Ivy Geranium (Pelargonium peltatum (L.) L’Hér.) Plant Growth and Flowering as Affected by Mineral or Biofertilizer with or without Compost Amendment
Abstract
1. Introduction
2. Materials and Methods
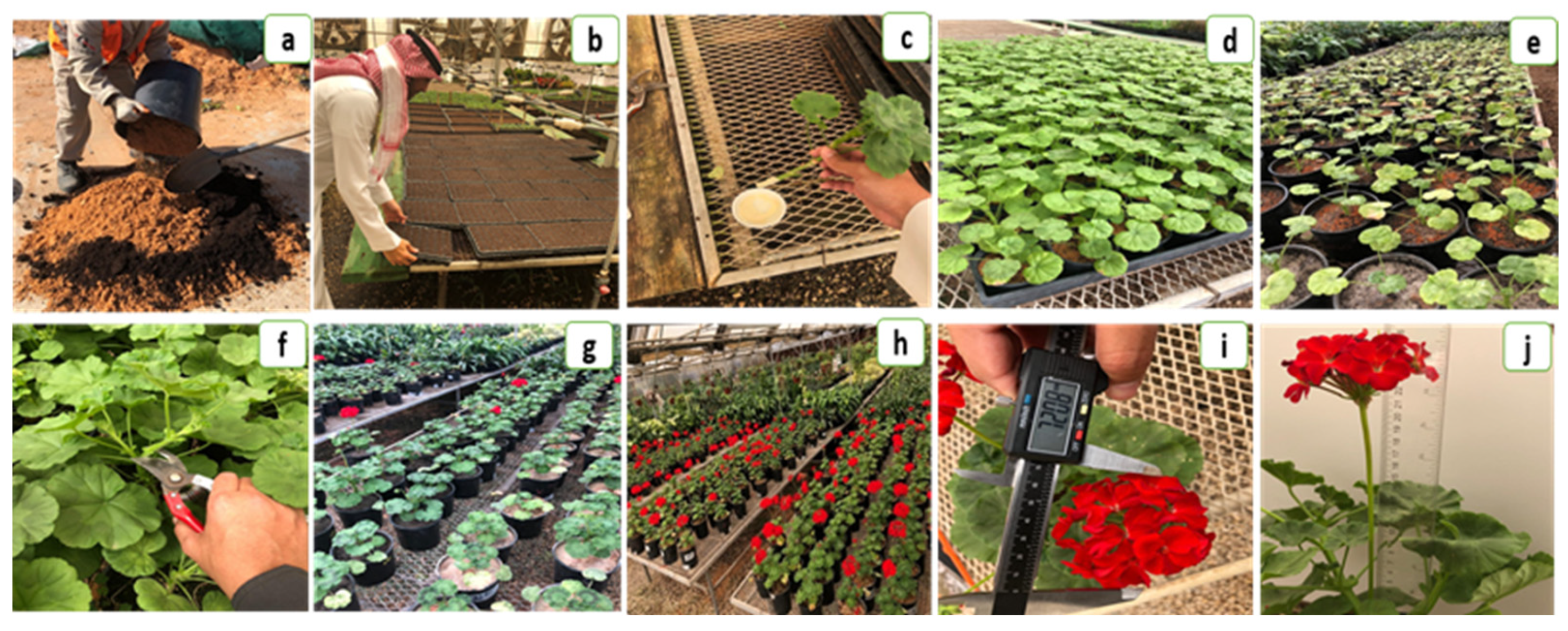
2.1. Plant Materials and Study Site
2.2. Experimental Design
2.3. Experimental Layout
2.4. Data Recorded
2.5. Statistical Analysis
3. Results
3.1. Growth and Flowering Attributes
3.2. Ion Percentage and Chlorophyll Concentrations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- UN (United Nations). World Urbanization Prospects: The 2018 Revision; United Nations, Department of Economic and Social Affairs, Population Division: New York, NY, USA, 2018; pp. 1–126. [Google Scholar]
- IPCC (Intergovernmental Panel on Climate Change). Urban Areas Climate Change 2014—Impacts, Adaptation and Vulnerability: Part A: Global and Sectoral Aspects: Working Group II Contribution to the IPCC Fifth Assessment Report; Cambridge University Press: Cambridge, UK, 2014; pp. 535–612. [Google Scholar]
- WHO (World Health Organization); UN-Habitat. Global Report on urban Health: Equitable Healthier Cities for Sustainable Development; World Health Organization: Geneva, Switzerland, 2016; pp. 1–239. Available online: https://apps.who.int/iris/handle/10665/204715 (accessed on 4 October 2022).
- Kendal, D.; Williams, N.S.; Williams, K.J. A cultivated environment: Exploring the global distribution of plants in gardens, parks and streetscapes. Urban Ecosyst. 2012, 15, 637–652. [Google Scholar] [CrossRef]
- Wilson, A.; Kendal, D.; Moore, J.L. Humans and ornamental plants: A mutualism? Ecopsychology 2016, 8, 257–263. [Google Scholar] [CrossRef]
- Savé, R. What is stress and how to deal with it in ornamental plants? Acta Hortic. 2009, 13, 241–254. [Google Scholar] [CrossRef]
- Keles, B.; Ertürk, Y. Advantages of microorganism containing biological fertilizers and evaluation of their use in ornamental plants. Int. J. Agric. For. Life Sci. 2021, 5, 189–197. [Google Scholar]
- Mamba, B.; Wahome, P.K. Propagation of geranium (Pelargonium hortorum) using different rooting medium components. Am.-Eurasian J. Agric. Environ. Sci. 2010, 7, 497–500. [Google Scholar] [CrossRef]
- Abedini Aboksari, H.; Hashemabadi, D.; Kaviani, B. Application of biofertilizer for Pelargonium peltatum growth in new organic substrates. J. Plant Nutr. 2018, 41, 137–149. [Google Scholar] [CrossRef]
- Krishnaraj, S.; Dan, T.; Saxena, P. A fragrant solution to soil remediation. Int. J. Phytoremediation 2000, 2, 117–132. [Google Scholar] [CrossRef]
- De Lucia, B.; Cristiano, G.; Vecchietti, L.; Rea, E.; Russo, G. Nursery growing media: Agronomic and environmental quality assessment of sewage sludge-based compost. Appl. Environ. Soil Sci. 2013, 10, 565139. [Google Scholar] [CrossRef]
- Merida, D.; Pivetta, K.F.L.; Mazzini-Guedes, R.B.; de Castro, C.E.F.; Purquerio, L.F.V. Effects of nitrogen fertilization on development, flowering, and mineral nutrition of potted Costus productus Gleason ex Maas. J. Plant Nutr. 2017, 40, 1045–1052. [Google Scholar] [CrossRef]
- Bhatla, S.C.; Lal, M.A. Plant Physiology, Development, and Metabolic; Springer Nature Singapore Pte Ltd.: Singapore, 2018. [Google Scholar] [CrossRef]
- Hassan, A.A.; Abd El-Azeim, M.M. Impacts of compost, biofertilizer and/or some antioxidant treatments on gladiolus (Gladiolus grandifloras). B. Corms and cormels production and some chemical constituents. Sci. J. Flowers Ornam. Plants 2020, 7, 285–301. [Google Scholar] [CrossRef]
- Esringü, A.; Turan, M.; Sushkova, S.; Minkina, T.; Rajput, V.D.; Glinushkin, A.; Kalinitchenko, V. Influence of vermicompost application on the growth of Vinca rosea valiant, Pelargonium peltatum L. and Pegasus patio rose. Horticulturae 2022, 8, 534. [Google Scholar] [CrossRef]
- Zhang, R.H.; Quan, Z.Q.; Li, Z.G. Use of spent mushroom substrate as growing media for tomato and cucumber seedling. Pedosphere 2012, 22, 333–342. [Google Scholar] [CrossRef]
- Abdou, M.A.H.; Taha, R.A.; Gahory, A.M.O.; Hassan, A.A.A. Effect of compost and some natural growth promoting on chamomile. Sci. J. Flowers Ornam. Plants 2021, 8, 447–459. [Google Scholar] [CrossRef]
- Ngo, P.T.; Rumpel, C.; Ngo, Q.A.; Alexis, M.; Vargas, G.V.; Gil, M.; Dang, D.K.; Jouquet, P. Biological and chemical reactivity and phosphorus forms of buffalo manure compost, vermicompost and their mixture with biochar. Bioresour. Technol. 2013, 148, 401–407. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, A.H.; El-Kiey, T.M.; Koreish, E.; Zaid, N.M. Physiological response of gazania plants to growing media and organic compost. Sci. J. Flowers Ornam. Plants 2020, 7, 11–26. [Google Scholar] [CrossRef]
- Yasin, M.; Ahmad, K.; Mussarat, W.; Tanveer, A. Bio-fertilizers, substitution of synthetic fertilizers in cereals for leveraging agriculture. Crop Environ. 2012, 3, 62–66. [Google Scholar]
- Ghatas, Y.A.A.; Mohamed, Y.F.Y. Influence of some phosphorus source and biofertilizers (EM and phosphorein) on vegetative growth, fixed oil productivity and chemical constituents of Oenothera biennis L. plant. Sci. J. Flowers Ornam. Plants 2020, 7, 247–268. [Google Scholar] [CrossRef]
- Pandey, D.K.; Singh, K.K.; Pandey, A. Effect of bio-fertilizer on various growth parameters of Gladiolus cv. American Beauty. Ind. J. Pure App. Biosci. 2020, 8, 737–741. [Google Scholar] [CrossRef]
- Motsara, M.R.; Roy, R.N. Guide to Laboratory Establishment for Plant Nutrient Analysis; FAO fertilizer and plant Nutrition Bulletin No., 19; FAO: Rome, Italy, 2008. [Google Scholar]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Latimer, G.W. Official Methods of Analysis of AOAC, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2019. [Google Scholar]
- Hargreaves, J.C.; Adl, M.S.; Warman, P.R.A. Review of the use of composted municipal solid waste in agriculture. Agric. Ecosyst. Environ. 2008, 123, 1–14. [Google Scholar] [CrossRef]
- Van Der Heijden, M.G.; Bardgett, R.D.; Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Gaber, M.K. Vegetative and flowering growth of geranium as affected by mineral fertilization and ascorbic acid foliar application. Middle East J. Appl. Sci. 2019, 9, 220–230. [Google Scholar]
- Nofal, E.M.S.; EL-Mahrouk, M.E.; EL-Sayed, B.A.; Radwan, A.M.M. Effect of NPK fertilizer and some natural extract treatments on the vegetative growth and flowering of African marigold (Tagetes erecta L. var. Dwarf Chrysanthemum). Appl. Ecol. Environ. Res. 2021, 19, 3167–3179. [Google Scholar] [CrossRef]
- Inugraha, I.; Maghfoer, M.D.; Widaryanto, E. Response of stevia (Stevia rebaudiana Bertonim) to nitrogen and potassium fertilization. IOSR J. Agric. Veter. Sci. 2014, 7, 47–55. [Google Scholar] [CrossRef]
- Abdou, M.A.H.; Ibrahim, T.I.E. Effect of mineral and biofertilization treatments on: 1- Vegetative growth and flowering of gladiolus plants. Sci. J. Flowers Ornam. Plants 2016, 3, 1–8. [Google Scholar] [CrossRef]
- Singh, L.; Gurjar, P.K.S.; Barholia, A.K.; Haldar, A.; Shrivastava, A. Effect of organic manures and inorganic fertilizers on growth and flower yield of marigold (Tagetes erecta L.) var. Pusa Narangi Gainda. Plant Arch. 2015, 15, 779–783. [Google Scholar]
- Acharya, M.M.; Dashora, L.K. Response of graded level of N and P on vegetative growth and flowering in African marigold. J. Ornam. Hortic. 2004, 7, 179–183. [Google Scholar]
- Csirzinsky, A.A. Yield response of herbs to N and K rates in multiple harvests on sand. Proc. Fla. State Hort. Soc. 1997, 110, 385–388. [Google Scholar]
- Nayak, D.; Mandal, T.; Roychowdhury, N. Effect of NPK nutrition on growth and flowering of Gerbera jamesonii L. c.v. Constance Orissa. J. Hortic. 2005, 33, 11–15. [Google Scholar]
- Sarkar, B.; Mondal, S.S.; Nayak, S.S.; Saha, M.; Biswas, S. Integrated nutrient management for the productivity and quality improvement of potato under irrigated condition. Potato J. 2007, 34, 99–100. [Google Scholar]
- Hamid, N.S.; Siddique, M.A.A.; Fahmeeda, S.; Shameen, I.; Ahmad, W.M. Influence of different organic manures and biofertilizers on morphological, floral and bulb traits of Narcissus (Daffodil cv. “Salome”). Int. J. Agric. Sci. 2017, 9, 3989–3992. [Google Scholar]
- Bi, G.; Evans, B.W.; Spiers, J.M.; Witcher, A.L. Effects of organic and inorganic fertilizers on marigold growth and flowering. Hortic. Sci. 2010, 45, 1373–1377. [Google Scholar] [CrossRef]
- Chand, S.; Pande, P.; Prasad, A.; Anwar, M.; Patra, D.D. Influence of integrated supply of vermicompost and zinc-enriched compost with two graded levels of iron and zinc on the productivity of geranium. Commun. Soil Sci. Plant Anal. 2007, 38, 2581–2599. [Google Scholar] [CrossRef]
- Singh, R.P.; Varshney, G. Effects of carbofuran on availability of macronutrients and growth of tomato plants in natural soils and soils amended with inorganic fertilizers and vermicompost. Commun. Soil Sci. Plant Anal. 2013, 44, 2571–2586. [Google Scholar] [CrossRef]
- Herencia, J.F.; Ruiz-Porras, J.C.; Melero, S.; Garcia-Galavis, P.A.; Morillo, E.; Maqueda, C. Comparison between organic and mineral fertilization for soil fertility levels. Crop Macronutr. Conc. J. Agron. 2006, 99, 973–983. [Google Scholar]
- Taha, R.A.; Hassan, A.H. Response of gladiolus plants to mineral bio-fertilization and bio-fertilization treatments. 2- Corm production and chemical constituents. Alex. J. Agric. Res. 2008, 53, 87–95. [Google Scholar]
- Bordoloi, S.; Talukdar, M.C. Effect of GA3 and biofertilizer on growth and yield parameters of Anthurium (Anthurium andreanum Lindex Ex Andre) cv. Tropical in Soilless culture. Int. J. Curr. Microbiol. App. Sci. 2019, 8, 1157–1165. [Google Scholar] [CrossRef]
- Attia, K.E.; Elbohy, N.F.S.; Ashour, N.A.M. Response of tuberose plants (Polianthes tuberosa L.) to chemical and bio-fertilization and their effect on vegetative growth, flowering and chemical composition under sandy soil conditions. Sci. J. Flowers Ornam. Plants 2018, 5, 261–273. [Google Scholar] [CrossRef]
- Castagno, L.N.; Estrella, M.J.; Sannazzaro, A.I.; Grassano, A.E.; Ruiz, O.A. Phosphate solubilization mechanism and in vitro plant growth promotion activity mediated by Pantoea eucalypti isolated from Lotus tenuis rhizosphere in the Salado River Basin (Argentina). J. Appl. Microbiol. 2011, 110, 1151–1165. [Google Scholar] [CrossRef]
- Senthil-Kumar, T.; Swaminathan, V.; Kumar, S. Influence of nitrogen, phosphorus and biofertilizers on growth, yield and essential oil constituents in ratoon crop of davana (Artemisia pallens Wall.). Electron. J. Environ. Agric. Food Chem. 2009, 8, 86–95. [Google Scholar]
- Qasim, M.; Younis, A.; Zahir, Z.A.; Riaz, A.; Raza, H.; Tariq, U. Microbial inoculation increases the nutrient uptake efficiency for quality production of Gladiolus grandiflorus. Pak. J. Agric. Sci. 2014, 51, 875–880. [Google Scholar]
- Khan, S.M.; Zaidi, P.; Wani, A. Role of phosphate-solubilizing microorganisms in sustainable agriculture-A review. Agron. Sustain. Dev. 2007, 27, 29–43. [Google Scholar] [CrossRef]
- Salman, N.D.; Ali, N.S.; Majeed, N.H. Effect of Azotobacter inoculation on potassium availability of two different soil texture cultivated with maize. Basrah J. Agric. Sci. 2008, 21, 167–185. [Google Scholar]
- Pansuriya, P.B.; Varu, D.K.; Viradia, R.R. Effect of biostimulants and biofertilizers on growth, flowering and quality of gladiolus (Gladiolus grandiflorus L.) cv. American beauty under greenhouse conditions. Int. J. Chem Stud. 2018, 6, 2191–2196. [Google Scholar]
- Scher, F.M.; Baker, R. Effect of Pseudomonas putida and a synthetic iron chelator on induction of soil suppressiveness to Fusarium wilt pathogens. Phytopathology 1982, 72, 1567–1573. [Google Scholar] [CrossRef]
- Flaishman, M.A.; Eyal, Z.A.; Zilberstein, A.; Voisard, C.; Hass, D. Suppression of Septoria tritci blotch and leaf rust of wheat by recombinant cyanide producing strains of Pseudomonas putida. Mol. Plant Microbes Interact. 1996, 9, 642–645. [Google Scholar] [CrossRef]
- Megali, L.; Glauser, G.; Rasmann, S. Fertilization with beneficial microorganisms decreases tomato defenses against insect pests. Agron. Sustain. Dev. 2013, 34, 649–656. [Google Scholar] [CrossRef]
- Brijendra, S.; Singh, B. Foliar application of fertilizer mixtures for Chrysanthemum. South Indian Hort. 1986, 34, 367–369. [Google Scholar]
- El-Ghandour, I.A.; Desouky, E.M.; Galal, Y.G.M.; Arafa, R.A.; Abou Seer, A.M.M. Effect of biofertilizers and organic phosphorus amendments on growth and essential oil of marjoram (Majorana hortensis L.). Egypt Acad. J. Biol. Sci. 2009, 1, 29–36. [Google Scholar] [CrossRef]
- Puente, M.E.; Bashan, Y.; Lebsky, V.K. Microbial population and activities in rhizoplane of rockweathering desert plants, I. Root colonization and weathering of igneous rocks. Plant Biol. 2004, 6, 629–642. [Google Scholar] [CrossRef]
- Parassana, A.; Deepa, V.; Murthy, P.B.; Deecaraman, M.; Sridhar, R.; Dhandapani, P. Insoluble phosphate solubilization by bacterial strains isolated from rice rhizosphere soils from Southern India. Int. J. Soil Sci. 2011, 6, 134–141. [Google Scholar] [CrossRef]
- Zakaria, A.A.B. Growth Optimization of Potassium Solubilizing Bacteria Isolated from Biofertilizer. Bachelor’s Thesis, Universiti Malaysia Pahang, Pahang, Malaysia, 2009. Available online: https://core.ac.uk/download/159177375.pdf (accessed on 23 April 2023).
- Dubey, R.K.; Mishra, R.L. Response of chemical and bio-fertilizers on corm and cormel production in gladiolus. Progress. Hortic. Today 2005, 5, 36–40. [Google Scholar]
- Abou El-Ghait, E.M.; Gomaa, A.O.; Youssef, A.S.M.; Atiea, E.M.; AbdAllah, W.H. Effect of sowing dates, bio, organic and chemical fertilization treatments on growth and production of Indian fennel under north Sinai conditions. Bull. Fac. Cairo Univ. 2012, 63, 52–68. [Google Scholar]
- Balakrishnan, V.; Venkatesan, K.; Ravindran, K.C. The influence of halophytic compost, farmyard manure, and phosphor-bacteria on soil microflora and enzyme activities. Plant Soil Environ. 2007, 53, 186–192. [Google Scholar] [CrossRef]
| Physical Attributes | Value | Chemical Attributes | Value |
|---|---|---|---|
| Silt (%) | 88.22 | pH | 7.64 |
| Clay (%) | 8.78 | EC (dSm−1) | 1.47 |
| Sand (%) | 3.00 | Cation Exchange Capacity (meq 100 g−1 soil) | 35.94 |
| Texture soil | Sandy | Organic matter (%) | 1.82 |
| Bulk density (g cm−3) | 1.40 | Available N (mg kg−1 soil) | 57.24 |
| Available P (mg kg−1 soil) | 7.93 | ||
| Available K (mg kg−1 soil) | 120.16 |
| Characteristics | Value | Characteristics | Value |
|---|---|---|---|
| Moisture (%) | 25–50 | P (%) | 0.5–0.75 |
| EC (dSm−1) | 3–4 | K (%) | 1.25–1.75 |
| pH | 7–7.5 | Fe (mg kg−1) | 50–70 |
| Organic matter (%) | 45.56 | Mn (mg kg−1) | 10–15 |
| C/N ratio | 14.2/1 | Cu (mg kg−1) | 5–10 |
| Total N (%) | 1.5–1.8 | Zn (mg kg−1) | 15–22 |
| Treatments | Plant Height (cm) | Leaves Number/Plant | Branches Number/Plant | Leaf Area (cm2) | Shoot Dry Weight (g) | Root Dry Weight (g) |
|---|---|---|---|---|---|---|
| Compost treatment | ||||||
| − Comp (C1) | 26.32 ± 1.98 b | 41.05 ± 2.56 b | 7.08 ± 0.97 b | 95.29 ± 9.65 b | 5.46 ± 1.06 b | 1.79 ± 0.25 b |
| + Comp (C2) | 31.6 ± 2.32 a | 43.52 ± 3.32 a | 7.51 ± 1.43 a | 122.6 ± 16.0 a | 7.23 ± 1.43 a | 1.89 ± 0.32 a |
| ANOVA p-values | *** | *** | *** | *** | *** | *** |
| Fertilization treatments | ||||||
| Control (without fertilization) (T1) | 22.58 ± 0.54 f | 35.33 ± 0.43 f | 5.58 ± 0.34 e | 88.58 ± 0.69 b | 4.03 ± 0.22 e | 1.45 ± 0.02 g |
| Osmocote (T2) | 31.45 ± 0.50 b | 39.02 ± 0.38 e | 6.98 ± 0.13 cd | 117.1 ± 20.0 b | 6.83 ± 0.78 b | 1.85 ± 0.03 cd |
| Bio-Fert (T3) | 26.51 ± 0.98 c | 42.38 ± 1.30 c | 7.56 ± 0.48 b | 103.8 ± 13.3 e | 6.21 ± 1.26 c | 1.58 ± 0.08 f |
| Bio-Phos (T4) | 28.04 ± 1.06 d | 41.96 ± 0.50 d | 6.76 ± 0.42 d | 101.8 ± 13.9 g | 5.54 ± 0.89 d | 1.68 ± 0.06 e |
| Bio-Potas (T5) | 29.09 ± 0.81 e | 42.01 ± 0.70 d | 6.70 ± 0.41 d | 102.0 ± 12.4 g | 5.48 ± 1.09 d | 1.79 ± 0.05 d |
| Bio-Fert + Bio-Phos (T6) | 30.95 ± 0.94 b | 43.74 ± 1.87 b | 7.75 ± 0.35 b | 112.4 ± 21.1 c | 7.12 ± 1.33 b | 1.89 ± 0.04 c |
| Bio-Fert + Bio-Potas (T7) | 30.02 ± 0.92 c | 43.05 ± 1.19 c | 7.86 ± 0.19 b | 111.1 ± 19.6 d | 6.96 ± 1.01 b | 1.88 ± 0.05 c |
| Bio-Phos + Bio-Potas (T8) | 27.87 ± 0.97 c | 42.47 ± 0.66 cd | 7.20 ± 0.25 c | 110.3 ± 20.1 e | 6.45 ± 1.39 c | 1.96 ± 0.09 b |
| Bio-Fert + Bio-Phos + Bio-Potas (T9) | 34.20 ± 1.18 a | 50.16 ± 3.38 a | 9.30 ± 1.21 a | 133.6 ± 14.0 a | 8.46 ± 1.03 a | 2.50 ± 0.22 a |
| ANOVA p-values | *** | *** | *** | *** | *** | *** |
| Interaction Effects | ||||||
| C1T1 | 21.39 ± 0.23 i | 35.18 ± 0.03 i | 5.56 ± 0.20 i | 88.33 ± 0.46 m | 3.92 ± 0.14 k | 1.44 ± 0.01 h |
| C1T2 | 29.36 ± 0.20 jk | 38.78 ± 0.16 h | 6.93 ± 0.08 fg | 98.86 ± 0.54 i | 6.13 ± 0.10 ef | 1.83 ± 0.01 de |
| C1T3 | 22.75 ± 0.21 g–i | 42.01 ± 0.75 e–g | 7.20 ± 0.05 ef | 91.66 ± 0.31 k | 5.07 ± 0.05 gh | 1.51 ± 0.02 h |
| C1T4 | 24.85 ± 0.57 ij | 41.62 ± 0.22 fg | 6.40 ± 0.05 h | 89.3 ± 0.29 m | 4.74 ± 0.10 hi | 1.63 ± 0.01 g |
| C1T5 | 25.66 ± 0.18 k | 41.40 ± 0.08 g | 6.50 ± 0.05 gh | 90.6 ± 0.30 l | 4.49 ± 0.09 ij | 1.75 ± 0.01 c |
| C1T6 | 28.72 ± 0.11 d–f | 42.09 ± 0.22 e–g | 7.16 ± 0.08 de | 93.1 ± 0.40 j | 5.91 ± 0.13 f | 1.88 ± 0.01 d |
| C1T7 | 26.65 ± 0.14 f–j | 42.15 ± 0.51 e–g | 7.76 ± 0.08 cd | 93.16 ± 0.14 j | 6.06 ± 0.20 ef | 1.86 ± 0.03 de |
| C1T8 | 25.81 ± 0.43 f–h | 41.92 ± 0.17 fg | 7.20 ± 0.15 ef | 91.96 ± 0.17 k | 5.23 ± 0.20 g | 1.90 ± 0.06 d |
| C1T9 | 31.71 ± 0.19 b | 44.35 ± 0.43 c | 8.70 ± 0.26 b | 120.8 ± 0.36 e | 7.57 ± 0.24 cd | 2.32 ± 0.08 b |
| C2T1 | 23.77 ± 0.16 i | 35.47 ± 0.37 i | 5.60 ± 0.20 i | 88.83 ± 0.34 m | 4.14 ± 0.09 jk | 1.47 ± 0.01 h |
| C2T2 | 33.53 ± 0.21 ij | 39.26 ± 0.19 b | 7.03 ± 0.06 ef | 135.5 ± 0.37 b | 7.53 ± 0.10 cd | 1.87 ± 0.02 d |
| C2T3 | 30.26 ± 0.32 de | 43.75 ± 0.29 cd | 7.93 ± 0.08 c | 116.0 ± 0.36 f | 7.36 ± 0.13 d | 1.65 ± 0.02 fg |
| C2T4 | 31.22 ± 0.29 e–g | 42.30 ± 0.20 e–g | 7.13 ± 0.12 ef | 114.5 ± 0.17 g | 6.33 ± 0.16 ef | 1.73 ± 0.03 ef |
| C2T5 | 32.50 ± 0.41 h–j | 42.62 ± 0.18 ef | 6.90 ± 0.17 ef | 113.4 ± 0.11 h | 6.47 ± 0.09 e | 1.83 ± 0.03 de |
| C2T6 | 33.17 ± 0.28 bc | 45.39 ± 0.39 b | 8.03 ± 0.12 c | 131.7 ± 0.38 c | 8.33 ± 0.06 b | 1.91 ± 0.02 d |
| C2T7 | 33.38 ± 0.22 cd | 43.96 ± 0.32 cd | 7.96 ± 0.12 c | 129.1 ± 0.36 d | 7.86 ± 0.10 c | 1.89 ± 0.02 d |
| C2T8 | 29.92 ± 0.59 de | 43.01 ± 0.20 de | 7.20 ± 0.17 ef | 128.6 ± 0.46 d | 7.67 ± 0.29 cd | 2.03 ± 0.01 c |
| C2T9 | 36.69 ± 0.37 a | 55.96 ± 0.20 a | 9.80 ± 0.29 a | 146.4 ± 0.63 a | 9.36 ± 0.15 a | 2.69 ± 0.03 a |
| ANOVA p-values | *** | *** | *** | *** | *** | *** |
| Treatments | Number of Days to Flowering | Flowering Stock Length (cm) | Inflorescence No/Plant | Flower Number /Plant | Inflorescence Diameter (cm) | Inflorescence Age (Day) | Inflorescence Dry Weight (g) |
|---|---|---|---|---|---|---|---|
| Compost treatment (Comp, at a ratio of 1:2 v/v soil/compost) | |||||||
| − Comp (C1) | 43.09 ± 2.87 b | 14.69 ± 2.96 b | 2.11 ± 0.67 b | 32.41 ± 3.94 b | 5.18 ± 0.65 b | 33.40 ± 4.36 b | 0.36 ± 0.10 b |
| + Comp (C2) | 44.48 ± 3.65 a | 16.59 ± 3.68 a | 2.67 ± 0.85 a | 35.41 ± 6.38 a | 5.61 ± 0.11 a | 34.35 ± 5.06 a | 0.99 ± 0.62 a |
| ANOVA p-values | *** | *** | *** | *** | *** | *** | *** |
| Fertilization treatments | |||||||
| Control (without fertilization) (T1) | 38.57 ± 0.27 f | 10.06 ± 0.22 b | 1.40 ± 0.14 f | 24.00 ± 0.37 g | 4.10 ± 0.05 b | 25.56 ± 0.65 g | 0.22 ± 0.01 g |
| Osmocote (T2) | 44.59 ± 0.57 c | 15.83 ± 0.90 e | 2.05 ± 0.28 de | 36.75 ± 1.14 b | 5.45 ± 0.08 c | 37.71 ± 1.48 b | 0.40 ± 0.01 e |
| Bio-Fert (T3) | 42.48 ± 2.66 e | 12.30 ± 1.30 g | 2.20 ± 0.59 d | 29.41 ± 1.04 f | 4.89 ± 0.16 g | 30.30 ± 1.46 f | 0.31 ± 0.05 f |
| Bio-Phos (T4) | 42.92 ± 2.16 e | 12.86 ± 0.98 f | 1.86 ± 0.37 e | 31.81 ± 1.07 e | 5.01 ± 0.18 f | 30.13 ± 1.17 f | 0.32 ± 0.05 f |
| Bio-Potas (T5) | 43.39 ± 1.87 cd | 15.31 ± 0.99 e | 2.83 ± 0.50 b | 34.41 ± 1.89 d | 5.62 ± 0.15 b | 33.38 ± 0.73 e | 0.82 ± 0.44 d |
| Bio-Fert + Bio-Phos (T6) | 44.66 ± 2.50 c | 16.58 ± 1.26 d | 1.88 ± 0.17 e | 34.03 ± 0.77 d | 5.25 ± 0.25 e | 34.60 ± 1.01 d | 0.79 ± 0.48 d |
| Bio-Fert + Bio-Potas (T7) | 44.65 ± 2.80 c | 18.06 ± 1.22 c | 2.63 ± 0.28 c | 36.90 ± 1.76 b | 5.36 ± 0.10 d | 35.01 ± 1.16 d | 1.03 ± 0.64 c |
| Bio-Phos + Bio-Potas (T8) | 45.26 ± 2.72 b | 19.06 ± 1.39 b | 2.66 ± 0.21 bc | 35.16 ± 1.52 c | 5.35 ± 0.19 d | 36.53 ± 0.91 c | 1.07 ± 0.69 b |
| Bio-Fert+Bio-Phos+Bio-Potas (T9) | 47.82 ± 2.23 a | 20.70 ± 1.94 a | 4.03 ± 0.58 a | 42.75 ± 6.30 a | 7.56 ± 0.10 a | 41.63 ± 2.81 a | 1.19 ± 0.69 a |
| ANOVA p-values | *** | *** | *** | *** | *** | *** | *** |
| Interaction Effects | |||||||
| C1T1 | 37.97 ± 0.12 hi | 9.66 ± 0.12 n | 1.30 ± 0.05 k | 23.90 ± 0.20 l | 4.10 ± 0.21 k | 25.50 ± 0.45 j | 0.21 ± 0.01 j |
| C1T2 | 43.58 ± 0.12 ef | 15.10 ± 0.32 ij | 1.80 ± 0.05 hi | 35.73 ± 0.08 ef | 5.38 ± 0.21 ef | 36.16 ± 0.54 cd | 0.38 ± 0.01 f–j |
| C1T3 | 41.54 ± 0.62 g | 11.30 ± 0.63 m | 1.66 ± 0.06 ij | 28.66 ± 0.46 b | 4.75 ± 0.32 j | 29.30 ± 0.58 h | 0.26 ± 0.01 j |
| C1T4 | 42.51 ± 0.31 fg | 12.10 ± 0.36 l | 1.53 ± 0.06 i–k | 30.93 ± 0.24 j | 4.84 ± 0.44 j | 29.16 ± 0.14 h | 0.26 ± 0.01 j |
| C1T5 | 42.76 ± 0.37 f | 14.50 ± 0.17 j | 2.40 ± 0.11 ef | 32.80 ± 0.41 i | 5.49 ± 0.35 de | 32.93 ± 0.38 f | 0.41 ± 0.01 fg |
| C1T6 | 43.30 ± 0.24 f | 15.56 ± 0.53 hi | 1.80 ± 0.05 hi | 33.40 ± 0.11 hi | 5.03 ± 0.49 i | 35.23 ± 0.54 de | 0.35 ± 0.01 i |
| C1T7 | 43.60 ± 0.05 e | 16.96 ± 0.12 ef | 2.46 ± 0.17 d–f | 35.40 ± 0.11 ef | 5.28 ± 0.42 fg | 35.93 ± 0.43 cd | 0.42 ± 0.01 fg |
| C1T8 | 44.83 ± 0.05 cd | 17.80 ± 0.05 d | 2.56 ± 0.14 c–e | 33.90 ± 0.17 gh | 5.18 ± 0.44 gh | 37.30 ± 0.15 c | 0.44 ± 0.01 f |
| C1T9 | 47.24 ± 0.38 b | 18.93 ± 0.14 c | 3.50 ± 0.05 b | 37.03 ± 0.41 cd | 6.60 ± 0.44 b | 39.06 ± 0.08 b | 0.56 ± 0.01 e |
| C2T1 | 39.17 ± 0.05 h | 10.16 ± 0.13 n | 1.50 ± 0.05 jk | 24.10 ± 0.25 l | 4.11 ± 0.40 k | 25.63 ± 0.38 i | 0.24 ± 0.01 j |
| C2T2 | 45.60 ± 0.38 c | 16.56 ± 0.18 fg | 2.30 ± 0.05 ef | 37.76 ± 0.21 bc | 5.52 ± 0.34 d | 39.26 ± 0.38 b | 0.41 ± 0.01 f–h |
| C2T3 | 43.43 ± 0.29 e | 13.30 ± 0.10 k | 2.73 ± 0.08 cd | 30.16 ± 0.35 j | 5.03 ± 0.49 i | 31.30 ± 0.66 g | 0.36 ± 0.01 hi |
| C2T4 | 43.34 ± 0.42 ef | 13.63 ± 0.29 k | 2.20 ± 0.05 fg | 32.70 ± 0.36 i | 5.16 ± 0.29 h | 31.10 ± 0.45 g | 0.37 ± 0.01 g–i |
| C2T5 | 44.03 ± 0.62 d | 16.13 ± 0.34 gh | 3.26 ± 0.12 b | 36.03 ± 0.44 de | 5.76 ± 0.29 c | 32.83 ± 1.13 f | 1.23 ± 0.01 d |
| C2T6 | 45.02 ± 0.20 c | 17.60 ± 0.10 de | 1.96 ± 0.12 gh | 34.66 ± 0.29 fg | 5.47 ± 0.45 de | 33.83 ± 0.31 ef | 1.24 ± 0.01 d |
| C2T7 | 45.71 ± 0.37 c | 19.16 ± 0.14 c | 2.80 ± 0.10 c | 38.40 ± 0.58 a | 5.43 ± 0.37 de | 34.10 ± 0.32 ef | 1.58 ± 0.03 c |
| C2T8 | 45.70 ± 0.59 c | 20.33 ± 0.08 b | 2.76 ± 0.08 c | 36.43 ± 0.56 de | 5.52 ± 0.50 d | 35.76 ± 0.29 cd | 1.70 ± 0.05 b |
| C2T9 | 48.40 ± 0.37 a | 22.46 ± 0.08 a | 4.56 ± 0.03 a | 48.46 ± 0.52 a | 8.52 ± 0.34 a | 44.20 ± 0.00 a | 1.83 ± 0.03 a |
| ANOVA p-values | *** | *** | *** | *** | *** | *** | *** |
| Treatments | N (%) | P (%) | K (%) | Chlorophyll (mg·g−1 FW) |
|---|---|---|---|---|
| Compost treatment | ||||
| − Comp (C1) | 1.91 ± 0.41 b | 0.17 ± 0.04 b | 1.25 ± 0.26 b | 5.02 ± 0.27 b |
| + Comp (C2) | 2.13 ± 0.37 a | 0.25 ± 0.08 a | 1.46 ± 0.32 a | 5.93 ± 0.13 a |
| ANOVA p-values | *** | *** | *** | *** |
| Fertilization treatments | ||||
| Control (without fertilization) (T1) | 1.73 ± 0.24 ef | 0.120.01 f | 1.15 ± 0.03 f | 3.92 ± 0.27 g |
| Osmocote (T2) | 2.20 ± 0.31 bc | 0.19 ± 0.04 d | 1.76 ± 0.18 a | 6.07 ± 0.18 b |
| Bio-Fert (T3) | 2.31 ± 0.10 b | 0.24 ± 0.05 c | 1.08 ± 0.12 g | 5.05 ± 0.19 e |
| Bio-Phos (T4) | 1.79 ± 0.14 e | 0.15 ± 0.03 e | 0.98 ± 0.05 h | 5.02 ± 0.21 e |
| Bio-Potas (T5) | 1.57 ± 0.08 f | 0.17 ± 0.05 d | 1.59 ± 0.07 e | 4.73 ± 0.23 f |
| Bio-Fert + Bio-Phos (T6) | 2.07 ± 0.06 cd | 0.25 ± 0.03 bc | 1.03 ± 0.14 g | 5.64 ± 0.17 cd |
| Bio-Fert + Bio-Potas (T7) | 1.93 ± 0.45 de | 0.27 ± 0.06 b | 1.39 ± 0.17 e | 5.80 ± 0.15 c |
| Bio-Phos + Bio-Potas (T8) | 1.81 ± 0.08 e | 0.18 ± 0.04 d | 1.50 ± 0.09 d | 5.14 ± 0.20 d |
| Bio-Fert + Bio-Phos + Bio-Potas (T9) | 2.78 ± 0.15 a | 0.34 ± 0.10 a | 1.79 ± 0.24 b | 7.89 ± 0.19 a |
| ANOVA p-values | *** | *** | *** | *** |
| Interaction Effects | ||||
| C1T1 | 1.52 ± 0.05 i | 0.12 ± 0.01 h | 1.14 ± 0.02 g | 3.86 ± 0.51 hi |
| C1T2 | 1.94 ± 0.08 e–h | 0.15 ± 0.01 g | 1.60 ± 0.02 bc | 5.74 ± 0.91 d |
| C1T3 | 2.22 ± 0.02 c–e | 0.21 ± 0.01 d–f | 0.97 ± 0.01 hi | 4.70 ± 0.25 e |
| C1T4 | 1.71 ± 0.03 g–i | 0.11 ± 0.01 b | 0.93 ± 0.01 i | 4.55 ± 0.10 f |
| C1T5 | 1.52 ± 0.04 f | 0.13 ± 0.01 gh | 1.53 ± 0.01 cd | 4.34 ± 0.50 g |
| C1T6 | 2.05 ± 0.04 e–g | 0.22 ± 0.01 de | 0.91 ± 0.02 i | 4.88 ± 0.21 e |
| C1T7 | 1.75 ± 0.37 f–i | 0.21 ± 0.01 ef | 1.24 ± 0.01 f | 5.48 ± 0.24 d |
| C1T8 | 1.78 ± 0.06 f–i | 0.14 ± 0.01 gh | 1.43 ± 0.02 e | 4.55 ± 0.58 f |
| C1T9 | 2.70 ± 0.03 ab | 0.24 ± 0.01 d | 1.48 ± 0.03 de | 7.08 ± 0.72 b |
| C2T1 | 1.95 ± 0.03 e–h | 0.120.01 gh | 1.17 ± 0.01 fg | 3.98 ± 0.09 h |
| C2T2 | 2.47 ± 0.07 bc | 0.23 ± 0.01 de | 1.92 ± 0.04 a | 6.39 ± 0.21 c |
| C2T3 | 2.40 ± 0.10 b–d | 0.28 ± 0.02 c | 1.20 ± 0.01 fg | 5.39 ± 0.42 de |
| C2T4 | 1.88 ± 0.09 e–h | 0.18 ± 0.01 f | 1.03 ± 0.01 h | 5.50 ± 0.81 d |
| C2T5 | 1.62 ± 0.04 hi | 0.22 ± 0.01 de | 1.66 ± 0.01 b | 5.13 ± 0.61 de |
| C2T6 | 2.10 ± 0.01 df | 0.28 ± 0.01 c | 1.16 ± 0.01 fg | 6.40 ± 0.80 c |
| C2T7 | 2.10 ± 0.05 d–f | 0.33 ± 0.01 b | 1.55 ± 0.04 cd | 6.13 ± 0.22 cd |
| C2T8 | 1.85 ± 0.01 f–i | 0.22 ± 0.01 de | 1.58 ± 0.03 c | 5.73 ± 0.34 d |
| C2T9 | 2.85 ± 0.04 a | 0.44 ± 0.01 a | 1.92 ± 0.01 a | 8.70 ± 0.62 a |
| ANOVA p-values | *** | *** | *** | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altobaishi, S.F.; Almana, F.A.; Abd-ElGawad, A.M.; Al-Yafrsi, M.A.; Elhindi, K.M. Ivy Geranium (Pelargonium peltatum (L.) L’Hér.) Plant Growth and Flowering as Affected by Mineral or Biofertilizer with or without Compost Amendment. Agriculture 2023, 13, 1106. https://doi.org/10.3390/agriculture13051106
Altobaishi SF, Almana FA, Abd-ElGawad AM, Al-Yafrsi MA, Elhindi KM. Ivy Geranium (Pelargonium peltatum (L.) L’Hér.) Plant Growth and Flowering as Affected by Mineral or Biofertilizer with or without Compost Amendment. Agriculture. 2023; 13(5):1106. https://doi.org/10.3390/agriculture13051106
Chicago/Turabian StyleAltobaishi, Sultan F., Fahed A. Almana, Ahmed M. Abd-ElGawad, Mohammed A. Al-Yafrsi, and Khalid M. Elhindi. 2023. "Ivy Geranium (Pelargonium peltatum (L.) L’Hér.) Plant Growth and Flowering as Affected by Mineral or Biofertilizer with or without Compost Amendment" Agriculture 13, no. 5: 1106. https://doi.org/10.3390/agriculture13051106
APA StyleAltobaishi, S. F., Almana, F. A., Abd-ElGawad, A. M., Al-Yafrsi, M. A., & Elhindi, K. M. (2023). Ivy Geranium (Pelargonium peltatum (L.) L’Hér.) Plant Growth and Flowering as Affected by Mineral or Biofertilizer with or without Compost Amendment. Agriculture, 13(5), 1106. https://doi.org/10.3390/agriculture13051106








