Dissolved Organic Matter from Earthworm Casts Restrained the Phytotoxicity of Soil Glyphosate to Citrus (Poncirus trifoliata (L.) Raf.) Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plant Cultivation and Treatment
2.3. Plant Growth Parameters
2.4. Plant Physiological Parameters
2.5. Antioxidant Enzyme and Detoxification Enzyme Activity Assay
2.6. Statistical Analysis
3. Results
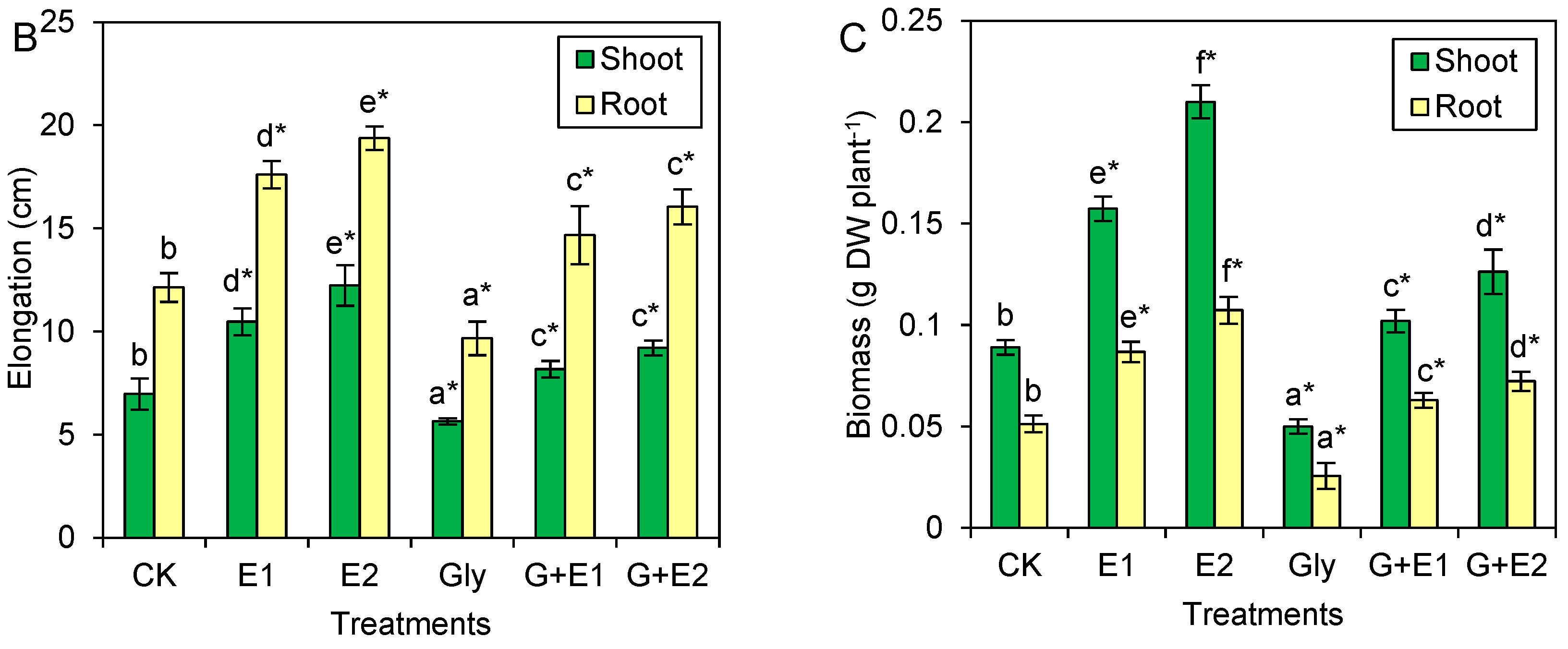
3.1. EWC-DOM Reversed Glyphosate Stress on the Growth of Citrus Plants
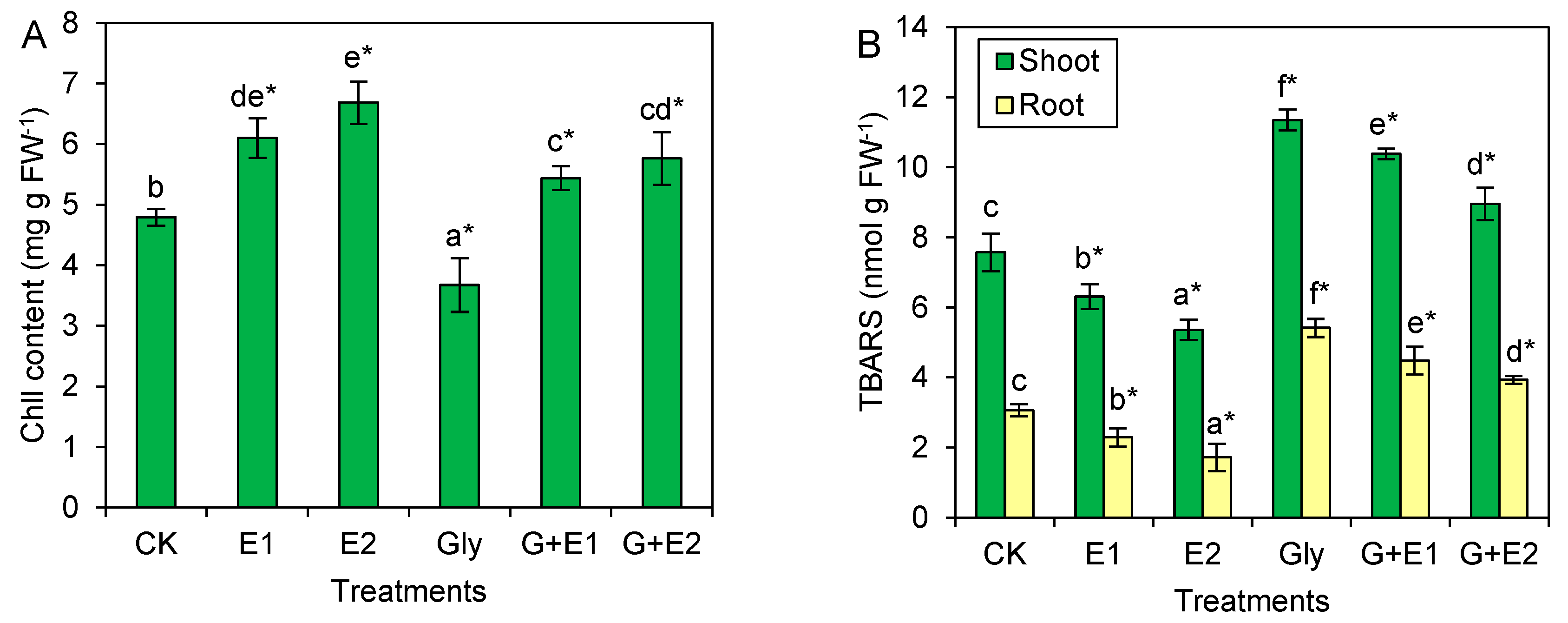
3.2. EWC-DOM Adjusted Physiological Responses of Citrus Plants under Glyphosate Stress
3.3. Effect of EWC-DOM on the Activities of Detoxifying Enzymes in Citrus Seedlings under Glyphosate Stress
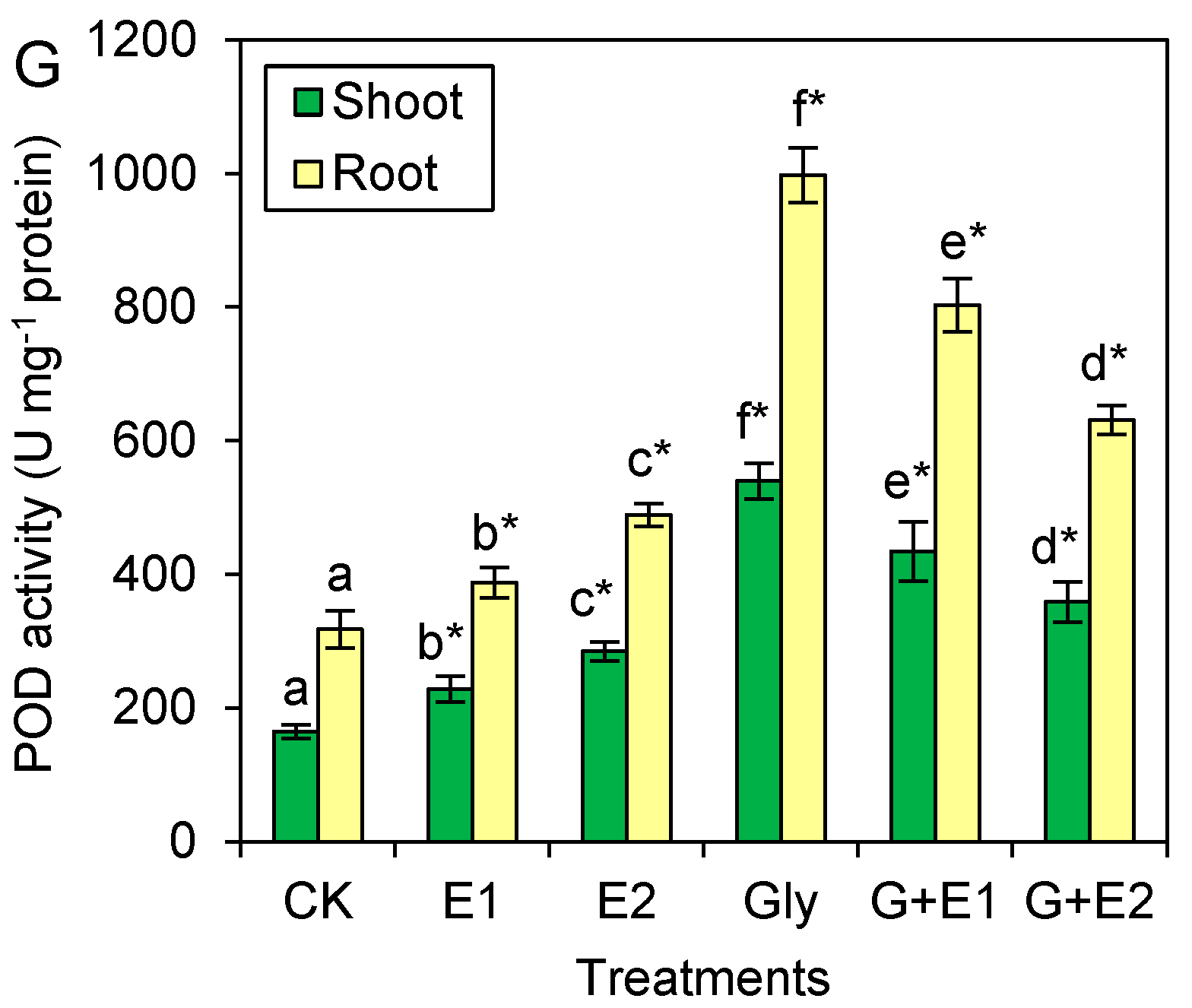
3.4. EWC-DOM Adjusted the Activities of Antioxidant Enzymes in Citrus Seedlings under Glyphosate Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zabaloy, M.C.; Allegrini, M.; Hernandez Guijarro, K.; Behrends Kraemer, F.; Morrás, H.; Erijman, L. Microbiomes and glyphosate biodegradation in edaphic and aquatic environments: Recent issues and trends. World J. Microbiol. Biotechnol. 2022, 38, 98. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, R.; Rufino, L.R., Jr.; Alcántara-de la Cruz, R.; da Conceição, P.M.; Monquero, P.A.; de Azevedo, F.A. Glyphosate Excessive Use Affects Citrus Growth and Yield, The Vicious (and Unsustainable) Circle in Brazilian Orchards. Agronomy 2022, 12, 453. [Google Scholar] [CrossRef]
- Palma-Bautista, C.; Gherekhloo, J.; Domínguez-Martínez, P.A.; Domínguez-Valenzuela, J.A.; Cruz-Hipolito, H.E.; Alcántara-de la Cruz, R.; De Prado, R. Characterization of three glyphosate resistant Parthenium hysterophorus populations collected in citrus groves from Mexico. Pestic. Biochem. Physiol. 2019, 155, 1–7. [Google Scholar] [CrossRef]
- Benbrook, C.M. Trends in glyphosate herbicide use in the United States and globally. Environ. Sci. Eur. 2016, 28, 3. [Google Scholar] [CrossRef] [PubMed]
- Primost, J.E.; Marino, D.J.; Aparicio, V.C.; Costa, J.L.; Carriquiriborde, P. Glyphosate and AMPA, “pseudo-persistent” pollutants under real-world agricultural management practices in the Mesopotamic Pampas agroecosystem Argentina. Environ. Pollut. 2017, 229, 771–779. [Google Scholar] [CrossRef]
- Guo, X.; Li, G.; Ding, X.; Zhang, J.; Ren, B.; Liu, P.; Zhang, S.; Zhao, B. Response of Leaf Senescence, Photosynthetic Characteristics, and Yield of Summer Maize to Controlled-Release Urea-Based Application Depth. Agronomy 2022, 12, 687. [Google Scholar] [CrossRef]
- Gill, J.P.K.; Sethi, N.; Mohan, A.; Datta, S.; Girdhar, M. Glyphosate toxicity for animals. Environ. Chem. Lett. 2018, 16, 401–426. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, Y.; Huang, Q.; Li, B.; Ma, T.; Qin, X.; Zhao, L.; Sun, Y.; Xu, Y. Effects of sepiolite and biochar on the photosynthetic and antioxidant systems of pakchoi under Cd and atrazine stress. J. Environ. Sci. Health Part B 2022, 57, 897–904. [Google Scholar] [CrossRef]
- Van Bruggen, A.H.; He, M.M.; Shin, K.; Mai, V.; Jeong, K.C.; Finckh, M.R.; Morris, J.G., Jr. Environmental and health effects of the herbicide glyphosate. Sci. Total Environ. 2018, 616, 255–268. [Google Scholar] [CrossRef]
- Aira, M.; Lazcano, C.; Gómez-Brandón, M.; Domínguez, J. Ageing effects of casts of Aporrectodea caliginosa on soil microbial community structure and activity. Appl. Soil Ecol. 2010, 46, 143–146. [Google Scholar] [CrossRef]
- Mupambwa, H.A.; Mnkeni, P.N.S. Optimizing the vermicomposting of organic wastes amended with inorganic materials for production of nutrient-rich organic fertilizers, a review. Environ. Sci. Pollut. Res. 2018, 25, 10577–10595. [Google Scholar] [CrossRef]
- Liu, H.; Yang, X.; Liang, C.; Li, Y.; Qiao, L.; Ai, Z.; Liu, G. Interactive effects of microplastics and glyphosate on the dynamics of soil dissolved organic matter in a Chinese loess soil. Catena 2019, 182, 104177. [Google Scholar] [CrossRef]
- Luo, S.; Ren, L.; Wu, W.; Chen, Y.; Li, G.; Zhang, W.; Lin, Z. Impacts of earthworm casts on atrazine catabolism and bacterial community structure in laterite soil. J. Hazard. Mater. 2022, 425, 127778. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.J.; Bai, J.; Li, W.; Murrell, J.C.; Zhang, Y.; Wang, J.; Li, Y. Mechanisms of the enhanced DDT removal from soils by earthworms, Identification of DDT degraders in drilosphere and non-drilosphere matrices. J. Hazard. Mater. 2021, 404, 124006. [Google Scholar] [CrossRef] [PubMed]
- Bhadauria, T.; Saxena, K.G. Role of earthworms in soil fertility maintenance through the production of biogenic structures. Appl. Environ. Soil Sci. 2010, 2010, 816073. [Google Scholar] [CrossRef]
- Tian, B.B.; Zhou, J.H.; Xie, F.; Guo, Q.N.; Zhang, A.P.; Wang, X.Q.; Yang, H. Impact of surfactant and dissolved organic matter on uptake of atrazine in maize and its mobility in soil. J. Soils Sediments 2019, 19, 599–608. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, B.J.; Liang, J.Q.; Pan, B.; Yang, Y.; Lin, Y. Reduced phytotoxicity of nonylphenol on tomato (Solanum lycopersicum L.) plants by earthworm casts. Environ. Pollut. 2020, 265, 115020. [Google Scholar] [CrossRef]
- Lechinovski, L.; Bados, M.; Rosa, J.; Moda, D.B.; Bueno Krawczyk, A.C.D. Ecotoxicological effects of conventional herbicides and a natural herbicide on freshwater fish (Danio rerio). J. Environ. Sci. Health Part B 2022, 57, 812–820. [Google Scholar] [CrossRef]
- Jiang, L.; Pan, B.; Liang, J.Q.; Wang, B.J.; Yang, Y.; Lin, Y. Earthworm casts restrained the accumulation and phytotoxicity of soil glyphosate to cowpea (Vigna unguiculata (L.) Walp.) plants. Chemosphere 2021, 279, 130571. [Google Scholar] [CrossRef]
- Jiang, L.; Yang, H. Prometryne-induced oxidative stress and impact on antioxidant enzymes in wheat. Ecotoxicol. Environ. Saf. 2009, 72, 1687–1693. [Google Scholar] [CrossRef]
- Xu, X.; Yang, B.; Qin, G.; Wang, H.; Zhu, Y.; Zhang, K.; Yang, H. Growth, accumulation, and antioxidative responses of two Salix genotypes exposed to cadmium and lead in hydroponic culture. Environ. Sci. Pollut. Res. 2019, 26, 19770–19784. [Google Scholar] [CrossRef] [PubMed]
- Banik, S.P.; Pal, S.; Ghorai, S.; Chowdhury, S.; Khowala, S. Interference of sugars in the Coomassie Blue G dye binding assay of proteins. Anal. Biochem. 2009, 386, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Strother, S. The role of free radicals in leaf senescence. Gerontology 1988, 34, 151–156. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Yamamoto, K.; Tawaratsumida, T.; Yuasa, T.; Iwaya-Inoue, M. Hydrogen peroxide scavenging regulates germination ability during wheat (Triticum aestivum L.) seed maturation. Plant Signal. Behav. 2008, 3, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Castillo, F.J.; Penel, C.; Greppin, H. Peroxidase Release Induced by Ozone in Sedum album Leaves: Involvement of Ca. Plant Physiol. 1984, 74, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Qiu, C.B.; Zhou, Y.; Jin, Z.P.; Yang, H. Bioaccumulation and degradation of pesticide fluroxypyr are associated with toxic tolerance in green alga Chlamydomonas reinhardtii. Ecotoxicology 2011, 20, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Johannes, C.; Majcherczyk, A. Laccase activity tests and laccase inhibitors. J. Biotechnol. 2000, 78, 193–199. [Google Scholar] [CrossRef]
- Guengerich, F.P.; Martin, M.V.; Sohl, C.D.; Cheng, Q. Measurement of cytochrome P450 and NADPH-cytochrome P450 reductase. Nat. Protoc. 2009, 4, 1245–1251. [Google Scholar] [CrossRef]
- Yang, C.Q.; Lu, S.; Mao, Y.B.; Wang, L.J.; Chen, X.Y. Characterization of two NADPH, cytochrome P450 reductases from cotton (Gossypium hirsutum). Phytochemistry 2010, 71, 27–35. [Google Scholar] [CrossRef]
- Suzuki, T.; Kim, S.J.; Yamauchi, H.; Takigawa, S.; Honda, Y.; Mukasa, Y. Characterization of a flavonoid 3-O-glucosyltransferase and its activity during cotyledon growth in buckwheat (Fagopyrum esculentum). Plant Sci. 2005, 169, 943–948. [Google Scholar] [CrossRef]
- Feckler, A.; Rakovic, J.; Kahlert, M.; Tröger, R.; Bundschuh, M. Blinded by the light, Increased chlorophyll fluorescence of herbicide-exposed periphyton masks unfavorable structural responses during exposure and recovery. Aquat. Toxicol. 2018, 203, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.A.; Niazi, A.K.; Akhtar, J.; Farooq, M.; Souri, Z.; Karimi, N.; Rengel, Z. Acquiring control: The evolution of ROS-Induced oxidative stress and redox signaling pathways in plant stress responses. Plant Physiol. Biochem. 2019, 141, 353–369. [Google Scholar] [CrossRef]
- Iwakami, S.; Uchino, A.; Kataoka, Y.; Shibaike, H.; Watanabe, H.; Inamura, T. Cytochrome P450 genes induced by bispyribac-sodium treatment in a multiple-herbicide-resistant biotype of Echinochloa phyllopogon. Pest Manag. Sci. 2014, 70, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.F.; Liu, J.T.; Zhang, N.; Chen, Z.J.; Yang, H. OsPAL as a key salicylic acid synthetic component is a critical factor involved in mediation of isoproturon degradation in a paddy crop. J. Clean. Prod. 2020, 262, 121476. [Google Scholar] [CrossRef]
- Nykiel-Szymańska, J.; Bernat, P.; Słaba, M. Potential of Trichoderma koningii to eliminate alachlor in the presence of copper ions. Ecotoxicol. Environ. Saf. 2018, 162, 1–9. [Google Scholar] [CrossRef]
- Zhang, J.J.; Yang, H. Metabolism and detoxification of pesticides in plants. Sci. Total Environ. 2021, 790, 148034. [Google Scholar] [CrossRef]
- Vipotnik, Z.; Michelin, M.; Tavares, T. Rehabilitation of a historically contaminated soil by different laccases and laccase-mediator system. J. Soils Sediments 2022, 22, 1546–1554. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, X.Y.; Qiu, X.M.; Li, Z.G. Methylglyoxal triggers the heat tolerance in maize seedlings by driving AsA-GSH cycle and reactive oxygen species-methylglyoxal-scavenging system. Plant Physiol. Biochem. 2019, 138, 91–99. [Google Scholar] [CrossRef]
- Shu, S.; Yuan, L.Y.; Guo, S.R.; Sun, J.; Yuan, Y.H. Effects of exogenous spermine on chlorophyll fluorescence, antioxidant system and ultrastructure of chloroplasts in Cucumis sativus L. under salt stress. Plant Physiol. Biochem. 2013, 63, 209–216. [Google Scholar] [CrossRef]
- Li, L.; Yi, H. Effect of sulfur dioxide on ROS production, gene expression and antioxidant enzyme activity in Arabidopsis plants. Plant Physiol. Biochem. 2012, 58, 46–53. [Google Scholar] [CrossRef]
- Lukacova, Z.; Bokor, B.; Vavrova, S.; Soltys, K.; Vaculik, M. Divergence of reactions to arsenic (As) toxicity in tobacco (Nicotiana benthamiana) plants: A lesson from peroxidase involvement. J. Hazard. Mater. 2021, 417, 126049. [Google Scholar] [CrossRef]
- Singh, A.; Karmegam, N.; Singh, G.S.; Bhadauria, T.; Chang, S.W.; Awasthi, M.K.; Ravindran, B. Earthworms and vermicompost, an eco-friendly approach for repaying nature’s debt. Environ. Geochem. Health 2020, 42, 1617–1642. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Garg, V.K. Industrial wastes and sludges management by vermicomposting. Rev. Environ. Sci. Bio/Technol. 2011, 10, 243–276. [Google Scholar] [CrossRef]
- Le Mer, G.; Bottinelli, N.; Dignac, M.F.; Capowiez, Y.; Jouquet, P.; Mazurier, A.; Rumpel, C. Exploring the control of earthworm cast macro-and micro-scale features on soil organic carbon mineralization across species and ecological categories. Geoderma 2022, 427, 116151. [Google Scholar] [CrossRef]
- Jin, D.; Lu, W.; Ping, S.; Zhang, W.; Chen, J.; Dun, B.; Lin, M. Identification of a new gene encoding EPSPs with high glyphosate resistance from the metagenomic library. Curr. Microbiol. 2007, 55, 350–355. [Google Scholar] [CrossRef]
- Biswas, J.K.; Banerjee, A.; Rai, M.; Naidu, R.; Biswas, B.; Vithanage, M.; Meers, E. Potential application of selected metal resistant phosphate solubilizing bacteria isolated from the gut of earthworm (Metaphire posthuma) in plant growth promotion. Geoderma 2018, 330, 117–124. [Google Scholar] [CrossRef]
- Morel, C.; Tunney, H.; Plénet, D.; Pellerin, S. Transfer of phosphate ions between soil and solution: Perspectives in soil testing. J. Environ. Qual. 2000, 29, 50–59. [Google Scholar] [CrossRef]
- Vos, H.M.; Koopmans, G.F.; Beezemer, L.; de Goede, R.G.; Hiemstra, T.; van Groenigen, J.W. Large variations in readily-available phosphorus in casts of eight earthworm species are linked to cast properties. Soil Biol. Biochem. 2019, 138, 107583. [Google Scholar] [CrossRef]
- Chen, Y.; Duan, T.; Li, W.; Zhang, J.; Dong, Y.; Zhou, Y.; Zhou, Y. The effect of dissolved natural organic matter on adsorption of phenolic compounds on suspended sediments. Environ. Technol. 2022, 43, 3366–3377. [Google Scholar] [CrossRef] [PubMed]
- Botten, N.; Wood, L.J.; Werner, J.R. Glyphosate remains in forest plant tissues for a decade or more. For. Ecol. Manag. 2021, 493, 119259. [Google Scholar] [CrossRef]
- Mahapatra, K.; De, S.; Banerjee, S.; Roy, S. Pesticide mediated oxidative stress induces genotoxicity and disrupts chromatin structure in fenugreek (Trigonella foenum-graecum L.) seedlings. J. Hazard. Mater. 2019, 369, 362–374. [Google Scholar] [CrossRef]
- Sandermann, H., Jr. Higher plant metabolism of xenobiotics: The green liver’ concept. Pharmacogenetics 1994, 4, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.; Blake-Kalff, M.; Davies, E. Detoxification of xenobiotics by plants: Chemical modification and vacuolar compartmentation. Trends Plant Sci. 1997, 2, 144–151. [Google Scholar] [CrossRef]
- Schäffner, A.; Messner, B.; Langebartels, C.; Sandermann, H. Genes and enzymes for in-planta phytoremediation of air, water and soil. Acta Biotechnol. 2002, 22, 141–151. [Google Scholar] [CrossRef]
- Dimaano, N.G.; Iwakami, S. Cytochrome P450-mediated herbicide metabolism in plants, current understanding and prospects. Pest Manag. Sci. 2021, 77, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Price, C.L.; Warrilow, A.G.; Parker, J.E.; Mullins, J.G.; Nes, W.D.; Kelly, D.E.; Kelly, S.L. Novel Substrate Specificity and Temperature-Sensitive Activity of Mycosphaerella graminicola CYP51 Supported by the Native NADPH Cytochrome P450 Reductase. Appl. Environ. Microbiol. 2015, 81, 3379–3386. [Google Scholar] [CrossRef]
- Huang, M.T.; Lu, Y.C.; Zhang, S.; Luo, F.; Yang, H. Rice (Oryza sativa) laccases involved in modification and detoxification of herbicides atrazine and isoproturon residues in plants. J. Agric. Food Chem. 2016, 64, 6397–6406. [Google Scholar] [CrossRef] [PubMed]
- Devireddy, A.R.; Zandalinas, S.I.; Fichman, Y.; Mittler, R. Integration of reactive oxygen species and hormone signaling during abiotic stress. Plant J. 2021, 105, 459–476. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Aibibu, N.; Liu, Y.; Zeng, G.; Wang, X.; Chen, B.; Song, H.; Xu, L. Cadmium accumulation in vetiveria zizanioides and its effects on growth, physiological and biochemical characters. Bioresour. Technol. 2010, 101, 6297–6303. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, H.; Jiang, L.; Wang, B.; Pan, B.; Lin, Y. Dissolved Organic Matter from Earthworm Casts Restrained the Phytotoxicity of Soil Glyphosate to Citrus (Poncirus trifoliata (L.) Raf.) Plants. Agriculture 2023, 13, 1148. https://doi.org/10.3390/agriculture13061148
Feng H, Jiang L, Wang B, Pan B, Lin Y. Dissolved Organic Matter from Earthworm Casts Restrained the Phytotoxicity of Soil Glyphosate to Citrus (Poncirus trifoliata (L.) Raf.) Plants. Agriculture. 2023; 13(6):1148. https://doi.org/10.3390/agriculture13061148
Chicago/Turabian StyleFeng, Huan, Lei Jiang, Bingjie Wang, Bo Pan, and Yong Lin. 2023. "Dissolved Organic Matter from Earthworm Casts Restrained the Phytotoxicity of Soil Glyphosate to Citrus (Poncirus trifoliata (L.) Raf.) Plants" Agriculture 13, no. 6: 1148. https://doi.org/10.3390/agriculture13061148
APA StyleFeng, H., Jiang, L., Wang, B., Pan, B., & Lin, Y. (2023). Dissolved Organic Matter from Earthworm Casts Restrained the Phytotoxicity of Soil Glyphosate to Citrus (Poncirus trifoliata (L.) Raf.) Plants. Agriculture, 13(6), 1148. https://doi.org/10.3390/agriculture13061148




