Abstract
In the management of eco-tea gardens, the recycling of weathered leaves of the configured tree species plays an important role in the nutrients of the tea garden ecosystem. The in situ decomposition method was used to analyze the decomposition rate, elemental release, and enzymatic activity of different combinations of leaf litter in tea garden soil. The results showed that mixed decomposition accelerates the decomposition turnover period of Jiu’an ecological tea garden. The higher release rate of the litter improved the nutrient effectiveness in the ecological tea garden. The trends of CAT (catalase), AP (acid phosphatase), and PPO (polyphenol oxidase) activities were similar during the decomposition of different mixed leaf litters, while all other enzyme activities showed some differences. There were also some differences in the relationship between the enzyme activity and decomposition rate of leaf litter and the nutrient release rate related to the substrate mass content during the decomposition of leaf litter. Overall, the changes in the nutrient content of the leaf litter during decomposition promoted an increase in enzyme activity, which in turn promoted the release of leaf litter elements, shortened the turnover period of leaf litter decomposition, and accelerated the recycling of elements in the ecological tea garden.
1. Introduction
Litter is the product of plant metabolism and is a general term for organic matter that can provide a source of material and energy for decomposers and some consumers [1]. Litter decomposition is one of the main sources of organic matter and nutrients in forest soils and is of great significance in maintaining soil fertility and promoting normal material cycling and nutrient balance in forest ecosystems [2]. In ecosystems, litter generally exists as a mixture, and this mixed condition not only forms the decomposition environment but also affects the specific microbial community that feeds on litter, ultimately affecting the decomposition rate of litter as well as the nutrient cycling rate. Related studies have shown that the mixed decomposition of different litter materials can promote the decomposition rate and element release [3], which is more effective in improving soil nutrients than the decomposition of litter materials alone [4,5]. In addition to environmental conditions and the nature of litter, microorganisms and the enzymes they produce are closely related to the decomposition of litter, and their types and activities vary depending on the composition of litter and environmental conditions [6,7,8]. Enzyme activity quickly responds to changes in litter decomposition conditions and reflects the decomposition speed to a certain extent, which also plays an important role in the processes of material cycling and energy flow in forest ecosystems. The complete degradation of litter is completed under the comprehensive action of the litter and soil enzyme system, and the increase in enzyme activity is beneficial to litter decomposition and nutrient release. Therefore, study of the changes in element release and enzyme activity during the mixed decomposition of leaf litter is helpful to better understand the change mechanism of the internal material cycle in the processes of species combination and allocation and is of great significance for the maintenance of soil fertility and the sustainable management of woodland [9].
An ecological tea garden refers to a highly efficient artificial agricultural ecosystem established with tea as the main tree species and is guided by the principles of ecology and economics [10]. Guizhou is a rugged province with a crisscrossed distribution of plateau mountains, canyons, and river valleys. The operation and management of ecological tea gardens is an important opportunity for Guizhou to adjust measures to local conditions and take advantage of the situation to promote the green revitalization of superior industries in mountainous areas and to develop the characteristic resources of mountain forestry. Compound ecological tea gardens are one of the main types of green tea gardens in Guizhou [11]. As the management of tea gardens is a long-term process, single planting leads to a decline in soil fertility, a reduction in species diversity, serious diseases and insect pests, ecological environment degradation and other problems. The cultivation of different tree species and tea can enrich the species diversity in the ecosystem, improve the chemical composition of litter, and more effectively improve the soil fertility of woodlands and the structure and function of the ecosystem. However, how to select and match tree species, optimize the leaf litter of different tree species in tea garden management, and realize the sustainable development of the tea industry are the main problems in the management of ecological tea gardens. In this study, the leaf litters of Cinnamomum glanduliferum, Betula luminifera, Cunninghamia lanceolata, and Pinus massoniana in ecological tea gardens were taken as the research object. By simulating the decomposition process of leaf litter buried in the tillage layer, the changes in decomposition rate, element release dynamics, and enzyme activity were analyzed. In order to deeply understand the mixed decomposition characteristics of associated tree species in an ecological tea garden and enrich the understanding of ecological material and nutrient cycling. And the coupling adaptation mechanism and inherent potential of different leaf litters in the soil system were revealed. The purpose of this paper was to explore the soil fertility maintenance and virtuous circle of ecological tea gardens from the aspect of ecological conservation to provide a theoretical basis for the revitalization of the tea industry in mountain areas and regional green development.
2. Materials and Methods
2.1. Study Area
The experimental area is located in Jiu’an Township, Huaxi District, Guiyang City, Guizhou Province, China (N 26°31′8″–N 26°31′12″, E 106°36′47″–E 106°36′50″)(Figure 1). The average annual temperature is 13.6 °C, the average temperature of the coldest month (January) is 3–4 °C, the average temperature of the hottest month (July) is 22 °C, the frost-free period is 260 d, the average annual rainfall is 1000–1150 mm, the elevation range is 1100–1446 m, the area has a subtropical plateau monsoon climate, and the soil type is mainly Orthic Acrisols.
Figure 1.
Experimental study area.
2.2. Experimental Design and Arrangement
Sample collection and treatment: In July 2019, the leaves of Pinus massoniana, Cunninghamia lanceolata, Cinnamomum glanduliferum, and Betula luminifera were collected by shaking branches in the Jiu’an ecological tea garden in Huaxi, Guizhou Province. The litter of the same tree species was evenly mixed and brought back to the laboratory to dry at 85 °C to a constant weight. Using the field decomposition bag method [12], 35 cm × 25 cm and 1 mm pore diameter decomposition bags were selected, and the dried litter leaves were put into the decomposition bags. The leaf litter was uniformly mixed at a 1:1 mass ratio. The mixing methods included Cinnamomum glanduliferum + Betula luminifera (CGB), Cinnamomum glanduliferum+Pinus massoniana (CGP), Cinnamomum glanduliferum + Cunninghamia lanceolata (CGC), Betula luminifera + Pinus massoniana (BLP) and Betula luminifera+Cunninghamia lanceolata (BLC). Each bag was 40 g (20 g of each tree species), and each sample was repeated 3 times. Before burying the samples, three bags were left for initial nutrient analysis, and the nutrients in the leaf litter are shown in Table 1.

Table 1.
Basic properties of nutrients in leaf litter for testing.
Test arrangement: A 10 m × 15 m decomposition field was set up in the flat area of the experimental area, and decomposition bags containing samples were laid and buried in the soil layer between the rows of the tea plantation in the ecological tea garden. Each row was laid with the same kind of treatment. The landfill depth was 20 cm. The decomposition bags were laid parallel and did not overlap each other, making them close to the natural state (Figure 2).
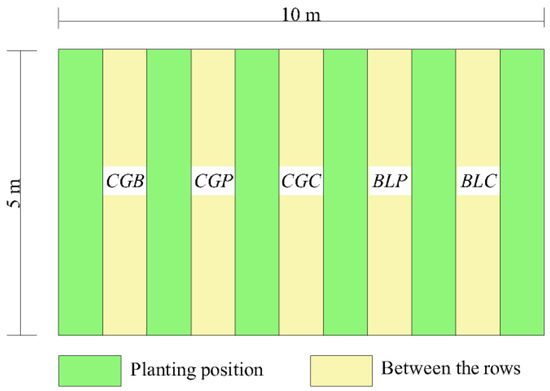
Figure 2.
Schematic diagram of the experimental layout.
2.3. Sample Recovery and Testing
Leaf litter recovery: From July 2019 to September 2020, samples were collected every two months (when decomposed for 8 months, the fourth sample should have been taken in March 2020, and the fourth stage of decomposition was 4 months, i.e., 6–10 months due to the influence of the epidemic). Six bags were randomly taken from each treatment, impurities were removed from the samples, and the samples were brought back to the laboratory. Among them, 3 bags were dried in an oven at 65 °C until the mass was constant, and then the litter leaves were crushed according to the determination requirements to determine their chemical properties (total carbon (TC), total nitrogen (TN), total phosphorus (TP), total potassium (TK), lignin, and cellulose). The other 3 bags of fresh samples were stored at 4 °C for enzyme activity determination.
Testing method: TC was determined using the concentrated sulfuric acid-potassium dichromate method [13]; the semi-micro Kjeldahl method was used to determine TN; the molybdenum-antimony resistance colorimetric method was used to determine TP; and flame photometry was used to determine TK [14,15,16]. The contents of lignin and cellulose and the activities of cellulase (CL), sucrase (SC), polyphenol oxidase (PPO), catalase (CAT), urease (UE), and acid phosphatase (AP) were determined by a biochemical kit from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China).
2.4. Data Analysis
Leaf litter residue rate:
where R represents the leaf litter residue rate, %; Mt represents the dry mass of the remaining sample of leaf litter in the bag during sampling at time t, g; and M0 represents the initial dry mass of the leaf litter without decomposition, g.
Leaf litter decomposition rate [17]:
where L represents the decomposition rate of leaf litter, %; Mt represents the dry mass of the remaining sample of leaf litter in the bag during sampling at time t, g; and M0 represents the initial dry mass of the leaf litter without decomposition, g.
The decomposition coefficient in the Olson exponential model [18] is often used to describe the leaf litter decomposition rate, where y represents the leaf litter residue rate, %; k represents the decomposition coefficient; α is a fit parameter; and t represents the decomposition time of the leaf litter, m.
Decomposition half-life (50% decomposition):
Decomposition turnover period (95% decomposition):
Nutrient release rate of leaf litter [19]:
where E represents the nutrient release rate of leaf litter, %; C0 represents the initial nutrient content of the leaf litter when it is not decomposed, g·kg−1; Ct represents the nutrient content of the remaining sample of leaf litter in the bag during sampling at time t, g·kg−1; M0 represents the initial dry mass of the leaf litter without decomposition, g; and Mt represents the dry mass of the remaining sample of plant residue in the bag during sampling at time t, g. When E is positive, net release occurs, and when E is negative, net enrichment occurs.
2.5. Data Processing
The data statistics and analysis were completed by SPSS 22.0 software, the chart was drawn by Origin 2018, the leaf litter decomposition curve was fitted by nonlinear regression analysis (nonlinear regression), and the significance of leaf litter decomposition rate and nutrient content was tested by single-factor analysis of variance (one-way ANOVA).
3. Results
3.1. Decomposition Characteristics of Different Mixed Leaf Litters
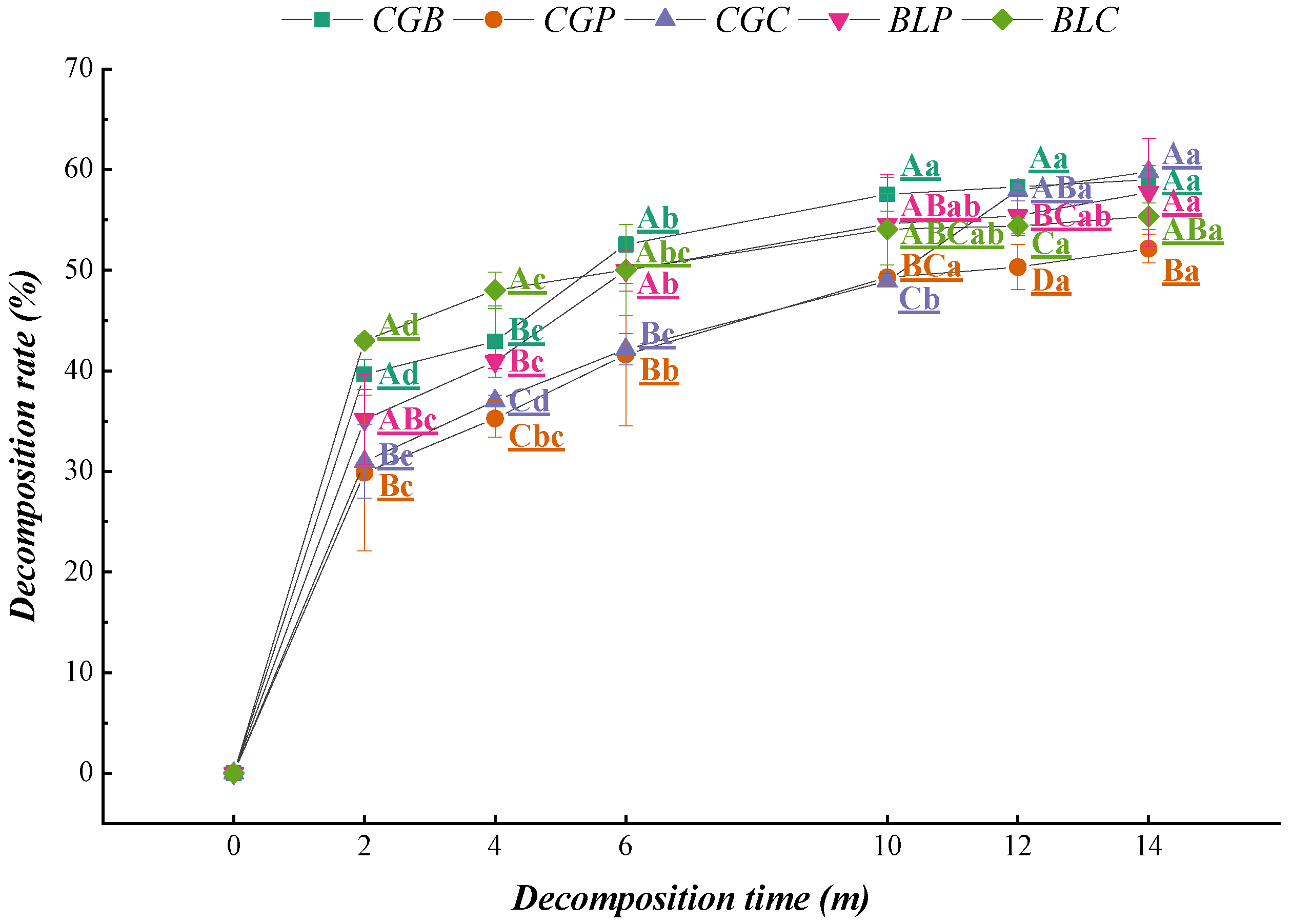
The decomposition rates of the five leaf litter types were faster at the initial stage of decomposition and decreased significantly after 2 months (Figure 3), and the decomposition rates of various litter types were relatively uniform in the later stage of decomposition (10–14 months).
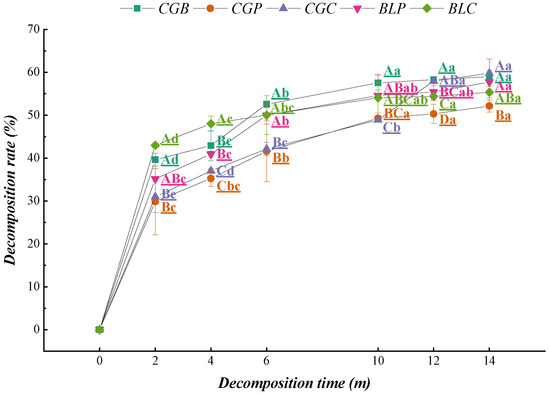
Figure 3.
Characteristics of the decomposition rate of leaf litter with decomposition time.
Regression analysis showed that the correlation coefficients of the five leaf litter types all reached a significant level (Table 2). The decomposition coefficients of the five leaf litter types were CGC > CGB > BLP > CGP > BLC. The time range of 50% decomposition of the five leaf litter types was 1.07–1.37 a, and the time range of 95% decomposition was 4.41–5.92 a. There were some differences between the half-lives and turnover periods of the different leaf litter types. The decomposition coefficient k of CGC was the largest, and the decomposition rate was the fastest. The decomposition rate of BLC was the slowest, at 1.37 a for 50% and 5.92 a for 95%.

Table 2.
Regression decomposition model of dry matter mass loss of different leaf litters.
3.2. Dynamics of Element Release from Different Mixed Litter Leaves
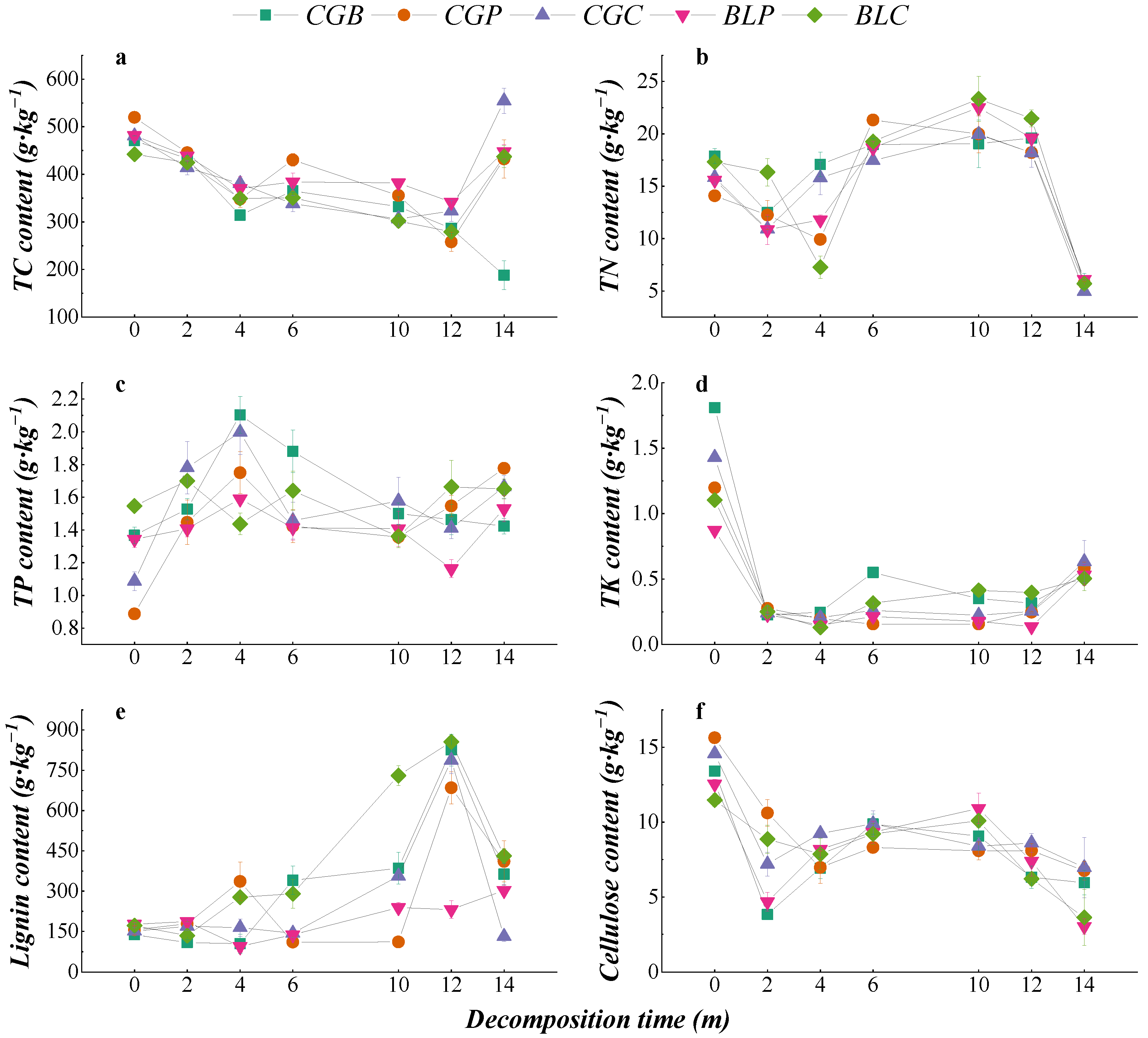
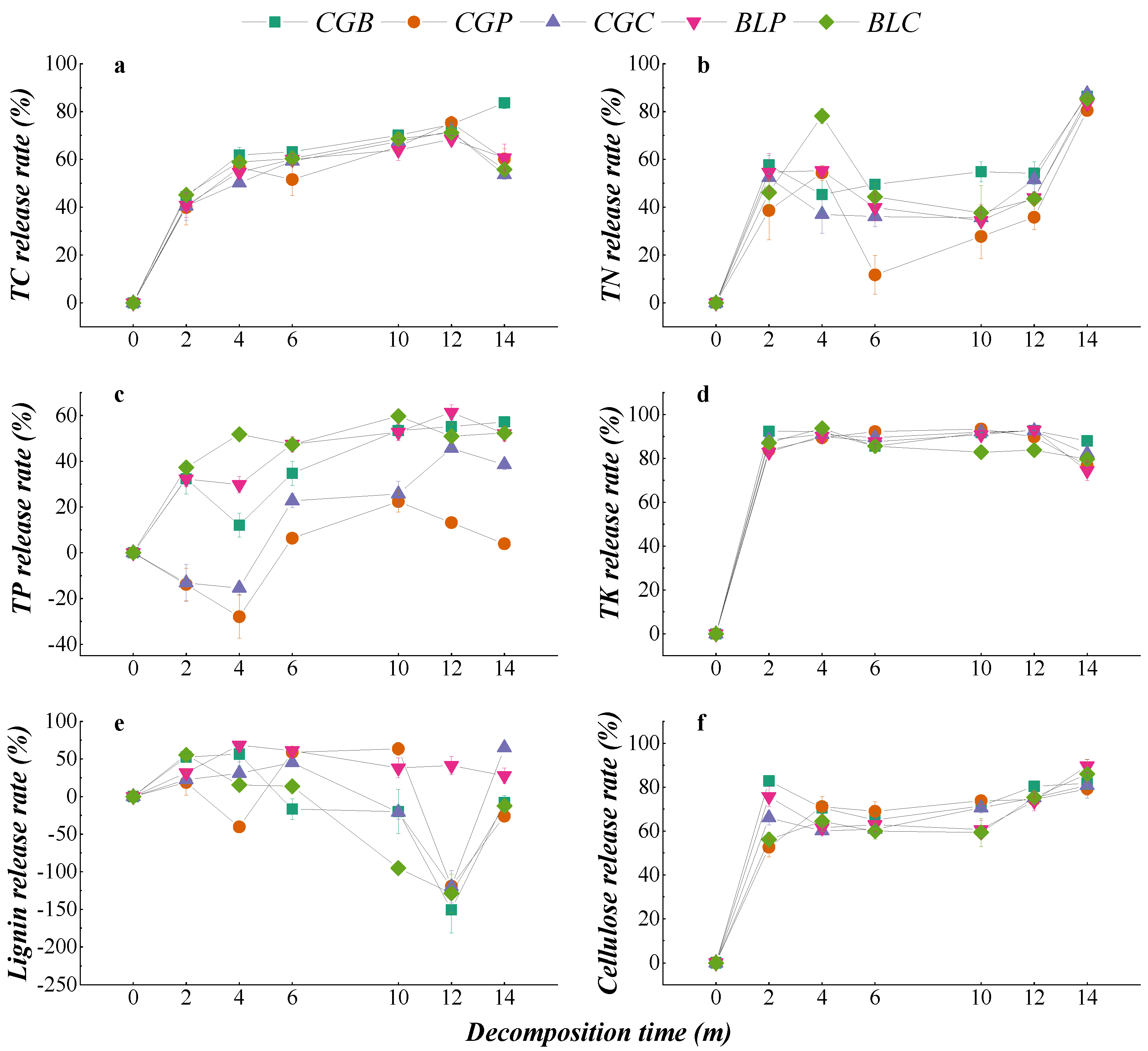
The change in nutrient content during the decomposition of different leaf litter types is shown in Figure 4. The change trend of the TC content in the five leaf litter types was similar. From the whole decomposition process, the TC content of the CGC type was significantly higher than the initial TC content after 14 months of decomposition (p < 0.05), while the TC content of the CGB, CGP, and BLP leaf litter types was significantly lower than the initial content (p < 0.05). The change trends of the TN, TK, and cellulose contents were also similar under different treatments, and the TN, TK, and cellulose contents of the five leaf litter types were significantly lower than the initial contents after 14 months of decomposition (p < 0.05). In the process of decomposition, there were some differences in the contents of TP and lignin among the five leaf litter types. From the whole decomposition process, the TP content of CGP, CGC, BLP, and BLC leaf litter types after 14 months of decomposition was significantly higher than that of nondecomposition (p < 0.05). The lignin content of the five kinds of leaf litter increased in the late stage of decomposition, and the lignin content of CGB, CGP, BLP, and BLC was significantly higher than that of nondecomposition (p < 0.05).
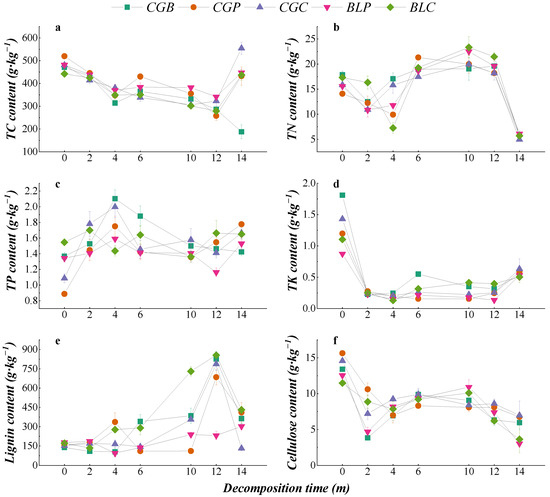
Figure 4.
Changes in the nutrient contents of different leaf litters during decomposition. (a–f) represents the TC content, TN content, TP content, TK content, Lignin content and Cellulose content.
The change in the nutrient release rate during the decomposition of different leaf litter types is shown in Figure 5. The release patterns of TC, TN, TK, and cellulose in the five leaf litter types all showed direct releases, and the release trend was similar in the process of decomposition. There were some differences in the release patterns of TP and lignin under the different treatments. Among them, the TP release of five leaf litter types showed two patterns: that is, the CGB, BLP, and BLC leaf litter TP release was a direct release, while the CGP and CGC had a leaching-enrichment-release. There were four modes of lignin release from different leaf litters, among which CGB and BLC had leaching-enrichment, BLP had direct release, CGC had leaching-enrichment-release, and CGP had leaching-enrichment-release-enrichment.
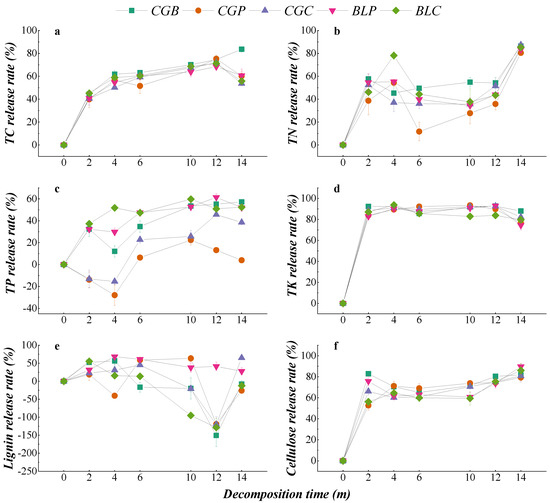
Figure 5.
Nutrient release rates of different leaf litters in the decomposition process. (a–f) represents the TC release rate, TN release rate, TP release rate, TK release rate, Lignin release rate and Cellulose release rate.
3.3. Dynamic Changes in Leaf Litter Decomposition Enzyme Activity in Different Leaf Mixtures
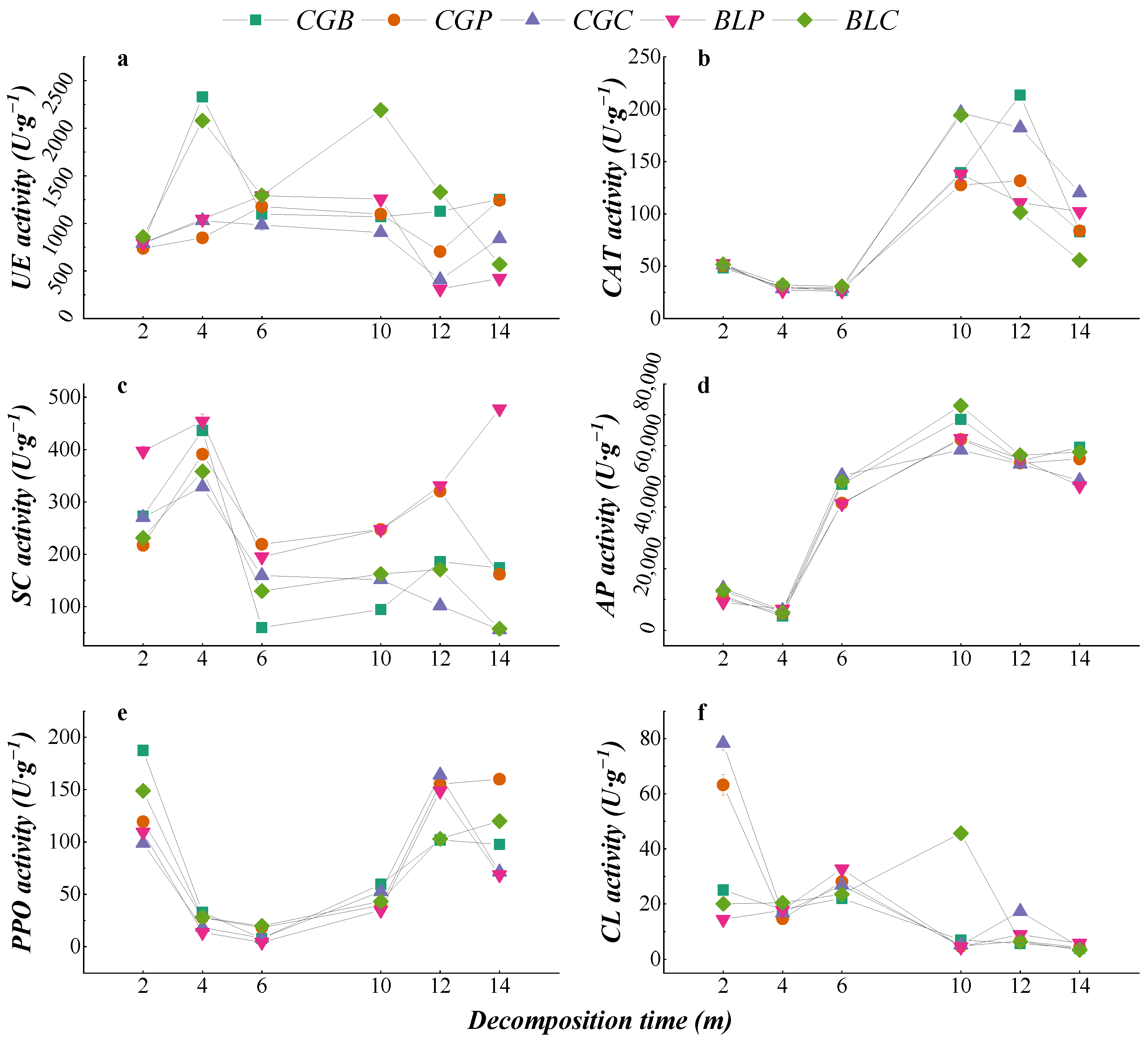
The dynamic changes in different enzyme activities during leaf litter decomposition are shown in Figure 6. The dynamic changes in the CAT, AP, and PPO activities of the five leaf litter types were similar, decreasing in the early stage of decomposition and increasing in the middle stage of decomposition. The dynamic changes in UE activity in the different leaf litter types were different, in which the CGB and BLC leaf litters showed higher urease activity in the early stage of decomposition (2–4 months), which was different from the other leaf litter types. In the early stage of decomposition, the SC activity of the five kinds of litter leaves increased gradually and decreased after 4 months of decomposition. There were great differences in the CL activity of the five kinds of leaf litter leaves during decomposition. The CL activity of BLP and BLC increased gradually in the early stage of decomposition, while the CL activity of CGB, CGP, and CGC decreased gradually. After 4 months of decomposition, the cellulase activity increased gradually. In the late stage of decomposition (14 months), the CL activity of the five leaf litter types was lower than that in the early stage of decomposition (2 months).
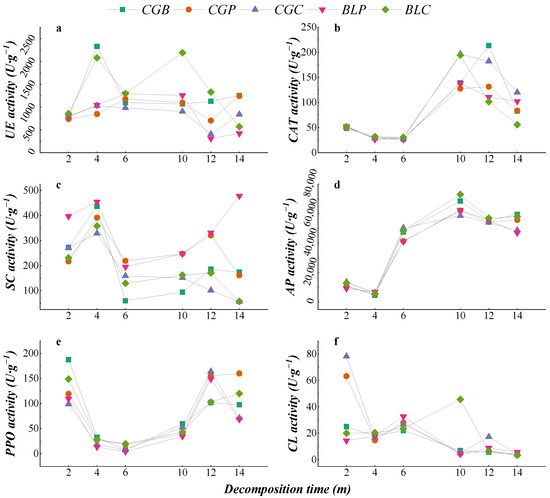
Figure 6.
Dynamics of enzyme activities of different leaf litters in the decomposition process. (a–f) represents the Urease activity, Catalase activity, Sucrase activity, Acid phosphatase activity, Polyphenol oxidase activity and Cellulase activity.
3.4. Correlation between Leaf Litter Decomposition Rate, Nutrient Release Rate and Enzyme Activity
The correlation between the leaf litter decomposition rate, nutrient release rate, and enzyme activity is shown in Table 3. There was a very significant positive correlation between the decomposition rate of the five kinds of leaf litter and AP activity (p < 0.01). The decomposition rates of CGB, CGC, CGP, and BLP were also positively correlated with CAT activity (p < 0.01). The decomposition rates of CGB, CGP, and CGC were significantly negatively correlated with CL activity (p < 0.01). There was a significant positive correlation between the TC release rate and the catalase activity in the five kinds of leaf litter. According to the TN release rate of leaf litter, only BLP was negatively correlated with UE activity. There was a very significant positive correlation between the TP release rate and the AP activity in the five kinds of leaf litter (p < 0.01). There was a certain correlation between the lignin release rate and the CAT activity and PPO activity in the five kinds of leaf litter, and there was a very significant negative correlation between the lignin release rate and the CAT activity in CGB and BLC (p < 0.01). There was a very significant negative correlation between the CGP lignin release rate and the PPO activity (p < 0.01). The lignin release rates of CGC and BLP were significantly negatively correlated with the CAT activity and PPO activity. The cellulose release rate of CGP and BLC was negatively correlated with the CL activity (p < 0.01); there was a certain correlation between the cellulose release rate of CGC and BLP and the SC activity. Among them, the cellulose release rate of CGC was significantly negatively correlated with the SC activity (p < 0.01), while BLP was positively correlated with the SC activity (p < 0.05).

Table 3.
Correlation of decomposition rate, nutrient release rate and enzyme activity of different leaf.
4. Discussion
Related studies have shown that the litter decomposition rate has two stages: faster and slower during the decomposition period [20]. In this study, the decomposition rate of different mixed leaf litters showed a trend of being fast at first and then slow, which was consistent with the results of previous studies [21]. Our previous research results showed that the mixed decomposition of CGB, CGC, BLP, and BLC produced a certain synergistic effect. Compared with the individual decomposition of leaf litter in tea gardens, the mixture of different species promoted the decomposition of leaf litter, and mixed decomposition accelerated the decomposition turnover period by 1.65 years [22]. This result shows that different combinations of leaf litter decomposition can accelerate the circular management and utilization of leaf litter in ecological tea gardens.
The speed of litter decomposition is usually affected by many factors, such as environmental factors, litter properties, and biological factors [19,23,24,25,26,27]. Aerts believed that climate was the most important factor affecting litter decomposition [27] and had a significant impact on litter decomposition on a large scale [17]. However, under the same environmental conditions, the chemical properties of litter were the main factors affecting the speed of litter decomposition [28]. Some studies have shown that the litter of broad-leaved trees decomposes more easily than that of coniferous trees [29]. In this study, the decomposition rates of the five kinds of mixed leaf litter were CGC > CGB > BLP > CGP > BLC. The decomposition rate of needle-broad mixed leaf litter (CGC) was higher than that of broad-leaf mixed leaf litter (CGB). In terms of litter matrix quality, early studies showed that the litter decomposition rate was proportional to the initial N concentration [18,30], and the C/N ratio and lignin/N ratio were significantly negatively correlated with the litter decomposition rate [17,18]. In this study, CGB had a higher initial N content and a lower C/N ratio and lignin/N ratio than that of CGC, indicating that the decomposition of mixed litter was not a case of simple addition between species; rather, the mixture drove an interaction between species.
In mixed litter, the individual components of the mixed litter are generally called component litter [31]. There were differences in nutrient contents among the different components of the mixed litter. High-quality components will be preferentially utilized and decomposed in the process of decomposition, releasing nutrients and thus reducing their nutrient content. As a result, the low-quality components have higher nutrient availability, and a nutrient content difference is formed between the litter components. Again, decomposers prioritize the decomposition of high-quality component litter.
This process speeds up the decomposition rate of the original low-quality litter and then speeds up the decomposition rate of the mixed litter as a whole [32]. Litter with a high N content, a low C/N ratio, and a low lignin content is usually called high-quality litter; in contrast, litter with a low N content, a high C/N ratio, and a high lignin content is called low-quality litter [33,34]. In this study, there were significant differences in the lignin content, C/N ratio, and lignin/N ratio between Cinnamomum glanduliferum and Cunninghamia lanceolata, and there was a large difference in the nutrient content between the components of CGC mixed leaf litter. At the initial stage of decomposition, the decomposers will prioritize decomposing the high-quality component litter, that is, the Cinnamomum glanduliferum component litter, and release its nutrients so that the low-quality component litter, that is, the Cunninghamia lanceolata component litter, has higher nutrient availability, thus forming a certain difference in nutrient content and promoting its decomposition. However, the difference in nutrient content among the CGB component litter was small, so the decomposition rate of CGB was lower than that of CGC, and the litter decomposition of BLP, CGP, and BLC also followed this law. In our previous studies, we found that the mixed decomposition of CGC, CGB, BLP, and BLC produced a synergistic effect [22], and the mixed effect was more likely to occur among the component species with different litter nutrient contents, which was consistent with the conclusion of previous studies [35,36,37,38]. The species richness and species composition of mixed litter have a significant impact on the decomposition of litter. The interaction between high-quality litter and low-quality litter increases the decomposition rate and can play a positive role in improving soil nutrients.
In addition to environmental conditions and litter properties, microorganisms and their enzymes are closely related to litter decomposition, and their types and activities vary with litter composition and environmental conditions [6,7,8]. In the process of decomposition, enzyme activity involves various biochemical processes that are directly related to litter decomposition, and these processes reflect the cycling of soil nutrients to a large extent and vary due to different litter types and environmental conditions [7,39]. Catalase and polyphenol oxidase are not only the main enzymes that decompose lignin [40] but also the key enzymes that maintain the carbon cycle in the ecosystem [39]. In this study, the change trends of CAT activity and PPO activity in the five kinds of leaf litter were similar. In the early stage of decomposition, the TC content of the five kinds of leaf litter decreased gradually, showing a net release state, so the activities of CAT and PPO decreased gradually in the early stage of decomposition. In the later stage of decomposition, lignin, which is difficult to decompose, accumulated continuously [41] and became the main factor affecting the rate in the later stage [40]. The lignin content of the five kinds of leaf litter increased in the later stage of decomposition, which was one of the reasons for the slow decomposition rate in the later stage of decomposition. Additionally, the activities of CAT and PPO increased gradually, and the increase in substrate content led to the need to increase the activity of related decomposing enzymes to maintain the decomposition process. In terms of correlation, there was a significant positive correlation between the TC release rate and the CAT activity in the five kinds of leaf litter, which was due to the continuous accumulation of lignin in the process of decomposition. CAT, as the main enzyme that decomposes lignin, needs to be improved to participate in the decomposition of lignin. The correlation between the lignin release rate and the decomposition enzyme activity of the five kinds of leaf litter leaves was different, but all showed a significant negative correlation, and the difference was due to the level of enzyme activity in the process of decomposition. For example, the lignin release rate of CGB was significantly negatively correlated with the CAT activity but not with the PPO activity. This is because CAT has higher enzyme activity and PPO activity is lower during decomposition, so CAT is involved in lignin decomposition. In the process of CGC decomposition, the activities of CAT and PPO were higher, so the lignin release rate was significantly negatively correlated with the activities of the two enzymes, indicating that the enzymes acting on the same substance were mixed with each other in the process of decomposition, and the needle-broad mixture significantly increased the activity of PPO, which was consistent with the results of Song Ying et al. [42]. Studies by Waring [6] and Allison et al. [43] showed that the activities of enzymes related to the C cycle were closely related to the mass loss of litter decomposition. In this study, the decomposition rates of CGB, CGP, CGC, and BLP were significantly positively correlated with the CAT activity, while there was no significant correlation between the BLC decomposition rate and the CAT activity. The reason may be related to the initial C content of BLC. Compared with the other four kinds of leaf litter, BLC had a lower initial C content, and the C content of BLC leaf litter decreased before 12 months, so it did not need the trapping process of carbon decomposition microflora, which led to the increase in CAT activity. BLC had higher PPO activity in the early stage of leaf litter decomposition, which could be used as a compensation for CAT participation in carbon decomposition. This shows that litter decomposition is not the result of the participation of an enzyme but is rather the result of the joint action of a variety of enzymes. There was a very significant positive correlation between the TP release rate and the AP activity in the five kinds of leaf litter. Some studies have shown that the addition of N can promote the synthesis of phosphatase to a certain extent, thus increasing the activity of AP [44]. In this study, the TN content of five kinds of leaf litter increased during the decomposition process (4–12 months), which led to the gradual increase in AP activity in this decomposition stage, indicating that during the decomposition process, the enzyme activity affected the nutrient release of litter, and the nutrient content of litter also affected the activity of decomposing enzymes [45].
5. Conclusions
The ecological significance of litter is to return nutrients to the soil and provide nutrients for forest growth, and thus, litter is an important mechanism of forest self-fertilization. The mixed effect caused by the mixed decomposition of litter can be applied to the process of forest cultivation, such as the relationship among tree species and afforestation tree species. The results showed that in the process of mixed leaf litter decomposition, the decomposition of CGC, CGB, BLC, and BLP all produced a synergistic effect and promoted decomposition, in which CGC and CGB shortened the litter decomposition turnover period in the ecological tea garden to a large extent, accelerated the nutrient cycle in the ecological tea garden, and increased nutrient availability. Therefore, in the process of ecological tea garden management, to promote the sustainable management of modern ecological tea gardens, realize the compound management of forests and tea, and solve the ecological problems of existing tea gardens, selecting Betula luminifera, Cinnamomum glanduliferum, Cunninghamia lanceolata, and Pinus massoniana to adjust the tree species allocation of ecological tea gardens can improve litter decomposition and produce a positive mixed effect, increase its nutrient return ability, maintain tea garden fertility, and realize the ecological development of tea gardens. However, the optimal allocation ratio still needs to be studied.
Author Contributions
Methodology, R.Y.; software, S.L.; investigation, C.H.; writing—original draft preparation, S.L.; writing—review and editing, R.Y. and S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science and Technology Program of Guizhou Province: Integration and demonstration of key technologies of species configuration in ecological tea gardens in mountainous areas of Guizhou, grant number Qian Ke He support [2020]1Y011 and the Forestry Scientific Research Project of Guizhou Province: Study on dynamic mechanism of plant residue decomposition in ecological compound management, grant number Qian Lin Ke He [2020]22.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors gratefully acknowledge the Forestry College of Guizhou University for the technical support, especially appreciate Rui Yang for the valuable advice.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, T.; Xi, M.; Kong, F.; Li, Y.; Pang, L. A review on litter decomposition and influence factors. Chin. J. Ecol. 2016, 35, 1927–1935. [Google Scholar] [CrossRef]
- Manzoni, S.; Jackson, R.B.; Trofymow, J.A.; Porporato, A. The Global Stoichiometry of Litter Nitrogen Mineralization. Science 2008, 321, 684–686. [Google Scholar] [CrossRef]
- Kaimin, L.; Zhiqin, Z.; Guanqiu, C.; Zongming, H.; Xiangqing, M. Decomposition characteristics and its nutrient dynamics of leaf litter mixtures of both Chinese fir and Phoeba bournei. Acta Ecol. Sin. 2006, 26, 2732–2738. [Google Scholar] [CrossRef]
- Hongyue, H.; Changming, M.; Hongxiang, M.; Yuanyuan, S. The Decomposition Characteristics and Soil Nutrient Dynamics of Leaf Litter Mixture of Laric principis-rupprechtii and Betula platyphylla. For. Resour. Manag. 2018, 0, 93–100. [Google Scholar] [CrossRef]
- Hongyue, H.; Hongxiang, M.; Changming, M.; Yuanyuan, S. The decomposition characteristics and their effect on soil nutrients of leaf litter mixtures of Larix principis-rupprechtii and Lespedeza bicolor. J. Hebei Agric. Univ. 2018, 41, 55–61. [Google Scholar] [CrossRef]
- Waring, B.G. Exploring relationships between enzyme activities and leaf litter decomposition in a wet tropical forest. Soil Biol. Biochem. 2013, 64, 89. [Google Scholar] [CrossRef]
- Aubert, M.; Margerie, P.; Trap, J.; Bureau, F. Aboveground–belowground relationships in temperate forests: Plant litter composes and microbiota orchestrates. For. Ecol. Manag. 2010, 259, 563–572. [Google Scholar] [CrossRef]
- Criquet, S.; Ferre, E.; Farnet, A.M.; Le Petit, J. Annual dynamics of phosphatase activities in an evergreen oak litter: Influence of biotic and abiotic factors. Soil Biol. Biochem. 2004, 36, 1111–1118. [Google Scholar] [CrossRef]
- Yaling, Y.; Danjie, Z.; Yan, Z.; Xun, L.; Yamei, C.; Yu, Q.; Jian, Z. Enzyme activities in the early stage of mixed leaf litter decomposition from Pinus massoniana and broad-leaved tree species. Chin. J. Appl. Environ. Biol. 2018, 24, 508–517. [Google Scholar] [CrossRef]
- Yonghui, T.; Yongjun, T. Model of Ecological Tea Plantation and Benefical Analysis in Guizhou. Guizhou Agric. Sci. 2000, 28, 46–48. [Google Scholar]
- Jun, L.; Shengchuan, L.; Yuanfa, L.; Jialun, W.; Huajian, H.; Zhengwu, C.; Xueyi, D. Construction of Ecological Tea Plantation in Guizhou and its Development Model. J. Mt. Agric. Biol. 2012, 31, 250–254. [Google Scholar] [CrossRef]
- Bocock, K.L.; Gilbert, O.J.W. The Disappearance of Leaf Litter under Different Woodland Conditions; Springer: Berlin/Heidelberg, Germany, 1957; Volume 9, pp. 179–185. [Google Scholar] [CrossRef]
- Ming, D.; Yifeng, W.; Fanzhi, K.; Gaoming, J.; Zhibin, Z. Survey, Observation and Analysis of Terrestrial Biocommunities; Standards Press of China: Beijing, China, 1997; pp. 152–153. [Google Scholar]
- LY/T 1271-1999; Determination of Total Nitrogen, Phosphorus, Potassium, Sodium, Calcium, Magnesium in Forest Plant and Forest Floor. The State Forestry Administration of the People’s Republic of China: Beijing, China, 1999.
- LY/T 1269-1999; Determination of Total Nitrogen in Forest Plant and Forest Floor. The State Forestry Administration of the People’s Republic of China: Beijing, China, 1999.
- LY/T 1270-1999; Determination of Total Silica, Iron, Aluminium, Calcium, Magnesium, Potassium, Sodium, Phosphorus, Sulphur, Manganese, Copper and Zinc in Forest Plant and Forest Floor. The State Forestry Administration of the People’s Republic of China: Beijing, China, 1999.
- Jingjing, Y. Litter Decomposition Characteristics in Different Habitats in Extreme Arid Area. Master’s Thesis, Tarim Universitry, Tarim, China, 2020. [Google Scholar]
- Olson, J.S. Energy Storage and the Balance of Producers and Decomposers in Ecological Systems. Ecol. Soc. Am. 1963, 44, 322–331. [Google Scholar]
- Berg, B.; Berg, M.P.; Bottner, P.; Box, E.; Breymeyer, A.; De Anta, R.C.; Couteaux, M.; Escudero, A.; Gallardo, A.; Kratz, W.; et al. Litter Mass Loss Rates in Pine Forests of Europe and Eastern United States: Some Relationships with Climate and Litter Quality. Biogeochemistry 1993, 20, 127–159. [Google Scholar] [CrossRef]
- Zar, A.M.N.; Ez, S.N.N.; Rquez, A.B. Leaf litter decomposition in a southern Sonoran Desert ecosystem, northwestern Mexico: Effects of habitat and litter quality. Acta Oecologica 2007, 32, 291–300. [Google Scholar] [CrossRef]
- Lulu, G.; Zongming, H.; Qingquan, M.; Yu, L. Mixed Decomposition Process of Litterfall of Acacia aulacocarpa and Casuarina equisetifolia Plantations in Southeast Coastal Area. J. Northeast. For. Univ. 2019, 47, 35–40. [Google Scholar] [CrossRef]
- Shaqian, L.; Rui, Y.; Chunlan, H.; Juebing, M.; Jiarui, G. Decomposition Characteristics of Lignin and Cellulose in Different Litters of Ecological Tea Gardens in Mountainous Areas of Guizhou. J. Tea. Sci. 2021, 41, 654–668. [Google Scholar] [CrossRef]
- Xinjian, X.; Jingyao, C.; Xintuo, Y. Studies on the Litter Nutrient Returns of Six Maior Chinese Fir Assoeiated Species in Wuyi Moutains. J. Fujian Coll. For. 1995, 15, 213–217. [Google Scholar] [CrossRef]
- Yihui, H.; Lingzhi, C.; Qinglang, C.; Fanzhi, K.; Yougui, M. Studies on the litter decomposition rates of several plants. Acta Phytoecologica Geobotanica Sinica 1987, 11, 124–132. [Google Scholar]
- Garcia-Pausas, J.; Casals, P.; Romanyà, J. Litter decomposition and faunal activity in Mediterranean forest soils: Effects of N content and the moss layer. Soil Biol. Biochem. 2004, 36, 989–997. [Google Scholar] [CrossRef]
- Singhk, P.; Singhp, K.; Tripathis, K. Litterfall, litter decomposition and nutrient release patterns in four native tree species raised on coal mine spoil at Singrauli, India. Biol. Fertil. Soils 1999, 29, 371–378. [Google Scholar] [CrossRef]
- Aerts, R. Climate, Leaf Litter Chemistry and Leaf Litter Decomposition in Terrestrial Ecosystems: A Triangular Relationship. Oikos 1997, 79, 439–449. [Google Scholar]
- Zhouyu, L.; Yang, H.; Shaojun, W.; Peng, C. Leaf litter decomposition and nutrient return characteristics of Pinus yunnanensis at different forest ages. Ecol. Environ. Sci. 2018, 27, 1981–1986. [Google Scholar] [CrossRef]
- Peipei, G.; Hong, J.; Shuquan, Y.; Yuandan, M.; Rongpeng, D.; Xinzhang, S. Comparison of Litter Decomposition of Six Species of Coniferous and Broad-leaved Trees in Subtropical China. Chin. J. Appl. Environ. Biol. 2009, 15, 655–659. [Google Scholar] [CrossRef]
- Xuhu, G.; Derong, X.; Kun, T.; Hongzhong, Y. Biomass production and litter decomposition of lakeshore plants in Napahai wetland, Northwestern Yunnan Plateau, China. Acta Ecol. Sin. 2013, 33, 1425–1432. [Google Scholar] [CrossRef]
- Ying, L. Analysis of Mixed Effects of Leaf Litter Decomposition and Its Main Affecting Factors of Dominant Tree Species in Yaoxiang Forest Farm; Shandong Agricultural University: Tai’an, China, 2020. [Google Scholar]
- Yinong, L.; Xiaomei, Z.; Naili, Z.; Keping, M. The research of mixed litter effects on litter decomposition in terrestrial ecosystems. Acta Ecol. Sin. 2016, 36, 4977–4987. [Google Scholar] [CrossRef]
- Haiyan, G.; Mei, H.; Lixia, H.; Zhao, Y.H. Bayinnamula and DE Haishan. Effects of exogenous nitrogen input and water change on litter decomposition in a desert grassland. Chin. J. Appl. Ecol. 2018, 29, 3167–3174. [Google Scholar] [CrossRef]
- Steffensen, J.P.; Andersen, K.K.; Bigler, M.; Clausen, H.B.; Dahl-Jensen, D.; Fischer, H.; Goto-Azuma, K.; Hansson, M.; Johnsen, S.J.; Jouzel, J.; et al. High-Resolution Greenland Ice Core Data Show Abrupt Climate Change Happens in Few Years. Science 2008, 321, 680–684. [Google Scholar] [CrossRef]
- Leroy, C.J.; Marks, J.C. Litter quality, stream characteristics and litter diversity influence decomposition rates and macroinvertebrates. Freshw. Biol. 2006, 51, 605–617. [Google Scholar] [CrossRef]
- Gartner, T.B.; Cardon, Z.G. Site of leaf origin affects how mixed litter decomposes. Soil Biol. Biochem. 2006, 38, 2307–2317. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Gasser, P. Soil Animals Alter Plant Litter Diversity Effects on Decomposition. Natl. Acad. Sci. 2005, 102, 1519–1524. [Google Scholar] [CrossRef]
- Wardle, D.A.; Bonner, K.I.; Nicholson, K.S. Biodiversity and Plant Litter: Experimental Evidence Which Does Not Support the View That Enhanced Species Richness Improves Ecosystem Function. Wiley Behalf Nord. Soc. Oikos 1997, 79, 247–258. [Google Scholar]
- Keelerb, L.; Hobbies, E.; Kelloggl, E. Effects of Long-Term Nitrogen Addition on Microbial Enzyme Activity in Eight Forested and Grassland Sites: Implications for Litter and Soil Organic Matter Decomposition. Ecosystems 2009, 12, 1–15. [Google Scholar] [CrossRef]
- Kai, H.; Qian, L.; Zhongfa, Z.; Wei, W. Research Progress of Litter Decomposition Enzyme. North. Hortic. 2021, 45, 134–140. [Google Scholar]
- Chung, H.; Zak, D.R.; Reich, P.B.; Ellsworth, D.S. Plant species richness, elevated CO2, and atmospheric nitrogen deposition alter soil microbial community composition and function. Glob. Chang. Biol. 2007, 13, 980–989. [Google Scholar] [CrossRef]
- Ying, S.; Xirong, G.; Mao, Y.H.; Xuelian, W.W.; Yuxuan, W. Dynamics of Microbes and Enzyme Activities During Litter Decomposition of Pinus massoniana Forest in Mid-subtropical Area. Environ. Sci. 2014, 35, 1151–1158. [Google Scholar] [CrossRef]
- Allison, S.D.; Vitousek, P.M. Extracellular Enzyme Activities and Carbon Chemistry as Drivers of Tropical Plant Litter Decomposition. Blotroplca 2004, 36, 285–296. [Google Scholar] [CrossRef]
- Marklein, A.R.; Houlton, B.Z. Nitrogen inputs accelerate phosphorus cycling rates across a wide variety of terrestrial ecosystems. N. Phytol. 2012, 193, 696–704. [Google Scholar] [CrossRef]
- Xiaogai, G.; Wenfa, X.; Lixiong, Z.; Zhilin, H.; Benzhi, Z. Effect of soil-litter layer enzyme activities on litter decomposition in Pinus massoniana plantation in Three Gorges Reservoir Area. Acta Ecol. Sin. 2014, 34, 2228–2237. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).