Complex Profiling of Roasted Coffee Based on Origin and Production Scale
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Sample Extraction
2.2.2. Folin Ciocalteu Method for the Determination of the Total Polyphenolic Content
2.2.3. Total Antioxidant Capacity
2.2.4. FT-NIR Analysis
2.2.5. B Vitamins Analysis
2.2.6. Tocopherols Analysis
2.2.7. PAHs Analysis
2.2.8. Polyphenols Analysis
2.2.9. Moister Content Analysis
2.2.10. Statistical Evaluation
3. Results and Discussion
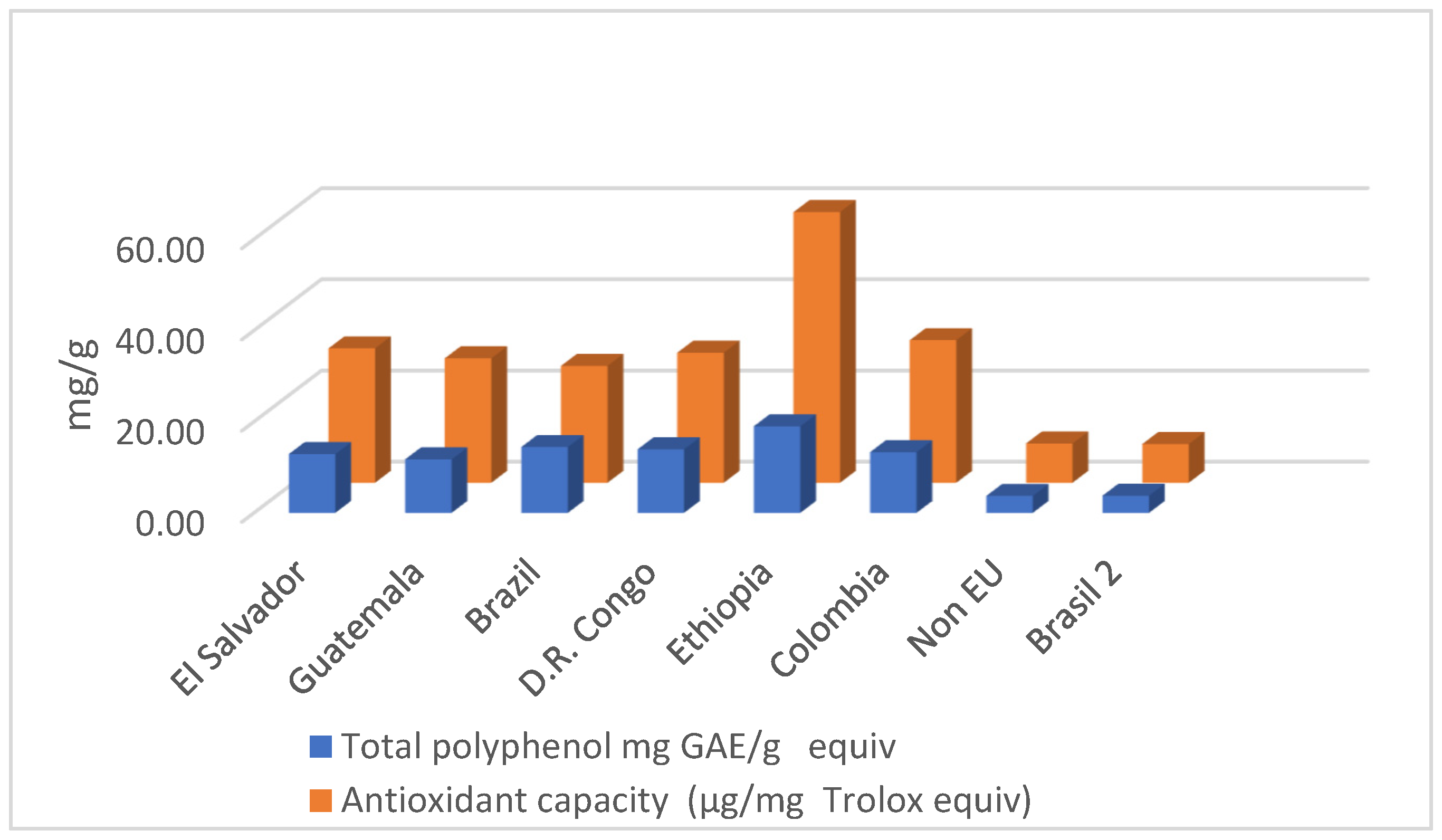
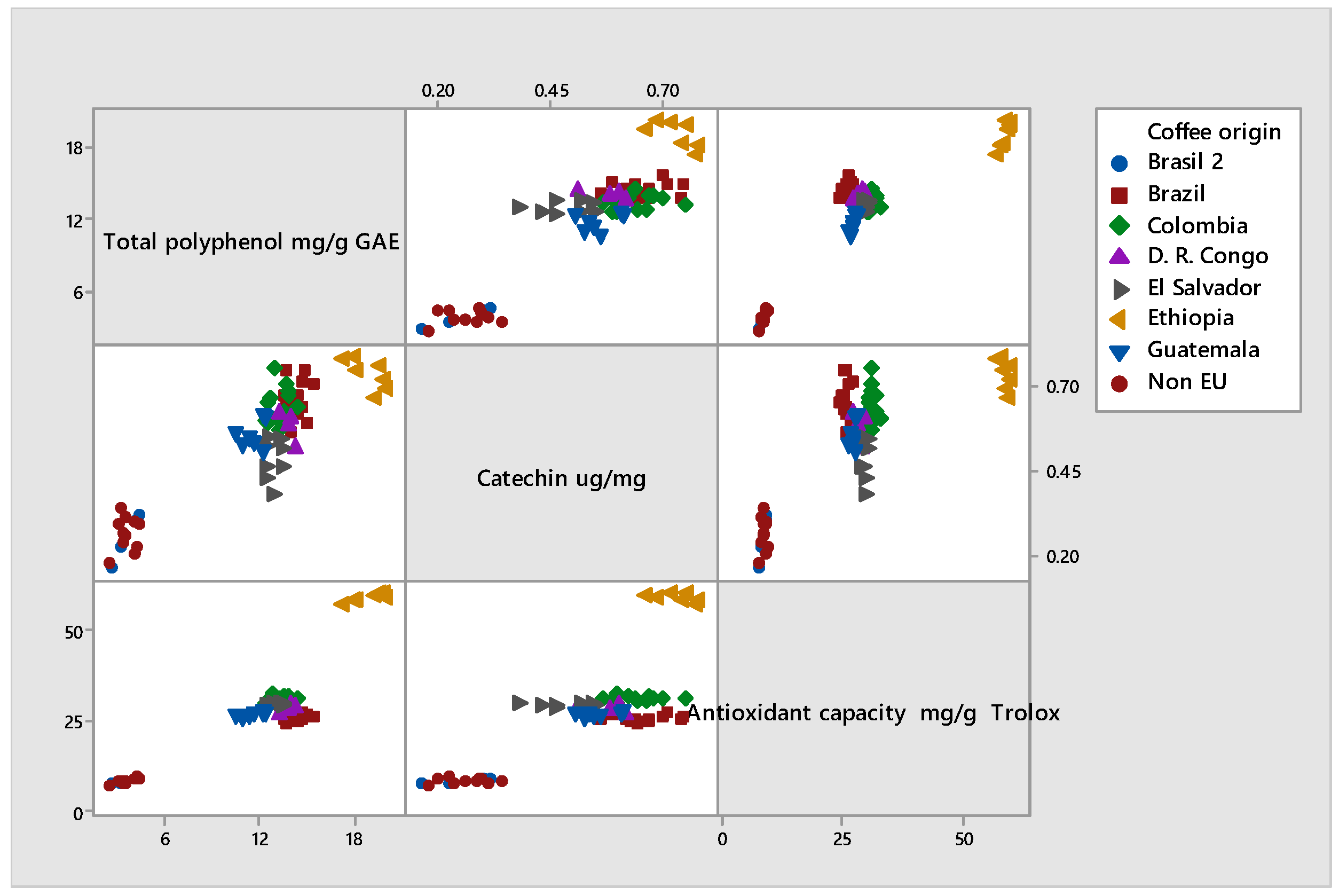
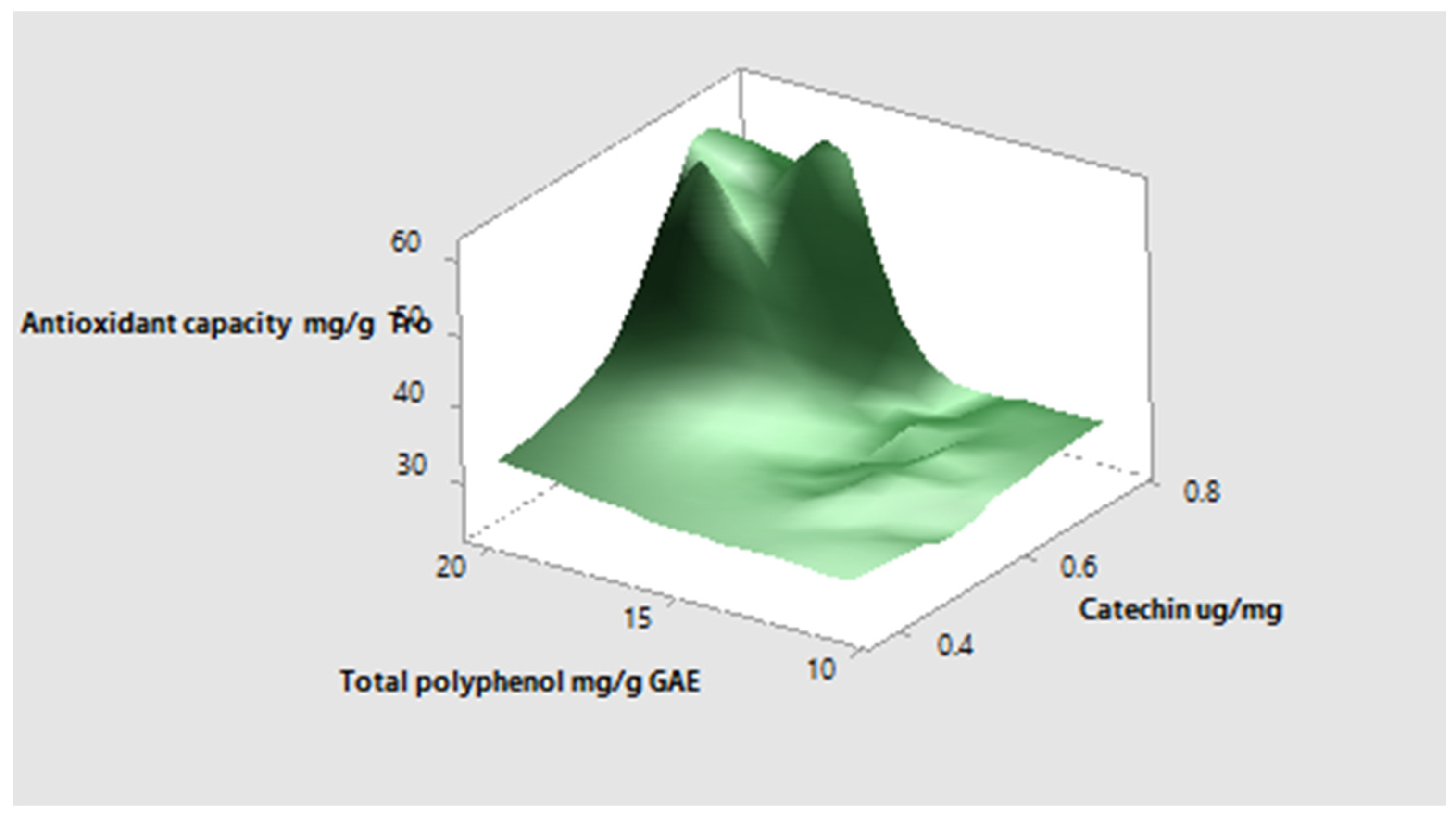
3.1. Total Phenols, Polyphenols, and Total Antioxidant Capacity
3.2. FT-NIR Analysis
3.3. Hydro- and Liposoluble Vitamins
3.4. PAHs Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Myhrvold, N. “Coffee”. Encyclopedia Britannica, Invalid Date. Available online: https://www.britannica.com/topic/coffee (accessed on 18 February 2023).
- Eurostat Database DS-016894, DS-066341. Available online: https://ec.europa.eu/eurostat/data/database (accessed on 18 February 2023).
- Palm, V. Key Figures on the European Food Chain; Publications Office of the European Union: Luxembourg, 2021; pp. 68–71. [Google Scholar] [CrossRef]
- International coffee Organization, Coffee Market Report–May 2022. Available online: https://www.ico.org/documents/cy2021-22/cmr-0522-e.pdf (accessed on 10 February 2023).
- European Coffee Federation (ECF), European Coffee Report 2018/2019. Available online: https://www.ecf-coffee.org/wp-content/uploads/2020/09/European-Coffee-Report-2018-2019.pdf (accessed on 5 February 2023).
- European Market Potential for Speciality Coffee. Available online: https://www.cbi.eu/market-information/coffee/specialty-coffee/market-potential (accessed on 25 March 2023).
- de Melo Pereira, G.V.; de Carvalho Neto, D.P.; Júnior, A.I.M.; do Prado, F.G.; Pagnoncelli, M.G.B.; Karp, S.G.; Soccol, C.R. Chapter Three—Chemical composition and health properties of coffee and coffee by-products. In Advances in Food and Nutrition Research; Toldrá, F., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 91, pp. 65–96. [Google Scholar] [CrossRef]
- Basdeki, E.D.; Argyris, A.A.; Efthymiou, O.; Athanasopoulou, E.; Sfikakis, P.P.; Protogerou, A.D.; Karatzi, K. Systematic Breakfast Consumption of Medium-Quantity and High-Quality Food Choices Is Associated with Better Vascular Health in Individuals with Cardiovascular Disease Risk Factors. Nutrients 2023, 15, 1025. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Li, C.; Wei, W.; He, D.; Zhao, Y.; Cai, Q.; Zhang, N.; Chu, X.; Shi, S.; Zhang, F. Assessing the association of coffee consumption on the relationship of chronic pain with depression and anxiety. Nutr. Neurosci. 2023. [Google Scholar] [CrossRef] [PubMed]
- Moeenfard, M.; Alves, A. New trends in coffee diterpenes research from technological to health aspects. Int. Food Res. 2020, 134, 109207. [Google Scholar] [CrossRef] [PubMed]
- Voskoboinik, A.; Koh, Y.; Kistler, P.M. Cardiovascular effects of caffeinated beverages. Trends Cardiovasc. Med. 2019, 29, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.K.; Mhd Rodzi, N.A.R. Addressing the Neuroprotective Actions of Coffee in Parkinson’s Disease: An Emerging Nutrigenomic Analysis. Antioxidants 2022, 11, 1587. [Google Scholar] [CrossRef]
- Safe, S.; Kothari, J.; Hailemariam, A.; Upadhyay, S.; Davidson, L.A.; Chapkin, R.S. Health Benefits of Coffee Consumption for Cancer and Other Diseases and Mechanisms of Action. Int. J. Mol. Sci. 2023, 24, 2706. [Google Scholar] [CrossRef]
- Hegde, S.; Shi, D.W.; Johnson, J.C.; Geesala, R.; Zhang, K.; Lin, Y.-M.; Shi, X.-Z. Mechanistic Study of Coffee Effects on Gut Microbiota and Motility in Rats. Nutrients 2022, 14, 4877. [Google Scholar] [CrossRef]
- Viencz, T.; Bonfanti Acre, L.; Barros Rocha, R.; Anastácio Alves, E.; Rostand Ramalho, A.; de Toledo Benassi, M. Caffeine, trigonelline, chlorogenic acids, melanoidins, and diterpenes contents of Coffea canephora coffees produced in the Amazon. J. Food Compos. Anal. 2023, 117, 105140. [Google Scholar] [CrossRef]
- Yamagata, K. Do Coffee Polyphenols Have a Preventive Action on Metabolic Syndrome Associated Endothelial Dysfunctions? An Assessment of the Current Evidence. Antioxidants 2018, 7, 26. [Google Scholar] [CrossRef]
- Socała, K.; Szopa, A.; Serefko, A.; Poleszak, E.; Wlaź, P. Neuroprotective Effects of Coffee Bioactive Compounds: A Review. Int. J. Mol. Sci. 2021, 22, 107. [Google Scholar] [CrossRef]
- Maksimowski, D.; Pachura, N.; Oziembłowski, M.; Nawirska-Olszańska, A.; Szumny, A. Coffee Roasting and Extraction as a Factor in Cold Brew Coffee Quality. Appl. Sci. 2022, 12, 2582. [Google Scholar] [CrossRef]
- Tarigan, E.B.; Wardiana, E.; Hilmi, Y.S.; Komarudin, N.A. The changes in chemical properties of coffee during roasting: A review. IOP Conf. Ser. Earth Environ. Sci. 2022, 974, 012115. [Google Scholar] [CrossRef]
- Muluken, B.; Shimelis, E. Effects of coffee roasting technologies on cup quality and bioactive compounds of specialty coffee beans. Food Sci. Nutr. 2020, 8, 6120–6130. [Google Scholar]
- Heo, J.; Adhikari, K.; Choi, K.S.; Lee, J. Analysis of Caffeine, Chlorogenic Acid, Trigonelline, and Volatile Compounds in Cold Brew Coffee Using High-Performance Liquid Chromatography and Solid-Phase Microextraction—Gas Chromatography-Mass Spectrometry. Foods 2020, 9, 1746. [Google Scholar] [CrossRef]
- Fuller, M.; Rao, N.Z. The Effect of Time, Roasting Temperature, and Grind Size on Caffeine and Chlorogenic Acid Concentrations in Cold Brew Coffee. Sci. Rep. 2017, 7, 17979. [Google Scholar] [CrossRef]
- El-Hawary, E.A.; Zayed, A.; Laub, A.; Modolo, L.V.; Wessjohann, L.; Farag, M.A. How Does LC/MS Compare to UV in Coffee Authentication and Determination of Antioxidant Effects? Brazilian and Middle Eastern Coffee as Case Studies. Antioxidants 2022, 11, 131. [Google Scholar] [CrossRef]
- Manwaring, C.W.; Cravino, J.A.; Patel, M.; Stathakis, J.G.H.; Soliven, A.; Suktham, T.; Shalliker, R.A. Using HPLC with In-Column Derivatization to Authenticate Coffee Samples. Molecules 2023, 28, 1651. [Google Scholar] [CrossRef]
- Núñez, N.; Collado, X.; Martínez, C.; Saurina, J.; Núñez, O. Authentication of the Origin, Variety and Roasting Degree of Coffee Samples by Non-Targeted HPLC-UV Fingerprinting and Chemometrics. Application to the Detection and Quantitation of Adulterated Coffee Samples. Foods 2020, 9, 378. [Google Scholar] [CrossRef]
- Xie, X.; Zhang, Y.; Yue, Z.; Wang, K.; Mai, X.; Liu, Y.; Zhu, M.; Fan, H.; Zhang, W. Multi-fingerprint profiling analysis for screening and quantification of illegal adulterated antidiabetics in a functional food using HPLC coupled to diode array detection/fluorescence detection. Microchem. J. 2019, 149, 103995. [Google Scholar] [CrossRef]
- Klikarová, J.; Česlová, L. Targeted and Non-Targeted HPLC Analysis of Coffee-Based Products as Effective Tools for Evaluating the Coffee Authenticity. Molecules 2022, 27, 7419. [Google Scholar] [CrossRef]
- Belchior, V.; Botelho, B.G.; Franca, A.S. Comparison of Spectroscopy-Based Methods and Chemometrics to Confirm Classification of Specialty Coffees. Foods 2022, 11, 1655. [Google Scholar] [CrossRef] [PubMed]
- Rocha Baqueta, M.; Coqueiro, A.; Henrique Março, P.; Valderrama, P. Multivariate classification for the direct determination of cup profile in coffee blends via handheld near-infrared spectroscopy. Talanta 2021, 222, 121526. [Google Scholar] [CrossRef] [PubMed]
- Milani, M.I.; Rossini, E.L.; Catelani, T.A.; Pezza, L.; Toci, A.T.; Redigolo Pezza, H. Authentication of roasted and ground coffee samples containing multiple adulterants using NMR and a chemometric approach. Food Control. 2022, 112, 112–107104. [Google Scholar] [CrossRef]
- Núñez, N.; Pons, J.; Saurina, J.; Núñez, O. FIA-ESI-MS Fingerprinting Method with Chemometrics for the Characterization of Adulterated Coffee Samples. Biol. Life Sci. Forum 2021, 6, 102. [Google Scholar] [CrossRef]
- Núñez, N.; Saurina, J.; Núñez, O. Authenticity Assessment and Fraud Quantitation of Coffee Adulterated with Chicory, Barley, and Flours by Untargeted HPLC-UV-FLD Fingerprinting and Chemometrics. Foods 2021, 10, 840. [Google Scholar] [CrossRef]
- Bobková, A.; Hudáček, M.; Jakabová, S.; Belej, Ľ.; Capcarová, M.; Čurlej, J.; Bobko, M.; Árvay, J.; Jakab, I.; Čapla, J.; et al. The effect of roasting on the total polyphenols and antioxidant activity of coffee. J. Environ. Sci. Health 2020, 55, 495–500. [Google Scholar] [CrossRef]
- Zhu, M.; Long, Y.; Chen, Y.; Huang, Y.; Tang, L.; Gan, B.; Yu, Q.; Xie, J. Fast determination of lipid and protein content in green coffee beans from different origins using NIR spectroscopy and chemometrics. J. Food Compost. Anal. 2021, 102, 104055. [Google Scholar] [CrossRef]
- Grotzkyj Giorgi, M.; Howland, K.; Martin, C.; Bonner, A.B. A Novel HPLC Method for the Concurrent Analysis and Quantitation of Seven Water-Soluble Vitamins in Biological Fluids (Plasma and Urine): A Validation Study and Application. Sci. World J. 2012, 2012, 359721. [Google Scholar] [CrossRef]
- ISO 11294:1994; Roasted Ground Coffee—Determination of Moisture Content—Method by Determination of Loss in Mass at 103 °C (Routine Method). ISO: Geneva, Switzerland, 1994.
- Climate Knowled Geportal. Available online: https://climateknowledgeportal.worldbank.org/ (accessed on 2 April 2023).
- Ali, A.; Zahid, H.F.; Cottrell, J.J.; Dunshea, F.R. A Comparative Study for Nutritional and Phytochemical Profiling of Coffea arabica (C. arabica) from Different Origins and Their Antioxidant Potential and Molecular Docking. Molecules 2022, 27, 5126. [Google Scholar] [CrossRef]
- Good Kitzberger, C.S.; dos Santos Scholz, M.B.; Protasio Pereira, L.F.; Gonçalves Dias da Silva, J.B.; de Toledo Benassi, M. Profile of the diterpenes, lipid and protein content of different coffee cultivars of three consecutive harvests. Agric. Food 2016, 1, 254–264. [Google Scholar] [CrossRef]
- Nzekoue, F.K.; Borsetta, G.; Navarini, L.; Abouelenein, D.; Xiao, J.; Sagratini, G.; Vittori, S.; Caprioli, G.; Angeloni, S. Coffee silverskin: Characterization of B-vitamins, macronutrients, minerals and phytosterols. Food Chem. 2022, 32, 131188. [Google Scholar] [CrossRef]
- Mansur Tavares, K.; Ribeiro Lima, A.; Nunes, C.A.; Almeida Silva, V.; Mendes, E.; Casal, S.; Alvarenga Pereira, R.G.F. Free tocopherols as chemical markers for Arabica coffee adulteration with maize and coffee by-products. Food Control. 2016, 70, 318–324. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuff. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32006R1881 (accessed on 2 April 2023).
- Jimenez, A.; Adisa, A.; Woodham, C.; Saleh, M. Determination of polycyclic aromatic hydrocarbons in roasted coffee. J. Environ. Sci. Health B 2014, 49, 828–835. [Google Scholar] [CrossRef]

| Coffee Origin | Number of Samples | Purchased From |
|---|---|---|
| El Salvador | 8 | Specialty coffee shops |
| Guatemala | 7 | Specialty coffee shops |
| Brazil 1 | 13 | Specialty coffee shops |
| D.R. Congo | 4 | Specialty coffee shops |
| Ethiopia | 7 | Specialty coffee shops |
| Colombia | 13 | Specialty coffee shops |
| Non-EU origin | 11 | General store |
| Brazil 2 | 4 | General store |
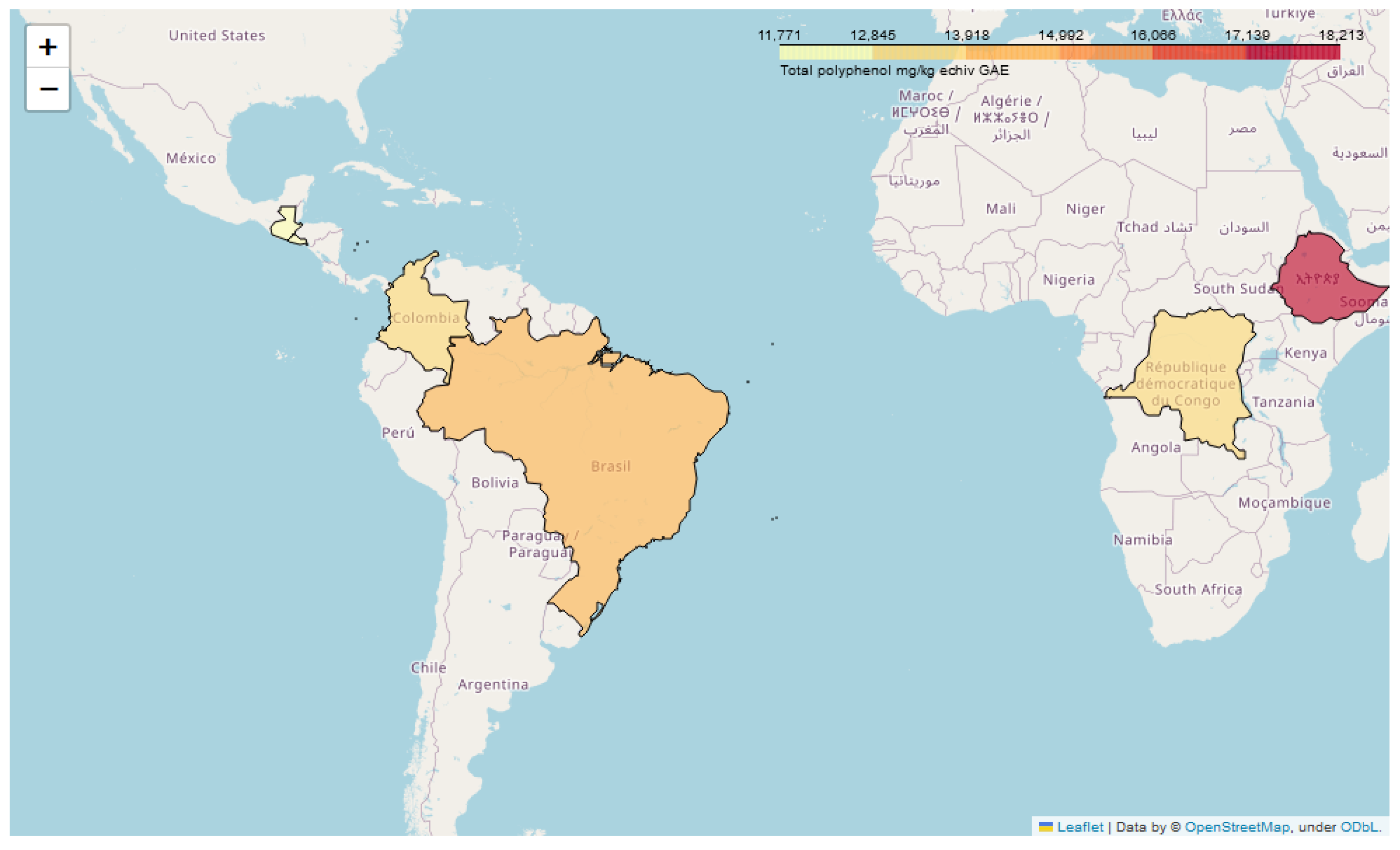
| Coffee Origin | Soil Type | Mean Annual Precipitation (mm) | Mean Annual Temperature (°C) | Altitude (m) |
|---|---|---|---|---|
| El Salvador | volcanic | 1703.54 | 25.24 | 1200 |
| Guatemala | clay-and-limestone | 2171.74 | 23.66 | 1750 |
| Brazil | volcanic loam | 1778.87 | 25.45 | 1000 |
| D.R. Congo | clay-sandy volcanic | 1500.77 | 24.35 | 1800 |
| Ethiopia | volcanic Nitosols | 850.14 | 23.37 | 1950 |
| Colombia | volcanic | 2562.17 | 25 | 1750 |
| Coffee Origin | Total Polyphenol mg GAE/g | Catechin µg/mg | Antioxidant Capacity mg/g Trolox Equiv. |
|---|---|---|---|
| El Salvador | 12.899 ±0.472 | 0.482 ± 0.060 | 29.493 ± 0.559 |
| Guatemala | 11.731 ± 0.737 | 0.558 ± 0.042 | 27.304 ± 0.635 |
| Brazil 1 | 14.447 ± 0.575 | 0.660 ± 0.056 | 25.652 ± 0.804 |
| D.R. Congo | 13.933 ± 0.392 | 0.580 ± 0.048 | 28.543 ± 1.030 |
| Ethiopia | 19.00 ± 1.087 | 0.74 ± 0.045 | 59.37 ± 1.107 |
| Colombia | 13.31 ± 0.614 | 0.64 ± 0.053 | 31.28 ± 0.737 |
| Non-EU origin | 3.72 ± 0.564 | 0.26 ± 0.050 | 8.62 ± 0.596 |
| Brazil 2 | 3.78 ± 0.822 | 0.25 ± 0.071 | 8.50 ± 0.824 |
| Coffee Origin | Total Lipids % | Total Protein % |
|---|---|---|
| El Salvador | 14.610 ± 0.303 | 12.474 ± 0.858 |
| Guatemala | 13.541 ± 0.371 | 12.834 ± 0.970 |
| Brazil 1 | 9.482 ± 0.789 | 9.777 ± 0.639 |
| D.R. Congo | 13.543 ± 0.562 | 12.223 ± 0.808 |
| Ethiopia | 10.39 ± 0.754 | 11.02 ± 0.698 |
| Colombia | 12.76 ± 1.119 | 12.94 ± 1.389 |
| Non-EU origin | 9.85 ± 0.843 | 9.93 ± 0.591 |
| Brazil 2 | 9.00 ± 0.816 | 10.48 ± 0.248 |
| Coffee Origin | B1 µg/g | B2 µg/g | B3 µg/g | B6 µg/g | α-Tocopherol µg/g | β-Tocopherol µg/g | γ-Tocopherol µg/g | δ-Tocopherol µg/g |
|---|---|---|---|---|---|---|---|---|
| El Salvador | 0.136 ± 0.012 | 0.116 ± 0.038 | 8.881 ± 0.56 | 0.015 ± 0.002 | 30.7 ± 1.3 | 50.8 ± 0.7 | 0.016 ± 0.003 | 0.936 ± 0.206 |
| Guatemala | 0.063 ± 0.011 | 0.027 ± 0.011 | 8.124 ± 0.31 | 0.014 ± 0.001 | 31.2 ± 1.5 | 51.5 ± 1.1 | 0.029 ± 0.005 | 0.853 ± 0.063 |
| Brazil 1 | 0.339 ± 0.048 | 0.215 ± 0.055 | 10.311 ± 0.73 | 0.075 ± 0.04 | 47.1 ± 2.5 | 110.4 ± 4.8 | 0.114 ± 0.150 | 1.335 ± 0.202 |
| D.R. Congo | 0.163 ± 0.028 | 0.158 ± 0.022 | 10.353 ± 0.36 | 0.058 ± 0.008 | 44.2 ± 0.7 | 99.1 ± 5.6 | 0.023 ± 0.002 | 1.154 ± 0.083 |
| Ethiopia | 0.22 ± 0.040 | 0.07 ± 0.013 | 11.40 ± 0.67 | 0.08 ± 0.010 | 45.2 ± 0.4 | 98.7 ± 4.5 | 0.03 ± 0.004 | 1.19 ± 0.179 |
| Colombia | 0.25 ± 0.030 | 0.13 ± 0.029 | 9.58 ± 0.65 | 0.07 ± 0.008 | 32.6 ± 0.8 | 52.7 ± 1.0 | 0.03 ± 0.005 | 0.92 ± 0.094 |
| Non-EU origin | 0.03 ± 0.019 | 0.02 ± 0.015 | 2.34 ± 0.56 | 0.01 ± 0.003 | 22.5 ± 0.7 | 39.3 ± 5.0 | 0.02 ± 0.016 | 0.24 ± 0.049 |
| Brazil 2 | <LQ * | <LQ | 2.01 ± 0.31 | <LQ | 23.0 ± 0.7 | 40.9 ± 1.6 | <LQ ** | 0.24 ± 0.044 |
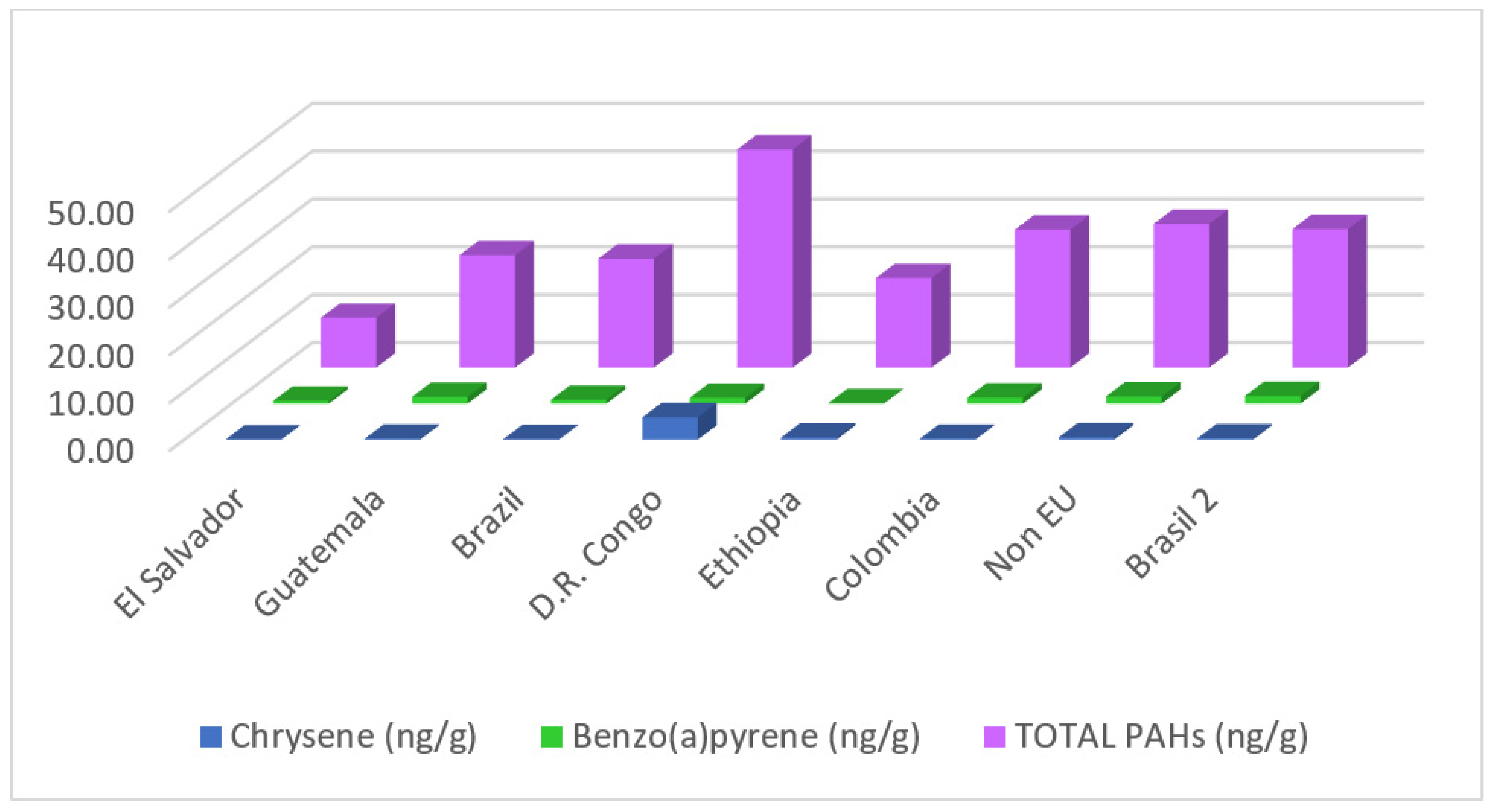
| Coffee Origin | Value | Nap | Phen | Anthr | Fluor | Pyrene | Benzo (a)antr | Chry | Benzo (b)f | Benzo (a)p | Dibenzo (a,h)a | Total PAHs |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| El Salvador | Avg. | 5.21 | 1.25 | 1.11 | 0.59 | 0.87 | 0.22 | 0.08 | 0.11 | 0.59 | 0.09 | 10.43 |
| SD | 0.72 | 0.02 | 0.09 | 0.13 | 0.38 | 0.04 | 0.01 | 0.12 | 0.23 | n.a. | 3.52 | |
| Guatemala | Avg. | 7.11 | 12.64 | 1.12 | 0.68 | 0.18 | 0.14 | 0.20 | 0.04 | 1.43 | 0.12 | 23.50 |
| SD | 1.66 | 1.18 | 0.10 | 0.07 | 0.10 | 0.03 | 0.02 | 0.01 | 0.24 | n.a. | 2.28 | |
| Brazil 1 | Avg. | 6.35 | 13.84 | 0.76 | 0.56 | 0.22 | 0.15 | 0.13 | 0.07 | 0.81 | 0.07 | 22.79 |
| SD | 0.89 | 1.00 | 0.09 | 0.10 | 0.06 | 0.24 | 0.03 | 0.04 | 0.07 | 0.05 | 1.72 | |
| D.R. Congo | Avg. | 10.34 | 26.71 | 0.50 | 1.94 | 0.12 | 0.13 | 4.65 | <LQ * | 1.22 | <LQ | 45.61 |
| SD | 1.09 | 7.57 | 0.08 | 0.36 | 0.03 | 0.04 | 8.90 | n.a. | 0.12 | n.a. | 7.72 | |
| Ethiopia | Avg. | 8.74 | 6.68 | 0.71 | 1.75 | 0.20 | 0.04 | 0.45 | <LQ | 0.13 | 0.24 | 18.75 |
| SD | 0.62 | 1.15 | 0.08 | 0.35 | 0.04 | 0.02 | 0.10 | n.a. | 0.04 | n.a. | 0.99 | |
| Colombia | Avg. | 10.47 | 10.41 | 3.53 | 1.95 | 0.95 | 0.03 | 0.20 | 0.02 | 1.26 | 0.22 | 28.86 |
| SD | 0.61 | 0.79 | 0.40 | 0.66 | 3.02 | 0.01 | 0.09 | 0.01 | 0.18 | 0.05 | n.a | |
| Non-EU origin | Avg. | 12.55 | 10.91 | 2.08 | 1.87 | 0.39 | 0.09 | 0.49 | 0.04 | 1.53 | 0.37 | 30.04 |
| SD | 1.49 | 0.88 | 0.37 | 0.53 | 0.04 | 0.03 | 0.15 | 0.02 | 0.27 | 0.08 | 2.12 | |
| Brazil 2 | Avg. | 11.70 | 10.91 | 1.81 | 2.03 | 0.37 | 0.09 | 0.30 | 0.04 | 1.61 | 0.33 | 28.99 |
| SD | 1.75 | 0.89 | 0.46 | 0.45 | 0.05 | 0.01 | 0.22 | 0.01 | 0.06 | 0.12 | 2.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simedru, D.; Becze, A. Complex Profiling of Roasted Coffee Based on Origin and Production Scale. Agriculture 2023, 13, 1146. https://doi.org/10.3390/agriculture13061146
Simedru D, Becze A. Complex Profiling of Roasted Coffee Based on Origin and Production Scale. Agriculture. 2023; 13(6):1146. https://doi.org/10.3390/agriculture13061146
Chicago/Turabian StyleSimedru, Dorina, and Anca Becze. 2023. "Complex Profiling of Roasted Coffee Based on Origin and Production Scale" Agriculture 13, no. 6: 1146. https://doi.org/10.3390/agriculture13061146
APA StyleSimedru, D., & Becze, A. (2023). Complex Profiling of Roasted Coffee Based on Origin and Production Scale. Agriculture, 13(6), 1146. https://doi.org/10.3390/agriculture13061146








