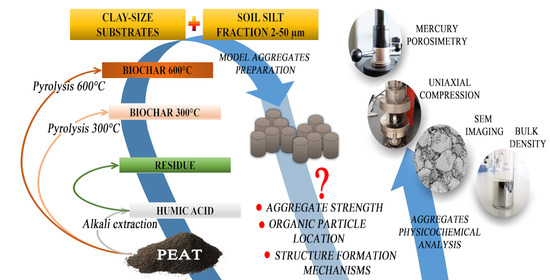
New Insight into Organomineral Interactions in Soils. The Impact of Clay-Size Peat-Derived Organic Species on the Structure and the Strength of Soil Silt Aggregates
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrates
- SILT: Silt fraction with a particle size range of 2–50 µm coming from the upper 0–10 cm layer of Haplic Luvisol containing 66% sand (2–0.02 mm), 28% silt (0.02–0.002 mm), and 6% clay (<0.002 mm), described in detail by Lipiec et al. [40], was used as skeletal material. Clay particles were discarded from the soil by sedimentation (4:100 solid: liquid w/w ratio), and sand particles by wet sieving. Then, the material was treated with hydrogen peroxide to remove native organic matter (if any), intensively washed with NaCl, and then with water. The silt was composed mainly from feldspars and quartz.
- PEAT: An acidic peat bought in a garden shop (pH ≈ 4), fragmented by a 3-min treatment with an ordinary kitchen blender and depleted from sand by sedimentation, was intensively washed with 0.1 mol·L−1 hydrochloric acid to remove inorganic substances and next with distilled water by centrifuging to pH around 4, air dried, and finely milled in a ball mill.
- HA: Humic acids extracted from the PEAT using 0.5 mol·L−1 sodium hydroxide, precipitated with HCl at pH = 1 and washed with distilled water by centrifuging to pH around 4.
- RES: The residue remaining after HA extraction was washed with 0.1 mol·L−1 HCl and next with distilled water by centrifuging to pH around 4. Material was lyophilized and finely ground in a ball mill.
- BC300: Biochar produced by heating the PEAT at 300 °C under limited air access, washed with 0.1 mol·L−1 HCl and next with distilled water by centrifuging to a pH of around 4, air dried and finely milled in a ball mill.
- BC600: Biochar produced by heating the PEAT at 600 °C under limited air access, washed with 0.1 mol·L−1 HCl and next with distilled water by centrifuging to a pH of around 4, air dried and finely milled in a ball mill.
2.2. Characteristics of the Substrates
- Substrates (solid phase) densities, SPD [kg·m−3], were estimated by helium pycnometry using an automatic device Ultrapycnometer 1000 (Quantachrome Instruments, Boynton Beach, FL, USA) in five replicates.
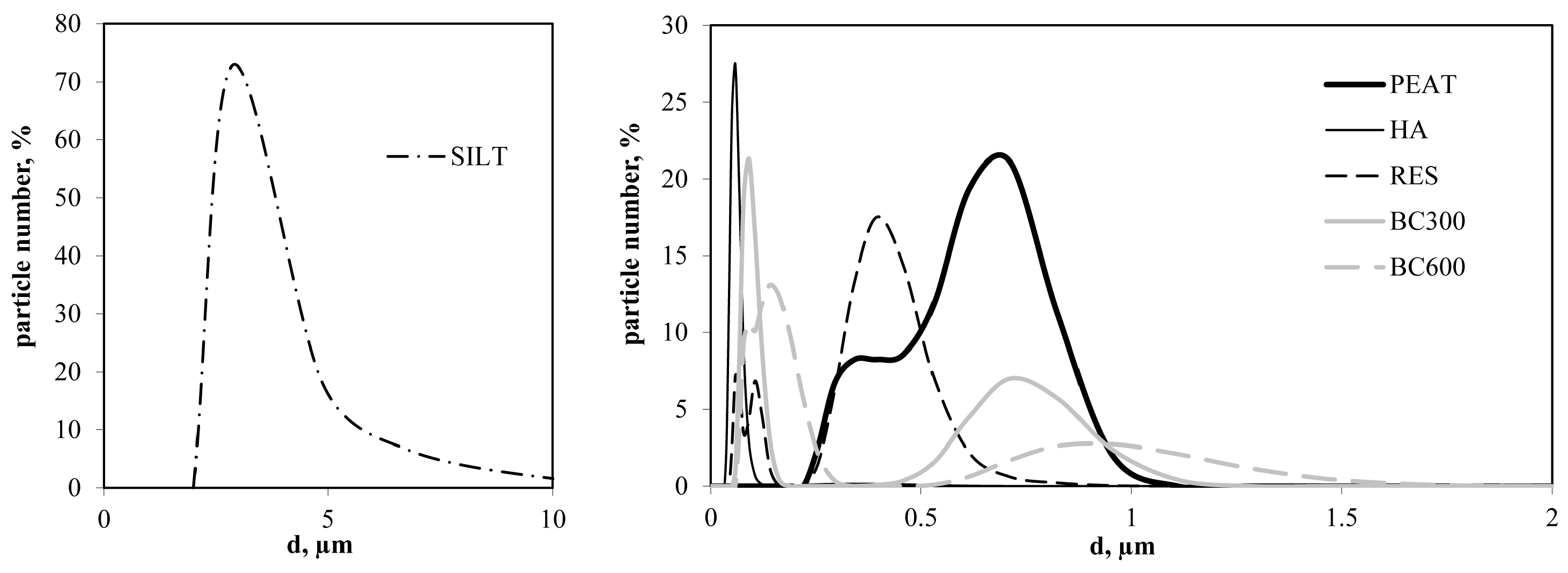
- Organic amendments were ground intensively to uniformize their particle sizes and remove impurities, which allowed the joining of the aggregate properties with the measured surface and physicochemical parameters. Particle size distribution, PSD, of the organic substrates was determined at 25 °C for suspensions of 100 mg·dm−3 materials in 1 L of 0.01 mol·L−1 sodium hexametaphosphate/0.015 mol·L−1 sodium bicarbonate solution using a ZetaSizer Nano ZS instrument (Malvern Ltd., Leamington, UK) device in six replicates. The distribution of the silt was not measured with the ZetaSizer due to large particles sedimented in the electric field. Therefore, this characteristic was determined using a laser diffraction method using Malvern Mastersizer 2000, as described by Ryzak and Bieganowski [45]. The PSD functions were expressed as the number of particles of a given radius in the total number of particles. The average particle diameter, d [m], was then calculated from PSD functions.
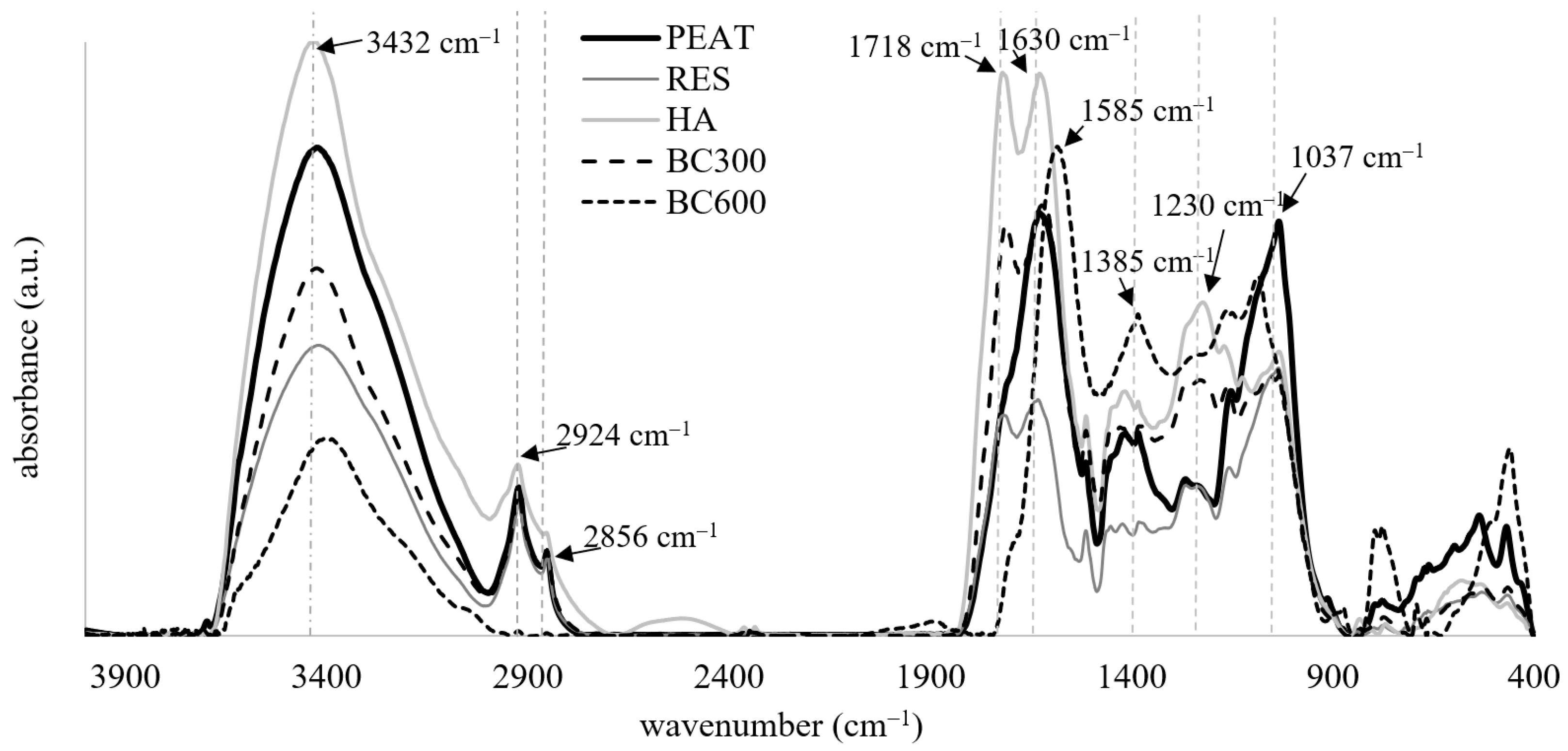
- FTIR characteristics were recorded for dried organic substrates (105 °C) using a Tensor 27 spectrometer (Bruker Corporation, Billerica, MA, USA) in 400–4000 cm−1 range. Briefly, 1 mg of each powdered sample was homogenised with 200 mg of spectral purity bromide potassium and pelletized. The spectra were obtained as an average of three measurements with 256 scans at 2 cm−1 resolution each. The transmittance signals were converted to absorbance, and the characteristics were prepared using concave rubber band correction of the baseline, vector normalization, and smoothing function.
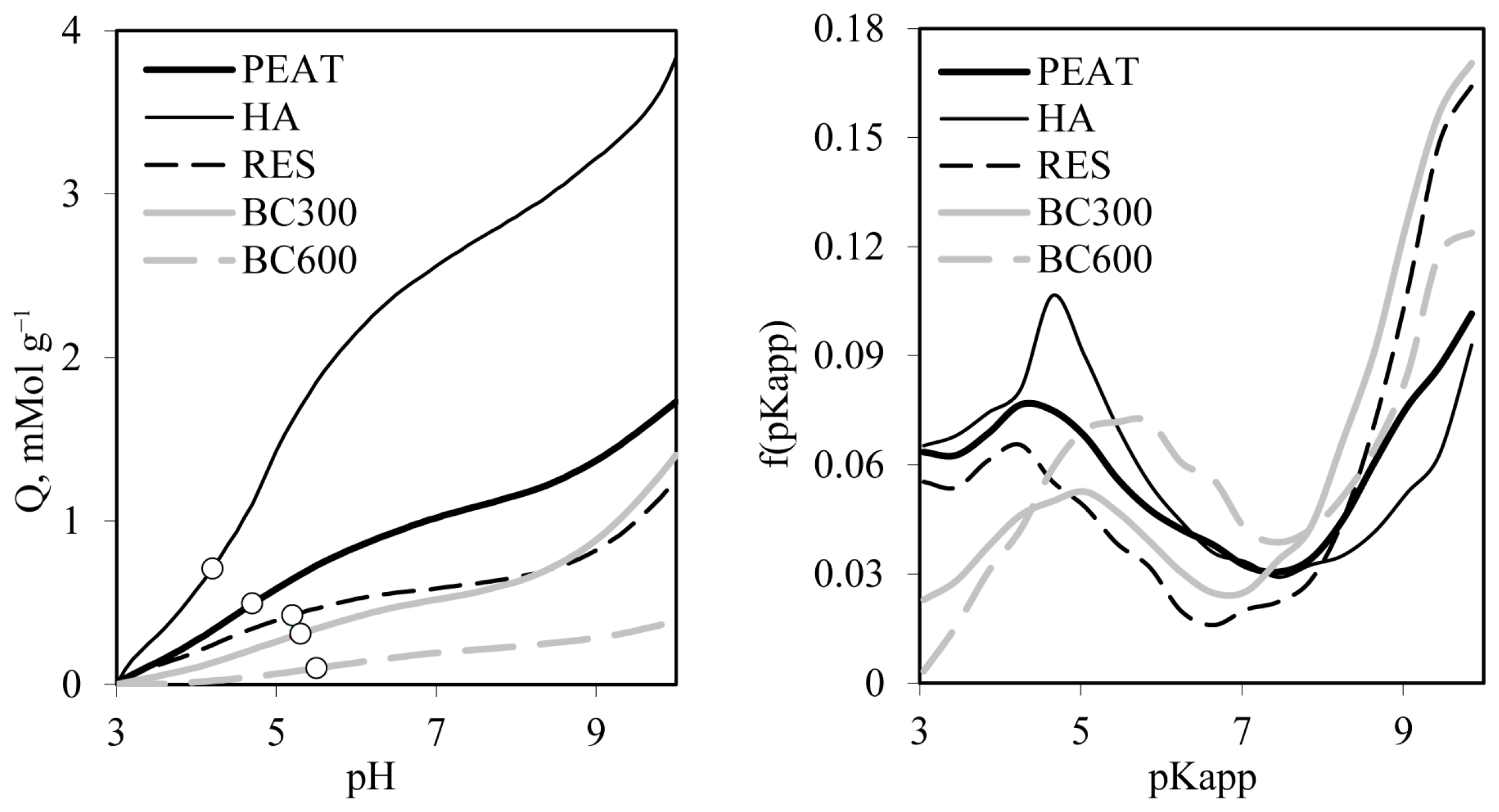
- Dependencies of variable surface charge—Q [mol·g−1] on pH, distribution functions of apparent surface dissociation constants, and their average values were determined from titration curves measured using Titrino 702SM autotitration unit (Metrohm A.G., Herisau, Switzerland). A suspension of a particular material was equilibrated overnight with 1 mol·L−1 NaCl solution and then adjusted to pH =3.0 and titrated by 60 s increments of 1 μL 0.100 mol·L−1 NaOH/1 mol·L−1 NaCl solution to pH = 10 under N2 flux. The measurements were performed in triplicate. From the averaged titration curve of the sample suspended in liquid, the titration curve of its equilibrium solution (1 mol·L−1 NaCl) was subtracted to obtain the titration curve of the solid phase. The data of the latter curve were expressed as the variable surface charge pH dependence. Under the assumption that surface acids dissociate stepwise, the actual pH is −log of the apparent surface dissociation constant, pKapp. The first derivative of the Q(pKapp) curve on pKapp gives the distribution function of apparent surface dissociation constants, f(pKapp), and the average value of pKapp in the whole experimental window, pKapp,av, was calculated as ∫f(pKapp)dpKapp. More details on the method and calculations are presented in Jozefaciuk et al. [46].
2.3. Aggregates
2.4. Characteristics of the Aggregates
- Magnified images (1000×) of the surfaces of the aggregates broken by hand were taken using the Phenom ProX desktop SEM provided by Thermo Fisher Scientific (Waltham, MA, USA). Samples were placed on aluminium stubs by double-sided carbon tape and sputtered with a 5 nm gold layer (sputter coater, CCU-010 LV, Safematic GmbH, Zizers, Switzerland). The imaging was conducted in BSE mode at an accelerating voltage of 10 kV. Representative images were selected.
- The bulk density, BD [g·cm−3], was estimated for air-dry specimens. The aggregate mass (minus its water content) was divided by the aggregate volume measured by its compulsive immersion in mercury. The water content in the aggregates was measured by weighing them after overnight heating at 105 °C. The measurements were replicated five times.
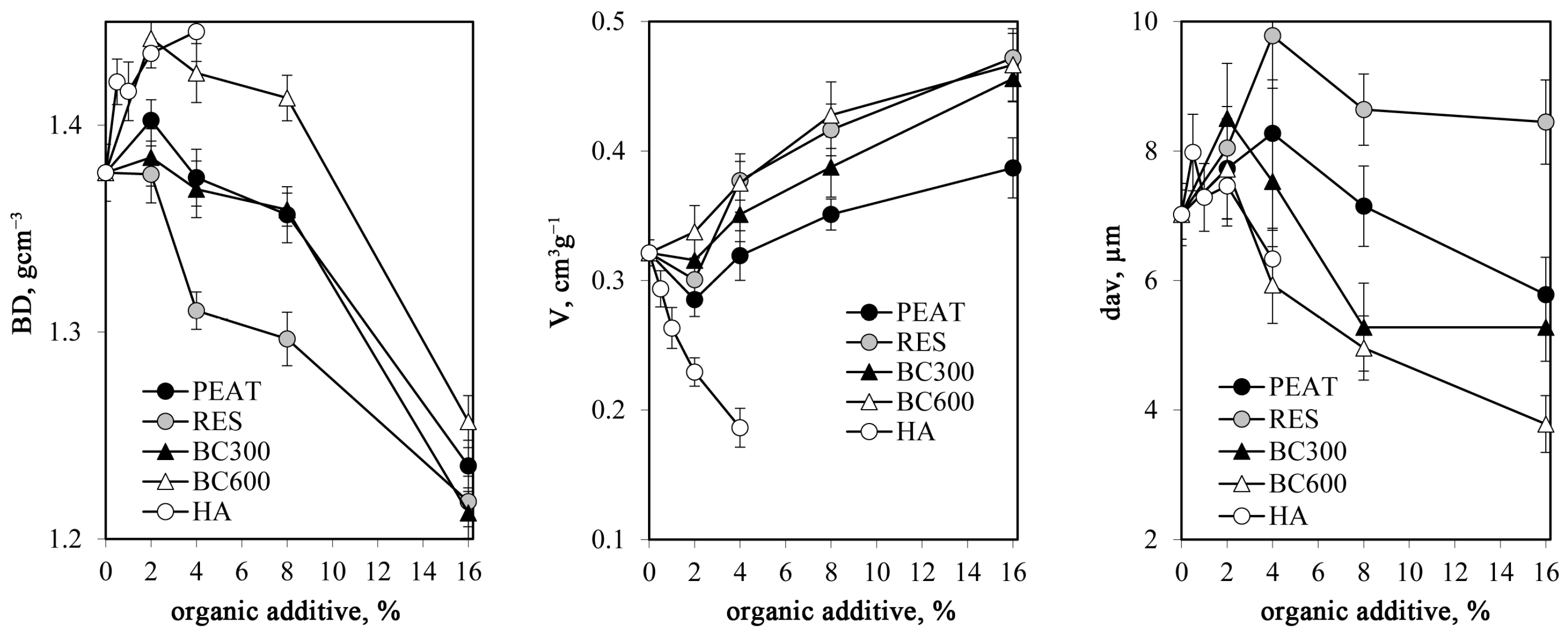
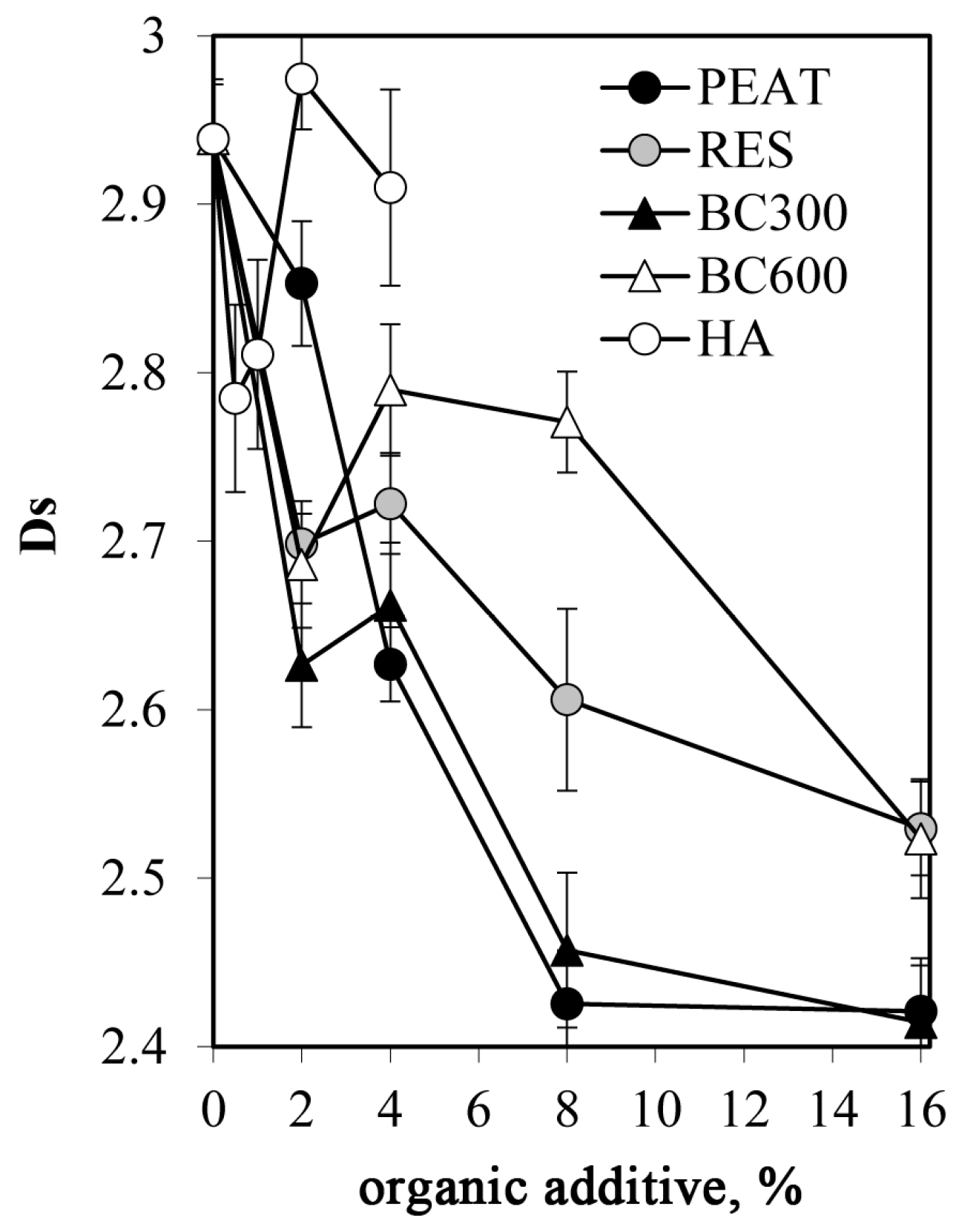
- Mercury intrusion porosimetry (MIP) measurements for the studied aggregates were performed using the Autopore IV 9500 porosimeter (Micromeritics, Norcross, GA, USA) for three replicates of each material. The total pore volumes Vt [m3·g−1], pore size distribution functions, and average pore diameter, dav [m], were calculated from the mercury intrusion curves that show the dependence between the pore volume (intruded mercury) and the pore diameter d [m].. The pressures at which mercury started to enter the pores inside the aggregates (penetration thresholds), PT [m], were approximated by the pore diameter at which the second derivative of the pore volume on logd equals zero [48]. The intrinsic pore volume within an aggregate, Via, was taken as the volume of mercury intruded into the pores at higher pressures than those corresponding to PT, and the average intrinsic pore diameter in that range, dint,av [m], was calculated. The pore surface fractal dimension, Ds, was calculated from the slope of the linear part (if any) of the dependence of log(dV/dd) against logd. All details of the method and the calculations are presented by Jozefaciuk [49]. MIP tests were also performed for the dried HA paste in order to have some insight into the structure of pure HA.
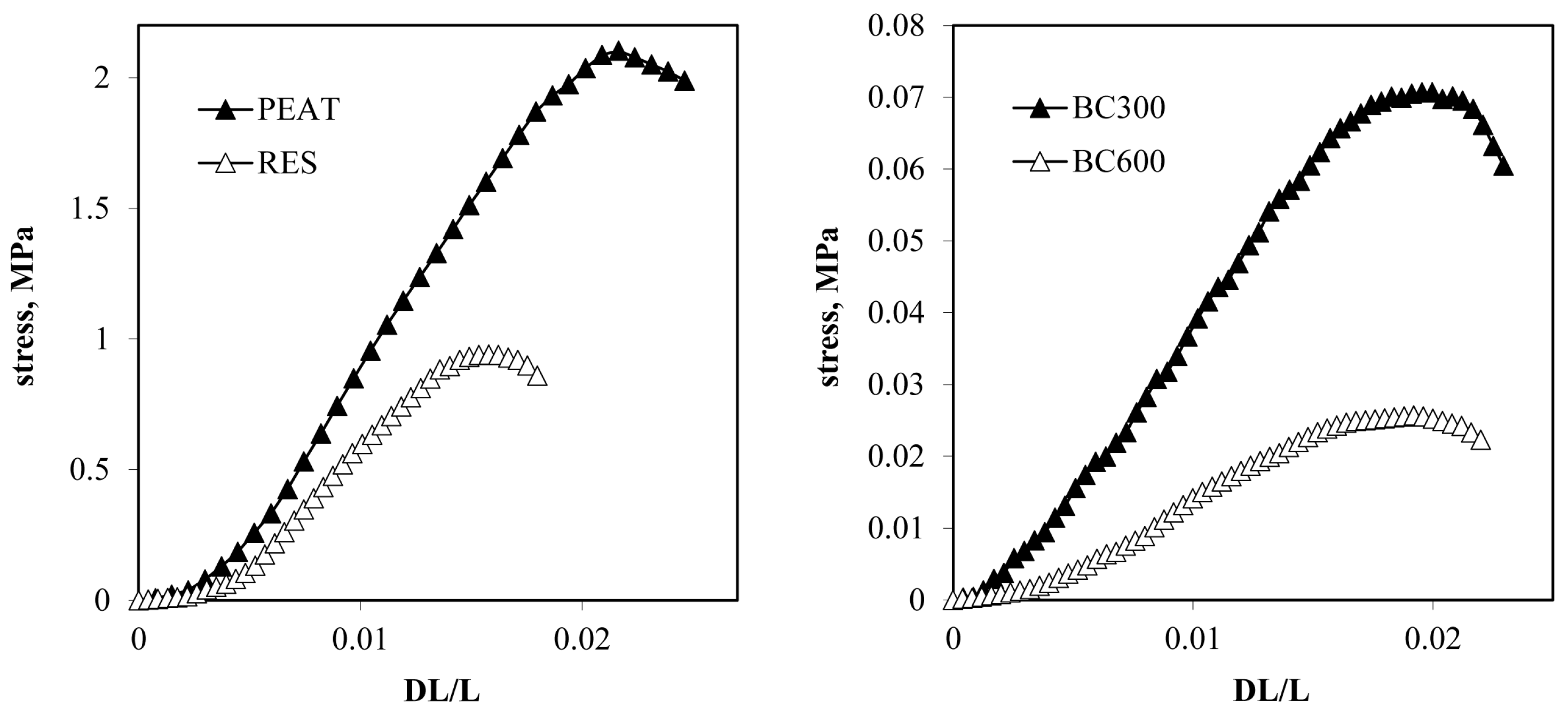
- Uniaxial compression measurements were performed using Lloyd LRX (Lloyd Instruments Ltd., Bognor Regis, UK). The aggregate was placed vertically on the machine basement and forced by a piston. The force estimated with the accuracy of ±0.05 N against displacement of the piston passed with 10−5 m·s−1 was examined for ten replicates for aggregates of a given organic-mineral composition. The averaged breakage curve was calculated from at least six curves, most similar among ten replicates. Different curves, measured most probably for aggregates having structural artifacts, were discarded. From the average breakage curves, the dependencies of the compression stress, σ [MPa], (load divided by the aggregate cross-section area) versus strain, and ∆L/L (relative aggregate deformation, equal to piston displacement divided by the aggregate height) were calculated.
3. Results and Discussion
3.1. Properties of Substrates
3.2. Structure of the Aggregates
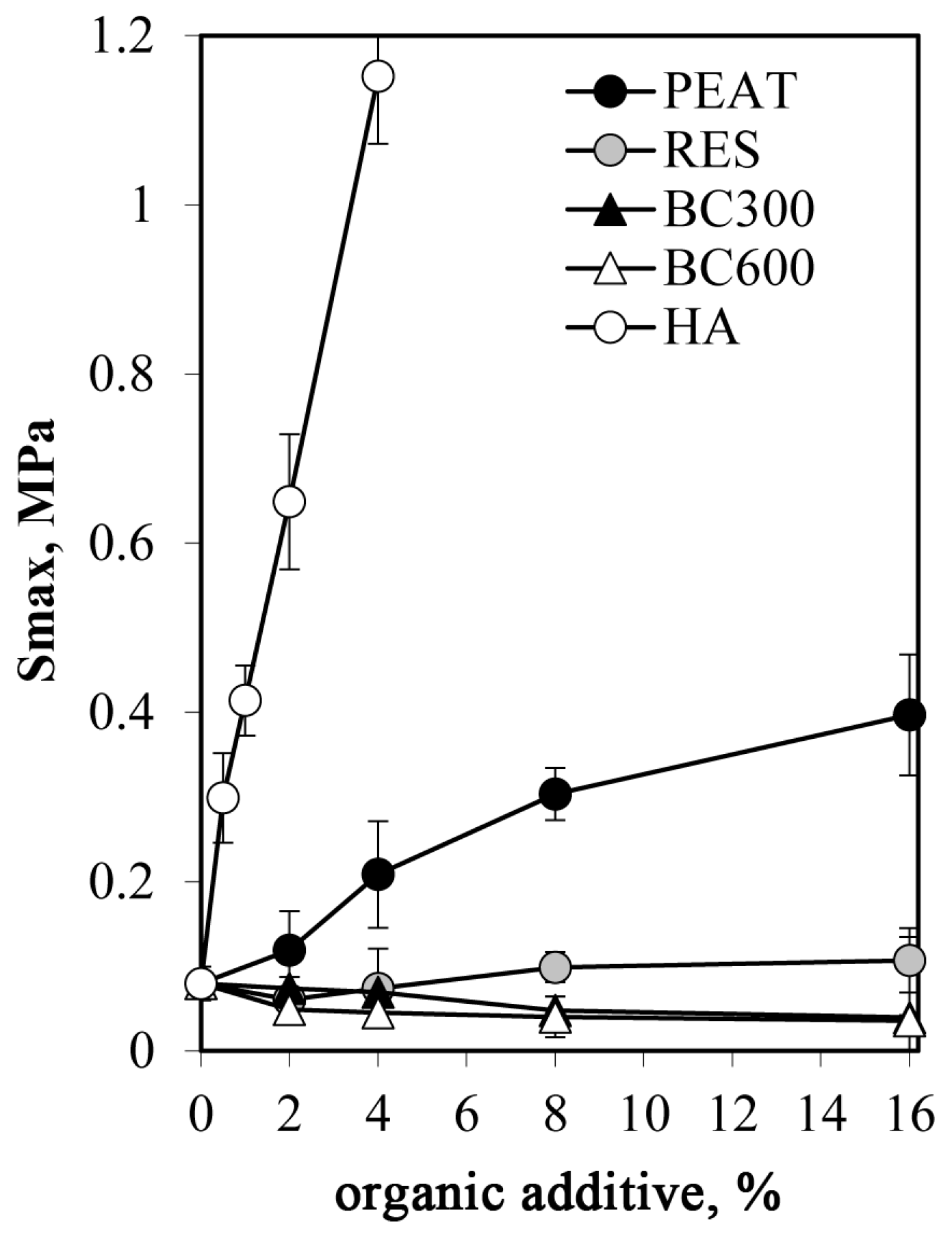
3.3. Strength of Aggregates
3.4. General Remarks and Implications
4. Conclusions
- Humic acid exerted an extremely high effect on the mechanical strength of silt aggregates. At similar concentrations, it increased the aggregate strength a few times more than the peat and over ten times more than the residue after HA extraction from the peat (RES).
- Despite similar particle sizes to PEAT and RES, biochars obtained from the peat after 300 and 600°C heating weakened the aggregates.
- Bulk density of the silt aggregates increased due to HA addition and decreased due to other amendments, whereas the total volume of pores behaved in the opposite direction.
- HA smoothed the surfaces of the pore system, which was indicated by a decrease in pore surface fractal dimension.
- The Langmuir-like curve was proposed to relate mechanical strength to the aggregate composition (a given organic additive content). A satisfactory model fit to the experimental data was reached.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costa, O.Y.A.; Raaijmakers, J.M.; Kuramae, E.E. Microbial Extracellular Polymeric Substances: Ecological Function and Impact on Soil Aggregation. Front. Microbiol. 2018, 9, 1636. [Google Scholar] [CrossRef] [PubMed]
- Szatanik-Kloc, A.; Horn, R.; Lipiec, J.; Siczek, A.; Boguta, P. Initial growth and root surface properties of dicotyledonous plants in structurally intact field soil and compacted headland soil. Soil Tillage Res. 2019, 195, 104387. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, M.; Tian, Y.; Shi, X.; Li, X.; Xu, L.; Xie, X.; Shi, Y.; Zhu, Y. Effects of porous clay ceramic rates on aeration porosity characteristics in a structurally degraded soil under greenhouse vegetable production. Pedosphere 2021, 31, 606–614. [Google Scholar] [CrossRef]
- Grave, R.A.; da Silveira Nicoloso, R.; Cassol, P.C.; Aita, C.; Corrêa, J.C.; Dalla Costa, M.; Fritz, D.D. Short-term carbon dioxide emission under contrasting soil disturbance levels and organic amendments. Soil Tillage Res. 2015, 146, 184–192. [Google Scholar] [CrossRef][Green Version]
- Yazdanpanah, N.; Mahmoodabadi, M.; Cerda, A. The impact of organic amendments on soil hydrology, structure and microbial respiration in semiarid lands. Geoderma 2016, 266, 58–65. [Google Scholar] [CrossRef]
- Munkholm, L.J.; Heck, R.J.; Deen, B.; Zidar, T. Relationship between soil aggregate strength, shape and porosity for soils under different long-term management. Geoderma 2016, 268, 52–59. [Google Scholar] [CrossRef]
- Yusof, N.Q.A.M.; Zabidi, H. Correlation of mineralogical and textural characteristics with engineering properties of granitic rock from Hulu Langat, Selangor. Procedia Chem. 2016, 19, 975–980. [Google Scholar] [CrossRef]
- Wei, S.; Tan, W.; Zhao, W.; Yu, Y.; Liu, F.; Koopal, L.K. Microstructure, interaction mechanisms, and stability of binary systems containing goethite and kaolinite. Soil Sci. Soc. Am. J. 2012, 76, 389–398. [Google Scholar] [CrossRef]
- Ghadr, S.; Assadi-Langroudi, A. Effect of grain size and shape on undrained behaviour of sands. Int. J. Geosynth. Ground Eng. 2019, 5, 18. [Google Scholar] [CrossRef]
- Li, D.; Wentong, Z.; Yunwu, X.; Jiaye, Z.; Quanzhong, H.; Xu, X.; Ping, R.; Guanhua, H. Impact of short-term organic amendments incorporation on soil structure and hydrology in semiarid agricultural lands. Int. Soil Water Conserv. Res. 2022, 10, 457–469. [Google Scholar] [CrossRef]
- Arthur, E.; Schjønning, P.; Moldrup, P.; de Jonge, L.W. Soil resistance and resilience to mechanical stresses for three differently managed sandy loam soils. Geoderma 2012, 173, 50–60. [Google Scholar] [CrossRef]
- Imbufe, A.U.; Patti, A.F.; Burrow, D.; Surapaneni, A.; Jackson, W.R.; Milner, A.D. Effects of potassium humate on aggregate stability of two soils from Victoria, Australia. Geoderma 2005, 125, 321–330. [Google Scholar] [CrossRef]
- Sarker, T.C.; Incerti, G.; Spaccini, R.; Piccolo, A.; Mazzoleni, S.; Bonanomi, G. Linking organic matter chemistry with soil aggregate stability: Insight from 13C NMR spectroscopy. Soil Biol. Biochem. 2018, 117, 175–184. [Google Scholar] [CrossRef]
- Han, M.; Wang, M.; Zhai, G.; Li, Y.; Yu, S.; Wang, E. Difference of soil aggregates composition, stability, and organic carbon content between eroded and depositional areas after adding exogenous organic materials. Sustainability 2022, 14, 2143. [Google Scholar] [CrossRef]
- Kitir, N.; Yildirim, E.; Şahin, Ü.; Turan, M.; Ekinci, M.; Ors, S.; Ünlü, H. Peat use in horticulture. In Peat; Topcuoglu, B., Turan, M., Eds.; IntechOpen: London, UK, 2018; pp. 75–90. [Google Scholar] [CrossRef]
- Cao, S. Research and application of peat in agriculture. IOP Conf. Ser. Earth Environ. Sci. 2019, 384, 012174. [Google Scholar] [CrossRef]
- Kazemian, S.; Huat, B.B.K.; Prasad, A.; Barghchi, M. A state of art review of peat: Geotechnical engineering perspective. Int. J. Phys. Sci. 2011, 6, 1974–1981. [Google Scholar] [CrossRef]
- Wang, L.; Sun, X.; Li, S.; Zhang, T.; Zhang, W.; Zhai, P. Application of organic amendments to a coastal saline soil in north China: Effects on soil physical and chemical properties and tree growth. PLoS ONE 2014, 9, e89185. [Google Scholar] [CrossRef]
- Kowalczyk-Jusko, A.; Onuch, J.; Koscik, B.; Skowron, P.; Cholody, M.; Kosidlo, A.; Rawski, J. Environmental and practical aspects of the use of peat for agriculture and energy aims. J. Ecol. Eng. 2016, 17, 138–142. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Prasad, M.; Kavanagh, A.; Tzortzakis, N. Biochar type and ratio as a peat additive/partial peat replacement in growing media for cabbage seedling production. Agronomy 2019, 9, 693. [Google Scholar] [CrossRef]
- Rex, P.; Mohammed Ismail, K.R.; Meenakshisundaram, N.; Barmavatu, P.; Sai Bharadwaj, A.V.S.L. Agricultural biomass waste to biochar: A review on biochar applications using machine learning approach and circular economy. Chem. Eng. 2023, 7, 50. [Google Scholar] [CrossRef]
- Xiao, Y.; Igalavithana, A.D.; Oh, S.-E.; Nam, H.; Zhang, M.; Wang, C.-H.; Kwon, E.E.; Tsang, D.C.W.; Ok, Y.S. Characterization of bioenergy biochar and its utilization for metal/metalloid immobilization in contaminated soil. Sci. Total Environ. 2018, 640–641, 704–713. [Google Scholar] [CrossRef]
- Juriga, M.; Simansky, V. Effect of biochar on soil structure—Review. Acta Fytotech. Zootech. 2018, 21, 11–19. [Google Scholar] [CrossRef]
- Sun, F.; Lu, S. Biochars improve aggregate stability, water retention, and pore-space properties of clayey soil. J. Plant. Nutr. Soil Sci. 2014, 177, 26–33. [Google Scholar] [CrossRef]
- Adekiya, A.O.; Agbede, T.M.; Olayanju, A.; Ejue, W.S.; Adekanye, T.A.; Adenusi, T.T.; Ayeni, J.F. Effect of biochar on soil properties, soil loss, and cocoyam yield on a tropical sandy loam alfisol. Sci. World J. 2020, 2020, 9391630. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, X.; Jing, Y.; Li, Q.; Zhang, J.; Huang, Q. Effects of biochar amendment on rapeseed and sweet potato yields and water stable aggregate in upland red soil. Catena 2014, 123, 45–51. [Google Scholar] [CrossRef]
- Zong, Y.; Chen, D.; Lu, S. Impact of biochars on swell-shrinkage behavior, mechanical strength, and surface cracking of clayey soil. J. Plant. Nutr. Soil Sci. 2014, 177, 920–926. [Google Scholar] [CrossRef]
- Hardie, M.; Clothier, B.; Bound, S.; Oliver, G.; Close, D. Does biochar influence soil physical properties and soil water availability? Plant Soil 2014, 376, 347–361. [Google Scholar] [CrossRef]
- Simansky, V.; Horak, J.; Igaz, D.; Jonczak, J.; Markiewicz, M.; Felber, R.; Rizhiya, E.Y.; Lukac, M. How dose of biochar and biochar with nitrogen can improve the parameters of soil organic matter and soil structure? Biologia 2016, 71, 989–995. [Google Scholar] [CrossRef]
- Yang, F.; Tang, C.; Antonietti, M. Natural and artificial humic substances to manage minerals, ions, water, and soil microorganisms. Chem. Soc. Rev. 2021, 50, 6221–6239. [Google Scholar] [CrossRef]
- Sodhi, G.P.S.; Beri, V.; Benbi, D.K. Soil aggregation and distribution of carbon and nitrogen in different fractions under long-term application of compost in rice-wheat system. Soil Tillage Res. 2009, 103, 412–418. [Google Scholar] [CrossRef]
- Zhou, L.; Monreal, C.M.; Xu, S.; McLaughlin, N.B.; Zhang, H.; Hao, G.; Liu, J. Effect of bentonite-humic acid application on the improvement of soil structure and maize yield in a sandy soil of a semi-arid region. Geoderma 2019, 338, 269–280. [Google Scholar] [CrossRef]
- Boechat, C.L.; Pistóia, V.C.; Ludtke, A.C.; Gianello, C.; Camargo, F.A.O. Solubility of heavy metals/metalloid on multi-metal contaminate soil samples from a gold ore processing area: Effects of humic substances. Rev. Bras. Cienc. Solo 2016, 40, 0150383. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lal, R.; Zimmerman, A.R. Impacts of 1.5-year field aging on biochar, humic acid, and water treatment residual amended soil. Soil Sci. 2014, 179, 333–339. [Google Scholar] [CrossRef]
- Tobiasova, E.; Barancikova, G.; Gomoryova, E.; Debska, B.; Banach-Szott, M. Humus substances and soil aggregates in the soils with different texture. Soil Water Res. 2018, 13, 44–50. [Google Scholar] [CrossRef]
- Boguta, P.; Sokolowska, Z. Statistical relationship between selected physicochemical properties of peaty-muck soils and their fraction of humic acids. Int. Agrophys. 2014, 28, 269–278. [Google Scholar] [CrossRef]
- Ampong, K.; Thilakaranthna, M.S.; Yuya, G.L. Understanding the role of humic acids on crop performance and soil health. Frontiers 2022, 4, 1–14. [Google Scholar] [CrossRef]
- Klucakova, M. Size and charge evaluation of standard humic and fulvic acids as crucial factors to determine their environmental behavior and impact. Front. Chem. 2018, 6, 235. [Google Scholar] [CrossRef]
- Angelico, R.; Colombo, C.; Di Iorio, E.; Brtnický, M.; Fojt, J.; Conte, P. Humic substances: From supramolecular aggregation to fractal conformation—Is there time for a new paradigm? Appl. Sci. 2023, 13, 2236. [Google Scholar] [CrossRef]
- Lipiec, J.; Turski, M.; Hajnos, M.; Swieboda, R. Pore structure, stability and water repellency of earthworm casts and natural aggregates in loess soil. Geoderma 2015, 243, 124–129. [Google Scholar] [CrossRef]
- Jouhara, H.; Ahmad, D.; Boogaert, I.; Katsou, E.; Simons, S.; Spencer, N. Pyrolysis of domestic based feedstock at temperatures up to 300 °C. Therm. Sci. Eng. Prog. 2018, 5, 117–143. [Google Scholar] [CrossRef]
- Ahmad, M.; Lee, S.S.; Dou, X.; Mohan, D.; Sung, J.K.; Yang, J.E.; Sik Ok, Y. Effects of pyrolysis temperature on soybean stover- and peanut shell-derived biochar properties and TCE adsorption in water. Bioresour. Technol. 2012, 118, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Boguta, P.; Sokołowska, Z.; Skic, K. Use of thermal analysis coupled with differential scanning calorimetry, quadrupole mass spectrometry and infrared spectroscopy (TG-DSC-QMS-FTIR) to monitor chemical properties and thermal stability of fulvic and humic acids. PLoS ONE 2017, 12, e0189653. [Google Scholar] [CrossRef] [PubMed]
- Moradi-Choghamarani, F.; Moosavi, A.A.; Baghernejad, M. Determining organo-chemical composition of sugarcane bagasse-derived biochar as a function of pyrolysis temperature using proximate and Fourier transform infrared analyses. J. Therm. Anal. Calorim. 2019, 138, 331–342. [Google Scholar] [CrossRef]
- Ryzak, M.; Bieganowski, A. Methodological aspects of determining soil particle-size distribution using the laser diffraction method. J. Plant. Nutr. Soil Sci. 2011, 174, 624–633. [Google Scholar] [CrossRef]
- Jozefaciuk, G.; Szatanik-Kloc, A.; Lukowska, M.; Szerement, J. Pitfalls and uncertainties of using potentiometric titration for estimation of plant roots surface charge and acid-base properties. Am. J. Plant Sci. 2014, 5, 1862–1876. [Google Scholar] [CrossRef]
- Jozefaciuk, G.; Skic, K.; Adamczuk, A.; Boguta, P.; Lamorski, K. Structure and strength of artificial soils containing monomineral clay fractions. Materials 2021, 14, 4688. [Google Scholar] [CrossRef]
- Bartoli, F.; Bird, N.R.A.; Gomendy, V.; Vivier, H.; Niquet, S. The relation between silty soil structures and their mercury porosimetry curve counterparts: Fractals and percolation. Eur. J. Soil Sci. 1999, 50, 9–22. [Google Scholar] [CrossRef]
- Jozefaciuk, G. Effect of the size of aggregates on pore characteristics of minerals measured by mercury intrusion and water-vapor desorption techniques. Clays Clay Miner. 2009, 57, 586–601. [Google Scholar] [CrossRef]
- Das, O.; Sarmah, A.K. The love–hate relationship of pyrolysis biochar and water: A perspective. Sci. Total Environ. 2015, 512, 682–685. [Google Scholar] [CrossRef]
- Omran, E.S.; Shorafa, M.; Zolfaghari, A.A.; Toolarood, A.A.S. The effect of biochar on severity of soil water repellency of crude oil-contaminated soil. Environ. Sci. Pollut. Res. 2020, 27, 6022–6032. [Google Scholar] [CrossRef]
- Liu, Z.; Dugan, B.; Masiello, C.A.; Barnes, R.T.; Gallagher, M.E.; Gonnermann, H. Impacts of biochar concentration and particle size on hydraulic conductivity and DOC leaching of biochar–sand mixtures. J. Hydrol. 2016, 533, 461–472. [Google Scholar] [CrossRef]
- Whittington, P.; Koiter, A.; Watts, D.; Brewer, A.; Golubev, V. Bulk density, particle density, and porosity of two species of Sphagnum: Variability in measurement techniques and spatial distribution. Soil Sci. Soc. Am. J. 2021, 85, 2220–2233. [Google Scholar] [CrossRef]
- Bartley, P.C., III; Amoozegar, A.; Fonteno, W.C.; Jackson, B.E. Particle densities of horticultural substrates. Hortic. Sci. 2022, 57, 379–383. [Google Scholar] [CrossRef]
- Jindo, K.; Mizumoto, H.; Sawada, Y.; Sanchez-Monedero, M.A.; Sonoki, T. Physical and chemical characterization of biochars derived from different agricultural residues. Biogeosciences 2014, 11, 6613–6621. [Google Scholar] [CrossRef]
- Boguta, P.; Sokołowska, Z.; Skic, K.; Tomczyk, A. Chemically engineered biochar—Effect of concentration and type of modifier on sorption and structural properties of biochar from wood waste. Fuel 2019, 256, 115893. [Google Scholar] [CrossRef]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of pyrolysis temperature and manure source on physicochemical characteristics of Biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Cen, K.; Zhuang, X.; Gan, Z.; Zhou, J.; Zhang, Y.; Zhang, H. Insight into biomass pyrolysis mechanism based on cellulose, hemicellulose, and lignin: Evolution of volatiles and kinetics, elucidation of reaction pathways, and characterization of gas, biochar and bio-oil. Combust. Flame 2022, 242, 112142. [Google Scholar] [CrossRef]
- Shoulaifar, T.K.; DeMartini, N.; Ivaska, A.; Fardim, P.; Hupa, M. Measuring the concentration of carboxylic acid groups in torrefied spruce wood. Bioresour. Technol. 2012, 123, 338–343. [Google Scholar] [CrossRef]
- Kelly, C.N.; Benjamin, J.; Calderon, F.C.; Mikha, M.M.; Rutherford, D.W.; Rostad, C.E. The incorporation of biochar carbon into stable soil aggregates: The role of clay mineralogy and other soil characteristics. Pedosphere 2017, 27, 694–704. [Google Scholar] [CrossRef]
- Munkholm, L.J. Soil friability: A review of the concept, assessment and effects of soil properties and management. Geoderma 2011, 167, 236–246. [Google Scholar] [CrossRef]
- Ajayi, A.E.; Horn, R. Modification of chemical and hydrophysical properties of two texturally differentiated soils due to varying magnitudes of added biochar. Soil Tillage Res. 2016, 164, 34–44. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lal, R. Biochar impacts on soil physical properties and greenhouse gas emissions. Agronomy 2013, 3, 313–339. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, K.; Sun, C.; Yang, K.; Zheng, J. Differences in soil physical properties caused by applying three organic amendments to loamy clay soil under field conditions. J. Soils Sediments 2022, 22, 43–55. [Google Scholar] [CrossRef]
- Tsadilas, C.D.; Mitsios, I.K.; Golia, E. Influence of biosolids application on some soil physical properties. Commun. Soil Sci. Plant Anal. 2005, 36, 709–716. [Google Scholar] [CrossRef]
- Benites, V.M.; Machado, L.O.A.; Fidalgo, C.C.; Coelho, M.R.; Madari, B.E. Pedotransfer functions for estimating soil bulk density from existing soil survey reports in Brazil. Geoderma 2007, 139, 90–97. [Google Scholar] [CrossRef]
- Celik, I.; Gunal, H.; Budak, M.; Akpinar, C. Effects of long-term organic and mineral fertilizers on bulk density and penetration resistance in semi-arid Mediterranean soil conditions. Geoderma 2010, 160, 236–243. [Google Scholar] [CrossRef]
- Brahim, N.; Bernoux, M.; Gallali, T. Pedotransfer functions to estimate soil bulk density for Northern Africa: Tunisia case. J. Arid Environ. 2012, 81, 77–83. [Google Scholar] [CrossRef]
- Bai, Y.; Tao, T.; Gu, C.; Wang, L.; Feng, K.; Shan, Y. Mudflat soil amendment by sewage sludge: Soil physicochemical properties, perennial ryegrass growth, and metal uptake. Soil Sci. Plant Nutr. 2013, 59, 942–952. [Google Scholar] [CrossRef]
- Toková, L.; Igaz, D.; Horák, J.; Aydın, E. Can application of biochar improve the soil water characteristics of silty loam soil? J. Soils Sediments 2023, 23, 2832–2847. [Google Scholar] [CrossRef]
- Kamali, M.; Sweygers, N.; Al-Salem, S.; Appels, L.; Aminabhavi, T.M.; Dewil, R. Biochar for soil applications-sustainability aspects, challenges and future prospects. Chem. Eng. 2022, 428, 131189. [Google Scholar] [CrossRef]
- Skic, K.; Sokołowska, Z.; Boguta, P.; Skic, A. The effect of application of digestate and agro-food industry sludges on dystric cambisol porosity. PLoS ONE 2020, 15, e0238469. [Google Scholar] [CrossRef] [PubMed]
- Dlapa, P.; Hrinik, D.; Hrabovsky, A.; Simkovic, I.; Zarnovican, H.; Sekucia, F.; Kollár, J. The impact of land-use on the hierarchical pore size loamy soils. Water 2020, 12, 339. [Google Scholar] [CrossRef]
- Li, Y.; Hong, C.; Wang, Z.; Xing, Y.; Yang, J.; Feng, L.; Hu, J.; Li, Z.; Zhang, Z.; Zhao, H.; et al. Fractal characteristics of biochars derived from Penicillin v potassium residue pyrolysis. J. Anal. Appl. Pyrolysis 2019, 141, 104636. [Google Scholar] [CrossRef]
- Franus, W.; Jozefaciuk, G.; Bandura, L.; Franus, M. Use of spent zeolite sorbents for the preparation of lightweight aggregates differing in microstructure. Minerals 2017, 7, 25. [Google Scholar] [CrossRef]
- Rezanezhad, F.; Quinton, W.; Price, J.; Elliot, T.; Elrick, D.; Shook, K. Influence of pore size and geometry on peat unsaturated hydraulic conductivity computed from 3D computed tomography image analysis. Hydrol. Process. 2010, 24, 2983–2994. [Google Scholar] [CrossRef]
- Sokołowska, Z. Fractal dimension of the peat-muck soils at different state of their secondary transformation. Acta Agroph. 2002, 68, 193–204. [Google Scholar]
- Sokołowska, Z.; Warchulska, P.; Sokołowski, S. Trends in soil fractal parameters caused by accumulation of soil organic matter as resulting from the analysis of water vapor adsorption isotherms. Ecol. Complex. 2009, 6, 254–262. [Google Scholar] [CrossRef]
- Lipetzky, P.; Wanner, A. On the problem of interpreting flexure experiments on semi-brittle materials. Mater. Sci. Eng. 1996, 211, 87–94. [Google Scholar] [CrossRef]
- Long, M. Review of peat strength, peat characterisation and constitutive modelling of peat with reference to landslides. Stud. Geotech. Mech. 2005, 27, 67–90. Available online: https://api.semanticscholar.org/CorpusID:54977320 (accessed on 21 November 2023).
- Boylan, N.; Long, M. Evaluation of peat strength for stability assessments. Geotech. Eng. 2012, 167, 421–430. [Google Scholar] [CrossRef]
- Julianto, F.E.; Harwadi, F.R. Characteristics of Palangkaraya fibrous peat. MATEC Web Conf. 2019, 276, 05008. [Google Scholar] [CrossRef]
- Assi, L.N.; Deaver, E.E.; Ziehl, P. Effect of source and particle size distribution on the mechanical and microstructural properties of fly ash-based geopolymer concrete. Constr. Build Mater. 2018, 167, 372–380. [Google Scholar] [CrossRef]
- Traven, K.; Cesnovar, M.; Ducman, V. Particle size manipulation as an influential parameter in the development of mechanical properties in electric arc furnace slag-based AAM. Ceram. Int. 2019, 45, 22632–22641. [Google Scholar] [CrossRef]
- Horvat, B.; Ducman, V. Influence of particle size on compressive strength of alkali activated refractory materials. Materials 2020, 13, 2227. [Google Scholar] [CrossRef] [PubMed]
- Gili, A.; Alonso, E.E. Microstructural deformation mechanisms of unsaturated granular soils. Int. J. Numer. Anal. Methods Geomech. 2002, 26, 433–468. [Google Scholar] [CrossRef]
- Abiven, S.; Menasseri, S.; Angers, D.A.; Leterme, P. Dynamics of aggregate stability and biological binding agents during decomposition of organic materials. Eur. J. Soil Sci. 2007, 58, 239–247. [Google Scholar] [CrossRef]
- Byun, Y.H.; Tran, K.; Yun, T.S.; Lee, J.S. Strength and stiffness characteristics of unsaturated hydrophobic granular media. Geotech. Test. J. 2011, 35, 193–200. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, H. Shear behavior of hydrophilic and hydrophobic unsaturated sands. Sci. Technol. Stud. 2019, 59, 961–966. [Google Scholar] [CrossRef]
- de Blas, E.; Rodriguez-Alleres, M.; Almendros, G. Speciation of lipid and humic fractions in soils under pine and eucalyptus forest in northwest Spain and its effect on water repellency. Geoderma 2010, 155, 242–248. [Google Scholar] [CrossRef]
- Kleber, M.; Eusterhues, K.; Keiluweit, M.; Mikutta, C.; Mikutta, R.; Nico, P.S. Mineral–organic associations: Formation, properties, and relevance in soil environments. In Advances in Agronomy, 2nd ed.; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 130, pp. 1–140. [Google Scholar] [CrossRef]
- Jozefaciuk, G.; Czachor, H. Impact of organic matter, iron oxides, alumina, silica and drying on mechanical and water stability of artificial soil aggregates. Assessment of new method to study water stability. Geoderma 2014, 221, 1–10. [Google Scholar] [CrossRef]
- Sokołowska, Z.; Szewczuk-Karpisz, K.; Turski, M.; Tomczyk, A.; Cybulak, M.; Skic, K. Effect of wood waste and sunflower husk biochar on tensile strength and porosity of dystric cambisol artificial aggregates. Agronomy 2020, 10, 244. [Google Scholar] [CrossRef]
- Chan, K.Y.; Zwieten, L.V.; Meszaros, I.; Downie, A.; Joseph, S. Agronomic values of greenwaste biochar as a soil amendment. Aust. J. Soil Res. 2007, 45, 629–634. [Google Scholar] [CrossRef]
- Busscher, W.J.; Novak, J.M.; Ahmedna, M. Physical effects of organic matter amendment of a southeastern us coastal loamy sand. Soil Sci. 2011, 176, 661–667. [Google Scholar] [CrossRef]
- Zong, Y.; Xiao, Q.; Lu, S. Acidity, water retention, and mechanical physical quality of a strongly acidic Ultisol amended with biochars derived from different feedstocks. J. Soils Sediments 2016, 16, 177–190. [Google Scholar] [CrossRef]
- Blanco-Canqui, H. Biochar and soil physical properties. Soil Sci. Soc. Am. J. 2017, 81, 687–711. [Google Scholar] [CrossRef]
- Joseph, S.D.; Camps-Arbestain, M.; Lin, Y.; Munroe, P.; Chia, C.H.; Hook, J.; van Zwieten, L.; Kimber, S.; Cowie, A.; Singh, B.P.; et al. An investigation into the reactions of biochar in soil. Aust. J. Soil Res. 2010, 48, 501–515. [Google Scholar] [CrossRef]
- Horabik, J.; Jozefaciuk, G. Structure and strength of kaolinite-soil silt aggregates: Measurements and modelling. Geoderma 2021, 382, 114687. [Google Scholar] [CrossRef]




| SILT | PEAT | HA | RES | BC300 | BC600 | |
|---|---|---|---|---|---|---|
| Average particle diameter; d [µm] | 3.70 | 0.57 | 0.06 | 0.29 | 0.24 | 0.23 |
| Particle density; SPD [g·cm−3] | 2.73 | 1.61 | 1.49 | 1.50 | 1.59 | 1.82 |
| PEAT | HA | RES | BC300 | BC600 | |
|---|---|---|---|---|---|
| Average particle diameter; d [µm] | 0.49 | 0.71 | 0.42 | 0.32 | 0.10 |
| Particle density; SPD [g·cm−3] | 6.47 | 6.18 | 6.98 | 7.15 | 7.52 |
| SILT | PEAT | HA 1 | RES | BC300 | BC600 | |
|---|---|---|---|---|---|---|
| Bulk density; BD [cm3·g−1] | 1.38 | 0.69 | n.d. | 0.65 | 0.82 | 0.85 |
| Total pore volume; Vt [cm3·g−1] | 0.32 | 0.50 | 0.51 | 0.73 | 0.75 | 0.60 |
| Average pore diameter; dav [µm] | 7.02 | 1.97 | 13.1 | 3.85 | 2.35 | 1.10 |
| SILT | PEAT | HA 1 | RES | BC300 | BC600 | |
|---|---|---|---|---|---|---|
| Intrinsic pore volume; Vint [cm3·g−1] | 0.18 | 0.23 | 0.03 | 0.38 | 0.44 | 0.37 |
| Average intrinsic pore diameter; dav,int [µm] | 1.33 | 0.86 | 0.01 | 1.07 | 0.97 | 0.77 |
| PEAT | HA | RES | BC300 | BC600 | |
|---|---|---|---|---|---|
| A, [MPa] | 0.592 | 3.887 | 0.386 | 0.085 | 0.045 |
| k | 0.063 | 0.090 | 0.004 | 0.051 | 0.955 |
| Smax of aggregate of pure substrate, [MPa] | 2.10 | n.d. | 0.940 | 0.071 | 0.026 |
| Initial Changes | Further Trends | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | PEAT | HA | RES | BC300 | BC600 | PEAT | HA | RES | BC300 | BC600 |
| Maximum strength | + | + | − | − | − | + | + | + | − | − |
| Bulk density | + | + | − | − | + | − | + | − | − | − |
| Total pore volume | − | − | − | − | + | + | − | + | + | + |
| Average pore diameter | + | + | + | + | + | − | − | − | − | − |
| Intrinsic pore volume | − | − | − | + | + | + | − | + | + | − |
| Intrinsic pore diameter | − | − | + | + | + | + | − | + | − | − |
| Fractal dimension | − | − | − | − | − | − | + | − | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skic, K.; Adamczuk, A.; Boguta, P.; Gryta, A.; Masoudi Soltani, S.; Ignatova, S.; Józefaciuk, G. New Insight into Organomineral Interactions in Soils. The Impact of Clay-Size Peat-Derived Organic Species on the Structure and the Strength of Soil Silt Aggregates. Agriculture 2023, 13, 2241. https://doi.org/10.3390/agriculture13122241
Skic K, Adamczuk A, Boguta P, Gryta A, Masoudi Soltani S, Ignatova S, Józefaciuk G. New Insight into Organomineral Interactions in Soils. The Impact of Clay-Size Peat-Derived Organic Species on the Structure and the Strength of Soil Silt Aggregates. Agriculture. 2023; 13(12):2241. https://doi.org/10.3390/agriculture13122241
Chicago/Turabian StyleSkic, Kamil, Agnieszka Adamczuk, Patrycja Boguta, Angelika Gryta, Salman Masoudi Soltani, Svetlana Ignatova, and Grzegorz Józefaciuk. 2023. "New Insight into Organomineral Interactions in Soils. The Impact of Clay-Size Peat-Derived Organic Species on the Structure and the Strength of Soil Silt Aggregates" Agriculture 13, no. 12: 2241. https://doi.org/10.3390/agriculture13122241
APA StyleSkic, K., Adamczuk, A., Boguta, P., Gryta, A., Masoudi Soltani, S., Ignatova, S., & Józefaciuk, G. (2023). New Insight into Organomineral Interactions in Soils. The Impact of Clay-Size Peat-Derived Organic Species on the Structure and the Strength of Soil Silt Aggregates. Agriculture, 13(12), 2241. https://doi.org/10.3390/agriculture13122241