Abstract
Geophytes are a very important group among ornamental plants, for which more and more plant growth regulators (PGRs) are being used to improve the plant quality, flowering intensity, and vase life of flowers and leaves. PGRs constitute a large group of naturally occurring or synthetically produced organic chemical compounds. There are many factors that influence the efficiency of PGRs, and the method of their application plays a key role in determining their success. In the case of geophytes, the most common method of application is spraying and soaking the storage organs before planting. This article presents information on the application of PGRs to different species of geophytes, both at the cultivation stage and during the post-harvest treatment of flowers and leaves.
1. Introduction
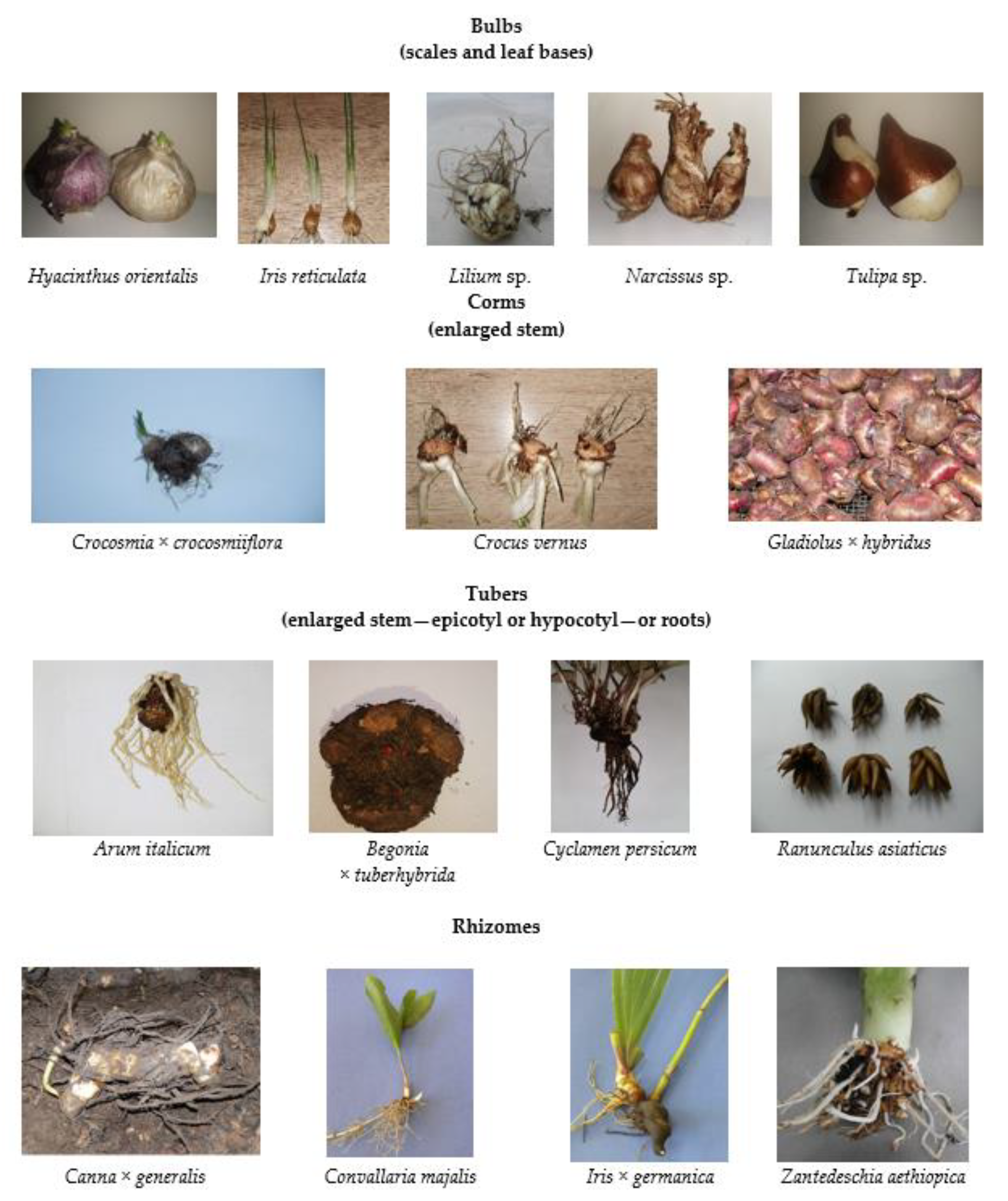
Geophytes are herbaceous terrestrial plants that usually go through a state of dormancy, during which they “go to sleep” and form underground storage organs in the form of bulbs, corms, tubers, or rhizomes (Figure 1) [1]. These organs enable the plants to survive periods of drought and other environmental stresses by protecting the regenerating buds underground and providing stored nutrients. Geophytes have a wide geographic range but are particularly diverse in Mediterranean ecosystems [2,3,4,5]. Among geophytes, there are both monocotyledonous and dicotyledonous species [6,7,8,9]. Regardless of the storage organ, the species that belong to geophytes (Figure 2) are commonly referred to as bulbous plants [10].

Figure 1.
Storage organs in geophytes.

Figure 2.
The different species of geophytes.
Geophytes occupy a very important position in floriculture. They are grown to be used as cut flowers and potted plants for interior ornamentation. They have also valued plants for gardens and landscaped areas. Thanks to forcing, it is possible to produce flowering geophytes in containers or to use them to grow cut flowers all year round. Many species are used for culinary, medicinal, and industrial purposes. The leaves, flowers, and underground organs are edible as a fresh, dried, frozen, or sugar-preserved product. Some species are used as spices, for medicinal purposes, or as a source of essential oils for the pharmaceutical industry. Out of approximately 800 types of geophytes, seven accounts for 90% of bulb production, including Tulipa, Lilium, and Narcissus. In the case of the remaining 10%, the assortment of species introduced to the global flower market is constantly increasing [11].
As in the case of other ornamental plants, plant growth regulators (PGRs) are more and more often used in this group to improve the quality of plants, the intensity of flowering, and the post-harvest longevity of flowers and leaves. PGRs are organic substances that are naturally produced by tall plants, controlling growth in a place distant from the place of their production. They are active in trace amounts or as synthetic products administered exogenously [12,13].
The most important PGRs include auxins, gibberellins (GAs), cytokinins (CKs), ethylene, and growth retardants. The effects of PGRs in plants depend on various factors, which play an important role in achieving the expected results. These factors include the method of application, the timing of application, the concentration of the PGRs, the plant species, and the environmental conditions in which the plants are grown [14]. The intensity of application is also considered an important factor influencing the effectiveness of PGRs, as some plants respond well to a single application while multiple applications are beneficial for others [15]. Other complementary factors may include the chemical properties of the solution that contains the PGRs, in particular the pH, which plays a crucial role in the absorption of the PGRs by plants [13]. Various methods are used for the application of PGRs, including foliar applications [16], drenching [17], pre-plant sowing [18], seed priming [19], pasting [20], capillary string [21] and injection [22]. In the case of ornamental plants, including geophytes, leaf spraying [13], soaking storage organs [23,24,25,26,27,28,29,30,31,32,33,34], and spraying them [35] are the most popular methods of application.
Research is being conducted worldwide to determine the effects of PGRs on ornamental plants from different groups. In this paper, the results obtained for geophytes are presented, where PGRs were applied at the cultivation stage and in the post-harvest period for the treatment of flowers and leaves.
2. Plant Growth Regulators (PGRs) Used in Geophytes
2.1. Gibberellins (GAs)
Among the PGRs, the most numerous are gibberellins (GAs), which were discovered and isolated by Japanese researchers from the fungus Giberella fujikuroi, which belongs to Ascomycetes. This pathogen infects rice seedlings, causing their excessive elongation. The plants growing from such seedlings are thinner and more fragile. The disease was named bakanae—“crazy seedling”. In 1926, Kurosawa observed that, in sterile extracts from cultures of Giberella fujikuroi (the perfect sexually reproducing stage) and Fusarium moniliforme (the imperfect conidial stage), there is a compound that causes excessive elongation of the internodes of rice. In 1935, Yabuta, Hayashi, and Sumiki isolated chemical compounds from the metabolic products of the fungus, which they named GAs [36,37]. The research on GAs was interrupted by World War II. It continued in the early 1950s.
GAs are acids with a structure based on a giban ring. They are denoted by the symbol GA followed by the appropriate sequence number. GAs are formed in the youngest parts of plants [38]. They are translocated by the xylem and phloem [39]. GAs interrupt hereditary plant dwarfism [40]. Under their influence, shoots elongate, and plants flower more abundantly [33]. When GAs is applied, plant dormancy can be interrupted [41], seed germination can be improved [42], flowering can be changed [31], and flower and leaf vase life can be improved [33], as GAs inhibit chlorophyll and protein degradation in leaves [43,44]. Gibberellic acid (GA3) is the most commonly used GA [40].
2.1.1. Mechanism of GA Action
Through the use of biochemical, genetic, and molecular research techniques, the mechanism of GA signal perception and transduction in plants has been elucidated. In the first step, the GA signal is received by GA-insensitive dwarf 1 (GID1) located in the cytoplasm and nucleus. In the Oryza sativa genome, a single GID1 gene was identified, while in the Arabidopsis thaliana genome, three were discovered—GID1a, GID1b, and GID1c—with partially overlapping functions. The binding of bioactive GAs to the GID1 receptor promotes interactions between GID1 and the DELLA domain present in DELLA proteins, which are the main repressors of the GA pathway. The rapid degradation of DELLA proteins involving ubiquitin-protein ligases is a universal mode of GA function. Gas in a non-dissociated form can penetrate into the cell, as the cell membrane does not provide a barrier to limit their diffusion. An increase in the concentration of bioactive Gas in cells results in the formation of the GA–GID1 complex. Interactions between these proteins occur through hydrophilic (direct hydrogen bonds or indirect via water molecules), hydrophobic, and van der Waals interactions. This allows one of the DELLA proteins to attach via the highly conserved N-terminal DELLA and TVHYNP motifs. Eventually, a polyubiquitin chain is attached to the DELLA proteins, which is the signal for their degradation in proteasomes. The entire cycle of events leads to the release of specific transcription factors that activate or inhibit target genes [45].
2.1.2. The Effect of Gas on Flowering and Quality of Geophytes
As in the case of other species of ornamental plants, the most commonly used GA in geophytes is GA3 (Table 1).

Table 1.
The effect of PGRs on flowering and quality of geophytes and on the vase life of flowers and leaves.
The beneficial effects of GA3 have been used in the field cultivation of Anemone coronaria, inter alia. Piskornik and Piskornik [50] showed that, in the case of that species, the effect of GA3 depends not only on the concentration but also on the timing of application, which the authors explained by the different thermal conditions prevailing after the application of this PGR. According to the authors, with respect to that species, the use of GA3 at a concentration of 100 mg·dm−3 effectively improves the quality of flowers expressed by the weight and length of flowering shoots. Later studies by Janowska et al. [51] indicated that GA3 is also worth using in Anemone coronaria when grown while covered. According to the authors, in the case of the ‘Sylphide’ cultivar, the use of GA3 at 50–150 mg·dm−3 speeds up flowering by 11–16 days and stimulates the elongation of flowering shoots (Figure 3).

Figure 3.
Anemone coronaria ‘Sylphide’: from the left, a control plant and plants grown from tubers soaked in GA3 at a concentration of 50 mg·dm−3, 100 mg·dm−3, and 150 mg·dm−3.
However, when GA3 is applied at concentrations of 100 or 150 mg·dm−3, the flower yield doubles. Furthermore, according to a study by Janowska et al. [52], GA3 in this cultivar stimulates the formation of chlorophyll and carotenoids and the accumulation of sugars in the leaves. GA3 is also useful in the cultivation of Cyclamen persicum. Spraying the leaves with GA3 at a concentration of 10 or 50 mgˑdm−3 not only initiates the growth of flower stalks but also significantly accelerates flowering and increases the number of flowers [57,58,59]. GA3 is used in the cultivation of Zantedeschia with colorful spathes cultivars. The results of the tests carried out indicated a differential response of the cultivars, which was closely related to the concentrations of GA3 used. In Zantedeschia, the intensity of flowering depends not only on the cultivar but also on the size of the rhizomes and the length of their storage [88]. However, the size of the rhizomes is not correlated with intensive flowering, as even from the largest ones, without the use of GA3, a very good yield of flowers was not obtained. The research conducted showed that GA3 could be used at concentrations from 25 to 500 mg∙dm−3 [23,81,82,83,84,89,90,91,92]. However, too high a concentration of GA3 causes inflorescence deformation [84,89]. The use of GA3 in colored Zantedeschia cultivars delays flowering but also prolongs it [23,84]. In addition to soaking rhizomes in water–GA3 solutions, the spraying of leaves and rhizomes is used [25,35]. Such methods protect Zantedeschia from Pectobacterium carotovorum subsp. carotovorum [93]. Jerzy and Janowska [94] conducted a study in which they evaluated the subsequent effect of GA3 used at the in vitro propagation stage on the flowering and quality of two Zantedeschia cultivars. GA3 was applied at the final stage of in vitro plant micropropagation [95] by introducing it at 50 mg·dm−3 into pre-sterilized rooting medium. Prior sterilization of the medium was necessary because the GA3 activity in the autoclave could be drastically reduced to as low as 10% [96]. In regenerated plants from GA3-treated cuttings, the authors [94] observed an altered leaf shape and a reduced rhizome size and weight. In contrast, Andrzejak and Janowska [33] demonstrated that in Z. albomaculata ‘Albomaculata’, GA3 could be replaced by mycorrhiza. The addition of arbuscular mycorrhizal fungi (AMF) stimulated flowering in this cultivar, probably because AMF produces PGRs, including GAs [93,97].
Attempts were made to improve the quality of Iris × hollandica with GA3. In the cultivars Wedgewood and ‘Prof. Blaauw’, however, no large effect of GA3 on flower shoot elongation was found [72]. In Tulipa sp., on the other hand, GA3 was shown to have a stimulating effect on flower shoot elongation [23,75], the inhibition of flower aging, and earlier flowering [98].
However, the use of GAs does not always have the desired effect. For example, in Hyacinthus orientalis, there was an inhibitory effect of GA3 on the formation of adventitious bulbs in hollowed-out parent bulbs, while at the same time, there was very intensive leaf growth [70,71]. However, soaking Gladiolus corms in GA3 at 100 or 500 mgˑ·dm−3 had a beneficial effect on the number of adventitious corms. GA3 also stimulated photosynthetic intensity as the chlorophyll levels increased. Moreover, plants grown under short-day conditions with GA3 flowered; however, the forming spikes were shorter and had fewer flowers than control plants grown under natural day-length conditions. Consequently, short days and low light levels are limiting factors for GAs [62]. Janowska et al. [31] reported, however, that in G. hybridus ‘Black Velvet’, GA3 at a concentration of 100–600 mg·dm−3 inhibited inflorescence shoot elongation but stimulated spike elongation. Moreover, it had a beneficial effect on the uptake of calcium (Ca) and manganese (Mn). Sajid et al. [61] reported that spraying G. grandiflorus leaves with GA3 at a concentration of 25, 50, or 100 mg∙dm−3 stimulated inflorescence shoot growth. The longest inflorescence stems were recorded by the authors for the treatment in which GA3 was applied at a concentration of 100 mg∙dm−3. Moreover, GA3 at 50 or 100 mg∙dm−3 stimulated spike elongation and flower development. Shoot and inflorescence elongation after GA3 application in Gladiolus ‘H.B.Pitt’ was reported by Sable et al. [64]. The authors further reported that, in this cultivar, GA3 at 100–200 mg∙dm−3 stimulated flower development, and at 200 mg∙dm−3, it caused significant inflorescence elongation. The beneficial effect of GA3 on the shoot length, inflorescence development, and flower development in Gladiolus ‘White Prosperity’ was reported by Sajjad et al. [16]. On the other hand, in Muscari armeniacum, GA3 not only accelerated flowering but also stimulated inflorescence stalk and leaf growth in partially and fully cooled bulbs [74]. GA3 was also used for Freesia reflacta. Żurawik and Placek [60] reported that, in three Freesia cultivars from the Easy Pot group, soaking corms in a GA3 solution with a concentration of 10, 20, 40, 80, or 160 mg·dm−3 for 24 h stimulated inflorescence shoot elongation and leaf development, but reduced flower diameter. The application of GA3 increased the weight of offspring corms. The highest effect among the concentrations assessed was recorded at 160 mg·dm−3. In contrast, the GA3 used in the experiment had no effect on the number of new corms obtained. An interesting study was conducted by Janowska et al. [56]. This study assessed the effect of GA3 on the content of biologically active substances in the corms of Crocosmia × crocosmiiflora ‘Lucifer.’ Four groups of biologically active substances with antioxidant properties were extracted from C. × crocosmiiflora ‘Lucifer’ corms: saponins (medicagenic acid, medicagenic acid 3-O-triglucoside, and polygalic acid), phenolic acids (caffeic acid, p-coumaric acid, and gallic acid), flavonoids (kaempferol, kaempferol 3-O-rhamnosylglucoside, quercetin, and quercetin 3-O-glucoside) and carotenoids (crocin and β-carotene). The corms of the ‘Lucifer’ cultivar proved to be a rich source of antioxidants. After treatment with GA3, the antioxidative activity increased in proportion to the concentration of GA3 used in the experiment. GA3 increased the content of medicagenic acid, polygalic acid, caffeic acid, p-coumaric acid, gallic acid, kaempferol, quercetin, quercetin 3-O-glucoside, kaempferol 3-O-rhamnosylglucoside, and β-carotene without affecting the content of medicagenic acid 3-O-triglucoside and crocin.
2.2. Cytokinins (CKs)
Naturally occurring cytokinins (CKs) are derivatives of the adenine purine base, with an alkyl chain or aryl group attached to the amino group. In plants, CKs with aliphatic substituents are common, especially zeatin and dihydrozeatin. CKs with an aromatic benzyl substituent are less common. They are responsible for cell division and differentiation [96]. In horticulture, they are especially used to extend the vase life of cut flowers and florists’ greens [26,27,28]. CKs are produced by a variety of creative tissues, but the main sites of their synthesis include the apical meristems of the root system, young fruits and seeds during intensive growth, and callus tissue [99].
2.2.1. Mechanism of CK Action
CKs play a key role in different phases of plant growth and development. The underlying molecular mechanisms of their biosynthesis and signal transduction have recently been elucidated [100]. To date, several CK-binding proteins—CKI1, CRE1, and AHK2/3/4—have been identified as the most likely receptors for these PGRs, and the genes encoding them are known. A common feature of the receptors is that these proteins have catalytic histidine kinase activity. The proposed CK signal transduction pathway resembles the bacterial two-component response system and is based on the transfer of a phosphate group between protein components. AHP proteins mediate CK signal transduction to the cell nucleus. Genes encoding ARR proteins, or so-called response regulators, act as early response genes, the induction of which generates typical plant cell responses to CKs. CK-binding proteins are located primarily on the thylakoid membranes of plant cells and in the microsomal fraction. When CKs bind to a specific protein, a specific physiological response of the cell is initiated [100,101,102]. Research on CKs is closely linked to the development of in vitro cultures. In the 1950s, a compound that strongly stimulated cell division was isolated from immature maize kernels. It was given the name zeatin [103,104]. Benzyladenine (BA), which is a synthetic CK, is widely used in floriculture [105]. BA is used primarily as an ingredient in media for the in vitro propagation of plants. In recent years, it has also been used for the cultivation of ornamental plants in the ground and under covers [38]. BA can be applied as a solution to soak storage organs or spray leaves. It should be noted that BA added to water does not dissolve in it but forms a suspension. Therefore, it should be first dissolved in a small amount of ethanol (C₂H₆O), and only then should water be added [106].
2.2.2. The Effect of CKs on Flowering and Quality of Geophytes
In horticultural practice, CKs are used to a small extent (Table 1). However, ongoing research indicates that CKs in certain species have a beneficial effect on flowering intensity, as shown by Luria et al. [78] in Z. aethiopica following the application of BA at 350 mg·dm−3. In Zantedeschia with colorful spathes, BA also effectively increases the intensity of flowering. An application of BA at 350 or 600 mg·dm−3 in a solution for soaking the rhizomes improves the flowering of the cultivars ‘Black Magic’, ‘Mango’, and ‘Albomaculata’ [27,85]. Janowska and Stanecki [27] further showed that BA slightly delayed the flowering time of these cultivars. However, the authors noted that the application of BA at the lowest concentration in the cultivars ‘Mango’ and ‘Albomaculata’ resulted in earlier flowering of the plants. This is partially supported by the research of Tjia and Funnell [107], who achieved earlier flowering by soaking Z. elliottiana rhizomes in BA at 50–100 mg∙dm−3. In contrast, Ngamau [79] obtained no yield increase in Z. aethiopica ‘Green Goddess’ after a BA application. There was also no increase in the yield of A. coronaria ‘Sylphide’ after the application of BA at 50–150 mg∙dm−3 in a solution for soaking the tubers (Figure 4) [51].

Figure 4.
Anemone coronaria ‘Sylphide’: from the left, a control plant and plants are grown from tubers soaked in BA at 50 mg·dm−3, 100 mg·dm−3, and 150 mg·dm−3.
CKs can affect flower quality traits expressed in terms of the stalk length and the flower size and weight, as well as leaf development, with either positive or negative effects. In F. reflacta, BA at a concentration of 50 mg·dm−3 causes plants to grow vigorously and have the longest inflorescences and the largest leaf area [61]. On the other hand, in colored Zantedeschia, BA inhibits the growth of the inflorescence stalks, with the response to the concentrations used depending on the cultivar. In addition, BA affects the formation of longer spathes in the cultivar ‘Albomaculata’, while in the cultivars ‘Black Magic’ and ‘Mango’, it results in the growth of flowers with a smaller weight [27,85]. However, as reported by Janowska and Stanecki [86], BA at 100–600 mg·dm−3 in the ‘Mango’ cultivar and at 350–600 mg·dm−3 in the ‘Albomaculata’ cultivar inhibited leaf development but had a positive effect on leaf quality, for which a higher greening index and a higher protein and sugar content were recorded. The formation of shorter flower stalks following a BA application was reported by Janowska et al. [51] in A. coronaria ‘Sylphide’, in which, additionally, the smallest flowers developed in plants whose tubers were soaked in BA at 50 mg·dm−3. Sajjad et al. [16] reported that BA had a beneficial effect on the inflorescence shoot length in Gladiolus ‘White Prosperity’, while Sajjad et al. [63] reported a similar phenomenon for G. grandiflorus. In contrast, Janowska et al. [32] reported that BA at 100–600 mg·dm−3 inhibited the inflorescence shoot elongation of G. hybridus ‘Black Velvet’, but stimulated inflorescence elongation and flower development. Moreover, BA at 100–600 mg·dm−3 in the ‘Black Velvet’ cultivar stimulated Ca uptake without affecting the uptake of the other macronutrients Mn, zinc (Zn) (600 mg·dm−3), and copper (Cu) (100–600 mg·dm−3), but it inhibited the boron (B) uptake.
2.3. Effect of a Mixture of CKs and GAs on Flowering and Plant Quality of Geophytes
In the West, ready-made preparations containing PGRs of various compositions are often used in floricultural production. These include Promalin (100 mg·dm−3 GA4+7 + 100 mg·dm−3 BA) [108,109]. Unfortunately, this preparation is expensive due to the costly synthesis of GA4+7. Therefore, e.g., in nursery production, it is replaced by the cheaper GA3- and BA-containing Arbolin [110]. However, it should be mentioned that these preparations are not registered in Poland, and consequently, combined PGRs from different groups are used in studies (Table 1). Janowska and Stanecki [28] found that based on studies evaluating the effect of the combined application of GA3 and BA on the flowering of Zantedeschia with colorful spathes, soaking the rhizomes in a mixture of those PGRs increased the inflorescence yield in the cultivars ‘Black Magic’ and ‘Albomaculata,’ which confirmed an earlier study by Funnell et al. [83], who found an increase in the cut inflorescence yield of up to 469% in Zantedeschia ‘Galaxy’ after treatment with Promalin compared to control plants. In this cultivar, GA3 also caused an increase in yield, but only by half as much. Similarly, in Z. aethiopica ‘Green Goddess,’ the cut flower yield increased after the application of the BA + GA3 mixture [79]. According to Janowska and Stanecki [26], in the Zantedeschia with colorful spathes cultivar, the application of the BA + GA3 mixture influenced the growth of inflorescences with shorter stalks from the rhizomes. On the contrary, Ngamau [79] claimed that slightly longer stalks were obtained in Z. aethiopica ‘Green Goddess’ after an application of BA + GA3; however, these differences were not statistically significant. Interesting results were obtained by Janowska et al. [29]. The authors evaluated the effect of a mixture of BA and GA3 on the number and size of stomata in the epidermis of Zantedeschia leaves. They found that in the cultivar ‘Albomaculata’, after an application of the BA + GA3 mixture (100 + 100 or 350 + 350 mg·dm−3), the stomata in the upper leaf epidermis were larger, and their number decreased. In the lower epidermis, the BA + GA3 at the concentrations used affected the formation of larger stomata, with their abundance decreasing when the mixture was applied at concentrations of 350 + 350 mg·dm−3.
2.4. Auxins
Auxins are synthesized in shoot and root apices. The natural auxin is indole acetic acid (IAA). Synthetic auxins include indole butyric acid (IBA), naphthalene acetic acid (NAA), methyl ester of naphthalene acetic acid (MENA), 2-methyl-4-chlorophenoxyacetic acid (MCPA), 2,3,5-triiodobenzoic acid (TIBA), 2,4-dichlorophenoxyacetic acid (2,4-D) and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T). Natural auxins can be in the form of free auxins, which move freely or diffuse readily from plant tissues, or bound auxins, which are only released from plant tissues after hydrolysis, autolysis, or enzymolysis. Auxins promote growth at low concentrations, while they inhibit growth at high concentrations. [111]. The rooting of cuttings is the most important role played by auxins [112]. IBA [113] and IAA [114] are most commonly used for this purpose. Auxins influence the formation of primary, secondary, and adventitious roots [115]. They are used for the rooting of many ornamental plant species from different groups [116,117,118,119,120]. The stimulation of the rooting of cuttings involves reducing the number of days required for rooting [117], increasing the percentage of rooting, and increasing the length, number, and weight of roots [118].
Effect of Auxins on Flowering and Quality of Geophytes
Apart from rooting cuttings, auxins are rarely used in the cultivation of ornamental plants. However, a few studies have shown that interesting results can be obtained in geophytes treated with auxins (Table 1). According to Sharma et al. [121], auxins in Gladiolus ‘Friendship’ NAA (100 mg·dm−3) had a positive effect on corm size and weight. In Tulipa gesneriana ‘Cassini’, a similar effect was obtained when IAA was applied, but at a very high concentration of 5000 mg·dm−3 [64]. Later studies by Kumar et al. [122] on two Gladiolus cultivars, ‘Iyotsna’ and ‘Shabnum’, did not confirm the beneficial effect of NAA on corm size. On the other hand, Sudhan and Kumar [67] reported that in G. grandiflorus ‘White Friendship’, an NAA application increased not only the number and weight of corms but also the plant height, leaf number, length, width, and spike length.
3. Vase Life of Geophyte Flowers
PGRs are used to extend the post-harvest shelf life of horticultural products: vegetables [123,124], cut flowers [70,125], and florists’ greens [80]. Flowers and florists’ greens are delicate and not very durable products, and their lifespans vary from a few to several days according to the type, species, and cultivar. They have a large external surface area from which water evaporates, and they have no protection against water loss. For post-harvest longevity, the cutting stage is very important. Some flowers, such as those of Iris, Narcissus, and Tulipa, need to be harvested at the closed but colored bud stage. Other flowers, such as Zantedeschia, should be cut in full bloom. The vase life of flowers and florists’ greens is largely determined by their water balance, which is influenced by the following factors: water uptake and conduction, transpiration, the ability of cells to retain water (which determines turgor), and competition for water under stress conditions. A negative water balance, indicative of a decrease in the fresh matter, occurs when the water losses due to transpiration are greater than the water uptake [71,126,127,128,129]. Extending the vase life of flowers and florists’ greens should start at the stage of the producer, whose task it is to carry out conditioning [130,131]. This treatment can be carried out at both low and high temperatures, following the principle that the lower the temperature, the longer the conditioning. Various compounds, chemicals, PGRs, and ready-made formulations available on the market are used for conditioning. After harvesting, however, flowers and florists’ greens cannot be treated equally. In the case of flowers, sugar is often added to water, and in geophytes, this often does not work. Examples of species for which sugar cannot be added to water include Cyclamen, Z. aethiopica, and Narcissus [128,132]. In addition to sugar, hydroxyquinoline esters such as sulfate (8HQS) and citrate (8HQC) are often added to the cut flower medium. They inhibit microbial growth, lower the pH of the solution, and chelate some metal ions, which prevents the formation of physiological blockages and causes closure of the stomata [71,126]. For geophytes, the efficacy of a medium containing sugar and hydroxyquinoline esters was demonstrated in Hippeastrum × chmielii [133], H. × hybridum [134], H. vittatum [135], Alstroemeria aurantiaca [48] and Z. albomaculata ’Albomaculata’ [43], among others. Sometimes, PGRs are used to extend the vase life of cut geophyte flowers. Janowska et al. [44] found that BA at 50–150 mg·dm−3 extended the post-harvest longevity of Z. albomaculata ‘Albomaculata’ inflorescences by 7–14 days.
4. Vase Life of Geophyte Leaves
Aging leaves lose turgor, lose color or dry out. Enzymes appear in the cells that destroy cell walls [136]. In cells, proteins [137,138] and chlorophyll [68,69,139] break down, but free radicals are formed, which are structures that destroy cell components [140]. In response to free radicals, reactive oxygen species (ROS) are formed, which inhibit the aging process [141,142,143,144,145,146,147]. The most important ROS is hydrogen peroxide (H2O2). Large amounts of it indicate oxidative stress [148].
As the aging processes of florists’ greens are different from those of cut flowers, standard nutrient solutions are usually not very effective for them [80]. In contrast, numerous studies have shown that, in florists’ greens, the extension of post-harvest longevity is caused by CKs and GAs (Table 1), which effectively inhibit chlorophyll and protein degradation. However, the response to PGRs among geophytes is closely species-related [86]. Skutnik et al. [80] reported a beneficial effect of GA3 on the vase life of the leaves of Z. aetiophica. The authors reported as much as a six-fold increase in the vase life of the leaves following the application of this PGR, with concomitant inhibition of chlorophyll degradation. A similar response was reported in Zantedeschia leaves with colorful spathes [43,44,86,87]. In H. × hybridum, on the other hand, this PGR caused as much as an eight-fold increase in post-harvest leaf longevity [69]. The beneficial effect of this regulator is also used in practice in Alstroemeria aurantiaca [46,47,48] and Lilium sp. [73], for which it effectively prevents leaf yellowing, as it inhibits chlorophyll breakdown. Arum italicum leaves also respond positively to GA3 [53]. In this species, Janowska and Schroeter-Zakrzewska [54] further showed that BA was also effective.
In addition to GA3 and BA, other regulators from the CK group are also used to extend the vase life of florists’ greens. In a study by Janowska et al. [44], topolins (Ts) were used to extend the leaf vase life of Z. albomaculata ‘Albomaculata’. Meta-methoxytopolin (MemT) and its riboside (MemTR) were found to affect the post-harvest longevity and leaf quality of the cultivar under study. Recent studies have shown that MemTR at 50 and 100 mg·dm−3 also extends the leaf vase life of Hemerocallis × hybrida ‘Agata’ [68].
New regulators used for the post-harvest treatment of leaves are quaternary ammonium salts with selected organic cations and GA3 anions (2-hydroxyethyl)dimethylethylammonium gibberellinate ([Chol][Gib]), acetylcholine gibberellinate ([Gib][Ach]), 1-ethyl quinine gibberellinate ([Q-C2][Gib]) and 1-dodecyl acetylcholine gibberellinate ([Q-C12][Gib]) [55,68]. The few studies to date suggest that these compounds extend the vase life of the leaves of Convallaria majalis (Figure 5) [55] and H. × hybrida ‘Agata’ [68].

Figure 5.
Convallaria majalis leaves. From the left: control leaves; leaves conditioned in GA3 (100 mg·dm−3) and conditioned in [Gib][Ach] (100 mg·dm−3).
5. Conclusions
As in the case of other ornamental plants, plant growth regulators (PGRs) are more and more often used in the geophyte group to improve the quality of plants, the intensity of flowering, and the vase life of flowers and leaves. In the case of geophytes, the most common method of application is spraying and soaking the storage organs before planting. In geophytes, gibberellic acid (GA3) and benzyladenine (BA) are the most commonly used, although scientists tend to focus most of their attention on GA3, as this regulator significantly increases the flowering intensity in many species, which is important for horticultural practice. In addition, PGRs have an impact on early flowering. After their application, plants start flowering earlier or later. PGRs also affect plant quality as expressed in plant height, length of flower or inflorescence stems, flower size, number of leaves, and yield of storage organs (bulbs, tubers, corms). PGRs also stimulate the uptake of macro- and micronutrients so that the use of mineral fertilizers can be reduced. GAs and CKs have a strong effect on the vase life, particularly on leaves, with the response of individual species depending on the regulator used and its concentration. Further research in geophytes is necessary as the response to PGRs depends not only on the species but also on the cultivar. Therefore, a uniform formula cannot be given for all cultivated species. Furthermore, it is worth focusing not only on the use of known and popular PGRs and the introduction of new, often equally effective regulators into the research, as evidenced by studies with topolins (Ts) and ionic liquids. It is worth concentrating future research efforts on determining changes in the content of biologically active compounds in geophytes after the application of PGRs, as many species contain very valuable substances with diverse uses.
Author Contributions
Conceptualization, B.J. and R.A.; writing—original draft, B.J. and R.A.; writing—review and editing, B.J. and R.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Raunkiaer, C. The Life Forms of Plants and Statistical Plant Geography; Oxford University Press: London, UK, 1934. [Google Scholar]
- Rundel, P.W. Monocotyledonous geophytes in the California flora. Madroño 1996, 43, 355–368. [Google Scholar]
- Hoffmann, A.J.; Liberona, F.; Hoffmann, E.A. Distribution and ecology of geophytes in Chile. Conservation threats to geophytes in Mediterranean-type regions. In Landscape Disturbance and Biodiversity in Mediterranean-Type Ecosystems; Rundel, P.W., Montenegro, G., Jaksic, F.M., Eds.; Springer Nature: New York, NY, USA, 1998; pp. 231–253. [Google Scholar]
- Parsons, R.F.; Hopper, S.D. Monocotyledonous geophytes: Comparison of south-western Australia with other areas of mediterranean climate. Aust. J. Bot. 2003, 51, 129–133. [Google Scholar] [CrossRef]
- Procheş, Ş.; Cowling, R.M.; Goldblatt, P.; Manning, J.C.; Snijman, D.A. An overview of the Cape geophytes. Biol. J. Linn. Soc. 2006, 87, 27–43. [Google Scholar] [CrossRef]
- Bryan, J.E. Bulbs; Timber Press: Portland, OR, USA, 1989. [Google Scholar]
- Genders, R. Bulbs, a Complete Handbook of Bulbs, Corms and Tubers; Robert Hale and Company: London, UK, 1973. [Google Scholar]
- Mathew, B. Dwarf Bulbs; B.T. Batsford Limited: London, UK, 1973. [Google Scholar]
- Mathew, B. The Larger Bulbs; Batsford Limited: London, UK, 1978. [Google Scholar]
- Halevy, A.H. Recent advances in control of flowering, and growth habit of geophytes. Acta Hortic. 1990, 266, 35–42. [Google Scholar] [CrossRef]
- Sochacki, D.; Rabiza-Świder, J.; Skutnik, E. Ozdobne Rośliny Cebulowe–Produkcja i Zastosowaniem; Katedra Roślin Ozdobnych, SGGW: Warszawa, Poland, 2018; p. 5. [Google Scholar]
- Rademacher, W. Plant growth regulators: Backgrounds and uses in plant production. J. Plant Growth Regul. 2015, 34, 845–872. [Google Scholar] [CrossRef]
- Sathyanarayana, E.; Divya, K. Role of plant growth regulators in flower crops. In Advances in Horticulture; Lodhi, S.K., Kumar, V., Eds.; AkiNik Publications: New Delhi, India, 2021; pp. 3–15. [Google Scholar]
- Grzesik, M. Factors influencing the effectiveness of growth regulators in nursery production. Acta Hort. 1989, 251, 371–375. [Google Scholar] [CrossRef]
- Carey, D.J.; Whipker, B.E.; McCall, I.; Buhler, W. Cytokinin based PGR affects growth of vegetative petunia. In Proceedings of the 35th Annual Meeting of the Plant Growth Regulation Society of America, San Francisco, CA, USA, 3–7 August 2008; Plant Growth Regulation Society of America: Atlanta, GA, USA, 2008. [Google Scholar]
- Sajjad, Y.; Jaskani, M.J.; Ashraf, M.Y.; Qasim, M.; Ahmad, R. Response of morphological and physiological growth attributes to foliar application of plant growth regulators in Gladiolus ‘White Prosperity’. Pak. J. Agri. Sci. 2014, 51, 123–129. [Google Scholar]
- Matsumoto, T.K. Gibberellic acid and benzyladenine promote early flowering and vegetative growth of Miltoniopsis orchid hybrids. Hort. Sci. 2006, 41, 131–135. [Google Scholar] [CrossRef]
- Patil, M.S.; Bhoite, A.; Gaikwad, D. Effect of pre-sowing treatments of PGRs on the activity of enzyme peroxidase in Simarouba glauca seedlings. ICCRCLS 2015, 8, 2320–9593. [Google Scholar]
- Pill, W.G.; Gunter, J.A. Emergence and shoot growth of Cosmos and Marigold from paclobutrazol treated seed. J. Environ. Hortic. 2001, 19, 11–14. [Google Scholar] [CrossRef]
- Saniewski, M.; Goraj, J.; Lesiak, E.W.; Okubo, H.; Miyamoto, K.; Ueda, J. Different growth of excised and intact fourth internode after removal of the flower bud in growing tulips: Focus on. J. Fruit Ornam. Plant Res. 2010, 18, 297–308. [Google Scholar]
- Carswell, F.E.; Day, J.S.; Gould, K.S. Cytokinins and the regulation of plant form in three species of Sephora. N. Z. J. Bot. 1996, 34, 123–130. [Google Scholar] [CrossRef]
- de Vries, D.P.; Dubois, L.A.M. The effect of BAP and IBA on sprouting and adventitious root formation of ‘Amanda’ rose single-node softwood cuttings. Sci. Hortic. 1988, 34, 115–121. [Google Scholar] [CrossRef]
- Janowska, B.; Schroeter, A. Wpływ kwasu giberelinowego na kwitnienie cantedeskii Elliota (Zantedeschia elliottiana/W. Wats./Engl. ’Black Magic’. Zesz. Probl. Post. Nauk Rol. 2002, 483, 93–99. [Google Scholar]
- Janowska, B.; Zakrzewski, P. Wpływ fluropirimidolu na wzrost i kwitnienie cantedeskii (Zantedeschia Spreng.) uprawianej w doniczkach. Zesz. Probl. Post. Nauk Roln. Part II 2005, 504, 611–621. [Google Scholar]
- Janowska, B.; Zakrzewski, P. Wpływ kwasu giberelinowego i sposobu przygotowania kłączy na kwitnienie cantedeskii (Zantedeschia Spreng.). Zesz. Probl. Post. Nauk Roln. Part I 2006, 510, 223–233. [Google Scholar]
- Janowska, B. Effect of growth regulators on flower and leaf yield of the calla lily (Zantedeschia Spreng.). Hortic. Sci. 2013, 40, 78–82. [Google Scholar] [CrossRef]
- Janowska, B.; Stanecki, M. Effect of benzyladenine on the abundance and quality of flower yield in the Calla lily (Zantedeschia Spreng.). Acta Agrobot. 2012, 65, 109–116. [Google Scholar] [CrossRef]
- Janowska, B.; Stanecki, M. Effect of rhizome soaking in a mixture of BA and GA3 on the earliness of flowering and quality of the yield of flowers and leaves in the Calla lily (Zantedeschia Spreng.). Acta Sci. Pol. Hortorum Cultus 2013, 13, 3–12. [Google Scholar]
- Janowska, B.; Mansfeld, N.; Andrzejak, R. Effect of BA and GA3 on the morphological features of stomata in the leaf epidermis of the Zantedeschia albomaculata cv. ‘Albomaculata’. Not. Bot. Horti Agrobot. Cluj-Napoca 2014, 42, 104–108. [Google Scholar] [CrossRef]
- Janowska, B. Regulatory wzrostu w uprawie i przedłużaniu trwałości kwiatów i liści cantedeskii (Zantedeschia Spreng.) o barwnych pochwach kwiatostanowych. Zesz. Probl. Post. Nauk Rol. 2016, 585, 87–96. [Google Scholar]
- Janowska, B.; Andrzejak, R.; Kosiada, T.; Kwiatkowska, M.; Smolińska, D. The flowering and nutritional status of Gladiolus hybridus ‘Black Velvet’ following gibberellin treatment. Hortic. Sci. 2018, 45, 205–210. [Google Scholar] [CrossRef]
- Janowska, B.; Andrzejak, R.; Kosiada, T.; Kwiatkowska, M.; Smolińska, D. The flowering and nutritional status of Gladiolus hybridus cv. Black Velvet following a cytokinin treatment. J. Element. 2018, 23, 1119–1128. [Google Scholar] [CrossRef]
- Andrzejak, R.; Janowska, B. Yield and quality of inflorescences in the Zantedeschia albomaculata (Hook.) Baill. ‘Albomaculata’ after the treatment with AMF and GA3. Agronomy 2021, 11, 644. [Google Scholar] [CrossRef]
- Janowska, B.; Andrzejak, R. Cytokinins and gibberellins stimulate the flowering and post-harvest longevity of flowers and leaves of Calla lilies (Zantedeschia Spreng.) with colourful inflorescence spathes. Agronomy 2022, 12, 1859. [Google Scholar] [CrossRef]
- Janowska, B.; Andrzejak, R. Effect of gibberellic acid spraying and soaking of rhizomes on the growth and flowering of calla lily (Zantedeschia Spreng.). Acta Agrobot. 2010, 63, 155–160. [Google Scholar] [CrossRef]
- Stowe, B.B.; Yamaki, T. The history and physiological action of the gibberellins. Ann. Rev. Plant Physiol. 1957, 8, 181–216. [Google Scholar] [CrossRef]
- Stanisławski, J. Wpływ gibereliny A3 na kiełkowanie, wzrost i plonowanie roślin. Wiad. Bot. 1977, 21, 167–281. [Google Scholar]
- Kobayashi, M.; Yamaguchi, I.; Murofushi, N.; Ota, Y.; Takahashi, N. Fluctuation and localization of endogenous gibberellins in rice. Agric. Biol. Chem. 1988, 52, 1189–1194. [Google Scholar]
- Hoad, G.V. Transport of hormones in the phloem of higher plants. Plant Growth Regul. 1995, 16, 173–182. [Google Scholar] [CrossRef]
- Gupta, R.; Chakrabarty, S.K. Gibberellic acid in plant. Still a mystery unresolved. Plant Signal. Behav. 2013, 8, e25504. [Google Scholar] [CrossRef]
- Debeaujon, I.; Koornneef, M. Gibberellin requirement for Arabidopsis seed germination is determined both by testa characteristics and embryonic abscisic acid. Plant Physiol. 2000, 122, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, J.; Murase, K.; Rieu, I.; Zentella, R.; Zhang, Z.-L.; Powers, S.J.; Gong, F.; Phillips, A.L.; Hedden, P.; Sun, T.-P.; et al. Genetic characterization and functional analysis of the GID1 gibberellin receptors in Arabidopsis. Plant Cell 2006, 18, 3399–3414. [Google Scholar] [CrossRef]
- Janowska, B.; Stanecka, A. Effect of growth regulators on the postharvest longevity of cut flowers and leaves of the Calla lily (Zantedeschia Spreng.). Acta Agrobot. 2011, 64, 91–98. [Google Scholar] [CrossRef][Green Version]
- Janowska, B.; Stanecka, A.; Czarnecka, B. Postharvest longevity of the leaves of the Calla lily (Zantedeschia Spreng.). Acta Sci. Pol. Hortorum Cultus 2012, 11, 121–131. [Google Scholar]
- Marciniak, K.; Świeżawska, B.; Kęsy, J.; Tretyn, A.; Kopcewicz, J. Gibereliny–percepcja i transdukcja sygnału u roślin. PBK 2012, 39, 24–47. [Google Scholar]
- Dai, J.W.; Paull, R.E. Postharvest handling of Alstroemeria. HortScience 1991, 26, 314. [Google Scholar] [CrossRef]
- Hicklenton, P.R. GA3 and benzylaminopurine delay leaf chlorosis in cut Alstroemeria stems. HortScience 1991, 26, 1198–1199. [Google Scholar] [CrossRef]
- Yeat, C.S.; Szydlik, M.; Łukaszewska, A.J. The effect of postharvest treatments on flower quality and vase life of cut Alstroemeria ‘Dancing Queen’. J. Fruit Ornam. Plant Res. 2012, 20, 147–160. [Google Scholar] [CrossRef]
- Mishra, S.K.; Mishra, S.; Bahadur, V. Effect of growth regulators on growth, yield and shelf life in amaryllis lily (Amaryllis belladona) cv. Zephyranthes. J. Pharmacogn. Phytochem. 2019, 8, 1217–1219. [Google Scholar]
- Piskornik, M.; Piskornik, Z. Wpływ dawki i terminu stosowania kwasu giberelinowego (GA3) na jakość kwiatów zawilca wieńcowatego (Anemone coronaria L.) uprawianego w gruncie. In Fizjologiczne Aspekty Produkcji Ogrodniczej; Akademia Rolnicza w Krakowie: Kraków, Poland, 1995. [Google Scholar]
- Janowska, B.; Schroeter-Zakrzewska, A.; Rybus-Zając, M. Effect of benzyladenine and gibberellic acid on the growth and flowering of Anemone coronaria L. ‘Sylphide’. EJPAU 2009, 12, 08. [Google Scholar]
- Janowska, B.; Rybus-Zając, M.; Schroeter-Zakrzewska, A. Content of chloroplast pigments and saccharides in leaves of poppy anemone (Anemone coronaria L.) ‘Sylphide’ after application of benzyladenine and gibberellic acid. Nauka Przyr. Technol. 2012, 6, 44. [Google Scholar]
- Janowska, B. Effect of conditioning on the longevity of leaves of the Italian arum (Arum italicum Mill.) kept at a low temperature. Nauka Przyr. Technol. 2010, 4, 12. [Google Scholar]
- Janowska, B.; Schroeter-Zakrzewska, A. Effect of gibberellic acid, benzyladenine and 8-hydroxyquinoline sulphate on post-harvest leaf longevity of Arum italicum Mill. Zesz. Probl. Post. Nauk Rol. 2008, 525, 181–187. [Google Scholar]
- Szymaniak, D.; Pernak, J.; Rzemieniecki, T.; Kaczmarek, D.K.; Andrzejak, R.; Kosiada, T.; Janowska, B. Synthesis and characterization of bio-based quaternary ammonium salts with gibberellate or α-tryptophanate anion. Monatsh. Chem. Chem. Mon. 2020, 151, 1365–1373. [Google Scholar] [CrossRef]
- Janowska, B.; Andrzejak, R.; Szwajkowska-Michałek, L.; Stuper-Szablewska, K. The content of biologically active substances in Crocosmia × crocosmiiflora ‘Lucifer’ tubers after treatment with GA3. Agronomy 2021, 11, 553. [Google Scholar] [CrossRef]
- Thomas, P.A.; Coorts, G.D.; Preece, J.E. Phthalimides, gibberellic acid, and flowering of Cyclamen. HortScience 1983, 18, 752–753. [Google Scholar] [CrossRef]
- Treder, J.; Matysiak, B.; Nowak, J. Przyspieszanie kwitnienia cyklamenów przy użyciu kwasu giberelinowego. In Postęp w Produkcji Roślin Doniczkowych i Rabatowych; Insytut Sadownictwa i Kwiaciarstwa w Skierniewicach: Skierniewice, Poland, 1999. [Google Scholar]
- Nowak, J. Wpływ różnych preparatów zawierających kwas giberelinowy na wzrost i kwitnienie cyklamena i gerbery. Zesz. Nauk. ISiK Skiern. 2000, 7, 259–263. [Google Scholar]
- Żurawik, P.; Placek, M. The influence of selected growth regulators on development, decorative value and yield of corms of Easy Pot freesia (Freesia Eckl. ex Klatt). Part I. Gibberellin A3. Acta Sci. Pol. Hortorum Cultus 2013, 12, 55–64. [Google Scholar]
- Ibrahim, M.M. Influence of corms size and spraying with benzyladenine and paclobutrazol on growth and flowering characteristics of Freesia sp. L. plants. SJSR 2014, 1, 1–10. [Google Scholar]
- Tonecki, J. Światło i Hormony Roślinne we Wzroście i Rozwoju Mieczyka (Gladiolus × hortorum cv. Acca Laurentia). Postdoctoral Dissertation, SGGW w Warszawie: Warszawa, Poland, 1988. [Google Scholar]
- Sajiad, M.; Anjum, M.A.; Hussain, S. Foliar application of plant growth regulators affect growth, flowering, vase life and corm production of Gladiuolus grandiflorus L. under calcareous soil. Bul. J. Agric Sci. 2015, 21, 982–989. [Google Scholar]
- Sable, P.B.; Ransingh, U.R.; Waskar, D.P. Effect of foliar application of plant growth regulators on growth and flower quality of Gladiolus cv. ‘H.B.Pitt’. J. Hortic. 2015, 2, 1000141. [Google Scholar] [CrossRef]
- Rahman, A.; Hussain, I.; Ziaullah, N.G. Exogenous gibberellic acid application influences on vegetative and reproductive aspects in gladiolus. Ornam. Hortic. 2020, 26, 244–250. [Google Scholar] [CrossRef]
- Kurtar, E.S.; Ayan, A.K. Effect of gibberellic acid and indole-3-aciedic acid on flowering, stalk elongation and bulb characteristics of Tulip (Tulipa gesneriana var. Cassini). Pak. J. Biol. Sci. 2005, 8, 273–277. [Google Scholar]
- Sudhakar, M.; Kumar, S.R. Effect of growth regulators on growth, flowering and corm production of Gladiolus (Gladiolus grandiflorus L.) cv. White Friendship. Indian J. Plant Sci. 2012, 1, 133–136. [Google Scholar]
- Janowska, B.; Nowińska, M.; Andrzejak, R. The vase life of the leaves of selected perennial species after the application of growth regulators. Agronomy 2022, 12, 805. [Google Scholar] [CrossRef]
- Łukaszewska, A.; Skutnik, E. Przewodnik Florysty; Wydawnictwo SGGW: Warszawa, Poland, 2003. [Google Scholar]
- Saniewski, M.; Mynett, k.; Nowak, J.; Rudnicki, M.R. Badania nad fizjologią cebul hiacyntów (Hyacinthus orientalis L.) Część II. Badania nad wpływem hormonów roslinnych na rozmnazanie hiacyntów. Prace Inst. Sadow. 1975, 2, 81–89. [Google Scholar]
- Nowak, J.; Rudnicki, R.M. Hyacinthus. In The Physiology of Flower Bulbs; de Hertogh, A., le Nard, M., Eds.; Elsevier Sciences: Amsterdam, The Netherlands, 1993; pp. 335–347. [Google Scholar]
- Halevy, A.H.; Shoub, J. The effect of cold storage and treatment with gibberellic acid on flowering and bulb yields of Dutch iris. J. Hort. Sci. 1964, 39, 120–129. [Google Scholar] [CrossRef]
- Han, S.S. Growth regulators delay foliar chlorosis of Easter lily leaves. J. Am. Soc. Hortic. Sci. 1995, 120, 254–258. [Google Scholar] [CrossRef]
- Saniewski, M.; Tymoszczuk, J.; Rudnicki, R.M. Hormonal control of flowering and the growth of the inflorescence stalk and leaves of Muscari armeniacum Leichtl. Prace ISiK Skiern. 1978, 3, 57–67. [Google Scholar]
- Pisulewski, T.R.; Goszczyńska, D.M.; Rudnicki, R.M. The effect of gibberellic acid and ethrel on quality and longevity of cut tulip flowers. Prace ISiK Skiern. 1989, 14, 155–160. [Google Scholar]
- Saniewski, M.; Kawa, L.; Węgrzynowicz, E. The effect of abscisic acid on pistil and stem growth in tulips. Prace ISiK Skiern. 1990, 15, 95–103. [Google Scholar]
- Salachna, P.; Mikiciuk, M.; Zawadzińska, A.; Piechocki, R.; Ptak, P.; Mikiciuk, G.; Pietrak, A.; Łopusiewicz, Ł. Changes in growth and physiological parameters of ×Amarine following an exogenous application of gibberellic acid and methyl jasmonate. Agronomy 2020, 10, 980. [Google Scholar] [CrossRef]
- Luria, G.; Weiss, D.; Ziv, O.; Borochov, A. Effect of planting depth and density, leaf removal, cytokinin ang gibberellic acid treatments on flowering and rhizome production in Zantedeschia aethiopica. Acta Hortic. 2005, 673, 725–730. [Google Scholar] [CrossRef]
- Ngamau, K. Promoting side shoot development in Zantedeschia aethiopica ‘Green Goddess’. Gartenbauwissenschaft 2001, 66, 85–92. [Google Scholar]
- Skutnik, E.; Łukaszewska, A.J.; Serek, M.; Rabiza, J. Effect of growth regulators on postharvest characteristics of Zantedeschia aethiopica. Post. Biol. Technol. 2001, 21, 241–246. [Google Scholar] [CrossRef]
- Bent, E.N.; Croci, A. Calla: Una antica novita. Flortecnica 1989, 6, 25–29. [Google Scholar]
- Dennis, D.J.; Doreen, J.; Ohteki, T. Effect of a gibberellic acid ‘quick-dip’ and storage on the yield and quality of blooms from hybrid Zantedeschia tubers. Sci. Hortic. 1994, 57, 133–142. [Google Scholar] [CrossRef]
- Funnell, K.A.; MacKay, B.R.; Lawoko, C.R.O. Comparative effects of Promalin® and GA3 on flowering and development of Zantedeschia ‘Galaxy’. Acta Hortic. 1992, 292, 173–179. [Google Scholar] [CrossRef]
- Janowska, B.; Krause, J. Wpływ traktowania bulw kwasem giberelinowym na kwitnienie cantedeskii. Rocz. Akad. Rol. Pozn. Ogrod. 2001, 33, 61–67. [Google Scholar]
- Janowska, B.; Stanecki, M. Effect of benzyladenine on the abundance and quality of the leaf yield in the calla lily (Zantedeschia Spreng.). Nauka Przyr. Technol. 2013, 7, 6. [Google Scholar]
- Janowska, B.; Andrzejak, R. The Role of Cytokinins and gibberellins on post-harvest longevity of florists’ greens. Agriculture 2022, 12, 1375. [Google Scholar] [CrossRef]
- Janowska, B.; Jerzy, M. Effect of gibberellic acid on the post-harvest Zantedeschia elliottiana (W.Wats) Engl. leaf longevity. J. Fruit Ornam. Plant Res. 2003, 11, 69–76. [Google Scholar]
- Funnell, K.A. Zantedeschia. In The Physiology of Flower Bulbs; de Hertogh, A., le Nard, M., Eds.; Elsevier Sciences: Amsterdam, The Netherlands, 1993; pp. 683–704. [Google Scholar]
- Corr, B.E.; Widmer, R.E. Paclobutrazol, gibberellic acid and rhizome size affect growth and flowering of Zantedeschia. HortScience 1991, 262, 133–135. [Google Scholar] [CrossRef]
- Funnell, K.A.; Tjia, B.O. Effect of storage temperature, duration and gibberellic acid on the flowering of Zantedeschia elliottiana and Z. ‘Pink Satin’. J. Am. Soc. Hortic. Sci. 1998, 113, 860–863. [Google Scholar] [CrossRef]
- Ali, Y.S.; Elkiey, T. Effect of chloromequat and GA3 on growth and flowering of calla (Zantedeschia rehmanii). J. King Saud Univ. Agric. Sci. 1995, 7, 271–282. [Google Scholar]
- Treder, J. Wzrost i kwitnienie cantedeskii uprawianej w szklarni i w polu. ZPPNR 2003, 491, 283–291. [Google Scholar]
- Ravensdale, M.; Blom, T.J.; Gracia-Garza, J.A.; Svirce, A.M.; Smith, R.J. Bacteriophages and the control of Erwinia carotovora subsp. Carotovora. Can. J. Plant Pathol. 2007, 29, 121–130. [Google Scholar] [CrossRef]
- Jerzy, M.; Janowska, B. Wzrost i kwitnienie cantedeskii Elliota (Zantedeschia elliottiana/W. Wats./Engl.) uprawianej z sadzonek traktowanych kwasem giberelinowym in vitro. ZPPNR 2003, 491, 125–130. [Google Scholar]
- Jerzy, M.; Pawlak-Anhalt, A. Regeneracja cantedeskii Elliota in vitro z eksplantatów izolowanych z kłączy. ZPPNR 2002, 483, 101–107. [Google Scholar]
- van Bragt, J.; Pierik, R.L.M. The effect of autoclaving on the gibberellin activity of aqueous solutions containing gibberellin A3. Misc. Papers 1971, 9, 133–137. [Google Scholar]
- Matysiak, B. Effect of endomycorrhizal inocula during propagation on the growth following transplanting of Ilex × meserveae ‘Blue Boy’ cutting. ZPPNR 2009, 539, 499–506. [Google Scholar]
- le Nard, M.; de Hertogh, A.A. Tulipa. In The Physiology of Flower Bulbs; de Hertogh, A., le Nard, M., Eds.; Elsevier Sciences: Amsterdam, The Netherlands, 1993; pp. 617–682. [Google Scholar]
- Dawande, V.; Gurav, R. Effect of cytokinins on shoot induction from seed derived. Int. J. Curr. Res. 2015, 7, 16383–16386. [Google Scholar]
- Sakakibara, H. Cytokinins: Activity, biosynthesis, and translocation. Annu. Rev. Plant Biol. 2006, 57, 431–449. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, A.; Czerpak, P. Molekularne mechanizmy działania cytokinin. PBK 2004, 31, 93–115. [Google Scholar]
- Murai, N. Plant growth hormone cytokinins control the crop seed yield. Am. J. Plant Sci. 2014, 5, 2178–2187. [Google Scholar] [CrossRef]
- Borkowska, B. Cytokininy. In Regulatory Wzrostu i Rozwoju Roślin; Jankiewicz, L., Ed.; PWN: Warszawa, Poland, 1997; pp. 60–61. [Google Scholar]
- Svolacchia, N.; Sabatini, S. Cytokinins. Current Biol. 2013, 33, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Kupke, B.M.; Tucker, M.R.; Able, J.A.; Porker, K.D. Manipulation of barley development and flowering time by exogenous application of plant growth regulators. Front. Plant Sci. 2022, 12, 694424. [Google Scholar] [CrossRef]
- Janowska, B. Cantedeskie Nie Tylko Białe; Wydawnictwo UP w Poznaniu: Poznań, Poland, 2014. [Google Scholar]
- Tjia, B.O.; Funnell, K.A. Postharvest studies of cut Zantedeschia inflorescences. Acta Hortic. 1986, 181, 451–458. [Google Scholar] [CrossRef]
- Ranwala, A.P.; Miller, W.B. Gibberellin4+7, benzyladenine and supplemental light improve postharvest leaf and flower quality of cold-stored ‘Star Gazer’ hybrid lilies. J. Amer. Soc. Hortic. Sci. 1998, 123, 563–568. [Google Scholar] [CrossRef]
- Rodriguez-Pérez, J.A.; de Leon-Hernández, A.M.; Vera-Batista, M.C.; Rodriguez-Hernández, J.; Alberto-Rodriguez, P. Effect of pretreatment with gibberellic acid (GA3) and Promalin (GA4+7 + BA) on germination of Protea aristata and P. repens. Acta Hortic. 2009, 813, 441–444. [Google Scholar] [CrossRef]
- Jaumień, F.; Dziuba, N.R.; Nowakowski, R. Rozgałęzianie drzewek jabłoni w szkółce. Szkółkarstwo 2004, 3, 54–60. [Google Scholar]
- Pal, S.L. Role of plant growth regulators in floriculture: An overview. J. Pharmacogn. Phytochem. 2019, 8, 789–796. [Google Scholar]
- Hartmann, H.T.; Kester, D.E.; Davis, J.F.T.; Geneve, R.L. Plant Propagation: Principles and Practices; Prentice Hall: State College, PA, USA, 2002. [Google Scholar]
- Nickel, L.G. Plant Growth Regulators. Agricultural Uses; Springer: NY, NY, USA, 1990; pp. 4–5. [Google Scholar]
- Pop, T.I.; Pamfil, D.; Bellini, C. Auxin control in the formation of adventitious roots. Not. Bot. Horti Agrobot. Cluj-Napoca 2011, 39, 307–316. [Google Scholar] [CrossRef]
- Šebánek, J. Vegetative Physiology Tree Propagation; Mendel University of Agriculture and Forestry in Brno: Brno, Czech Republic, 2008. [Google Scholar]
- Gowda, P.; Dhananjaya, M.V.; Kumar, R. Effect of indole butyric acid (IBA) on rooting of different carnation (Dianthus caryophyllus L.) genotypes. Int. J. Pure Appl. Biosci. 2017, 5, 1075–1080. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, R. Influence of growth regulating substances on rooting of cuttings of Poinsettia cv. Flaming Sphere. Progr. Hortic. 2005, 37, 85. [Google Scholar]
- Saini, S.; Sharma, I.; Kaur, N.; Pati, P. Auxin: A master regulator in plant root development. Plant Cell Rep. 2013, 32, 741–757. [Google Scholar] [CrossRef]
- Dawa, S.; Rather, Z.A.; Sheikh, M.Q.; Nazki, I.T.; Hussain, A. Influence of growth regulators on rhizogenesis in semi-hardwood cuttings of some cut flower Roses. App. Biol. Res. 2013, 15, 1–7. [Google Scholar]
- Akhtar, G.; Akram, A.; Sajjad, Y.; Balal, R.M.; Shahid, M.A.; Sardar, H.; Naseem, K.; Shah, S.M. Potential of plant growth regulators on modulating rooting of Rosa centifolia. Am. J. Plant Sci. 2015, 6, 659–665. [Google Scholar] [CrossRef]
- Sharma, J.R.; Gupta, R.; Panwar, R.D. Growth, flowering and corm production of Gladiolus cv. Friendship as influenced by foliar application of nutrients and growth regulators. J. Ornam. Hort. 2004, 7, 154–158. [Google Scholar]
- Kumar, N.P.; Reddy, Y.N.; Chandrashekar, R. Effect of growth regulators on flowering and corm production in Gladiolus. Indian J. Hort. 2008, 65, 73–78. [Google Scholar]
- Guzman, V.L. Concentration of N6 benzyladenine, temperature, and time effects on retardation of senescence in celery and endive. Proc. Fla. State Hortic. Soc. 1963, 75, 196–201. [Google Scholar]
- Wittwerm, S.H.; Dedolphm, R.R. “Youth” hormone keeps vegetables fresh longer. Am. Veg. Grower 1962, 10, 54–55. [Google Scholar]
- Heide, O.M.; Oydvin, J. Effects of 6-benzylamino-purine on the keeping quality and respiration of glasshouse Carnations. Hortic. Res. 1969, 9, 26–36. [Google Scholar]
- Dzięcioł, J.; Rudnicki, R. Badania nad trwałością kwiatów ciętych. Post. Nauk Rol. 1971, 3, 105–119. [Google Scholar]
- Łukaszewska, A.J. Wpływ Etylenu i Niskiej Temperatury na Niektóre Procesy Zachodzące w Ciętych Różach. Postdoctoral Dissertation, SGGW-AR, Warszawa, Poland, 1988. [Google Scholar]
- Łukaszewska, A.J. Dlaczego cięte kwiaty więdną i jak temu zapobiegać. In Najnowsze Metody Przedłużania Trwałości Ciętych Kwiatów; SGGW w Warszawie: Warszawa, Poland, 1998. [Google Scholar]
- Rudnicki, R.M.; Nowak, J. Jak Przedłużyć Trwałość Kwiatów Ciętych i Roślin Doniczkowych; PHU Mutual Benefit: Skierniewice, Poland, 1992; p. 76. [Google Scholar]
- Lisiecka, A.; Janowska, B. Przedłużanie trwałości kwiatów róż. In Najnowsze Metody Przedłużania Trwałości Ciętych Kwiatów; SGGW w Warszawie: Warszawa, Poland, 1998. [Google Scholar]
- Skutnik, E. Wpływ pożywek i egzogennego etylenu na trwałość ciętych pędów Molucella laevis (dzwonki irlandzkie). In Najnowsze Metody Przedłużania Trwałości Ciętych Kwiatów; SGGW w Warszawie: Warszawa, Poland, 1998. [Google Scholar]
- Łukaszewska, A.J.; Kokosa, A. Przedłużanie trwałości ciętych kwiatów geofitów. Ogrodnictwo 1997, 2, 19–20. [Google Scholar]
- Rabiza-Świder, J.; Skutnik, E. Wpływ regulatorów wzrostu na trwałość ciętych liści zwartnicy Chmiela (Hippeastrum × chmielii Chm.). Zesz. Probl. Post. Nauk Rol. 2006, 510, 551–557. [Google Scholar]
- AbdelKader, H.H. Postharvest physiology of cut Hippeastrum (Hippeastrum hybridum Herb.) inflorescences. World App. Sci. J. 2012, 19, 943–995. [Google Scholar]
- Hassan, F. Influence of 8-hydroxyquinoline sulphate and sucrose treatments on the post-harvest quality of cut flowers of Strelitzia reginae and Hippeastrum vittatum. Acta Agron. Hung. 2009, 57, 165–174. [Google Scholar] [CrossRef]
- Janowska, B.; Trelka, T. Effect of preparations from the Chrysal series and benzyladenine on the postharvest longevity of shoots of the St. John’s wort (Hypericum calycinum L.). Nauka Przyr. Technol. 2010, 4, 8. [Google Scholar]
- Nam, H.G. The molecular genetic analysis of leaf senescence. Curr. Opin. Biotechnol. 1997, 8, 200–207. [Google Scholar] [CrossRef]
- Breeze, E.; Harrison, E.; McHattie, S.; Hughes, L.; Hickman, R.; Hill, C.; Kiddle, S.; Kim, Y.-S.; Penfold, C.A.; Jenkins, D.; et al. High-resolution temporal profiling of transcripts during Arabidopsis leaf senescence reveals a distinct chronology of processes and regulation. Plant Cell 2011, 23, 873–894. [Google Scholar] [CrossRef]
- Pacifici, S.; Burchi, G.; del Carlo, A.; Ferrante, A. Effect of storage temperature and duration on vase life of cut Ruscus racemosus L. foliage. Acta Hortic. 2013, 970, 69–74. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Lee, S.; Seo, P.J.; Lee, H.J.; Park, C.M. A NAC transcription factor ntl4 promotes reactive oxygen species production during drought-induced leaf senescence in Arabidopsis. Plant J. Cell Mol. Biol. 2012, 70, 831–844. [Google Scholar] [CrossRef]
- Prochazkova, D.; Sairam, R.K.; Srivastava, G.C.; Singh, D.V. Oxidative stress and antioxidant activity as the basis of senescence in maize leaves. Plant Sci. 2001, 161, 765–771. [Google Scholar] [CrossRef]
- Selote, D.S.; Khanna-Chopra, R. Drought acclimation confers oxidative stress tolerance by inducing co-ordinated antioxidant defense at cellular and subcellular level in leaves of wheat seedlings. Physiol. Plant. 2006, 127, 494–506. [Google Scholar] [CrossRef]
- Silva, E.N.; Ferreira-Silva, S.L.; Fontenele, A.D.V.; Ribeiro, R.V.; Viégas, R.A.; Silveira, J.A.G. Photosynthetic changes and protective mechanisms against oxidative damage subjected to isolated and combined drought and heat stresses in Jatropha curcas plants. J. Plant Phsiol. 2010, 167, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.; Panda, P.; Sahoo, L.; Panda, S.K. Reactive oxygen species signaling in plants under abiotic stress. Plant Signal. Behav. 2013, 8, e23681. [Google Scholar] [CrossRef]
- Río, L.A.D.; Pastori, G.M.; Palma, J.M.; Sandalio, L.M.; Sevilla, F.; Corpas, F.J.; Jiménez, A.; López-Huertas, E.; Hernández, J.A. The activated oxygen role of peroxisomes in senescence. Plant Physiol. 1998, 116, 1195–1200. [Google Scholar]
- Lushchak, V.I. Adaptive response to oxidative stress: Bacteria, fungi, plants and animals. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2011, 153, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Jajic, I.; Sarna, T.; Strzalka, K. Senescence, stress, and reactive oxygen species. Plants 2015, 4, 393–411. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).