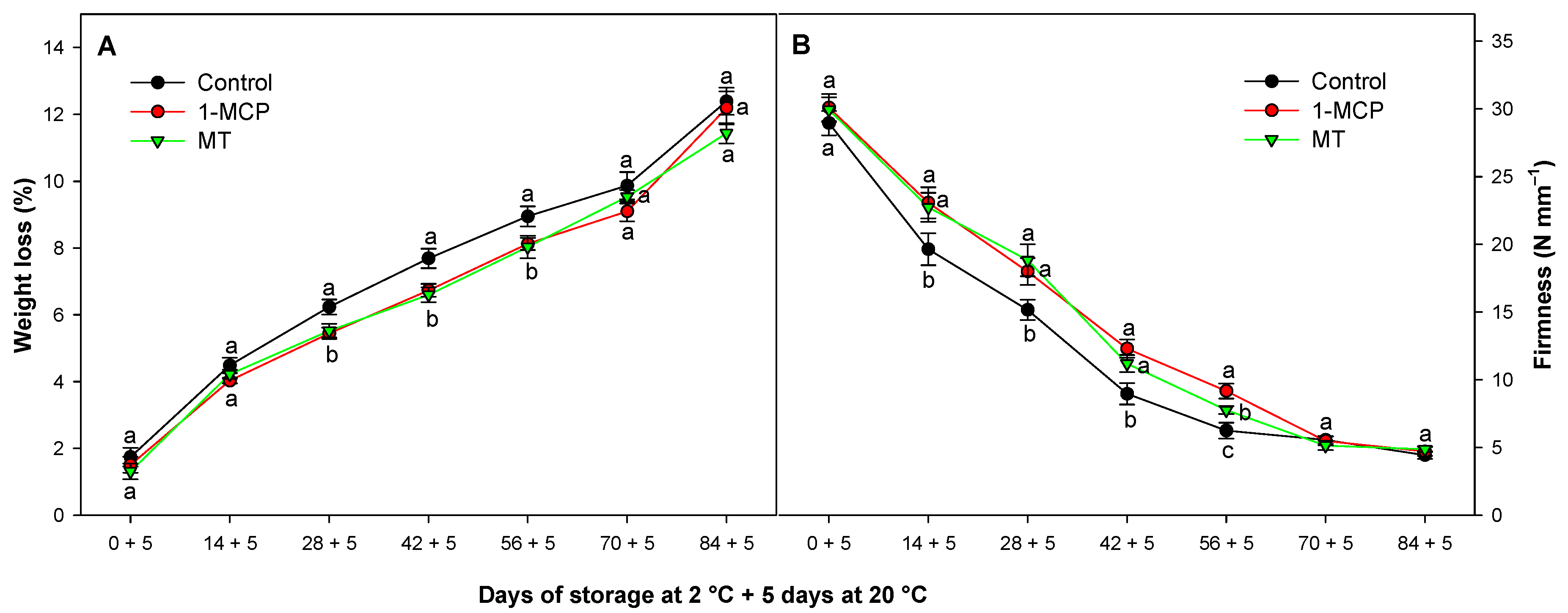
3.1. Effect of Exogenous 1-MCP and MT on Weight Loss and Fruit Firmness
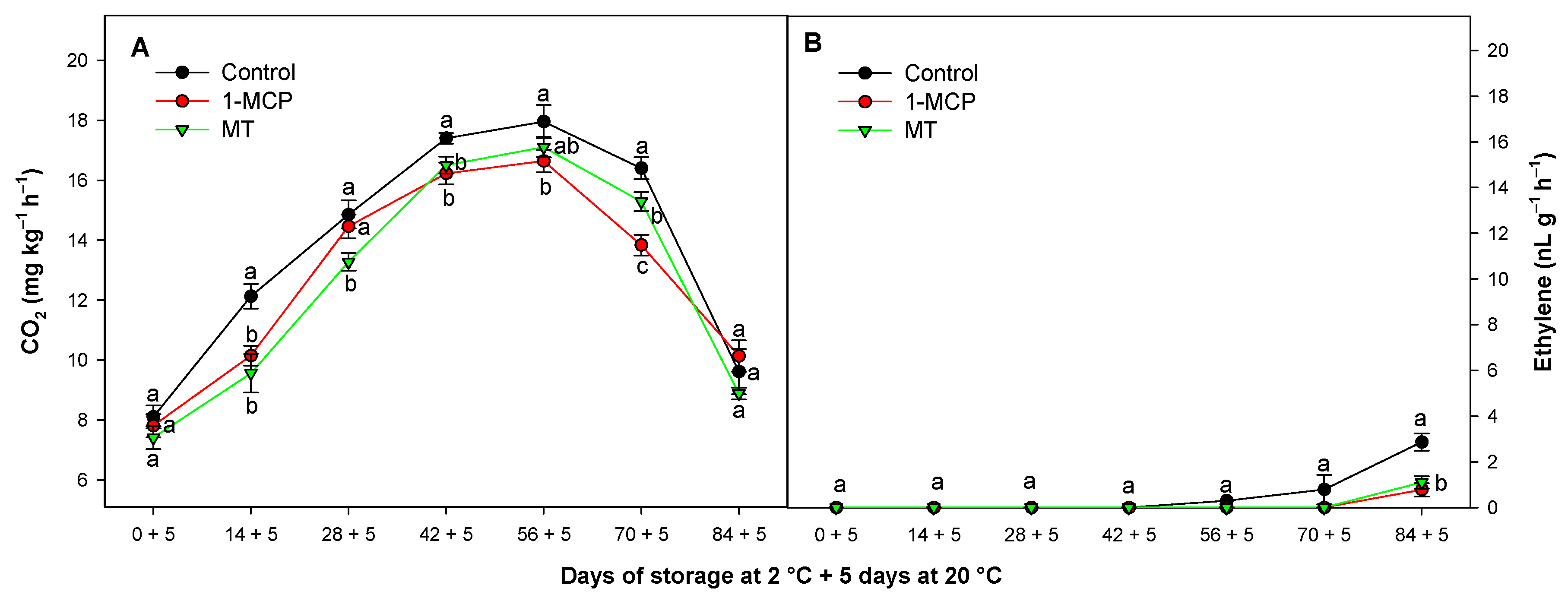
3.2. Effect of Exogenous 1-MCP and MT on Respiration Rate and Ethylene Production
3.3. Effect of Exogenous 1-MCP and MT on TSS and TA
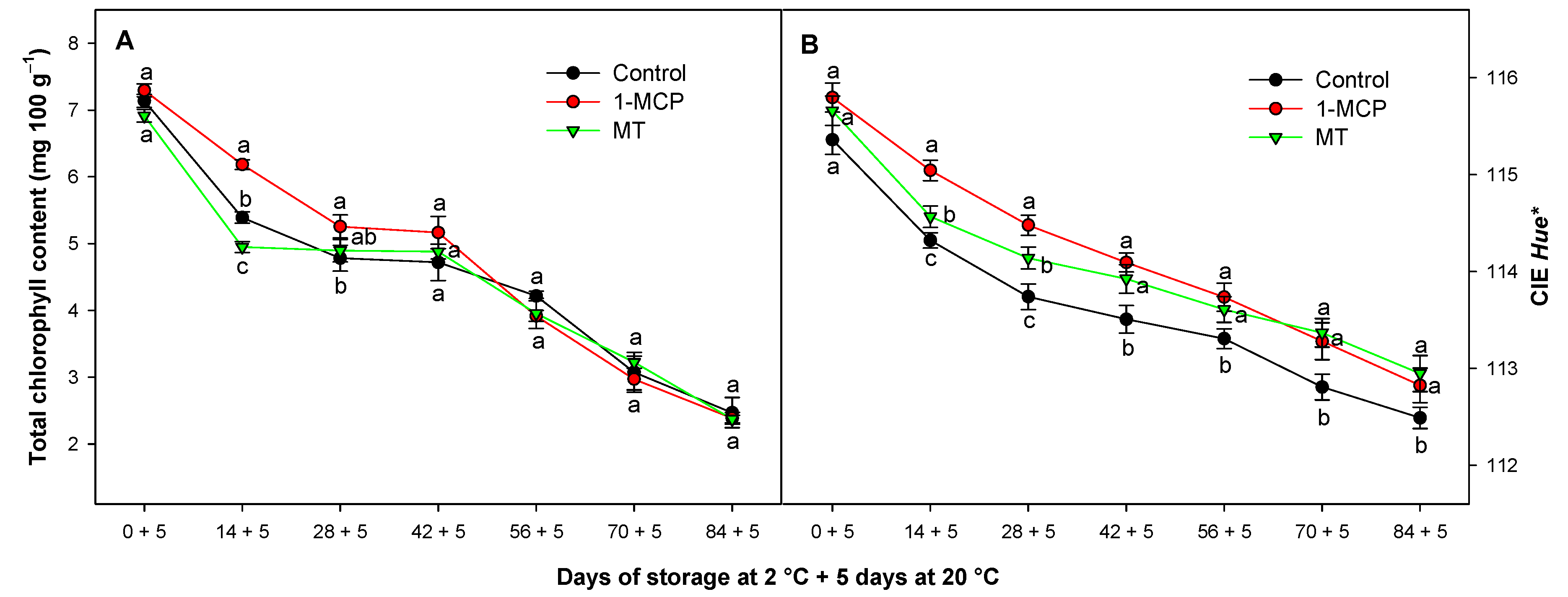
3.4. Effect of Exogenous 1-MCP and MT on Chlorophyll Content and Internal Colour
3.5. Effect of Exogenous 1-MCP and MT on EL and Chilling Injury
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Satpal, D.; Kaur, J.; Bhadariya, V.; Sharma, K. Actinidia deliciosa (Kiwi fruit): A comprehensive review on the nutritional composition, health benefits, traditional utilization, and commercialization. J. Food Process. Preserv. 2021, 45, e15588. [Google Scholar] [CrossRef]
- Wang, S.; Qiu, Y.; Zhu, F. Kiwifruit (Actinidia spp.): A review of chemical diversity and biological activities. Food Chem. 2021, 350, 128469. [Google Scholar] [CrossRef]
- Richardson, D.P.; Ansell, J.; Drummond, L.N. The nutritional and health attributes of kiwifruit: A review. Eur. J. Nutr. 2018, 57, 2659–2676. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Wang, J.; Vidyarthi, S.K.; Wang, H.; Zhang, X.G.; Gao, L.; Yang, K.W.; Zhang, J.S.; Xiao, H.W. Effects of postharvest ripening on water status and distribution, drying characteristics, volatile profiles, phytochemical contents, antioxidant capacity and microstructure of kiwifruit (Actinidia deliciosa). Food Control 2022, 139, 109062. [Google Scholar] [CrossRef]
- Yi, J.; Kebede, B.T.; Grauwet, T.; Loey, A.V.; Hu, X.; Hendrickx, M. A multivariate approach into physicochemical, biochemical and aromatic quality changes of purée based on ’Hayward’ kiwifruit during the final phase of ripening. Postharvest Biol. Technol. 2016, 117, 206–216. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, Z.; Leng, J.; Sui, Y.; Jiang, M.; Wisniewski, M.; Liu, J.; Wang, Q. Eco-friendly management of postharvest fungal decays in kiwifruit. Crit. Rev. Food Sci. Nutr. 2022, 62, 8307–8318. [Google Scholar] [CrossRef]
- Mitalo, O.W.; Tokiwa, S.; Kondo, Y.; Otsuki, T.; Galis, I.; Suezawa, K.; Kataoka, I.; Doan, A.T.; Nakano, R.; Ushijima, K.; et al. Low temperature storage stimulates fruit softening and sugar accumulation without ethylene and aroma volatile production in kiwifruit. Front. Plant Sci. 2019, 10, 888. [Google Scholar] [CrossRef] [PubMed]
- Cha, G.H.; Kumarihami, H.P.C.; Kim, H.L.; Kwack, Y.B.; Kim, J.G. Storage temperature influences fruit ripening and changes in organic acids of kiwifruit treated with exogenous ethylene. Hortic. Sci. Technol. 2019, 37, 618–629. [Google Scholar]
- Chai, J.; Wang, Y.; Liu, Y.; Gu, Z.; Liu, Z. High O2/N2 controlled atmosphere accelerates postharvest ripening of ‘Hayward’ kiwifruit. Sci. Hortic. 2022, 300, 111073. [Google Scholar] [CrossRef]
- Han, Y.; East, A.; Nicholson, S.; Jeffery, P.; Glowacz, M.; Heyes, J. Benefits of modified atmosphere packaging in maintaining ‘Hayward’ kiwifruit quality at room temperature retail conditions. N. Z. J. Crop Hortic. Sci. 2022, 50, 242–258. [Google Scholar] [CrossRef]
- Kim, K.H.; Yook, H.S. Effect of gamma irradiation on quality of kiwifruit (Actinidia deliciosa var. deliciosa cv. Hayward). Radiat. Phys. Chem. 2009, 78, 414–421. [Google Scholar] [CrossRef]
- Kumarihami, H.P.C.; Kim, Y.H.; Kwack, Y.B.; Kim, J.; Kim, J.G. Application of chitosan as edible coating to enhance storability and fruit quality of Kiwifruit: A Review. Sci. Hortic. 2022, 292, 110647. [Google Scholar] [CrossRef]
- Öztürk, B.; Yücedağ, F. Effects of methyl jasmonate on quality properties and phytochemical compounds of kiwifruit (Actinidia deliciosa cv. Hayward) during cold storage and shelf life. Turk. J. Agric. For. 2021, 45, 154–164. [Google Scholar] [CrossRef]
- Liu, Q.; Li, X.; Jin, S.; Dong, W.; Zhang, Y.; Chen, W.; Shi, L.; Cao, S.; Yang, Z. γ-Aminobutyric acid treatment induced chilling tolerance in postharvest kiwifruit (Actinidia chinensis cv. Hongyang) via regulating ascorbic acid metabolism. Food Chem. 2023, 404, 134661. [Google Scholar] [CrossRef]
- Minas, I.S.; Karaoglanidis, G.S.; Manganaris, G.A.; Vasilakakis, M. Effect of ozone application during cold storage of kiwifruit on the Development of stem-end rot caused by Botrytis cinerea. Postharvest Biol. Technol. 2010, 58, 203–210. [Google Scholar] [CrossRef]
- Luo, A.; Bai, J.; Li, R.; Fang, Y.; Li, L.; Wang, D.; Zhang, L.; Liang, J.; Huang, T.; Kou, L. Effects of ozone treatment on the quality of kiwifruit during postharvest storage affected by Botrytis cinerea and Penicillium expansum. J. Phytopathol. 2019, 167, 470–478. [Google Scholar] [CrossRef]
- Cantin, C.M.; Holcroft, D.; Crisosto, C.H. Postharvest application of 1-methylcyclopropene (1-MCP) extends shelf life of kiwifruit. Acta Hortic. 2011, 913, 621–626. [Google Scholar] [CrossRef]
- Xu, F.; Liu, S.; Liu, Y.; Xu, J.; Liu, T.; Dong, S. Effectiveness of lysozyme coatings and 1-MCP treatments on storage and preservation of kiwifruit. Food Chem. 2019, 288, 201–207. [Google Scholar] [CrossRef]
- Krupa, T.; Tomala, K.; Zaraś-Januszkiewicz, E. Evaluation of Storage Quality of ‘Hardy’ Kiwifruit (Actinidia arguta): Effect of 1-MCP and Maturity Stage. Agriculture 2022, 12, 2062. [Google Scholar] [CrossRef]
- Cornacchia, R.; Amodio, M.L.; Rinaldi, R.; Colelli, G. Effect of 1-methylcyclopropene and controlled atmosphere on storage of kiwifruits. Fresh Prod. 2008, 2, 22–25. [Google Scholar]
- Koukounaras, A.; Sfakiotakis, E. Effect of 1-MCP prestorage treatment on ethylene and CO2 production and quality of ‘Hayward’ kiwifruit during shelf-life after short, medium and long term cold storage. Postharvest Biol. Technol. 2007, 46, 174–180. [Google Scholar] [CrossRef]
- Watkins, C.B. The use of 1-methylcyclopropene (1-MCP) on fruits and vegetables. Biotechnol. Adv. 2006, 24, 389–409. [Google Scholar] [CrossRef]
- Boquete, E.J.; Trinchero, G.D.; Fraschina, A.A.; Vilella, F.; Sozzi, G.O. Ripening of ‘Hayward’ kiwifruit treated with 1-methylcyclopropene after cold storage. Postharvest Biol. Technol. 2004, 32, 57–65. [Google Scholar] [CrossRef]
- Salazar, J.; Jorquera, C.; Campos-Vargas, R.; Jorgensen, C.; Zapata, P.; Infante, R. Effect of the application timing of 1-MCP on postharvest traits and sensory quality of a yellow-fleshed kiwifruit. Sci. Hortic. 2019, 244, 82–87. [Google Scholar] [CrossRef]
- Algul, B.E.; Al Shoffe, Y.; Park, D.; Miller, W.B.; Watkins, C.B. Preharvest 1-methylcyclopropene treatment enhances ‘stress-associated watercore’ dissipation in ‘Jonagold’ apples. Postharvest Biol. Technol. 2021, 181, 111689. [Google Scholar] [CrossRef]
- Huang, H.; Guo, L.; Wang, L.; Wang, H.; Ma, S.; Jiang, Y.; Qu, H. 1-Methylcyclopropene (1-MCP) slows ripening of kiwifruit and affects energy status, membrane fatty acid contents and cell membrane integrity. Postharvest Biol. Technol. 2019, 156, 110941. [Google Scholar] [CrossRef]
- Park, Y.S.; Im, M.H.; Gorinstein, S. Shelf-life extension and antioxidant activity of ‘Hayward’ kiwifruit as a result of prestorage conditioning and 1-methylcyclopropene treatment. J. Food Sci. Technol. 2015, 52, 2711–2720. [Google Scholar] [CrossRef]
- Lim, S.; Han, S.H.; Kim, J.; Lee, H.J.; Lee, J.G.; Lee, E.J. Inhibition of hardy kiwifruit (Actinidia aruguta) ripening by 1-methylcyclopropene during cold storage and anticancer properties of the fruit extract. Food Chem. 2016, 190, 150–157. [Google Scholar] [CrossRef]
- Huan, C.; Zhang, J.; Jia, Y.; Jiang, T.; Shen, S.; Zheng, X. Effect of 1-methylcyclopropene treatment on quality, volatile production and ethanol metabolism in kiwifruit during storage at room temperature. Sci. Hortic. 2020, 265, 109266. [Google Scholar] [CrossRef]
- Blankenship, S.M.; Dole, J.M. 1-Methylcyclopropene: A review. Postharvest Biol. Technol. 2003, 28, 1–25. [Google Scholar] [CrossRef]
- Zhang, A.; Zhang, Q.; Li, J.; Gong, H.; Fan, X.; Yang, Y.; Liu, X.; Yin, X. Transcriptome co-expression network analysis identifies key genes and regulators of ripening kiwifruit ester biosynthesis. BMC Plant Biol. 2020, 20, 103. [Google Scholar] [CrossRef] [PubMed]
- Debnath, B.; Islam, W.; Li, M.; Sun, Y.; Lu, X.; Mitra, S.; Hussain, M.; Liu, S.; Qiu, D. Melatonin mediates enhancement of stress tolerance in plants. Int. J. Mol. Sci. 2019, 20, 1040. [Google Scholar] [CrossRef]
- Madebo, M.P.; Hu, S.; Zheng, Y.; Jin, P. Mechanisms of chilling tolerance in melatonin treated postharvest fruits and vegetables: A review. J. Future Foods 2021, 1, 156–167. [Google Scholar] [CrossRef]
- Cao, S.; Qu, G.; Ma, C.; Ba, L.; Ji, N.; Meng, L.; Lei, J.; Wang, R. Effects of melatonin treatment on the physiological quality and cell wall metabolites in kiwifruit. Food Sci. Technol. 2021, 42, e85421. [Google Scholar] [CrossRef]
- Hu, M.; Li, J.; Rao, J. Effect of melatonin on ripening and senescence of postharvest kiwifruits. Sh. Kexue/Food Sci. 2018, 39, 226–232. [Google Scholar]
- Wang, X.; Liang, D.; Xie, Y.; Lv, X.; Wang, J.; Xia, H. Melatonin application increases accumulation of phenol substances in kiwifruit during storage. Emir. J. Food Agric. 2019, 31, 361–367. [Google Scholar]
- Liang, D.; Shen, Y.; Ni, Z.; Wang, Q.; Lei, Z.; Xu, N.; Deng, Q.; Lin, L.; Wang, J.; Lv, X.; et al. Exogenous melatonin application delays senescence of kiwifruit leaves by regulating the antioxidant capacity and biosynthesis of flavonoids. Front. Plant Sci. 2018, 9, 426. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Mujumdar, A.S.; Jin, X.; Liu, Z.L.; Zhang, Y.; Xiao, H.W. Effects of postharvest ripening on physicochemical properties, microstructure, cell wall polysaccharides contents (pectin, hemicellulose, cellulose) and nanostructure of kiwifruit (Actinidia deliciosa). Food Hydrocoll. 2021, 118, 106808. [Google Scholar] [CrossRef]
- Tavarini, S.; Degl’Innocenti, E.; Remorini, D.; Massai, R.; Guidi, L. Antioxidant capacity, ascorbic acid, total phenols and carotenoids changes during harvest and after storage of ‘Hayward’ kiwifruit. Food Chem. 2008, 107, 282–288. [Google Scholar] [CrossRef]
- Jiao, J.; Jin, M.; Liu, H.; Suo, J.; Yin, X.; Zhu, Q.; Rao, J. Application of melatonin in kiwifruit (Actinidia chinensis) alleviated chilling injury during cold storage. Sci. Hortic. 2022, 296, 110876. [Google Scholar] [CrossRef]
- Cheng, J.; Zheng, A.; Li, H.; Huan, C.; Jiang, T.; Shen, S.; Zheng, X. Effects of melatonin treatment on ethanol fermentation and ERF expression in kiwifruit cv. Bruno during postharvest. Sci. Hortic. 2022, 293, 110696. [Google Scholar] [CrossRef]
- Almeida, D.P.; Gomes, M.H. Uncoupling the sensory effects of 1-Methylcyclopropene and ripening stage on ‘Hayward’ kiwifruit. HortScience 2009, 44, 1936–1940. [Google Scholar] [CrossRef]
- Martínez-Romero, D.; Serrano, M.; Carbonell, A.; Burgos, L.; Riquelme, F.; Valero, D. Effects of postharvest putrescine treatment on extending shelf life and reducing mechanical damage in apricot. J. Food Sci. 2002, 67, 1706–1712. [Google Scholar] [CrossRef]
- Mao, L.C.; Wang, G.Z.; Zhu, C.G.; Pang, H.Q. Involvement of phospholipase D and lipoxygenase in response to chilling stress in postharvest cucumber fruits. Plant Sci. 2007, 172, 400–405. [Google Scholar] [CrossRef]
- McCollum, T.G.; McDonald, R.E. Electrolyte leakage, respiration, and ethylene production as indices of chilling injury in grapefruit. HortScience 1991, 26, 1191–1192. [Google Scholar] [CrossRef]
- Vu, H.T.; Scarlett, C.J.; Vuong, Q.V. Changes of phytochemicals and antioxidant capacity of banana peel during the ripening process; with and without ethylene treatment. Sci. Hortic. 2019, 253, 255–262. [Google Scholar] [CrossRef]
- Celano, G.; Minnocci, A.; Sebastiani, L.; D’auria, M.; Xiloyannis, C. Changes in the structure of the skin of kiwifruit in relation to water loss. J. Hortic. Sci. Biotechnol. 2009, 84, 41–46. [Google Scholar] [CrossRef]
- Burdon, J.; Clark, C. Effect of postharvest water loss on ’Hayward’ kiwifruit water status. Postharvest Biol. Technol. 2001, 22, 215–225. [Google Scholar] [CrossRef]
- Valero, D.; Serrano, M. Postharvest Biology and Technology for Preserving Fruit Quality, 1st ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Jeong, J.; Huber, D.J.; Sargent, S.A. Influence of 1-methylcyclopropene (1-MCP) on ripening and cell-wall matrix polysaccharides of avocado (Persea americana) fruit. Postharvest Biol. Technol. 2002, 25, 241–256. [Google Scholar] [CrossRef]
- Guillén, F.; Castillo, S.; Zapata, P.J.; Martínez-Romero, D.; Serrano, M.; Valero, D. Efficacy of 1-MCP treatment in tomato fruit: 1. Duration and concentration of 1-MCP treatment to gain an effective delay of postharvest ripening. Postharvest Biol. Technol. 2007, 43, 23–27. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, Y.; Chen, X.; Xu, W.; Wang, N.; Xu, F.; Wang, H.; Shao, X. Postharvest strategy combining maturity and storage temperature for 1-MCP-treated peach fruit. J. Food Process. Preserv. 2020, 44, e14388. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, Z.K.; Chai, H.K.; Cheng, N.; Yang, Y.; Wang, D.N.; Yang, T.; Cao, W. Melatonin treatment delays postharvest senescence and regulates reactive oxygen species metabolism in peach fruit. Postharvest Biol. Technol. 2016, 118, 103–110. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, H.; Sheng, K.; Liu, W.; Zheng, L. Effects of melatonin treatment on the postharvest quality of strawberry fruit. Postharvest Biol. Technol. 2018, 139, 47–55. [Google Scholar] [CrossRef]
- Onik, J.C.; Wai, S.C.; Li, A.; Lin, Q.; Sun, Q.; Wang, Z.; Duan, Y. Melatonin treatment reduces ethylene production and maintains fruit quality in apple during postharvest storage. Food Chem. 2021, 337, 127753. [Google Scholar] [CrossRef]
- Latocha, P.; Krupa, T.; Jankowski, P.; Radzanowska, J. Changes in postharvest physicochemical and sensory characteristics of hardy kiwifruit (Actinidia arguta and its hybrid) after cold storage under normal versus controlled atmosphere. Postharvest Biol. Technol. 2014, 88, 21–33. [Google Scholar] [CrossRef]
- Paniagua, A.C.; East, A.R.; Hindmarsh, J.P.; Heyes, J. Moisture loss is the major cause of firmness change during postharvest storage of blueberry. Postharvest Biol. Technol. 2013, 79, 13–19. [Google Scholar] [CrossRef]
- Gwanpua, S.G.; Zhao, M.; Jabbar, A.; Bronlund, J.E.; East, A.R. A model for firmness and low temperature-induced storage breakdown disorder of ‘Hayward’ kiwifruit in supply chain. Postharvest Biol. Technol. 2022, 185, 111789. [Google Scholar] [CrossRef]
- Mahajan, B.V.C.; Singh, K.; Dhillon, W.S. Effect of 1-methylcyclopropene (1-MCP) on storage life and quality of pear fruits. J. Food Sci. Technol. 2010, 47, 351–354. [Google Scholar] [CrossRef]
- Xu, F.; Liu, Y.; Dong, S.; Wang, S. Effect of 1-methylcyclopropene (1-MCP) on ripening and volatile compounds of blueberry fruit. J. Food Process. Preserv. 2020, 44, e14840. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, T.; Zhang, P.; Wang, Z.Y. Melatonin attenuates postharvest physiological deterioration of cassava storage roots. J. Pineal Res. 2016, 60, 424–434. [Google Scholar] [CrossRef]
- Sabban-Amin, R.; Feygenberg, O.; Belausov, E.; Pesis, E. Low oxygen and 1-MCP pretreatments delay superficial scald development by reducing reactive oxygen species (ROS) accumulation in stored ‘Granny Smith’apples. Postharvest Biol. Technol. 2011, 62, 295–304. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, H.; Jiang, H.; Xu, Y.; Cao, J.; Jiang, W. Multiple 1-MCP treatment more effectively alleviated postharvest nectarine chilling injury than conventional one-time 1-MCP treatment by regulating ROS and energy metabolism. Food Chem. 2020, 330, 127256. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Pei, H.; Jiao, J.; Jin, M.; Li, H.; Zhu, Q.; Ma, Y.; Rao, J. 1-Methylcyclopropene treatment followed with ethylene treatment alleviates postharvest chilling injury of ‘Xuxiang’ kiwifruit during low-temperature storage. Food Control 2021, 130, 108340. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Fard, J.R. Melatonin treatment attenuates postharvest decay and maintains nutritional quality of strawberry fruits (Fragaria × anannasa cv. Selva) by enhancing GABA shunt activity. Food Chem. 2017, 221, 1650–1657. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, R.; Aghdam, M.S.; Arnao, M.B.; Brecht, J.K.; Fawole, O.A.; Pareek, S. Melatonin alleviates chilling injury symptom development in mango fruit by maintaining intracellular energy and cell wall and membrane stability. Front. Nutr. 2022, 9, 936932. [Google Scholar] [CrossRef]
- Matsumoto, S.; Obara, T.; Luh, B.S. Changes in chemical constituents of kiwifruit during post-harvest ripening. J. Food Sci. 1983, 48, 607–611. [Google Scholar] [CrossRef]
- Burdon, J.; Lallu, N.; Pidakala, P.; Barnett, A. Soluble solids accumulation and postharvest performance of ‘Hayward’ kiwifruit. Postharvest Biol. Technol. 2013, 80, 1–8. [Google Scholar] [CrossRef]
- Goldberg, T.; Agra, H.; Ben-Arie, R. Quality of ‘Hayward’ kiwifruit in prolonged cold storage as affected by the stage of maturity at Harvest. Horticulturae 2021, 7, 358. [Google Scholar] [CrossRef]
- Yaman, Ö.; Bayoιndιrlι, L. Effects of an edible coating and cold storage on shelf-life and quality of cherries. LWT-Food Sci. Technol. 2002, 35, 146–150. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Jannatizadeh, A.; Luo, Z.; Paliyath, G. Ensuring sufficient intracellular ATP supplying and friendly extracellular ATP signaling attenuates stresses, delays senescence and maintains quality in horticultural crops during postharvest life. Trends Food Sci. Technol. 2018, 76, 67–81. [Google Scholar] [CrossRef]
- Gao, S.P.; Hu, K.D.; Hu, L.Y.; Li, Y.H.; Han, Y.; Wang, H.L.; Lv, K.; Liu, Y.S.; Zhang, H. Hydrogen sulfide delays postharvest senescence and plays an antioxidative role in fresh-cut kiwifruit. HortScience 2013, 48, 1385–1392. [Google Scholar] [CrossRef]
- Du, Y.; Jin, T.; Zhao, H.; Han, C.; Sun, F.; Chen, Q.; Yue, F.; Luo, Z.; Fu, M. Synergistic inhibitory effect of 1-methylcyclopropene (1-MCP) and chlorine dioxide (ClO2) treatment on chlorophyll degradation of green pepper fruit during storage. Postharvest Biol. Technol. 2021, 171, 111363. [Google Scholar] [CrossRef]
- Khanna-Chopra, R. Leaf senescence and abiotic stresses share reactive oxygen species-mediated chloroplast degradation. Protoplasma 2012, 249, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Meng, Q.K.; Liu, X.H.; Xia, X.D.; Li, R.; Zhu, X.H.; Li, S.J. Effect of 1-MCP treatment on ascorbic acid content and its quality, physiological of Actinidia chinensis. J. Chin. Inst. Food Sci. Technol. 2014, 14, 134–141. [Google Scholar]
- Fuke, Y.; Sasago, K.; Matsuoka, H. Determination of chlorophylls in kiwi fruit and their changes during ripening. J. Food Sci. 1985, 50, 1220–1223. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Yang, Q.; Zhao, Q. Exogenous melatonin delays postharvest fruit senescence and maintains the quality of sweet cherries. Food Chem. 2019, 301, 125311. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, G.; Chai, J.; Yang, Y.; Ma, F.; Liu, Z. The effect of 1-MCP on the expression of carotenoid, chlorophyll degradation, and ethylene response factors in ‘Qihong’ kiwifruit. Foods 2021, 10, 3017. [Google Scholar] [CrossRef]
- Shiri, M.A.; Ghasemnezhad, M.; Moghadam, J.F.; Ebrahimi, R. Effect of CaCl2 sprays at different fruit development stages on postharvest keeping quality of “Hayward” kiwifruit. J. Food Process. Preserv. 2016, 40, 624–635. [Google Scholar] [CrossRef]
- Malekzadeh, P.; Khosravi-Nejad, F.; Hatamnia, A.A.; Mehr, R.S. Impact of postharvest exogenous γ-aminobutyric acid treatment on cucumber fruit in response to chilling tolerance. Physiol. Mol. Biol. Plants 2017, 23, 827–836. [Google Scholar] [CrossRef]
- Wang, S.Y.; Shi, X.C.; Wang, R.; Wang, H.L.; Liu, F.; Laborda, P. Melatonin in fruit production and postharvest preservation: A review. Food Chem. 2020, 320, 126642. [Google Scholar] [CrossRef] [PubMed]
- Parkin, K.L.; Marangoni, A.; Jackman, R.L.; Yada, R.Y.; Stanley, D.W. Chilling injury. A review of possible mechanisms. J. Food Biochem. 1989, 13, 127–153. [Google Scholar] [CrossRef]
- Gwanpua, S.G.; Jabbar, A.; Zhao, M.; Heyes, J.A.; East, A.R. Investigating the potential of dual temperature storage as a postharvest management practice to mitigate chilling injury in kiwifruit. Int. J. Refrig. 2018, 86, 62–72. [Google Scholar] [CrossRef]
- Azadshahraki, F.; Jamshidi, B.; Mohebbi, S. Postharvest melatonin treatment reduces chilling injury and enhances antioxidant capacity of tomato fruit during cold storage. Adv. Hortic. Sci. 2018, 32, 299–310. [Google Scholar]
- Gao, H.; Lu, Z.; Yang, Y.; Wang, D.; Yang, T.; Cao, M.; Cao, W. Melatonin treatment reduces chilling injury in peach fruit through its regulation of membrane fatty acid contents and phenolic metabolism. Food Chem. 2018, 245, 659–666. [Google Scholar] [CrossRef]
- Jiao, W.; Xi, Y.; Cao, J.; Fan, X.; Jiang, W. Regulatory effects of CaCl2, sodium isoascorbate, and 1-methylcyclopropene on chilling injury of banana fruit at two ripening stages and the mechanisms involved. J. Food Process. Preserv. 2018, 42, e13442. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Aracil, M.C.; Guillén, F.; Ilea, M.I.M.; Martínez-Romero, D.; Lorente-Mento, J.M.; Valverde, J.M. Comparative Effect of Melatonin and 1-Methylcyclopropene Postharvest Applications for Extending ‘Hayward’ Kiwifruit Storage Life. Agriculture 2023, 13, 806. https://doi.org/10.3390/agriculture13040806
Ruiz-Aracil MC, Guillén F, Ilea MIM, Martínez-Romero D, Lorente-Mento JM, Valverde JM. Comparative Effect of Melatonin and 1-Methylcyclopropene Postharvest Applications for Extending ‘Hayward’ Kiwifruit Storage Life. Agriculture. 2023; 13(4):806. https://doi.org/10.3390/agriculture13040806
Chicago/Turabian StyleRuiz-Aracil, María Celeste, Fabián Guillén, Mihaela Iasmina Madalina Ilea, Domingo Martínez-Romero, José Manuel Lorente-Mento, and Juan Miguel Valverde. 2023. "Comparative Effect of Melatonin and 1-Methylcyclopropene Postharvest Applications for Extending ‘Hayward’ Kiwifruit Storage Life" Agriculture 13, no. 4: 806. https://doi.org/10.3390/agriculture13040806
APA StyleRuiz-Aracil, M. C., Guillén, F., Ilea, M. I. M., Martínez-Romero, D., Lorente-Mento, J. M., & Valverde, J. M. (2023). Comparative Effect of Melatonin and 1-Methylcyclopropene Postharvest Applications for Extending ‘Hayward’ Kiwifruit Storage Life. Agriculture, 13(4), 806. https://doi.org/10.3390/agriculture13040806