Loving the Alien: The Contribution of the Wild in Securing the Breeding of Cultivated Hexaploid Wheat and Oats
Abstract
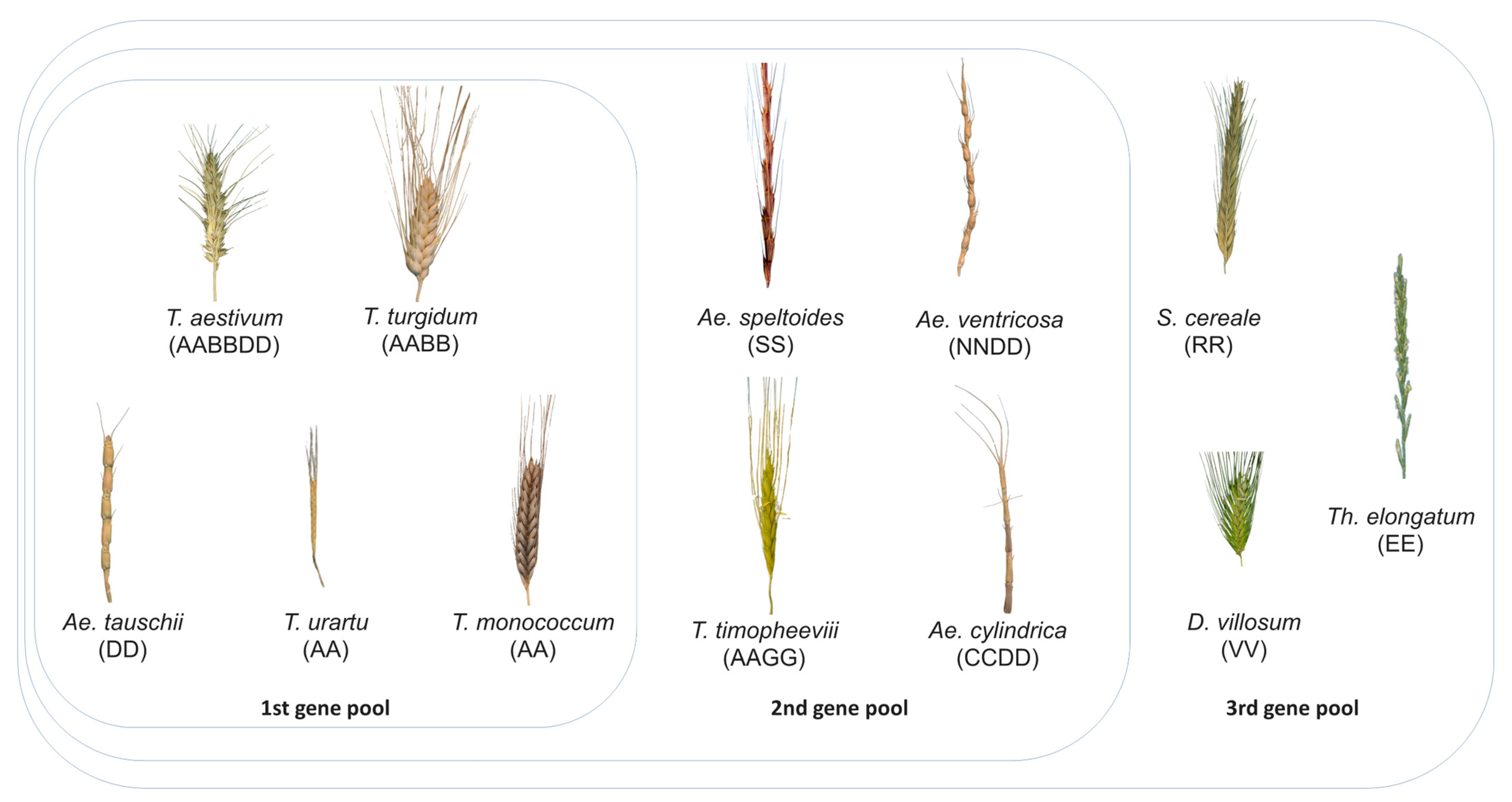
:1. Introduction
2. Wheat
2.1. Wheat, An Outstanding Grass Species
2.2. Synthetic Hexaploid Wheat and Examples of Re-Synthesized Polyploids from Other Crops
2.3. Direct and Bridge Crosses
2.3.1. Waiting for Rare Gametes: The Challenge Associated with the Exploitation of Lower Ploidy Level Species That Share Genomes with Common Wheat
| Gene | Origin | Introgression Method | Reference |
|---|---|---|---|
| Pm1b | T. monococcum | Direct cross | [44,45] |
| Pm4a | T. dicoccum | Direct cross | [46,47] |
| Pm4d | T. monococcum | Direct cross | [48] |
| Pm16 = Pm30 | T. dicoccoides | Direct cross | [49,50] |
| Pm26 | T. dicoccoides | Direct cross | [51] |
| Pm31 | T. dicoccoides | Direct cross | [52] |
| Pm34 | Ae. tauschii | Direct cross | [53,54] |
| Pm35 | Ae. tauschii | Direct cross | [55,56] |
| Pm50 | T. dicoccum | Direct cross | [57] |
| Pm60 | T. urartu | Triploid hybrid bridge | [58] |
| Pm60b | T. urartu | Triploid hybrid bridge | [58] |
| Pm64 | T. dicoccoides | Direct cross | [59] |
| Pm69 | T. dicoccoides | Three-way cross | [60] |
| MlZec1 | T. dicoccoides | Direct cross | [61] |
| PmG16 | T. dicoccoides | Three-way cross | [62] |
| Yr15 | T. dicoccoides | Direct cross Three-way cross | [43,63,64] |
| Yr35 | T. dicoccoides | Direct cross | [65] |
| YrAS2388R | Ae. tauschii | Direct cross | [66,67] |
| Lr14a | T. dicoccum | Direct cross | [4,68,69] |
| Lr21 1 | Ae. tauschii | Direct cross | [70] |
| Lr39 | Ae. tauschii | Direct cross | [71] |
| Lr42 | Ae. tauschii | Direct cross | [72,73] |
| Lr53 | T. dicoccoides | Direct cross | [65] |
| Sr21 | T. monococcum | Direct cross Triploid hybrid bridge | [74,75] |
| Sr22 | T. boeoticum | Direct cross Triploid hybrid bridge | [74,75] |
| Sr22b | T. monococcum | Triploid hybrid bridge | [76] |
| Sr35 | T. monococcum | Direct cross | [77,78] |
| Sr60 | T. monococcum | Triploid hybrid bridge | [79] |
| SrTA1662 | Ae. tauschii | Direct cross | [80] |
| GPC-B1 2 | T. dicoccoides | Direct cross | [81] |
2.3.2. The Induction of Homoeologous Pairing
2.4. Impact of Genomics and Cloned Genes on the Advanced Utilization of Genetic Resources in Wheat
3. Oat
3.1. Oat—Common and Unique
3.2. Introduction to the Genus Avena
3.3. Use of Wild Relatives in Oat Improvement
3.4. Limitations in the Use of Wild Relatives of the Oat
3.5. Cultivar Enhancement through Direct Crosses with Hexaploid Species
3.6. Non-Hexaploid Species as a Source of Desirable Genes
3.7. Synthetic Polyploids
3.8. The Oat in the Genomic Era
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Langridge, P.; Alaux, M.; Almeida, N.F.; Ammar, K.; Baum, M.; Bekkaoui, F.; Bentley, A.R.; Beres, B.L.; Berger, B.; Braun, H.-J.; et al. Meeting the challenges facing wheat production: The strategic research agenda of the Global Wheat Initiative. Agronomy 2022, 12, 2767. [Google Scholar] [CrossRef]
- Kihara, H. Genomanalyse bei Triticum und Aegilops. Cytologia 1930, 1, 263–284. [Google Scholar] [CrossRef]
- Nagaharu, U. Genome analysis in Brassica with special reference to the experimental formation of B. napus and peculiar mode of fertilization. Japan. J. Bot. 1935, 7, 389–452. [Google Scholar]
- McFadden, E.S. A successful transfer of emmer characteristics to vulgare wheat. J. Am. Soc. Agron. 1930, 22, 1020–1034. [Google Scholar] [CrossRef]
- Mago, R.; Tabe, L.; McIntosh, R.A.; Pretorius, Z.; Kota, R.; Paux, E.; Wicker, T.; Breen, J.; Lagudah, E.S.; Ellis, J.G.; et al. A multiple resistance locus on chromosome arm 3BS in wheat confers resistance to stem rust (Sr2), leaf rust (Lr27) and powdery mildew. Theor. Appl. Genet. 2011, 123, 615–623. [Google Scholar] [CrossRef]
- Tabe, L.; Samuel, S.; Dunn, M.; White, R.; Mago, R.; Estavillo, G.; Spielmeyer, W. Phenotypes conferred by wheat multiple pathogen resistance locus, Sr2, include cell death in response to biotic and abiotic stresses. Phytopathology 2019, 109, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Harlan, J.R.; de Wet, J.M.J. Toward a rational classification of cultivated plants. Taxon 1971, 20, 509–517. [Google Scholar] [CrossRef]
- Tanksley, S.D.; Nelson, J.C. Advanced backcross QTL analysis: A method for the simultaneous discovery and transfer of valuable QTLs from unadapted germplasm into elite breeding lines. Theor. Appl. Genet. 1996, 92, 191–203. [Google Scholar] [CrossRef]
- Eshed, Y.; Zamir, D. An introgression line population of Lycopersicon pennellii in the cultivated tomato enables the identification and fine mapping of yield-associated QTL. Genetics 1995, 141, 1147–1162. [Google Scholar] [CrossRef]
- Bayer, P.E.; Golicz, A.A.; Scheben, A.; Batley, J.; Edwards, D. Plant pan-genomes are the new reference. Nat. Plants 2020, 6, 914–920. [Google Scholar] [CrossRef]
- Elshire, R.J.; Glaubitz, J.C.; Sun, Q.; Poland, J.A.; Kawamoto, K.; Buckler, E.S.; Mitchell, S.E. A robust, simple genotyping-by-sequencing (GBS) approach for high diversity cpecies. PLoS ONE 2011, 6, e19379. [Google Scholar] [CrossRef]
- Hajjar, R.; Hodgkin, T. The use of wild relatives in crop improvement: A survey of developments over the last 20 years. Euphytica 2007, 156, 1–13. [Google Scholar] [CrossRef]
- Watson, A.; Ghosh, S.; Williams, M.J.; Cuddy, W.S.; Simmonds, J.; Rey, M.D.; Asyraf Md Hatta, M.; Hinchliffe, A.; Steed, A.; Reynolds, D.; et al. Speed breeding is a powerful tool to accelerate crop research and breeding. Nat. Plants 2018, 4, 23–29. [Google Scholar] [CrossRef]
- Lemmon, Z.H.; Reem, N.T.; Dalrymple, J.; Soyk, S.; Swartwood, K.E.; Rodriguez-Leal, D.; Van Eck, J.; Lippman, Z.B. Rapid improvement of domestication traits in an orphan crop by genome editing. Nat. Plants 2018, 4, 766–770. [Google Scholar] [CrossRef]
- Li, T.; Yang, X.; Yu, Y.; Si, X.; Zhai, X.; Zhang, H.; Dong, W.; Gao, C.; Xu, C. Domestication of wild tomato is accelerated by genome editing. Nat. Biotechnol. 2018, 36, 1160–1163. [Google Scholar] [CrossRef]
- Zsögön, A.; Čermák, T.; Naves, E.; Notini, M.M.; Edel, K.H.; Weinl, S.; Freschi, L.; Voytas, D.F.; Kudla, J.; Peres, L.E.P. De novo domestication of wild tomato using genome editing. Nat. Biotechnol. 2018, 36, 1211–1216. [Google Scholar] [CrossRef]
- Yu, H.; Lin, T.; Meng, X.; Du, H.; Zhang, J.; Liu, G.; Chen, M.; Jing, Y.; Kou, L.; Li, X.; et al. A route to de novo domestication of wild allotetraploid rice. Cell 2021, 184, 1156–1170. [Google Scholar] [CrossRef]
- FAOSTAT. 2021. Available online: https://www.fao.org/faostat/en/#data (accessed on 24 September 2023).
- Erenstein, O.; Jaleta, M.; Mottaleb, K.A.; Sonder, K.; Donovan, J.; Braun, H.-J. Global Trends in Wheat Production, Consumption and Trade. In Wheat Improvement; Reynolds, M.P., Braun, H.-J., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 47–66. [Google Scholar]
- International Grains Council. 2021. Available online: https://www.igc.int/en/default.aspx (accessed on 24 September 2023).
- Raj, S.; Brinkley, C.; Ulimwengu, J. Connected and extracted: Understanding how centrality in the global wheat supply chain affects global hunger using a network approach. PLoS ONE 2022, 17, e0269891. [Google Scholar] [CrossRef]
- Wang, R.; Lu, B. Biosystematics and evolutionary relationships of perennial Triticeae species revealed by genomic analyses. J. Syst. Evol. 2014, 52, 697–705. [Google Scholar] [CrossRef]
- Soltis, P.S.; Soltis, D.E. The role of genetic and genomic attributes in the success of polyploids. Proc. Natl. Acad. Sci. USA 2000, 97, 7051–7057. [Google Scholar] [CrossRef]
- Pennington, P.D.; Costa, L.M.; Gutierrez-Marcos, J.F.; Greenland, A.J.; Dickinson, H.G. When genomes collide: Aberrant seed development following maize interploidy crosses. Ann. Bot. 2008, 101, 833–843. [Google Scholar] [CrossRef]
- Monnier, M. Culture of zygotic embryos of higher plants. In Plant Cell and Tissue Culture. Methods in Molecular Biology; Pollard, J.W., Walker, J.M., Eds.; Humana Press: Totowa, NJ, USA, 1990; Volume 6, pp. 129–139. [Google Scholar] [CrossRef]
- McFadden, E.S.; Sears, E.R. The artificial synthesis of Triticum spelta. Records Genet. Soc. Am. 1944, 13, 26–27. [Google Scholar]
- Olsson, G. Species crosses within the genus Brassica. II. Artificial Brassica napus L. Hereditas 1960, 45, 351–386. [Google Scholar] [CrossRef]
- Sundberg, E.; Glimelius, K. Resynthesis of Brassica napus via somatic hybridization: A model for production of interspecific hybrids within Brassiceae. In Genetic Manipulation in Plant Breeding: Proceedings International Symposium Organized by EUCARPIA, Berlin (West), Germany, 8–13 September 1985; Horn, W., Jensen, C.J., Odenbach, W., Schieder, O., Eds.; De Gruyter: Berlin, Germany, 1986; pp. 709–712. [Google Scholar] [CrossRef]
- Trethowan, R.; van Ginkel, M. Synthetic wheat—An emerging genetic resource. In Wheat Science and Trade; Carver, B.F., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2009; pp. 369–385. [Google Scholar] [CrossRef]
- Aberkane, H.; Payne, T.; Kishi, M.; Smale, M.; Amri, A.; Jamora, N. Transferring diversity of goat grass to farmer’s fields through the development of synthetic hexaploid wheat. Food Sec. 2020, 12, 1017–1033. [Google Scholar] [CrossRef]
- Joppa, L.R.; Williams, N.D. Registration of Largo, a greenbug resistant hexaploid wheat. Crop Sci. 1982, 22, 901–902. [Google Scholar] [CrossRef]
- Hollenhorst, M.W.; Joppa, L.R. Chromosomal location of genes for resistance to greenbug in ‘Largo’ and ‘Amigo’ wheats. Crop Sci. 1983, 23, 91–93. [Google Scholar] [CrossRef]
- Rudd, J.D.; Devkota, R.N.; Baker, J.A.; Peterson, G.L.; Lazar, M.D.; Bean, B.; Worrall, D.; Baughman, D.; Marshall, D.; Sutton, R.; et al. ‘TAM 112’ wheat, resistant to greenbug and wheat curl mite and adapted to the dryland production system in the southern high plains. J. Plant Reg. 2014, 8, 291–297. [Google Scholar] [CrossRef]
- Dhakal, S.; Tan, C.-T.; Anderson, V.; Yu, H.; Fuentealba, M.P.; Rudd, J.C.; Haley, S.D.; Xue, Q.; Ibrahim, A.M.; Garza, L.; et al. Mapping and KASP marker development for wheat curl mite resistance in “TAM 112” wheat using linkage and association analysis. Mol. Breed. 2018, 38, 119. [Google Scholar] [CrossRef]
- Sharma, J.S.; Overlander, M.; Faris, J.D.; Klindworth, D.L.; Rouse, M.N.; Kang, H.; Long, Y.; Jin, Y.; Lagudah, E.S.; Xu, S.S. Characterization of synthetic wheat line Largo for resistance to stem rust. G3 Genes Genomes Genet. 2021, 11, jkab193. [Google Scholar] [CrossRef]
- Molero, G.; Coombes, B.; Joynson, R.; Pinto, F.; Piñera-Chávez, F.J.; Rivera-Amado, C.; Hall, A.; Reynolds, M.P. Exotic alleles contribute to heat tolerance in wheat under field conditions. Commun. Biol. 2023, 6, 21. [Google Scholar] [CrossRef]
- Kishii, M. An update of recent use of Aegilops species in wheat breeding. Front. Plant Sci. 2019, 10, 585. [Google Scholar] [CrossRef]
- Bertioli, D.J.; Gao, D.; Ballen-Taborda, C.; Chu, Y.; Ozias-Akins, P.; Jackson, S.A.; Holbrook, C.C.; Leal-Bertioli, S.C.M. Registration of GA-BatSten1 and GA-MagSten1, two induced allotetraploids derived from peanut wild relatives with superior resistance to leaf spots, rust, and root-knot nematode. J. Plant Regist. 2021, 15, 372–378. [Google Scholar] [CrossRef]
- Chu, Y.; Stalker, H.T.; Marasigan, K.; Levinson, C.M.; Gao, D.; Bertioli, D.J.; Leal-Bertioli, S.C.M.; Corley Holbrook, C.; Jackson, S.A.; Ozias-Akins, P. Registration of three peanut allotetraploid interspecific hybrids resistant to late leaf spot disease and tomato spotted wilt. J. Plant Regist. 2021, 15, 562–572. [Google Scholar] [CrossRef]
- Gill, B.S.; Raupp, W.J. Direct genetic transfers from Aegilops squarrosa L. to hexaploid wheat. Crop Sci. 1987, 27, 445–450. [Google Scholar] [CrossRef]
- Joppa, L.R.; Cantrell, R.G. Chromosomal location of genes for grain protein content of wild tetraploid wheat. Crop Sci. 1990, 30, 1059–1064. [Google Scholar] [CrossRef]
- Joppa, L.R.; Du, C.; Hart, G.E.; Hareland, G.A. Mapping gene(s) for grain protein in tetraploid wheat (Triticum turgidum L.) using a population of recombinant inbred chromosome lines. Crop Sci. 1997, 37, 1586–1589. [Google Scholar] [CrossRef]
- Grama, A.; Gerechter-Amitai, Z.K. Inheritance of resistance to stripe rust (Puccinia striiformis) in crosses between wild emmer (Triticum dicoccoides) and cultivated tetraploid and hexaploid wheats. II. Triticum aestivum. Euphytica 1974, 23, 393–398. [Google Scholar] [CrossRef]
- Valkoun, J.; Kučerova, D.; Bartoŝ, P. Transfer of a new gene for stem rust resistance from Triticum monococcum L. to hexaploid wheat, T. aestivum L. Genet. Ŝlecht 1989, 25, 209–214. [Google Scholar]
- Hsam, S.L.K.; Huang, X.Q.; Ernst, F.; Hartl, L.; Zeller, F.J. Chromosomal location of genes for resistance to powdery mildew in common wheat (Triticum aestivum L. em Thell.). Alleles at the Pm1 Locus. Theor. Appl. Genet. 1998, 96, 1129–1134. [Google Scholar] [CrossRef]
- Briggle, L.W. Transfer of resistance to Erysiphe graminis f. sp. tritici from Khapli emmer and Yuma durum to hexaploid wheat. Crop Sci. 1966, 6, 459–461. [Google Scholar] [CrossRef]
- Sánchez-Martín, J.; Widrig, V.; Herren, G.; Wicker, T.; Zbinden, H.; Gronnier, J.; Spörri, L.; Praz, C.R.; Heuberger, M.; Kolodziej, M.C.; et al. Wheat Pm4 resistance to powdery mildew is controlled by alternative splice variants encoding chimeric proteins. Nat. Plants 2021, 7, 327–341. [Google Scholar] [CrossRef]
- Schmolke, M.; Mohler, V.; Hartl, L.; Zeller, F.J.; Hsam, S.L.K. A new powdery mildew resistance allele at the Pm4 wheat locus transferred from einkorn (Triticum monococcum). Mol. Breed. 2012, 29, 449–456. [Google Scholar] [CrossRef]
- Reader, S.M.; Miller, T.E. The introduction into bread wheat of a major gene for resistance to powdery mildew from wild emmer wheat. Euphytica 1991, 53, 57–60. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Sun, Q.X.; Ni, Z.F.; Nevo, E.; Yang, T.M. Molecular characterization of a novel powdery mildew resistance gene Pm30 in wheat originating from wild emmer. Euphytica 2002, 123, 21–29. [Google Scholar] [CrossRef]
- Rong, J.K.; Millet, E.; Manisterski, J.; Feldman, M. A new powdery mildew resistance gene: Introgression from wild emmer into common wheat and RFLP-based mapping. Euphytica 2000, 115, 121–126. [Google Scholar] [CrossRef]
- Xie, C.J.; Sun, Q.X.; Ni, Z.F.; Yang, T.M.; Nevo, E.; Fahima, T. Chromosomal location of a Triticum dicoccoides-derived powdery mildew resistance gene in common wheat by using microsatellite markers. Theor. Appl. Genet. 2003, 106, 341–345. [Google Scholar] [CrossRef]
- Murphy, J.P.; Leath, S.; Huynh, D.; Navarro, R.A.; Shi, A. Registration of NC97BGTD7 and NC97BGTD8 wheat germplasms resistant to powdery mildew. Crop Sci. 1999, 39, 884–885. [Google Scholar] [CrossRef]
- Miranda, L.M.; Murphy, J.P.; Marshall, D.; Leath, S. Pm34: A new powdery mildew resistance gene transferred from Aegilops tauschii Coss. to common wheat (Triticum aestivum L.). Theor. Appl. Genet. 2006, 113, 1497–1504. [Google Scholar] [CrossRef]
- Murphy, J.P.; Leath, S.; Huynh, D.; Navarro, R.A.; Shi, A. Registration of NC96BGTD1, NC96BGTD2 and NC96BGTD3 wheat germplasm resistant to powdery mildew. Crop Sci. 1998, 38, 570–571. [Google Scholar] [CrossRef]
- Miranda, L.M.; Murphy, J.P.; Marshall, D.; Cowger, C.; Leath, S. Chromosomal location of Pm35, a novel Aegilops tauschii derived powdery mildew resistance gene introgressed into common wheat (Triticum aestivum L.). Theor. Appl. Genet. 2007, 114, 1451–1456. [Google Scholar] [CrossRef]
- Mohler, V.; Bauer, C.; Schweizer, G.; Kempf, H.; Hartl, L. Pm50: A new powdery mildew resistance gene in common wheat derived from cultivated Emmer. J. Appl. Genet. 2013, 54, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, Y.; Li, Y.; Fahima, T.; Shen, Q.; Xie, C. Introgression of the powdery mildew resistance genes Pm60 and Pm60b from Triticum urartu to common wheat using durum as a ‘bridge’. Pathogens 2022, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhu, K.; Dong, L.; Liang, Y.; Li, G.; Fang, T.; Guo, G.; Wu, Q.; Xie, J.; Chen, Y.; et al. Wheat powdery mildew resistance gene Pm64 derived from wild emmer (Triticum turgidum var. dicoccoides) is tightly linked in repulsion with stripe rust resistance gene Yr5. Crop J. 2019, 7, 761–770. [Google Scholar] [CrossRef]
- Li, Y.; Wei, Z.; Sela, H.; Govta, L.; Klymiuk, V.; Roychowdhury, R.; Chawla, H.S.; Ens, J.; Wiebe, K.; Bocharova, V.; et al. Long-read genome sequencing accelerated the dissection of a rapidly evolving resistance gene cluster and the cloning of Pm69. In Proceedings of the 2nd International Wheat Congress, Beijing, China, 11–15 September 2022. [Google Scholar]
- Mohler, V.; Zeller, F.J.; Wenzel, G.; Hsam, S.L.K. Chromosomal location of genes for resistance to powdery mildew in common wheat (Triticum aestivum L. em Thell.). 9. Gene MlZec1 from the Triticum dicoccoides-derived wheat line Zecoi-1. Euphytica 2005, 142, 161–167. [Google Scholar] [CrossRef]
- Li, Y.; Wei, Z.; Fatiukha, A.; Jaiwar, S.; Wang, H.; Hasan, S.; Liu, Z.; Sela, H.; Krugman, T.; Fahima, T. TdPm60 identified in wild emmer wheat is an ortholog of Pm60 and constitutes a strong candidate for PmG16 powdery mildew resistance. Theor. Appl. Genet. 2021, 134, 2777–2793. [Google Scholar] [CrossRef]
- Gerechter-Amitai, Z.K.; van Silfhout, C.H.; Grama, A.; Kleitman, F. Yr15—A new gene for resistance to Puccinia striiformis in Triticum dicoccoides sel. G-25. Euphytica 1989, 43, 187–190. [Google Scholar] [CrossRef]
- Klymiuk, V.; Yaniv, E.; Huang, L.; Raats, D.; Fatiukha, A.; Chen, S.; Feng, L.; Frenkel, Z.; Krugman, T.; Lidzbarsky, G.; et al. Cloning of the wheat Yr15 resistance gene sheds light on the plant tandem kinase-pseudokinase family. Nat. Commun. 2018, 9, 3735. [Google Scholar] [CrossRef]
- Marais, G.F.; Pretorius, Z.A.; Wellings, C.R.; McCallum, B.M.; Marais, A.S. Leaf rust and stripe rust resistance genes transferred to common wheat from Triticum dicoccoides. Euphytica 2005, 143, 115–123. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, L.Q.; Liu, B.L.; Yan, Z.H.; Zhang, B.; Zhang, H.G.; Zheng, Y.L.; Liu, D.C. Molecular tagging of a stripe rust resistance gene in Aegilops tauschii. Euphytica 2011, 179, 313–318. [Google Scholar] [CrossRef]
- Lopez, S.R.; Wiersma, A.T.; Strauss, N.M.; Watkins, T.; Baik, B.-K.; Zhang, G.; Sehgal, S.K.; Kolb, F.L.; Poland, J.A.; Esten Mason, R.; et al. Description of U6719-004 wheat germplasm with YrAS2388R stripe rust resistance introgression from Aegilops tauschii. J. Plant Regist. 2022, 17, 26–33. [Google Scholar] [CrossRef]
- Dyck, P.L.; Samborski, D.J. The genetics of two alleles for leaf rust resistance at the Lr14 locus in wheat. Can. J. Genet. Cytol. 1970, 12, 689–694. [Google Scholar] [CrossRef]
- Kolodziej, M.C.; Singla, J.; Sanchez-Martin, J.; Zbinden, H.; Simkova, H.; Karafiatova, M.; Dolezel, J.; Gronnier, J.; Poretti, M.; Glauser, G.; et al. A membrane-bound ankyrin repeat protein confers race-specific leaf rust disease resistance in wheat. Nat. Commun. 2021, 12, 956. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Brooks, S.A.; Li, W.; Fellers, J.P.; Trick, H.N.; Gill, B.S. Map-based cloning of leaf rust resistance gene Lr21 from the large and polyploid genome of bread wheat. Genetics 2003, 164, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Raupp, W.J.; Singh, S.; Brown-Guedira, G.L.; Gill, B.S. Cytogenetic and molecular mapping of the leaf rust resistance gene Lr39 in wheat. Theor. Appl. Genet. 2001, 102, 347–352. [Google Scholar] [CrossRef]
- Cox, T.S.; Raupp, W.J.; Gill, B.S. Leaf rust-resistance genes Lr41, Lr42 and Lr43 transferred from Triticum tauschii to common wheat. Crop Sci. 1993, 34, 339–343. [Google Scholar] [CrossRef]
- Lin, G.; Chen, H.; Tian, B.; Sehgal, S.K.; Singh, L.; Xie, J.; Rawat, N.; Juliana, P.; Singh, N.; Shresta, S.; et al. Cloning of the broadly effective wheat leaf rust resistance gene Lr42 transferred from Aegilops tauschii. Nat. Commun. 2022, 13, 3044. [Google Scholar] [CrossRef]
- The, T.T. Chromosome location of genes conditioning stem rust resistance transferred from diploid to hexaploid wheat. Nat. New Biol. 1973, 241, 256. [Google Scholar] [CrossRef]
- Steuernagel, B.; Periyannan, S.; Hernández-Pinzón, I.; Witek, K.; Rouse, M.N.; Yu, G.; Hatta, A.; Ayliffe, M.; Bariana, H.; Jones, J.D.; et al. Rapid cloning of disease resistance genes in plants using mutagenesis and sequence capture. Nat. Biotechnol. 2016, 34, 652–655. [Google Scholar] [CrossRef]
- Luo, J.; Rouse, M.N.; Hua, L.; Li, H.; Li, B.; Li, T.; Zhang, W.; Gao, C.; Wang, Y.; Dubcovsky, J.; et al. Identification and characterization of Sr22b, a new allele of the wheat stem rust resistance gene Sr22 effective against the Ug99 race group. Plant Biotechnol. J. 2022, 20, 554–563. [Google Scholar] [CrossRef]
- McIntosh, R.; Dyck, P.; Cusick, J.; Milne, D. Cytogenetical studies in wheat XIII. Sr35, a third gene from Triticum monococcum for resistance to Puccinia graminis tritici. Z. Pflanzenzuecht. 1984, 92, 1–14. [Google Scholar]
- Saintenac, C.; Zhang, W.; Salcedo, A.; Rousse, M.; Trick, H.; Akhunov, E.; Dubcovsky, J. Identification of wheat gene Sr35 that confers resistance to Ug99 stem rust race group. Science 2013, 341, 783–786. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Rouse, M.N.; Zhang, W.; Zhang, X.; Guo, Y.; Briggs, J.; Dubcovsky, J. Wheat gene Sr60 encodes a protein with two putative kinase domains that confers resistance to stem rust. New Phytol. 2020, 225, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Olson, E.L.; Rouse, M.N.; Pumphrey, M.O.; Bowden, R.L.; Gill, B.S.; Poland, J.A. Simultaneous transfer, introgression, and genomic localization of genes for resistance to stem rust race TTKSK (Ug99) from Aegilops tauschii to wheat. Theor. Appl. Genet. 2013, 126, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Mesfin, A.; Frohberg, R.; Anderson, J.A. RFLP markers associated with high grain protein from Triticum turgidum L. var. dicoccoides introgressed into hard red spring wheat. Crop Sci. 1999, 39, 508–513. [Google Scholar] [CrossRef]
- Rowland, G.G.; Kerber, E.R. Telocentric mapping in hexaploid wheat of genes for leaf rust resistance and other characters derived from Aegilops squarrosa. Can. J. Genet. Cytol. 1974, 16, 137–144. [Google Scholar] [CrossRef]
- Uauy, C.; Distelfeld, A.; Fahima, T.; Blechl, A.; Dubcovsky, J. A NAC gene regulating senescence improves grain protein, zinc, and iron content in wheat. Science 2006, 314, 1298–1301. [Google Scholar] [CrossRef] [PubMed]
- Friebe, B.; Jiang, J.; Raupp, W.J.; McIntosh, R.A.; Gill, B.S. Characterization of wheat-alien translocations conferring resistance to diseases and pests: Current status. Euphytica 1996, 91, 59–87. [Google Scholar] [CrossRef]
- Okamoto, M. Asynaptic effect of chromosome V. Wheat Inf. Serv. 1957, 5, 19–58. [Google Scholar]
- Riley, R.; Chapman, V. Genetic control of the cytologically diploid behavior of hexaploid wheat. Nature 1958, 182, 713–715. [Google Scholar] [CrossRef]
- Sears, E.R. An induced mutant with homoeologous pairing in common wheat. Can. J. Genet. Cytol. 1977, 19, 585–593. [Google Scholar] [CrossRef]
- Dover, G.A.; Riley, R. Prevention of pairing of homoeologous meiotic chromosomes of wheat by an activity of supernumerary chromosomes of Aegilops. Nature 1972, 240, 159–161. [Google Scholar] [CrossRef]
- Riley, R.; Chapman, V.; Miller, T. The determination of meiotic chromosome pairing. In Proceedings of the 4th International Wheat Genetics Symposium, Columbia, MO, USA, 6–11 August 1973. [Google Scholar]
- Dvořák, J. Chromosomal distribution of genes in diploid Elytrigia elongata that promote or suppress pairing of wheat homoeologous chromosomes. Genome 1987, 29, 34–40. [Google Scholar] [CrossRef]
- Koo, D.-H.; Liu, W.; Friebe, B.; Gill, B.S. Homoeologous recombination in the presence of Ph1 gene in wheat. Chromosoma 2017, 126, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Koo, D.-H.; Friebe, B.; Gill, B.S. Homoeologous recombination: A novel and efficient system for broadening the genetic variability in wheat. Agronomy 2020, 10, 1059. [Google Scholar] [CrossRef]
- Li, L.-F.; Zhang, Z.-B.; Wang, Z.-H.; Li, N.; Sha, Y.; Wang, X.-F.; Ding, N.; Li, Y.; Zhao, J.; Wu, Y.; et al. Genome sequences of the five Sitopsis species of Aegilops and the origin of polyploid wheat B-subgenome. Mol. Plant 2022, 15, 488–503. [Google Scholar] [CrossRef]
- Zhang, P.; Dundas, I.S.; McIntosh, R.A.; Xu, S.S.; Park, R.F.; Gill, B.S.; Friebe, B. Wheat– Aegilops introgressions. In Alien Introgression in Wheat. Cytogenetics, Molecular Biology, and Genomics; Molnár-Láng, M., Ceoloni, C., Doležel, J., Eds.; Springer: Cham, Switzerland, 2015; pp. 221–243. [Google Scholar]
- Li, H.; Dong, Z.; Ma, C.; Xia, Q.; Tian, X.; Sehgal, S.; Koo, D.H.; Friebe, B.; Ma, P.; Liu, W. A spontaneous wheat-Aegilops longissima translocation carrying Pm66 confers resistance to powdery mildew. Theor. Appl. Genet. 2020, 133, 1149–1159. [Google Scholar] [CrossRef]
- Yu, G.T.; Matny, O.; Champouret, N.; Steuernagel, B.; Moscou, M.J.; Hernández-Pinzón, I.; Green, P.; Hayta, S.; Smedley, M.; Harwood, W.; et al. Aegilops sharonensis genome-assisted identification of stem rust resistance gene Sr62. Nat. Commun. 2022, 13, 1607. [Google Scholar] [CrossRef]
- Li, S.; Wang, J.; Wang, K.; Chen, J.; Wang, K.; Du, L.; Ye, X. Development of PCR markers specific to Dasypyrum villosum genome based on transcriptome data and their application in breeding Triticum aestivum-D. villosum#4 alien chromosome lines. BMC Genom. 2019, 20, 289. [Google Scholar] [CrossRef]
- Li, H.; Xin, Z.Y.; Ma, Y.Z.; Xu, H.J.; Jia, X. Development and identification of wheat–Haynaldia villosa T6DL.6VS chromosome translocation lines conferring resistance to powdery mildew. Plant Breed. 2005, 124, 203–205. [Google Scholar] [CrossRef]
- Xing, L.; Hu, P.; Liu, J.; Witek, K.; Zhou, S.; Xu, J.; Zhou, W.; Gao, L.; Huang, Z.; Zhang, R.; et al. Pm21 from Haynaldia villosa encodes a CC-NBS-LRR protein conferring powdery mildew resistance in wheat. Mol. Plant 2018, 11, 874–878. [Google Scholar] [CrossRef]
- He, H.; Zhu, S.; Zhao, R.; Jiang, Z.; Ji, Y.; Ji, J.; Qiu, D.; Li, H.-J.; Bie, T. Pm21, encoding a typical CC-NBS-LRR protein, confers broad-spectrum resistance to wheat powdery mildew disease. Mol. Plant 2018, 11, 879–882. [Google Scholar] [CrossRef]
- Zhang, R.Q.; Sun, B.X.; Cheng, J.; Cao, A.Z.; Xing, L.P.; Feng, Y.G.; Lan, C.X.; Chen, P.D. Pm55, a developmental-stage and tissue-specific powdery mildew resistance gene introgressed from Dasypyrum villosum into common wheat. Theor. Appl. Genet. 2016, 129, 1975–1984. [Google Scholar] [CrossRef]
- Zhang, R.Q.; Fan, Y.L.; Kong, L.N.; Wang, Z.J.; Wu, J.Z.; Xing, L.P.; Cao, A.Z.; Feng, Y.G. Pm62, an adult-plant powdery mildew resistance gene introgressed from Dasypyrum villosum chromosome arm 2VL into wheat. Theor. Appl. Genet. 2018, 131, 2613–2620. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xiong, C.; Mu, H.; Yao, R.; Meng, X.; Kong, L.; Xing, L.; Wu, J.; Feng, Y.; Cao, A. Pm67, a new powdery mildew resistance gene transferred from Dasypyrum villosum chromosome 1V to common wheat (Triticum aestivum L.). Crop J. 2021, 9, 882–888. [Google Scholar] [CrossRef]
- Li, S.; Jia, Z.; Wang, K.; Du, L.; Li, H.; Lin, Z.; Ye, X. Screening and functional characterization of candidate resistance genes to powdery mildew from Dasypyrum villosum#4 in a wheat line Pm97033. Theor. Appl. Genet. 2020, 133, 3067–3083. [Google Scholar]
- Zhao, R.; Liu, B.; Jiang, Z.; Chen, T.; Wang, L.; Ji, Y.; Hu, Z.; He, H.; Bie, T. Comparative analysis of genetic efects of wheat-Dasypyrum villosum translocations T6V#2S·6AL and T6V#4S·6DL. Plant Breed. 2019, 138, 503–512. [Google Scholar] [CrossRef]
- Wan, W.; Zhao, R.; Chen, T.; Wang, L.; Zhang, X.; Li, H.; Wang, X.; Bie, T. Rapid development of wheat-Dasypyrum villosum compensating translocations resistant to powdery mildew using a triple marker strategy conducted on a large ph1b-induced population. Theor. Appl. Genet. 2023, 136, 148. [Google Scholar] [CrossRef]
- Zhang, R.; Lu, C.; Meng, X.; Fan, Y.; Du, J.; Liu, R.; Feng, Y.; Xing, L.; Cápal, P.; Holušová, K.; et al. Fine mapping of powdery mildew and stripe rust resistance genes Pm5V/Yr5V transferred from Dasypyrum villosum into wheat without yield penalty. Theor. Appl. Genet. 2022, 135, 3629–3642. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, S.; Lang, T.; Wang, Y.; Long, H.; Deng, G.; Chen, Q.; Guo, Y.; Xuan, P.; Xiao, J.; et al. Molecular cytogenetic identification of the wheat–Dasypyrum villosum T3DL·3V#3S translocation line with resistance against stripe rust. Plants 2022, 11, 1329. [Google Scholar] [CrossRef]
- Qi, L.L.; Pumphrey, M.O.; Friebe, B.; Zhang, P.; Qian, C.; Bowden, R.L.; Rouse, M.N.; Jin, Y.; Gill, B.S. A novel Robertsonian translocation event leads to transfer of a stem rust resistance gene (Sr52) effective against race Ug99 from Dasypyrum villosum into bread wheat. Theor. Appl. Genet. 2011, 123, 159–167. [Google Scholar] [CrossRef]
- Liu, C.; Guo, W.; Wang, Y.; Fu, B.; Doležel, J.; Liu, Y.; Zhai, W.; Said, M.; Molnár, I.; Holušová, K.; et al. Introgression of sharp eyespot resistance from Dasypyrum villosum chromosome 2VL into bread wheat. Crop J. 2023, 11, 1512–1520. [Google Scholar] [CrossRef]
- Zhang, R.Q.; Feng, Y.X.; Li, H.F.; Yuan, H.X.; Dai, J.L.; Cao, A.Z.; Xing, X.P.; Li, H.L. Cereal cyst nematode resistance gene CreV effective against Heterodera filipjevi transferred from chromosome 6VL of Dasypyrum villosum to bread wheat. Mol. Breed. 2016, 36, 122. [Google Scholar] [CrossRef]
- Zhang, Q.P.; Li, Q.; Wang, X.E.; Wang, H.Y.; Lang, S.P.; Wang, Y.L.; Wang, S.L.; Chen, P.D.; Liu, D.J. Development and characterization of a Triticum aestivum-Haynaldia villosa translocation line T4VS.4DL conferring resistance to wheat spindle streak mosaic virus. Euphytica 2005, 145, 317–320. [Google Scholar] [CrossRef]
- Wu, N.; Lei, Y.; Pei, D.; Wu, H.; Liu, X.; Fang, J.; Guo, J.; Wang, C.; Guo, J.; Zhang, J.; et al. Predominant wheat-alien chromosome translocations in newly developed wheat of China. Mol. Breed. 2021, 41, 30. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Ji, J.; Li, H.; Tong, J.; Feng, Y.; Wang, X.; Han, R.; Bie, T.; Liu, C.; Zhu, S. Genetic diversity and evolutionary analyses reveal the powdery mildew resistance gene Pm21 undergoing diversifying selection. Front. Genet. 2020, 11, 489. [Google Scholar] [CrossRef]
- Friebe, B.; Raupp, W.J.; Gill, B.S. Alien genes in wheat improvement. In Wheat in a Global Environment; Bedö, Z., Láng, L., Eds.; Kluwer Academic Publisher: Dordrecht, The Netherlands, 2001; Developments in Plant Breeding; Volume 9, pp. 709–720. [Google Scholar] [CrossRef]
- Ceoloni, C.; Kuzmanović, L.; Forte, P.; Virili, M.E.; Bitti, A. Wheat-perennial Triticeae introgressions: Major achievements and prospects. In Alien Introgression in Wheat. Cytogenetics, Molecular Biology, and Genomics; Molnár-Láng, M., Ceoloni, C., Doležel, J., Eds.; Springer: Cham, Switzerland, 2015; pp. 273–313. [Google Scholar]
- Grewal, S.; Hubbart-Edwards, S.; Yang, C.; Devi, U.; Baker, L.; Heath, J.; Ashling, S.; Scholefield, D.; Howells, C.; Yarde, J.; et al. Rapid identification of homozygosity and site of wild relative introgressions in wheat through chromosome-specific KASP genotyping assays. Plant Biotechnol. J. 2020, 18, 743–755. [Google Scholar] [CrossRef]
- Grewal, S.; Othmeni, M.; Walker, J.; Hubbart-Edwards, S.; Yang, C.; Scholefield, D.; Ashling, S.; Isaac, P.; King, I.P.; King, J. Development of wheat-Aegilops caudata introgression lines and their characterisation using genome-specific KASP markers. Front. Plant Sci. 2020, 11, 606. [Google Scholar] [CrossRef]
- Grewal, S.; Coombes, B.; Joynson, R.; Hall, A.; Fellers, J.; Yang, C.; Scholefield, D.; Ashling, S.; Isaac, P.; King, I.P.; et al. Chromosome-specific KASP markers for detecting Amblyopyrum muticum segments in wheat introgression lines. Plant Genome 2022, 15, e20193. [Google Scholar] [CrossRef]
- Coombes, B.; Fellers, J.P.; Grewal, S.; Rusholme-Pilcher, R.; Hubbart-Edwards, S.; Yang, C.; Joynson, R.; King, I.P.; King, J.; Hall, A. Whole genome sequencing uncovers the structural and transcriptomic landscape of hexaploid wheat/Ambylopyrum muticum introgression lines. Plant Biotechnol. J. 2022, 21, 482–496. [Google Scholar] [CrossRef]
- Grewal, S.; Yang, C.; Hubbart-Edwards, S.; Scholefield, D.; Ashling, S.; Burridge, A.; King, I.P.; King, J. Characterisation of Thinopyrum bessarabicum chromosomes through genome-wide introgressions into wheat. Theor. Appl. Genet. 2017, 131, 389–406. [Google Scholar] [CrossRef]
- Baker, L.; Grewal, S.; Yang, C.-Y.; Hubbart-Edwards, S.; Scholefield, D.; Ashling, S.; Burridge, A.J.; Przewieslik-Allen, A.M.; Wilkinson, P.A.; King, I.P.; et al. Exploiting the genome of Thinopyrum elongatum to expand the gene pool of hexaploid wheat. Theor. Appl. Genet. 2020, 133, 2213–2226. [Google Scholar] [CrossRef] [PubMed]
- The, T.T.; Latter, B.D.H.; McIntosh, R.A.; Ellison, F.W.; Brennan, P.S.; Fisher, J.; Hollamby, G.J.; Rathjen, A.J.; Wilson, R.E. Grain Yields of near-isogenic lines with added genes for stem rust resistance. In Proceedings of the 7th International Wheat Genetic Symposium, Cambridge, UK, 13–19 July 1988. [Google Scholar]
- Olson, E.L.; Brown-Guedira, G.; Marshall, D.; Stack, E.; Bowden, R.L.; Jin, Y.; Rouse, M.; Pumphrey, M.O. Development of wheat lines having a small introgressed segment carrying stem rust resistance gene Sr22. Crop. Sci. 2010, 540, 1823–1830. [Google Scholar] [CrossRef]
- Hatta, M.A.M.; Ghosh, S.; Athiyannan, N.; Richardson, T.; Steuernagel, B.; Yu, G.; Rouse, M.N.; Ayliffe, M.; Lagudah, E.S.; Radhakrishnan, G.V.; et al. Extensive genetic variation at the Sr22 wheat stem rust resistance gene locus in the grasses revealed through evolutionary genomics and functional analyses. Mol. Plant Microbe Interact. 2020, 33, 1286–1298. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Knott, D.R. The transfer of leaf-rust resistance from Agropyron to Triticum by irradiation. Can. J. Genet. Cytol. 1966, 8, 137–143. [Google Scholar] [CrossRef]
- Reynolds, M.P.; Calderini, D.F.; Condon, A.G.; Rajaram, S. Physiological basis of yield gains in wheat associated with the Lr19 translocation from Agropyron elongatum. Euphytica 2001, 119, 139–144. [Google Scholar] [CrossRef]
- Shen, X.; Kong, L.; Ohm, H. Fusarium head blight resistance in hexaploid wheat (Triticum aestivum)-Lophopyrum genetic lines and tagging of the alien chromatin by PCR markers. Theor. Appl. Genet. 2004, 108, 808–813. [Google Scholar] [CrossRef]
- Wang, H.; Sun, S.; Ge, W.; Zhao, L.; Hou, B.; Wang, K.; Lyu, Z.; Chen, L.; Xu, S.; Guo, J.; et al. Horizontal gene transfer of Fhb7 from fungus underlies Fusarium head blight resistance in wheat. Science 2020, 368, eaba5435. [Google Scholar] [CrossRef]
- Zhang, W.; Dubcovsky, J. Association between allelic variation at the Phytoene synthase 1 gene and yellow pigment content in the wheat grain. Theor. Appl. Genet. 2008, 116, 635–645. [Google Scholar] [CrossRef]
- Li, X.; Li, D.; Xuan, Y.; He, Z.; Zhao, L.; Hao, Y.; Ge, W.; Xu, S.; Hou, B.; Wang, B.; et al. Elimination of the yellow pigment gene PSY-E2 tightly linked to the Fusarium head blight resistance gene Fhb7 from Thinopyrum ponticum. Crop J. 2023, 11, 957–962. [Google Scholar] [CrossRef]
- Zhang, W.; Danilova, T.; Zhang, M.; Ren, S.; Zhu, X.; Zhang, Q.; Zhong, S.; Dykes, L.; Fiedler, J.; Xu, S.; et al. Cytogenetic and genomic characterization of a novel tall wheatgrass-derived Fhb7 allele integrated into wheat B genome. Theor. Appl. Genet. 2022, 135, 4409–4419. [Google Scholar] [CrossRef]
- Xu, S.; Lyu, Z.; Zhang, N.; Li, M.; Wei, X.; Gao, Y.; Cheng, X.; Ge, W.; Li, X.; Bao, Y.; et al. Genetic mapping of the wheat leaf rust resistance gene Lr19 and development of translocation lines to break the linkage with yellow pigment. Theor. Appl. Genet. 2023, 136, 200. [Google Scholar] [CrossRef]
- Kim, N.S.; Armstrong, K.; Knott, D.R. Molecular detection of Lophopyrum chromatin in wheat-Lophopyrum recombinants and their use in the physical mapping of chromosome 7D. Theor. Appl. Genet. 1993, 85, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Athiyannan, N.; Zhang, P.; McIntosh, R.; Chakraborty, S.; Hewitt, T.; Bhatt, D.; Forrest, K.; Upadhyaya, N.; Steuernagel, B.; Arora, S.; et al. Haplotype variants of the stripe rust resistance gene Yr28 in Aegilops tauschii. Theor. Appl. Genet. 2022, 135, 4327–4336. [Google Scholar] [CrossRef]
- Kerber, E.R.; Green, G.J. Suppression of stem rust resistance in the hexaploid wheat cv. Canthatch by chromosome 7DL. Can. J. Bot. 1980, 58, 1347–1350. [Google Scholar] [CrossRef]
- Hiebert, C.W.; Moscou, M.J.; Hewitt, T.; Steuernagel, B.; Hernández-Pinzón, I.; Green, P.; Pujol, V.; Zhang, P.; Rouse, M.N.; Jin, Y.; et al. Stem rust resistance in wheat is suppressed by a subunit of the mediator complex. Nat. Commun. 2020, 11, 1123. [Google Scholar] [CrossRef]
- Yahiaoui, N.; Brunner, S.; Keller, B. Rapid generation of new powdery mildew resistance genes after wheat domestication. Plant J. 2006, 47, 85–98. [Google Scholar] [CrossRef]
- Xie, J.; Guo, G.; Wang, Y.; Hu, T.; Wang, L.; Li, J.; Qiu, D.; Li, Y.; Wu, Q.; Lu, P.; et al. A rare single nucleotide variant in Pm5e confers powdery mildew resistance in common wheat. New Phytol. 2020, 228, 1011–1026. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Guo, L.; Wang, Z.; Li, B.; Li, J.; Li, Y.; Qiu, D.; Shi, W.; Yang, L.; Wang, N.; et al. A rare gain of function mutation in a wheat tandem kinase confers resistance to powdery mildew. Nat. Commun. 2020, 11, 680. [Google Scholar] [CrossRef]
- Hewitt, T.; Mueller, M.C.; Molnár, I.; Mascher, M.; Holušová, K.; Šimková, H.; Kunz, L.; Zhang, J.; Li, J.; Bhatt, D.; et al. Highly differentiated region of wheat chromosome 7AL encodes a Pm1a immune receptor that recognizes its corresponding AvrPm1a effector from Blumeria graminis. New Phytol. 2021, 229, 2812–2826. [Google Scholar] [CrossRef]
- Klymiuk, V.; Fatiukha, A.; Raats, D.; Bocharova, V.; Huang, L.; Feng, L.; Jaiwar, S.; Pozniak, C.; Coaker, G.; Dubcovsky, J.; et al. Three previously characterized resistances to yellow rust are encoded by a single locus Wtk1. J. Exp. Bot. 2020, 71, 2561–2572. [Google Scholar] [CrossRef]
- Brueggeman, R.; Rostoks, N.; Kudrna, D.; Kilian, A.; Han, F.; Chen, J.; Druka, A.; Steffenson, B.; Kleinhofs, A. The barley stem rust-resistance gene Rpg1 is a novel disease-resistance gene with homology to receptor kinases. Proc. Natl. Acad. Sci. USA 2002, 99, 9328–9333. [Google Scholar] [CrossRef] [PubMed]
- Sharma Poudel, R.; Al-Hashel, A.F.; Gross, T.; Gross, P.; Brueggeman, R. Pyramiding rpg4- and Rpg1-mediated stem rust resistance in barley requires the Rrr1 gene for both to function. Front. Plant Sci. 2018, 871, 1789. [Google Scholar] [CrossRef] [PubMed]
- Kempf, H.; (Secobra Saatzucht GmbH, Moosburg a. d., Isar, Germany). Personal communication, 2022.
- Clayton, W.D.; Renvoize, S.A. Genera Gramineum, Grasses of the World, Kew Bulletin Additional Series XIII; Her Majesty’s Stationary Office: London, UK, 1986. [Google Scholar]
- Jellen, E.N.; Leggett, M. Cytogenetic Manipulation in Oat Improvement Genetic Resources, Chromosome Engineering, and Crop Improvement. In Cereals; Singh, R.J., Jauhar, P.P., Eds.; Taylor & Francis: London, UK, 2006; pp. 199–231. ISBN 978-1859723760. [Google Scholar]
- Leggett, J.M.; Thomas, H. Oat evolution and cytogenetics. In Oat Crop Production and Utilization; Welch, R., Ed.; Chapman & Hall: London, UK, 1995. [Google Scholar]
- Thomas, H. Cytogenetics of Avena. In Oat Science and Technology; Agronomy Monograf No.33; Marshall, H.G., Sorrells, M.E., Eds.; Merican Society of Agronomy: Madison, WI, USA, 1992; pp. 473–507. [Google Scholar]
- Rajharthy, T.; Thomas, H. Cytogenetics of oats (Avena L.); no. 2; Miscellaneous publications of the Genetics Society of Canada: Ottawa, ON, USA, 1974. [Google Scholar]
- Baum, B.R. Oats: Wild and Cultivated. A monograph of the genus Avena L. (Poaceae); Monogr. No. 14.; Department of Agriculture Supply and Services Canada: Ottawa, ON, Canada, 1977.
- Legget, J.M. Classification and Speciation in Avena. In Oat Science and Technology; Agronomy Monograf, No.33; Marshall, H.G., Sorrells, M.E., Eds.; American Society of Agronomy: Madison, WI, USA, 1992; pp. 29–52. [Google Scholar]
- Malzev, A.I. Wild and Cutivated Oats. Section Euavena Griseb; Works Appl. Bot. Plant. Breed. Suppl No: 38: Leningrad, Russia, 1930. [Google Scholar]
- Zeller, F.J. Nutzung des genetischen Potentials der Avena-Wildarten zur Verbesserung des Saathafers (Avena sativa L.). J. Appl. Bot. 1998, 72, 180–185. [Google Scholar]
- Loskutov, I.G.; Rines, H.W. Avena. In Wild Crop Relatives: Genomic and Breeding Resources: Cereals; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 109–183. [Google Scholar]
- Leggett, J.M. Using and conserving Avena genetic resources. In Proceedings of the 5th International Oats Conference, Saskatoon, SK, Canada, 29 July–6 August 1996; pp. 128–132. [Google Scholar]
- Jellen, E.N.; Phillips, R.L.; Rines, H.W. C-banded karyotypes and polymorphisms in hexaploid oat accessions (Avena spp.) using Wright’s stain. Genome 1993, 36, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska, P.; Kosina, R. Cytogenetic events in the endosperm of amphiploid Avena magna × A. longiglumis. J. Plant Res. 2021, 134, 1047–1060. [Google Scholar] [CrossRef]
- Yan, H.; Bekele, W.A.; Wight, C.P.; Peng, Y.; Langdon, T.; Latta, R.G.; Fu, Y.-B.; Diederichsen, A.; Howarth, C.J.; Jellen, E.N.; et al. High-density marker profiling confirms ancestral genomes of Avena species and identifies D-genome chromosomes of hexaploid oat. Theor. Appl. Genet. 2016, 129, 2133–2149. [Google Scholar] [CrossRef]
- Yan, H.; Ren, Z.; Deng, D.; Yang, K.; Yang, C.; Zhou, P.; Wight, C.P.; Ren, C.; Peng, Y. New evidence confirming the CD genomic constitutions of the tetraploid Avena species in the section Pachycarpa Baum. PLoS ONE 2021, 16, e0240703. [Google Scholar] [CrossRef]
- Katsiotis, A.; Hagidimitriou, M.; Heslop-Harrison, J.S. The close relationship between the A and B genomes in Avena L. (Poaceae) determined by molecular cytogenetic analysis of total genomic, tandemly and dispersed repetitive DNA sequences. Ann. Bot. 1997, 79, 103–109. [Google Scholar] [CrossRef]
- Hutchinson, J.; Postoyko, J. C-banding of Avena species. In Genetic Manipulation in Plant Breeding; De Gruyter: Berlin, Germany; New York, NY, USA, 1986; pp. 157–159. [Google Scholar]
- Jellen, E.N.; Gill, B.S.; Cox, T.S. Genomic in situ hybridization differentiates between A/D and C-genome chromatin and detects intergenomic translocations in polyploid species (genus Avena). Genome 1994, 37, 613–618. [Google Scholar] [CrossRef]
- Ladizinsky, G. A new species of Avena from Sicily, possibly progenitor of hexaploid oats. Genet. Resour. Crop Evol. 1998, 45, 263–269. [Google Scholar] [CrossRef]
- Tomaszewska, P.; Schwarzacher, T.; Heslop-Harrison, J.S.P. Oat chromosome and genome evolution defined by widespread terminal intergenomic translocations in polyploids. Front Plant Sci. 2022, 13, 1026364. [Google Scholar] [CrossRef] [PubMed]
- Markhand, G.S.; Leggett, J.M. The genomes of A. lusitanica, A. hispanica and A. matritensis confirmed using GISH. In Proceedings of the 5th International Oats Conference, Saskatoon, SK, Canada, 29 July–6 August 1996; pp. 347–349. [Google Scholar]
- Kamal, N.; Tsardakas Renhuldt, N.; Bentzer, J.; Gundlach, H.; Haberer, G.; Juhász, A.; Lux, T.; Bose, U.; Tye-Din, J.A.; Lang, D.; et al. The mosaic oat genome gives insights into a uniquely healthy cereal crop. Nature 2022, 606, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Ladizinsky, G.; Zohary, D. Notes of species delimination, species relationships and polyploidy in Avena. Euphytica 1971, 20, 380–395. [Google Scholar] [CrossRef]
- Rajhathy, T. The chromosomes of Avena. In Chromosome Engineering in Plants: Genetics, Breeding and Evolution; Gupta, P.K., Tsuchiya, T., Eds.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1991; pp. 449–467. [Google Scholar]
- Boczkowska, M.; Tarczyk, E. Genetic diversity among Polish landraces of common oat (Avena sativa L.). Genet. Resour. Crop Evol. 2013, 60, 2157–2169. [Google Scholar] [CrossRef]
- Diederichsen, A. Assessments of genetic diversity within a world collection of cultivated hexaploid oat (Avena sativa L.) based on qualitative morphological characters. Genet. Resour. Crop Evol. 2007, 55, 419–440. [Google Scholar] [CrossRef]
- Fu, Y.B.; Peterson, G.W.; Williams, D.; Richards, K.W.; Fetch, J.M. Patterns of AFLP variation in a core subset of cultivated hexaploid oat germplasm. Theor. Appl. Genet. 2005, 111, 530–539. [Google Scholar] [CrossRef]
- Frey, K.J. Genetic resources and their use in oat breeding. In Proceedings of the Second International Oats Conference; Lawes, D.A., Thomas, H., Eds.; Springer: Aberystwyth, UK, 1985; pp. 9–15. [Google Scholar]
- Langer, I.; Frey, K.J.; Bailey, T.B. Production response and stability characteristics of oat cultivars developed in different eras. Crop Sci. 1978, 18, 938–942. [Google Scholar] [CrossRef]
- Rodgers, D.M.; Murphy, J.P.; Frey, K.J. Impact of plant breeding on the grain yield and genetic diversity of spring oats. Crop Sci. 1983, 23, 737–740. [Google Scholar] [CrossRef]
- Burrows, V.D. Breeding oats for food and feed: Conventional and new techniques and materials. In Oats: Chemistry and Technology; Webster, F.H., Ed.; American Association of Cereal Chemists: St. Paul, MN, USA, 1986; pp. 13–46. [Google Scholar]
- Comeau, A. Barley yellow dwarf virus resistance in the genus Avena. Euphytica 1984, 33, 49–55. [Google Scholar] [CrossRef]
- Cox, T.S.; Frey, K.J. Complementarity of genes for high groat-protein percentage from Avena sativa L. and A. sterilis L. Crop Sci. 1985, 25, 106–109. [Google Scholar] [CrossRef]
- Cox, D.J.; Frey, K.J. Improving cultivated oats (Avena sativa L.) with allels for vegetative growth index from A. sterilis L. Theor. Appl. Genet. 1984, 68, 239–245. [Google Scholar] [CrossRef]
- Frey, K.J. Heritability of groat-protein percentage of hexaploid oats. Crop Sci. 1975, 15, 227–228. [Google Scholar] [CrossRef]
- Hsam, S.L.K.; Paderina, E.V.; Gordei, S.; Zeller, F.J. Genetic studies of powdery mildew resistance in cultivated oat (Avena sativa L.) II. Cultivars and breeding lines grown in Northen and Eastern Europe. Hereditas 1998, 129, 227–230. [Google Scholar] [CrossRef]
- Lawrence, P.K.; Frey, K.J. Backcross variability for grain yield in oat species crosses (Avena sativa L. × A. sterilis L.). Euphytica 1975, 24, 77–85. [Google Scholar] [CrossRef]
- Leggett, J.M. The conservation and exploitation of wild oat species. In Proceedings of the 4th International Oats Conference, Adelaide, Australia, 19–23 October 1992; pp. 85–87. [Google Scholar]
- Loskutov, I.G. The collection of wild oat species of C.I.S. as a source of diversity in agricultural traits. Genet. Resour. Crop Evol. 1998, 45, 291–295. [Google Scholar] [CrossRef]
- Ohm, H.W.; Patterson, F.L. A six-parent diallel cross analysis for protein in Avena sterilis L. Crop Sci. 1973, 13, 27–30. [Google Scholar] [CrossRef]
- Rines, H.W.; Stuthman, D.D.; Briggle, L.W.; Youngs, V.L.; Jedlinski, H.; Smith, D.H.; Webster, J.A.; Rothman, P.G. Collection and evaluation of Avena fatua for use in oat improvement. Crop Sci. 1980, 20, 65–68. [Google Scholar] [CrossRef]
- Thro, A.M.; Frey, K.J. Inheritance of groat-oil content and high-oil selection in oats (Avena sativa L.). Euphytica 1985, 34, 251–263. [Google Scholar] [CrossRef]
- Welch, R.W.; Brown, J.C.W.; Leggett, J.M. Interspecific and intraspecific variation in grain and groat characteristics of wild oat (Avena) species: Very high groat (1,3),(1,4)-β-D-glucan in an Avena atlantica genotype. J. Cereal Sci. 2000, 31, 273–279. [Google Scholar] [CrossRef]
- Welch, R.W.; Leggett, J.M. Nitrogen content, oil content, oil composition of oat cultivars (A. sativa) and wild Avena species in relation to nitrogen fertility, yield and partioning of assimilates. J. Cereal Sci. 1997, 26, 105–120. [Google Scholar] [CrossRef]
- Boczkowska, M.; Podyma, W.; Łapiński, B. Oat. In Genetic and Genomic Resources for Grain Cereals Improvement; Elsevier: London, UK, 2016; pp. 159–225. [Google Scholar]
- Ladizinsky, G. Genetic resources and their use in the breeding of oats. In Proceedings of the Second International Oats Conference; Lawes, D.A., Thomas, H., Eds.; Springer: Aberystwyth, UK, 1986; pp. 52–53. [Google Scholar]
- Ohm, H.W.; Shaner, G. Breeding oat for resistance to diseases. In Oat Science and Technology; Agronomy Monograf No.33; Marshall, H.G., Sorrells, M.E., Eds.; American Society of Agronomy: Madison, WI, USA, 1992; pp. 657–683. [Google Scholar]
- Stalker, H.T. Utilization of Wild Species for Crop Improvement; Advances in Agronomy No. 33; Elsevier: Amsterdam, The Netherlands, 1980; pp. 111–147. [Google Scholar]
- Jellen, E.N.; Jackson, E.W.; Maughan, P.J. Oat Improvement and Innovation Using Wild Genetic Resources (Poaceae, Avena spp.): Elevating “Oats” to a New Level and Stature. In Polyploidy and Hybridization for Crop Improvement; Mason, A.S., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 364–376. [Google Scholar]
- Jellen, E.N.; Beard, J. Geographical distribution of a chromosome 7C and 17 intergenomic translocation in cultivated oat. Crop Sci. 2000, 40, 256–263. [Google Scholar] [CrossRef]
- Jellen, E.N.; Rines, H.W.; Fox, S.L.; Davis, D.W.; Phillips, R.L.; Gill, B.S. Characterization of SUN II oat monosomics through C-banding and identification of eight new Sun II monosomics. Theor. Appl. Genet. 1997, 95, 1190–1195. [Google Scholar] [CrossRef]
- Oliver, R.E.; Tinker, N.A.; Lazo, G.R.; Chao, S.; Jellen, E.N.; Carson, M.L.; Rines, H.W.; Obert, D.E.; Lutz, J.D.; Shackelford, I.; et al. SNP discovery and chromosome anchoring provide the first physically-anchored hexaploid oat map and reveal synteny with model species. PLoS ONE 2013, 8, e58068. [Google Scholar] [CrossRef]
- Sanz, M.J.; Jellen, E.N.; Loarce, Y.; Irigoyen, M.L.; Ferrer, E.; Fominaya, A. A new chromosome nomenclature system for oat (Avena sativa L. and A. byzantina C. Koch) based on FISH analysis of monosomic lines. Theor. Appl. Genet. 2010, 121, 1541–1552. [Google Scholar] [CrossRef] [PubMed]
- Kianian, S.F.; Wu, B.-C.; Fox, S.L.; Rines, H.W.; Phillips, R.L. Aneuploid marker assignment in hexaploid oat with the C genome as a reference for determining remnant homoeology. Genome 1997, 40, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Tinker, N.A.; Wight, C.P.; Bekele, W.A.; Yan, W.; Jellen, E.N.; Renhuldt, N.T.; Sirijovski, N.; Lux, T.; Spannagl, M.; Mascher, M. Genome analysis in Avena sativa reveals hidden breeding barriers and opportunities for oat improvement. Commun. Biol. 2022, 5, 474. [Google Scholar] [CrossRef]
- Clamot, G.; Rivoal, R. Genetic resistance to cereal cyst nematode Heterodera avenae in wild oat A. sterilis. Euphytica 1984, 33, 27–32. [Google Scholar] [CrossRef]
- Hsam, S.L.K.; Peters, N.; Paderina, E.V.; Felsenstein, F.; Oppitz, K.; Zeller, F.J. Genetic studies of powdery mildew resistance in common oat (Avena sativa L.) I. Cultivars and breeding lines grown in Western Europe and North America. Euphytica 1997, 96, 421–427. [Google Scholar] [CrossRef]
- Lyrene, P.M.; Shands, H.L. Heading dates in six A. sativa × A. sterilis crosses. Crop Sci. 1975, 15, 359–360. [Google Scholar] [CrossRef]
- Lyrene, P.M.; Shands, H.L. Associations among traits in progenies from A. sativa × A. sterilis crosses. Crop Sci. 1975, 15, 361–363. [Google Scholar] [CrossRef]
- Bacon, R.K. Registration of ‘Ozark’ oat. Crop Sci. 1991, 31, 1383–1384. [Google Scholar] [CrossRef]
- Frey, K.J. Oat improvement with genes from Avena species. In Proceedings of the 4th International Oat Conference, Vol 2: Wild Oats in Agriculture, Adelaide, SA, Australia, 19–23 October 1992; pp. 61–64. [Google Scholar]
- Fox, S.L.; Brown, P.D.; Chong, J. Inheritance of crown rust resistance in four accessions of Avena sterilis L. Crop Sci. 1997, 37, 342–345. [Google Scholar] [CrossRef]
- Gregory, J.W.; Wise, R.P. Linkage of genes conferring specific resistance to crown rust in diploid Avena. Genome 1994, 37, 92–96. [Google Scholar] [CrossRef] [PubMed]
- O’Donoughue, L.S. The identification, localization and utilization of molecular markers for rust resistance genes in oat. In Proceedings of the 5th International Oats Conference, Saskatoon, SK, Canada, 29 July–6 August 1996; pp. 150–155. [Google Scholar]
- Parker, J.H. A preliminary study of the inheritance of rust resistance in oats. Agron. J. 1920, 12, 23–38. [Google Scholar] [CrossRef]
- Marshall, H.G.; Shaner, G.E. Genetics and inheritance in oat. In Oat Science and Technology; Agronomy Monograph, No.33; Marshall, H.G., Sorrells, M.E., Eds.; American Society of Agronomy: Madison, WI, USA, 1992; pp. 509–570. [Google Scholar]
- Gnanesh, B.N.; Mitchell Fetch, J.; Zegeye, T.; McCartney, C.A.; Fetch, T. Oat. In Alien Gene Transfer in Crop Plants; Pratap, A., Kumar, J., Eds.; Springer: New York, NY, USA, 2014; Volume 2, pp. 51–73. [Google Scholar] [CrossRef]
- Park, R.F.; Boshoff, W.H.P.; Cabral, A.L.; Chong, J.; Martinelli, J.A.; McMullen, M.S.; Mitchell Fetch, J.W.; Paczos-Grzęda, E.; Prats, E.; Roake, J.; et al. Breeding oat for resistance to the crown rust pathogen Puccinia coronata f. sp. avenae: Achievements and prospects. Theor. Appl. Genet. 2022, 135, 3709–3734. [Google Scholar] [CrossRef]
- Simons, M.D. Transfer of field resistance to Puccinia coronata from Avena sterilis to cultivated oats by backcrossing. Phytopathology 1985, 75, 314–317. [Google Scholar] [CrossRef]
- Chong, J.; Gruenke, J.; Dueck, R.; Mayert, W.; Woods, S. Virulence of oat crown rust [Puccinia coronata f. sp. avenae] in Canada during 2002–2006. Can. J. Plant Pathol. 2008, 30, 115–123. [Google Scholar] [CrossRef]
- Leonard, K.; Martinelli, J.A. Virulence of oat crown rust in Brazil and Uruguay. Plant Dis. 2005, 89, 802–808. [Google Scholar] [CrossRef]
- Admassu-Yimer, B.; Esvelt-Klos, K.; Griffiths, I.; Cowan, A.; Howarth, C. Mapping of crown rust (Puccinia coronata f. sp. avenae) resistance gene Pc54 and a novel quantitative trait locus effective against powdery mildew (Blumeria graminis f. sp. avenae) in the oat (Avena sativa) line Pc54. Phytopathology 2022, 112, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Ociepa, T.; Okoń, S. Chromosomal location of Pm12—A novel powdery mildew resistance gene from Avena sterilis. Genes 2022, 13, 2409. [Google Scholar] [CrossRef]
- Ociepa, T.; Okoń, S.M.; Nucia, A.; Leśniowska-Nowak, J.; Paczos-Grzęda, E.; Bisaga, M. Molecular identification and chromosomal localization of new powdery mildew resistance gene Pm11 in oat. Theor. Appl. Genet. 2020, 133, 179–185. [Google Scholar] [CrossRef]
- Roderick, H.W.; Jones, E.R.L.; Šebesta, J. Resistance to oat powdery mildew in Britain and Europe: A review. Ann. Appl. Biol. 2000, 136, 85–91. [Google Scholar] [CrossRef]
- Fetch, T.G.; Jin, Y. Letter code system of nomenclature for Puccinia graminis f. sp. avenae. Plant Dis. 2007, 91, 763–766. [Google Scholar] [CrossRef]
- Takeda, K.; Frey, K.J. Simultaneous selection for grain yield and protein percentage in backcross populations from A. sterilis × A. sativa matings by using independent culling levels procedure. Theor. Appl. Genet. 1985, 69, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Frey, K.J. Protein yield and its relationship to other traits in backcross population in from an A. sativa × A. sterilis cross. Crop Sci. 1979, 19, 623–628. [Google Scholar] [CrossRef]
- Rossnagel, B.G.; Bhatty, R.S. Use of A. sterilis and A. maroccana derived A. sativa germplasm to increase groat protein concetration in oat for Western Canada. In Proceedings of the 4th International Oats Conference, Adelaide, Australia, 19–23 October 1992; pp. 134–137. [Google Scholar]
- Frey, K.J.; Holland, J.B. Nine cycles of recurrent selection for increased groat-oil content in oat. Crop Sci. 1999, 39, 1636–1641. [Google Scholar] [CrossRef]
- Schipper, H.; Frey, K.J. Observed gains from three recurrent selection regimes for increased groat-oil content of oat. Crop Sci. 1991, 31, 1505–1510. [Google Scholar] [CrossRef]
- Morikawa, T. Genetic analysis on dwarfness of wild oats, Avena fatua. Jpn. J. Genet. 1989, 64, 363–371. [Google Scholar] [CrossRef]
- Morikawa, T.; Sumiya, M.; Kuriyama, S. Transfer of new dwarfing genes from the weed species Avena fatua into cultivated oat A. byzantina. Plant Breed. 2007, 126, 30–35. [Google Scholar] [CrossRef]
- Milach, S.C.; Rines, H.W.; Phillips, R.L.; Stuthman, D.D.; Morikawa, T. Inheritance of a new dwarfing gene in oat. Crop Sci. 1997, 38, 356–360. [Google Scholar] [CrossRef]
- Suneson, C.A. Registration of Montezuma oats. Crop Sci. 1969, 9, 527. [Google Scholar] [CrossRef]
- Thompson, R.K. Registration of Mesa oats. Crop Sci. 1967, 7, 167. [Google Scholar] [CrossRef]
- Dyck, P.L.; Rajharthy, T. Cytogenetics of a hexaploid oat with an extra pair of chromosomes. Can. J. Genet. Cytol. 1963, 5, 408–413. [Google Scholar] [CrossRef]
- Sharma, D.C. Disomic alien chromosome substitution and addition in hexaploid oat. Euphytica 1978, 27, 581–586. [Google Scholar] [CrossRef]
- Thomas, H. Evaluation of the use of species hybrids and synthetic amphiploids in the improvement of the oat crop. Euphytica 1968, 17, 404–413. [Google Scholar] [CrossRef]
- Thomas, H.; Bahatti, I.M. Notes on the cytogenetic structure of the cultivated oat Avena sativa (2n = 6x = 42). Euphytica 1975, 24, 149–157. [Google Scholar] [CrossRef]
- Sharma, D.C. Chromosome pairing problems in interploidy transfer of leaf rust resistance in oats. Euphytica 1975, 24, 503–510. [Google Scholar] [CrossRef]
- Brown, P.D.; Forsberg, R.A.; McKenzie, R.I.H.; Martens, J.W. The use of disomic addition lines in the transfer of oat stem resistance to hexaploid oats. In Proceedings of the Second International Oats Conference; Lawes, D.A., Thomas, H., Eds.; Springer: Aberystwyth, UK, 1985; pp. 16–20. [Google Scholar]
- Aung, T.; Thomas, H.; Jones, I.T. The transfer of the gene for mildew resistance from Avena barbata (4x) into the cultivated oat A. sativa by an induced translocation. Euphytica 1977, 26, 623–632. [Google Scholar] [CrossRef]
- Sharma, D.C.; Forsberg, R.A. Spontaneous and induced interspecific gene transfer for crown rust resistance in Avena. Crop Sci. 1977, 17, 855–860. [Google Scholar] [CrossRef]
- Rajhathy, T.; Thomas, H. Genetic control of chromosome pairing in hexaploid oats. Nat. New Biol. 1972, 239, 217–219. [Google Scholar] [CrossRef]
- Riley, R.; Chapman, V. The effects of the deficiency of chromosom V (5B) of Triticum aestivum on the meiosis of synthetic amphiploids. Heredity 1963, 18, 473–484. [Google Scholar] [CrossRef]
- Jauhar, P.P. Genetic regulation of diploid-like chromosome pairing in Avena. Theor. Appl. Genet. 1977, 49, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, F.M.; McGinnis, R.C. The meiotic behavior of a nulliploid plant in Avena sativa L. Can. J. Genet. Cytol. 1968, 10, 186–189. [Google Scholar] [CrossRef]
- Rothman, P.G. Registration of four stem rust and crown rust resistant oat germplasm lines. Crop Sci. 1984, 24, 1217–1218. [Google Scholar] [CrossRef]
- Aung, T.; Chong, J.; Leggett, J.M. The transfer of crown rust resistance gene Pc94 from a wild diploid to cultivated hexaploid oat. In Proceedings of the 9th European and Mediterranean Cereal Rusts and Powdery Mildews Conference, Lunteren, The Netherlands, 2–6 September 1996; p. 3. [Google Scholar]
- Dyck, P.L.; Zillinsky, F.L. Inheritance of crown rust resistance transferred from diploid to hexaploid oats. Can. J. Genet. Cytol. 1963, 5, 398–407. [Google Scholar] [CrossRef]
- Rooney, W.L.; Rines, H.R.; Phillips, R.L. Identification of RFLP markers linked to crown rust resistance genes Pc91 and Pc92 in oat. Crop Sci. 1994, 34, 940–944. [Google Scholar] [CrossRef]
- Hsam, S.L.K.; Mohler, V.; Zeller, F.J. The genetics of resistance to powdery mildew in cultivated oats (Avena sativa L.): Current status of major genes. J. Appl. Genet. 2014, 55, 155–162. [Google Scholar] [CrossRef]
- Brown, P.D. The transfer of oat stem rust resistance gene Pg16 from tetraploid Avena barbata Pott. to hexaploidy Avena sativa L. Dissert. Abstr. Int. B Sci. Eng. 1985, 45, 2036B. [Google Scholar]
- Pohler, W.; Hoppe, H.D. Avena macrostachya—A potential gene source for oat breeding. Vortr Pflanzenzüchtg 1991, 20, 66–71. [Google Scholar]
- Yu, J.; Herrmann, M. Inheritance and mapping of a powdery mildew resistance gene introgressed from Avena macrostachya in cultivated oat. Theor. Appl. Genet. 2006, 113, 429–443. [Google Scholar] [CrossRef]
- Hoppe, H.D.; Kummer, M. New productive hexaploid derivatives after introgression from A. pilosa features. Vortr Pflanzenzüchtg 1991, 20, 56–61. [Google Scholar]
- Cabral, A.L.; Park, R.F. Seedling resistances to Puccinia coronata f. sp. avenae in Avena strigosa, A. barbata and A. sativa. Euphytica 2014, 196, 385–395. [Google Scholar] [CrossRef]
- Cabral, A.L.; Singh, D.; Park, R.F. Identification and genetic characterisation of adult plant resistance to crown rust in diploid and tetraploid accessions of Avena. Ann. Appl. Biol. 2011, 159, 220–228. [Google Scholar] [CrossRef]
- Okoń, S.; Paczos-Grzęda, E.; Ociepa, T.; Koroluk, A.; Sowa, S.; Kowalczyk, K.; Chrząstek, M. Avena sterilis L. Genotypes as a Potential Source of Resistance to Oat Powdery Mildew. Plant Dis. 2016, 100, 2145–2151. [Google Scholar] [CrossRef]
- Okoń, S.; Ociepa, T.; Paczos-Grzęda, E.; Ladizinsky, G. Evaluation of resistance to Blumeria graminis (DC.) f. sp. avenae, in Avena murphyi and A. magna genotypes. Crop Prot. 2018, 106, 177–181. [Google Scholar] [CrossRef]
- Paczos-Grzęda, E.; Sowa, S.; Koroluk, A.; Langdon, T. Characteristics of resistance to Puccinia coronata f. sp. avenae in Avena fatua L. Plant Dis. 2018, 102, 2616–2624. [Google Scholar] [CrossRef] [PubMed]
- Paczos-Grzęda, E.; Sowa, S.; Boczkowska, M.; Langdon, T. Detached leaf assays for resistance to crown rust reveal diversity within populations of Avena sterilis L. Plant Dis. 2019, 105, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Paczos-Grzęda, E.; Boczkowska, M.; Sowa, S.; Koroluk, A.; Toporowska, J. Hidden Diversity of Crown Rust Resistance within Genebank Resources of Avena sterilis L. Agronomy 2021, 11, 315. [Google Scholar] [CrossRef]
- Sowa, S.; Paczos-Grzęda, E.; Koroluk, A.; Okoń, S.; Ostrowska, A.; Ociepa, T.; Chrząstek, M.; Kowalczyk, K. Resistance to Puccinia coronata f. sp. avenae in Avena magna, A. murphyi, and A. insularis. Plant Dis. 2016, 100, 1184–1191. [Google Scholar] [CrossRef]
- Sowa, S.; Mohler, V.; Paczos-Grzęda, E. Searching for novel oat crown rust resistance in diploid oat Avena strigosa Schreb. reveals the complexity and heterogeneity of the analyzed genebank accessions. Agriculture 2023, 13, 296. [Google Scholar] [CrossRef]
- Rines, H.W.; Porter, H.L.; Carson, M.L.; Ochocki, G.E. Introgression of crown rust resistance from diploid oat Avena strigosa into hexaploid cultivated oat A. sativa by two methods: Direct crosses and through an initial 2x 4x synthetic hybrid. Euphytica 2007, 158, 67–79. [Google Scholar] [CrossRef]
- Ladizinsky, G.; Fainstein, R. Introgression between the cultivated hexaploid oat A. sativa and the tetraploid wild A. magna and A. murphyi. Can. J. Genet. Cytol. 1977, 19, 59–60. [Google Scholar] [CrossRef]
- Ladizinsky, G. A synthetic hexaploid (2n=42) oat from the cross of Avena strigosa (2n = 14) and domesticated A. magna (2x = 28). Euphytica 2000, 116, 231–235. [Google Scholar] [CrossRef]
- Ladizinsky, G. Domestication via hybridization of the wild tetraploid oats Avena magna and A. murphyi. Theor. Appl. Genet. 1995, 91, 639–646. [Google Scholar] [CrossRef]
- Jellen, E.N.; Jackson, E.W.; Elhadji, T.; Young, L.K.; El Mouttaqi, A.; Al Halfa, I.; El Fartassi, I.; Sanata Katile, L.; Linchangco, R.; Klassen, K.; et al. Adaptation and agronomic performance of domesticated moroccan oat (Avena magna ssp. domestica). Lines under subsistence farming conditions at multiple locations in Morocco. Agronomy 2021, 11, 1037. [Google Scholar] [CrossRef]
- Thiam, E.; Jellen, E.N.; Jackson, E.W.; Nelson, M.; Rogers, W.; El Mouttaqi, A.; Benlhabib, O. Productivity and stability evaluation of 12 selected Avena magna ssp. domestica lines based on multi-location experiments during three cropping seasons in Morocco. Agriculture 2023, 13, 1486. [Google Scholar] [CrossRef]
- McCartney, C.A.; Stonehouse, R.G.; Rossnagel, B.G.; Eckstein, P.E.; Scoles, G.J.; Zatorski, T.; Beattie, A.D.; Chong, J. Mapping of the oat crown rust resistance gene Pc91. Theor. Appl. Genet. 2011, 122, 317–325. [Google Scholar] [CrossRef]
- Łapiński, B.; Nita, Z.; Szołkowska, A.; Wieczorek, P. A hybrid of cultivated oat with the wild species Avena macrostachya as a source of new variation for yield quality improvement in naked oats. Biuletyn IHAR 2013, 270, 43–54. [Google Scholar] [CrossRef]
- Chaffin, A.S.; Huang, Y.-F.; Smith, S.; Bekele, W.A.; Babiker, E.; Gnanesh, B.N.; Foresman, B.J.; Blanchard, S.G.; Jay, J.J.; Reid, R.W.; et al. A consensus map in cultivated hexaploid oat reveals conserved grass synteny with substantial subgenome rearrangement. Plant Genome 2016, 9, 1–21. [Google Scholar] [CrossRef]
- GrainGenes: A Database for Triticeae and Avena, Avena sativa, OT3098 v2, PepsiCo. Available online: https://wheat.pw.usda.gov/jb?data=/ggds/oat-ot3098v2-pepsico (accessed on 25 January 2023).
- Peng, Y.; Yan, H.; Guo, L.; Deng, C.; Wang, C.; Wang, Y.; Kang, L.; Zhou, P.; Yu, K.; Dong, X.; et al. Reference genome assemblies reveal the origin and evolution of allohexaploid oat. Nat. Genet. 2022, 54, 1248–1258. [Google Scholar] [CrossRef]

| Disease | Gene | Line(s) | Chromosome Constitution | Reference |
|---|---|---|---|---|
| Powdery mildew | Pm21 | Several NAU427 DvRes-1 | T6AL.6V#2S Cryptic 6V#2S introgression not published | [98] [99] [100] |
| Pm55 | NAU421 | T5AL.5V#4S | [101] | |
| Pm62 | NAU1823 | T2BS.2V#5L | [102] | |
| Pm67 | NAU1817 | T1DL.1V#5S | [103] | |
| PmV | Pm97033 RIL 12401 Dv6T25 Dv6T31 | T6DL.6V#4S T6AL.6V#4S-6V#2S short distal 6VS segment short proximal 6VS segment | [104] [105] [106] [106] | |
| Pm5V | NAU1908 | T5DL.5V#5S | [107] | |
| Stripe rust | YrCD-3 | 22-12 | T3DL.3V#3S | [108] |
| Yr5V | NAU1908 | T5DL.5V#5S | [107] | |
| Stem rust | Sr52 | Several | T6AS.6V#3L | [109] |
| Sharp eyespot | - | NAU2V–8 | T2DS.2V#4L | [110] |
| Cereal cyst nematode | CreV | NAU423 | T6AS.6V#4L | [111] |
| Wheat spindle streak mosaic virus | Wss1 | NAU413 | T4VS.4DL | [112] |
| Section/Species | Chromosome Number | Genomic Constitution |
|---|---|---|
| Section: Avenotrichon | ||
| A. macrostachya Bal. ex Coss. et Dur. | 2n = 4x = 28 | CmCmCmCm |
| Section: Ventricosa | ||
| A. clauda Dur. | 2n = 2x = 14 | CpCp |
| A. eriantha Dur. | 2n = 2x = 14 | CpCp |
| A. ventricosa Bal. ex Coss. | 2n = 2x = 14 | CvCv |
| Section: Agraria | ||
| A. brevis Roth. | 2n = 2x = 14 | AsAs |
| A. hispanica Lag. | 2n = 2x = 14 | AsAs |
| A. nuda L. | 2n = 2x = 14 | AsAs |
| A. strigosa Schreb. | 2n = 2x = 14 | AsAs |
| Section: Tenuicarpa | ||
| A. atlantica Baum et Fedak | 2n = 2x = 14 | AsAs |
| A. canariensis Baum Rajhathy et Sampson | 2n = 2x = 14 | AcAc |
| A. damascena Rajhathy et Baum | 2n = 2x = 14 | AdAd |
| A. hirtula Lag. | 2n = 2x = 14 | AsAs |
| A. longiglumis Dur. | 2n = 2x = 14 | AlAl |
| A. lusitanica (Table Mar.) Baum Comb et Stat. | 2n = 2x = 14 | AsAs |
| A. matritensis Baum Sp. Nov | 2n = 2x = 14 | AA? |
| A. prostrata Ladiz. | 2n = 2x = 14 | ApAp |
| A. wiestii Steud | 2n = 2x = 14 | AsAs |
| A. agadiriana Baum et Fedak | 2n = 4x = 28 | AABB (DDDD) |
| A. barbata Pott. ex Link. | 2n = 4x = 28 | AABB |
| Section: Ethiopica | ||
| A. abyssinica Hochst | 2n = 4x = 28 | AABB |
| A. vaviloviana (Malz.) Mordv. | 2n = 4x = 28 | AABB |
| Section: Pachycarpa | ||
| A. magna Murphy et Terrell | 2n = 4x = 28 | CCDD |
| A. murphyi Ladiz. | 2n = 4x = 28 | CCDD |
| A. insularis Ladiz. | 2n = 4x = 28 | CCDD |
| Section: Avena | ||
| A. byzantina Koch. | 2n = 6x = 42 | AACCDD |
| A. fatua L. | 2n = 6x = 42 | AACCDD |
| A. ludoviciana Dur. | 2n = 6x = 42 | AACCDD |
| A. occidentalis Dur. | 2n = 6x = 42 | AACCDD |
| A. sativa L. | 2n = 6x = 42 | AACCDD |
| A. sterilis L. | 2n = 6x = 42 | AACCDD |
| Disease | Gene | Origin | Introgression Method | Reference |
|---|---|---|---|---|
| Crown rust | Pc15 | A. strigosa | Triploid hybrid bridge, monosomic substitution line irradiation | [239] |
| Pc23 | A. strigosa | Synthetic octoploid backcrosses | [246] | |
| Pc91 | A. magna | Triploid hybrid bridge | [244] | |
| Pc92 | A. strigosa | Autoteraploid, Triploid hybrid bridge | [247] | |
| Pc94 | A. strigosa | Autoteraploid, Triploid hybrid bridge | [245] | |
| Stem rust | Pg6 | A. strigosa | Direct crosses Synthetic octoploid backcrosses | [244] |
| Pg16 | A. barbata | Direct crosses irradiation | [237,249] | |
| Powdery mildew | Pm2 | A. hirtula | - | [248] |
| Pm4 | A. barbata | Direct crosses, Disomic addition line irradiation | [238] | |
| Pm5 | A. macrostachya | Direct crosses with A. magna, backcrosses with A. sativa | [250,251] | |
| Pm7 | A. eriantha | Direct crosses with A. sativa, embryo rescue, backcrosses with A. sativa | [252] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohler, V.; Paczos-Grzęda, E.; Sowa, S. Loving the Alien: The Contribution of the Wild in Securing the Breeding of Cultivated Hexaploid Wheat and Oats. Agriculture 2023, 13, 2060. https://doi.org/10.3390/agriculture13112060
Mohler V, Paczos-Grzęda E, Sowa S. Loving the Alien: The Contribution of the Wild in Securing the Breeding of Cultivated Hexaploid Wheat and Oats. Agriculture. 2023; 13(11):2060. https://doi.org/10.3390/agriculture13112060
Chicago/Turabian StyleMohler, Volker, Edyta Paczos-Grzęda, and Sylwia Sowa. 2023. "Loving the Alien: The Contribution of the Wild in Securing the Breeding of Cultivated Hexaploid Wheat and Oats" Agriculture 13, no. 11: 2060. https://doi.org/10.3390/agriculture13112060
APA StyleMohler, V., Paczos-Grzęda, E., & Sowa, S. (2023). Loving the Alien: The Contribution of the Wild in Securing the Breeding of Cultivated Hexaploid Wheat and Oats. Agriculture, 13(11), 2060. https://doi.org/10.3390/agriculture13112060








