Abstract
Peat organic soils play a major role in the accumulation of soil organic matter (SOM) and the mercury (Hg) cycle. Large mercury resources in peatlands can be a source of methylmercury for many decades and centuries, even if deposition is significantly reduced. The organic matter of peatland soils drained for agricultural use is subject to secondary transformation, which may affect the accumulation and resources of mercury. The aim of our work is to assess the secondary transformation of organic matter in the soils of drained peatlands of the temperate climate zone and to examine whether it affects mercury resources and profile distribution in organic soils. Field research was conducted in peatlands located in eastern Poland. In the present study, evaluation of secondary transformations occurring after drainage was based on observations of soil morphological characteristics, physical and chemical properties as well as fractional composition of organic matter of the identified soil horizons (to depth 70 cm). Standard cold vapor atomic absorption spectrometry (CV-AAS) was used to measure the total mercury content. In our research, we found a significant effect of the secondary transformation of organic matter occurring in drained peatlands of the temperate climate zone on the total mercury content and stock in soils. The highest content and differentiation of mercury occurred in murshic horizons (up to a maximum depth of 43 cm). The average mercury content of the distinguished soil horizons is grouped in the following series (in μg kg−1): M1 (212.0) > M2 (182.8) > M3 (126.3) > Pt (84.9). The mercury stock, up to a depth of 70 cm in the tested soils, ranged from 17.5 to 39.6 mg m−2. As much as 82.2% of soil mercury was found in the upper murshic horizons. We found strong correlations between soil properties characteristically variable in the secondary transformation process and total mercury content. The increased content of humic substances in murshic horizons caused a significant increase in the total mercury content. Our research is of great importance for soil monitoring, as the amount of determined mercury was greatly influenced by the depth of sampling (up to 25 cm). The results of the research should be taken into account when planning the restoration of peatlands of the temperate climate zone. There is a potential risk that elevated mercury concentrations in the upper murshic horizons may be a source of methylmercury for a long period of time. In peat soils with a high concentration of mercury, the risk of contamination with this toxic metal should be determined before re-irrigation.
1. Introduction
Soil is a basic component of the natural environment and it plays a key role in the geochemistry of elements and food production security. Therefore, it has to be regularly checked for potential contamination with trace elements. In this context, special importance is attributed to mercury, which is one of the most geochemically active and toxic metals [1,2,3,4,5]. In the natural environment, mercury is converted to methylmercury which is easily bioaccumulated [6,7,8,9]. Elevated mercury contents in the food chain markedly increase the risk of health issues in animals and people [10,11,12]. As regards mercury, the European Food Safety Authority (EFSA) adopted on 24 February 2004 an opinion related to mercury and methylmercury in food and endorsed the provisional tolerable weekly intake of 1.6 μg/kg body weight [13]. Methylmercury can make up more than 90% of the total mercury in fish and seafood. Forms of mercury present in other foods than fish and seafood are mainly not methylmercury and they are therefore considered to be of lower risk. Maximum levels of mercury in fish and seafood range between from 0.30 to 1.0 mg/kg wet weight.
Global mercury emissions from anthropogenic sources have increased 3–4 times compared with the pre-industrial period [14]. Major anthropogenic mercury sources since the beginning of the industrial era have included metal production, fossil fuel (mainly coal) combustion, waste incineration and sewage dumping [15,16]. The time when the elemental form of mercury is present in the atmosphere, spanning 0.8 to 1.7 years (from the beginning of emission to deposition on the soil surface), means the element is likely to be transported by way of the atmosphere over long distances, and indicates a global source of soil contamination with this metal [17,18,19,20]. Despite the reduction in global mercury emissions, it still poses a serious threat to the environment and human health, especially where there are favourable conditions for the production of methylmercury [21,22,23].
After being released into the atmosphere, a mercury atom can remain in circulation for >1000 years [2].
Estimated mercury pools in the European Union soils amount to around 44.8 Gg, the average concentration being 103 g ha−1 [4,5]. Agricultural soils account for 85% of mercury released into the environment [5]. The concentration of mercury in soil varies greatly and depends on many factors, including its origin. In regions free of anthropopressure, it has been assumed that deposition from the atmosphere is the major mercury source in the soil [2,24,25]. The share of mercury inputs from the atmosphere reaches 50% [26,27], the highest mercury content being recorded in the topsoil layer of soils [20,28]. The availability of mercury to plants is low because there is a tendency for mercury bioaccumulation in their roots which form a specific barrier against translocation to the above-ground parts [29]. The concentration of mercury in soils is associated with organic matter content and is affected by temperature and vegetation type [4]. Mercury sequestration in soil is positively correlated with organic matter content but the amount of mercury in terrestrial ecosystems is generally determined by atmospheric mercury deposition [30,31]. Most soil mercury received from the atmosphere is associated with organic carbon and does not form a permanent mercury sink because it is released as a result of decomposition of soil organic matter.
Soils of raised bogs (ombrotrophic bogs) are mainly fed by rainwater and spread above the groundwater level. These are the reasons why they are commonly chosen for research into contamination with airborne mercury [20,32,33]. Much less scientific attention has been paid to the issues of mercury accumulation in fen peatlands although quite a number of these peatlands are used as grasslands to produce biomass for fodder purposes. Organic soils of peatland areas used for farming purposes, in the context of mercury accumulation and circulation, have the following characteristics:
- (i)
- They are a component linking terrestrial habitats with bodies of surface water. Organic soils are a significant source of methylmercury in ecosystems adjacent to bodies of water in which it can be accumulated in fish [34,35,36]. In general, peatlands are potentially highly productive providers of methylmercury and are its main source for the aquatic food chain [37,38,39]. Anaerobic conditions in soil are conductive to the production of this toxic compound [39,40,41]. In the studies of Busaan et al. [42] the amounts of methylmercury in wetland sediments ranged from 0.36 to 1.43 ng g−1. In addition, the authors in pioneering in situ studies proved that the indicators mercury methylation and demethylation rates vary seasonally (highest in summer and lowest in winter). Moreover, the risk exists of mercury accumulation in plants followed by its accumulation in milk, meat and other foodstuffs [43,44,45];
- (ii)
- They are fed not only directly by rainwater but to a great extent by river water and surface water within the catchment area, which creates another possibility for mercury transport and accumulation in the soils of river valleys [17,46,47]. An inflow of mercury through natural watercourses is estimated to account for 25% of its current content [35,48]. Mason et al. [26] estimated that as much as 90% of mercury derived from the atmosphere is retained in soils in the areas of river catchments. Due to water erosion of European and UK soils, an approximate annual amount of 43 Mg mercury is released, of which around 6 Mg mercury reaches rivers [5];
- (iii)
- The solid phase of organic soils mainly consists of soil organic matter (SOM). Strong binding of mercury in soils is due to its high chemical affinity for organic matter [4,6,18,49,50,51]. Peatland drainage (very frequent in case of utilisation of organic soils) stimulates aerobic organic matter decomposition, production of dissolved organic matter (DOM) and humic substances (HS) [52,53,54,55]. Dissolved organic matter affects mercury speciation, mobility and toxicity in the environment [8,9,19,50,56,57,58,59]. DOM increases mercury mobility in the environment by, e.g., reducing precipitation of mercury sulphide [9,60]. HS are an example of the soil parameters which enhance mercury methylation [61];
- (iv)
- Soils of peatland areas (particularly drained ones) are characterised by great dynamics of oxidation-reduction conditions. Fluctuating groundwater levels result in, e.g., thermal changes in the soil and in pH values; they also affect vegetation cover [62,63,64]. These factors may affect mercury accumulation in soil [1,4,7,38,47,49,57,65,66];
- (v)
- Peatland drainage and agricultural utilisation result in increased soil trophicity which is followed by the enhanced microbiological activity of the soil [54,67,68]. In the study by Wang et al. [66], net production of methylmercury was positively correlated with the trophic requirements of vegetation and an increased availability of electron acceptors and donors for mercury-methylating microorganisms. Moreover, the authors believe that ecosystem characteristics which intensify microbial processes involved in mercury methylation also contribute to mercury reduction, which may lead to mercury re-emission into the atmosphere. Busaan et al. [42] showed that in wetland sediments with increasing temperature the rate of mercury methylation increases.
Peatland drainage for farming purposes is a major cause of fen peatland degradation in the temperate climate [69]. Peat degradation is followed by the formation of a murshic horizons which has an aggregate structure and a greater quantity of humic substances [52,54,68,70]. The process of peatland drainage is progressing due to climatic change which contributes to a negative water balance that is unfavourable for peatland development [71,72]. Disturbing forecasts of climate change in Central Europe point to declining annual precipitation and increased air temperature [73,74].
The aim of this research is to assess the secondary transformation of organic matter in soils of drained peatlands of the temperate climate zone and to investigate whether this affects the total mercury content and distribution in the soil profile. We pose the hypothesis that the process of secondary transformation of organic matter, intensified by organic soil drainage, significantly affects the distribution of total mercury in the soil profile.
2. Materials and Methods
2.1. Location and Soil Sampling
Field research was conducted in peatlands located in eastern Poland (the river Bug catchment):
- −
- The valley of the river Liwiec on the Siedlecka Upland (the number of physio-geographical region is 318.94) [75]. The region is a poorly peaty old glacial moraine upland with a 3.1% share of peatlands (region I) [76];
- −
- Peatlands in the area of the Wieprz-Krzna Canal (the number of physio-geographical region is 845.11) [75]. The region is a swampy sandy proglacial stream valley in Western Polesie (Pripet Marshes) with a high share (13.4%) of peatlands (region II) [76].
The study of peatlands in region I were drained in the 1960s whereas drainage of the soils in region II was performed somewhat later, that is in the 1970s. At present, they are intensively managed for agricultural purposes to produce cattle feed. According to the Köppen–Geiger’s climate classification [77], the peatlands are located in the fully humid warm temperate climatic zone with warm summers.
Nine soil profiles of grassland soils were chosen for laboratory analyses (4 from region I, 5 from region II). According to the FAO WBR system of soil classification [78], the examined soils are classified as Drainic Histosols. The study material comprised soil samples collected from 36 soil horizons that were determined according to the Polish Soil Classification System [79]. The soil samples were taken to the depth of 70 cm. The overall description of the examined soil horizons is presented in Table 1.

Table 1.
Soil morphological characteristics.
2.2. Laboratory Analysis
The following parameters were determined in the samples of fresh soil material:
- −
- Bulk density (ρa) by the gravimetric method. Soil samples, taken so as to preserve the original layout, were placed in cylinders (v = 100 cm3) and dried at 105 °C;
- −
- pH value by the potentiometric method, using a pH meter, in a suspension of H2Odist. (soil/water = 1/5).
The remaining analyses were performed in soil samples dried at room temperature and ground in a porcelain mortar (ø < 0.25 mm). The following analyses were carried out:
- −
- Ash content (ash) by the weighing method, after decrepitation in a muff furnace (T = 600 °C);
- −
- Total carbon (TC) and nitrogen (TN) contents using the PerkinElmer® 2400 Series II elemental analyzer with thermal conductivity detection (TCD) and acetanilide (C = 71.09%; N = 10.34%) as reference calibration standard;
- −
- Sequential fractioning of organic matter [80] (Table 2). Carbon content in extraction solutions was determined by the oxidation-titration method. The fractional composition of organic matter was expressed as the share of fraction carbon in TC (% TC);
 Table 2. Fractionation methods for soil organic matter.
Table 2. Fractionation methods for soil organic matter. - −
- Standard cold vapour atomic absorption spectrometry (CV-AAS) was used to measure the total mercury content. Based on in situ dry ashing followed by gold amalgamation cold vapour, AAS was evaluated. Using the mercury analyser AMA 254 (Altec, Prague, Czech Republic), the total content of mercury was determined in 3 replications. The detection limit was 0.01 ng Hg. Measurement conditions: wavelength 253.65 nm, gas–oxygen (purity ≥ 99.5%), with pressure 200–250 kPa. Times of the individual stages of the analysis were: 120, 150 (decomposition at 550 °C) and 60 s.
The analytical procedure of mercury determination was controlled with the use of the TILL-3 certified reference material (Table 3). Mercury content was expressed in units of mass per soil mass (Hgm/m, µg∙kg−1) and units of mass per soil volume (Hgm/V, µg∙dm−3).

Table 3.
Analysis of certified reference material (Geological Survey of Canada, Ottawa, Ontario).
Chemical analyses were replicated 3 times. The obtained results were related to absolute dry mass of the soil sample. The content of absolute dry mass was determined after the sample was dried at 105 °C.
2.3. Calculations and Formulas
- −
- Humin acid fraction content was calculated following the formula:
HAs-C = HS-C − FAs-C,
(see Table 2).
- −
- Residual fraction content (post-extraction residue) was computed following the formula:
Res-C = TC − (Lab-C + HS-C).
(see Table 2).
- −
- The values of the index of mercury distribution in the profile (Hg(DI)) were calculated following the formula:
- (a)
- Hg(DIm/m) = Hgm/m(M)/Hgm/m(Pt);
- (b)
- Hg(DIm/V) = Hgm/V(M)/Hgm/V(Pt);
where:
Hgm/m—mercury content (in µg·kg−1) in murshic (M) and peat (Pt) horizons;
Hgm/V—mercury content (in µg·dm−3) in murshic (M) and peat (Pt) horizons.
- −
- Mercury pool in the examined soils (to the depth of 70 cm) was calculated following the formula:
Hgstock—soil mercury pool (w mg·m−2);
i—number of soil horizon;
V = h·m2—soil horizon volume (in m3) whose thickness is h (in m) and area is 1 m2;
Hgm/V—mercury content (in μg·dm−3) in the soil horizon.
2.4. Statistical Analysis
Statistical calculations were performed using the statistical software STATISTICA 13 PL (TIBCO Software Inc., Palo Alto, CA, USA). Mean values of the examined parameters were compared using a one-way variance analysis and the post-hoc Tukey test. Statistically significant differences were determined at the significance level of p < 0.05. Relationships between the examined parameters were checked using a linear correlation coefficient (r). Selected relationships were presented as linear regression equations.
3. Results and Discussion
3.1. Evaluation of Secondary Transformations
In the present study, evaluation of secondary transformations occurring after drainage was based on observations of soil morphological characteristics, physical and chemical properties as well as fractional composition of organic matter of the identified soil horizons.
3.1.1. Soil Properties
During field studies, there were determined murshic horizons (M1, M2) with poorly diversified thickness, regardless of soil profile location. They contained stable soil aggregates with granular structure. Parental material layers of the study soils were made up of peat with a fibrous structure (Pt). An M3 horizon was determined between the M2 murshic horizon and peat horizon (Pt), which has morphological properties of both these horizons. A clear morphological distinction of top murshic horizons, compared to peat horizons, were confirmed by results of laboratory analyses as well as ANOVA (Table 4). The M1 and M2 horizons were found to form a uniform group compared to the layers below them; the group was characterised by a significantly higher ash content, greater density (higher ρa values), lower TC content and lower values of the TC/TN ratio. The obtained variation in the properties of the examined profiles is typical of drained organic soils of fern peatlands [52,54,55,67,68,70]. Processes of rapid organic matter decomposition contributed to a significant decline in organic matter content and nitrogen accumulation and, as a consequence, to lowering the values of the TC/TN ratio. The properties of the examined soils, in particular the carbon-to-nitrogen ratio, relatively low acidification and potentially good aeration of the M1 and M2 horizons are indicative of the fact that they are biologically active soils. This may increase the intensity of processes of organic matter decomposition and more rapid eutrophication of the soil environment [53,55,81,82,83].

Table 4.
Soil parameters.
3.1.2. Soil Organic Matter Fractions
Analysis of the carbon content in the identified organic matter fractions confirmed distinctiveness of the M1 and M2 horizons compared with the M3 and Pt horizons of the study soils (Table 5). The M1 and M2 horizons had a significantly higher share of labile organic matter forms (Lab-C) and humic substances extracted with 0.1 M NaOH (HS-C), both the fulvic acid fraction (FAs-C) and humic acid fraction (HAs-C). Simultaneously, no significant differences were found between values of the HAs-C/FAs-C ratio. Moreover, the M1 surface horizon had significantly the highest share of Lab-C fraction compared with the remaining horizons. It may be the effect of considerable biological activity as well as the inflow of fresh organic matter from the existing vegetation. Hence, we confirmed the association reported in the literature that peatland soil drainage and intensification of the process of secondary transformations enhance organic matter humification and increase the share of humic substances in the soil matter [52,54,68,84].

Table 5.
Share of organic carbon fraction (% TC).
An increase in the ash content of the study soils, and a simultaneous decline in TC content and increase in bulk density, was accompanied by significantly lower values of the TC/TN ratio (Table 6). Enhanced decomposition of organic matter is followed by lowering of the TC/TN values in the soil matter, as indicated by a significant negative correlation of the TC/TN ratio with Lab-C and HS-C, and a significant positive association with Res-C. Values of the Pearson coefficient of linear correlation undoubtedly indicate there were favourable conditions in the examined soils for organic matter transformations due to drainage and oxygenation of surface soil layers. Organic matter transformation under such conditions results in an increase in the amount of labile forms of organic matter and humic substances.

Table 6.
Significant correlation coefficient p < 0.05.
3.2. Mercury Content and Distribution in the Profile
Variation was noted in the total mercury content in the soil profiles of both the study regions (Table 7). Mercury concentration declined with an increasing depth in the profiles.

Table 7.
Total mercury content and values of enrichment factors in murshic horizons.
Peat in the soil parent material of both the regions had a very similar total mercury content (p = 0.999). The M1, M2 and M3 horizons in region I soils had a higher mercury content compared with surface horizons of soils in region II. No significant variation was found between them. The length of the period following drainage had no effect on the mercury concentration in the surface layer of soils in both the regions.
As soils of both the regions had a similar total mercury content (Table 8), the need was recognised to present the mechanism of mercury accumulation as a universal phenomenon in the soils. Taking into account the average total mercury content in the examined profiles, their horizons can be ranked in the following order: M1 > M2 > M3 > Pt. The highest values of enrichment factor indicate a substantial mercury accumulation in the M1 and M2 horizons, that is in the rhizosphere zone of the study soils. In particular, this applies to values of the factor calculated using soil volume (μg Hg·dm−3). Mercury accumulation in the M1 and M2 horizons was over four times as high as the values for M3 and Pt. The M1 and M2 murshic horizons formed uniform groups with a significantly higher total mercury contents compared with M3 and Pt (Table 9).

Table 8.
Results of statistical analysis (Anova, the Tukey test).

Table 9.
Values of descriptive statistics for mercury content and values of distribution index of mercury.
Mercury pools calculated to the depth of 0.7 m of the study soils were within the range of 17.5 to 39.6 mg·m−2 (Table 10). A varied mercury accumulation in profiles resulted in distinctively higher pools of this element in the M1, M2 and M3 horizons (on average, 82.2% of total mercury pool), the M2 horizon having 32.5% of the total mercury pool. The mercury pool in the soils of region I was significantly higher compared with the pool in region II soils (Table 11).

Table 10.
Mercury stock in soils.

Table 11.
Result of statistical analysis of mercury stocks in the regions (Anova, Tukey test).
Estimation of mercury pools in soils made it possible to evaluate interactions between the total mercury content and other soil parameters and was used to determine the degree of its accumulation. It should be stressed that mercury contents in the examined soils were multiple times lower than the permissible content in Poland which poses a risk of contamination [85], and which is 5.5 Hg kg−1 dry soil mass for the 0–25 cm layer of organic soils. The regulatory depth of 0.25 cm corresponds approximately to the combined thickness of the M1 and M2 horizons which form the rhizosphere zone of the examined soils. The mercury contents determined in the horizons of the study soils are far higher than those reported for European Union soils by Ballabio et al. [4] who estimated the content’s median to be around 38.3 μg·kg−1. Data reported for various soils on a worldwide basis show that mean concentrations of mercury in surface soils do not exceed 400 µg/kg [24]. The organic soils and paddy soils are likely to retain more than any other soils. The mean content of total mercury in agricultural surface soils and forest soils of Poland is estimated at 61 µg/kg, within the range 3.4 to 284.4 µg/kg.
The total mercury content in the analysed soils may be associated with both natural and anthropogenic sources. It is difficult to distinguish between these two sources. Many researchers point to the atmosphere as the main source of peatland contamination with mercury, it being either dry or wet deposition [14,20,25,86,87]. The phenomenon is global in character [1,19]. According to research by Benoit et al. [88], pre-industrial deposition in Minnesota (the USA) was approximately 4 μg·m−2·year−1 and, in the 1990s, it exceeded 190 μg·m−2·year−1. In the work by Coggins et al. [89], the index of mercury accumulation from 1950 to 1970 in various Irish peatlands was from 6 to 24 μg·m−2·year−1. The large amount of mercury in peatlands compared to deposition and export in surface water runoff indicates that they have accumulated mercury from past atmospheric deposition [6].
Mercury accumulation in surface horizons of the study soils was the result of direct atmospheric deposition of this metal from local/global anthropogenic sources of its emission. Moreover, the murshic horizon (M1) is rich in humus colloids and it can bind more mercury whereas the existing vegetation may exacerbate the interception of this metal from the atmosphere. Osterwalder et al. [17] point out that mercury pools in peatlands can be a source of this metal for decades even if deposition decreases. The store of mercury in peat and organic soils is very large compared to runoff fluxes [17]. Changes in metal concentration in peats are associated with bulk density and ash content in the peat [89]. Numerous studies point to the trend of higher heavy metal concentrations in upper peat layers compared with lower ones [65,90,91,92].
In our study, values of correlation coefficients indicate that mercury accumulation in drained organic soils to a large extent may have resulted from organic matter transformation (Table 12). Quantitative changes in physical and chemical parameters of the study soils during the process of secondary transformation are significantly correlated with mercury content. Organic matter mineralisation and increased ash content had a significant positive effect on mercury accumulation in the examined surface layer of the soils (Figure 1). Many works have confirmed that organic matter mineralisation in the process of secondary transformation results in an increase in the concentration of ash components in surface soil horizons [54,67,93]. In addition, research by Golovatskaya and Lyapina [49] has demonstrated a higher mercury content in surface and oxygenated layers of peat. Plants can act as pathways for the transfer of mercury from the geosphere to the atmosphere or as a source of mercury in the air–plant–soil system after the decomposition of organic matter [94,95].

Table 12.
Significant correlation coefficients between soil properties and mercury content, p < 0.05.
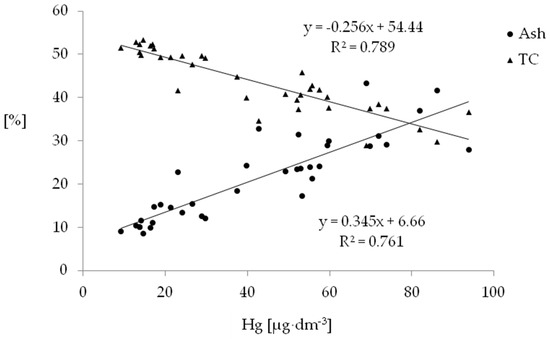
Figure 1.
Relationship between mercury content, ash content and TC content in the studied soil horizons.
A significant relationship between mercury accumulation and share of humic substances in the study soils is presented in Figure 2. Many researchers believe the extent of SOM decomposition and an increased share of humic substances in the soil mass account for the higher mercury content in drained organic soils compared with natural peatlands [8,49]. It is these substances that play an important role in the process of mercury binding in this type of soils [49]. Concentration of mercury in soil is a function of deposition rate and carbon turnover time. Mercury accumulation in soil is strongly influenced by the organic matter decomposition degree [96].
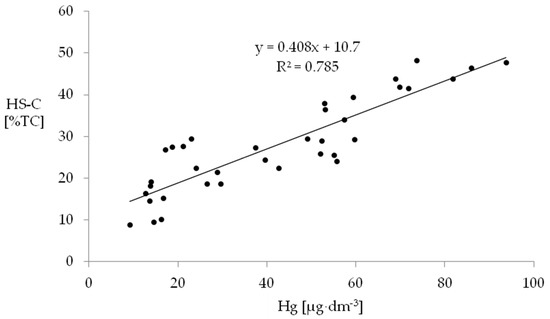
Figure 2.
Relationship between mercury content and carbon content of humic substances (HS-C) in the studied soil horizons.
4. Conclusions
In our research, we found a significant effect of the secondary transformation of organic matter occurring in drained peatlands of the temperate climate zone on the total mercury content and stock in soils. The highest content and differentiation of mercury occurred in murshic horizons (up to a maximum depth of 43 cm). The average mercury content of the distinguished soil horizons is grouped in the following series (in μg kg−1): M1 (212.0) > M2 (182.8) > M3 (126.3) > Pt (84.9). The mercury stock, up to a depth of 70 cm in the tested soils, ranged from 17.5 to 39.6 mg m−2. As much as 82.2% of soil mercury was found in the upper murshic horizons. We found strong correlations between soil properties characteristically variable in the secondary transformation process and total mercury content. The increased content of humic substances in murshic horizons caused a significant increase in the total mercury content.
Our research is of great importance for soil monitoring, as the amount of determined mercury was greatly influenced by the depth of sampling (up to 25 cm). The results of the research should be taken into account when planning the restoration of peatlands of the temperate climate zone. There is a potential risk that elevated mercury concentrations in the upper murshic horizons may be a source of methylmercury for a long period of time. In peat soils with a high concentration of mercury, the risk of contamination with this toxic metal should be determined before re-irrigation.
Conscious actions should be taken to slow down the murshic process and thus reduce the emission of significant amounts of methylmercury and CO2 into the atmosphere. Organic carbon contained in peatlands and natural meadows is a significant share in the circulation of this element in the environment. Mercury reserves in peat soils can be a reservoir of this metal for many decades and centuries. The assessment of this risk requires clarification and comprehensive scientific research in the future.
Author Contributions
Conceptualisation, M.B. and M.K.; methodology, M.B. and M.K.; validation, M.B. and M.K.; formal analysis, M.B., M.K., K.P. and D.J.; investigation, M.B., M.K. and D.J.; resources, M.B., M.K. and K.P.; data curation, M.B. and M.K.; writing—original draft preparation, M.B. and M.K.; writing—review and editing, M.B., M.K., K.P. and D.J.; visualisation, M.B., M.K., K.P. and. D.J.; supervision, M.B. and M.K.; project administration, M.B. and K.P.; funding acquisition, M.B. and K.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported under the research theme no. 158/23/B by the Ministry of Science and Higher Education.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- O’Connor, D.; Houa, D.; Ok, Y.S.; Mulderd, J.; Duana, L.; Wua, Q.; Wanga, S.; Tacke, F.M.G.; Rinklebef, J. Mercury speciation, transformation, and transportation in soils, atmospheric flux, and implications for risk management: A critical review. Environ. Int. 2019, 126, 747–761. [Google Scholar] [CrossRef]
- Gustin, M.S.; Bank, M.S.; Bishop, K.; Bowman, K.; Branfireun, B.; Chételat, J.; Eckley, C.S.; Hammerschmidt, C.R.; Lamborg, C.; Lyman, S.; et al. Mercury biogeochemical cycling: A synthesis of recent scientific advances. Sci. Total Environ. 2020, 737, 139619. [Google Scholar] [CrossRef] [PubMed]
- Gworek, B.; Dmuchowski, W.; Baczewska-Dąbrowska, A.H. Mercury in the terrestrial environment: A review. Environ. Sci. Eur. 2020, 32, 128. [Google Scholar] [CrossRef]
- Ballabio, C.; Jiskra, M.; Osterwalder, S.; Borrelli, P.; Montanarella, L.; Panagos, P. A spatial assessment of mercury content in the European Union topsoil. Sci. Total Environ. 2021, 769, 144755. [Google Scholar] [CrossRef]
- Panagos, P.; Jiskra, M.; Borrelli, P.; Liakos, L.; Ballabio, C. Mercury in European topsoils: Anthropogenic sources, stocks and fluxes. Environ. Res. 2021, 201, 111556. [Google Scholar] [CrossRef] [PubMed]
- Grigal, D.F. Mercury Sequestration in Forests and Peatlands: A Review. J. Environ. Qual. 2003, 32, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Beckers, F.; Rinklebe, J. Cycling of mercury in the environment: Sources, fate, and human health implications-a review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 693–794. [Google Scholar] [CrossRef]
- Sysalova, J.; Kucera, J.; Drtinova, B.; Cervenka, R.; Zverina, O.; Komarek, J.; Kamenik, J. Mercury species in formerly contaminated soils and released soil gases. Sci. Total Environ. 2017, 584, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.; Zhang, F.; Chen, X.; Ge, S.; Mu, M.; Jia, L.; Wu, Q.; Zhang, T. Carbon and mercury export from the Arctic rivers and response to permafrost degradation. Water Res. 2019, 161, 54–60. [Google Scholar] [CrossRef] [PubMed]
- de Vries, W.; Römkens, P.F.A.M.; Schütze, G. Critical soil concentrations of cadmium, lead, and mercury in view of health effects on humans and animals. Rev. Environ. Contamin Toxic 2007, 191, 91–130. [Google Scholar] [CrossRef]
- Hong, Y.S.; Kim, Y.M.; Lee, K.E. Methylmercury exposure and health effects. J. Prev. Med. Public Health 2012, 45, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Eagles-Smith, C.A.; Silbergeld, E.K.; Basu, N.; Bustamante, P.; Diaz-Barriga, F.; Hopkins, W.A.; Karen, A.; Kidd, K.A.; Nyland, J.F. Modulators of mercury risk to wildlife and humans in the context of rapid global change. Ambio 2018, 47, 170–197. [Google Scholar] [CrossRef] [PubMed]
- European Union. Official Journal of the European Union, L 364/5. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs (Text with EEA relevance). Document 02006R1881-20230101. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32006R1881 (accessed on 27 April 2023).
- Cooke, C.A.; Martínez-Cortizas, A.; Bindler, R.; Gustin, M.S. Environmental archives of atmospheric Hg deposition–A review. Sci. Total Environ. 2020, 709, 134800. [Google Scholar] [CrossRef]
- Pacyna, E.G.; Pacyna, J.M.; Steenhuisen, F.; Wilson, S. Global anthropogenic mercury emission inventory for 2000. Atmos. Environ. 2006, 40, 4048–4063. [Google Scholar] [CrossRef]
- Streets, D.G.; Zhang, Q.; Wu, Y. Projections of global mercury emissions in 2050. Environ. Sci. Technol. 2009, 43, 2983–2988. [Google Scholar] [CrossRef]
- Osterwalder, S.; Bishop, K.; Alewell, C.; Fritsche, J.; Laudon, H.; Åkerblom, S.; Nilsson, M.B. Mercury evasion from a boreal peatland shortens the timeline for recovery from legacy pollution. Sci. Rep. 2017, 7, 16022. [Google Scholar] [CrossRef]
- Kohlenberg, A.J.; Turetsky, M.R.; Thompson, D.K.; Branfireun, B.A.; Mitchell, C.P.J. Controls on boreal peat combustion and resulting emissions of carbon and mercury. Environ. Res. Lett. 2018, 13, 035005. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, S.; Li, Y.; Han, D.; Liu, H.; Wang, G. Holocene mercury accumulation calibrated by peat decomposition in a peat sequence from the Sanjiang Plain, northeast China. Quatern. Int. 2019, 527, 19–28. [Google Scholar] [CrossRef]
- Miszczak, E.; Stefaniak, S.; Michczyński, A.; Steinnes, E.; Twardowska, I. A novel approach to peatlands as archives of total cumulative spatial pollution loads from atmospheric deposition of airborne elements complementary to EMEP data: Priority pollutants (Pb, Cd, Hg). Sci. Total Environ. 2020, 705, 135776. [Google Scholar] [CrossRef] [PubMed]
- Obrist, D.; Kirk, J.L.; Zhang, L.; Sunderland, E.M.; Jiskra, M.; Selin, N.E. A review of global environmental mercury processes in response to human and natural perturbations: Changes of emissions, climate, and land use. Ambio 2018, 47, 116–140. [Google Scholar] [CrossRef]
- Zhang, Y.; Jacob, D.J.; Horowitz, H.M.; Chen, L.; Amos, H.M.; Krabbenhoft, D.P. Observed decrease in atmospheric mercury explained by global decline in anthropogenic emissions. Proc. Natl. Acad. Sci. USA 2016, 113, 526–531. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, Z.; Huang, S.; Zhang, P.; Peng, Y.; Wu, P.; Gu, J.; Dutkiewicz, S.; Zhang, H.; Wu, S.; et al. Global health effects of future atmospheric mercury emissions. Nat. Commun. 2021, 12, 3035. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Pandias, H. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Kobierski, M.; Malczyk, P. Rtęć w glebach poletek łowieckich oraz otaczających je lasów. Mercury in the soils of hunting plots and surrounding forests. Sylwan 2016, 160, 433–440. [Google Scholar]
- Mason, R.P.; Lawson, N.M.; Sullivan, K.A. Atmospheric deposition to the Chesapeake Bay watershed—Regional and local sources. Atmos. Environ. 1997, 31, 3531–3540. [Google Scholar] [CrossRef]
- Rossmann, R. Lake Michigan 1994–1996 surficial sediment mercury. J. Great Lakes Res. 2002, 28, 65–76. [Google Scholar] [CrossRef]
- Ruggiero, P.; Terzano, R.; Spagnuolo, M.; Cavalca, L.; Colombo, M.; Andreoni, V.; Rao, M.A.; Perucci, P.; Monaci, E. Hg bioavailability and impact on bacterial communities in a long-term polluted soil. J. Environ. Monit. 2011, 13, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Patra, M.; Sharma, A. Mercury toxicity in plants. Bot. Rev. 2000, 66, 379–422. [Google Scholar] [CrossRef]
- Selin, N.E. Global biogeochemical cycling of mercury: A review. Ann. Rev. Environ. Res. 2009, 34, 43–63. [Google Scholar] [CrossRef]
- Smith-Downey, N.V.; Sunderland, E.M.; Jacob, D.J. Anthropogenic impacts on global storage and emissions of mercury from terrestrial soils: Insights from a new global model. J. Geophys. Res. Biogeosci. 2010, 115, G03008. [Google Scholar] [CrossRef]
- Wojtuń, B.; Samecka-Cymerman, A.; Kolon, K.; Kempers, A.J.; Skrzypek, G. Metals in some dominant vascular plants, mosses, lichens, algae, and the biological soil crust in various types of terrestrial tundra, SW Spitsbergen, Norway. Polar Biol. 2013, 36, 1799–1809. [Google Scholar] [CrossRef]
- Shotyk, W.; Goodsite, M.E.; Roos-Barraclough, F.; Frel, R.; Heinemeier, J.; Asmund, G.; Lohse, C.; Hansen, T.S. Anthropogenic contributions to atmospheric Hg, Pb and As accumulation recorded by peat cores from southern Greenland and Denmark dated using the 14C “bomb pulse curve”. Geochim. Cosmochim. Acta. 2003, 67, 3991–4011. [Google Scholar] [CrossRef]
- Rudd, J.W.M. Sources of methyl mercury to freshwater ecosystems: A review. Water Air Soil Pollut. 1995, 80, 697–713. [Google Scholar] [CrossRef]
- St. Louis, V.L.; Rudd, J.W.M.; Kelly, C.A.; Beaty, K.G.; Flett, R.J.; Roulet, N.T. Production and loss of methylmercury from boreal forest catchments containing different types of wetland. Environ. Sci. Technol. 1996, 30, 2719–2729. [Google Scholar] [CrossRef]
- Branfireun, B.A.; Roulet, N.T. Controls on the fate and transport of methylmercury in a boreal headwater catchment, northwestern Ontario, Canada. Hydrol. Earth Syst. Sci. 2002, 6, 785–794. [Google Scholar] [CrossRef]
- Mitchell, C.P.J.; Branfireun, B.A.; Kolka, R.K. Total mercury and methylmercury dynamics in upland–peatland watersheds during snowmelt. Biogeochemistry 2008, 90, 225–241. [Google Scholar] [CrossRef]
- Braaten, H.F.V.; de Wit, H.A. Effects of disturbance and vegetation type on total and methylmercury in boreal peatland and forest soils. Environ. Pollut. 2016, 218, 140–149. [Google Scholar] [CrossRef]
- Bishop, K.; Shanley, J.B.; Riscassi, A.; de Wit, H.A.; Eklöf, K.; Meng, B. Recent advances in understanding and measurement of mercury in the environment: Terrestrial Hg cycling. Sci. Total Environ. 2020, 721, 137647. [Google Scholar] [CrossRef]
- Branfireun, B.A.; Cosio, C.; Poulain, A.J.; Riise, G.; Bravo, A.G. Mercury cycling in freshwater systems—An updated conceptual model. Sci. Total Environ. 2020, 745, 140906. [Google Scholar] [CrossRef]
- Wu, P.; Kainz, M.J.; Bravo, A.G.; Åkerblom, S.; Sonesten, L.; Bishop, K. The importance of bioconcentration into the pelagic food web base for methylmercury biomagnification: A meta-analysis. Sci. Total Environ. 2019, 646, 357–367. [Google Scholar] [CrossRef]
- Bussan, D.D.; Douvris, C.; Cizdziel, J.V. Mercury Methylation Potentials in Sediments of an Ancient Cypress Wetland Using Species-Specific Isotope Dilution GC-ICP-MS. Molecules 2022, 27, 4911. [Google Scholar] [CrossRef]
- Barej, R.; Kwaśnicki, R.; Chojnacka, K.; Bolanowski, J.; Dobrzański, P.; Pokorny, P. Mercury content in rural and industrial regions in Lower Silesia. Polish J. Environ. Stud. 2009, 18, 547–552. [Google Scholar]
- Czaban, S.; Kolacz, R.; Dobrzanski, Z.; Bubel, F.; Opalinski, S.; Durkalec, M.; Gruszczynski, M.F. Mercury content in the area of the” Zelazny Most” tailings pond. Przemysl. Chem. 2013, 92, 1268–1271. [Google Scholar]
- Dobrzański, Z.; Kołacz, R.; Czaban, S.; Bubel, F.; Malczewski, M.; Kupczyński, R.; Opaliński, S. Assessing mercury content in plant and animal raw materials in an area impacted by the copper industry. Polish J. Environ. Stud. 2017, 26, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Boszke, L.; Kowalski, A.; Głosińska, G.; Szarek, R.; Siepak, J. Environmental factors affecting speciation of mercury in the bottom sediments; an overview. Polish J. Environ. Stud. 2003, 12, 5–13. [Google Scholar]
- Haynes, K.M.; Kane, E.S.; Potvin, L.; Lilleskov, E.A.; Kolka, R.K.; Mitchell, C.P. Gaseous mercury fluxes in peatlands and the potential influence of climate change. Atmos. Environ. 2017, 154, 247–259. [Google Scholar]
- Bigham, G.N.; Vandal, G.M. A drainage basin perspective of mercury transport and bioaccumulation: Onondaga Lake, New York. Neurotoxicology 1996, 17, 279–290. [Google Scholar]
- Golovatskaya, E.A.; Lyapina, E.E. Distribution of total mercury in peat soil profiles in West Siberia. Contemp. Probl. Ecol. 2009, 2, 156–161. [Google Scholar]
- He, M.; Tian, L.; Braaten, H.F.V.; Wu, Q.; Luo, J.; Cai, L.M.; Meng, J.H.; Lin, Y. Mercury–Organic Matter Interactions in Soils and Sediments: Angel or Devil? Bull. Environ. Contam. Toxicol. 2019, 102, 621–627. [Google Scholar] [CrossRef]
- Hesterberg, D.; Chou, J.W.; Hutchison, K.J.; Sayers, D.E. Bonding of Hg(II) to reduced organic sulfur in humic acid as affected by S/Hg ratio. Environ. Sci. Technol. 2001, 35, 2741–2745. [Google Scholar] [CrossRef]
- Becher, M.; Tołoczko, W.; Godlewska, A.; Pakuła, K.; Żukowski, E. Fractional Composition of Organic Matter and Properties of Humic Acids in the Soils of Drained Bogs of the Siedlce Heights in Eastern Poland. J. Ecol. Eng. 2022, 23, 208–222. [Google Scholar] [CrossRef]
- Glina, B.; Piernik, A.; Mocek-Płóciniak, A.; Maier, A.; Glatzel, S. Drivers controlling spatial and temporal variation of microbial properties and dissolved organic forms (DOC and DON) in fen soils with persistently low water tables. Glob. Ecol. Conserv. 2021, 27, 1–14. [Google Scholar] [CrossRef]
- Łachacz, A.; Kalisz, B.; Sowiński, P.; Smreczak, B.; Niedźwiecki, J. Transformation of Organic Soils Due to Artificial Drainage and Agricultural Use in Poland. Agriculture 2023, 13, 634. [Google Scholar] [CrossRef]
- Becher, M.; Kalembasa, D.; Kalembasa, S.; Symanowicz, B.; Jaremko, D.; Matyszczak, A. A New Method for Sequential Fractionation of Nitrogen in Drained Organic (Peat) Soils. Int. J. Environ. Res. Public Health 2023, 20, 2367. [Google Scholar] [CrossRef]
- Fahnestock, M.F.; Bryce, J.G.; McCalley, C.K.; Montesdeoca, M.; Bai, S.; Li, Y.; Driscoll, C.T.; Crill, P.M.; Rich, V.I.; Varner, R.K. Mercury reallocation in thawing subarctic peatlands. Geochem. Perspect. Let. 2019, 11, 33–38. [Google Scholar] [CrossRef]
- Skyllberg, U.; Xia, K.; Bloom, P.; Nater, E.; Bleam, W. Binding of mercury (II) to reduced sulphur groups in soil organic matter along upland-peat soil transects. J. Environ. Qual. 2000, 29, 855–865. [Google Scholar] [CrossRef]
- Kolka, R.K.; Grigal, D.F.; Nater, E.A.; Verry, E.S. Hydrologic cycling of mercury and organic carbon in a forested upland–bog watershed. Soil Sci. Soc. Am. J. 2001, 65, 897–905. [Google Scholar] [CrossRef]
- Veretennikova, E.E.; Golovatskaya, E.A. Lead and mercury distribution in the peat deposits of West Siberia (Vasyuganye Peat Bogs). Chem. Sustain. Dev. 2012, 20, 143–149. [Google Scholar]
- Ravichandran, M. Interactions between mercury and dissolved organic matter—A review. Chemosphere 2004, 55, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.H. Review of possible paths for abiotic methylation of mercury(II) in aquatic environment. Chemosphere 1993, 26, 2063–2077. [Google Scholar] [CrossRef]
- Gnatowski, T.; Szatyłowicz, J.; Brandyk, T.; Kechavarzi, C. Hydraulic properties of fen peat soils in Poland. Geoderma 2010, 154, 188–195. [Google Scholar] [CrossRef]
- Schindler, U.; Behrendt, A.; Müller, L. Change of soil hydrological properties of fens as a result of soil development. J. Plant Nutr. Soil Sci. 2003, 166, 357–363. [Google Scholar] [CrossRef]
- Schwärzel, K.; Renger, M.; Sauerbrey, R.; Wessolek, G. Soil physical characteristics of peat soils. J. Plant Nutrit. Soil Sci. 2002, 165, 479–486. [Google Scholar] [CrossRef]
- Roos-Barraclough, F.; Givelet, N.; Martinez-Cortizas, A.; Goodsite, M.E.; Biester, H.; Shotyk, W. An analytical protocol for the determination of total mercury concentrations in solid peat samples. Sci Total Environ. 2002, 292, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Nilsson, M.B.; Eklöf, K.; Hu, H.; Ehnvall, B.; Bravo, A.G.; Zhong, S.; Åkeblom, S.; Björn, E.; Bertilsson, S.; et al. Opposing spatial trends in methylmercury and total mercury along a peatland chronosequence trophic gradient. Sci. Total Environ. 2020, 718, 137306. [Google Scholar] [CrossRef] [PubMed]
- Becher, M.; Pakuła, K.; Jaremko, D. Phosphorus Accumulation in the Dehydrated Peat Soils of the Liwiec River Valley. J. Ecol. Eng. 2020, 21, 213–220. [Google Scholar] [CrossRef]
- Okruszko, H. Transformation of fen-peat soils under the impact of draining. Adv. Agri. Sci. Probl. 1993, 406, 3–73. [Google Scholar]
- Joosten, H.; Tanneberger, F.; Moen, A. Mires and Peatlands of Europe Status, Distribution and Conservation; Schweizerbart Science Publishers: Stuttgart, Germany, 2017. [Google Scholar]
- Glina, B.; Gajewski, P.; Kaczmarek, Z.; Owczarzak, W.; Rybczyński, P. Current state of peatland soils as an effect of long-term drainage—Preliminary results of peat-land ecosytems investigations in the Grójecka Valley (central Poland). Soil Sci. Ann. 2016, 67, 3–9. [Google Scholar] [CrossRef]
- Song, L.; Guanter, L.; Guan, K.; You, L.; Huete, A.; Ju, W.Y. Satellite suninduced chlorophyll fluorescence detects early response of winter wheat to heat Zhang stress in the Indian Indo-Gangetic Plains Glob. Chang. Biol. 2018, 24, 4023–4037. [Google Scholar] [CrossRef] [PubMed]
- Buras, A.R.; Zang, C.S. Quantifying impacts of the 2018 drought on European ecosystems in comparison to 2003. Biogeosciences 2020, 17, 1655–1672. [Google Scholar] [CrossRef]
- Europe Is Getting Warmer, and It’s Not Looking Like It’s Going to Cool Down Anytime Soon. Available online: https://www.europeandatajournalism.eu/News/Data-news/Europe-is-getting-warmer-and-it-s-not-looking-like-it-s-going-to-cool-down-anytime-soon (accessed on 21 December 2022).
- Orth, R.; Zscheischler, J.; Seneviratne, S.I. Record dry summer in 2015 challenges precipitation projections in Central Europe. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Solon, J.; Borzyszkowski, J.; Bidłasik, M.; Richling, A.; Badora, K.; Balon, J.; Brzezińska-Wójcik, T.; Chabudziński, Ł.; Dobrowolski, R.; Grzegorczyk, I.; et al. Physicogeographical mesoregions of Poland: Verifi cation and adjustment of boundaries on the basis of contemporary spatial data. Geogr. Pol. 2018, 91, 143–170. [Google Scholar] [CrossRef]
- Dembek, W.; Piorkowski, H.; Rycharski, M. Wetlands against the Background of Physico-Geograpfical Regionalization of Poland; The Institute for Land Reclamation and Grassland Farming: Falenty, Poland, 2000. [Google Scholar]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger Climate Classification Updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- IUSS World Reference Base for Soil Resources 2014. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; FAO: Rome, Italy, 2015.
- Kabała, C.; Charzyński, P.; Chodorowski, J.; Drewnik, M.; Glina, B.; Greinert, A.; Hulisz, P.; Jankowski, M.; Jonczak, J.; Łabaz, B.; et al. Polish Soil Classification, 6th Edition—Principles, Classification Scheme and Correlations. Soil Sci. Ann. 2019, 70, 71–97. [Google Scholar] [CrossRef]
- Becher, M.; Banach-Szott, M.; Godlewska, A. Organic Matter Properties of Spent Button Mushroom Substrate in the Context of Soil Organic Matter Reproduction. Agronomy 2021, 11, 204. [Google Scholar] [CrossRef]
- Banach-Szott, M.; Dziamski, A. Kwasy huminowe wieloletnich użytków zielonych Kompleksu Łąk Czerskich. Soil Sci. Ann. 2022, 73, 156099. [Google Scholar] [CrossRef]
- Kalisz, B.; Łachacz, A.; Glazewski, R. Transformation of some organic matter components in organic soils exposed to drainage. Turk. J. Agric. For. 2010, 34, 245–256. [Google Scholar] [CrossRef]
- Leifeld, J.; Klein, K.; Wüst-Galley, C. Soil organic matter stoichiometry as indicator for peatland degradation. Sci. Rep. 2020, 10, 7634. [Google Scholar] [CrossRef] [PubMed]
- Glina, B.; Bogacz, A.; Mendyk, Ł.; Boiko, O.; Nowak, M. Effect of restoration of a degraded shallow mountain fen after five years. Mires Peat 2018, 21, 1–15. [Google Scholar]
- Minister of the Environment. Regulation of the Minister of the Environment of 1 September 2016 on the method of conducting the assessment of land surface pollution. J. Laws 2016 2016, 1395. Available online: https://faolex.fao.org/docs/pdf/pol182531.pdf (accessed on 27 April 2023).
- Liu, R.; Wang, Q.; Lu, X.; Fang, F.; Wang, Y. Distribution and speciation of mercury in the peat bog of Xiaoxing’an Mountain, northeastern China. Environ. Pollut. 2003, 124, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Enrico, M.; Le Roux, G.; Heimbürger, L.E.; Van Beek, P.; Souhaut, M.; Chmeleff, J.; Sonke, J.E. Holocene atmospheric mercury levels reconstructed from peat bog mercury stable isotopes. Environ. Sci. Technol. 2017, 51, 5899–5906. [Google Scholar] [CrossRef] [PubMed]
- Benoit, J.M.; Fitzgerald, W.F.; Damman, A.W.H. The biogeochemistry of an ombrotrophic bog: Evaluation of use as an archive of atmospheric mercury deposition. Environ. Res. 1998, 178, 118–133. [Google Scholar] [CrossRef] [PubMed]
- Coggins, A.M.; Jennings, S.G.; Ebinghaus, R. Accumulation rates of the heavy metals lead, mercury and cadmium in ombrotrophic peatlands in the west of Ireland. Atmos. Environ. 2006, 40, 260–278. [Google Scholar] [CrossRef]
- Weis, D.; Shotyk, W.; Boyle, E.A.; Kramers, J.D.; Appleby, P.G.; Cheburkin, A.K. Comparative study of the temporal evolution of atmospheric lead deposition in Scotland and eastern Canada using blanket peat bogs. Sci Total Environ. 2002, 292, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Cortizas, A.M.; Garcίa-Rodeja Gayoso, E.; Weiss, D. Peat bog archives of atmospheric metal deposition. Sci. Total Environ. 2002, 292, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Talbot, J.; Moore, T.R.; Wang, M.; Ouellet Dallaire, C.; Riley, J.L. Distribution of lead and mercury in Ontario peatlands. Environ. Pollut. 2017, 231, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Silamikele, I.; Klavins, M.; Nikodemus, O. Major and trace element distribution in the peat from ombrotrophic bogs in Latvia. J. Environ. Sci. Health A Tox Hazard Subst. Environ. Eng. 2011, 46, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Schwesig, D.; Krebs, O. The role of ground vegetation in the uptake of mercury and methylmercury in a forest ecosystem. Plant Soil 2003, 253, 445–455. [Google Scholar] [CrossRef]
- Fay, L.; Gustin, M. Assessing the influence of different atmospheric and soil mercury concentrations on foliar mercury concentrations in a controlled environment. Water Air Soil Pollut. 2007, 181, 373–384. [Google Scholar] [CrossRef]
- Obrist, D.; Johnson, D.W.; Lindberg, S.E. Mercury concentrations and pools in four Sierra Nevada Forest sites and relationships to organic carbon and nitrogen. Biogeosciences 2009, 6, 765–777. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).