Abstract
Thyroid hormones mediate the interaction between the metabolic and reproductive systems, while their metabolism is controlled by different deiodinases. The present study aimed to search for associations of cow genotypes with SNPs in the deiodinase type 1 gene (DIO1) with thyroid profiles and reproductive traits. The blood was sampled from Russian black-and-white cows 2–6 weeks before calving and 1–13 weeks after calving to measure the hormonal levels by ELISA. RT-PCR analysis was performed for known mutations in the bovine DIO1 gene, and a polymorphism at position 13,149 was found. In animals with the CG genotype, the blood concentration of reverse triiodothyronine 6 weeks prepartum was higher and decreased much earlier than in animals with the CC genotype. Furthermore, 1 week after calving, the total triiodothyronine to reverse triiodothyronine ratio in cows with the CG genotype was higher than in cows with the CC genotype. A higher proportion of animals with better values of fertility traits was revealed in the CC group compared to the CG group. Thus, cows with the CC genotype of the DIO1 gene more often have a high reproductive ability, which may be associated with the rT3 profile features during the prepartum and early postpartum periods.
1. Introduction
During the transition from pregnancy to lactation, the body of dairy cows is subjected to an increased load on the metabolic system [1]. This phenomenon is especially evident in the animals of the Holstein breed. During the final stage of preparation for lactation and at its early stage, the body of dairy cows experiences an increased need for energy and nutrients that cannot be replenished by the incoming feed. Under these conditions, a negative energy balance is formed, resulting in homeorhetic changes aimed at maintaining lactation. These changes complicate the normal functioning of other physiological systems, including the reproductive one. The energy deficit is partially compensated by the mobilization of the body’s internal reserves, primarily fat depots [2]. As a result, the blood content of non-esterified fatty acids (NEFA) and ketone bodies, the intermediate products of their oxidation, increases [3,4], which has a negative effect on reproductive function and reduces overall immunity, thereby impairing the reproductive health of the animals [1,4,5]. In addition, postpartum cows are in a state of insulin resistance when the uptake of glucose by extramammary tissues is reduced, with this state being enhanced by NEFA [1].
Thyroid hormones are involved in the regulation of metabolism [6], and thus, they serve as an important link in the interaction of the metabolic and reproductive systems. Concurrently, the concentration of circulating thyroid hormones in many species, including cattle, is positively associated with energy balance [7,8]. In dairy cows, a decline in the content of thyroxine (T4) in the blood has been found one week after calving [9,10]. Such a decline in the activity of the thyroid system in the early postpartum period is considered one of the adaptive mechanisms that cause a deceleration in the metabolism under conditions of nutrient deficiency [11]. Furthermore, thyroid hormones contribute significantly to the endocrine control of lipid metabolism [6]. In cattle, the ratio between T4 and triiodothyronine (T3) is associated with fatty liver and with the concentration of NEFA in the blood [12,13]. Thus, thyroid hormones can affect the reproductive function of cows, especially during the transition from pregnancy to lactation, through the regulation of various metabolic pathways. Indeed, the concentration of T4 and T3 in the blood of dairy cows during the postpartum period is connected with the state of their reproductive system [10,14,15].
In addition to regulating general metabolic pathways, thyroid hormones can directly affect the ovary. In cattle, the presence of free T4 and T3 in the follicular fluid and their specific receptors in oocytes and cumulus cells indicates the possibility of a direct action of thyroid hormones on the follicle and oocyte maturation [16,17]. This assumption is also supported by the data on the ability of thyroid hormones to modulate in vitro the steroidogenic activity of bovine follicular cells and the oocyte developmental competence [17,18].
The activity of the thyroid system is highly dependent on the metabolism of thyroid hormones, which is under the control of three types of deiodinases, being the products of different genes [19]. All deiodinases are dimeric integral membrane proteins of about 60 kDa, containing the modified amino acid selenocysteine in the active center [20]. The conversion of T4 into a more biologically active form, T3, is catalyzed by the types 1 and 2 deiodinases (DIO1 and DIO2). Inactivation of T4 by its conversion to reverse T3 (rT3) is under the control of DIO1 and the type 3 deiodinase (DIO3), while the latter also inactivates T3, converting it to diiodothyronine. Meanwhile, outer-ring deiodination leads to T3, whereas inner-ring deiodination leads to rT3.
In humans, a number of polymorphisms have been identified in the genes for these three types of deiodinases, and some of them are connected with alterations in the thyroid hormone levels and, apparently, are of clinical importance [21,22]. Cattle, like other mammals, have several genetic variants for the DIO1 and DIO3 genes [23,24]. Studies on pigs have shown associations between the single nucleotide polymorphism (SNP) of the DIO3 gene and the reproductive performance of sows [25,26]. Similar associations may exist in other species, including cows.
In connection with the above, the present study was aimed to search for associations of cow genotypes with SNPs in the DIO1 gene with thyroid profiles and reproductive traits. We performed a DNA analysis for known mutations in the DIO1 gene in Russian black-and-white cows, as well as studied prepartum and postpartum thyroid hormone levels, the postpartum ovarian state, the open days period, and the first service conception rate in the animals with different polymorphic variants of this gene. In general, the findings suggest that the polymorphism at positions 13,149 of the DIO1 gene determines the prepartum and postpartum thyroid profiles, thus affecting the adaptive capacity and reproductive performance in dairy cows.
2. Materials and Methods
2.1. Animals
The study was carried out on Russian black-and-white cows of the 2nd to 4th calving with a productivity level of 5.5 to 7.5 thousand kg of milk for 305 days of lactation, kept in one commercial farm. The diet of the animals corresponded to their productivity and the stage of the reproductive cycle according to zootechnical standards. During the dry period, animals were fed with a diet composed of 5 kg of grass hay, 18 kg of perennial herbs haylage, 0.3 kg of syrup, 2 kg of a commercial concentrate (generally, 14.8 kg DM, 8.75 MJ/kg DM, 1.61 kg crude protein (CP), 3.76 kg crude fiber, 0.46 kg crude fat (CF), 0.75 kg starch, 1.38 kg sugar). The postpartum diet composition was as follows: 2 kg of grass hay, 14 kg of perennial herbs haylage, 14 kg of corn silage, 1.5 kg of syrup, 12 kg of a commercial concentrate (generally, 23.4 kg DM, 10.86 MJ/kg DM, 3.53 kg CP, 3.92 kg crude fiber, 1.00 kg CF, 3.75 kg starch, 2.29 kg sugar).
Cows calved from May to July were used in the survey. Only animals without calving difficulties and clinical signs of postpartum gynecological or metabolic diseases (palpably detectable endometritis, metritis, mastitis, abnormal genital discharges, parturient paresis, laminitis, ketosis) during the interval from the 1st to the 13th week postpartum were selected for further analysis (n = 68). The body condition score (BCS; 5-point scale according to Wildman et al. [27]) was assessed 2 weeks before calving and 3 weeks after calving.
2.2. Blood Sampling and Reproductive Traits Assessment
All procedures were conducted according to the ethical guidelines of the L.K. Ernst Federal Research Center for Animal Husbandry (protocol no. 2, dated 18 January 2021).
Thyroid profiles of individuals were studied during the prepartum and postpartum periods, which are critical for the subsequent reproductive ability of cows. For this purpose, the blood was taken from animals 6, 4, and 2 weeks before calving and 1, 3, 7, and 13 weeks after calving. All the blood samples were withdrawn from the coccygeal vein using serum vacuum tubes prior to the main morning meal. After serum was obtained, the respective samples were stored at −70 °C until assay.
To characterize the reproductive potential of animals, different reproductive traits were used: the period of recovery of the ovarian luteal activity, the duration of the open days period, and the first service conception rate. The luteal activity of the ovaries was assessed on the basis of an ultrasound study using a CTS-800 scanner (SIUI, Shantou, China) and the progesterone content in the blood of cows 3, 7, and 13 weeks after calving. The results of the ovarian ultrasonography (the presence of corpus lutea and/or large follicles, the size of ovaries [28]) were usually consistent with the data of the hormonal analysis. Meanwhile, the concentration of progesterone in the blood of animals of at least 1 ng/mL served as the main confirmation of ovarian activation [29].
By the end of August, a group of 30 animals was randomly formed, in which the ovarian cycle resumed by the 7th or 13th week after calving. The animals were simultaneously synchronized according to the Ovsynch protocol and artificially inseminated (AI) using the semen of one bull. Blood samples from the cows were collected on days 7, 14, 21, and 33 after AI to measure progesterone levels. Pregnancy was confirmed by ultrasonography on Day 33 and progesterone concentrations throughout the entire examined period.
The duration of the open days period for all cows studied was determined based on the records of artificial insemination and rectal and ultrasound examination data, confirmed by the birth of calves. The number of animals by the end of the experiment decreased to 61 due to withdrawal for different reasons (injuries, diseases of various etiologies).
2.3. Hormone Determination
The concentration of hormones in the blood serum was determined by enzyme immunoassay (ELISA) applying commercial kits preliminary validated for analyzing bovine serum. The assays were performed according to the manufacturer’s instructions, and the samples were analyzed in duplicate. All serum samples for each hormone were measured in a single assay. The following kits were used: Hema, Russia (progesterone), DRG Instruments GmbH, Germany (total T4 and total T3), and Diagnostics Biochem Canada, Inc., Canada (rT3). The hormone concentrations were measured on a microplate spectrophotometer Uniplan AIFR-01 (Pikon, Moscow, Russia). The lowest level of detection was 0.25 nmol/L for progesterone, 6.4 nmol/L for T4, 0.31 nmol/L for T3, and 0.03 nmol/L for rT3. The intra-assay coefficient of variation in all assays did not exceed 14%.
2.4. Genomic DNA Extraction
Blood samples assigned for DNA analysis were collected from the studied animals at the beginning of the experiment, preserved with EDTA-K3, and stored at −20 °C. Genomic DNA was extracted from a thawed blood aliquot (50 μL) using a commercial DNA-Extran-1 kit (Syntol, Moscow, Russia) according to the manufacturer’s protocol. The quantity and quality of DNA were assessed using a Qubit 2.0 fluorometer (Invitrogen/Life Technologies, Waltham, MA, USA) and a NanoDrop8000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The concentration of isolated DNA varied between 15 and 125 µg/µL.
2.5. Primer Design
Based on the analysis of international databases by comparing different sequences of the DIO1 gene, 4 point mutations were identified at positions 13,149, 16,767, 16,790, 16,970 (No. NC_037330, NCBI):
- 13,141 gagtgaaac/gg gcggctctag caatgtgtga gatccatcct aaggctctcc
- 16,741 gctgcttaac agcttacaac acacaaa/caca gcccccacaa caagaattac/t
- 16,921 catcaagaat caccggaatc tccaggaccg cctgcgggca gcccacctgc/a.
The list of primers and probes selected for amplification, as well as their annealing temperature, are shown in Table 1. All oligonucleotides were synthesized by Evrogen (Moscow, Russia).

Table 1.
Primers and probes for the analysis of regions of the DIO1 gene.
2.6. Real-Time PCR Analysis
For amplification, a PCR mixture was prepared with the following composition (20 µL of the mixture per 1 sample): 20 mM (NH4)2SO4; 75 mM Tris-HCl; pH = 8.8; 0.1% (v/v) Tween 20; 2.5 mM MgCl2; 0.25 mM dNTP; 10 pM of each primer. The mixture was supplemented with 0.2 units of SmarTaq DNA polymerase (Dialat, Moscow, Russia) per sample and 1 µL of genomic DNA. To detect polymorphism within the amplified sequences, 5 pM of fluorescently labeled probes were added to the PCR mixture. PCR was performed according to the following protocol: primary denaturation (8 min), denaturation (30 s), primer annealing (45 s), and elongation (5 s) × 40 cycles. The SNPs were genotyped with a QuantStudio 5 Real-Time PCR System and QuantStudio Design and Analysis Software v1.5.1 (Applied Biosystems, Foster City, CA, USA).
2.7. Statistical Analysis
Statistical processing of the results was performed using the SigmaStat 4.0 software package (Systat Software, Inc., San Jose, CA, USA). Data on the content of thyroid hormones in the blood were expressed as means ± SEM and subjected to one-way and two-way analysis of variance with repeated measurements. Time relative to calving served as an intragroup factor, and the genotype of animals or reproductive traits within the genotypes served as intergroup factors. The significance of differences between the compared mean values was assessed using Tukey’s test. Fisher’s test was used to analyze differences in the frequency of occurrence of cows with different reproductive traits between groups with polymorphic variants of the DIO1 gene. A probability of p < 0.05 was considered to be statistically significant.
Effects of polymorphic variants of the DIO1 gene, thyroid hormones, and their interaction on the reproductive traits were assessed with the help of a general linear model (GLM) with a binomial distribution. The reproductive traits studied were the open days period and the first service conception rate. In the case of the open days period, two groups were compared: cows with a period duration of fewer than 120 days and cows with a period duration of more than 120 days. In the case of the first service conception rate, the two compared groups consisted of animals that became pregnant or did not become pregnant, respectively. The statistical software R package lme4 version 1.1-21 was used for these calculations.
3. Results
3.1. Polymorphic Variants of the DIO1 Gene
Genotyping of DNA samples using the developed test systems showed the absence of polymorphism at positions 16,767/16,790 and 16,970 in the studied group of animals. All individuals were carriers of the AA/CC/CC genotypes at positions 16,767/16,790 and 16,970, respectively. At position 13,149, a polymorphism was found with the frequency of the C allele, 0.625, and the G allele, 0.375. The frequency of CC, CG, and GG genotypes was 44.12%, 36.76%, and 19.12%, respectively. Amplification plots for animal genotyping by real-time PCR for polymorphic position 13,149 are shown in Figure 1.
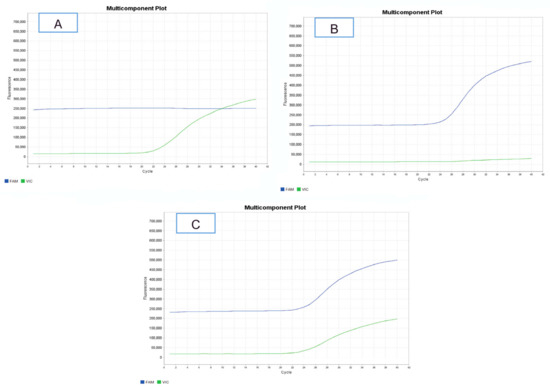
Figure 1.
Plots of amplification during genotyping of animals by real-time PCR at position 13,149 of the DIO1 gene. (A), CC genotype; (B), GG genotype; (C), CG genotype.
3.2. Thyroid Profiles in Cows with Polymorphic Variants of the DIO1 Gene
When analyzing the dependence of blood thyroid profiles on the genotype of cows with SNP in the DIO1 gene, an integrated approach was used, taking into account not only the concentration of total T4 and total T3 but also the concentration of rT3, an inactive T4 metabolite, as well as the ratio of the corresponding hormones.
In cows with the CC and GG genotypes, a decrease in the blood concentration of T4 by 1.4 times (p < 0.01–0.05) 2 weeks before calving, compared with that for 4 weeks, and its subsequent decline by 1.7–1.8 times to the first week of lactation were found (Figure 2). At the same time, in animals with the CG genotype, this concentration was already reduced by the fourth week of the prepartum period (by 1.2 times, p < 0.05) relative to that for 6 weeks and then reached its minimum by the first week of lactation. Meanwhile, 6 weeks before parturition, the content of T4 in the blood of individuals with the homozygous genotype for the G allele was 1.4 times lower (p < 0.05) than in individuals with the heterozygous genotype.
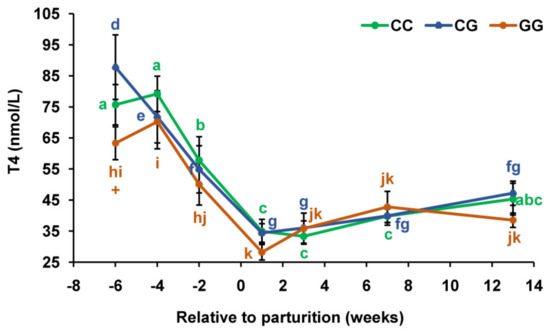
Figure 2.
Serum total thyroxine (T4) concentrations during the prepartum and postpartum periods in dairy cows with polymorphic variants of the DIO1 gene. Means with different letters differ significantly within each group (at least p < 0.05). + p < 0.05 (between CG and GG genotypes).
The content of T3 in the blood of cows with the CC and CG genotypes decreased (p < 0.001) between the second week before calving and the first week of lactation (Figure 3). However, in carriers of the CC genotype, this decrease was more acute (by 7.4 times) than in those of the CG genotype (by 4.3 times). In animals with the GG genotype, a significant decline in the concentration of T3 (by 1.6 times) was noted earlier, between the fourth and second weeks of the prepartum period, but the maximum decrease (by 6.8 times) also occurred by the first week of lactation.
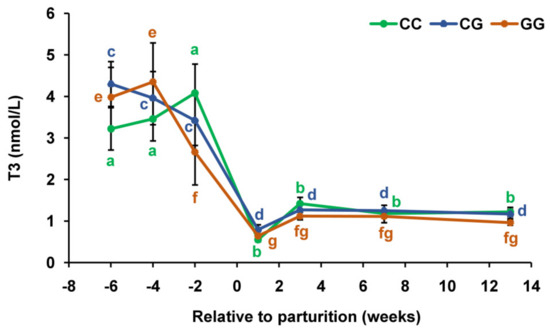
Figure 3.
Serum total triiodothyronine (T3) concentrations during the prepartum and postpartum periods in dairy cows with polymorphic variants of the DIO1 gene. Means with different letters differ significantly within each group (at least p < 0.05).
Throughout the study period, there were no statistically significant differences in the T4/T3 ratio between animals with the compared genotypes (Figure 4).
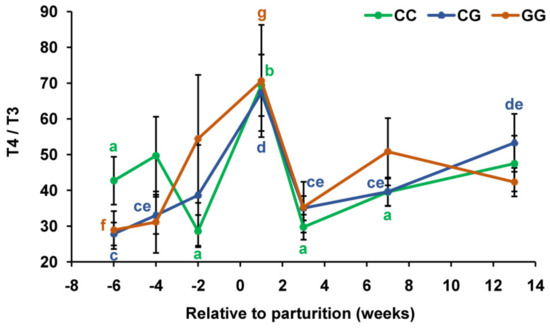
Figure 4.
Total thyroxine to total triiodothyronine ratio (T4/T3) during the prepartum and postpartum periods in the serum of dairy cows with polymorphic variants of the DIO1 gene. Means with different letters differ significantly within each group (at least p < 0.05).
The greatest differences between cows with different DIO1 gene genotypes were found in the temporal profile for rT3 (Figure 5). In animals with the CC and CG genotypes, the concentration of rT3 in the blood decreased during the transition period by 1.4 times (p < 0.01–0.05). However, this decrease occurred at different times: in individuals with the homozygous genotype, between the fourth week before calving and the third week of lactation, and in individuals with the heterozygous genotype, much earlier, between the sixth and second weeks of the prepartum period. At the same time, in cows with the GG genotype, the content of rT3 in the blood was relatively constant throughout the entire observation period. Concurrently, 6 weeks before parturition, the concentration of rT3 in animals with the homozygous for allele C genotype was 1.3 times lower (p < 0.05) than in animals with the heterozygous genotype.
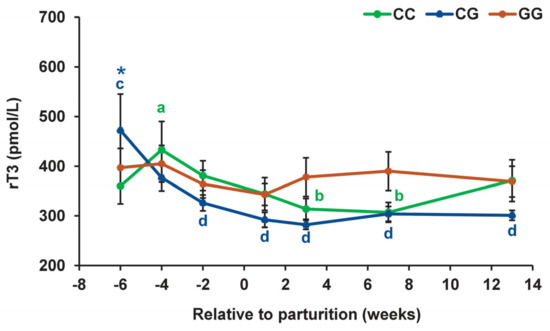
Figure 5.
Serum reverse triiodothyronine (rT3) concentrations during the prepartum and postpartum periods in dairy cows with polymorphic variants of the DIO1 gene. Means with different letters differ significantly within each group (at least p < 0.05). * p < 0.05 (between CC and CG genotypes).
The dynamics of changes in the T3/rT3 ratio were similar in the compared groups during the transition from late pregnancy to lactation (Figure 6). However, in cows with the CG genotype, this ratio was 1.6 times higher (p < 0.05) than in cows with the CC genotype (1 week after calving) and 1.4 times higher (p < 0.05) than in individuals with the GG genotype (13 weeks after calving).
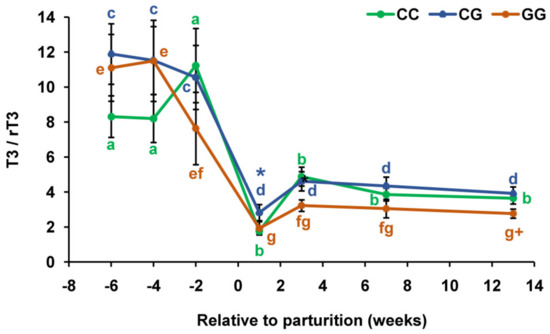
Figure 6.
Total triiodothyronine to reverse triiodothyronine ratio (T3/rT3) during the prepartum and postpartum periods in the serum of dairy cows with polymorphic variants of the DIO1 gene. Means with different letters differ significantly within each group (at least p < 0.05). * p < 0.05 (between CC and CG genotypes). + p < 0.05 (between CG and GG genotypes).
Overall, the data obtained show that the changes in levels of thyroid hormones with various activities during the prepartum and postpartum periods depend on the genotype of cows with SNP in the DIO1 gene at position 13,149.
3.3. Reproductive Traits, Their Association with Genotypes and Thyroid Profiles, and BCS in Cows with Polymorphic Variants of the DIO1 Gene
An analysis of the frequency of occurrence of cows with various reproductive traits in groups with the polymorphic variants of the DIO1 gene revealed a relationship between some indicators of reproductive ability and the genotype of animals (Table 2). In the compared groups, individuals with different durations of the recovery period of the ovarian luteal activity met with a similar frequency. At the same time, in the group with the CC genotype, the proportion of animals with a short open days period (<120 days) was higher than in the group with the CG genotype (48.3% versus 19.1%, p < 0.05), but did not differ from that in the group with the GG genotype (45.5%). In turn, the duration of the open days period of 120–240 days was more common in cows with the CG genotype (61.9%) than with the GG genotype (9.1%, p < 0.01) and also tended to occur more often in carriers of the CG genotype than the CC genotype (34.5%, p < 0.1). Moreover, the first service conception rate was the biggest in the CC animals, being two times higher than in the CG animals (p < 0.05).

Table 2.
The frequency of occurrence of cows with different reproductive traits in groups with polymorphic variants of the DIO1 gene.
Effects of polymorphic variants of the DIO1 gene, thyroid hormones, and their interaction on the reproductive traits were examined using GLM with a binomial distribution (Supplementary Table S1). Meanwhile, the recovery period of the ovarian luteal activity was not tested since this trait did not differ between the compared genotypes. In this analysis, some significant effects were identified: for T4 (2 weeks before calving) and T3 (7 weeks after calving) in the case of the open days period and for the CG genotype and T3 (13 weeks after calving) in the case of the first service conception rate. One can also note a trend toward dependence of the open days period on the CG genotype and the first service conception rate on the GG genotype. In addition, the results of the analysis suggest that 2 weeks before calving is an important time when the effect of thyroid hormones on the open days period is most pronounced.
Thyroid profiles were further divided according to the reproductive traits into subgroups within each genotype. In the case of the first service conception rate, this analysis was not performed due to the too-small size of the subgroups. The most numerous subgroup differences in thyroid levels and ratios were found for the recovery period of the ovarian luteal activity (Supplementary Figures S1–S5), which was not associated with the DIO1 gene polymorphisms. Concurrently, the serum content of rT3 was the only hormonal parameter that did not differ between the subgroups within the genotypes. For the open days period, which was associated with genotypes, subgroup distinctions were rare and solely observed for the CC genotype (T4) and CG genotype (T3, rT3, and T3/rT3) 2 or 4 weeks before calving (Supplementary Figures S6–S10). Moreover, these distinctions indicated that the activity of the thyroid system during the prepartum period in cows with the short open days period was lower than in the other two groups. It should also be noticed that the presence and pattern of differences in thyroid profiles between the subgroups depended on the polymorphic variants of the DIO1 gene in the case of both reproductive traits.
Two weeks before calving, BCS was similar in cows of the compared groups: 3.64 ± 0.06 (the CC genotype), 3.66 ± 0.09 (the CG genotype), and 3.74 ± 0.07 (the GG genotype). Three weeks after calving, BCS was also identified in the three groups and amounted to 3.28 ± 0.07, 3.24 ± 0.07, and 3.13 ± 0.05, respectively.
4. Discussion
The study of the polymorphism of iodothyronine deiodinase genes attracts the close attention of researchers due to their functional role in the regulation of thyroid hormone metabolism, in particular, T3, which is involved in milk synthesis and energy metabolism [7,30]. One of the first studies aimed at investigating the polymorphism of the DIO1 gene in cattle was carried out by Connor et al. [23]. In this work, four mutations were identified: one of them was found at position 357 b.p. (transversion C→G, corresponds to the mutation we studied at position 13,149) in direct sequence (BV005735, NCBI), the second at position 260 b.p. (transversion C→A) in direct sequence and two more at positions 130 b.p. (replacement C→T) and 200 b.p. (replacement A→G, corresponds to the mutation we studied at position 16,790) in reverse order (BV005736, NCBI). At the same time, the DIO1 gene of cattle is located in the region of chromosome 3, where loci of quantitative traits associated with clinical mastitis in dairy cattle and protein content in milk were previously identified [23].
Interesting data were obtained in human research that showed a relationship between a mutation in the DIO1 gene, rs2235544, and a change in the balance between the content of free T3 and free T4 in the blood [31,32]. Moreover, the C allele of this SNP was associated not only with a rise in the ratio of free T3 to free T4 but also with an increase in the concentration of free T3 and a decrease in the concentration of free T4 and rT3 in the blood [31]. Another study found a highly significant association of the rs11206244 mutation in the DIO1 gene with the ratio between total T3 and reverse T3 [33]. In addition, polymorphisms in the DIO1 gene have been shown to be associated with an increase in insulin resistance [21] as well as with impaired thyroid homeostasis in patients with hypothyroidism [34]. Therefore, various mutations in the DIO1 gene can determine thyroid status and pathological functional changes in the human body.
In the available literature, there is no information on the association of polymorphism in the DIO1 gene with fertility in mammals, whereas an association has been found between the SNP in the DIO3 gene and the reproductive performance of sows [25,26]. It has been previously shown that in Russian black-and-white cows, the ovarian functional state and reproductive ability depend on changes in the blood concentrations of T4 and T3 and their ratio in the dynamics of the postpartum period [10,15]. Therefore, we hypothesized that these animals have a link between polymorphism in the DIO1 gene, responsible for the conversion of T4 to T3 and rT3, and the activity of the thyroid system during the transition from pregnancy to lactation, as well as the subsequent state of the reproductive function.
Our study showed the dependence of changes in thyroid profiles during the prepartum and postpartum periods on polymorphic variants of the DIO1 gene in cows. In the course of the transition from pregnancy to lactation, the CC genotype was characterized by a later decrease in the serum concentration of T4 and T3 than the CG or GG genotypes, respectively. The content of rT3 in the blood decreased before calving in animals with the CG genotype and after calving in animals with the CC genotype but remained unchanged in animals with the GG genotype. Six weeks before parturition, cows with the heterozygous genotype were found to have higher levels of T4 and rT3 in the blood than cows with the homozygous for alleles G and C genotypes, respectively. Furthermore, in animals with the CG genotype, the T3/rT3 ratio was higher than in animals with the CC and GG genotypes after 1 and 13 weeks of lactation, respectively.
Concurrently with the differences in thyroid profiles, there were differences in reproductive performance between cows with various genotypes. Among animals homozygous for the C allele, individuals with the short open days period or those who became pregnant at the first service were more common than among animals with the heterozygous genotype. The influence of the CG genotype on the reproductive ability of cows, especially their ability to conceive, is also evidenced by the data obtained using GLM.
The most pronounced hormonal differences between these groups were associated with the level of rT3. In animals with the CG genotype, the blood concentration of rT3 6 weeks prepartum was higher and decreased much earlier than in animals with the CC genotype. Furthermore, 1 week after calving, the T3/rT3 ratio in cows with the CG genotype was higher than in cows with the CC genotype. According to the current notions, the support of metabolic adaptation to the transition period is finely tuned by the activity of the thyroid system [35]. Therefore, even small but significant changes in the levels of thyroid hormones may be enough to affect the metabolic system. In this regard, it can be assumed that increased conversion of T4 to rT3, the negative regulator of the thyroid system, 6 weeks before calving, a premature decline of that conversion, and insufficient inactivation of the thyroid system (a higher T3/rT3 ratio) at the beginning of lactation may lead to negative consequences for the adaptive ability of CG cows and thereby impair their fertility.
Our study also demonstrated that if the reproductive parameter does not depend on the DIO1 gene polymorphism, then differences in thyroid profiles between the respective reproductive subgroups within each genotype can occur quite often, pointing to other pathways regulating the hormonal levels. In its turn, the division of thyroid profiles into subgroups according to the duration of the open days period indicated that, in the case of the CG genotype, cows with the high reproductive ability had elevated rT3 levels and reduced T3 levels and T3/rT3 ratios 2–4 weeks before calving. Thus, a decrease in the activity of the thyroid system during the late prepartum period in CG animals can be considered a compensatory mechanism leading to an improvement in their fertility. These alterations in thyroid profiles were somewhat similar to those observed in cows with the CC genotype, associated with an increase in the proportion of animals with the short open days period. At the same time, the absence of subgroup distinctions within the GG genotype could be due to the small size of the respective subgroups (n = 1–5). Overall, these data suggest that a decrease in thyroid activity during the peripartum period may be necessary to maintain the fertility of dairy cows.
It seems likely that alterations in prepartum and postpartum levels of rT3 associated with the CG genotype result in modification of the homeorhetic alterations aimed at maintaining lactation in dairy cows. However, an effect of SNP at position 13,149 in the DIO1 gene on the energy balance is improbable since we have not found any differences in the postpartum BCS between groups with various genotypes. At the same time, changes in lipid metabolism are very likely, leading to an excessive increase in levels of NEFA and ketone bodies just before calving and right after it, which is unfavorable for the transition from pregnancy to lactation [6,8]. An increased risk of enhancing insulin resistance also cannot be ruled out [21], which is one of the reasons for the deterioration of reproductive function in the postpartum period in dairy cows [36]. Therefore, further studies are required that will link the DIO1 gene polymorphism with the state of the metabolic system during the transition period.
5. Conclusions
In general, the results of this study show for the first time that the higher reproductive capacity of Russian black-and-white cows with the CC genotype of the DIO1 gene (at position 13,149, NC_037330, NCBI) may be associated with the rT3 profile features during the prepartum and early postpartum periods. Thus, the data obtained suggest that the ability of the animals to adapt to the metabolic state during the critical period of transition from late pregnancy to early lactation depends, at least in part, on polymorphic variants of the DIO1 gene. The results obtained indicate the prospects for further research into the relationship between deiodinase gene polymorphisms and fertility traits in dairy cows in order to identify molecular markers suitable for use in the breeding process.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture13020398/s1, Table S1: General linear model with binomial distribution—effects of polymorphic variants of the DIO1 gene, thyroid hormones, and their interaction on the reproductive traits in dairy cows; Figure S1: Serum total thyroxine (T4) concentrations during the prepartum and postpartum periods in dairy cows with polymorphic variants of the DIO1 gene in relation to the period of recovery of the ovarian luteal activity; Figure S2: Serum total triiodothyronine (T3) concentrations during the prepartum and postpartum periods in dairy cows with polymorphic variants of the DIO1 gene in relation to the period of recovery of the ovarian luteal activity; Figure S3: Total thyroxine to total triiodothyronine ratio (T4/T3) during the prepartum and postpartum periods in dairy cows with different polymorphic variants of the DIO1 gene in relation to the period of recovery of the ovarian luteal activity; Figure S4: Serum reverse triiodothyronine (rT3) concentrations during the prepartum and postpartum periods in dairy cows with different polymorphic variants of the DIO1 gene in relation to the period of recovery of the ovarian luteal activity; Figure S5: Total triiodothyronine to reverse triiodothyronine ratio (T3/rT3) during the prepartum and postpartum periods in dairy cows with different polymorphic variants of the DIO1 gene in relation to the period of recovery of the ovarian luteal activity; Figure S6: Serum total thyroxine (T4) concentrations during the prepartum and postpartum periods in dairy cows with different polymorphic variants of the DIO1 gene in relation to the open days period; Figure S7: Serum total triiodothyronine (T3) concentrations during the prepartum and postpartum periods in dairy cows with different polymorphic variants of the DIO1 gene in relation to the open days period; Figure S8: Total thyroxine to total triiodothyronine ratio (T4/T3) during the prepartum and postpartum periods in dairy cows with different polymorphic variants of the DIO1 gene in relation to the open days period; Figure S9: Serum reverse triiodothyronine (rT3) concentrations during the prepartum and postpartum periods in dairy cows with different polymorphic variants of the DIO1 gene in relation to the open days period; Figure S10: Total triiodothyronine to reverse triiodothyronine ratio (T3/rT3) during the prepartum and postpartum periods in dairy cows with different polymorphic variants of the DIO1 gene in relation to the open days period.
Author Contributions
Conceptualization, I.Y.L.; methodology, O.V.K. and I.Y.L.; validation, O.V.K., O.S.M. and I.Y.L.; formal analysis, O.S.M. and O.V.A.; investigation, O.V.K., O.S.M., N.V.B. and O.V.A.; resources, O.V.K., O.S.M. and I.Y.L.; data curation, O.V.K., N.V.B., O.V.A. and I.Y.L.; writing—original draft preparation, O.V.K. and I.Y.L.; writing—review and editing, O.V.K., O.S.M., N.V.B., O.V.A. and I.Y.L.; visualization, N.V.B.; supervision, O.V.K. and I.Y.L.; project administration, O.S.M.; funding acquisition, O.S.M. and I.Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Higher Education of the Russian Federation, theme no. 0445-2021-0004.
Institutional Review Board Statement
Ethical approval for the study was provided by the bioethical commission of the L.K. Ernst Federal Research Center for Animal Husbandry (protocol no. 2, dated 18 January 2021).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Roche, J.R.; Burke, C.R.; Crookenden, M.A.; Heiser, A.; Loor, J.; Meier, S.; Mitchell, M.; Phyn, C.V.C.; Turner, S.-A. Fertility and the transition dairy cow. Reprod. Fertil. Dev. 2018, 30, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Wathes, D.C.; Clempson, A.M.; Pollott, G.E. Associations between lipid metabolism and fertility in the dairy cow. Reprod. Fertil. Dev. 2012, 25, 48–61. [Google Scholar] [CrossRef] [PubMed]
- González, F.D.; Muiño, R.; Pereira, V.; Campos, R.; Benedito, J.L. Relationship among blood indicators of lipomobilization and hepatic function during early lactation in high-yielding dairy cows. J. Veter-Sci. 2011, 12, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.-K.; Jeong, J.-K.; Choi, I.-S.; Kang, H.-G.; Hur, T.-Y.; Jung, Y.-H.; Kim, I.-H. Relationships among ketosis, serum metabolites, body condition, and reproductive outcomes in dairy cows. Theriogenology 2015, 84, 252–260. [Google Scholar] [CrossRef]
- Wathes, D.C. Mechanisms Linking Metabolic Status and Disease with Reproductive Outcome in the Dairy Cow. Reprod. Domest. Anim. 2012, 47, 304–312. [Google Scholar] [CrossRef]
- Mullur, R.; Liu, Y.-Y.; Brent, G.A. Thyroid Hormone Regulation of Metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef]
- Capuco, A.; Wood, D.; Elsasser, T.; Kahl, S.; Erdman, R.; Van Tassell, C.; Lefcourt, A.; Piperova, L. Effect of Somatotropin on Thyroid Hormones and Cytokines in Lactating Dairy Cows During Ad Libitum and Restricted Feed Intake. J. Dairy Sci. 2001, 84, 2430–2439. [Google Scholar] [CrossRef]
- Mohebbi-Fani, M.; Nazifi, S.; Rowghani, E.; Bahrami, S.; Jamshidi, O. Thyroid hormones and their correlations with serum glucose, beta hydroxybutyrate, nonesterified fatty acids, cholesterol, and lipoproteins of high-yielding dairy cows at different stages of lactation cycle. Comp. Clin. Path. 2009, 18, 211–216. [Google Scholar] [CrossRef]
- Fiore, E.; Piccione, G.; Gianesella, M.; Praticò, V.; Vazzana, I.; Dara, S.; Morgante, M. Serum thyroid hormone evaluation during transition periods in dairy cows. Arch. Anim. Breed. 2015, 58, 403–406. [Google Scholar] [CrossRef]
- Mityashova, O.S.; Solomakhin, A.A.; Bogolyubova, N.V.; Montvila, E.K.; Lebedeva, I.Y. Lipid metabolism and thyroid status of heifers with different functional conditions of the ovaries. Dostizheniya Nauk. I Tekhniki APK 2020, 34, 69–74. (In Russian) [Google Scholar] [CrossRef]
- Vernon, R.G.; Denis, R.G.P.; Sorensen, A.; Williams, G. Leptin and the Adaptations of Lactation in Rodents and Ruminants. Horm. Metab. Res. 2002, 34, 678–685. [Google Scholar] [CrossRef]
- Šamanc, H.; Stojić, V.; Kirovski, D.; Jovanović, M.; Cernescu, H.; Vujanac, I. Thyroid Hormones Concentrations during the Mid-Dry Period: An Early Indicator of Fatty Liver in Holstein-Friesian Dairy Cows. J. Thyroid. Res. 2010, 2010, 897602. [Google Scholar] [CrossRef]
- Piechotta, M.; Holzhausen, L.; Araujo, G.M.; Heppelmann, M.; Sipka, A.; Pfarrer, C.; Schuberth, H.-J.; Bollwein, H. Antepartal insulin-like growth factor concentrations indicating differences in the metabolic adaptive capacity of dairy cows. J. Veter-Sci. 2014, 15, 343–352. [Google Scholar] [CrossRef]
- Kafi, M.; Tamadon, A.; Saeb, M.; Mirzaei, A.; Ansari-Lari, M. Relationships between thyroid hormones and serum energy metabolites with different patterns of postpartum luteal activity in high-producing dairy cows. Animal 2012, 6, 1253–1260. [Google Scholar] [CrossRef]
- Lebedeva, I.Y.; Mityashova, O.S. Dependence of reproductive ability of first-calf heifers on the dynamics of the content of thyroid hormones in the blood in the first trimester of lactation. Dostizheniya Nauk. I Tekhniki APK 2020, 34, 91–96. (In Russian) [Google Scholar] [CrossRef]
- Ashkar, F.A.; Bartlewski, P.M.; Singh, J.; Malhi, P.S.; Yates, K.M.; Singh, T.; King, W.A. Thyroid hormone concentrations in systemic circulation and ovarian follicular fluid of cows. Exp. Biol. Med. 2010, 235, 215–221. [Google Scholar] [CrossRef]
- Costa, N.; Cordeiro, M.; Silva, T.; Sastre, D.; Santana, P.; Sá, A.; Sampaio, R.; Santos, S.; Adona, P.; Miranda, M.; et al. Effect of triiodothyronine on developmental competence of bovine oocytes. Theriogenology 2013, 80, 295–301. [Google Scholar] [CrossRef]
- Spicer, L.; Alonso, J.; Chamberlain, C. Effects of Thyroid Hormones on Bovine Granulosa and Thecal Cell Function In Vitro: Dependence on Insulin and Gonadotropins. J. Dairy Sci. 2001, 84, 1069–1076. [Google Scholar] [CrossRef]
- Ortiga-Carvalho, T.M.; Chiamolera, M.I.; Pazos-Moura, C.C.; Wondisford, F.E. Hypothalamus-Pituitary-Thyroid Axis. Compr. Physiol. 2016, 6, 1387–1428. [Google Scholar] [CrossRef]
- Sabatino, L.; Vassalle, C.; Del Seppia, C.; Iervasi, G. Deiodinases and the Three Types of Thyroid Hormone Deiodination Reactions. Endocrinol. Metab. 2021, 36, 952–964. [Google Scholar] [CrossRef]
- Bos, M.M.; Smit, R.A.J.; Trompet, S.; Van Heemst, D.; Noordam, R. Thyroid Signaling, Insulin Resistance, and 2 Diabetes Mellitus: A Mendelian Randomization Study. J. Clin. Endocrinol. Metab. 2017, 102, 1960–1970. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.C.; Kim, B.S. Pathophysiological relevance of deiodinase polymorphism. Curr. Opin. Endocrinol. Diabetes Obes. 2018, 25, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Connor, E.E.; Sonstegard, T.S.; Kahl, S.; Bennett, G.L.; Snelling, W.M. The bovine type I iodothyronine deiodinase (DIO1) gene maps to chromosome 3. Anim. Genet. 2003, 34, 233–234. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, D.; Wang, G.; Wu, X.; Zhang, M.; Zhang, C.; Cui, Y.; Li, S. Expression and imprinting of DIO3 and DIO3OS genes in Holstein cattle. J. Genet. 2017, 96, 333–339. [Google Scholar] [CrossRef]
- Coster, A.; Madsen, O.; Heuven, H.C.M.; Dibbits, B.; Groenen, M.A.M.; van Arendonk, J.A.M.; Bovenhuis, H. The Imprinted Gene DIO3 Is a Candidate Gene for Litter Size in Pigs. PLoS ONE 2012, 7, e31825. [Google Scholar] [CrossRef]
- Oczkowicz, M.; Dunkowska, A.; Piórkowska, K.; Mucha, A.; Tyra, M.; Ropka-Molik, K. 7. Associations between Polymorphisms in the DIO3 Gene and Reproductive Traits and Carcass Performance in Pigs. Ann. Anim. Sci. 2016, 16, 399–413. [Google Scholar] [CrossRef]
- Wildman, E.E.; Jones, G.M.; Wagner, P.E.; Boman, R.L.; Troutt, H.F., Jr.; Lesch, T.N. A Dairy Cow Body Condition Scoring System and Its Relationship to Selected Production Characteristics. J. Dairy Sci. 1982, 65, 495–501. [Google Scholar] [CrossRef]
- Wiltbank, M.C.; Gümen, A.; Sartori, R. Physiological classification of anovulatory conditions in cattle. Theriogenology 2002, 57, 21–52. [Google Scholar] [CrossRef]
- Shrestha, H.K.; Nakao, T.; Suzuki, T.; Higaki, T.; Akita, M. Effects of abnormal ovarian cycles during pre-service period postpartum on subsequent reproductive performance of high-producing Holstein cows. Theriogenology 2004, 61, 1559–1571. [Google Scholar] [CrossRef]
- Motil, K.J.; Thotathuchery, M.; Montandon, C.M.; Hachey, D.L.; Boutton, T.W.; Klein, P.D.; Garza, C. Insulin, Cortisol and Thyroid Hormones Modulate Maternal Protein Status and Milk Production and Composition in Humans. J. Nutr. 1994, 124, 1248–1257. [Google Scholar] [CrossRef]
- Panicker, V.; Cluett, C.; Shields, B.; Murray, A.; Parnell, K.S.; Perry, J.R.B.; Weedon, M.N.; Singleton, A.; Hernandez, D.; Evans, J.; et al. A Common Variation in Deiodinase 1 Gene DIO1 Is Associated with the Relative Levels of Free Thyroxine and Triiodothyronine. J. Clin. Endocrinol. Metab. 2008, 93, 3075–3081. [Google Scholar] [CrossRef]
- Liang, S.-R.; Cai, J.; Yang, Y.; Zhang, L.; Taylor, P.; Ming, J.; Yu, X.-W.; Hu, R.-F.; Zhou, J.; Da-Yan, C.M.; et al. Relationship between thyroid hormones and metabolic syndrome in a normal thyroid function population in Western China: A cross-sectional study based on both epidemiological and genetic analysis. Chin. Med. J. 2021, 135, 350–352. [Google Scholar] [CrossRef]
- de Jong, F.J.; Peeters, R.P.; Heijer, T.D.; van der Deure, W.M.; Hofman, A.; Uitterlinden, A.G.; Visser, T.J.; Breteler, M.M.B. The Association of Polymorphisms in the Type 1 and 2 Deiodinase Genes with Circulating Thyroid Hormone Parameters and Atrophy of the Medial Temporal Lobe. J. Clin. Endocrinol. Metab. 2006, 92, 636–640. [Google Scholar] [CrossRef]
- Wolff, T.M.; Dietrich, J.W.; Müller, M.A. Optimal Hormone Replacement Therapy in Hypothyroidism—A Model Predictive Control Approach. Front. Endocrinol. 2022, 13, 884018. [Google Scholar] [CrossRef]
- Pascottini, O.B.; Leroy, J.L.M.R.; Opsomer, G. Maladaptation to the transition period and consequences on fertility of dairy cows. Reprod. Domest. Anim. 2022, 57 (Suppl. 4), 21–32. [Google Scholar] [CrossRef]
- De Koster, J.D.; Opsomer, G. Insulin Resistance in Dairy Cows. Veter-Clin. N. Am. Food Anim. Pract. 2013, 29, 299–322. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).