Single-Nucleotide Polymorphisms Identified within Exon 2 of Fertility-Associated Bone Morphogenetic Protein (BMP15) Gene in Three Romanian Sheep Breeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Standards
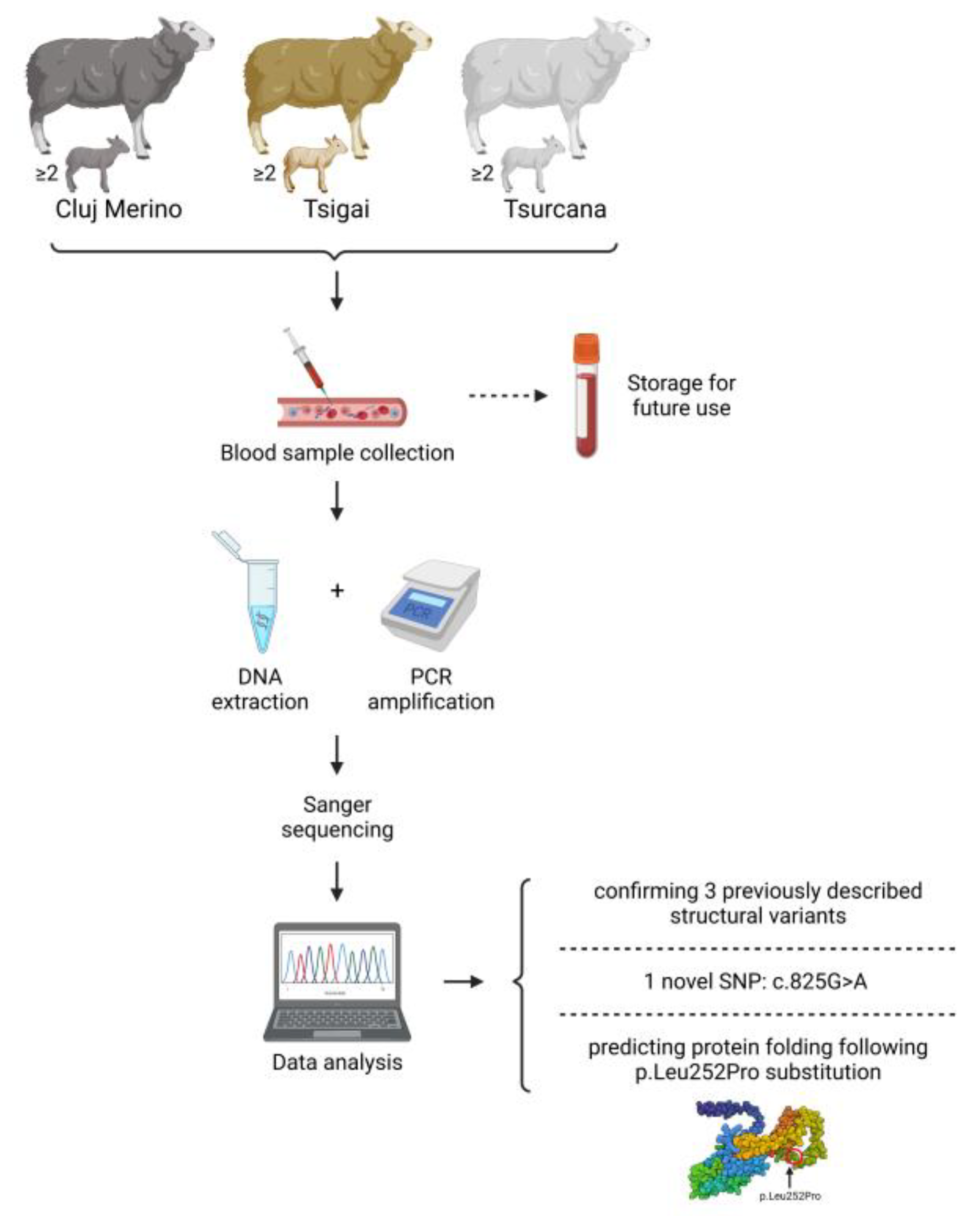
2.2. Animals and Sample Collection
2.3. DNA Extraction
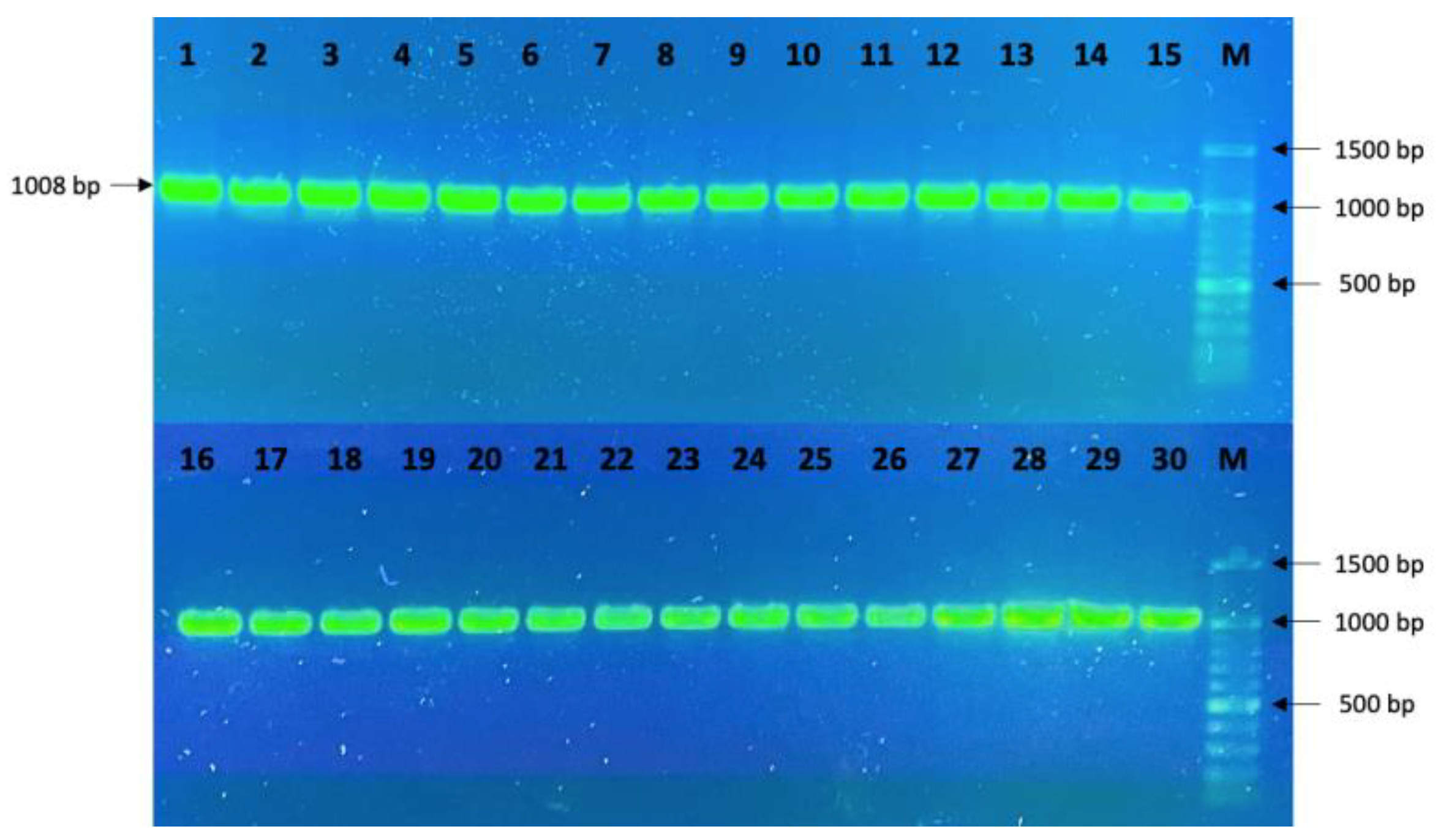
2.4. PCR Amplification
2.5. PCR Product Purification and Sequencing
2.6. Sequencing Data Analysis
2.7. SNPs Analysis
2.8. Protein Prediction and Modelling
3. Results
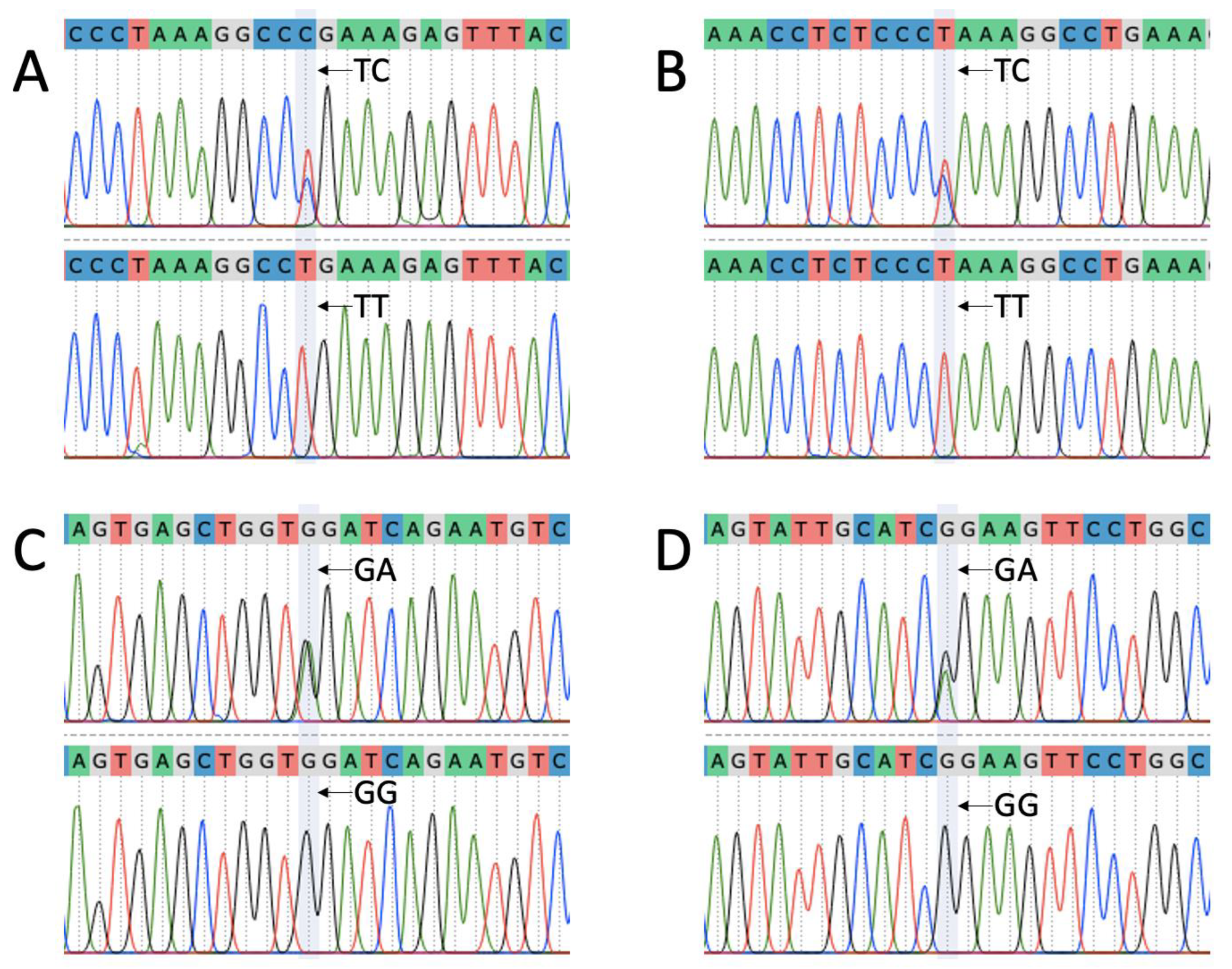
3.1. Sequencing Data Analysis
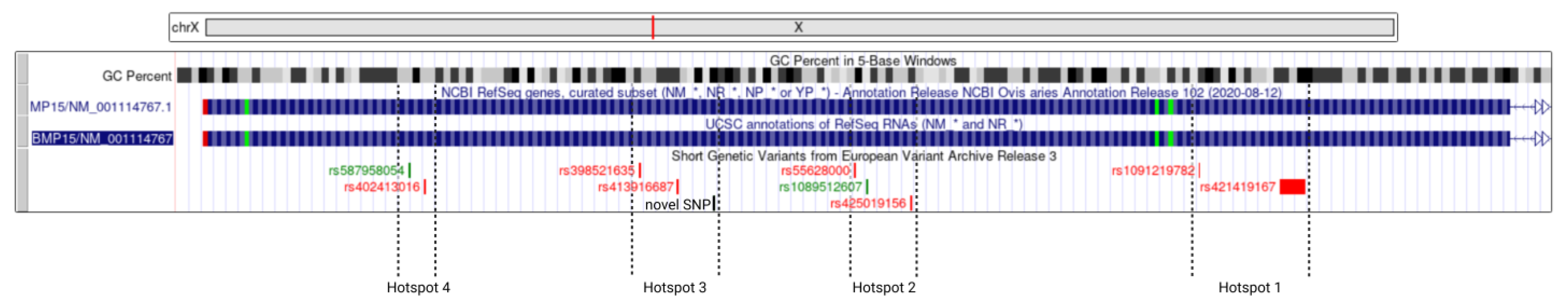
3.2. Putative Mutational Hotspots in Exon 2 of BMP15 Gene
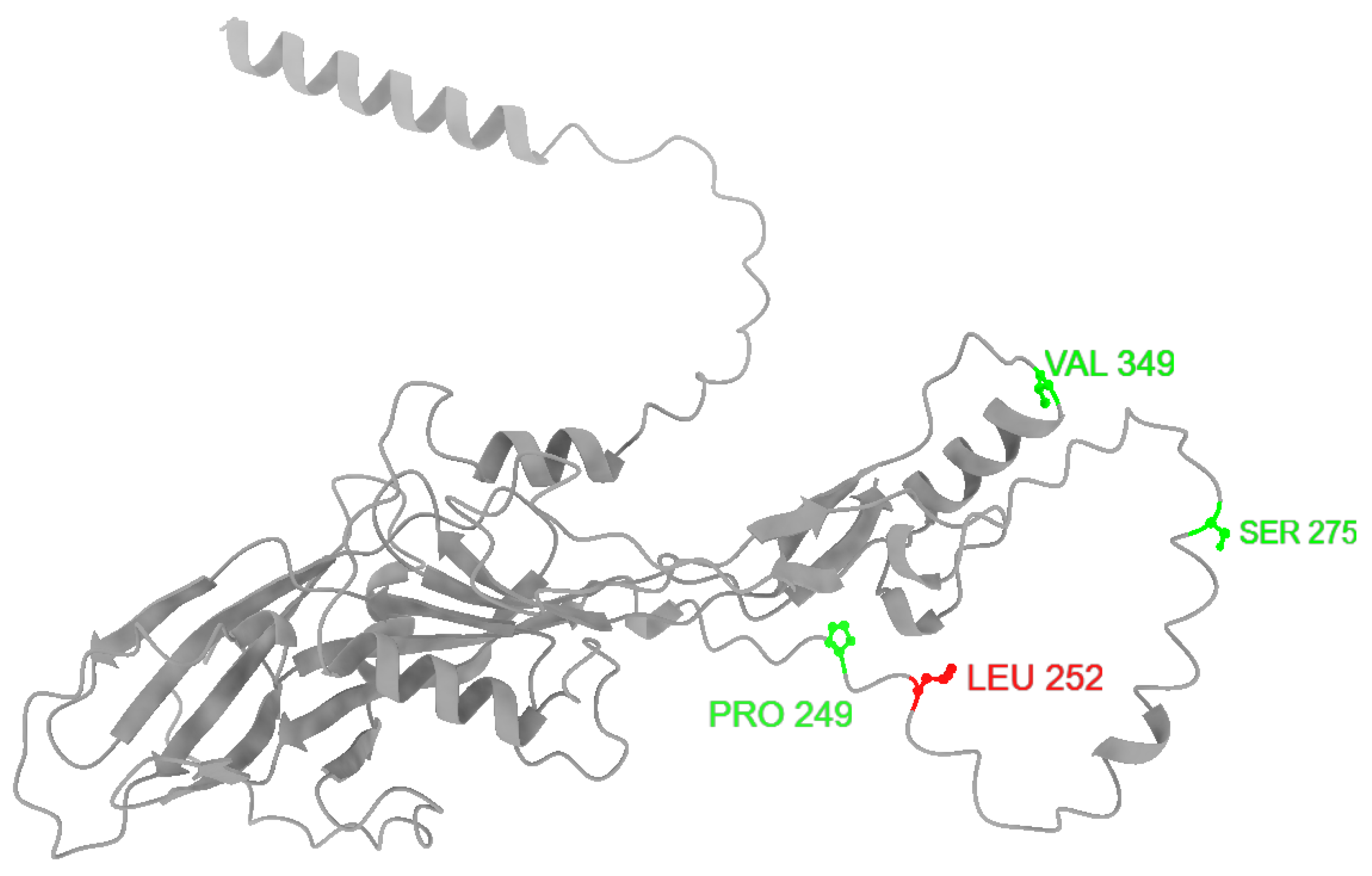
3.3. Predicting the Functional Impact of the Mutations Affecting BMP15 Protein
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miclea, V.; Zăhan, M.; Ilişiu, E.; Naghy, A.; Miclea, I. Reproductive performances of Tsigaie sheep. Bull. UASVM Cluj-Napoca Anim. Sci. Biotech. 2009, 66, 86–89. [Google Scholar]
- Pădurariu, A.; Dărăban, S.; Mireşan, V. The age and physiological state influence in Merino of Cluj Sheep on reproductive performance. Sci. Papers Anim. Sci. Biotechnol. 2018, 51, 166–171. [Google Scholar]
- Budai, C.; Gavojdian, D.; Kusza, S.; Cziszter, L.T.; Olah, J.; Pădeanu, I.; Kovacs, A.; Javor, A. Comparative study regarding reproductive performance in Gyimesi racka and Turcana sheep breeds. Sci. Papers Anim. Sci. Biotechnol. 2013, 46, 351–356. [Google Scholar]
- Vicovan, G. Genetic analysis of the sheep line with high prolificacy. Arch. Zooteh. 1995, 3, 47–51. [Google Scholar]
- Drăgănescu, C.; Grosu, H. Valachian (zackel) heritage philetic sheep group-A taxonomic problem. Sci. Papers Rom. Acad. 2010, 1, 1–7. [Google Scholar]
- Ilişiu, E.; Dărăban, S.; Radu, R.; Pădeanu, I.; Ilişiu, V.C.; Pascal, C.; Rahmann, G. The Romanian Tsigai sheep breed, their potential and the challenges for research. Appl. Agric. For. Res. 2013, 2, 161–170. [Google Scholar]
- Dãrãban, S.; Coroian, C.; Georgescu, B. Cluj Merino breeds’ potential for meat production. Anim. Biol. Anim. Husb. 2009, 1, 57–62. [Google Scholar]
- Davis, G.H. Major genes affecting ovulation rate in sheep. Genet. Sel. Evol. 2005, 37 (Suppl. 1), S11–S23. [Google Scholar] [CrossRef]
- Galloway, S.M.; Gregan, S.M.; Wilson, T.; McNatty, K.P.; Juengel, J.L.; Ritvos, O.; Davis, G.H. Bmp15 mutations and ovarian function. Mol. Cell Endocrinol. 2002, 191, 15–18. [Google Scholar] [CrossRef]
- Hanrahan, J.P.; Gregan, S.M.; Mulsant, P.; Mullen, M.; Davis, G.H.; Powell, R.; Galloway, S.M. Mutations in the Genes for Oocyte-Derived Growth Factors GDF9 and BMP15 Are Associated with Both Increased Ovulation Rate and Sterility in Cambridge and Belclare Sheep (Ovis aries). Biol. Reprod. 2004, 70, 900–909. [Google Scholar] [CrossRef]
- Mulsant, P.; Lecerf, F.; Fabre, S.; Schibler, L.; Monget, P.; Lanneluc, I.; Pisselet, C.; Riquet, J.; Monniaux, D.; Callebaut, I.; et al. Mutation in bone morphogenetic protein receptor-IB is associated with increased ovulation rate in Booroola Mérino ewes. Proc. Natl. Acad. Sci. USA 2001, 98, 5104–5109. [Google Scholar] [CrossRef] [PubMed]
- Drouilhet, L.; Mansanet, C.; Sarry, J.; Tabet, K.; Bardou, P.; Woloszyn, F.; Lluch, J.; Harichaux, G.; Viguié, C.; Monniaux, D.; et al. The Highly Prolific Phenotype of Lacaune Sheep Is Associated with an Ectopic Expression of the B4GALNT2 Gene within the Ovary. PLOS Genet. 2013, 9, e1003809. [Google Scholar] [CrossRef]
- Tapaloaga, D.; Tapaloaga, P.-R. Assessment of Some Morphometric Parameters in Ram Sperm Correlated with the Collection Method. Agric. Agric. Sci. Procedia 2016, 10, 340–345. [Google Scholar] [CrossRef]
- Di, R.; Wang, F.; Yu, P.; Wang, X.; He, X.; Mwacharo, J.M.; Pan, L.; Chu, M. Detection of Novel Variations Related to Litter Size in BMP15 Gene of Luzhong Mutton Sheep (Ovis aries). Animals 2021, 11, 3528. [Google Scholar] [CrossRef] [PubMed]
- Fabre, S.; Pierre, A.; Mulsant, P.; Bodin, L.; Di Pasquale, E.; Persani, L.; Monget, P.; Monniaux, D. Regulation of ovulation rate in mammals: Contribution of sheep genetic models. Reprod. Biol. Endocrinol. 2006, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J.; Attisano, L.; Wrana, J.L. The TGF-β family and its composite receptors. Trends Cell Biol. 1994, 4, 172–178. [Google Scholar] [CrossRef]
- Otsuka, F.; Yamamoto, S.; Erickson, G.F.; Shimasaki, S. Bone Morphogenetic Protein-15 Inhibits Follicle-stimulating Hormone (FSH) Action by Suppressing FSH Receptor Expression. J. Biol. Chem. 2001, 276, 11387–11392. [Google Scholar] [CrossRef]
- Regan, S.L.P.; Knight, P.G.; Yovich, J.L.; Leung, Y.; Arfuso, F.; Dharmarajan, A. Involvement of Bone Morphogenic Proteins (BMP) in the Regulation of Ovarian Function. Vitam. Horm. 2018, 107, 227–261. [Google Scholar] [CrossRef]
- Galloway, S.M.; McNatty, K.P.; Cambridge, L.M.; Laitinen, M.P.; Juengel, J.; Jokiranta, T.S.; McLaren, R.J.; Luiro, K.; Dodds, K.; Montgomery, G.; et al. Mutations in an oocyte-derived growth factor gene (BMP15) cause increased ovulation rate and infertility in a dosage-sensitive manner. Nat. Genet. 2000, 25, 279–283. [Google Scholar] [CrossRef]
- Chen, W.; Tian, Z.; Ma, L.; Gan, S.; Sun, W.; Chu, M. Expression Analysis of BMPR1B, BMP15, GDF9, Smad1, Smad5, and Smad9 in Rams with Different Fecundity. Pak. J. Zool. 2020, 52, 1665. [Google Scholar] [CrossRef]
- Kona, S.; Chakravarthi, V.P.; Kumar, A.S.; Srividya, D.; Padmaja, K.; Rao, V.H. Quantitative expression patterns of GDF9 and BMP15 genes in sheep ovarian follicles grown in vivo or cultured in vitro. Theriogenology 2016, 85, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Hu, W.; Di, R.; Liu, Q.; Wang, X.; Zhang, X.; Zhang, J.; Chu, M. Expression Analysis of the Prolific Candidate Genes, BMPR1B, BMP15, and GDF9 in Small Tail Han Ewes with Three Fecundity (FecB Gene) Genotypes. Anim. Basel 2018, 8, 166. [Google Scholar] [CrossRef] [PubMed]
- Juengel, J.L.; Quirke, L.D.; Lun, S.; Heath, D.A.; Johnstone, P.D.; McNatty, K.P. Effects of immunizing ewes against bone morphogenetic protein 15 on their responses to exogenous gonadotrophins to induce multiple ovulations. Reproduction 2011, 142, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Feary, E.S.; Juengel, J.L.; Smith, P.; French, M.C.; O’Connell, A.R.; Lawrence, S.B.; Galloway, S.M.; Davis, G.H.; McNatty, K.P. Patterns of Expression of Messenger RNAs Encoding GDF9, BMP15, TGFBR1, BMPR1B, and BMPR2 During Follicular Development and Characterization of Ovarian Follicular Populations in Ewes Carrying the Woodlands FecX2W Mutation1. Biol. Reprod. 2007, 77, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, F. Multifunctional bone morphogenetic protein system in endocrinology. Acta Med. Okayama 2013, 67, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Henderson, K.M.; Savage, L.C.; Ellen, R.L.; Ball, K.; McNatty, K.P. Consequences of increasing or decreasing plasma FSH concentrations during the preovulatory period in Romney ewes. J. Reprod. Fertil. 1988, 84, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Wang, P.; DeMayo, J.; DeMayo, F.J.; Elvin, J.A.; Carino, C.; Prasad, S.V.; Skinner, S.S.; Dunbar, B.S.; Dube, J.L.; et al. Synergistic Roles of Bone Morphogenetic Protein 15 and Growth Differentiation Factor 9 in Ovarian Function. Mol. Endocrinol. 2001, 15, 854–866. [Google Scholar] [CrossRef]
- Otsuka, F.; McTavish, K.J.; Shimasaki, S. Integral role of GDF-9 and BMP-15 in ovarian function. Mol. Reprod. Dev. 2011, 78, 9–21. [Google Scholar] [CrossRef]
- Bodin, L.; Di Pasquale, E.; Fabre, S.; Bontoux, M.; Monget, P.; Persani, L.; Mulsant, P. A Novel Mutation in the Bone Morphogenetic Protein 15 Gene Causing Defective Protein Secretion Is Associated with Both Increased Ovulation Rate and Sterility in Lacaune Sheep. Endocrinology 2007, 148, 393–400. [Google Scholar] [CrossRef]
- Martinez-Royo, A.; Jurado, J.J.; Smulders, J.P.; Martí, J.I.; Alabart, J.L.; Roche, A.; Fantova, E.; Bodin, L.; Mulsant, P.; Serrano, M.; et al. A deletion in the bone morphogenetic protein 15 gene causes sterility and increased prolificacy in Rasa Aragonesa sheep. Anim. Genet. 2008, 39, 294–297. [Google Scholar] [CrossRef]
- Monteagudo, L.V.; Ponz, R.; Tejedor, M.T.; Laviña, A.; Sierra, I. A 17bp deletion in the Bone Morphogenetic Protein 15 (BMP15) gene is associated to increased prolificacy in the Rasa Aragonesa sheep breed. Anim. Reprod. Sci. 2009, 110, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Barzegari, A.; Atashpaz, S.; Ghabili, K.; Nemati, Z.; Rustaei, M.; Azarbaijani, R. Polymorphisms in GDF9 and BMP15 Associated with Fertility and Ovulation Rate in Moghani and Ghezel Sheep in Iran. Reprod. Domest. Anim. 2010, 45, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Vacca, G.M.; Dhaouadi, A.; Rekik, M.; Carcangiu, V.; Pazzola, M.; Dettori, M.L. Prolificacy genotypes at BMPR 1B, BMP15 and GDF9 genes in North African sheep breeds. Small Rumin. Res. 2010, 88, 67–71. [Google Scholar] [CrossRef]
- Javanmard, A.; Azadzadeh, N.; Esmailizadeh, A.K. Mutations in bone morphogenetic protein 15 and growth differentiation factor 9 genes are associated with increased litter size in fat-tailed sheep breeds. Vet. Res. Commun. 2011, 35, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Demars, J.; Fabre, S.; Sarry, J.; Rossetti, R.; Gilbert, H.; Persani, L.; Tosser-Klopp, G.; Mulsant, P.; Nowak, Z.; Drobik, W.; et al. Genome-Wide Association Studies Identify Two Novel BMP15 Mutations Responsible for an Atypical Hyperprolificacy Phenotype in Sheep. PLoS Genet. 2013, 9, e1003482. [Google Scholar] [CrossRef]
- Wang, W.; Liu, S.; Li, F.; Pan, X.; Li, C.; Zhang, X.; Ma, Y.; La, Y.; Xi, R.; Li, T. Polymorphisms of the Ovine BMPR-IB, BMP-15 and FSHR and Their Associations with Litter Size in Two Chinese Indigenous Sheep Breeds. Int. J. Mol. Sci. 2015, 16, 11385–11397. [Google Scholar] [CrossRef]
- Nadri, S.; Zamani, P.; Ahmadi, A. Novel Mutation in Exon 1 of the BMP15 Gene and its Association with Reproduction Traits in Sheep. Anim. Biotechnol. 2016, 27, 256–261. [Google Scholar] [CrossRef]
- Lassoued, N.; Benkhlil, Z.; Woloszyn, F.; Rejeb, A.; Aouina, M.; Rekik, M.; Fabre, S.; Bedhiaf-Romdhani, S. FecX Bar a Novel BMP15 mutation responsible for prolificacy and female sterility in Tunisian Barbarine Sheep. BMC Genet. 2017, 18, 43. [Google Scholar] [CrossRef]
- Abdoli, R.; Mirhoseini, S.Z.; Hossein-Zadeh, N.G.; Zamani, P. Screening for Causative Mutations of Major Prolificacy Genes in Iranian Fat-Tailed Sheep. Int. J. Fertil. Steril. 2018, 12, 51–55. [Google Scholar] [CrossRef]
- Amini, H.-R.; Ajaki, A.; Farahi, M.; Heidari, M.; Pirali, A.; Forouzanfar, M.; Eghbalsaied, S. The novel T755C mutation in BMP15 is associated with the litter size of Iranian Afshari, Ghezel, and Shal breeds. Arch. Anim. Breed. 2018, 61, 153–160. [Google Scholar] [CrossRef]
- Chantepie, L.; Bodin, L.; Sarry, J.; Woloszyn, F.; Ruesche, J.; Drouilhet, L.; Fabre, S. Presence of causative mutations affecting prolificacy in the Noire du Velay and Mouton Vendéen sheep breeds. Livest. Sci. 2018, 216, 44–50. [Google Scholar] [CrossRef]
- Vera, M.; Aguion, M.; Bouza, C. Detection of Grivette BMP15 prolificacy variant (FecXGR) in different sheep breeds presented in Galicia (NW Spain). Gene Rep. 2018, 12, 109–114. [Google Scholar] [CrossRef]
- Chantepie, L.; Bodin, L.; Sarry, J.; Woloszyn, F.; Plisson-Petit, F.; Ruesche, J.; Drouilhet, L.; Fabre, S. Genome-Wide Identification of a Regulatory Mutation in BMP15 Controlling Prolificacy in Sheep. Front. Genet. 2020, 11, 585. [Google Scholar] [CrossRef]
- Calvo, J.H.; Chantepie, L.; Serrano, M.; Sarto, M.P.; Iguacel, L.P.; Jiménez, M.A.; Alabart, J.L.; Folch, J.; Fabre, S.; Lahoz, B. A new allele in the BMP15 gene (FecX(RA)) that affects prolificacy co-segregates with FecX(R) and FecX(GR) in Rasa aragonesa sheep. Theriogenology 2020, 144, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.-G.; Qin, J.; Jiang, Y.; Ding, X.-D.; Ding, Y.-G.; Tang, S.; Shi, H.-C. The Identification of Mutation in BMP15 Gene Associated with Litter Size in Xinjiang Cele Black Sheep. Animals 2021, 11, 668. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chi, Z.; Jia, S.; Zhao, S.; Cao, G.; Purev, C.; Cang, M.; Yu, H.; Li, X.; Bao, S.; et al. Effects of novel variants in BMP15 gene on litter size in Mongolia and Ujimqin sheep breeds. Theriogenology 2023, 198, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.H.; Dodds, K.G.; Wheeler, R.; Jay, N.P. Evidence That an Imprinted Gene on the X Chromosome Increases Ovulation Rate in Sheep. Biol. Reprod. 2001, 64, 216–221. [Google Scholar] [CrossRef]
- Chu, M.X.; Liu, Z.H.; Jiao, C.L.; He, Y.Q.; Fang, L.; Ye, S.C.; Chen, G.H.; Wang, J.Y. Mutations in BMPR-IB and BMP-15 genes are associated with litter size in Small Tailed Han sheep (Ovis aries). J. Anim. Sci. 2007, 85, 598–603. [Google Scholar] [CrossRef]
- Drouilhet, L.; Lecerf, F.; Bodin, L.; Fabre, S.; Mulsant, P. Fine mapping of the FecL locus influencing prolificacy in Lacaune sheep. Anim. Genet. 2009, 40, 804–812. [Google Scholar] [CrossRef]
- Haas, C.S.; Oliveira, F.C.; Rovani, M.T.; Ferst, J.G.; Vargas, S.F., Jr.; Vieira, A.D.; Mondadori, R.G.; Pegoraro, L.M.C.; Gonçalves, P.B.D.; Bordignon, V.; et al. Bone morphogenetic protein 15 intrafollicular injection inhibits ovulation in cattle. Theriogenology 2022, 182, 148–154. [Google Scholar] [CrossRef]
- Geneious Prime 2023.1. Available online: https://geneious.com (accessed on 15 January 2023).
- Okonechnikov, K.; Golosova, O.; Fursov, M.; The UGENE Team. Unipro UGENE: A unified bioinformatics toolkit. Bioinformatics 2012, 28, 1166–1167. [Google Scholar] [CrossRef] [PubMed]
- Parthiban, V.; Gromiha, M.M.; Schomburg, D. CUPSAT: Prediction of protein stability upon point mutations. Nucleic Acids Res. 2006, 34, W239–W242. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.H.M.; Pires, D.E.V.; Ascher, D.B. DynaMut: Predicting the impact of mutations on protein conformation, flexibility and stability. Nucleic Acids Res. 2018, 46, W350–W355. [Google Scholar] [CrossRef] [PubMed]
- Savojardo, C.; Fariselli, P.; Martelli, P.L.; Casadio, R. INPS-MD: A web server to predict stability of protein variants from sequence and structure. Bioinformatics 2016, 32, 2542–2544. [Google Scholar] [CrossRef]
- Laimer, J.; Hiebl-Flach, J.; Lengauer, D.; Lackner, P. MAESTROweb: A web server for structure-based protein stability prediction. Bioinformatics 2016, 32, 1414–1416. [Google Scholar] [CrossRef]
- Schrödinger, L.; DeLano, W. PyMOL. Available online: http://www.pymol.org/pymol2020 (accessed on 20 January 2023).
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef]
- Mirdita, M.; Schütze, K.; Moriwaki, Y.; Heo, L.; Ovchinnikov, S.; Steinegger, M. ColabFold: Making protein folding accessible to all. Nat. Methods 2022, 19, 679–682. [Google Scholar] [CrossRef]
- Marabotti, A.; Del Prete, E.; Scafuri, B.; Facchiano, A. Performance of Web tools for predicting changes in protein stability caused by mutations. BMC Bioinform. 2021, 22, 345. [Google Scholar] [CrossRef]
- Davis, G.H.; McEwan, J.C.; Fennessy, P.F.; Dodds, K.G.; McNatty, K.P.; Wai-Sum, O. Infertility Due to Bilateral Ovarian Hypoplasia in Sheep Homozygous (FecX1 FecX1) for the Inverdale Prolificacy Gene Located on the X Chromosome. Biol. Reprod. 1992, 46, 636–640. [Google Scholar] [CrossRef]
- Niciura, S.C.M.; Cruvinel, G.G.; Moraes, C.V.; Bressani, F.A.; Junior, W.M.; Benavides, M.V.; de Chagas, A.C.S. PCR-based genotyping of SNP markers in sheep. Mol. Biol. Rep. 2018, 45, 651–656. [Google Scholar] [CrossRef]
- Jones, A.K.; Jones, D.L.; Cross, P. The carbon footprint of lamb: Sources of variation and opportunities for mitigation. Agric. Syst. 2014, 123, 97–107. [Google Scholar] [CrossRef]
- Eurostat. Available online: https://ec.europa.eu/eurostat/databrowser/product/view/APRO_MT_LSSHEEP (accessed on 15 April 2023).
| Allele Symbol | DNA Change | Amino Acid Change | Reference |
|---|---|---|---|
| FecXR | c.525_541del | p.A101Cfs * 113 | [47] |
| FecXB | c.1100G>T | p.S367I | [10] |
| FecXI | c.896T>A | p.V299D | [8] |
| FecXH | c.871C>T | p.Q291 * | |
| FecXL | c.962G>A | p.C321Y | |
| FecXG | c.718C>T | p.Q238 * | [48] |
| FecXGr | c.950C>T | p.T317I | [49] |
| FecXO | c.1009A>C | p.N337H | |
| FecXBar | c.301_304delGCTAinsT | p.A101Cfs * 113 | [38] |
| - | c.755T>C | p.L252P | [40] |
| Primer Sequence (5′→3′) | Position | Amplicon Size |
|---|---|---|
| F: CATCTCAAGGCTGCTTGTCA | Exon 2 | 1008 bp |
| R: TTCGAATTTCTTGGGCAAAC |
| HGVS (GenBank: NC_056080.1) | WT Allele | Mutant Allele | Amino Acid Change | Breed | LS | Parity |
| c.747T>C | T | C | p.Pro249= | Tsurcana | 2 | 2 |
| c.755T>C | T | C | p.Leu252Pro | 2.66 | 3 | |
| c.1047G>A | G | A | p.Val349= | 2.33 | 3 | |
| c.755T>C | T | C | p.Leu252Pro | Cluj Merino | 2 | 2 |
| c.825G>A | G | A | p.Ser275= | 2 | 1 |
| Size (nt) | CDS Region | Variants | CDS Change | Variant Effect | Variant Type | |
|---|---|---|---|---|---|---|
| Hotspot 1 | 79 | c.455–534 | rs1091219782 | c.529C>G | p.Pro177Ala | missense |
| rs421419167 | c.460_476del | p.Trp153AsnfsTer55 | frameshift deletion | |||
| Hotspot 2 | 48 | c.713–760 | rs55628000 | c.755T>C | p.Leu252Pro | missense |
| rs1089512607 | c.747T>C | p.Pro249= | silent | |||
| rs425019156 | c.718C>T | p.Gln240Ter | nonsense | |||
| Hotspot 3 | 82 | c.820–901 | rs398521635 | c.896T>A | p.Val299Asp | missense |
| rs413916697 | c.871C>T | p.Gln291Ter | nonsense | |||
| Novel SNP | c.825G>A | p.Ser275= | silent | |||
| Hotspot 4 | 21 | c.1032–1052 | rs587958054 | c.1047G>A | p.Val349= | silent |
| rs402413016 | c.1037G>A | p.Ser346Asn | missense |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deac, A.M.; Musca, A.S.; Mesesan, S.D.; Aipatioaie, M.G.; Ionascu, A.; Cosier, V.; Ratiu, A.C.; Miclea, I.; Ladosi, I.; Zahan, M. Single-Nucleotide Polymorphisms Identified within Exon 2 of Fertility-Associated Bone Morphogenetic Protein (BMP15) Gene in Three Romanian Sheep Breeds. Agriculture 2023, 13, 996. https://doi.org/10.3390/agriculture13050996
Deac AM, Musca AS, Mesesan SD, Aipatioaie MG, Ionascu A, Cosier V, Ratiu AC, Miclea I, Ladosi I, Zahan M. Single-Nucleotide Polymorphisms Identified within Exon 2 of Fertility-Associated Bone Morphogenetic Protein (BMP15) Gene in Three Romanian Sheep Breeds. Agriculture. 2023; 13(5):996. https://doi.org/10.3390/agriculture13050996
Chicago/Turabian StyleDeac, Alexandru Marius, Adriana Sebastiana Musca, Stefania Dana Mesesan, Marius Gavril Aipatioaie, Adrian Ionascu, Viorica Cosier, Attila Cristian Ratiu, Ileana Miclea, Ioan Ladosi, and Marius Zahan. 2023. "Single-Nucleotide Polymorphisms Identified within Exon 2 of Fertility-Associated Bone Morphogenetic Protein (BMP15) Gene in Three Romanian Sheep Breeds" Agriculture 13, no. 5: 996. https://doi.org/10.3390/agriculture13050996
APA StyleDeac, A. M., Musca, A. S., Mesesan, S. D., Aipatioaie, M. G., Ionascu, A., Cosier, V., Ratiu, A. C., Miclea, I., Ladosi, I., & Zahan, M. (2023). Single-Nucleotide Polymorphisms Identified within Exon 2 of Fertility-Associated Bone Morphogenetic Protein (BMP15) Gene in Three Romanian Sheep Breeds. Agriculture, 13(5), 996. https://doi.org/10.3390/agriculture13050996








