Organic Amendment for Disinfecting Soil Alters the Metabolites in Spinacia oleracea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Experiments and Sampling
2.2. Determining of Nutrients/Analytical Techniques Used for Spinach Characterisation
2.3. Determination of Free Radical Scavenging and Natural Antioxidants
2.4. Analysis of Flavonoids
2.5. Non-Target Analysis Using High-Resolution Accurate Mass Spectrometry (HRAMS)
Analysis and Data Processing
2.6. Statistical Analysis
3. Results
3.1. Mineral Elements in Spinach Leaves
3.2. Antioxidant Capacity, Total Flavonoids, and Phenol Contents in Spinach Leaves
3.3. Total Contents of Carotenoids, Chlorophyll A, and Chlorophyll B in Spinach Leaves
3.4. Glycosilated Flavonoid Identification and Quantitation in Spinach Leaves
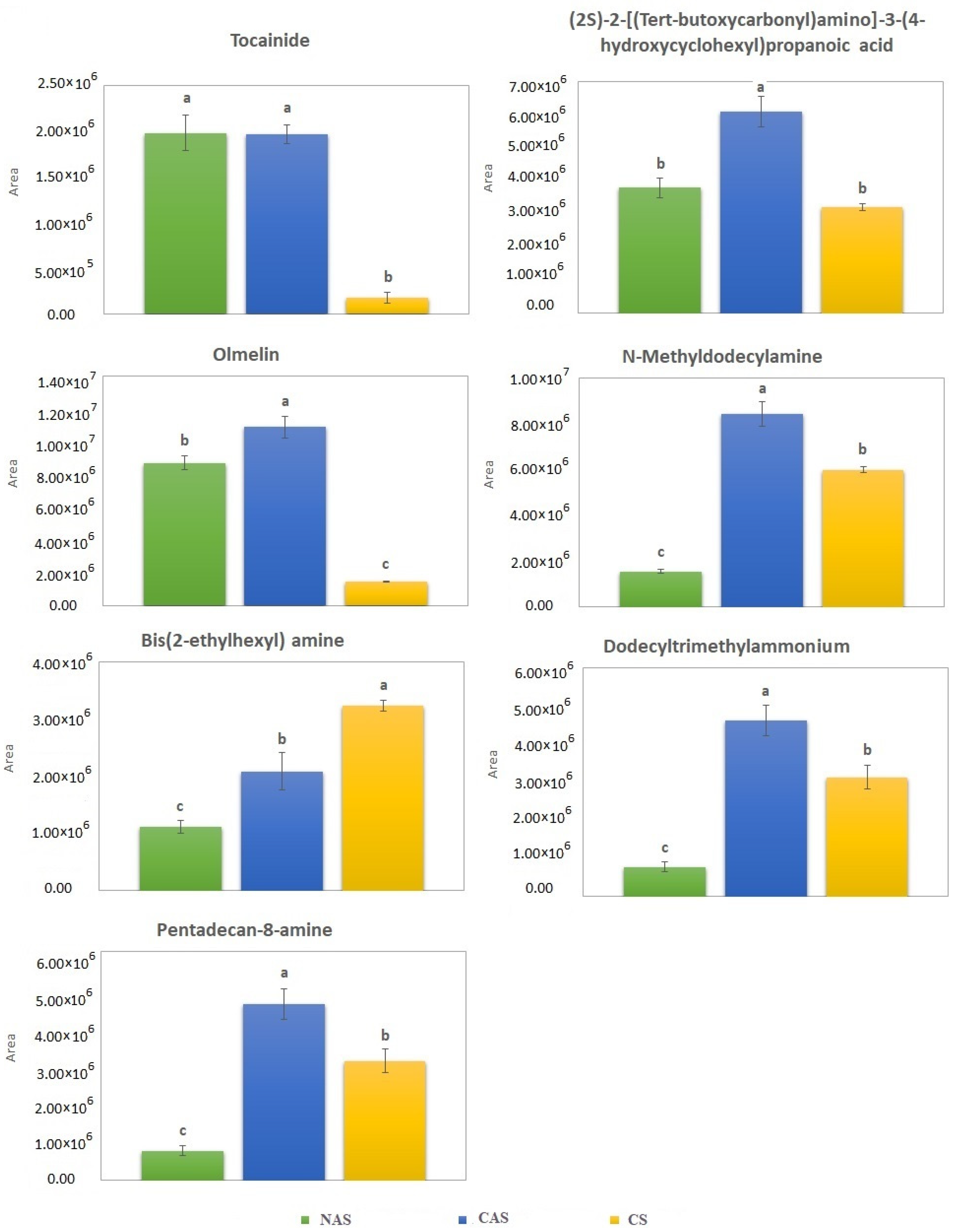
3.5. Tentative Spinach Leaf Marker Identification Using HRAMS
4. Discussion
4.1. Effects of Radiation and Compost Disinfection on Spinach Quality
4.2. Free Radical Scavenging Capacity and Natural Antioxidant Tests
4.3. Glycosylated Flavonoid Identification in Spinach Leaves
4.4. Non-Target Metabolite Profiles in Spinach Leaves
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gisi, U.; Chet, I.; Gullino, M.L. Recent Developments in Management of Plant Diseases; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Achmon, Y.; Claypool, J.T.; Fernández-Bayo, J.D.; Hernandez, K.; McCurry, D.G.; Harrold, D.R.; Su, J.; Simmons, B.A.; Singer, S.W.; Dahlquist-Willard, R.M. Structural Changes in Bacterial and Fungal Soil Microbiome Components during Biosolarization as Related to Volatile Fatty Acid Accumulation. Appl. Soil Ecol. 2020, 153, 103602. [Google Scholar] [CrossRef]
- Gelsomino, A.; Badalucco, L.; Landi, L.; Cacco, G. Soil Carbon, Nitrogen and Phosphorus Dynamics as Affected by Solarization Alone or Combined with Organic Amendment. Plant Soil 2006, 279, 307–325. [Google Scholar] [CrossRef]
- Bonanomi, G.; Lorito, M.; Vinale, F.; Woo, S.L. Organic Amendments, Beneficial Microbes, and Soil Microbiota: Toward a Unified Framework for Disease Suppression. Annu. Rev. Phytopathol. 2018, 56, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Tamura, Y.; Mori, T.; Nakabayashi, R.; Kobayashi, M.; Saito, K.; Okazaki, S.; Wang, N.; Kusano, M. Metabolomic Evaluation of the Quality of Leaf Lettuce Grown in Practical Plant Factory to Capture Metabolite Signature. Front. Plant Sci. 2018, 9, 665. [Google Scholar] [CrossRef] [PubMed]
- Nemzer, B.; Al-Taher, F.; Abshiru, N. Extraction and Natural Bioactive Molecules Characterization in Spinach, Kale and Purslane: A Comparative Study. Molecules 2021, 26, 2515. [Google Scholar] [CrossRef] [PubMed]
- Mou, B. Evaluation of Oxalate Concentration in the US Spinach Germplasm Collection. HortScience 2008, 43, 1690–1693. [Google Scholar] [CrossRef]
- Santamaria, P. Nitrate in Vegetables: Toxicity, Content, Intake and EC Regulation. J. Sci. Food Agric. 2006, 86, 10–17. [Google Scholar] [CrossRef]
- Giménez, A.; Egea-Gilabert, C.; Pascual, J.A.; Ros, M.; Fernández, J.A. Effect of the Application of a Compost Tea in the Production of Baby Leaf Lettuce in a Floating System. In Proceedings of the XI International Symposium on Protected Cultivation in Mild Winter Climates and I International Symposium on Nettings and 1268, Tenerife, Spain, 27–31 January 2019; pp. 173–178. [Google Scholar]
- Bunea, A.; Andjelkovic, M.; Socaciu, C.; Bobis, O.; Neacsu, M.; Verhé, R.; Van Camp, J. Total and Individual Carotenoids and Phenolic Acids Content in Fresh, Refrigerated and Processed Spinach (Spinacia oleracea L.). Food Chem. 2008, 108, 649–656. [Google Scholar] [CrossRef]
- Pandjaitan, N.; Howard, L.R.; Morelock, T.; Gil, M.I. Antioxidant Capacity and Phenolic Content of Spinach as Affected by Genetics and Maturation. J. Agric. Food Chem. 2005, 53, 8618–8623. [Google Scholar] [CrossRef]
- Li, H.-B.; Wong, C.-C.; Cheng, K.-W.; Chen, F. Antioxidant Properties in Vitro and Total Phenolic Contents in Methanol Extracts from Medicinal Plants. LWT-Food Sci. Technol. 2008, 41, 385–390. [Google Scholar] [CrossRef]
- Bergquist, S. Bioactive Compounds in Baby Spinach (Spinacia oleracea L.). Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2006. [Google Scholar]
- Iqbal, N.; Tanzeem-ul-Haq, H.S.; Turan, V.; Iqbal, M. Soil Amendments and Foliar Melatonin Reduced Pb Uptake, and Oxidative Stress, and Improved Spinach Quality in Pb-Contaminated Soil. Plants 2023, 12, 1829. [Google Scholar] [CrossRef] [PubMed]
- Ali, U.; Shaaban, M.; Bashir, S.; Chhajro, M.A.; Qian, L.; Rizwan, M.S.; Fu, Q.; Zhu, J.; Hu, H. Potential of Organic and Inorganic Amendments for Stabilizing Nickel in Acidic Soil, and Improving the Nutritional Quality of Spinach. Environ. Sci. Pollut. Res. 2021, 28, 57769–57780. [Google Scholar] [CrossRef] [PubMed]
- Pohan, S.D. The Effect of Organic Fertilizers on Growth and Yield of Water Spinach (Ipomoea Reptans Poir). JERAMI Indones. J. Crop Sci. 2021, 3, 37–44. [Google Scholar] [CrossRef]
- Xu, C.; Mou, B. Vermicompost Affects Soil Properties and Spinach Growth, Physiology, and Nutritional Value. HortScience 2016, 51, 847–855. [Google Scholar] [CrossRef]
- Lara, L.J.; Egea-Gilabert, C.; Niñirola, D.; Conesa, E.; Fernández, J.A. Effect of Aeration of the Nutrient Solution on the Growth and Quality of Purslane (Portulaca oleracea). J. Hortic. Sci. Biotechnol. 2011, 86, 603–610. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Pérez-Tortosa, V.; López-Orenes, A.; Martínez-Pérez, A.; Ferrer, M.A.; Calderón, A.A. Antioxidant Activity and Rosmarinic Acid Changes in Salicylic Acid-Treated Thymus Membranaceus Shoots. Food Chem. 2012, 130, 362–369. [Google Scholar] [CrossRef]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the Total Phenolic, Flavonoid and Proline Contents in Burkina Fasan Honey, as Well as Their Radical Scavenging Activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Martínez-Hernández, G.B.; Gómez, P.A.; Pradas, I.; Artés, F.; Artés-Hernández, F. Moderate UV-C Pretreatment as a Quality Enhancement Tool in Fresh-Cut Bimi® Broccoli. Postharvest Biol. Technol. 2011, 62, 327–337. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Noperi-Mosqueda, L.C.; López-Moreno, F.J.; Navarro-León, E.; Sánchez, E.; Blasco, B.; Moreno, D.A.; Soriano, T.; Ruiz, J.M. Effects of Asparagus Decline on Nutrients and Phenolic Compounds, Spear Quality, and Allelopathy. Sci. Hortic. 2020, 261, 109029. [Google Scholar] [CrossRef]
- Bueno, M.J.M.; Díaz-Galiano, F.J.; Rajski, Ł.; Cutillas, V.; Fernández-Alba, A.R. A Non-Targeted Metabolomic Approach to Identify Food Markers to Support Discrimination between Organic and Conventional Tomato Crops. J. Chromatogr. A 2018, 1546, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Lara, A.; Ros, M.; Cuartero, J.; Vivo, J.-M.; Lozano-Pastor, P.; Pascual, J.A. Effects of Solarisation Combined with Compost on Soil Pathogens and the Microbial Community in a Spinach Cropping System. Agric. Ecosyst. Environ. 2023, 346, 108359. [Google Scholar] [CrossRef]
- El-Nakhel, C.; Pannico, A.; Graziani, G.; Kyriacou, M.C.; Giordano, M.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Variation in Macronutrient Content, Phytochemical Constitution and in Vitro Antioxidant Capacity of Green and Red Butterhead Lettuce Dictated by Different Developmental Stages of Harvest Maturity. Antioxidants 2020, 9, 300. [Google Scholar] [CrossRef]
- Stagnari, F.; Di Bitetto, V.; Pisante, M. Effects of N Fertilizers and Rates on Yield, Safety and Nutrients in Processing Spinach Genotypes. Sci. Hortic. 2007, 114, 225–233. [Google Scholar] [CrossRef]
- Libutti, A.; Trotta, V.; Rivelli, A.R. Biochar, Vermicompost, and Compost as Soil Organic Amendments: Influence on Growth Parameters, Nitrate and Chlorophyll Content of Swiss Chard (Beta vulgaris L. var. Cycla). Agronomy 2020, 10, 346. [Google Scholar] [CrossRef]
- Yamazaki, A.; Watanabe, T.; Tsunogai, U.; Iwase, F.; Yamano, H. A 150-year Variation of the Kuroshio Transport Inferred from Coral Nitrogen Isotope Signature. Paleoceanography 2016, 31, 838–846. [Google Scholar] [CrossRef]
- Roberts, J.L.; Moreau, R. Functional Properties of Spinach (Spinacia oleracea L.) Phytochemicals and Bioactives. Food Funct. 2016, 7, 3337–3353. [Google Scholar] [CrossRef]
- Ghoora, M.D.; Babu, D.R.; Srividya, N. Nutrient Composition, Oxalate Content and Nutritional Ranking of Ten Culinary Microgreens. J. Food Compos. Anal. 2020, 91, 103495. [Google Scholar] [CrossRef]
- López-Moreno, M.; Garcés-Rimón, M.; Miguel, M. Antinutrients: Lectins, Goitrogens, Phytates and Oxalates, Friends or Foe? J. Funct. Foods 2022, 89, 104938. [Google Scholar] [CrossRef]
- Giménez, A.; Gómez, P.A.; Bustamante, M.Á.; Pérez-Murcia, M.D.; Martínez-Sabater, E.; Ros, M.; Pascual, J.A.; Egea-Gilabert, C.; Fernández, J.A. Effect of Compost Extract Addition to Different Types of Fertilizers on Quality at Harvest and Shelf Life of Spinach. Agronomy 2021, 11, 632. [Google Scholar] [CrossRef]
- Wang, X.; Cai, X.; Xu, C.; Zhao, Q.; Ge, C.; Dai, S.; Wang, Q. Diversity of Nitrate, Oxalate, Vitamin C and Carotenoid Contents in Different Spinach Accessions and Their Correlation with Various Morphological Traits. J. Hortic. Sci. Biotechnol. 2018, 93, 409–415. [Google Scholar] [CrossRef]
- Bottino, A.; Degl’Innocenti, E.; Guidi, L.; Graziani, G.; Fogliano, V. Bioactive Compounds during Storage of Fresh-Cut Spinach: The Role of Endogenous Ascorbic Acid in the Improvement of Product Quality. J. Agric. Food Chem. 2009, 57, 2925–2931. [Google Scholar] [CrossRef] [PubMed]
- Serri, F.; Souri, M.K.; Rezapanah, M. Growth, Biochemical Quality and Antioxidant Capacity of Coriander Leaves under Organic and Inorganic Fertilization Programs. Chem. Biol. Technol. Agric. 2021, 8, 33. [Google Scholar] [CrossRef]
- Conant, R.T.; Ryan, M.G.; Ågren, G.I.; Birge, H.E.; Davidson, E.A.; Eliasson, P.E.; Evans, S.E.; Frey, S.D.; Giardina, C.P.; Hopkins, F.M. Temperature and Soil Organic Matter Decomposition Rates–Synthesis of Current Knowledge and a Way Forward. Glob. Chang. Biol. 2011, 17, 3392–3404. [Google Scholar] [CrossRef]
- Ros, M.; Hurtado-Navarro, M.; Giménez, A.; Fernández, J.A.; Egea-Gilabert, C.; Lozano-Pastor, P.; Pascual, J.A. Spraying Agro-Industrial Compost Tea on Baby Spinach Crops: Evaluation of Yield, Plant Quality and Soil Health in Field Experiments. Agronomy 2020, 10, 440. [Google Scholar] [CrossRef]
- Lakhdar, A.; Falleh, H.; Ouni, Y.; Oueslati, S.; Debez, A.; Ksouri, R.; Abdelly, C. Municipal Solid Waste Compost Application Improves Productivity, Polyphenol Content, and Antioxidant Capacity of Mesembryanthemum Edule. J. Hazard. Mater. 2011, 191, 373–379. [Google Scholar] [CrossRef]
- Bergquist, S.Å.M.; Gertsson, U.E.; Knuthsen, P.; Olsson, M.E. Flavonoids in Baby Spinach (Spinacia oleracea L.): Changes during Plant Growth and Storage. J. Agric. Food Chem. 2005, 53, 9459–9464. [Google Scholar] [CrossRef]
- Cho, M.J.; Howard, L.R.; Prior, R.L.; Morelock, T. Flavonoid Content and Antioxidant Capacity of Spinach Genotypes Determined by High-performance Liquid Chromatography/Mass Spectrometry. J. Sci. Food Agric. 2008, 88, 1099–1106. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and Bioefficacy of Polyphenols in Humans. I. Review of 97 Bioavailability Studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Rive-Evans, C.A.; Miller, N.J. Structure-Antioxidant Activity Relationships of Flavonoids and phenolic acids. Free. Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, K.; Oka, N.; Shinano, T.; Osaki, M.; Takebe, M. Differences in the Metabolite Profiles of Spinach (Spinacia oleracea L.) Leaf in Different Concentrations of Nitrate in the Culture Solution. Plant Cell Physiol. 2008, 49, 170–177. [Google Scholar] [CrossRef] [PubMed]
- More, A.S.; Ranadheera, C.S.; Fang, Z.; Zhang, P.; Warner, R.; Ajlouni, S. Using Biological Metabolites as Biomarkers to Predict Safety and Quality of Whole and Minimally Processed Spinach. Food Chem. 2022, 375, 131870. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, X.; Wang, L.; Duan, Q.; Huang, D. Comparative Analysis of Metabolites Profile in Spinach (Spinacia oleracea L.) Affected by Different Concentrations of Gly and Nitrate. Sci. Hortic. 2016, 204, 8–15. [Google Scholar] [CrossRef]
- Kim, B.; Lee, H.; Song, Y.H.; Kim, H. Effect of Salt Stress on the Growth, Mineral Contents, and Metabolite Profiles of Spinach. J. Sci. Food Agric. 2021, 101, 3787–3794. [Google Scholar] [CrossRef]
- Martin, S.E.; Nguyen, C.M.; Basaraba, R.J.; Melander, C. Analogue Synthesis Reveals Decoupling of Antibiofilm and Β-lactam Potentiation Activities of a Lead 2-aminoimidazole Adjuvant against Mycobacterium Smegmatis. Chem. Biol. Drug Des. 2018, 92, 1403–1408. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Qiu, P.; Zhang, X.; Zhang, Y.; Mi, L.; Peng, C.; Pan, X.; Peng, F. Biochanin A from Chinese Medicine: An Isoflavone with Diverse Pharmacological Properties. Am. J. Chin. Med. 2021, 49, 1623–1643. [Google Scholar] [CrossRef]
- Alarcón-Flores, M.I.; Romero-González, R.; Martínez Vidal, J.L.; Garrido Frenich, A. Determination of Phenolic Compounds in Artichoke, Garlic and Spinach by Ultra-High-Performance Liquid Chromatography Coupled to Tandem Mass Spectrometry. Food Anal. Methods 2014, 7, 2095–2106. [Google Scholar] [CrossRef]
- Mirgorodskaya, A.B.; Kushnazarova, R.A.; Lukashenko, S.S.; Voloshina, A.D.; Lenina, O.A.; Zakharova, L.Y.; Sinyashin, O.G. Carbamate-Bearing Surfactants: Micellization, Solubilization, and Biological Activity. J. Mol. Liq. 2018, 269, 203–210. [Google Scholar] [CrossRef]
- Mirgorodskaya, A.B.; Kushnazarova, R.A.; Lukashenko, S.S.; Zakharova, L.Y. Self-Assembly of Mixed Systems Based on Nonionic and Carbamate-Bearing Cationic Surfactants as a Tool for Fabrication of Biocompatible Nanocontainers. J. Mol. Liq. 2019, 292, 111407. [Google Scholar] [CrossRef]
- Mirgorodskaya, A.B.; Kushnazarova, R.A.; Lukashenko, S.S.; Nikitin, E.N.; Sinyashin, K.O.; Nesterova, L.M.; Zakharova, L.Y. Carbamate-Bearing Surfactants as Effective Adjuvants Promoted the Penetration of the Herbicide into the Plant. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124252. [Google Scholar] [CrossRef]
- Rangel-Huerta, O.D.; Ivanova, L.; Uhlig, S.; Sivertsvik, M.; Sone, I.; Fernández, E.N.; Fæste, C.K. Impact of Plasma-Activated Water Treatment on Quality and Shelf-Life of Fresh Spinach Leaves Evaluated by Comprehensive Metabolomic Analysis. Foods 2021, 10, 3067. [Google Scholar] [CrossRef] [PubMed]
- Bektašević, M.; Politeo, O.; Roje, M.; Jurin, M. Polyphenol Composition, Anticholinesterase and Antioxidant Potential of the Extracts of Clinopodium vulgare L. Chem. Biodivers. 2022, 19, e202101002. [Google Scholar] [CrossRef] [PubMed]
- Mitharwal, S.; Kumar, A.; Chauhan, K.; Taneja, N.K. Nutritional, Phytochemical Composition and Potential Health Benefits of Taro (Colocasia esculenta L.) Leaves: A Review. Food Chem. 2022, 383, 132406. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.; Srivastava, V.; Singh, A. Multipurpose Benefits of an Underexplored Species Purslane (Portulaca oleracea L.): A Critical Review. Environ. Manag. 2023, 72, 309–320. [Google Scholar] [CrossRef]
- Zhou, B.; Chen, Y.; Zeng, L.; Cui, Y.; Li, J.; Tang, H.; Liu, J.; Tang, J. Soil Nutrient Deficiency Decreases the Postharvest Quality-Related Metabolite Contents of Tea (Camellia sinensis (L.) Kuntze) Leaves. Food Chem. 2022, 377, 132003. [Google Scholar] [CrossRef]


| CS | NAS | CAS | p-Value | |
|---|---|---|---|---|
| Total N (g kg −1) | 48.81 ± 0.08 | 48.21 ± 0.46 | 51.61 ± 3.09 | NS |
| TOC (g kg −1) | 331.47 ± 3.04 | 329.43 ± 0.32 | 329.90 ± 10.66 | NS |
| NO3− (mg kg −1 FW) | 228.35 ± 30.64 a | 101.77 ± 8.90 c | 161.22 ± 18.88 b | *** |
| NO2− (mg kg −1 FW) | 114.39 ± 15.61 a | 49.99 ± 13.33 c | 65.74 ± 3.29 b | *** |
| NH4+ (mg kg −1 FW) | 4.27 ± 0.1 | 3.99 ± 0.22 | 4.11 ± 0.42 | NS |
| C2O42− (mg kg −1 FW) | 3328 ± 207 | 3331 ± 139 | 3494 ± 379 | NS |
| SO42− (mg kg −1 FW) | 219 ± 25 a | 134 ± 8 c | 179 ± 20 b | *** |
| PO42− (mg kg −1 FW) | 623 ± 34 | 567 ± 60 | 602 ± 82 | NS |
| Na+ (mg kg −1 FW) | 332 ± 26 a | 248 ± 49 b | 220 ± 35 b | *** |
| K+ (mg kg −1 FW) | 5114 ± 667 | 4524 ± 188 | 5102 ± 740 | NS |
| Ca2+ (mg kg −1 FW) | 121 ± 6 a | 111 ± 13 ab | 107 ± 13 b | * |
| Mg2+ (mg kg −1 FW) | 494 ± 21 a | 422 ± 24 b | 441 ± 63 b | ** |
| CS | NAS | CAS | p-Value | |
|---|---|---|---|---|
| Antioxidant capacity (mg DPPH Kg −1 FW) | 51.21 ± 6.32 | 61.31 ± 4.57 | 58.12 ± 3.39 | NS |
| Total Flavonoids (mg Rutin kg −1 FW) | 4114 ± 97 | 4095 ± 195 | 3865 ± 128 | NS |
| Total Phenols (mg GA kg −1 FW) | 856 ± 53 b | 974 ± 48 a | 932 ± 30 ab | * |
| Carotenoids Total (mg kg −1 FW) | 185 ± 9 | 185 ± 12 | 177 ± 3 | NS |
| Chlorophyll a (mg kg −1 FW) | 548 ± 18 | 557 ± 27 | 509 ± 7 | NS |
| Chlorophyll b (mg kg −1 FW) | 77.13 ± 2.65 b | 89.83 ± 5.12 a | 91.45 ± 7.74 a | * |
| CS | NAS | CAS | p-Value | |
|---|---|---|---|---|
| Patuletin 3-glucosyl-(1-6)[apiosyl(1-2)]glucoside | 0.445 ± 0.006 b | 0.530 ± 0.016 a | 0.489 ± 0.018 a | *** |
| Patuletin 3-O-gentiobioside | 0.214 ± 0.003 c | 0.243 ± 0.008 a | 0.223 ± 0.009 b | *** |
| Spinacetin-3-glucosyl-(1-6)[apiosyl(1-2)-glucoside] | 0.325 ± 0.006 b | 0.394 ± 0.014 a | 0.382 ± 0.015 a | *** |
| Spinacetin 3-O-gentiobioside | 0.530 ± 0.012 b | 0.646 ± 0.022 a | 0.612 ± 0.024 a | *** |
| Spinacetin glucuronide (Spinatoside) | 0.887 ± 0.064 b | 1.065 ± 0.029 a | 1.006 ± 0.043 a | ** |
| Jaceidin glucuronide | 0.281 ± 0.007 c | 0.350 ± 0.012 a | 0.324 ± 0.013 b | *** |
| 5,3′,4′-trihydroxy-3-methoxy-6:7-methylenedioxyflavone-4′-glucuronide | 0.476 ± 0.010 c | 0.580 ± 0.017 a | 0.526 ± 0.024 b | *** |
| 5,4′-dihydroxy-3,3′-dimethoxy-6:7-methylenedioxyflavone-4′-glucuronide | 0.127 ± 0.011 c | 0.172 ± 0.006 a | 0.152 ± 0.007 b | *** |
| tR | Precursor Ion | Fragment Ions | |||||
|---|---|---|---|---|---|---|---|
| Accurate Mass Value (m/z) | Proposed Formula | Mass Deviation a (ppm) | Accurate Mass Value (m/z) | Proposed Formula | Mass Deviation a (ppm) | Tentatively Identified Compounds b | |
| 1.7 | 164.0473 | C9 H8 O3 | −0.2 | 51.0229 | C4H3 | −1.4 | (E)-p-coumaric acid (Phenolic acid) |
| 63.0230 | C5H3 | 0.9 | |||||
| 65.0386 | C5H5 | 0.4 | |||||
| 77.0386 | C6H5 | −0.2 | |||||
| 91.0541 | C7H7 | −1.4 | |||||
| 95.0490 | C6H7O | −1.5 | |||||
| 103.0541 | C8H7 | −1.4 | |||||
| 107.0491 | C7H7O | −0.7 | |||||
| 109.0648 | C7H9O | 0.5 | |||||
| 123.0440 | C7H7O2 | −0.4 | |||||
| 147.0441 | C9H7O2 | 0.1 | |||||
| 3.2 | 317.2547 | C15 H33 N4 O3 | 0.2 | 58.0651 | C3H8N | −0.2 | 2-Methyl-2-propanyl (2-aminoethyl) {[3-(diethylamino) propyl] carbamoyl} carbamate (Other compounds) |
| 74.0236 | C2H4O2N | −0.4 | |||||
| 86.0963 | C5H12N | −1.3 | |||||
| 102.0549 | C4H8O2N | −0.6 | |||||
| 113.0711 | C5H9ON2 | 1.3 | |||||
| 129.1022 | C6H13ON2 | 0.4 | |||||
| 144.1022 | C7H14O2N | 1.8 | |||||
| 189.1709 | C9H21O2N2 | 0.9 | |||||
| 3.3 | 165.0789 | C9 H11 N O2 | −0.3 | 51.0229 | C4H3 | −0.7 | L-Phenylalanine (Amino acid) |
| 65.0386 | C5H5 | 0.4 | |||||
| 77.0385 | C6H5 | −0.8 | |||||
| 79.0543 | C6H7 | 0.5 | |||||
| 91.0541 | C7H7 | −1.1 | |||||
| 95.0490 | C6H7O | −1.9 | |||||
| 102.0463 | C8H6 | −1.1 | |||||
| 103.0541 | C8H7 | −1.2 | |||||
| 120.0807 | C8H10N | −0.7 | |||||
| 131.0491 | C9H7O | −0.3 | |||||
| 3.5 | 203.1754 | C10 H23 N2 O2 | −0.1 | 58.0651 | C3H8N | −0.8 | 1,1-Dimethylethyl N-(4-aminobutyl)-N-methylcarbamate (Other compounds) |
| 74.0236 | C2H4O2N | −0.4 | |||||
| 102.0548 | C4H8O2N | −1.5 | |||||
| 144.1019 | C7H14O2N | −0.2 | |||||
| 203.1753 | C10H23O2N2 | −0.5 | |||||
| 3.7 | 219.1105 | C9 H17 N O5 | −0.4 | 56.0130 | C2H2ON | −1.7 | D-Pantothenic acid (Vitamin) |
| 59.0490 | C3H7O | −2.3 | |||||
| 70.0287 | C3H4ON | −0.9 | |||||
| 85.0647 | C5H9O | −1.1 | |||||
| 90.0547 | C3H8O2N | −2.3 | |||||
| 95.0490 | C6H7O | −1.3 | |||||
| 98.0235 | C4H4O2N | −1.9 | |||||
| 116.0338 | C4H6O3N | −3.3 | |||||
| 142.0861 | C7H12O2N | −0.9 | |||||
| 166.0862 | C9H12O2N | −0.6 | |||||
| 184.0967 | C9H14O3N | −0.4 | |||||
| 202.1075 | C9H16O4N | 0.7 | |||||
| 3.9 | 204.0898 | C11 H12 N2 O2 | −0.2 | 74.0237 | C6H4O2N | 0.5 | DL-Tryptophan (Amino acid) |
| 91.0542 | C7H7 | −0.4 | |||||
| 102.0549 | C4H8O2N | −0.9 | |||||
| 118.0651 | C8H8N | −0.1 | |||||
| 130.0652 | C9H8N | 0.8 | |||||
| 159.0919 | C10H11N2 | 1.2 | |||||
| 170.0606 | C11H8ON | 3.1 | |||||
| 188.0707 | C11H10O2N | 0.3 | |||||
| 4.8 | 287.1369 | C13 H21 N O6 | 0.3 | 69.0335 | C4H5O | −0.2 | BOC-D-GLU(OALL)-OH (Amino acid) |
| 85.0282 | C4H5O2 | −2.9 | |||||
| 97.0283 | C7H7 | −0.6 | |||||
| 145.0499 | C6H9O4 | 2.6 | |||||
| 4.9 | 153.0790 | C8 H11 N O2 | 0.3 | 53.0386 | C4H5 | −0.2 | Dopamine (Other compounds) |
| 65.0384 | C5H5 | −2.5 | |||||
| 81.0699 | C6H9 | 0.9 | |||||
| 91.0541 | C7H7 | −0.9 | |||||
| 108.0806 | C7H10N | −1.2 | |||||
| 5.0 | 269.0685 | C11 H16 N2 O | −0.2 | 79.0543 | C6H7 | 1.3 | Tocainide (Other compounds) |
| 91.0540 | C7H7 | −2.1 | |||||
| 105.0698 | C8H9 | −0.5 | |||||
| 150.0909 | C9H12ON | −3.2 | |||||
| 176.1067 | C11H14ON | −1.7 | |||||
| 8.1 | 288.1806 | C14 H26 N O5 | −0.9 | 55.0542 | C4H7 | −0.8 | (2S)-2-[(Tert-butoxycarbonyl)amino]-3-(4-hydroxycyclohexyl)propanoic acid (Other compounds) |
| 69.0700 | C5H9 | 2.1 | |||||
| 72.0569 | C4H10N | −0.1 | |||||
| 86.0726 | C5H12N | −3.8 | |||||
| 118.0858 | C5H12O2N | −3.7 | |||||
| 224.1646 | C13H22O2N | 0.6 | |||||
| 242.1751 | C13H24O3N | 0.3 | |||||
| 10.4 | 284.0686 | C16 H12 O5 | 0.7 | 68.9970 | C3HO2 | −1.0 | Olmelin (Phenolic acid) |
| 84.0206 | C4H4O2 | 0.2 | |||||
| 112.0155 | C5H4O3 | 0.3 | |||||
| 270.0527 | C15H10O5 | 1.7 | |||||
| 11.1 | 200.2373 | C13 H30 N | −0.1 | 57.0698 | C4H9 | 1.7 | N-Methyldodecylamine (Other compounds) |
| 71.0856 | C5H11 | 4.2 | |||||
| 85.1013 | C6H13 | 4.6 | |||||
| 12.0 | 242.2842 | C16 H36 N | 0.5 | 57.0698 | C4H9 | 1.7 | Bis(2-ethylhexyl) amine (Other compounds) |
| 71.0852 | C5H11 | −1.4 | |||||
| 12.1 | 228.2686 | C15 H34 N | −0.1 | 57.0697 | C4H9 | 0.1 | Dodecyltrimethylammonium (Other compounds) |
| 71.0854 | C5H11 | 1.4 | |||||
| 95.0853 | C7H11 | 0.1 | |||||
| 12.2 | 228.2686 | C15 H34 N | −0.1 | 57.0697 | C4H9 | 0.1 | Pentadecan-8-amine (Other compounds) |
| 71.0854 | C5H11 | 1.4 | |||||
| 95.0853 | C7H11 | 0.1 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Lara, A.; Ros, M.; Giménez, A.; Moreno, D.A.; Díaz-Galiano, F.J.; Martínez-Bueno, M.J.; Lozano-Pastor, P.; Pascual, J.A. Organic Amendment for Disinfecting Soil Alters the Metabolites in Spinacia oleracea. Agriculture 2023, 13, 2227. https://doi.org/10.3390/agriculture13122227
Hernández-Lara A, Ros M, Giménez A, Moreno DA, Díaz-Galiano FJ, Martínez-Bueno MJ, Lozano-Pastor P, Pascual JA. Organic Amendment for Disinfecting Soil Alters the Metabolites in Spinacia oleracea. Agriculture. 2023; 13(12):2227. https://doi.org/10.3390/agriculture13122227
Chicago/Turabian StyleHernández-Lara, Alicia, Margarita Ros, Almudena Giménez, Diego A. Moreno, Francisco J. Díaz-Galiano, María Jesús Martínez-Bueno, Pedro Lozano-Pastor, and José Antonio Pascual. 2023. "Organic Amendment for Disinfecting Soil Alters the Metabolites in Spinacia oleracea" Agriculture 13, no. 12: 2227. https://doi.org/10.3390/agriculture13122227
APA StyleHernández-Lara, A., Ros, M., Giménez, A., Moreno, D. A., Díaz-Galiano, F. J., Martínez-Bueno, M. J., Lozano-Pastor, P., & Pascual, J. A. (2023). Organic Amendment for Disinfecting Soil Alters the Metabolites in Spinacia oleracea. Agriculture, 13(12), 2227. https://doi.org/10.3390/agriculture13122227






