Abstract
Oilseed rape is frequently damaged by insect pests. Much attention is paid to the protection of oilseed rape against Brassicogethes aeneus (Coleoptera: Nitidulidae), which is one of the most significant pests of spring and winter oilseed rape. The presence of different pollen beetle species was monitored in the Czech Republic in the years 2011–2013. A minimum of 500 individuals were captured at each site. Morphometric characteristics and the morphology of male and/or female genitalia were used to determine species. B. aeneus, B. subaeneus, B. viridescens and B. coracinus were most abundant. Other species presented in oilseed rape were B. coeruleovirens, B. czwalinai, B. matronalis, B. anthracinus, Boragogethes symphyti, Cychramus luteus, Fabogethes nigrescens, Genistogethes carinulatus, Meligethes atratus, Sagitogethes maurus, and Lamiogethes atramentarius. Our main conclusion is that the reason for the presence of the pollen beetle species associated with their development into non-cruciferous plants in oilseeds is the sufficiency of pollen as food for beetles. In addition, they may occur here incidentally, as they can be transported relatively long distances by air. Accompanying species of pollen beetles probably also have a positive effect on abundance reduction in species considered to be harmful as they are hosts to parasitoids of the oilseed rape pest.
1. Introduction
Brassicogethes aeneus Fabricius, 1775 is one of the most significant pests of spring and winter oilseed rape. Treatment against this pest has been used regularly for several decades and is causing problems with resistance to the insecticide's active ingredients. Insecticide resistance testing is carried out in most European countries, including the Czech Republic (CZ). Pollen beetle populations were highly resistant to the pyrethroid lambda-cyhalothrin, according to 2014–2015 test results from CZ [1]. Relatively high proportions of the populations also showed resistance to the pyrethroid tau-fluvalinate [2]. At present, especially if the number of registered active ingredients is reduced, a reduction in the level of resistance cannot be expected. Many other pollen beetle species were present in the oilseed rape in addition to Brassicogethes spp. (Coleoptera: Nitidulidae). The larvae of all Brassicogethes species develop on Brassicaceae plants, and adults feed on the pollen of various family plants [3,4]. Usually, the pollen beetle (Brassicogethes aeneus) is considered a pest of oilseed rape, although there are several other pollen beetle species that may inflict damage as well [5].
The pollen beetles, which are the oilseed rape pests, formerly belonged to the genus Meligethes Stephens, 1830. Until recently, the genus Meligethes was one of the most numerous of the Nitidulidae family. Just in the Palearctic region, there are about 250 polinivorous species associated with plants of the families Brassicaceae, Lamiaceae, Fabaceae, and Rosaceae [6]. The classification of this genus, as well as the subfamily Meligethinae, has been revised. Currently, this genus contains many groups of species that are morphologically similar and hardly distinguishable [6]. Some species were excluded from the genus Meligethes and newly classified as separate genera: Boragogethes, Brassicogethes, Clypeogethes, Fabogethes, Genistogethes, Lamiogethes, Meligethes, Pria, and Sagittogethes [7].
The beetles of the Nitidulidae family overwinter as adults, in early spring they leave the wintering ground and there is a possibility to find them feeding on the pollen of different species of flowering plants [8]. There are many species of pollen beetles associated with Brassicaceae plants present in the Czech Republic. Namely: Brassicogethes aeneus, B. viridescens (Fabricius, 1787), B. anthracinus (C. Brisout de Barneville, 1863), B. coeruleovirens (Forster, 1849), B. coracinus (Sturm, 1845), B. czwalinai (Reitter, 1871), B. subaeneus (Sturm, 1845), B. matronalis (Audisio & Spornraft, 1990), and Clypeogethes lepidii (Miller, 1852). Other species whose larval development is not associated with Brassicaceae plants but whose adults feed on the pollen of oilseed rape are Boragogethes symphyti (Heer, 1841), Cychramus luteus (Fabricius 1787), Epurea aestiva (Linnaeus, 1758), Fabogethes nigrescens (Stephens, 1830), Genistogethes carinulatus (Förster, 1849), Lamiogethes atramentarius (Förster, 1849), Meligethes atratus (Olivier, 1790), Pria dulcamare (Scopoli, 1763), and Sagitogethes maurus (Sturm, 1845).
Brassicogethes aeneus biology is the best described. All developing stages are described according to Osborn [9]. Adults are 1.9–2.7 mm long, oval in shape, broadly rounded, and have a relatively broad head. The head and body are black with a metallic shine or green, blue, or purple. The legs are lighter, especially the front tibiae, up to dark yellow. The length of the antennae is almost as long as the width of the head. The anterior tibiae are finely toothed on the outer margin (Figure A1 in Appendix A). The middle femur lacks a tooth in the apical third. The eggs are cylindrical, 0.81 mm long, 0.29 mm wide, and whitish gray. The first instar larva is up to 4.4 mm long, and whitish with a brown to black scutellum. The pupa is about 2.35 mm long, oval-shaped, and creamy white colored. B. aeneus occurs in Europe, North Africa, and West Asia in fields and forests [10]. This species is oligophagous, their larvae are associated with species of the Brassicaceae family, especially the subfamilies Arabidae, Brassicae, and Hesperidae. It is a significant pest on all Brassicaceae plants, and its larvae develop on Brassica oleracea var. Capitata L., B. nigra (L.) W. D. J. Koch, Sinapis alba L., and S. arvensis L. [11]. They also develop on Barbarea verna (Mill.) Asch. and B. vulgaris W. T. Aiton [12] and the genera Cakile and Biscutella [10]. B. aeneus prefers Brassica rapa L. to B. napus L. after overwintering due to its earlier flowering, as stated by Cook et al. [13]. Borg [14] studied pollen beetle preferences to the host plants and shows this sequence: B. juncea (L.) Czern. > B. napus > S. alba. Kaasik et al. [15] found that beetles are more attracted to Sinapis alba than B. napus. According to Veroman et al. [16], females of the common pollen beetle prefer species of genus Brassica for laying eggs over other species of this family, and if the larvae develop on Raphanus sativus L., up to 35% of them remain undeveloped. Noronha and Mason [17], said that B. aeneus in Europe infests particularly winter oilseed rape, while spring oilseed rape is infested mainly by B. viridescens.
Brassicogethes viridescens (Fabricius, 1787) is 2.4–2.9 mm long. This species is similar to B. aeneus, and is metallic shine colored with yellowish legs and finer punctuation at the dorsal part [17]. Hoebeke & Wheeler [18] pointed out that the legs, mouthpart, and proximal antennal segments are yellowish or orange-yellow. The body is elongated-oval, and the color is variable, usually dark green, often with bluish, violet, or less often black, bronze, or copper shining. Pubescence is fine, sparse, silver or yellow, and uniformly distributed. This species is slightly distinguished from the others by the presence of a subapical tooth along the posterior margin of the middle femur [18]. Male and female genitalia bear important distinguishing marks (Figure A2). This species is oligophagous on plants of the Brassicaceae family, mainly on the genus Brassica spp. and Sinapis spp. Borg [14] shows the order of preferences for nutrient plants: B. napus > S. alba > B. juncea. The beetles were found on flowers of different plant species—Acer pseudoplatanus L., Tripleurospermum inodorum (L.) Sch.-Bip., Crataegus sp., and on plants of the family Ranunculaceae as well. Audisio et al. [19] present Diplotaxis as a host plant for larvae. Its larval biology is similar to B. aeneus [17,20] and it is associated with oilseed rape during its larval development [11] and also with species of the Brassicaceae family [21]. Females lay eggs on flower bud bases [22]. As stated by Mason et al. [23], this pollen beetle species is a significant pest of oilseed rape in the Palearctic region and an invasive pest on this plant in Canada. Osborne [9] and Sorauer [24] considered this species to be the second most important pest of oilseed rape, with B. aeneus in the first place. This species is widespread [8], especially in disturbed habitats [19]. B. viridescens (Figure A2) causes damage in North America [17]. B. aeneus and B. viridescens are considered to be the most damaging species to cruciferous crops [25]. Zimmer et al. [26] explain that the pollen beetles select pollen-rich plants in both periods—adults in the spring before they lay their eggs and new beetles in the summer after they have hatched—and also feed on pollen from plants outside the Brassicaceae family. For overwintering, they migrate to places outside the field, especially to forests or similar habitats that they prefer [27]. Although B. aeneus is considered to be a pest of oilseed rape, Alford [28] states that it can also damage the flowers of fruit species in large numbers in an attempt to obtain pollen. In contrast, the benefits of B. aeneus and B. viridescens for raspberry pollination are reported by Levesque and Levesque [29].
Brassicogethes anthracinus (C. Brisout de Barneville, 1863) adults are 1.6–2.6 mm long, black, with dense fine punctuation on the pronotum and elytra, rather matte [8]. The distinguishing features are shown in Figure A3. This species is associated with Isatis tinctoria L. by its larval development [8,21]. This species occurs mainly in dry grassland stands, dry meadows, and also in disturbed habitats [19].
Brassicogethes coeruleovirens (Forster, 1849) is a species whose bionomy is not completely known yet. The adult is 1.8–2.5 mm long, oval-shaped, and black with a blue or blue-green metallic shine [8]. Distinguishing features are shown in Figure A4.
Brassicogethes coracinus (Sturm, 1845) occurs in xerothermic habitats [30], fields, meadow edges, river banks, and shady stands [10]. Adults are 1.5–2.7 mm long [8]. The body is black or black-brown, never green, blue, or violet metallic shine [10]. The distinguishing features are shown in Figure A5. In the Brassicaceae family, it feeds on Barbarea spp., Sisymbrium spp., Brassica spp., and Sinapis spp. [10,11]. The adults were found on Sisymbrium loeselli L., Rorippa austriaca (Crantz) Besser, and also on species of the Apiaceae family [30]. Larval development takes place on Brassica spp., Sinapis spp., and Sisymbrium spp. Audisio et al. [31] and Kurochkin [32] show only S. loeselli as the larval food plant. The simultaneous presence of B. coracinus and B. aeneus adults on plants of the Rosaceae family is reported by Kurochkin [32]. B. coracinus is common in central and northern Europe [10], and Mancini et al. [10] report that it occurs throughout Europe, Turkey, Central Asia, and Siberia. Differentiation between B. coracinus and B. subaeneus is difficult due to the genital similarity of both species [31].
Brassicogethes czwalinai (Reitter, 1871) adults are 2.3–3.1 mm long and habitually very similar to B. viridescens. The anterior tibia is widest at the level of the third tooth [8]. The distinguishing features are shown in Figure A6. This species occurs in mountain and foothill areas and the larvae develop on Lunaria rediviva L. [8].
Brassicogethes subaeneus (Sturm, 1845) adults are 1.8–2.2 mm long. The body is oval-shaped, and black with a green metallic shine. The legs are dark brown, with fine teeth on the front leg's tibia [8]. The distinguishing features are shown in Figure A7. Larvae feed on Cardamine species [31], Cardaminopsis species [33], especially C. arenosa (L.) Hayek [10,34]. Adults are found on the flowers of various plants. According to Lasoń [30], they are found on Armoracia rusticana P. Gaertn., B. Mey. & Scherb., Geranium spp., Prunus spp., Filipendula ulmaria (L.) Maxim., Sorbus aucuparia L., Oenothera biennis L., and flowers of the Apiaceae family. It is abundant in lower and middle altitudes [34]. This species is considered to inhabit xerothermic habitats and is widespread, occurring in northern, central, and eastern Europe [10].
Brassicogethes matronalis (Audisio & Spornraft, 1990). The imago of this species is 2.6 mm long and dark brown [35]. It is similar to B. subaeneus [36]. During its development, larvae are associated with Hesperis matronalis L. [31]. It is similar in appearance to B. subaeneus [36]. The distinguishing features are shown in Figure A8.
Clypeogethes lepidii (Miller, 1852) is a fine-haired black beetle with a body length of 1.2–2.2 mm; antennae and legs are dark brown to black [8]. The distinguishing features are shown in Figure A9. The larvae develop on Lepidium draba L. [8]; Alyssum spp., Aurinia spp., Moricandia spp., Hesperis spp., and Mathiola spp. Baviera and Audisio [21] show it on Isatis tinctoria L. It occurs mainly in lowland meadows [19].
Boragogethes symphyti (Heer, 1841) adults are 2.2–3.0 mm long, the body is convex, oval-shaped, dark blue, rarely dark green colored, with brown legs, the front legs are lighter [8]. The distinguishing features are shown in Figure A10. This species is associated with plants of the Boraginaceae family, particularly Symphytum [7]. According to Nunberg [8], larvae develop on Symphytum officinale L.
The Cychramus luteus (Fabricius 1787) adult body is rusty brown; the pronotum and elytra with hairs in visible rows. [35]. The distinguishing features are shown in Figure A11. The larvae of this species develop in fungi of the Agaricaceae and Lycoperdaceae families, they are often present in species of the genus Armillaria and Lycoperdon, and less often, in fungi of the class Ascomycetes [37]. According to Rimšaité [38] and Schigel [39], adults are raised from larvae present in Armillaria spp. Jelínek [34] states that larvae develop in the fruiting bodies of softer wood-destroying fungi and adults are present on flowers, especially of Aruncus dioicus (Walter) Fernald. It is abundant in Europe, adults feed on the pollen on the flowers of plants around the beehives [40]. Neumann and Ritter [41] reported finding adults in hives. However, C. luteus (Fabricius, 1787) did not leave holes in the cells of the honeycomb, and neither oviposition nor the presence of larvae on the combs was detected [41].
Epurea aestiva (Linnaeus, 1758) is 3 mm long, and rusty yellow [35]. The distinguishing features are shown in Figure A12. This species is widespread throughout Europe, where adults are found on plant flowers at the edge of forests [21] and they are abundant in deciduous forests [34]. Kurochkin [32] observed adults most frequently on woody plants of the family Rosaceae—Cerasus fruticosa Pall., Malus domestica (Suckow) Borkh., Prunus avium L., Rosa majalis Herrm., Filipendula ulmaria and F. vulgaris Moench, rarely on Knautia arvensis (L.) J.M. Coult. Majka and Klimaszewski [42] consider adults to be sap-feeders. Larvae develop in the nests of bumblebees and small mammals [19,34] and probably also on moldy substrates [34]. As noted by Arbogast et al. [40], the larvae are mycetophagous. This species is widespread in Europe, Asia, and the USA, and it has also been found in Canada [42].
Fabogethes nigrescens (Stephens, 1830) adults are 1.7–2.5 mm long; black with fine punctuation on pronotum and elytra; antennae and the legs are pitch brown; and the first, second, and fourth teeth of forelegs tibia are bigger [8,11]. The distinguishing features are shown in Figure A13. The larvae are associated with plants of the Fabaceae family, in particular Trifolium spp. [43], Onobrychis spp., Ononis spp., Lotus spp. [5], Medicago spp. [44]. Niezgodziński [45] considers it to be an insignificant pest of Vicia faba L. in Poland. It is found in open habitats [46]. It is an important pollinator of Primula [47,48] and Rubus [29]. Mifsud and Audisio [44] consider it to be a pest of medicinal plants in North America.
Genistogethes carinulatus (Förster, 1849) is 1.4–2.2 mm long, with black antennae and brown legs, and the forelegs tibia are relatively narrow [8]. The distinguishing features are shown in Figure A14. This species is associated with Lotus corniculatus L. by their larval development [11,21]. It is mainly found in meadows [19]. Niezgodziński [45] considers it to be an insignificant bean pest. Duke [48] considers it to be a pest of Onobrychis viciifolia Scop.
Lamiogethes atramentarius (Förster, 1849). The imago is 2.0–2.8 mm long, oval-shaped, and black with metallic blue or green shine. Pronotum and elytra are finely punctuated. Antennae and legs are reddish-yellow. Tibiae of the foreleg are finely toothed [8]. The distinguishing features are shown in Figure A15. The nutrient plants of larvae are Galeobdolon luteum Huds., Lamium album L., and Galeopsis spp., adults are present on the flowers of various woody plants, e.g., Crataegus spp. and Prunus padus L. [49].
Meligethes atratus (Olivier, 1790) is 2.4–3.8 mm long and brownish-black. The first antennal segment is darker [8]. The distinguishing features are shown in Figure A16. It occurs at the edges of forests, road ditches, and grassy areas. Audisio et al. [19] stated its presence in disturbed habitats. Its larvae develop on species of the genus Rosa, including R. canina L. [11]. According to Audisio et al. [7], its larval development is strictly associated with the genus Rosa spp. and Rubus spp. It most likely also develops on Prunus spp., Pyrus spp., and Crataegus spp. [6]. Adults are polyphagous [11] and it is widespread throughout Europe [8,50].
The Pria dulcamare (Scopoli, 1763) imago is 1.6–1.8 mm long and rusty brown. Its body is egg-shaped or oval, less convex, elytra are shortened and 1–3 abdominal tergites are visible. The fourth tarsal segment is always shorter than the others [10,11]. This species is associated with the Solanum dulcamara L. [8,11,51,52], as well as with S. nigrum L. [11] during its larval development. Adults are also found on flower species other than Solanaceae [44].
The Sagitogethes maurus (Sturm, 1845) imago is 2.0–3.0 mm long, black colored, the legs and antennae are dark brown, and the distal part of the tibia of the front legs is distinctly widened [8]. The distinguishing features are shown in Figure A17. All species of this genus are associated with plants of the family Lamiaceae [7]. This species is associated with Salvia spp. [43]. Adults are found on the flower of Salvia pratensis L., S. verticillata L., Sambucus, Taraxacum, and Potentilla [49].
The main contribution of this work was the identification of the species spectrum of the genus Brassicogethes present in oilseed rape stands and its representation in the samples. Determining the proportion of B. aeneus and B. viridescens in the samples was particularly important. The results could help to refine the control of pollen beetles in OSR crops with insecticides, increase their effectiveness, and reduce the selection pressure inducing resistance to insecticides in pollen beetle populations. Until now, only a few authors have dealt with this issue and the available information is not very comprehensive.
2. Materials and Methods
2.1. Sampling Design and Collection of Beetles
Beetles for species identification and ecological analysis were obtained during the monitoring of pollen beetle populations for insecticide resistance in the Czech Republic. They were collected and tested according to International Resistance Action Committee (IRAC) methods for lambda-cyhalothrin No. 011 [53], BISCAYA 240 OD No. 021 [54], chlorpyrifos No. 025 [55], and indoxacarb No. 027 [56]. Beetles were collected from oilseed rape plants at the BBCH 50–60 growth stage. Samples were taken by digging diagonally across the field to include the edge of the plot and the interior. The sample from a tested site contained at least 500 individuals. The beetles were inserted in polyethylene bags with absorbent paper (to prevent water vapor from condensing on the bag walls) and oilseed rape inflorescence or other pollen sources. After collection, the bags were used to ensure adequate air supply. After laboratory testing for resistance, individuals from each site were preserved in 70% alcohol. The coordinates of the locations are shown in Table 1.

Table 1.
Coordinates of observed localities in the Czech Republic.
2.2. Species Identification
The identification of beetles was performed based on morphometric characteristics and/or using the method of dissection of the genitalia. Individuals that could not be distinguished on the basis of external characteristics were determined according to the species-specific morphology of the genitals. The Olympus SZX-61 stereomicroscope was used for the determination process. The publications by Oliverio et al. [57] and Kirk-Spriggs [11] were used as references for species identification. The scientific names of pests and other animals mentioned in this work are taken from the Fauna Europaea database [58], and the names of the pollen beetles are taken from Audisio [59] and Jelínek [35].
2.3. Ecological Analysis
The basic ecological characteristics of the monitored (sub) populations species were the presence and absence of the species in the given collection and are utilized for a simple comparison of individual localities [60]. The frequency and regularity of species occurrence are not taken into account. The basic index calculated for each site is the dominance index (D).
Furthermore, the Jaccard index (Ja’), Shannon–Weiner index (H’), Hill index (N2), E—evennes, and E’—corrected evennes index were calculated. The ComEcoPaC 1.0. software was used to calculate the ecological characteristics [61]. The species were divided into five classes of the Tischler scale determined by the percentage in the collections (localities) [60]: Eudominant—representation ˃ 10.1%, dominant—5.1–10%, subdominant—2.1–5%, recedent—1.1–2% and subrecedent—up to 1% of individuals. The dominance index makes it possible to determine whether there are strongly dominant species in the community or whether the community is more balanced. The abbreviations of indices are used by the ComEcoPack program. Explanation of abbreviations: S—number of species (species richness), N—number of specimens (abundance), SE—number of eudominant species, SD—number of dominant species, SSd—number of subdominant species, SR—number of recedent species, Ssr—number of subrecedent species, NE—abundance of eudominant species, ND—abundance of dominant species, Nsd—abundance of subdominant species, NR—abundance of recedent species, NSr—abundance of sub-recedent species, H’—Shannon—Wiener index, E—evennes, E’—corrected evennes, D—Simpson’s index, N2—Hill’s index (inverted Simpson’s index).
Eudominant and subrecedent species predominate in anthropogenically influenced biocenoses [62]. Lasoń [37], in his work on the composition of populations of the families Kateretidae and Nitidulidae, uses an extended range of dominance: this is a six-level scale and includes the following classes of dominance superdominant—over 30%, eudominant—10.0–29.9%, dominant—5.1–9.9%, subdominant—2.1–4.9%, recessive—1.1–2.0% and subrecessive—up to 1% of individuals. In this paper, this scale is only used as an alternative. The key factor in this work is the use of the Tischler scale, which is considered the standard. In the result tables, which are the output of the ComEcoPaC 1.0. software [61], the number of species represented by one (singleton), two (doubleton), or three (tripleton) individuals occurring in the collection at the given locality is also given. The Jaccard index is used to determine the faunistic similarity between pollen beetle samples collected at different localities [60]. The similarity of zoocenoses increases with an increasing index value, which can reach 1 or 100 when converted into percentages.
3. Results
3.1. Species Representation from 2011 to 2013
These pollen beetle species were present in all samples of (sub)populations collected during the years 2011 and 2013 in all collections at all localities: Brassicogethes aeneus (Fabricius, 1775), B. subaeneus (Sturm, 1845), B. coracinus (Sturm, 1845), and B. viridescens (Fabricius, 1787). B. anthracinus (C. Brisout de Barneville, 1863) was present in 2011 and 2013 years, B. coeruleovirens (Förster, 1849) in 2011–2013 years, and B. czwalinai (Reiter, 1871) only in 2013. Species with larval stages associated with non-cruciferous plants or hosted by non-plant organisms during their development were also present in some samples. Boragogethes symphyti (Heer 1841), Cychramus luteus (Fabricius 1787), Fabogethes nigrescens (Stephens, 1830), Genistogethes carinulatus (Förster, 1849), Meligethes atratus (Olivier, 1790) Sagitogethes maurus (Sturm, 1845) were present in all collections in each of the above years. In addition, Lamiogethes atramentarius (Förster, 1849) was present in 2011 and Epurea aestiva (Linnaeus, 1758) in 2012–2013.
3.1.1. Species Representation in 2011
In 2011, a total of 3348 individuals were identified. The representation of individual pollen beetle species in collections from 10 localities in 2011 is shown in Figure 1.
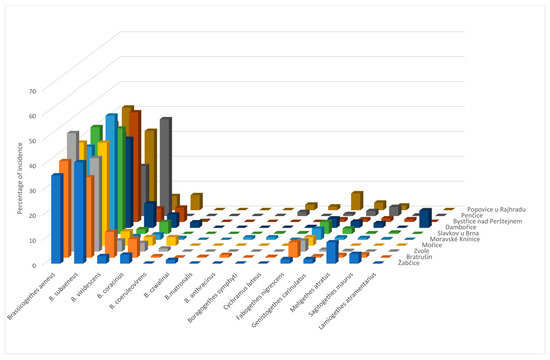
Figure 1.
Species spectrum of pollen beetles in individual localities in 2011.
The eudominant species, according to the Tischler classification, were Brassicogethes aeneus, B. subaeneus in all localities, and B. viridescens in the localities of Bratrušín and Pěnčice. The dominant species were B. viridescens in the localities of Mořice, Dambořice, Bystřice nad Pernštejnem, and Popovice u Rajhradu; B. coracinus in the localities Bratrušín, Dambořice, Popovice u Rajhradu; Fabogethes nigrescens in Bratrušín and Popovice u Rajhradu, Meligethes atratus in Žabčice and Lamiogethes atramentarius in Dambořice. Using Lason’s [37] alternative scale, the superdominant species is B. aeneus in all localities, B. subaeneus in all localities except for Pěnčice, where B. viridescens was superdominant. The values of ecological indicators and indices for individual sampling sites are presented in Table 2. The Jaccard index values calculated for individual localities (Table 3) show that the species composition is the most similar in the localities: Slavkov u Brna and Popovice u Rajhradu (0.9), Slavkov u Brna and Pěnčice (0.8), Zvole and Mořice (0.875), Zvole and Dambořice (0.8), Zvole and Bystřice nad Pernštejnem (0.889), Žabčice and Bystřice nad Pernštejnem (0.8). The lowest value of the Jaccard index (0), was calculated for the localities Bratrušín and Žabčice.

Table 2.
Ecological characteristics counted by ComEcoPaC 1.0 software for individual localities in the Czech Republic in 2011.

Table 3.
Jaccard index value of compared CZ localities in 2011.
3.1.2. Species Representation in 2012
A total of 5441 individuals were determined in 2012. Figure 2 shows the proportion of each species in the total sample of individual species. Eudominant species, according to Tischler’s classification, were Brassicogethes aeneus and B. subaeneus in all monitored localities, B. coracinus in localities Krhov and Rozsochy, Cychramus luteus in localities Popovice u Rajhradu and Kojetín, Meligethes atratus in localities Vedrovice and Němčice. The dominant species represented in the collections with 5.1–10% were B. viridescens in the localities Vranovice, Rozsíčka, Rozsochy, and Kojetín; B. coracinus at Vranovice, Radvanice, Bučovice, Rozsíčka, and Němčice; Boragogethes symphyti at Kojetín; Fabogethes nigrescens at Rozsochy and Cychramus luteus at Vranovice-Kelčice and Radvanice.
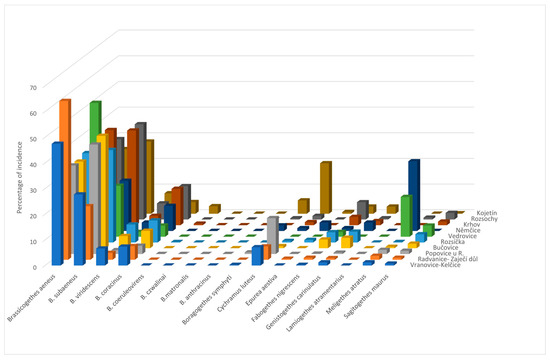
Figure 2.
Species spectrum of pollen beetles in individual localities in 2012.
Using the alternative Lason’s scale, the superdominant species (more than 30% of the collection) are B. aeneus in all localities except Němčice and Kojetín and B. subaeneus in the localities Popovice u Rajhradu, Bučovice, Rozsíčka, Krhov and Rozsochy. The values of the representation of individual species of pollen beetles and the values of ecological indicators and indices for individual collection sites are given in detail (Table 4). A comparison of Jaccard index values calculated for individual localities (Table 5) shows that the species composition clearly coincides with the localities with the index value 1, namely Vranovice-Kelčice with the localities Popovice u Rajhradu, Bučovice, and Rozsochy; then Popovice u Rajhradu with the localities Bučovice and Rozsochy. The lowest value of the Jaccard index is 0.5 for the localities Vedrovice and Kojetín.

Table 4.
Ecological characteristics counted by ComEcoPaC 1.0 software for individual localities in the Czech Republic in 2012.

Table 5.
Jaccard index value of compared CZ localities in 2012.
3.1.3. Species Representation in 2013
A total of 5779 individuals were identified in 2013. Figure 3 shows a representation of individual species in the total sample. Based on the Tischler’s classification, eudominant species in all monitored localities were Brassicogethes aeneus and B. subaeneus, B. viridescens in Prace u Brna, Rajhrad, Žabčice and Tuřany, B. coracinus in the localities Česká Třebová, Radvanice, Rosice u Brna, Vyškov, Popovice u Rajhradu and Rozsíčka and Genistogethes carinulatus in the locality Rozsíčka. The dominant species were B. coracinus in the locality of Mokrá Hora; Boragogethes symphyti in the localities of Rosice u Brna and Popovice u Rajhradu; Fabogthes nigrescens in the localities of Rosice u Brna, Vyškov, Mokrá Hora, Popovice u Rajhradu, Rajhrad, and Žabčice. The superdominant species using the alternative Lasoń’s scale [36] is B. aeneus in all localities except Rosice u Brna and Popovice u Rajhradu (there these species are eudominant according to Lasoń’s scale), B. subaeneus is superdominant in Česká Třebová, Radvanice, Rosice u Brna, Vyškov, Mokrá Hora and Popovice u Rajhradu. The values of the representation of individual species and ecological indicators and indices for individual collection sites are shown in Table 6. As the comparison of the values of the Jaccard index calculated for individual localities (Table 7) shows, it is clear that Radvanice and Rosice u Brna are identical with the value of index 1. The localities of Radvanice, Vyškov and Mokrá Hora (0.916), Rosice u Brna, Vyškov and Mokrá Hora (0.916), Popovice u Rajhradu and Vyškov (0.916) and Mokrá Hora and Žabčice (0.916) are very similar. The lowest value of the Jaccard index is 0.4 for localities Tuřany and Rozsíčka, and as the calculated values show, Tuřany is the locality with the lowest degree of similarity to other localities, Ja values reach 0.4–0.416, only when comparing Tuřany with Rajhrad is Ja 0.625.
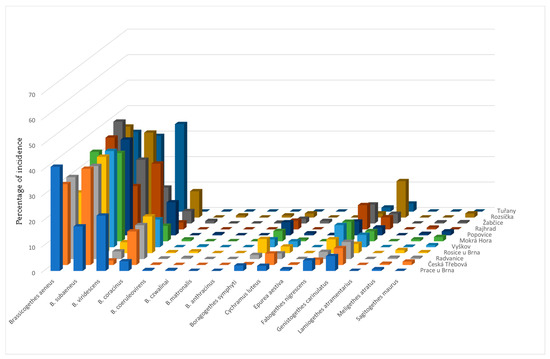
Figure 3.
Species spectrum of pollen beetles in individual localities in 2013.

Table 6.
Ecological characteristics counted by ComEcoPaC 1.0 software for individual localities in the Czech Republic in 2013.

Table 7.
Jaccard index value of compared CZ localities in 2013.
4. Discussion
Brassicogethes aeneus (Fabricius, 1775) is considered a key pest of oilseed rape and turnip rape (B. rapa) in Europe [4]; another significant species is B. viridescens (Fabricius, 1787), which is harmful in both Europe and North America. Not only is B. aeneus present in oilseeds, but other species are also common [62,63,64]. To our knowledge, few studies have focused on the species spectrum and abundance of individual pollen beetle species in oilseed rape. In Croatia Gotlin Čuljak and Juran [5] dealt with the species spectrum of pollen beetles in oilseed rape, they reported the presence of Brassicogethes aeneus, B. viridescens, B. coracinus (Sturm, 1845), Clypeogethes lepidii (L. Miller, 1851), and Fabogethes nigrescens (Stephens, 1830). B. aeneus was the eudominant species [5], the same results we obtained in 2011 and 2012. Interestingly, Clypeogethes lepidii was not present in any of the samples we analyzed. The reason for this remains unknown as we were unable to find any relevant information. B. aeneus and B. viridescens are among the most common species in the UK [3,9], both these species are also considered to be important in Sweden [65]. In Estonia, B. viridescens is very common in oilseed rape, more so in winter oilseed rape [66]. As stated by Finch et al. [67], both species mentioned above can also damage cauliflower and broccoli, reducing their market value. On the other hand, none of the listed pollen beetle species are known to be a pest of cruciferous vegetables in the Czech Republic. The species composition of pollen beetle populations present in oilseed rape fields after overwintering was studied in Hungary by Marczali [68], who found Brassicogethes aeneus in all collections, B. coracinus was in 78%, B. viridescens in 50%, M. picipes (Sturm, 1845) in 58%, M. nigrescens (Stephens, 1830) in 29%, Sagitogethes maurus (Sturm, 1845) in 43%, M. atratus (Olivier, 1790) in 21%, Odontogethes denticulatus (Heer, 1841) in 14% and Genistogethes carinulatus (Förster, 1849) in 7% of collections. Although M. picipes and M. nigrescens have been incorrectly reported as two species [68], they are in fact only one species—Fabogethes nigrescens [59]. As in the work of Marczali [68], B. aeneus was present in 100% of our collections in 2011–2013. The situation is slightly different for other species. B. coracinus was present in 90% of the samples in 2011 and 2013 and in 100% in 2012. B. viridescens was present in 100% of samples in 2011 and 2012 and in 90% of samples in 2013, Fabogethes nigrescens was present in 100% of samples in all the years observed. Sagitogethes maurus (Sturm, 1845) was present in 60% of samples in 2011, 90% in 2012, and 80% in 2013; M. atratus was present in 90% of samples in 2011, 80% in 2012, and 90% in 2013. Genistogethes carinulatus was present in 100% of samples in 2011 and 2013, and in 90% of samples in 2012. Meligethes denticulatus was absent in all samples during subsequent years. In their next study, Marczali and Keszthelyi [69] reported the presence of four pollen beetle species—M. aeneus, M. coracinus, M. viridescens and F. nigrescens in oilseed rape in Hungary. Thieme et al. [70] reported the presence of B. viridescens, F. nigrescens, B. coeruleovirens (Förster, 1849), and B. coracinus (Sturm, 1845). When studying the migration of pollen beetles from hibernacula to oilseed rape fields, Juhel et al. [71] found 99% B. aeneus and the presence of only two individuals of B. viridescens, they did not specify the species affiliation of the remaining proportion.
In other European countries, several pollen beetle species are found in oilseed rape fields like in the Czech Republic [62,63,64]. Brassicogethes aeneus is the eudominant species in the Czech Republic, but in some localities, B. subaeneus is also eudominant [62]. The treatment of oilseed rape against pollen beetles is based on reaching some of the economic threshold values. All specimens of pollen beetles present in oilseed rape are considered to be Brassicogethes aeneus (Fabricius, 1775). However, a whole complex of pollen beetles present in oilseed rape has been identified.
One of the first evaluations of the species spectrum of pollen beetles was carried out by us in 2010 [71], where B. aeneus (Fabricius, 1775) was found to be a eudominant species. B. subaeneus (Sturm, 1845), B. viridescens (Fabricius, 1787), and B. erythropus (Marsham, 1802) were also eudominant species in several localities as well. In most cases, B. subaeneus and B. viridescens were dominant or subdominant species. In the following years, pollen beetle populations were again analyzed to determine their species composition. Several pollen beetle species were present in the samples collected from 2011 to 2013 in oilseed rape in different regions of the Czech Republic. The eudominant species were usually B. aeneus, B. subaeneus, and B. viridescens. These results largely correspond to those obtained in subsequent years [62,64].
In reality, the presence of individual species is not taken into account, although the common occurrence of several pollen beetle species in oilseed rape fields has long been known, e.g., [72,73]. The determination of individual species is very time-consuming, and, with a few exceptions, requires the preparation of male and female genitalia. Knowledge of the species spectrum of pollen beetles and their bionomy could be one of the components of anti-resistance strategies. The degree of resistance of pollen beetles to insecticide active ingredients is investigated for population samples containing different species. Some authors claimed that it is not essential to know the level of resistance of other species [74]. Others, however, have considered this issue in more detail. B. viridescens is not resistant to insecticides [72]. According to Spaar et al. [22], B. aeneus is the most common species in cruciferous oilseeds fields, followed by B. viridescens, B. coracinus (Sturm, 1845), and Fabogethes nigrescens (Stephens, 1830). According to this author, only a few single specimens of B. coeruleovirens (Förster, 1849), Meligethes atratus (Olivier, 1790), Lamiogethes bidens (C. Brisout de Barneville, 1882), Sagittogethes maurus (Sturm, 1849), Meligethes flavimanus (Stephens, 1830), and Lamiogethes pedicularius (Gyllenhal, 1808) were found.
Brassicogethes aeneus is the dominant species together with B. subaeneus and B. viridescens. The genus Brassicogethes was represented in the collections from 81.8 to 90.5% of the pollen beetle population. A similar representation is also mentioned by some authors, e.g., Ouvrard et al. [3], where the representation of B. aeneus is 95%. In Lithuania, the representation is 98.2% to 98.8% [75]. In Romania, Talmaciu and Talmaciu [76] examined the fauna of pollen beetles on oilseed rape, and they reported only B. aeneus. Up to 1% of Brassicogethes coracinus and Fabogethes nigrescens were present in growth, as observed by Ouvrard et al. [3]. The presence of these species in spring and winter oilseed rape growths in Lithuania is reported—0.15% B. coracinus and 0.04% F. nigrescens [77]. In the populations collected from winter oilseed rape fields in the Czech Republic between 2011 and 2013, B. coracinus was represented by 4.21%, 7.72%, and 8.41%, (sorted by years ascending) and F. nigrescens was 3.75%, 2.42%, and 5.11%, 5.45 (sorted by years ascending). Brassicogethes viridescens occurs on all yellow-flowering cruciferous plants, but it is not as abundant as B. aeneus [11]. As stated by Derron et al. [72], its relative representation in the stands is highly variable. Ouvrard et al. [3] stated its representation in oilseed rape stands at 4%. Makūnas [77] showed its representation (average from winter and spring oilseed rape) at 0.35%, but later Makūnas [75] performed a genetic analysis of pollen beetles in Lithuania and stated the representation of this species in spring oilseed rape fields at 1.8%. The proportion of B. viridescens in our collections was 8.66% in 2011, 4.55% in 2012, and 10.51% in 2013. In 2012, it is close to the value that is reported by Derron et al. [72]. Overall, however, our results confirm the variability reported by Derron et al. [72]. In Spain, the presence of B. aeneus, B. viridescens, B. coeruleovirens, and B. coracinus in oilseed rape fields was reported by Moradillo [78].
The presence of adult Meligethes atratus in oilseed rape stands is probably due to the frequent occurrence of rose hips, a nutrient plant for larvae of this species, at field margins. In our collections, M. atratus was represented by 1.97% in 2011, 4.85% in 2012, and 0.48% in 2013. Pollen beetles actively fly into oilseed rape stands, but they also use the wind to occupy fields [79]. The pollen beetles can fly up to 13.5 km and can actively reach a distance of up to 300 m [80]. They are often more abundant at the edges of stands. They are also the first to be colonized by pollen beetles [81]. Pollen beetles are more abundant on the windward side of the field [82] and near overwintering sites [71]. The main reason for the presence of pollen beetle species associated with their development on non-cruciferous plants in oilseed stands is sufficient pollen as a food source for adults. Furthermore, as described by Ouvrard et al. [3], they can also occur here randomly if they can be transported over relatively long distances by air currents.
5. Conclusions
Several Brassicogethes species occur in oilseed rape stands. Among them, B. aeneus (Fabricius, 1775) is the eudominant species in all the sampling sites during all the years monitored. Other eudominant species, but with isolated fluctuations in abundance in some localities and some years, are B. subaeneus (Sturm, 1845), B. viridescens (Fabricius, 1787), and B. coracinus (Sturm, 1845). All species of this genus are associated with cruciferous plants by their larval development.
Thus, as our collection analysis shows, the pollen beetle species associated with cruciferous plants clearly predominated in individual years (representation in the collections was 83.7–93.4%), of which more than half were B. aeneus and B. viridescens. The pollen beetle species, whose larvae are not associated with the Brassicaceae family by their development, were represented in the collections between 9.6% and 16.3%.
From the point of view of protection of oilseed rape against pollen beetles, it is possible to ignore the fact that there are several species of pollen beetles and to work with this complex genus Brassicogethes as one species. To determine the critical number of beetles in the stand for protection purposes, it is not necessary to distinguish between pollen beetle species. On the other hand, when pollen beetle populations sampled in distinct localities are tested on susceptibility to insecticides, it would be good to know the differences in the species composition among them.
The presence of accompanying species can be beneficial, as they host some antagonists of harmful pollen beetle species, such as parasitoids, and can also be a reservoir for insect pathogens, thus indirectly contributing to bioregulation.
At the same time, as B. aeneus is treated with insecticides, other pollen beetle species present in the stand are also affected. However, as in the case of B. aeneus or the Brassicogethes species complex, it can be assumed that resistance to the insecticides used is also selected in the associated species.
Nowadays, it is important to monitor the spectrum of pollen beetle species in oilseed rape fields, but also in mustard or poppy fields. In particular, it provides early detection of harmful species of the Nitidulidae family, such as Aethina tumida Murray, 1867, an invasive species harmful to beehives. Species monitoring is also important from an ecological point of view, as it allows us to detect the effects of insecticides on non-target species and to take timely measures against the negative effects of pesticides.
Author Contributions
Conceptualization, E.H. and M.S.; methodology, E.H.; investigation, E.H. and P.T.; resources, E.H., M.S., J.P., P.T., P.K. and J.H.; writing—original draft preparation, E.H.; writing—E.H. and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Agriculture of the Czech Republic, Project No. QJ1230077.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available from the Department of Crop Science, Breeding and Plant Medicine, Mendel University in Brno.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A
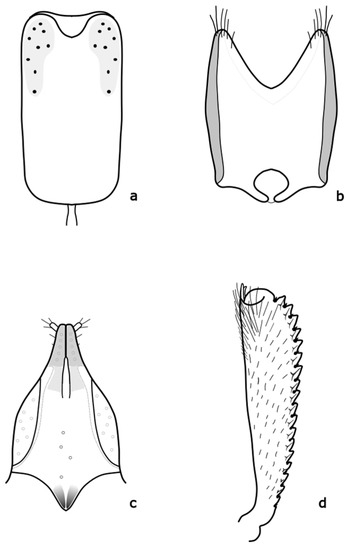
Figure A1.
Brassicogethes aeneus: (a) penis; (b) tegmen; (c) female genitalia; (d) foreleg tibia. Adapted after Kirk-Spriggs (1996) [11] and Nunberg (1976) [8].
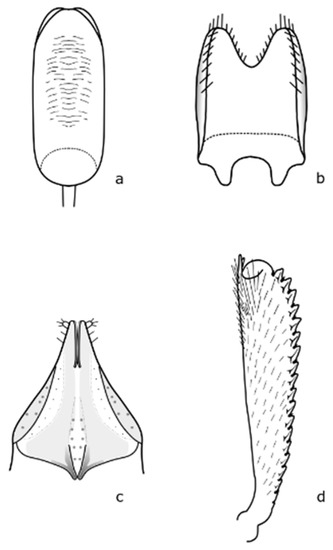
Figure A2.
B. viridescens: (a) penis; (b) tegmen; (c) female genitalia; (d) foreleg tibia. Adapted after Nunberg (1976) [8].
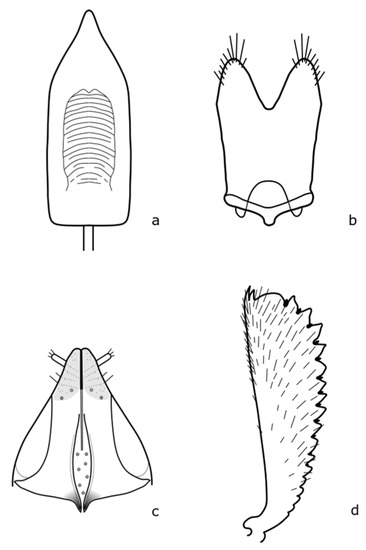
Figure A3.
B. anthracinus: (a) penis; (b) tegmen; (c) female genitalia; (d) foreleg. Adapted after Nunberg (1976) [8].
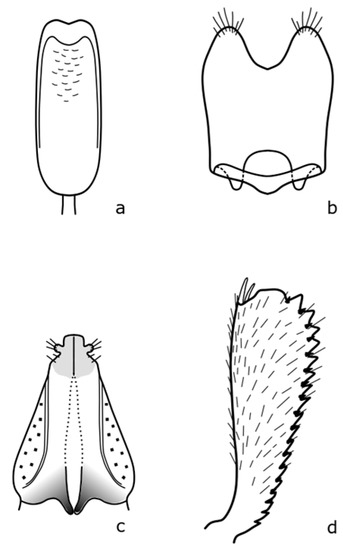
Figure A4.
B. coeruleovirens: (a) penis; (b) tegmen; (c) female genitalia; (d) foreleg. Adapted after Nunberg (1976) [8].
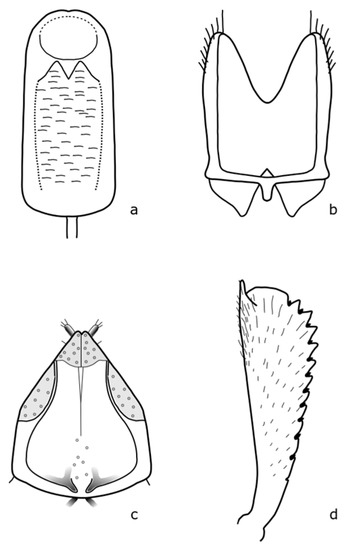
Figure A5.
B. coracinus: (a) penis; (b) tegmen; (c) female genitalia; (d) foreleg tibia. Adapted after Nunberg (1976) [8].
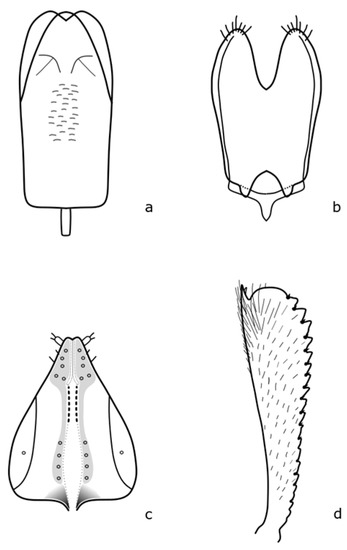
Figure A6.
B. czwalinai: (a) penis; (b) tegmen; (c) female genitalia; (d) foreleg tibia. Adapted after Nunberg (1976) [8].
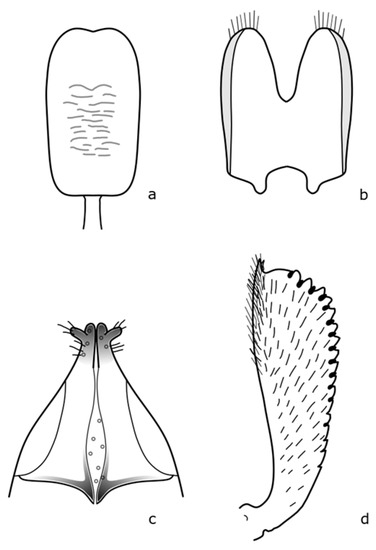
Figure A7.
B. subaeneus: (a) penis; (b) tegmen; (c) female genitalia; (d) foreleg tibia. Adapted after Nunberg (1976) [8].
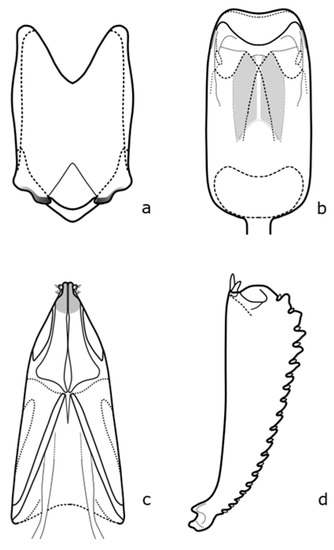
Figure A8.
B. matronalis: (a) penis; (b) tegmen; (c) female genitalia; (d) foreleg tibia. Adapted after Nunberg (1976) [8].
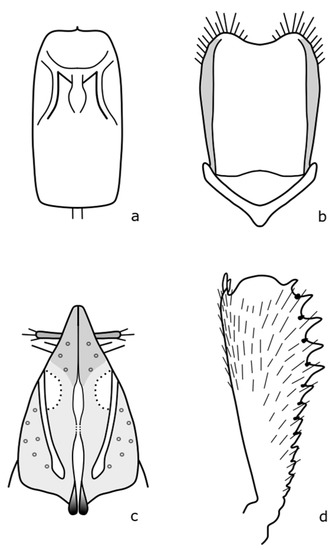
Figure A9.
B. lepidii: (a) penis; (b) tegmen; (c) female genitalia; (d) foreleg tibia. Adapted after Nunberg (1976) [8].
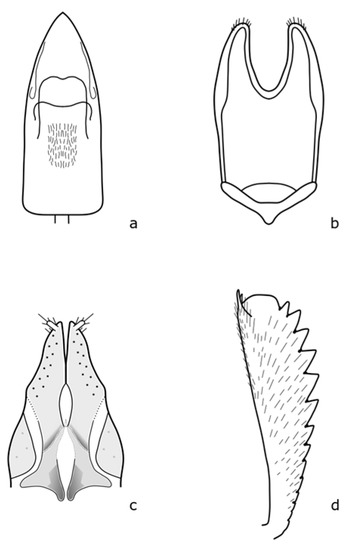
Figure A10.
Boragogethes symphyti: (a) penis; (b) tegmen; (c) antenna, (d) foreleg tibia. Adapted after Nunberg (1976) [8].
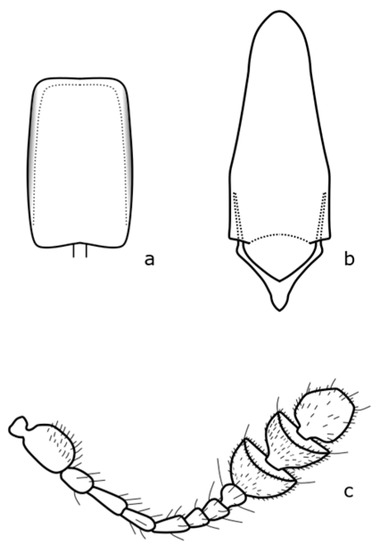
Figure A11.
Cychramus luteus: (a) penis; (b)tegmen; (c) antenna. Adapted after Nunberg (1976) [8].
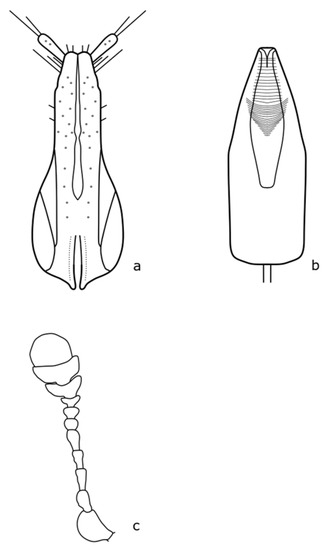
Figure A12.
Epuraea aestiva: (a) female genitalia; (b) tegmen; (c) antenna. Adapted after Nunberg (1976) [8].
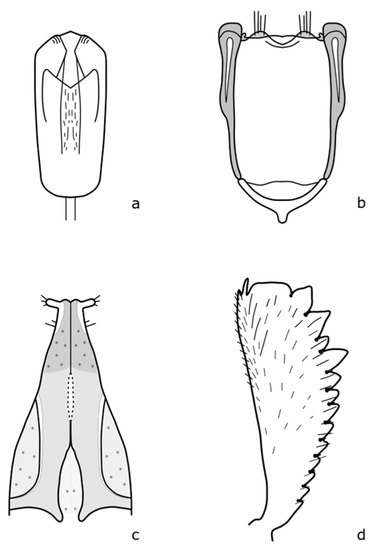
Figure A13.
Fabogethes nigrescens: (a) penis; (b) tegmen; (c) female genitalia; (d) foreleg tibia. Adapted after Nunberg (1976) [8].
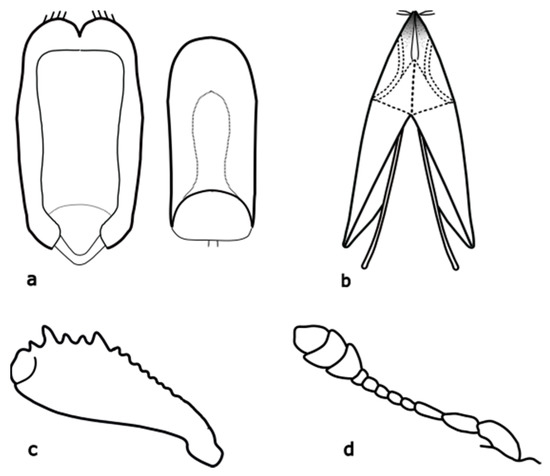
Figure A14.
Genistogethes carinulatus: (a) penis and tegmen; (b) female genitalia; (c) foreleg tibia; (d) antenna. Adapted after Von Arved Lompe [83].
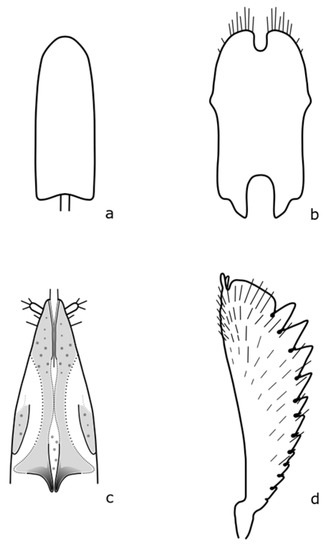
Figure A15.
Lamiogethes atramentarius: (a) penis; (b) tegmen; (c) female genitalia; (d) foreleg tibia. Adapted after Nunberg (1976) [8].
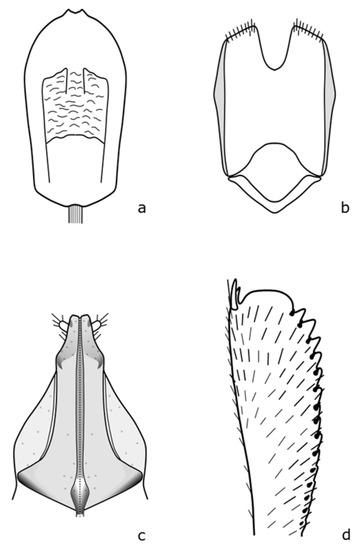
Figure A16.
Meligethes atratus: (a) penis; (b) tegmen; (c) female genitalia; (d) foreleg tibia. Adapted after Nunberg (1976) [8].
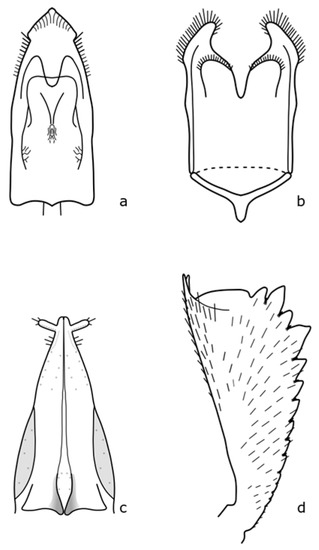
Figure A17.
Sagitogethes maurus: (a) penis; (b) tegmen; (c) female genitalia; (d) foreleg tibia. Adapted after Nunberg (1976) [8].
References
- Seidenglanz, M.; Poslušná, J.; Rotrekl, J.; Kolařík, P.; Hrudová, E.; Tóth, P.; Havel, J.; Plachká, E.; Táncik, J.; Hudec, K. Meligethes aeneus (Coleoptera: Nitidulidae) resistance to lambda-cyhalothrin in the Czech Republic in 2012 and 2013. Plant Protect. Sci. 2015, 51, 94–107. [Google Scholar] [CrossRef]
- Rubil, N.; Seidenglanz, M.; Hrudová, E.; Táncik, J.; Ruseňáková, M. Description of the situation regarding pollen beetle resistance to insecticides in the Czech Republic and Slovakia. IOBC-WPRS Bull. 2018, 36, 78–88. [Google Scholar]
- Ouvrard, P.; Hicks, D.M.; Mouland, M.; Nicholls, J.A.; Baldock, K.C.; Goddard, M.A.; Kunin, W.E.; Potts, S.G.; Thieme, T.; Veromann, E.; et al. Molecular taxonomic analysis of the plant associations of adult pollen beetles (Nitidulidae: Meligethinae), and the population structure of Brassicogethes aeneus. Genome 2016, 59, 1101–1116. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.V.P. Integrated Management of Insect Pests on Canola and Other Brassica Oilseed Crops; CABI: Wallingford, UK, 2017; Volume 408. [Google Scholar]
- Gotlin Čuljak, T.; Juran, I. Raznolikost vrsta potporodice Meligethinae u usjevima uljane repice u Hrvatskoj. Glas. Bilnje Zaštite 2014, 14, 443–449. [Google Scholar]
- Audisio, P.; Sabatelli, S.; Jelinek, J. Revision of the pollen beetle genus Meligethes (Coleoptera: Nitidulidae). Fragm. Entomol. 2014, 46, 19–112. [Google Scholar] [CrossRef]
- Audisio, P.; Cline, A.R.; De Biase, A.; Antonini, G.; Mancini, E.; Trizzino, M.; Costantini, L.; Strika, S.; Lamanna, F.; Cerretti, P. Preliminary re-examination of genus-level taxonomy of the pollen beetle subfamily Meligethinae (Coleoptera: Nitidulidae). Acta Entomol. Entomol. Entomol. Entomol. Musei Natl. Pragae 2009, 49, 341–504. [Google Scholar]
- Nunberg, M. Lyszczynkowate—Nitidulidae. Klucze do oznaczania owadów Polski. Chrzaszcze—Coleopt. 1976, XIX, 1–91. [Google Scholar]
- Osborne, P. Life History, Bionomics and Morphology of the Genus Meligethes (Coleoptera, Nitidulidae), Particularly M. aeneus (F.) and M. viri-descens (F.) and of Associated Parasites; The University of Edinburgh: Edinburgh, UK, 1957; p. 254. [Google Scholar]
- Mancini, E.; De Biase, A.; Cline, A.R.; Antonini, G.; Trizzino, M.; Clayhills, T.; Sabatelli, S.; Cerretti, P.; Audisio, P. Morphological, genetic and host-plant diversification in pollen-beetles of the Brassicogethes coracinus group (Coleoptera: Nitidulidae: Meligethinae). Rend. Lincei 2016, 27, 321–339. [Google Scholar] [CrossRef]
- Kirk-Spriggs, A.H. Pollen beetles Coleoptera: Kateretidae and Nitidulidae: Meligethinae. In Handbooks for indentification of British Insects; Royal Entomological Society: Wales, UK, 1996; Volume 5. [Google Scholar]
- Borjesdotter, D. Barbarea verna and Barbarea vulgaris as host plants for the pollen beetle (Meligethes aeneus). J. Agric. Sci. 2000, 134, 213–220. [Google Scholar] [CrossRef]
- Cook, S.M.; Rasmussen, H.B.; Birkett, M.A.; Murray, D.A.; Pye, B.J.; Watts, N.P.; Williams, I.H. Behavioural and chemical ecology underlying the success of turnip rape (Brassica rapa) trap crops in protecting oilseed rape (Brassica napus) from the pollen beetle (Meligethes aeneus). Arthropod-Plant Interact. 2007, 1, 57–67. [Google Scholar] [CrossRef]
- Borg, A. Oviposition Behaviour of Two Pollen Beetles (Meligethes aeneus and M. viridescens) on Different Host Plants. In Department of Entomology; Swedish University of Agricultural Sciences: Uppsala, Sweden, 1996. [Google Scholar]
- Kaasik, R.; Kovács, G.; Toome, M.; Metspalu, L.; Veromann, E. The relative attractiveness of Brassica napus, B. rapa, B. juncea and Sinapis alba to pollen beetles. BioControl 2014, 59, 19–28. [Google Scholar] [CrossRef]
- Veromann, E.; Kaasik, R.; Kovács, G.; Metspalu, L.; Williams, I.H.; Mänd, M. Fatal attraction: Search for a dead-end trap crop for the pollen beetle (Meligethes aeneus). Arthropod-Plant Interact. 2014, 8, 373–381. [Google Scholar] [CrossRef]
- Noronha, C.; Mason, P.G. Biology, Ecology and Management of Pollen Beetle Brassicogethes viridescens (Coleoptera: Nitidulidae). In Integrated Management of Insect Pests on Canola and Other Brassica Oilseed Crops; CABI: Wallingford, UK, 2017; p. 88. [Google Scholar]
- Hoebeke, E.; Wheeler, A. Meligethes viridescens (F.)(Coleoptera: Nitidulidae) in Maine, Nova Scotia, and Prince Edward Island: Diagnosis, distribution, and bionomics of a Palearctic species new to North America. Proc. Entomol. Soc. Wash. 1996, 98, 221–227, ISSN 0013-8797. [Google Scholar]
- Audisio, P.; Jelinek, J.; Mariotti, A.; De Biase, A. The Coleoptera Nitidulidae and Kateretidae from Anatolian, Caucasian and Middle East regions. Biogeogr.–J. Integr. Biogeogr. 2000, 21, 115. [Google Scholar] [CrossRef]
- Horowitz, A.R.; Ishaaya, I. Insect Pest Management: Field and Protected Crops; Springer: Berlin/Heidelberg, Germany, 2013; Volume 344. [Google Scholar]
- Baviera, C.; Audisio, P. The Nitidulidae and Kateretidae (Coleoptera: Cucujoidea) of Sicily: Recent records and updated checklist. Atti Della Accad. Peloritana Pericolanti-Cl. Sci. Fis. Mat. Nat. 2014, 92. [Google Scholar]
- Spaar, D.; Kleinhempel, H.; Fritzsche, R. Öl-und Faserpflanzen; Springer: Berlin/Heidelberg, Germany, 2013; Volume 248. [Google Scholar]
- Mason, P.; Olfert, O.; Sluchinski, L.; Weiss, R.; Boudreault, C.; Grossrieder, M.; Kuhlmann, U. Actual and potential distribution of an invasive canola pest, Meligethes viridescens (Coleoptera: Nitidulidae), in Canada. Can. Entomol. 2003, 135, 405–413. [Google Scholar] [CrossRef]
- Sorauer, P. Handbuch der Pflanzenkrankheiten: 3. Band-Die Tierischen Feinde; PACs Verlag GmbH: Breisgau, Germany, 2013. [Google Scholar]
- McKinlay, R.G. Vegetable Crop Pests; Palgrave Macmillan: London, UK, 1992. [Google Scholar]
- Zimmer, C.T.; Kohler, H.; Nauen, R. Baseline susceptibility and insecticide resistance monitoring in European populations of Meligethes aeneus and Ceutorhynchus assimilis collected in winter oilseed rape. Entomol. Exp. Appl. 2014, 150, 279–288. [Google Scholar] [CrossRef]
- Rusch, A.; Valantin-Morison, M.; Roger-Estrade, J.; Sarthou, J.-P. Local and landscape determinants of pollen beetle abundance in overwintering habitats. Agric. For. Entomol. 2011, 14, 37–47. [Google Scholar] [CrossRef]
- Alford, D.V. Pests of Fruit Crops: A Colour Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Levesque, C.; Levesque, G.-Y. Epigeal and flight activity of Coleoptera in a commercial raspberry plantation and adjacent sites in southern Quebec (Canada): Introduction and Nitidulidae. Great Lakes Entomol. 1992, 25, 271. [Google Scholar]
- Lasoń, A. Meligethes jelineki AUDISIO, 1976: Nowy dla fauny Polski gatunek chrzaszcza oraz nowe dane o rozsiedleniu przedstawicieli rodziny Nitidulidae (Coleoptera) na Podlasiu. Wiadomości Entomol. 2002, 21, 205–212. [Google Scholar]
- Audisio, P.; Belfiore, C.; De Biase, A.; Antonini, G. Identification of Meligethes matronalis and M. subaeneus based on morphometric and ecological characters (Coleoptera: Nitidulidae). Eur. J. Entomol. 2001, 98, 87–97. [Google Scholar] [CrossRef]
- Kurochkin, A.S. Fauna and bionomy of sap beetles (Coleoptera, Nitidulidae) and kateretid beetles (Coleoptera, Kateretidae) of Krasnosamarskoe forestry farm (Samara region, Russia). Vestn. Samara Univ. Nat. Sci. Ser. 2007, 8, 120–128. [Google Scholar]
- De Biase, A.; Antonini, G.; Mancini, E.; Trizzino, M.; Cline, A.; Audisio, P.A. Discordant patterns in the genetic, ecological, and morphological diversification of a recently radiated phytophagous beetle clade (Coleoptera: Nitidulidae: Meligethinae). Rendiconti Lince-Sci. Fis. Nat. 2012, 23, 207–215. [Google Scholar] [CrossRef]
- Jelínek, J. Lesknáčkovití brouci (Coleoptera: Brachypteridae, Nitidulidae) Chraněné krajinné oblasti Orlické hory. Acta Mus. Richnov. Sect. Natur. 1998, 5, 67–73. [Google Scholar]
- Jelínek, J. Icones Insectorum Europae Centralis (Coleoptera: Sphindidae, Kateretidae, Nitidulidae). Folia Heyrovskyana 2014, 21, 1–29. [Google Scholar]
- Lasoń, A. Epuraea (Epuraea) muehli REITTER, 1908 i Meligethes matronalis AUDISIO et SPORNRAFT, 1990—Nowe dla Polski gatunki chrząszczy oraz nowe dane o rozmieszczeniu Kateretidae i Nitidulidae (Coleoptera) w Polsce. Wiadomości Entomol. (Entomol. News) 2004, 23, 21–28. [Google Scholar]
- Lasoń, A. Zmiany skladu gatunkowego i liczebnosci Kateretidae i Nitidulidae [Coleoptera] jako element monitoringu ekologicznego na terenie Puszczy Bialowieskiej. Leśne Pr. Badaw. 2004, 4, 25–35. [Google Scholar]
- Rimšaitė, J. Contribution to the knowledge of insects humificators of fungi in Lithuania. Acta Zool. Litu. 2000, 10, 95–99. [Google Scholar] [CrossRef]
- Schigel, D.S. Fleshy fungi of the genera Armillaria, Pleurotus, and Grifola as habitats of Coleoptera. Karstenia 2007, 47, 37–48. [Google Scholar] [CrossRef]
- Arbogast, R.T.; Torto, B.; Willms, S.; Teal, P.E.A. Trophic Habits of Aethina tumida (Coleoptera: Nitidulidae): Their Adaptive Significance and Relevance to Dispersal. Environ. Entomol. 2009, 38, 561–568. [Google Scholar] [CrossRef]
- Neumann, P.; Ritter, W. A scientific note on the association of Cychramus luteus (Coleoptera: Nitidulidae) with honeybee (Apis mellifera) colonies. Apidologie 2004, 35, 665–666. [Google Scholar] [CrossRef]
- Majka, C.G.; Klimaszewski, J. Biodiversity, Biosystematics, and Ecology of Canadian Coleoptera; Pensoft Publishers: Sofia, Bulgaria, 2008; Volume 402. [Google Scholar]
- Audisio, P. The Nitidulidae and Kateretidae of Sardinia: Recent data and updated checklist (Coleoptera). In Biodiversity of Marganai and Montimannu (Sardinia). Research in the Framework of the ICP Forests Network; Nardi, G., Whitmore, D., Bardiani, M., Birtele, D., Mason, F., Spada, L., Cerretti, P., Eds.; Conservazione Habitat Invertebrati: Marmirolo, Italy, 2011; Volume 5, pp. 447–460. [Google Scholar]
- Mifsud, D.; Audisio, P. The Kateretidae and Nitidulidae of the Maltese Archipelago (Coleoptera). Bull. Entomol. Soc. Malta 2008, 1, 15–37. [Google Scholar]
- Niezgodziński, P. New possibilities of controlling some pests of field and broad beans. Ochr. Roślin 1988, 32, 6–9. [Google Scholar]
- Majka, C.G. Beetles in old growth forests: Perspectives from the Townshend Woodlot, Prince Edward Island. J. Acadian Entomol. Soc. 2010, 6, 39–43. [Google Scholar]
- Ford, E.B. Ecological Genetics; Springer: Dordrecht, The Netherlands, 2012; Volume 442. [Google Scholar]
- Duke, J. Handbook of LEGUMES of World Economic Importance; Springer: Greer, SC, USA, 2012; Volume 346. [Google Scholar]
- Burakowski, B.; Mroczkowski, M.; Stefanska, J. Chrzaszcze, Coleoptera, Cucujoidea, część 1. In Katalog Fauny Polski; PWN: Warszawa, Poland, 1986; Volume 23. [Google Scholar]
- Löbl, I.; Smetana, A. Elateroidea, Derodontoidea, Bostrichoidea, Lymexyloidea, Cleroidea and Cucujoidea; Brill: Leiden, The Netherlands, 2007. [Google Scholar]
- Kempenaar, C. Ecologie en Biologische Bestrijding van Bitterzoet (Solanum Dulcamara):[Deel I]; AB-DLO: Wageningen, The Netherlands, 1997. [Google Scholar]
- Calf, O.W.; van Dam, N.M. Bittersweet bugs: The Dutch insect community on the nightshade Solanum dulcamara. Entomol. Ber. 2012, 72, 193–198. [Google Scholar]
- IRAC. Method No. 011. Version 3. IRAC Susceptibility Test Methods Series. 2009. Available online: http://www.irac-online.org/content/uploads/Method_011_v3_june09.pdf (accessed on 5 May 2017).
- IRAC. Method No. 021. Version: 3.4. IRAC Susceptibility Test. 2011. Available online: https://irac-online.org/content/uploads/Method_021_v3.4_Oct11.pdf (accessed on 5 May 2017).
- IRAC. Method No. 025. Version: 2. IRAC Susceptibility Test. 2014. Available online: http://www.irac-online.org/methods/meligethes-species-adults-2/ (accessed on 5 May 2017).
- IRAC. Method No. 027. Version: 2.0. IRAC Susceptibility Test. 2012. Available online: http://www.irac-online.org/methods/meligethes-aeneus-adults-2/ (accessed on 5 May 2017).
- Oliverio, M.; Audisio, P.; Belfiore, C.; Antonini, G.; De Biase, A. Morphological, molecular and ecological evidence of a new Euro-Anatolian species of the Meligethes coracinus complex (Coleoptera: Nitidulidae). Insect Syst. Evol. 2000, 31, 361–385. [Google Scholar] [CrossRef]
- De Jong, Y. Fauna Europaea—All European animal species on the web. Biodivers. Data J. 2014, 2, e4034. [Google Scholar] [CrossRef]
- Audisio, P. Fauna Europaea: Coleoptera: Nitidulidae. Fauna Europaea Version 2.6. Available online: http://www.fauna-eu.org (accessed on 6 December 2016).
- Losos, B. Ekologie živočichů; Státní pedagogické nakladatelství: Czechoslovakia, Praha, Czech Republic, 1984. [Google Scholar]
- Drozd, P. ComEcoPaC—Community Ecology Parameter Calculator. Version 1. 2010. Available online: http://prf.osu.cz/kbe/dokumenty/sw/ComEcoPaC/ComEcoPaC.xls (accessed on 7 February 2017).
- Tóth, P.; Hrudová, E.; Sapáková, E.; Závadská, E.; Seidenglanz, M. Species of the genus Meligethes occurring in oil-seed crop fields in the Czech Republic. Plant Prot. Sci. 2013, 49, 177–186. [Google Scholar] [CrossRef]
- Tóth, P.; Hrudová, E.; Sapáková, E.; Seidenglanz, M.; Poslušná, J. Species spectrum of pollen beetles from genus Meligethes at selected localities of the Czech Republic in year 2010. In MendelNet 2011; Mendel University in Brno: Brno, Czech Republic, 2011. [Google Scholar]
- Hrudová, E.; Seidenglanz, M.; Kolařík, P.; Havel, J. Druhové spektrum blýskáčků v porostech řepky na jižní Moravě. The species spectrum of pollen beetles occuring in oilseed crops in the region of South Moravia. In Prosperující Olejniny; ČZU v Praze: Skalka, Czech Republic, 2016. [Google Scholar]
- Billqvist, A.; Ekbom, B. The influence of host plant species on parasitism of pollen beetles (Meligethes spp.) by Phradis morionellus. Entomol. Exp. Appl. 2001, 98, 41–47. [Google Scholar] [CrossRef]
- Veromann, E.; Luik, A.; Metspalu, L.; Williams, I. Key pests and their parasitoids on spring and winter oilseed rape in Estonia. Entomol. Fenn. 2006, 17, 400. [Google Scholar] [CrossRef]
- Finch, S.; Collier, R.H.; Elliot, M.S. Seasonal variations in the timing of attacks of bronzed blossom beetles (Meligethes aeneus/Meligethes viridescens) on horticultural brassicas. In Brighton Crop Protection Conference, Pests and Diseases; British Crop Protection Council: Brighton, UK, 1990; pp. 349–354. [Google Scholar]
- Marczali, Z.; Keszthelyi, S. A study on Meligethes species in Keszthely, 2002. J. Cent. Eur. Agric. (Online) 2003, 4, 237–244. [Google Scholar]
- Marczali, Z.; Keszthelyi, S. Data of the ecology of the four most common Hungarian Meligethes species. Novenyvedelem 2006, 42, 135–139. [Google Scholar]
- Thieme, T.; Drbal, U.; Gloyna, K.; Hoffmann, U. Different methods of monitoring susceptibility of oilseed rape beetles to insecticides. EPPO Bull. 2008, 38, 114–117. [Google Scholar] [CrossRef]
- Juhel, A.S.; Barbu, C.M.; Franck, P.; Roger-Estrade, J.; Butier, A.; Bazot, M.; Valantin-Morison, M. Characterization of the pollen beetle, Brassicogethes aeneus, dispersal from woodlands to winter oilseed rape fields. PLoS ONE 2017, 12, e0183878. [Google Scholar] [CrossRef] [PubMed]
- Derron, J.O.; Le Clech, E.; Bezencon, N.; Goy, G. Resistance of the pollen beetles (Melighetes spp.) to pyrethroids in Western Switzeland. Rev. Suisse D’agriculture 2004, 36, 237–242. [Google Scholar]
- Eickermann, M.; Delfosse, P.; Hoffmann, L.; Beyer, M. A note on the insecticide sensitivity status of Meligethes species (Coleoptera: Nitidulidae) in Luxemburg. J. Plant Dis. Prot. 2011, 118, 134–140. [Google Scholar] [CrossRef]
- Čuljak, T.G.; Pernar, R.; Juran, I.; Ančić, M.; Bažok, R. Impact of oilseed rape crop management systems on the spatial distribution of Brassicogethes aeneus (Fabricius 1775): Implications for integrated pest management. Crop. Prot. 2016, 89, 129–138. [Google Scholar] [CrossRef]
- Makūnas, V. Idetification of pollen beetle (Brassicogethes spp.) species using molecular (sequencing) method and GENETIC diversity assesment. Optim. Ornam. Gard. Plant Assortment Technol. Environ. 2014, 5, 94–103. [Google Scholar]
- Talmaciu, M.; Talmaciu, N. Aspects regarding the biodiversity of coleopterofauna in the rape crop. Cercet. Agron. Mold. 2011, 44, 51–58. [Google Scholar] [CrossRef]
- Makūnas, V. Species diversity of pollen beetles (Meligethes sl: Coleoptera, Nitidulidae) in oilseed rape and resistance of (Meligethes aeneus (F.) to pyrethroids. In Institute of Agriculture; Aleksandras Stulginskis University: Akademija, Lithuania, 2012. [Google Scholar]
- Moradillo, J.L.V. El Cultivo Rentable de la Colza: Como Recurso Sostenible del Biodiesel; Ediciones Agrotécnicas: Madrid, Spain, 2011. [Google Scholar]
- Kaasik, R.; Watts, N.P.; Murray, D.A.; Veromann, E.; Cook, S.M. Effects of Monitoring Position and Time of Day on Pollen Beetle Numbers in Crops of Oilseed Rape; IOBC-WPRS Bulletin: Göttingen, Germany, 2013; Volume 96, pp. 123–131. [Google Scholar]
- Mauchline, A.L.; Cook, S.M.; Powell, W.; Chapman, J.W.; Osborne, J.L. Migratory flight behaviour of the pollen beetle Meligethes aeneus. Pest Manag. Sci. 2017, 73, 1076–1082. [Google Scholar] [CrossRef]
- Williams, I.H. The Infestation of Oil-Seed Rape (Brassica napus L.) by the Pests Meligethes aeneus Fab. and Ceuthorhynchus assimilis Payk; University of London: London, UK, 1977; p. 409. [Google Scholar]
- Williams, I.H.; Frearson, D.; Barari, H.; McCartney, A. Migration to and dispersal from oilseed rape by the pollen beetle, Meligethes aeneus, in relation to wind direction. Agric. For. Entomol. 2007, 9, 279–286. [Google Scholar] [CrossRef]
- Von Lompe, A. Käfer Europas (Coleoptera, Nitidulidae) Gattung Meligethes—Untergattung Genistogethes (Meligethes-Erythropus-Gruppe). Available online: https://coleonet.de/coleo/texte/genistogethes.htm (accessed on 4 April 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).