Overview of SNPs Associated with Trans Fat Content in Cow’s Milk
Abstract
1. Introduction
2. SNPs Associated with Trans Fat Content in Cow’s Milk
3. Candidate Genes for Trans Fat Content in Cow’s Milk
3.1. Candidate Genes with Predicted Functions
3.2. Candidate Genes and Genomic Regions Identified by GWAS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, C.; Cobb, L.K.; Vesper, H.W.; Asma, S. Global Surveillance of trans-Fatty Acids. Prev. Chronic Dis. 2019, 16, E147. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Federico, E.; Jones, A.; Wu, J.H.Y. Presence of trans fatty acids containing ingredients in pre-packaged foods in Australia in 2018. Aust. N. Z. J. Public Health 2020, 44, 419–420. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Barbagallo, M. Chapter 3-Not All Fats Are Unhealthy. In The Prevention of Cardiovascular Disease through the Mediterranean Diet; Academic Press: Cambridge, MA, USA, 2018; pp. 35–58. [Google Scholar] [CrossRef]
- Vargas-Bello-Perez, E.; Garnsworthy, P.C. Trans fatty acids and their role in the milk of dairy cows. Cienc. Investig. Agrar. 2013, 40, 449–473. [Google Scholar] [CrossRef]
- Hanuš, O.; Samková, E.; Křížová, L.; Hasoňová, L.; Kala, R. Role of Fatty Acids in Milk Fat and the Influence of Selected Factors on Their Variability—A Review. Molecules 2018, 23, 1636. [Google Scholar] [CrossRef] [PubMed]
- Pipoyan, D.; Stepanyan, S.; Stepanyan, S.; Beglaryan, M.; Costantini, L.; Molinari, R.; Merendino, N. The Effect of Trans Fatty Acids on Human Health: Regulation and Consumption Patterns. Foods 2021, 10, 2452. [Google Scholar] [CrossRef]
- Cruz, V.A.R.; Oliveira, H.R.; Brito, L.F.; Fleming, A.; Larmer, S.; Miglior, F.; Schenkel, F.S. Genome-Wide Association Study for Milk Fatty Acids in Holstein Cattle Accounting for the DGAT1 Gene Effect. Animals 2019, 9, 997. [Google Scholar] [CrossRef]
- Shi, L.; Lv, X.; Liu, L.; Yang, Y.; Ma, Z.; Han, B.; Sun, D. A post-GWAS confirming effects of PRKG1 gene on milk fatty acids in a Chinese Holstein dairy population. BMC Genet. 2019, 20, 53. [Google Scholar] [CrossRef]
- Palombo, V.; Milanesi, M.; Sgorlon, S.; Capomaccio, S.; Mele, M.; Nicolazzi, E.; Ajmone-Marsan, P.; Pilla, F.; Stefanon, B.; D’Andrea, M. Genome-wide association study of milk fatty acid composition in Italian Simmental and Italian Holstein cows using single nucleotide polymorphism arrays. J. Dairy Sci. 2018, 101, 11004–11019. [Google Scholar] [CrossRef]
- Park, Y. Conjugated linoleic acid (CLA): Good or bad trans fat? J. Food Compos. Anal. 2009, 22, 4–12. [Google Scholar] [CrossRef]
- MacDonald, H.B. Conjugated linoleic acid and disease prevention: A review of current knowledge. J. Am. Coll. Nutr. 2000, 19, 111–118. [Google Scholar] [CrossRef]
- Pariza, M.W.; Park, Y.; Cook, M.E. The biologically active isomers of conjugated linoleic acid. Prog. Lipid Res. 2001, 40, 283–298. [Google Scholar] [CrossRef]
- Knutsen, T.M.; Olsen, H.G.; Ketto, I.A.; Sundsaasen, K.K.; Kohler, A.; Tafintseva, V.; Svendsen, M.; Kent, M.P.; Lien, S. Genetic variants associated with two major bovine milk fatty acids offer opportunities to breed for altered milk fat composition. Genet. Sel. Evol. 2022, 54, 35. [Google Scholar] [CrossRef]
- Yang, C.; Zhu, B.; Ye, S.; Fu, Z.; Li, J. Isomer-Specific Effects of cis-9,trans-11- and trans-10,cis-12-CLA on Immune Regulation in Ruminal Epithelial Cells. Animals 2021, 11, 1169. [Google Scholar] [CrossRef]
- Bessonov, V.V.; Zaitseva, L.V. Trans-isomers of fatty acids: Health risks and ways to reduce consumption. Food Issues 2016, 85, 6–17. [Google Scholar]
- Carroll, S.M.; DePeters, E.J.; Taylor, S.J.; Rosenberg, M.; Perez-Monti, H.; Capps, V.A. Milk composition of Holstein, Jersey, and Brown Swiss cows in response to increasing levels of dietary fat. Anim. Feed Sci. Technol. 2006, 131, 451–473. [Google Scholar] [CrossRef]
- Mele, M.; Macciotta, N.P.P.; Cecchinato, A.; Conte, G.; Schiavon, S.; Bittante, G. Multivariate factor analysis of detailed milk fatty acid profile: Effects of dairy system, feeding, herd, parity, and stage of lactation. J. Dairy Sci. 2016, 99, 9820–9833. [Google Scholar] [CrossRef]
- Maurice-Van Eijndhoven, M.H.T.; Hiemstra, S.J.; Calus, M.P.L. Short communication: Milk fat composition of 4 cattle breeds in the Netherlands. J. Dairy Sci. 2011, 94, 1021–1025. [Google Scholar] [CrossRef]
- Ramalho, H.M.; Campos, S.D.; Casal, S.; Alves, R.; Oliveira, M.B. Lipid fraction quality of milk produced by Minhota (Portuguese autochthonous breed) compared to Holstein Friesian cow’s. J. Sci. Food Agric. 2012, 92, 2994–3001. [Google Scholar] [CrossRef]
- Olsen, H.G.; Knutsen, T.M.; Kohler, A.; Svendsen, M.; Gidskehaug, L.; Grove, H.; Nome, T.; Sodeland, M.; Sundsaasen, K.K.; Kent, M.P.; et al. Genome-wide association mapping for milk fat composition and fine mapping of a QTL for de novo synthesis of milk fatty acids on bovine chromosome 13. Genet. Sel. Evol. 2017, 49, 20. [Google Scholar] [CrossRef]
- Ibeagha-Awemu, E.M.; Akwanji, K.A.; Beaudoin, F.; Zhao, X. Associations between variants of FADS genes and omega-3 and omega-6 milk fatty acids of Canadian Holstein cows. BMC Genet. 2014, 17, 25. [Google Scholar] [CrossRef]
- Bovenhuis, H.; Visker, M.H.P.W.; Poulsen, N.A.; Sehested, J.; van Valenberg, H.J.F.; van Arendonk, J.A.M.; Larsen, L.B.; Buitenhuis, A.J. Effects of the diacylglycerol o-acyltransferase 1 (DGAT1) K232A polymorphism on fatty acid, protein, and mineral composition of dairy cattle milk. J. Dairy Sci. 2016, 10, 3168. [Google Scholar] [CrossRef]
- Strillacci, M.G.; Frigo, E.; Canavesi, F.; Ungar, Y.; Schiavini, F.; Zaniboni, L.; Reghenzani, L.; Cozzi, M.C.; Samoré, A.B.; Kashi, Y.; et al. Quantitative trait loci mapping for conjugated linoleic acid, vaccenic acid and ∆(9) -desaturase in Italian Brown Swiss dairy cattle using selective DNA pooling. Anim. Genet. 2014, 45, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Pegolo, S.; Cecchinato, A.; Mele, M.; Conte, G.; Schiavon, S.; Bittante, G. Effects of candidate gene polymorphisms on the detailed fatty acids profile determined by gas chromatography in bovine milk. J. Dairy Sci. 2016, 99, 4558–4573. [Google Scholar] [CrossRef] [PubMed]
- Pegolo, S.; Dadousis, C.; Mach, N.; Ramayo-Caldas, Y.; Mele, M.; Conte, G.; Schiavon, S.; Bittante, G.; Cecchinato, A. SNP co-association and network analyses identify E2F3, KDM5A and BACH2 as key regulators of the bovine milk fatty acid profile. Sci. Rep. 2017, 7, 17317. [Google Scholar] [CrossRef] [PubMed]
- Rychtářová, J.; Sztankóová, Z.; Kyselová, J.; Zink, V.; Štípková, M.; Vacek, M.; Štolc, L. Effect of DGAT1, BTN1A1, OLR1, and STAT1 genes on milk production and reproduction traits in the Czech Fleckvieh breed. Czech J. Anim. Sci. 2014, 59, 45–53. [Google Scholar] [CrossRef]
- Bykova, O.A.; Chechenikhina, O.S.; Stepanov, A.V.; Kostyunina, O.V.; Shevkunov, O.A.; Kosilov, V.I. A Study on Milk Productivity of Black-and-white Cows Considering Genotypes of DNA Markers CSN2, LGB, CRH, STAT1, TFAM1, and TFAM2. Int. Trans. J. Eng. Manag. Appl. Sci. Technol. 2022, 13, 1–11. [Google Scholar] [CrossRef]
- Akhmetov, T.M.; Safina, N.Y.; Alimov, A.M.; Varlamova, M.I. Genetic parameters of milk productivity for three lactations of Holstein cattle with different genotypes of LEP gene. BIO Web Conf. 2020, 27, 00061. [Google Scholar] [CrossRef]
- Metin Kiyici, J.; Akyüz, B.; Kaliber, M.; Arslan, K.; Aksel, E.G.; Çinar, M.U. LEP and SCD polymorphisms are associated with milk somatic cell count, electrical conductivity and pH values in Holstein cows. Anim. Biotechnol. 2020, 31, 498–503. [Google Scholar] [CrossRef]
- Maletić, M.; Paprikić, N.; Lazarević, M.; Hodžić, A.; Davidović, V.; Stanišić, L.; Stanimirović, Z. Insight in Leptin gene polymorphism and impact on milk traits in autochtonous Busha cattle. Acta Vet.-Beogr. 2019, 69, 153–163. [Google Scholar] [CrossRef]
- Samková, E.; Čítek, J.; Brzáková, M.; Hanuš, O.; Večerek, L.; Jozová, E.; Hoštičková, I.; Trávníček, J.; Hasoňová, L.; Rost, M.; et al. Associations among Farm, Breed, Lactation Stage and Parity, Gene Polymorphisms and the Fatty Acid Profile of Milk from Holstein, Simmental and Their Crosses. Animals 2021, 11, 3284. [Google Scholar] [CrossRef]
- Haruna, I.L.; Zhou, H.; Hickford, J.G.H. Variation in bovine leptin gene affects milk fatty acid composition in New Zealand Holstein Friesian × Jersey dairy cows. Arch. Anim. Breed. 2021, 64, 245–256. [Google Scholar] [CrossRef]
- Ladyka, V.; Drevytska, T.; Pavlenko, Y.; Skliarenko, Y.; Lahuta, T.; Drevytskyi, O.; Dosenko, V. The Comparison of CSN2 (rs43703011) Beta-Casein Gene Options Frequencies in Different Breeds of Ukraine Cows and the Prospect of Creating Herds with the A2/A2 Genotype. Res. Sq. 2021, 1–12. [Google Scholar] [CrossRef]
- Vanvanhossou, S.F.U.; Giambra, I.J.; Yin, T.; Brügemann, K.; Dossa, L.H.; König, S. First DNA Sequencing in Beninese Indigenous Cattle Breeds Captures New Milk Protein Variants. Genes 2021, 12, 1702. [Google Scholar] [CrossRef]
- Amalfitano, N.; Macedo Mota, L.F.; Rosa, G.M.; Cecchinato, A.; Bittante, G. Role of CSN2, CSN3, and BLG genes and the polygenic background in the cattle milk protein profile. J. Dairy Sci. 2022, 105, 6001–6020. [Google Scholar] [CrossRef]
- Sørensen, B.M.; Chris Kazala, E.; Murdoch, G.K.; Keating, A.F.; Cruz-Hernandez, C.; Wegner, J.; Kennelly, J.J.; Okine, E.K.; Weselake, R.J. Effect of CLA and other C18 unsaturated fatty acids on DGAT in bovine milk fat biosynthetic systems. Lipids 2008, 43, 903–912. [Google Scholar] [CrossRef]
- Kühn, C.; Thaller, G.; Winter, A.; Bininda-Emonds, O.R.; Kaupe, B.; Erhardt, G.; Bennewitz, J.; Schwerin, M.; Fries, R. Evidence for multiple alleles at the DGAT1 locus better explains a quantitative trait locus with major effect on milk fat content in cattle. Genetics 2004, 167, 1873–1881. [Google Scholar] [CrossRef]
- Pausch, H.; Emmerling, R.; Gredler-Grandl, B.; Fries, R.; Daetwyler, H.D.; Goddard, M.E. Meta-analysis of sequence-based association studies across three cattle breeds reveals 25 QTL for fat and protein percentages in milk at nucleotide resolution. BMC Genom. 2017, 18, 853. [Google Scholar] [CrossRef]
- Elzaki, S.; Korkuć, P.; Arends, D.; Reissmann, M.; Brockmann, G.A. Effects of DGAT1 on milk performance in Sudanese Butana × Holstein crossbred cattle. Trop. Anim. Health Prod. 2022, 54, 142. [Google Scholar] [CrossRef]
- Dudásová, S.; Miluchová, M.; Gábor, M.; Candrák, J.; Dočkalová, K. Effects of the DGAT1 K232A polymorphism on milk production traits in Holstein cattle. Acta Fytotech. Zootech. 2021, 24, 233–237. [Google Scholar] [CrossRef]
- Tumino, S.; Criscione, A.; Moltisanti, V.; Marletta, D.; Bordonaro, S.; Avondo, M.; Valenti, B. Feeding System Resizes the Effects of DGAT1 Polymorphism on Milk Traits and Fatty Acids Composition in Modicana Cows. Animals 2021, 11, 1616. [Google Scholar] [CrossRef]
- Upadhyay, A.; Chakravarty, A.K.; De, S.; Gupta, A.K.; Singh, A.; Sakthivel Selvan, A. Detection of Single-Nucleotide Polymorphism in AGPAT6 Gene, Associated with Milk Fat Content, using Tetra-Primer ARMS PCR-Based Assay, in Karan Fries Breeding Bulls. Iran J. Biotechnol. 2019, 17, e2084. [Google Scholar] [CrossRef] [PubMed]
- Zaalberg, R.M.; Janss, L.; Buitenhuis, A.J. Genome-wide association study on Fourier transform infrared milk spectra for two Danish dairy cattle breeds. BMC Genet. 2020, 21, 9. [Google Scholar] [CrossRef] [PubMed]
- Viale, E.; Tiezz, F.; Maretto, F.; De Marchi, M.; Penasa, M.; Cassandro, M. Association of candidate gene polymorphisms with milk technological traits, yield, composition, and somatic cell score in Italian Holstein-Friesian sires. J. Dairy Sci. 2017, 100, 7271–7281. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, H.; Cheng, L.; Hodge, M.; Zhao, J.; Tung, R.; Edwards, G.; Hickford, J. Effects of FABP4 variation on milk fatty-acid composition for dairy cattle grazed on pasture in late lactation. J. Dairy Res. 2020, 87, 32–36. [Google Scholar] [CrossRef]
- Wang, X.; Maltecca, C.; Tal-Stein, R.; Lipkin, E.; Khatib, H. Association of bovine fibroblast growth factor 2 (FGF2) gene with milk fat and productive life: An example of the ability of the candidate pathway strategy to identify quantitative trait genes. J. Dairy Sci. 2008, 91, 2475–2480. [Google Scholar] [CrossRef]
- Li, M.; Gao, Q.; Wang, M.; Liang, Y.; Sun, Y.; Chen, Z.; Zhang, H.; Karrow, N.A.; Yang, Z.; Mao, Y. Polymorphisms in Fatty Acid Desaturase 2 Gene Are Associated with Milk Production Traits in Chinese Holstein Cows. Animals 2020, 10, 671. [Google Scholar] [CrossRef]
- Brzáková, M.; Hosnedlová, B.; Svitáková, A.; Vernerová, K.; Veselá, Z.; Čítek, J. Effect of the FGF2 SNP11646 on milk production and fertility traits of Holstein cattle. Czech J. Anim. Sci. 2016, 61, 377–382. [Google Scholar] [CrossRef]
- Pant, S.D.; Schenkel, F.S.; Leyva-Baca, I.; Sharma, B.S.; Karrow, N.A. Identification of single nucleotide polymorphisms in bovine CARD15 and their associations with health and production traits in Canadian Holsteins. BMC Genom. 2007, 8, 421. [Google Scholar] [CrossRef]
- Wang, W.; Cheng, L.; Yi, J.; Gan, J.; Tang, H.; Fu, M.Z.; Wang, H.; Lai, S.J. Health and production traits in bovine are associated with single nucleotide polymorphisms in the NOD2 gene. Genet. Mol. Res. 2015, 14, 3570–3578. [Google Scholar] [CrossRef]
- Selvaggi, M.; Albarella, S.; Dario, C.; Peretti, V.; Ciotola, F. Association of STAT5A Gene Variants with Milk Production Traits in Agerolese Cattle. Biochem. Genet. 2017, 55, 158–167. [Google Scholar] [CrossRef]
- Kiyici, J.M.; Akyüz, B.; Kaliber, M.; Arslan, K.; Aksel, E.G.; Cinar, M.U. Association of GH, STAT5A, MYF5 gene polymorphisms with milk somatic cell count, EC and pH levels of Holstein dairy cattle. Anim. Biotechnol. 2022, 33, 401–407. [Google Scholar] [CrossRef]
- Khatib, H.; Monson, R.L.; Schutzkus, V.; Kohl, D.M.; Rosa, G.J.; Rutledge, J.J. Mutations in the STAT5A gene are associated with embryonic survival and milk composition in cattle. J. Dairy Sci. 2008, 91, 784–793. [Google Scholar] [CrossRef]
- Yang, C.; Lim, W.; Bae, H.; Bazer, F.W.; Song, G. C-C motif chemokine ligand 2 induces proliferation and prevents lipopolysaccharide-induced inflammatory responses in bovine mammary epithelial cells. J. Dairy Sci. 2018, 101, 4527–4541. [Google Scholar] [CrossRef]
- Leyva-Baca, I.; Schenkel, F.; Sharma, B.S.; Jansen, G.B.; Karrow, N.A. Identification of single nucleotide polymorphisms in the bovine CCL2, IL8, CCR2 and IL8RA genes and their association with health and production in Canadian Holsteins. Anim. Genet. 2007, 38, 198–202. [Google Scholar] [CrossRef]
- Cobanoglu, O.; Kul, E.; Gurcan, E.K.; Abaci, S.H.; Cankaya, S. Determination of the association of GHR/AluI gene polymorphisms with milk yield traits in Holstein and Jersey cattle raised in Turkey. Arch. Anim. Breed. 2021, 64, 417–424. [Google Scholar] [CrossRef]
- Ma, Y.N.; He, P.J.; Zhu, J.; Lei, Z.M.; Liu, Z.; Wu, J.P. The effect of polymorphism F279Y of GHR gene on milk production trait in Chinese Holstein cattle. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2013, 29, 400–404. (In Chinese) [Google Scholar]
- Sun, D.; Jia, J.; Ma, Y.; Zhang, Y.; Wang, Y.; Yu, Y.; Zhang, Y. Effects of DGAT1 and GHR on milk yield and milk composition in the Chinese dairy population. Anim. Genet. 2009, 40, 997–1000. [Google Scholar] [CrossRef]
- Rahmatalla, S.A.; Müller, U.; Strucken, E.M.; Reissmann, M.; Brockmann, G.A. The F279Y polymorphism of the GHR gene and its relation to milk production and somatic cell score in German Holstein dairy cattle. J. Appl. Genet. 2011, 52, 459–465. [Google Scholar] [CrossRef]
- Lü, A.; Hu, X.; Chen, H.; Jiang, J.; Zhang, C.; Xu, H.; Gao, X. Single nucleotide polymorphisms in bovine PRL gene and their associations with milk production traits in Chinese Holsteins. Mol. Biol. Rep. 2010, 37, 547–551. [Google Scholar] [CrossRef]
- Patel, J.B.; Chauhan, J.B. Polymorphism of the Prolactin Gene and Its Relationship with Milk Production in Gir and Kankrej Cattle. J. Nat. Sci. Biol. Med. 2017, 8, 167–170. [Google Scholar] [CrossRef]
- Alipanah, M.; Rodbari, Z.; Esmailizadeh, A.; Javan, I.Y.; Qarari, F. The relationships between PRL/Rsa Ipolymorphism in prolactin gene and milk production in cattle: A Meta-analysis. Large Anim. Rev. 2023, 29, 9–14. [Google Scholar]
- Motmain, Z.; Özdemir, M.; Ekinci, K.; Saygili, E.; Bilgin, E. A Meta-Analysis of the Associations Between Prolactin (PRL) Gene Polymorphism and Milk Production Traits in Cattle. Kafkas Univ. Vet. Fak. Derg. 2022, 28, 627–631. [Google Scholar] [CrossRef]
- Li, C.; Sun, D.; Zhang, S.; Wang, S.; Wu, X.; Zhang, Q.; Liu, L.; Li, Y.; Qiao, L. Genome Wide Association Study Identifies 20 Novel Promising Genes Associated with Milk Fatty Acid Traits in Chinese Holstein. PLoS ONE 2014, 9, e96186. [Google Scholar] [CrossRef]
- Raven, L.A.; Cocks, B.G.; Hayes, B.J. Multibreed genome wide association can improve precision of mapping causative variants underlying milk production in dairy cattle. BMC Genom. 2014, 15, 62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Mi, S.; Brito, L.F.; Hu, L.; Wang, L.; Ma, L.; Xu, Q.; Guo, G.; Yu, Y.; Wang, Y. Genomic and transcriptomic analyses enable the identification of important genes associated with subcutaneous fat deposition in Holstein cows. J. Genet. Genom. 2023; in press. [Google Scholar] [CrossRef]
- Beak, S.H.; Lee, Y.; Lee, E.B.; Kim, K.H.; Kim, J.G.; Bok, J.D.; Kang, S.K. Study on the fatty acid profile of phospholipid and neutral lipid in Hanwoo beef and their relationship to genetic variation. J. Anim. Sci. Technol. 2019, 61, 69–76. [Google Scholar] [CrossRef]
- Li, M.; Lu, X.; Gao, Q.; Wang, M.; Arbab, A.A.I.; Sun, Y.; Chen, Z.; Zhang, H.; Karrow, N.A.; Yang, Z.; et al. A Functional 3′ UTR Polymorphism of FADS2 Affects Cow Milk Composition through Modifying Mir-744 Binding. Animals 2019, 9, 1090. [Google Scholar] [CrossRef]
- Bovenhuis, H.; Jibrila, I.; Dijkstra, J. Predicting milk phosphorus content based on genotypic and milk infrared data. In Proceedings of the World Congress on Genetics Applied to Livestock Production, Auckland, New Zealand, 7–11 February 2018; p. 534. Available online: http://www.wcgalp.org/proceedings/2018/predicting-milk-phosphorus-content-based-genotypic-and-milk-infrared-data (accessed on 15 April 2023).
- Wang, Q.; Bovenhuis, H. Genome-wide association study for milk infrared wavenumbers. J. Dairy Sci. 2018, 101, 2260–2272. [Google Scholar] [CrossRef]
- Sanchez, M.P.; Rocha, D.; Charles, M.; Boussaha, M.; Hozé, C.; Brochard, M.; Delacroix-Buchet, A.; Grosperrin, P.; Boichard, D. Sequence-based GWAS and post-GWAS analyses reveal a key role of SLC37A1, ANKH, and regulatory regions on bovine milk mineral content. Sci. Rep. 2021, 11, 7537. [Google Scholar] [CrossRef]
- Iung, L.H.S.; Petrini, J.; Ramírez-Díaz, J.; Salvian, M.; Rovadoscki, G.A.; Pilonetto, F.; Dauria, B.D.; Machado, P.F.; Coutinho, L.L.; Wiggans, G.R.; et al. Genome-wide association study for milk production traits in a Brazilian Holstein population. J. Dairy Sci. 2019, 102, 5305–5314. [Google Scholar] [CrossRef]
- An, B.; Xu, L.; Xia, J.; Wang, X.; Miao, J.; Chang, T.; Song, M.; Ni, J.; Xu, L.; Zhang, L.; et al. Multiple association analysis of loci and candidate genes that regulate body size at three growth stages in Simmental beef cattle. BMC Genet. 2020, 21, 32. [Google Scholar] [CrossRef]
- Hou, Y.; Liu, G.E.; Bickhart, D.M.; Cardone, M.F.; Wang, K.; Kim, E.S.; Matukumalli, L.K.; Ventura, M.; Song, J.; VanRaden, P.M.; et al. Genomic characteristics of cattle copy number variations. BMC Genom. 2011, 12, 127. [Google Scholar] [CrossRef]
- Purfield, D.C.; Bradley, D.G.; Evans, R.D.; Kearney, F.J.; Berry, D.P. Genome-wide association study for calving performance using high-density genotypes in dairy and beef cattle. Genet. Sel. Evol. 2015, 47, 47. [Google Scholar] [CrossRef]
- Chen, Z.; Brito, L.F.; Luo, H.; Shi, R.; Chang, Y.; Liu, L.; Guo, G.; Wang, Y. Genetic and Genomic Analyses of Service Sire Effect on Female Reproductive Traits in Holstein Cattle. Front. Genet. 2021, 3, 713575. [Google Scholar] [CrossRef]
- Raven, L.A.; Cocks, B.G.; Kemper, K.E.; Chamberlain, A.J.; Vander Jagt, C.J.; Goddard, M.E.; Hayes, B.J. Targeted imputation of sequence variants and gene expression profiling identifies twelve candidate genes associated with lactation volume, composition and calving interval in dairy cattle. Mamm. Genome 2016, 27, 81–97. [Google Scholar] [CrossRef]
- Kemper, K.E.; Hayes, B.J.; Daetwyler, H.D.; Goddard, M.E. How old are quantitative trait loci and how widely do they segregate? J. Anim. Breed. Genet. 2015, 132, 121–134. [Google Scholar] [CrossRef]
- Lopdell, T.J.; Tiplady, K.; Couldrey, C.; Johnson, T.J.J.; Keehan, M.; Davis, S.R.; Harris, B.L.; Spelman, R.J.; Snell, R.G.; Littlejohn, M.D. Multiple QTL underlie milk phenotypes at the CSF2RB locus. Genet. Sel. Evol. 2019, 51, 3. [Google Scholar] [CrossRef]
- Bierman, C.D.; Kim, E.; Shi, X.W.; Weigel, K.; Jeffrey Berger, P.; Kirkpatrick, B.W. Validation of whole genome linkage-linkage disequilibrium and association results, and identification of markers to predict genetic merit for twinning. Anim. Genet. 2010, 41, 406–416. [Google Scholar] [CrossRef]
- Mahmoudi, P.; Rostamzadeh, J.; Rashidi, A.; Zergani, E.; Razmkabir, M. A meta-analysis on association between CSN3 gene variants and milk yield and composition in cattle. Anim. Genet. 2020, 51, 369–381. [Google Scholar] [CrossRef]
- Alim, M.A.; Dong, T.; Xie, Y.; Wu, X.P.; Zhang, Y.; Zhang, S.; Sun, D.X. Effect of polymorphisms in the CSN3 (κ-casein) gene on milk production traits in Chinese Holstein Cattle. Mol. Biol. Rep. 2014, 41, 7585–7593. [Google Scholar] [CrossRef]
- Wang, Q.; Hulzebosch, A.; Bovenhuis, H. Genetic and environmental variation in bovine milk infrared spectra. J. Dairy Sci. 2016, 99, 6793–6803. [Google Scholar] [CrossRef]
- Pegolo, S.; Mach, N.; Ramayo-Caldas, Y.; Schiavon, S.; Bittante, G.; Cecchinato, A. Integration of GWAS, pathway and network analyses reveals novel mechanistic insights into the synthesis of milk proteins in dairy cows. Sci. Rep. 2018, 8, 566. [Google Scholar] [CrossRef] [PubMed]
- Dadousis, C.; Biffani, S.; Cipolat-Gotet, C.; Nicolazzi, E.L.; Rosa, G.J.M.; Gianola, D.; Rossoni, A.; Santus, E.; Bittante, G.; Cecchinato, A. Genome-wide association study for cheese yield and curd nutrient recovery in dairy cows. J. Dairy Sci. 2017, 100, 1259–1271. [Google Scholar] [CrossRef]
- Dadousis, C.; Biffani, S.; Cipolat-Gotet, C.; Nicolazzi, E.L.; Rossoni, A.; Santus, E.; Bittante, G.; Cecchinato, A. Genome-wide association of coagulation properties, curd firmness modeling, protein percentage, and acidity in milk from Brown Swiss cows. J. Dairy Sci. 2016, 99, 3654–3666. [Google Scholar] [CrossRef] [PubMed]
- Schopen, G.C.B. Genetic Analysis of Protein Composition of Bovine Milk; Wageningen University: Wageningen, The Netherlands, 2010; 189p, ISBN 9789085856450. [Google Scholar]
- Moravčíková, N.; Kasarda, R.; Kadlečík, O. Genetic improvement of cattle through low density SNP panels. In Proceedings of the VIII International Scientific Agriculture Symposium “V AgroSym.”, Jahorina, Bosnia and Herzegovina, 5–8 October 2017; Book of Proceedings. Volume 41, pp. 2212–2219. [Google Scholar]
- Moravčíková, N.; Kasarda, R.; Vostrý, L.; Krupová, Z.; Krupa, E.; Lehocká, K.; Olšanská, B.; Trakovická, A.; Nádaský, R.; Židek, R.; et al. Analysis of selection signatures in the beef cattle genome. Czech J. Anim. Sci. 2019, 64, 491–503. [Google Scholar] [CrossRef]
- Illa, S.K.; Mukherjee, S.; Nath, S.; Mukherjee, A. Genome-Wide Scanning for Signatures of Selection Revealed the Putative Genomic Regions and Candidate Genes Controlling Milk Composition and Coat Color Traits in Sahiwal Cattle. Front. Genet. 2021, 12, 699422. [Google Scholar] [CrossRef]
- Mengistie, D.; Edea, Z.; Tesema, T.; Dejene, G.; Dessie, T.; Jemal, J.; Kim, K.S.; Dadi, H. Genome-Wide Signature of Positive Selection and Linkage Disequilibrium in Ethiopian Indigenous and European Beef Cattle Breeds. Res. Sq. 2022, 1–14. [Google Scholar] [CrossRef]
- Ha, S.; Lee, D.; Lee, S.; Chae, J.; Seo, K. Genome-wide association study on immune-response for improving healthiness in Holstein dairy cattle. Korean J. Vet. Serv. 2019, 42, 217–225. [Google Scholar] [CrossRef]
- Tribout, T.; Croiseau, P.; Lefebvre, R.; Barbat, A.; Boussaha, M.; Fritz, S.; Boichard, D.; Hoze, C.; Sanchez, M.P. Confirmed effects of candidate variants for milk production, udder health, and udder morphology in dairy cattle. Genet. Sel. Evol. 2020, 52, 55. [Google Scholar] [CrossRef]
- Zhou, G.L.; Cao, Y.; Jin, H.G. Identification of SNPs in ME1 gene and association analysis with meat quality traits in Chinese Red cattle. Czech J. Anim. Sci. 2014, 59, 297–301. [Google Scholar] [CrossRef]
- Gill, J.L.; Bishop, S.C.; McCorquodale, C.; Williams, J.L.; Wiener, P. Identification of polymorphisms in the malic enzyme 1, NADP(+)-dependent, cytosolic and nuclear receptor subfamily 0, group B, member 2 genes and their associations with meat and carcass quality traits in commercial Angus cattle. Anim. Genet. 2012, 43, 88–92. [Google Scholar] [CrossRef]
- Tahir, M.S.; Porto-Neto, L.R.; Gondro, C.; Shittu, O.B.; Wockner, K.; Tan, A.W.L.; Smith, H.R.; Gouveia, G.C.; Kour, J.; Fortes, M.R.S. Meta-Analysis of Heifer Traits Identified Reproductive Pathways in Bos indicus Cattle. Genes 2021, 12, 768. [Google Scholar] [CrossRef]
- Guarini, A.R.; Lourenco, D.A.L.; Brito, L.F.; Sargolzaei, M.; Baes, C.F.; Miglior, F.; Misztal, I.; Schenkel, F.S. Genetics and genomics of reproductive disorders in Canadian Holstein cattle. J. Dairy Sci. 2019, 102, 1341–1353. [Google Scholar] [CrossRef]
- Salleh, M.S.; Mazzoni, G.; Hoglund, J.K.; Olijhoek, D.W.; Lund, P.; Lovendahl, P.; Kadarmideen, H.N. RNA-Seq transcriptomics and pathway analyses reveal potential regulatory genes and molecular mechanisms in high- and low-residual feed intake in Nordic dairy cattle. BMC Genom. 2017, 18, 258. [Google Scholar] [CrossRef]
- Laodim, T.; Elzo, M.A.; Koonawootrittriron, S.; Suwanasopee, T.; Jattawa, D. Pathway enrichment and protein interaction network analysis for milk yield, fat yield and age at first calving in a Thai multibreed dairy population. Asian-Australas. J. Anim. Sci. 2019, 32, 508–518. [Google Scholar] [CrossRef]
- Leal-Gutiérrez, J.D.; Elzo, M.A.; Carr, C.; Mateescu, R.G. RNA-seq analysis identifies cytoskeletal structural genes and pathways for meat quality in beef. PLoS ONE 2020, 15, e0240895. [Google Scholar] [CrossRef]
- Saatchi, M.; Schnabel, R.D.; Taylor, J.F.; Garrick, D.J. Large-effect pleiotropic or closely linked QTL segregate within and across ten US cattle breeds. BMC Genom. 2014, 15, 442. [Google Scholar] [CrossRef]
- Bovo, S.; Schiavo, G.; Kazemi, H.; Moscatelli, G.; Ribani, A.; Ballan, M.; Bonacini, M.; Prandi, M.; Dall’Olio, S.; Fontanesi, L. Exploiting within-breed variability in the autochthonous Reggiana breed identified several candidate genes affecting pigmentation-related traits, stature and udder defects in cattle. Anim. Genet. 2021, 52, 579–597. [Google Scholar] [CrossRef]
- Buaban, S.; Lengnudum, K.; Boonkum, W.; Phakdeedindan, P. Genome-wide association study on milk production and somatic cell score for Thai dairy cattle using weighted single-step approach with random regression test-day model. J. Dairy Sci. 2022, 105, 468–494. [Google Scholar] [CrossRef]
- Flori, L.; Fritz, S.; Jaffrézic, F.; Boussaha, M.; Gut, I.; Heath, S.; Foulley, J.L.; Gautier, M. The genome response to artificial selection: A case study in dairy cattle. PLoS ONE 2009, 4, e6595. [Google Scholar] [CrossRef]
- Li, G.; Yang, R.; Lu, X.; Liu, Y.; He, W.; Li, Y.; Yu, H.; Qin, L.; Cao, Y.; Zhao, Z.; et al. RNA-Seq Analysis Identifies Differentially Expressed Genes in the Longissimus dorsi of Wagyu and Chinese Red Steppe Cattle. Int. J. Mol. Sci. 2022, 24, 387. [Google Scholar] [CrossRef]
- Soares, R. Investigation of Genomic Regions Affecting Ketosis in Dairy Cattle. Master’s Thesis, The University of Guelph, Guelph, ON, Canada, 2020; 211p. Available online: https://hdl.handle.net/10214/21280 (accessed on 15 April 2023).
- Bögeholz, A.; Falker-Gieske, C.; Guélat, M.; Gurtner, C.; Hunziker, S.; Oevermann, A.; Thaller, G.; Drögemüller, C.; Tetens, J. GWAS Hits for Bilateral Convergent Strabismus with Exophthalmos in Holstein Cattle Using Imputed Sequence Level Genotypes. Genes 2021, 12, 1039. [Google Scholar] [CrossRef] [PubMed]
- Barber, A.M.; Helms, A.; Thompson, R.; Whitlock, B.K.; Steffen, D.J.; Petersen, J.L. Whole-genome sequencing to investigate a possible genetic basis of perosomus elumbis in a calf resulting from a consanguineous mating. Transl. Anim. Sci. 2021, 5, S1–S5. [Google Scholar] [CrossRef]
- Mastrangelo, S.; Ben Jemaa, S.; Ciani, E.; Sottile, G.; Moscarelli, A.; Boussaha, M.; Montedoro, M.; Pilla, F.; Cassandro, M. Genome-wide detection of signatures of selection in three Valdostana cattle populations. J. Anim. Breed. Genet. 2020, 137, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Do, D.N.; Bissonnette, N.; Lacasse, P.; Miglior, F.; Sargolzaei, M.; Zhao, X.; Ibeagha-Awemu, E.M. Genome-wide association analysis and pathways enrichment for lactation persistency in Canadian Holstein cattle. J. Dairy Sci. 2017, 100, 1955–1970. [Google Scholar] [CrossRef]
- Yu, S.-L.; Lee, S.-M.; Kang, M.-J.; Jeong, H.-J.; Sang, B.-C.; Jeon, J.-T.; Lee, J.-H. Identification of Differentially Expressed Genes between Preadipocytes and Adipocytes Using Affymetrix Bovine Genome Array. J. Anim. Sci. Technol. 2009, 51, 443–452. [Google Scholar] [CrossRef]
- Doyle, J.L.; Berry, D.P.; Veerkamp, R.F.; Carthy, T.R.; Evans, R.D.; Walsh, S.W.; Purfield, D.C. Genomic regions associated with muscularity in beef cattle differ in five contrasting cattle breeds. Genet. Sel. Evol. 2020, 52, 2. [Google Scholar] [CrossRef]
- Zeng, X. Angus Cattle at High Altitude: Pulmonary Arterial Pressure, Estimated Breeding Value and Genome-Wide Association Study. Ph.D. Thesis, Colorado State University, Fort Collins, CO, USA, 2016; 259p. Available online: https://mountainscholar.org/bitstream/handle/10217/178953/ZENG_colostate_0053A_13997.pdf (accessed on 19 April 2023).
- Nyman, S.; Duchemin, S.I.; de Koning, D.J.; Berglund, B. Genome-wide association study of normal and atypical progesterone profiles in Holstein-Friesian dairy cows. J. Dairy Sci. 2019, 102, 3204–3215. [Google Scholar] [CrossRef]
- Lu, X.; Arbab, A.A.I.; Abdalla, I.M.; Liu, D.; Zhang, Z.; Xu, T.; Su, G.; Yang, Z. Genetic Parameter Estimation and Genome-Wide Association Study-Based Loci Identification of Milk-Related Traits in Chinese Holstein. Front. Genet. 2022, 12, 799664. [Google Scholar] [CrossRef]
- Houlahan, K.L. Understanding the Genomic Architecture of Feed Efficiency and Implications of Selection for It in Dairy Cattle. Ph.D. Thesis, The University of Guelph, Guelph, ON, Canada, 2021; 137p. [Google Scholar]
- Aiken, G.E.; Klotz, J.L.; Looper, M.L.; Tabler, S.F.; Schrick, F.N. Disrupted hair follicle activity in cattle grazing endophyte-infected tall fescue in the summer insulates core body temperatures. Prof. Anim. Sci. 2011, 27, 336–343. [Google Scholar] [CrossRef]
- Rowan, T.N.; Durbin, H.J.; Seabury, C.M.; Schnabel, R.D.; Decker, J.E. Powerful detection of polygenic selection and evidence of environmental adaptation in US beef cattle. PLoS Genet. 2021, 17, e1009652. [Google Scholar] [CrossRef]
- Doyle, J.L.; Berry, D.P.; Veerkamp, R.F.; Carthy, T.R.; Walsh, S.W.; Evans, R.D.; Purfield, D.C. Genomic Regions Associated with Skeletal Type Traits in Beef and Dairy Cattle Are Common to Regions Associated with Carcass Traits, Feed Intake and Calving Difficulty. Front. Genet. 2020, 11, 20. [Google Scholar] [CrossRef]
- Fonseca, P.A.S.; Schenkel, F.S.; Cánovas, A. Genome-wide association study using haplotype libraries and repeated-measures model to identify candidate genomic regions for stillbirth in Holstein cattle. J. Dairy Sci. 2022, 105, 1314–1326. [Google Scholar] [CrossRef]
- Ryu, J.; Lee, C. Genetic association of marbling score with intragenic nucleotide variants at selection signals of the bovine genome. Animal 2016, 10, 566–570. [Google Scholar] [CrossRef]
- Ilska-Warner, J.J.; Psifidi, A.; Seeker, L.A.; Wilbourn, R.V.; Underwood, S.L.; Fairlie, J.; Whitelaw, B.; Nussey, D.H.; Coffey, M.P.; Banos, G. The Genetic Architecture of Bovine Telomere Length in Early Life and Association with Animal Fitness. Front. Genet. 2019, 10, 1048. [Google Scholar] [CrossRef]
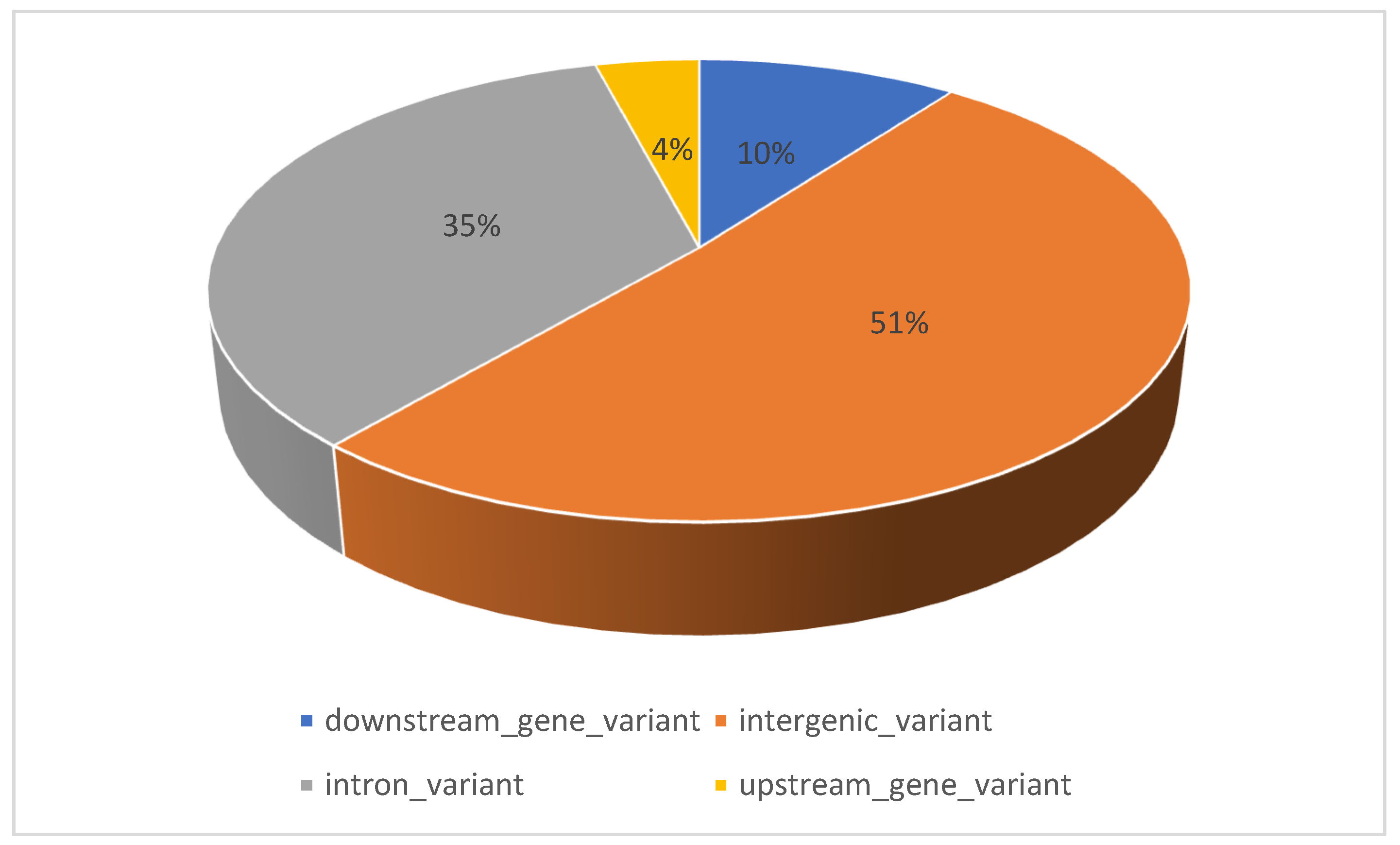
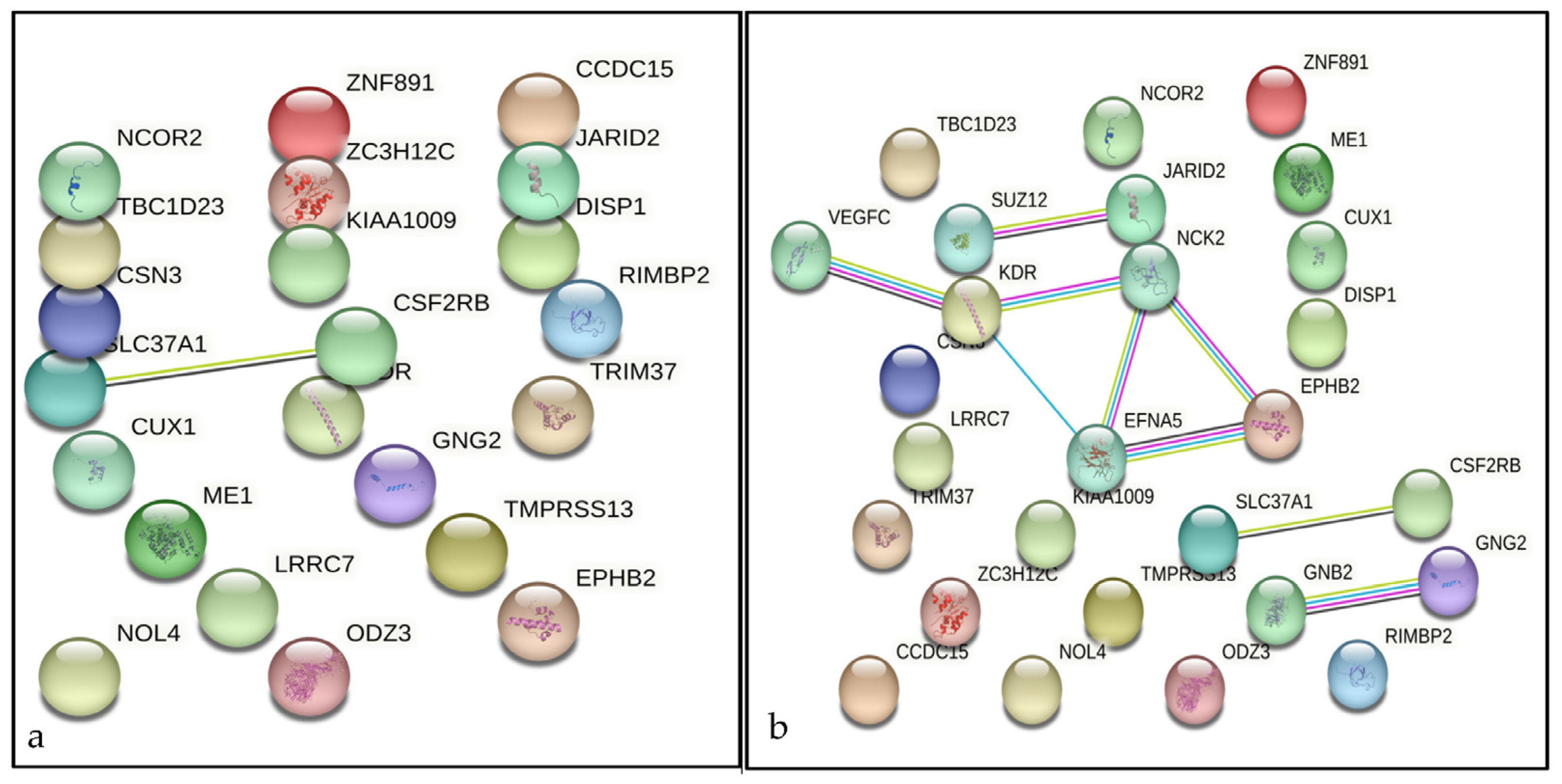
| No. | Traits | SNP | Breed | BTA | Position | Consequence | Candidate Gene | Allele | References |
|---|---|---|---|---|---|---|---|---|---|
| 1 | C18:1t11 | rs41569243 | Norwegian Red cattle | 1 | 45,564,535 | intergenic variant | T/A | [20] | |
| 2 | C18:1t11 | rs29019625 | Norwegian Red cattle | 1 | 142,894,021 | intergenic variant | SLC37A1 | A/G | [20] |
| 3 | C18:2t11,c15 | rs43232419 | Brown Swiss | 1 | 44,571,298 | intron variant | TBC1D23 | T/G | [25] |
| 4 | C18:1t11 | rs110831311 | Brown Swiss | 1 | 147,265,560 | intergenic variant | A/G | [25] | |
| 5 | C18:1t16, C18:1t6–8 | rs43705173 | Brown Swiss | 2 | 79,518,124 | downstream gene variant | STAT1 | G/A | [24] |
| 6 | C18:1t16 | rs43706906 | Brown Swiss | 2 | 79,553,228 | intron variant | STAT1 | G/C | [24] |
| 7 | C18:2c9,t11 | rs110614098 | Brown Swiss | 2 | 129,910,992 | intron variant | EPHB2 | G/A | [25] |
| 8 | C18:1t11 | rs110847444 | Brown Swiss | 4 | 88,230,965 | intergenic variant | T/C | [23] | |
| 9 | C18:2c9,t11, C18:2t11,c15 | rs29004170 | Brown Swiss | 4 | 92,436,328 | upstream gene variant | LEP | G/C | [24] |
| 10 | MTCFAR | rs41590819 | Norwegian Red cattle | 5 | 75,374,050 | downstream gene variant | CSF2RB | C/G | [20] |
| 11 | C18:1t10 | rs41623140 | Norwegian Red cattle | 5 | 92,068,616 | intergenic variant | T/G | [20] | |
| 12 | C18:1t10 | rs43703011 | Brown Swiss | 6 | 85,451,298 | missense variant | CSN2 | T/G | [24] |
| 13 | C18:2c9,t11 | rs43703013 | Brown Swiss | 6 | 85,451,132 | missense variant | CSN2 | C/G | [24] |
| 14 | C18:1t9 | rs29024681 | Norwegian Red cattle | 6 | 85,662,337 | upstream/downstream gene variant | CSN3 | G/A | [20] |
| 15 | C18:1t9 | rs29024683 | Norwegian Red cattle | 6 | 85,662,408 | upstream/downstream gene variant | CSN3 | A/G | [20] |
| 16 | C18:1t9 | rs29024684 | Norwegian Red cattle | 6 | 85,662,466 | upstream/downstream gene variant | CSN3 | C/A | [20] |
| 17 | C18:1t9 | rs29024685 | Norwegian Red cattle | 6 | 85,662,516 | upstream/downstream gene variant | CSN3 | A/G | [20] |
| 18 | C18:1t9 | rs41587868 | Norwegian Red cattle | 6 | 72,916,033 | intergenic variant | G/A | [20] | |
| 19 | C18:1t9 | rs41653769 | Norwegian Red cattle | 6 | 70,588,722 | intron variant | KDR | A/G | [20] |
| 20 | C18:1t16 | rs42960052 | Brown Swiss | 6 | 20,546,001 | intergenic variant | A/G | [25] | |
| 21 | C18:1t6–8 | rs41651324 | Brown Swiss | 6 | 76938995 | intergenic variant | C/T | [25] | |
| 22 | C18:2t11,c15 | rs41567758 | Brown Swiss | 6 | 69,216,812 | intergenic variant | A/C | [23] | |
| 23 | C18:1t16 | rs41573488 | Brown Swiss | 8 | 3,790,608 | intergenic variant | T/C | [25] | |
| 24 | C18:1t10 | rs41593428 | Norwegian Red cattle | 9 | 65,223,783 | intron variant | CEP162 | C/G | [20] |
| 25 | C18:1t10 | rs41611219 | Norwegian Red cattle | 9 | 23,244,267 | intron variant | ME1 | C/A | [20] |
| 26 | TFA | rs108973184 | Brown Swiss | 9 | 102,953,472 | intergenic variant | T/C | [25] | |
| 27 | C18:1t10 | rs41568929 | Norwegian Red cattle | 10 | 44,720,992 | intron variant | GNG2 | T/C | [18] |
| 28 | C18:1t11 | rs41575963 | Brown Swiss | 12 | 26,784,974 | intergenic variant | A/C | [21] | |
| 29 | MTRANSFA | rs41662646 | Norwegian Red cattle | 12 | 77,525,632 | intergenic variant | G/A | [20] | |
| 30 | C18:1t6–8 | rs109738802 | Brown Swiss | 12 | 78,577,324 | intergenic variant | C/T | [25] | |
| 31 | C18:1c9,t11 | rs109326954 | Jersey, Holstein | 14 | 611,020 | missense variant | DGAT1 | C/A | [22] |
| 32 | C18:1t9 | rs110445169 | Brown swiss | 14 | 20,267,955 | intron variant | AGPAT6 | T/C | [24] |
| 33 | C16:1t9, C18:1t4 | rs110757796 | Brown swiss | 14 | 44,677,959 | missense variant | FABP4 | T/C | [24] |
| 34 | C18:1T4 | rs110642420 | Brown Swiss | 14 | 5,880,036 | intergenic variant | C/T | [25] | |
| 35 | C18:1t11 | rs42357017 | Brown Swiss | 15 | 20,213,507 | intron variant | ZC3H12C | C/T | [23] |
| 36 | MTCFAR | rs29012314 | Norwegian Red cattle | 15 | 28,602,093 | upstream gene variant | TMPRSS13 | C/T | [20] |
| 37 | MTCFAR | rs29019684 | Norwegian Red cattle | 15 | 57,539,478 | intergenic variant | G/A | [20] | |
| 38 | C18:1t10 | rs41655008 | Brown Swiss | 15 | 69,449,535 | intergenic variant | C/T | [25] | |
| 39 | C18:1t11 | rs41578757 | Brown Swiss | 16 | 26,461,264 | intron variant | DISP1 | T/A | [23] |
| 40 | C18:1t11 | rs109178989 | Brown Swiss | 17 | 25,016,349 | intergenic variant | A/G | [23] | |
| 41 | C18:1T6–8 | rs110937773 | Brown Swiss | 17 | 34,849,206 | intron variant | FGF2 | T/C | [24] |
| 42 | MTCFAR | rs41633197 | Norwegian Red cattle | 17 | 46,687,151 | intron variant | RIMBP2 | A/G | [20] |
| 43 | MTCFAR | rs41637627 | Norwegian Red cattle | 17 | 44,150,664 | upstream gene variant | ZNF891 | G/T | [20] |
| 44 | MTCFAR | rs41660449 | Norwegian Red cattle | 17 | 51,342,347 | intron variant | NCOR2 | T/G | [20] |
| 45 | C18:1T4 | rs41596865 | Brown Swiss | 17 | 59,367,351 | intergenic variant | C/T | [25] | |
| 46 | C18:1T11 | rs41611446 | Brown Swiss | 17 | 20,279,742 | intergenic variant | G/A | [25] | |
| 47 | C18:1t16 | rs43710288 | Brown Swiss | 18 | 19,117,794 | missense variant | NOD2 | A/T | [24] |
| 48 | C18:1T4 | rs109578101 | Brown Swiss | 19 | 42,415,682 | 3 prime UTR variant | STAT5A | C/T | [24] |
| 49 | C18:3 | rs137182814 | Brown Swiss | 19 | 42,335,251 | splice region variant, synonymous variant | STAT5A | G/C | [24] |
| 50 | TFA, С18:1t11 | rs110773010 | Brown Swiss | 19 | 10,076,852 | intron variant | TRIM37 | G/A | [25] |
| 51 | C18:1t4 | rs41255713 | Brown Swiss | 19 | 15,905,377 | upstream gene variant | CCL2 | G/A | [24] |
| 52 | C18:1t10 | rs109136815 | Brown swiss | 20 | 31,870,046 | synonymous variant | GHR | A/G | [24] |
| 53 | C18:3c9,t11,c15 | rs109794490 | Brown Swiss | 22 | 21,455,728 | intergenic variant | C/T | [25] | |
| 54 | C18:2t9,t12 | rs211032652 | Brown Swiss | 23 | 35,333,764 | synonymous variant | PRL | C/T | [24] |
| 55 | C18:1t10 | rs41589759 | Norwegian Red cattle | 23 | 41,632,536 | intron variant | JARID2 | G/A | [20] |
| 56 | MTCFAR | rs41642031 | Norwegian Red cattle | 23 | 23,196,063 | intergenic variant | G/C | [20] | |
| 57 | C18:1t16 | rs41641235 | Brown Swiss | 23 | 22,032,364 | intergenic variant | T/G | [25] | |
| 58 | C18:1t9 | rs29024014 | Norwegian Red cattle | 24 | 23,015,448 | intron variant | NOL4 | A/G | [20] |
| 59 | C18:1t9 | rs41644943 | Norwegian Red cattle | 24 | 6,108,284 | intergenic variant | G/A | [20] | |
| 60 | MTRANSFA | rs41567529 | Norwegian Red cattle | 25 | 34,827,662 | intron variant | CUX1 | T/C | [20] |
| 61 | C18:2c9,t11 | rs41622946 | Brown Swiss | 25 | 32,138,950 | intergenic variant | T/C | [25] | |
| 62 | C18:3c9,t11,c15 | rs41624917 | Brown Swiss | 26 | 15,524,965 | intron variant | PLCE1 | G/A | [24] |
| 63 | C16:1t9 | rs41650170 | Brown Swiss | 27 | 13,628,042 | intron variant | TENM3 | G/A | [25] |
| 64 | C18:2c9,t11 | rs3423094014 | Brown Swiss | 28 | 4,467,528 | intron variant | DISC1 | G/T | [25] |
| 65 | C18:2t6 | rs42187261 | Holstein | 29 | 40,247,432 | synonymous variant | FADS1 | G/A | [21] |
| 66 | C18:2t10, C18:2t6 | rs210169303 | Holstein | 29 | 40,386,173 | 3 prime UTR variant | FADS2 | G/A | [21] |
| 67 | C14:1t | rs109772589 | Holstein | 29 | 40,387,345 | 3 prime UTR variant | FADS2 | G/A | [21] |
| 68 | C18:1t11 | rs42176310 | Brown Swiss | 29 | 28,440,651 | intron variant | CCDC15 | A/C | [23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bykova, O.; Shevkunov, O.; Kostyunina, O. Overview of SNPs Associated with Trans Fat Content in Cow’s Milk. Agriculture 2023, 13, 1151. https://doi.org/10.3390/agriculture13061151
Bykova O, Shevkunov O, Kostyunina O. Overview of SNPs Associated with Trans Fat Content in Cow’s Milk. Agriculture. 2023; 13(6):1151. https://doi.org/10.3390/agriculture13061151
Chicago/Turabian StyleBykova, Olga, Oleg Shevkunov, and Olga Kostyunina. 2023. "Overview of SNPs Associated with Trans Fat Content in Cow’s Milk" Agriculture 13, no. 6: 1151. https://doi.org/10.3390/agriculture13061151
APA StyleBykova, O., Shevkunov, O., & Kostyunina, O. (2023). Overview of SNPs Associated with Trans Fat Content in Cow’s Milk. Agriculture, 13(6), 1151. https://doi.org/10.3390/agriculture13061151









