Abstract
The objective of this study was to evaluate the impact of the genotype on the chemical composition, feeding value and in vitro rumen degradability of fresh and ensiled forage of four native maize varieties (Amarillo, Olotillo, Tampiqueño and Tuxpeño) from Tamaulipas, Mexico, and a commercial hybrid, as well as the stability and aerobic deterioration of the silage. In all genotypes, fresh forage consisted of whole plants of maize that were harvested when the grain reached a milky-mass state, and silage was fresh forage chopped and ensiled in plastic bags, where it fermented for 120 days. The hybrid presented the highest content (p < 0.05) of dry matter (DM), organic matter (OM), ether extract, non-fibrous carbohydrates (NFCs) and starch, as well as the lowest content (p < 0.05) of fibers (NDF and ADF), acid detergent lignin and water-soluble carbohydrates (WSCs). Furthermore, the hybrid and Amarillo genotypes obtained the lowest pH and ammoniacal nitrogen content (p < 0.05), intermediate values (p < 0.05) of lactic and butyric acid, and the lowest and highest acetic acid content (p < 0.05), respectively. Although OM did not differ (p > 0.05) between states of the forage, the fresh forage presented a higher (p < 0.05) content of DM, crude protein, NDF, ADF, WSCs, pH and butyric acid in all genotypes, while the rest of the parameters were higher (p < 0.05) in the silage. However, Amarillo obtained the highest feeding value (p < 0.05) in terms of DM intake, relative forage value, digestible energy, metabolizable energy and rumen degradability (DM, NDF and ADF), and between states of the forage, ensiled obtained the highest feeding value (p < 0.05). During the aerobic exposure, the Amarillo and hybrid silage showed greater (p < 0.05) stability (>38 h), and less (p < 0.05) deterioration, pH increase and loss of DM and OM, while Tuxpeño obtained less stability and greater deterioration. In conclusion, the genotype did influence the chemical composition of fresh and ensiled forage, which affected the feeding value and in vitro rumen degradability, and the Amarillo and hybrid genotypes presented the best values in the evaluated parameters.
1. Introduction
Maize (Zea mays L.) is the second most cultivated cereal and the most important in terms of grain production worldwide [1], and Mexico is considered to be one of the centers of origin, domestication, and diversification of this crop, since it has 29% of the 220 native maize breeds recognized in Latin America [2]. These breeds are constituted of varieties known as local or criollas, and although they lack formal genetic improvement, they have adapted to the instability of the agroecological conditions of their environment through evolutionary processes [3,4], which is why they have even been used to generate improved varieties. The above evidence shows that native varieties play an important role for the resilience of the agricultural sector facing climate change, and that they constitute a valuable plant genetic resource not only for grain production, but also for forage production [5]. In this sense, it has been shown that there are native varieties of maize with potential for forage production, and that they can respond better than improved varieties in terms of dry-matter yield and nutritional value [6].
As a source of forage for livestock, maize has stood out for its high energy value, potential for forage production and acceptance by livestock, but these factors are influenced by the genotype of the variety, whether native or improved [7,8]. This is because the genotype influences plant morphological traits and yield components, which in turn affects the chemical composition and digestibility of fresh maize forage [6,9]. In addition to the above, in some varieties digestibility is compromised by fibrosity, especially in varieties from hot-climate regions, since fibrous carbohydrates are mainly composed of cellulose and hemicellulose, and in the presence of lignin they can form recalcitrant lignocellulosic complexes [10]. In turn, these complexes cause difficulties for rumen microorganisms and enzymes to break down forage fiber and provide energy to animals [11], which decreases degradation efficiency and increases greenhouse gas emissions. Silage, which is actually an anaerobic fermentation, has traditionally been used for the conservation of forage with high moisture content [12], but it can also be useful as an alternative to improve the digestion of forage fibers, since during the transformation of water-soluble carbohydrates to organic acids there is a separation of the lignocellulosic structures [13], which improves the adhesion and colonization of the forage by ruminal microorganisms and, consequently, increases digestibility. However, deficient forage fermentation can cause high nutrient losses, low aerobic stability, and a reduction in silage quality [14], which negatively impacts dry matter intake, carbohydrate digestion and animal productivity [15].
Currently, there are numerous studies that have evaluated the nutritional value of ensiled maize forage, but most of them used improved varieties, which is why the information available for the use of native varieties in animal feed is limited, especially with regard to nutritional quality. Under these circumstances, it is important to evaluate local maize varieties so that livestock producers can have information that allows them to implement actions to improve livestock productivity and lower production costs, and thus obtain greater income. In addition, because these varieties are used mainly by small producers, these types of studies contribute to goal 2.3 of Sustainable Development Goal 2 of the United Nations 2030 Agenda, which establishes the aim of doubling agricultural productivity and the income of small-scale food producers, including those dedicated to livestock [16]. For this reason, the objective of this study was to evaluate the impact of the genotype on the chemical composition, feeding value and in vitro rumen degradability of fresh and ensiled forage of four genotypes of native maize varieties from Tamaulipas, Mexico, and a commercial hybrid, as well as the stability and aerobic deterioration of the silage.
2. Materials and Methods
2.1. Experimental Treatments
Four genotypes of native maize varieties from Tamaulipas, Mexico, locally known as Amarillo, Olotillo, Tampiqueño and Tuxpeño, and a commercial hybrid used as a control were evaluated. These genotypes are grown in regions with a warm climate, are used by livestock farmers who cannot acquire improved varieties and were chosen for their morphological characteristics (abundance of leaves, tall size, proportion of cob, etc.) and/or yield of forage. In addition to the comparison between genotypes, fresh forage was also compared with ensiled forage, and hereinafter referred to as “state of the forage”.
2.2. Forage Production and Silage
The forage was produced under rainfed conditions during the autumn/winter 2021 agricultural cycle, in Aldama, Tamaulipas, Mexico (22°59’09” N and 98°10’25” W, at 190 masl), and the climate of the site, according to the Köppen classification, is of the Aw0 type, which corresponds to the driest of warm subhumid types [17]. The sowing was carried out manually in quadruplicate in plots of 240 m2, and from each one 10 complete plants (leaf, stem, and cob) were harvested from the five central rows when the grain reached a milky-mass state, which was when the plants were approximately 90 ± 5 days old. These plants were chopped by plot in a hammer and knife mill (model THCF2000UK7, with Ukura® engine, Valles, SLP, Mexico) to reduce the particle size to 2 or 3 cm, and a sample (300 g) was obtained, which was called “fresh forage”. Likewise, 5 kg of chopped forage from each plot was weighed and placed in black polyethylene bags (30 × 50 cm, diameter, and height; caliber 500) that were vacuum sealed, and after 120 days of fermentation, they were opened and a sample (300 g) was obtained from each one, which was called “ensiled forage”.
2.3. Chemical Composition and Feeding Value of the Fresh and Ensiled Forage
At the time of taking the fresh and ensiled forage samples, the pH was measured following the Cherney and Cherney [18] methodology and using a potentiometer with a glass electrode (pH wireless electrode HALO® model HI11102, Hanna® Instruments, Woonsocket, RI, USA). Before analysis, the collected samples were dehydrated at 60 °C for 72 h and ground in a hammer mill (Thomas Wiley® Laboratory Mill model 4, Thomas Scientific™, Swedesboro, NJ, USA) with a 1 mm sieve. All analyses were performed in quadruplicate, and the content (g kg−1 DM) of dry matter (DM; method ID 934.01), ash (method ID 942.05), crude protein (CP; Kjeldahl method ID 920.87) and ether extract (EE; method ID 920.39) was determined according to the official methods of the Association of Official Analytical Chemists [19]. The neutral detergent (NDF) and acid detergent (ADF) fibers were analyzed in the ANKOM200 Fiber Analyzer Unit (ANKOM Technology Corp., Macedon, NY, USA) [20], while acid detergent lignin (method ID 973.18) agreed with the AOAC [19]. Sodium sulfite and thermostable α-amylase were used in the NDF analysis, and the NDF and ADF values were expressed without residual ash. The content (g kg−1 DM) of organic matter (OM) and non-fibrous carbohydrates (NFCs) was calculated according to the equations reported by Ferreira et al. [21] and Sniffen et al. [22]:
OM = 1000 − ash
NFC = 1000 − (CP + NDF + EE + ash)
The content (g kg−1 DM) of starch, water-soluble carbohydrates, ammoniacal nitrogen, and organic acids (lactic, acetic and butyric acids) was estimated by near-infrared reflectance spectroscopy (NIRS). Feeding value was evaluated based on dry matter intake (DMI; g kg−1 liveweight), total digestible nutrients (TDNs; g kg−1 DM), relative forage value (RFV; %), and digestible (DE; Mcal kg−1 DM) and metabolizable energy (ME; Mcal kg−1 DM). These variables were calculated on a dry basis with the equations reported by Horrocks and Valentine [23], and Ravhuhali et al. [24] as follows:
DMI = (120/NDF) × 10
TDN = [(−1.291 × ADF) + 101.35] × 10
RFV = DMD × DMI × 0.775
DE = 0.27 + 0.0428 (DMD)
ME = 0.821 × DE
In the cases of RVF and DE, the dry matter degradability (DMD) value obtained at 48 h in the in vitro rumen degradability test described below was used, and when calculating, the DMD and DMI were used in percentages.
2.4. In Vitro Rumen Degradability of the Fresh and Ensiled Forage
The degradability test was performed in the ANKOM DaysiII incubator (ANKOM Technology Corp., Macedon, NY, USA), which is equipped with four jars and simulates the temperature and ruminal movements of the animals. The nutritive medium used was prepared following the Goering and Van Soest [25] methodology, and the ruminal fluid was obtained from the rumen content of four sheep (30 ± 2.5 kg liveweight) slaughtered in a local slaughterhouse regulated by the Official Mexican Norm NOM-033-SAG/ZOO-2014, which establishes the methods of killing domestic and wild animals. Before slaughter, these animals were fed with commercial concentrate (Purina®, Victoria, Tam., Mexico) and were maintained with a constant supply of fresh water. The rumen content obtained was transferred to the laboratory in airtight thermoses, where it was filtered with four layers of gauze to extract the rumen liquid and kept at 39 °C until use.
Before incubation, 500 mg of dehydrated sample of fresh and ensiled forage was placed in ANKOM bags with 25 µm porosity (Filter bags F57, ANKOM Technology Corp., Macedon, NY, USA), and then heat sealed. In each jar of the incubator, 1600 mL of nutrient medium and 400 mL of rumen liquid were added to obtain a 4:1 (v/v) ratio, and then the ANKOM bags with the samples were placed as described by Vega-Zúñiga et al. [26]. The incubation was carried out at a temperature of 39 °C, and at 6, 12, 24 and 48 h the bags of each treatment with their repetitions were removed, rinsed with plenty of tap water and dehydrated at 60 °C for 72 h. The percentage of in vitro rumen degradability of DM, NDF and ADF was calculated as follows:
where DMD is dry matter degradability, DMi is the initial dry matter and DMr is the residual dry matter at time t (6, 12, 24 or 48 h).
where NDFD is neutral detergent fiber degradability, NDFi is the initial neutral detergent fiber, and NDFr is the residual neutral detergent fiber at time t (6, 12, 24, or 48 h).
where ADFD is acid detergent fiber degradability, ADFi is the initial acid detergent fiber, and ADFr is the residual acid detergent fiber at time t (6, 12, 24, or 48 h). The determinations of DM, NDF and ADF in the residues of the samples were carried out following the aforementioned methodologies.
DMD = [(DMi − DMr)/DMi] × 100
NDFD = [(NDFi − NDFr)/NDFi] × 100
ADFD = [(ADFi − ADFr)/ADFi] × 100
2.5. Stability and Aerobic Deterioration of the Ensiled Forage
This evaluation was carried out following the methodology of Del Valle et al. [27], which consists of placing 3 kg of ensiled forage in an expanded polystyrene container (28 × 25 cm, diameter, and height), covering it with two layers of cheesecloth and keeping it in a closed room with controlled temperature. The room temperature was maintained at 24 ± 0.5 °C during the 10 days of evaluation, every 8 h the temperature of the sample was recorded with a digital thermometer and every 24 h a sample (100 g) was taken to determine the pH, the DM and the OM. Aerobic stability was defined as the number of hours that the temperature of the ensiled forage and the ambient temperature were maintained with a difference of 2 °C or less [28], while deterioration was expressed as (i) increased maximum temperature, (ii) time to reach maximum temperature and (iii) the accumulation of daily temperature increases up to 240 h of aerobic exposure [29].
2.6. Statistical Analysis
The experimental design was completely randomized with a bifactorial arrangement, where factor 1 was the maize genotype and factor 2 the state of the forage, with four repetitions. Data were analyzed using the GLM procedure of SAS version 9.2 [30], and according to the following statistical model:
where Yijk is the response variable, µ is the general mean, Gi is the effect of the maize genotype (i = Amarillo, Olotillo, Tampico, Tuxpeño, Hybrid), Sj is the effect of the state of the forage (j = fresh, ensiled), (G × S)ij is the effect of the interaction between maize genotype and state of the forage, and εijk is the residual error. Means with statistical difference were compared using Tukey’s test, and they were considered significantly different when p < 0.05.
Yijk = µ + Gi + Sj + (G × S)ij + εijk
3. Results
3.1. Chemical Composition and Feeding Value of the Fresh and Ensiled Forage
The dry matter (DM) content, chemical composition and pH differed (p < 0.05) between genotypes and states of the forage, and there was an effect (p < 0.05) of the interaction of both on acid detergent lignin (ADL), water-soluble carbohydrates (WSCs), starch, ammonia nitrogen (NH3-N), and organic acids (Table 1 and Table 2). The hybrid presented the highest values (p < 0.05) of DM, ether extract (EE), non-fibrous carbohydrates (NFCs) and starch, as well as the lowest (p < 0.05) of neutral detergent fiber (NDF), acid detergent fiber (ADF), ADL and WSCs, and it did not differ (p > 0.05) from the rest in the crude protein (CP) content. In addition, the hybrid and Amarillo genotypes obtained the lowest pH and NH3-N content (p < 0.05), as well as the best organic acids profile in terms of lactic (LA), acetic (AA) and butyric (BA) acids. Regardless of the genotype, the fresh forage presented higher (p < 0.05) DM, CP, NDF, ADF, WSC, pH and BA content, while the rest of the parameters were higher (p < 0.05) in the silage, with the exception of organic matter (OM), which did not differ (p > 0.05) between states of the forage (Table 1 and Table 2).

Table 1.
Dry matter content and chemical composition of the fresh and ensiled forage of four genotypes of native maize (Zea mays L.) from Mexico and a commercial hybrid.

Table 2.
pH, ammonia nitrogen and organic acids of the fresh and ensiled forage of four genotypes of native maize (Zea mays L.) from Mexico and a commercial hybrid.
The feeding value was also influenced (p < 0.05) by the genotype and the state of the forage, and their interaction was significant (p < 0.05) in the relative forage value, digestible energy and metabolizable energy (Table 3). The Amarillo genotype obtained the highest values (p < 0.05) in all the parameters, except in total digestible nutrients, which corresponded to Olotillo, and between states of the forage, the ensiled was the one with the highest quality (p < 0.05; Table 3).

Table 3.
Feeding value of the fresh and ensiled forage of four genotypes of native maize (Zea mays L.) from Mexico and a commercial hybrid.
3.2. In Vitro Rumen Degradability of the Fresh and Ensiled Forage
The interaction (p < 0.05) of the genotype and the state of the forage with the incubation time was significant in the degradability of DM, NDF and ADF, and although there were variations during incubation, in the end the Amarillo genotype presented the highest values (p < 0.05) of degradability; it even surpassed the hybrid by up to 13.0% (Figure 1). In addition, the silage presented higher (p < 0.05) degradability compared to fresh forage in all genotypes (Figure 1).
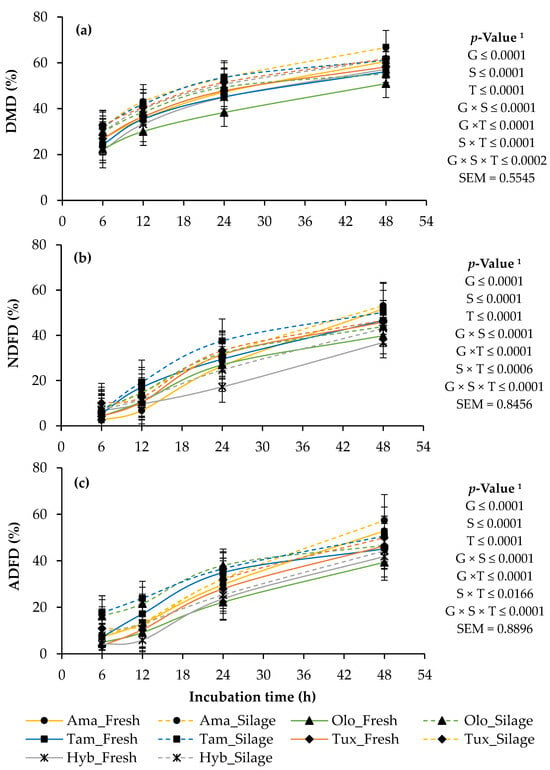
Figure 1.
In vitro rumen degradability of dry matter (DMD); (a), neutral detergent fiber (NDFD); (b) and acid detergent fiber (ADFD); (c) of the fresh and ensiled forage of four genotypes of native maize (Zea mays L.) from Mexico and a commercial hybrid, at 6, 12, 24 and 48 h of incubation. 1 G: genotypes of maize; S: states of the forage; T: incubation time; G × S: interaction between genotypes of maize and states of forage; G × T: interaction between genotypes of maize and incubation time; S × T: interaction between states of the forage and incubation time; G × S × T: interaction genotypes of maize, states of the forage and incubation time; SEM: standard error of the mean.
3.3. Stability and Aerobic Deterioration of the Ensiled Forage
The genotype influenced the stability and aerobic deterioration of the ensiled forage, and the Amarillo and hybrid genotypes showed higher (p < 0.05) aerobic stability, and lower (p < 0.05) maximum temperature and accumulation of daily temperature increases to 240 h. However, these genotypes did not differ significantly (p > 0.05) from the rest in time taken to reach the maximum temperature (Table 4).

Table 4.
Indices of stability and aerobic deterioration of the ensiled forage of four genotypes of native maize (Zea mays L.) from Mexico and a commercial hybrid, at 240 h of exposure aerobic.
During aerobic exposure, the genotype presented interaction (p < 0.05) with time, and the Amarillo and hybrid genotypes presented the lowest pH increases and DM and OM reductions (p < 0.05), while Tuxpeño obtained the highest values (p < 0.05; Figure 2).
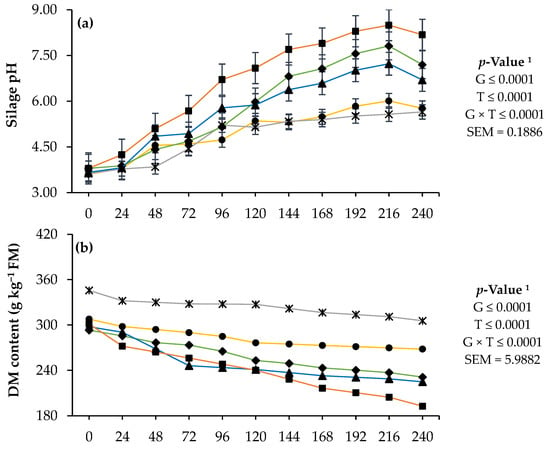
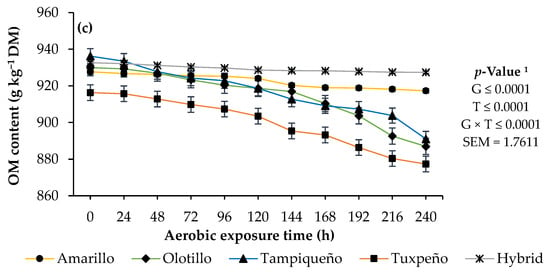
Figure 2.
pH (a) and dry matter (DM); (b) and organic matter (OM); (c) content of the ensiled forage of four genotypes of native maize (Zea mays L.) from Mexico and a commercial hybrid, at different times of aerobic exposure. 1 G: genotypes of maize; T: aerobic exposure time; G × T: interaction between genotypes of maize and aerobic exposure time; SEM: standard error of the mean.
4. Discussion
4.1. Chemical Composition and Feeding Value of the Fresh and Ensiled Forage
The dry matter (DM) content in fresh forage results from the proportion of each component, and generally, the stem provides a greater amount of DM than the cob and the leaves [6], which explains the variations between genotypes. Despite this, the DM content of the fresh forage was higher than recommended (300.0 g kg−1) for ensiling [31], and although there is a loss of DM that is inevitable during silage fermentation, in the current study it was relatively low (3.8 to 5.5%) in all genotypes, except in Tampiqueño, where it was 11.5%. In this case, the fermentation was probably dominated by heterolactic bacteria, since in addition to lactic acid (LA) they produce other compounds and generate gases [32], which implies greater degradation of DM. The organic matter (OM) content did not differ between states of the forage, but between genotypes, and in plants it accumulates mainly in the stem [33], so it can be assumed that the differences between genotypes in fresh forage were caused by the proportion of its components (stem, leaf, and cob), as in DM. Furthermore, in fresh forage the high content of crude protein (CP), ether extract (EE), non-fibrous carbohydrates (NFCs) and water-soluble carbohydrates (WSCs) is associated with a higher leaf: stem and cob: stem ratio, while a lower ratio of these components is associated with the high content of neutral detergent fiber (NDF), acid detergent fiber (ADF) and acid detergent lignin (ADL) [34]. However, the silage goes through a fermentation process that generates chemical changes in the forage, including the deamination of proteins, also called proteolysis [35], which justifies the lower CP content in silage. Although the exact reason for the increase in EE in silage is unknown, it is speculated that it is due to the loss of other nutrients since this causes the concentration of EE to increase if it does not present degradation. Furthermore, changes in fibrous carbohydrates are attributed to the acidic conditions of the forage, which causes acid hydrolysis [36], which mainly reduces the NDF content and, to a lesser extent, also the ADF content [37]. In Olotillo and Tampiqueño, the high ADL content could have acted as a protective layer and decreased the degradation rate of these carbohydrates [38], while in Tuxpeño it can be attributed to a low enzymatic activity. Consequently, the degradation of these fibers led to an increase in NFCs, including WSCs [39,40], which explains the increase in NFCs in all genotypes and WSCs in Olotillo, Tampiqueño and Tuxpeño. However, there are variations in the production of LA and WSCs between epiphytic strains of lactic acid bacteria (LAB) [41], so it cannot be ruled out that this also influenced the increase in NFC and WSC. The starch was lowest in the fresh forage and high in the ensiled, and the hybrid presented the highest amount, which is consistent because hybrids are bred to increase grain production, and it is in this part of the plant where it is found starch accumulates [42,43]. In native maize, variations are attributed to grain characteristics, since starch content is influenced by grain percentage and accumulation rate [44].
As expected, the pH was higher in the fresh forage and lower in the silage, while ammonia nitrogen (NH3-N) and organic acids (OAs) were higher in the silage and lower in the fresh forage. It is known that the pH depends on the content of organic acids, and that in the case of fresh forage the OA content is determined by epiphytic bacteria, especially LA, since fatty acids (FAs) such as acetic (AA) and butyric (BA) acids are constituted from lipids in the leaves, stems and grains of maize [45]. In addition, the FAs have a high positive correlation with chlorophyll content [46], which allows us to assume that the genotypes with higher FA content not only had a larger population of epiphytic bacteria, but also a greater quantity of green leaves at harvest time. Instead, in silage these acids are formed from the homolactic and/or heterolactic fermentation of the WSCs by epiphytic LAB, and although at the end of the fermentation the pH can be the same in several genotypes, the microbial populations involved in the acidification and fermentative pathways may differ, leading to variations in the final products [47], as is believed to have occurred in the current study. In the case of Tuxpeño, the low amount of LA and AA, and high BA, are attributed to a slow production of LA and acidification of the forage in the initial stage of silage, which was possibly caused by a low population of epiphytic LAB (<103 cfu g−1 FM) [40], since it presented a greater amount of WSCs than other genotypes such as Olotillo and Tampiqueño. As for NH3-N, as previously indicated, it is formed from the deamination of proteins, so its presence in the fresh forage and variations between genotypes are probably due to physiological processes of the plants, such as protein formation and nutrient translocation. Instead, in silage it is associated with the degradation of proteins by protease enzymes and proteolytic microorganisms [37], which explains the higher content of NH3-N in silage and the differences between genotypes. Furthermore, in the current study no genotype exceeded the maximum threshold (100 g kg−1 N) for good quality silage [48], indicating correct forage conservation.
The silage and the Amarillo and hybrid genotypes presented higher dry matter intake (DMI) and total digestible nutrient (TDN) values compared to the fresh forage and the rest of the genotypes, which is attributed to the NDF and ADF content they presented, since these components are negatively associated with the DMI and TDNs, respectively [23,37]. Although the TDN value improved in the silage, all genotypes, including the hybrid, were below the value (688 g kg−1 DM) reported by the National Research Council for maize silage [49]. However, the DM degradability (DMD) and DMI favorably impacted on the relative forage value (RFV), since it is derived from them and, therefore, increasing these parameters also increases the RFV [50]. In all genotypes, digestible energy and metabolizable energy were higher in silage, which is attributed to the increase in DMD, and remained within the range (0.57–2.78 Mcal kg−1) necessary for maintenance and high activity in ruminants [49].
4.2. In Vitro Rumen Degradability of the Fresh and Ensiled Forage
The degradability of forage is normally influenced by the carbohydrate and lignin content [24], and in this study the silage of all the genotypes, especially Amarillo, presented greater degradation than the fresh forage, which in the case of DMD could be related to the higher content of NFCs and lower content of NDF and ADF in the silage. Instead, the NDF and ADF degradability is attributed to a reduction in the complexity of the physicochemical structure of lignocellulose, which is possibly caused by acid hydrolysis during silage fermentation [51]. In turn, this provides greater accessibility to rumen microorganisms and enzymes and, consequently, there is greater degradation of NDF and ADF during rumen fermentation, as in the current study. However, in the silage of the genotypes in which the increase in degradability was low, lignin possibly influenced, since its structure is recalcitrant, branched, and dense [52], which limits degradation, especially of the fibers.
4.3. Stability and Aerobic Deterioration of the Ensiled Forage
In the current study, the pH and temperature increased rapidly in the Olotillo, Tampiqueño and Tuxpeño silage, which caused them to present less stability and a higher degree of deterioration and loss of DM and OM than the Amarillo and hybrid silage. Considering that the genotypes with less stability and greater spoilage presented high WSC and/or LA content, it is possible to suppose that they had a high yeast activity, since these microorganisms are the initiators of aerobic spoilage because they can oxidize WSCs and LA to carbon dioxide and water [53], which raises the pH and temperature [54]. Moreover, the oxidation of these carbohydrates and LA generates adequate conditions for the growth of aerobic bacteria and molds [55], and although AA has antifungal properties and is negatively correlated with yeasts [56], in the current study it possibly had little influence on stability, since it varied little between genotypes, and, since it is a weak gas, it is not ruled out that a part volatilized during aerobic exposure [57]. Instead, in more stable genotypes with less deterioration, such as Amarillo and the hybrid, the content of WSCs and LA did not compromise aerobic stability, and this was reflected in a lower pH and MT than the rest of the genotypes.
In relation to time to reach maximum temperature (TRMT) and accumulation of daily temperature increases (ADTI), both reflect the intensity of the activity of aerobic microorganisms, including molds and yeasts [58], which allows us to assume that when the TRMT was lower and ADTI higher, the microorganisms had a greater quantity of substrates for their activities, while when the TRMT was higher and ADTI lower, the opposite occurred. This may also be the origin of the higher DM and OM losses in the Olotillo, Tampiqueño and Tuxpeño genotypes compared to the Amarillo and the hybrid. It should be noted that, in the present study, the aerobic stability of the silage of all the genotypes was higher at 12 and 24 h, the time it that would possibly be exposed to air in the feeder.
5. Conclusions
It is concluded that all the genotypes, including the hybrid, and both states of the forage presented variability among themselves in chemical composition, the profile of organic acids and other compounds, which influenced the feeding value and in vitro ruminal degradability of the forage. Furthermore, of all the genotypes, the Amarillo and the hybrid are the ones that presented the best values, while between the states of the forage the ensiled was the best. However, the aerobic stability of the silage differed between genotypes, and although it depended on the chemical composition of the fresh forage, all presented acceptable stability. Therefore, native maize varieties have the potential to match the quality of improved ones, and silage is not only useful in forage conservation, but also as a pre-ingestive treatment to improve digestibility, especially of fiber.
Author Contributions
The study was designed and conducted by E.R.A.-R., G.B.-R., D.L.-A. and M.A.R.-J., while J.R.-H., J.H.-M. and A.G.L.-M. covered the financial costs. Data was collected by E.R.A.-R., J.R.-H. and M.A.R.-J., while A.Z.M.S., C.A.H.-C. and D.L.-A. statistically analyzed the data. The first draft of the manuscript was written by E.R.A.-R., A.Z.M.S., M.A.R.-J. and A.G.L.-M., while G.B.-R., D.L.-A., J.R.-H., C.A.H.-C. and J.H.-M. critically revised the manuscript for journal submission. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The present study did not require approval or ethical review by any Educational Institution since, as indicated in the Materials and Methods section, the rumen liquid was obtained from four sheep slaughtered in a local slaughterhouse that is legally regulated by the Official Mexican Norm NOM-033-SAG/ZOO-2014, which establishes the methods to kill domestic and wild animals.
Data Availability Statement
The data supporting this study will be made available upon reasonable request to the corresponding author.
Acknowledgments
All the authors thank the Faculty of Engineering and Sciences of the Autonomous University of Tamaulipas for the facilities granted to carry out this research in the Animal Nutrition Laboratory.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ocampo-Giraldo, V.; Camacho-Villa, C.; Costich, D.E.; Vidal, M.V.A.; Smale, M.; Jamora, N. Dynamic conservation of genetic resources: Rematriation of the maize landrace Jala. Food Secur. 2020, 12, 945–958. [Google Scholar] [CrossRef]
- Guzzon, F.; Arandia, R.L.W.; Caviedes, C.G.M.; Céspedes, P.M.; Chávez, C.A.; Muriel, F.J.; Medina, H.A.E.; Jara, C.T.V.; Molnar, T.L.; Narro, L.L.A.; et al. Conservation and use of Latin American maize diversity: Pillar of nutrition security and cultural heritage of humanity. Agronomy 2021, 11, 172. [Google Scholar] [CrossRef]
- McLean-Rodríguez, F.D.; Camacho-Villa, T.C.; Almekinders, C.J.M.; Pè, M.E.; Dell’Acqua, M.; Costich, D.E. The abandonment of maize landraces over the last 50 years in Morelos, Mexico: A tracing study using a multi-level perspective. Agric. Human Values 2019, 36, 651–668. [Google Scholar] [CrossRef]
- Janzen, G.M.; Aguilar-Rangel, M.R.; Cíntora-Martínez, C.; Blöcher-Juárez, K.A.; González-Segovia, E.; Studer, A.J.; Runcie, D.E.; Flint-Garcia, S.A.; Rellán-Álvarez, R.; Sawers, R.J.H.; et al. Demonstration of local adaptation in maize landraces by reciprocal transplantation. Evol. Appl. 2022, 15, 817–837. [Google Scholar] [CrossRef] [PubMed]
- Robles-Jimenez, L.E.; Rosas, D.M.; Osorio, A.J.; Chay-Canul, A.J.; Palacios, C.; Castelan, O.O.A.; González, R.M. Evaluation of mexican native and hybrid maize (Zea mays) silages for sustainable milk production. Trop. Subtrop. Agroecosyst. 2021, 24, 124. [Google Scholar] [CrossRef]
- Sánchez-Hernández, M.Á.; Morales-Terán, G.; Mendoza-Pedroza, S.I.; Hernández-Bautista, J.; Fraire-Cordero, S.; Rivas-Jacobo, M.A. Caracterización productiva de maíces nativos con aptitud forrajera en la cuenca baja del Papaloapan. Rev. Fitotec. Mex. 2021, 44, 755–764. [Google Scholar] [CrossRef]
- Horst, E.H.; López, S.; Neumann, M.; Giráldez, F.J.; Bumbieris Junior, V.H. Effects of Hybrid and Grain Maturity Stage on the Ruminal Degradation and the Nutritive Value of Maize Forage for Silage. Agriculture 2020, 10, 251. [Google Scholar] [CrossRef]
- Rivas-Jacobo, M.A.; Mendoza, P.S.I.; Sangerman-Jarquín, D.M.; Sánchez, H.M.Á.; Herrera, C.C.A.; Rojas, G.A.R. Evaluación forrajera de maíces de diversos orígenes de México en la región semiárida. REMEXCA 2020, 11, 93–104. [Google Scholar] [CrossRef]
- Joaquín, C.S.; Rocandio, R.M.; Álvarez, V.P.; Hernández, G.F.J.; Limas, M.A.G.; Garay, M.J.R. Rendimiento y valor nutritivo del forraje y ensilado de maíces nativos en condiciones subtropicales. REMEXCA 2022, 13, 873–881. [Google Scholar] [CrossRef]
- Bernardes, T.F.; Daniel, J.L.P.; Adesogan, A.T.; McAllister, T.A.; Drouin, P.; Nussio, L.G.; Cai, Y. Silage review: Unique challenges of silages made in hot and cold regions. J. Dairy Sci. 2018, 101, 4001–4019. [Google Scholar] [CrossRef]
- Jennings, J.S.; Lockard, C.L.; Tedeschi, L.O.; Lawrence, T.E. Effects of corn stalk inclusion rate on rumination and ruminal pH in finishing beef steers. AAS 2020, 36, 377–388. [Google Scholar] [CrossRef]
- Wilkinson, J.M.; Muck, R.E. Ensiling in 2050: Some challenges and opportunities. Grass Forage Sci. 2019, 74, 178–187. [Google Scholar] [CrossRef]
- Frydendal-Nielsen, S.; Hjorth, M.; Baby, S.; Felby, C.; Jørgensen, U.; Gislum, R. The effect of harvest time, dry matter content and mechanical pretreatments on anaerobic digestion and enzymatic hydrolysis of miscanthus. Bioresour. Technol. 2016, 218, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, Y.; Wang, S.; Zhao, L.; Zhang, B.; Jia, W.; Zhai, Z.; Zhao, L.; Li, Y. Effects of antibacterial peptide-producing Bacillus subtilis, gallic acid, and cellulase on fermentation quality and bacterial community of whole-plant corn silage. Front. Microbiol. 2022, 13, 1028001. [Google Scholar] [CrossRef]
- Ferraretto, L.F.; Fonseca, A.C.; Sniffen, C.J.; Formigoni, A.; Shaver, R.D. Effect of corn silage hybrids differing in starch and neutral detergent fiber digestibility on lactation performance and total-tract nutrient digestibility by dairy cows. J. Dairy Sci. 2015, 98, 395–405. [Google Scholar] [CrossRef]
- UN (United Nations). The Sustainable Development Agenda. Available online: https://www.un.org/sustainabledevelopment/development-agenda/ (accessed on 15 September 2023).
- Vargas, T.V.; Hernández, R.M.E.; Gutiérrez, L.J.; Plácido, D.C.J.; Jiménez, C.A. Climatic classification of the state of Tamaulipas, México. CienciaUAT 2007, 2, 15–19. [Google Scholar]
- Cherney, J.H.; Cherney, D.J.R. Assessing silage quality. In Silage Science and Technology; Buxton, D.R., Muck, R.E., Harrison, J.H., Eds.; American Society of Agronomy Inc.: Madison, WI, USA; Crop Science Society of America Inc.: Madison, WI, USA; Soil Science Society of America Inc.: Madison, WI, USA, 2003; pp. 141–198. [Google Scholar] [CrossRef]
- AOAC. Association of Official Analytical Chemists, 16th ed.; Association of Official Analytical Chemists International: Arlington, VA, USA, 1997. [Google Scholar]
- Van Soest, P.V.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.; Bandeira, D.; Zanine, A.; Parente, H.; Parente, M.; Rodrigues, R.; Santos, E.M.; Lima, A.G.; Ribeiro, M.; Pinho, R.; et al. Effects of Adding Agro-Industrial By-Products of Babassu to Guinea Grass Silage. Agriculture 2023, 13, 1697. [Google Scholar] [CrossRef]
- Sniffen, C.J.; O’connor, J.D.; Van Soest, P.J.; Fox, D.G.; Russell, J.B. A net carbohydrate and protein system for evaluating cattle diets: II. Carbohydrate and protein availability. J. Anim. Sci. 1992, 70, 3562–3577. [Google Scholar] [CrossRef] [PubMed]
- Horrocks, R.D.; Valentine, J.F. Harvested Forages; Academic Press: San Diego, CA, USA, 1999. [Google Scholar] [CrossRef]
- Ravhuhali, K.E.; Msiza, N.H.; Mudau, H.S. Seasonal dynamics on nutritive value, chemical estimates and in vitro dry matter degradability of some woody species found in rangelands of South Africa. Agrofor. Syst. 2022, 96, 23–33. [Google Scholar] [CrossRef]
- Goering, M.K.; Van Soest, P.J. Forage Fibre Analysis (Apparatus, Reagents, Procedures and Some Applications); Agricultural Research Service USDA: Washington, DC, USA, 1970; pp. 1–24. [Google Scholar]
- Vega-Zúñiga, M.A.; Vázquez-Armijo, J.F.; Martínez-González, J.C.; Quintanilla-Medina, J.J.; Lucero-Magaña, F.A.; López-Aguirre, D. Cinnamon extract on the characteristics of ruminal fermentation in in-vitro systems. ERA 2021, 8, e2763. [Google Scholar] [CrossRef]
- Del Valle, T.A.; Zenatti, T.F.; Antonio, G.; Campana, M.; Gandra, J.R.; Zilio, E.M.C.; de Mattos, L.F.A.; de Morais, J.G.P. Effect of chitosan on the preservation quality of sugarcane silage. Grass Forage Sci. 2018, 73, 630–638. [Google Scholar] [CrossRef]
- Ranjit, N.K.; Kung Jr, L. The effect of Lactobacillus buchneri, Lactobacillus plantarum, or a chemical preservative on the fermentation and aerobic stability of corn silage. J. Dairy Sci. 2000, 83, 526–535. [Google Scholar] [CrossRef]
- McEniry, J.; O’kiely, P.; Clipson, N.J.W.; Forristal, P.D.; Doyle, E.M. The relative impacts of wilting, chopping, compaction and air infiltration on the conservation characteristics of ensiled grass. Grass Forage Sci. 2007, 62, 470–484. [Google Scholar] [CrossRef]
- SAS (Statistical Analysis System). Institute, Cary SAS; User’s Guide: Statistics, Version 9.0; SAS Institute: Cary, NC, USA, 2002. [Google Scholar]
- Da Silva, É.B.; Liu, X.; Mellinger, C.; Gressley, T.F.; Stypinski, J.D.; Moyer, N.A.; Kung, L., Jr. Effect of dry matter content on the microbial community and on the effectiveness of a microbial inoculant to improve the aerobic stability of corn silage. J. Dairy Sci. 2022, 105, 5024–5043. [Google Scholar] [CrossRef] [PubMed]
- Ávila, C.L.S.; Carvalho, B.F. Silage fermentation-updates focusing on the performance of micro-organisms. J. Appl. Microbiol. 2020, 128, 966–984. [Google Scholar] [CrossRef] [PubMed]
- Horst, E.H.; Bumbieris Junior, V.H.; Neumann, M.; López, S. Effects of the harvest stage of maize hybrids on the chemical composition of plant fractions: An analysis of the different types of silage. Agriculture 2021, 11, 786. [Google Scholar] [CrossRef]
- Guyader, J.; Baron, V.S.; Beauchemin, K.A. Corn forage yield and quality for silage in short growing season areas of the Canadian prairies. Agronomy 2018, 8, 164. [Google Scholar] [CrossRef]
- Bağcık, C.; Koç, F.; Erten, K.; Esen, S.; Palangi, V.; Lackner, M. Lentilactobacillus buchneri preactivation affects the mitigation of methane emission in corn silage treated with or without urea. Fermentation 2022, 8, 747. [Google Scholar] [CrossRef]
- Desta, S.T.; Yuan, X.; Li, J.; Shao, T. Ensiling characteristics, structural and nonstructural carbohydrate composition and enzymatic digestibility of Napier grass ensiled with additives. Bioresour. Technol. 2016, 221, 447–454. [Google Scholar] [CrossRef]
- Contreras-Govea, F.; Marsalis, M.; Angadi, S.; Smith, G.; Lauriault, L.M.; VanLeeuwen, D. Fermentability and nutritive value of corn and forage sorghum silage when in mixture with lablab bean. Crop Sci. 2011, 51, 1307–1313. [Google Scholar] [CrossRef]
- Esen, S.; Cabi, E.; Koç, F. Effect of freeze-dried kefir culture inoculation on nutritional quality, in vitro digestibility, mineral concentrations, and fatty acid composition of white clover silages. Biomass Convers. Biorefin. 2022, 1–12. [Google Scholar] [CrossRef]
- Chen, L.; Yuan, X.J.; Li, J.F.; Dong, Z.H.; Wang, S.R.; Guo, G.; Shao, T. Effects of applying lactic acid bacteria and propionic acid on fermentation quality, aerobic stability and in vitro gas production of forage-based total mixed ration silage in Tibet. Anim. Prod. Sci. 2019, 59, 376–383. [Google Scholar] [CrossRef]
- Kaewpila, C.; Khota, W.; Gunun, P.; Kesorn, P.; Kimprasit, T.; Sarnklong, C.; Cherdthong, A. Characterization of green manure sunn hemp crop silage prepared with additives: Aerobic instability, nitrogen value, and in vitro rumen methane production. Fermentation 2022, 8, 104. [Google Scholar] [CrossRef]
- Khota, W.; Pholsen, S.; Higgs, D.; Cai, Y. Natural lactic acid bacteria population of tropical grasses and their fermentation factor analysis of silage prepared with cellulase and inoculant. J. Dairy Sci. 2016, 99, 9768–9781. [Google Scholar] [CrossRef] [PubMed]
- Rivas, J.M.A.; Carballo, C.A.; Quero, C.A.; Hernández, G.A.; Vaquera, H.H.; Rivas, Z.E.C.; Rivas, Z.M.A.; Rivas, Z.E.J. Productive behaviour of twelve threelineal forage corn hybrids in a dry tropical region. Trop. Subtrop. Agroecosyst. 2018, 21, 579–586. [Google Scholar] [CrossRef]
- Dong, Z.; Che, L.; Li, J.; Yuan, X.; Shao, T. Characterization of nitrogen transformation dynamics in alfalfa and red clover and their mixture silages. Grassl. Sci. 2019, 65, 109–115. [Google Scholar] [CrossRef]
- Gusmão, J.O.; Lima, L.M.; Ferraretto, L.F.; Casagrande, D.R.; Bernardes, T.F. Effects of hybrid and maturity on the conservation and nutritive value of snaplage. Anim. Feed Sci. Technol. 2021, 274, 114899. [Google Scholar] [CrossRef]
- Khan, N.A.; Cone, J.W.; Pellikaan, W.F.; Khan, M.A.; Struik, P.C.; Hendriks, W.H. Changes in fatty acid content and composition in silage maize during grain filling. J. Sci. Food Agric. 2011, 91, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Bolton, P.; Harwood, J.L. Lipid metabolism in green leaves of developing monocotyledons. Planta 1978, 139, 267–272. [Google Scholar] [CrossRef]
- Jančík, F.; Kubelková, P.; Loučka, R.; Jambor, V.; Kumprechtová, D.; Homolka, P.; Koukolová, V.; Tyrolová, Y.; Viborná, A. Shredlage processing affects the digestibility of maize silage. Agronomy 2022, 12, 1164. [Google Scholar] [CrossRef]
- Zhao, J.; Tao, X.; Wang, S.; Li, J.; Shao, T. Effect of sorbic acid and dual-purpose inoculants on the fermentation quality and aerobic stability of high dry matter rice straw silage. J. Appl. Microbiol. 2021, 130, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- NRC (National Research Council). Nutrient Requirements of Dairy Cattle, 7th ed.; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Lithourgidis, A.S.; Vasilakoglou, I.B.; Dhima, K.V.; Dordas, C.A.; Yiakoulaki, M.D. 2006. Forage yield and quality of common vetch mixtures with oat and triticale in two seeding ratios. Field Crops Res. 2006, 99, 106–113. [Google Scholar] [CrossRef]
- Nazar, M.; Xu, Q.; Ullah, M.W.; Khan, N.A.; Iqbal, B.; Zhu, D. Integrated laccase delignification with improved lignocellulose recalcitrance for enhancing enzymatic saccharification of ensiled rice straw. Ind. Crops Prod. 2023, 202, 116987. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, L.; Li, X.; Bai, S.; Xue, Y.; Li, Z.; Shang-Wen, T.; Wang, Y.; Wang, Y.; Hu, Z.; et al. Distinctively altered lignin biosynthesis by site-modification of OsCAD2 for enhanced biomass saccharification in rice. GCB-Bioenergy 2021, 13, 305–319. [Google Scholar] [CrossRef]
- Dos Santos, A.P.M.; Santos, E.M.; Araújo, G.G.L.; De Oliveira, J.S.; Zanine, A.M.; Pinho, R.M.A.; Cruz, G.F.L.; Ferreira, D.J.; Perazzo, A.F.; Pereira, D.M.; et al. Effect of inoculation with preactivated Lactobacillus buchneri and urea on fermentative profile, aerobic stability and nutritive value in corn silage. Agriculture 2020, 10, 335. [Google Scholar] [CrossRef]
- Dai, T.; Dong, D.; Wang, S.; Zong, C.; Yin, X.; Xu, G.; Jia, Y.; Shao, T. Assessment of organic acid salts on fermentation quality, aerobic stability, and in vitro rumen digestibility of total mixed ration silage. Trop. Anim. Health Prod. 2022, 54, 261. [Google Scholar] [CrossRef]
- Tabacco, E.; Righi, F.; Quarantelli, A.; Borreani, G. Dry matter and nutritional losses during aerobic deterioration of corn and sorghum silages as influenced by different lactic acid bacteria inocula. J. Dairy Sci. 2011, 94, 1409–1419. [Google Scholar] [CrossRef]
- Danner, H.; Holzer, M.; Mayrhuber, E.; Braun, R. Acetic acid increases stability of silage under aerobic conditions. Appl. Environ. Microbiol. 2003, 69, 562–567. [Google Scholar] [CrossRef]
- Shan, G.; Buescher, W.; Maack, C.; Lipski, A.; Acir, I.H.; Trimborn, M.; Kuellmer, F.; Wang, Y.; Grantz, D.A.; Sun, Y. Dual sensor measurement shows that temperature outperforms pH as an early sign of aerobic deterioration in maize silage. Sci. Rep. 2021, 11, 8686. [Google Scholar] [CrossRef]
- Borreani, G.; Tabacco, E.; Schmidt, R.J.; Holmes, B.J.; Muck, R.E. Silage review: Factors affecting dry matter and quality losses in silages. J. Dairy Sci. 2018, 101, 3952–3979. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).