Abstract
Chestnuts are multipurpose trees that grow mainly in the Northern Hemisphere due to their aptitude for fruit and wood production. These trees are vastly significant for the economy and wildlife. The widespread distribution of these trees demonstrates their genetic adaptability to many environmental conditions. The main varieties of European chestnut (Castanea sativa Miller) cultivated in Portugal, their productive challenges and breeding and biotechnological efforts developed over the last decades are described. This paper highlights the efforts focused on the improvement of varieties and rootstocks through selection and hybridization of European chestnut with the Asian species Castanea crenata Siebold and Zuccarini and Castanea mollissima Blume, which are resistant to ink disease, which have been the foundation of the Portuguese chestnut breeding programs. Breeding and biotechnological efforts developed over the last decades, focused on ink disease and chestnut blight resistance, are described. The potentialities of this research to stimulate the competitiveness of bioeconomy-based knowledge and innovation in the productive chestnut sector is also discussed.
1. Introduction
Chestnuts, genus Castanea, are multipurpose trees that grow mainly in the Northern Hemisphere due to their aptitude for fruit and wood production [1,2]. These are vastly significant for the economy and wildlife [1,2]. The name of the genus, Castanea, derived from “Kastah”, which means “dried fruit, seed” in eastern Asia. It is suspected that chestnuts were introduced in Europe by Greeks, where the Latin word Castanea is derived from the Greek “Kastanon” [3,4]. The chestnut is incorporated in the Fagaceae family, in the Castaneoidiae subfamily and in the genus Castanea, which includes sativa and 12 other species, all diploid (2n = 24) [3]. The species were distributed mainly in three regions of the world: Europe and the Mediterranean basin (C. sativa Miller), Asia (China (C. mollissima Blume)), North Korea (C. crenata Siebold and Zuccarini), Republic of Korea (C. seguinii Dode), Japan (C. davidii Dode), Vietnam (C. henryi Rehder and Wilson), and in North America (C. dentata (Marsh.) Borkh, C. pumila Miller, C. floridiana Ashe, C. ashei Sudworth, C. alnifolia Nuttal, C. paucispina Ashe and C. azarkensis) [5,6]. Each species clearly differs from one another in terms of vegetative habits, fruit and wood characteristics, sizes, resistance to biotic and abiotic factors and adaptability. Altogether, these factors have influenced their distribution throughout time.
As result of its dispersal and use in Europe, the species name acquired the epithet sativa (derived from the Latin “sativus”), which means “cultivated” [4]. The introduction and dispersion of the chestnut tree in Portugal, as in other European countries, was made by the Romans during their colonization [7]. C. sativa, which has hundreds of varieties with significant economic potential for fruit production, is one of the most-planted Castanea species in Europe [1]. According to official data made available by FAO [8], over the past few years, harvesting and production of chestnuts have expanded substantially around the world.
Although Castanea species have adapted to different environmental conditions, their production in Europe has been continuously threatened by biotic and abiotic stresses, presently exacerbated by climate change. To tackle this problem, the scientific community has been focusing on identifying the cellular, molecular and genetic interactions underlying all biotic and abiotic stresses in the chestnut tree [2]. Most efforts on this topic have been toward breeding and biotechnology to address the main chestnut’s biotic stresses. Numerous biotic stressors affect Castanea species, but the two most damaging pathogens are Cryphonectria parasitica (Murr.) Barr. (CP) and Phytophthora cinnamomi Rands (PC) [9,10]. C. parasitica causes chestnut blight or chestnut canker, while P. cinnamomi causes the ink disease, also known as root rot. Despite the availability of chemical treatments, they have proven to be ineffective and harmful for the environment [9,10]. Biological treatments based on hypovirulence have successfully controlled chestnut blight in some locations in Europe [10]. Other diseases affect chestnuts, such as the leaf spot (Marssonina ochroleuca), twig canker (Cryptodiaporthe castanea) and chestnut mosaic virus (ChMV), although the damage they inflict is not as severe as that from the above-mentioned diseases [9]. The Asian chestnut gall wasp Dryocosmus kuriphilus is another pest that caused serious impacts on chestnut production and orchard health, but the introduction of the biological control agent Torymus sinensis seems to have had positive results on the issue [10]. Other approaches include, as an example, the use of Castanea crenata (Sieb and Zucc), a rootstock for C. sativa that created hybrid rootstock plants that are advantageously used today due to their growth and disease resistance [11,12], which will be discussed in more detail in the following sections.
The purpose of this review is to provide a description of the main varieties of chestnut cultivated in Portugal, their productive challenges and a description of the most recent accomplishments, as well as challenges underlying their improvement regarding biotic stresses.
2. Chestnut Varieties in Portugal
Portugal occupies the seventh position worldwide in chestnut production, with a production of 37,876 tons, as result of the expansion of chestnut grove planting areas [8]. Portuguese chestnuts have an enhanced quality recognized nationally and internationally. To protect the specificity of this product, and due to its economic and social importance, protected designations of origin (PDO) (EU Regulation no. 1151/2012, article 5) for Portuguese chestnuts were created. This agrarian policy of the European Union outlines territorial quality products as components of local development, whose primary objective is the improvement and preservation of the genetic heritage of the product, setting guidelines, parameters and rules to distinguish the production and market in the various regions [13,14]. Four PDO for chestnut were established: two of them in Trás-os-Montes (“Castanha da Padrela” and “Castanha da Terra Fria”), one in the region of Portalegre (“Castanha de Marvão-Portalegre”) and another in Beira Alta (“Castanha dos Soutos da Lapa”) [10]. A schematic representation of the 4 PDOs in Portugal is depicted in Figure 1, highlighting the municipalities included. Each PDO for chestnut production has its own history, predominant varieties and production area. The most popular and representative varieties in Portugal are “Boa ventura”, “Judia”, “Longal” and “Martaínha”, but many more were identified and cultivated, namely “Amarelal”, “Aveleira”, “Bebim”, “Benfeita”, “Côta”, “Lada”, “Lamela”, “Negral” and “Trigueira”, among others [7,15].
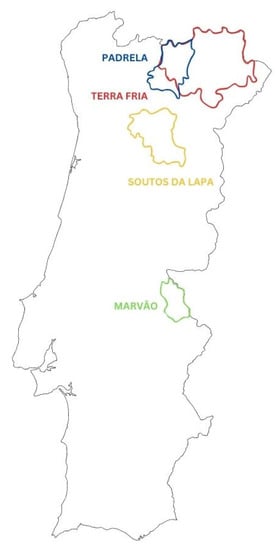
Figure 1.
Chestnut protected designations of origin (PDO) in Portugal. In each PDO, the municipalities included in the PDO production area are depicted. Legend: PADRELA PDO (blue) (Murça, Vila Pouca de Aguiar, Chaves and Valpaços municipalities), TERRA FRIA PDO (red) (Alfândega da Fé, Bragança, Chaves, Macedo de Cavaleiros, Mirandela, Valpaços, Vimioso and Vinhais municipalities), SOUTOS DA LAPA PDO (yellow) (Lamego, Trancoso, Tarouca, Tabuaço, Armamar, Aguiar da Beira, S.João da Pesqueira, Moimenta da Beira, Sernancelhe and Penedono municipalities) and MARVÃO PDO (green) (Castelo de Vide, Marvão and Portalegre municipalities).
In geographical terms, the main production areas are located in the most mountainous part of the North Centre “Soutos da Lapa” and Northeast region in Trás-os-Montes, which have optimal climatic characteristics to produce chestnuts [4]. Trás-os-Montes is the region with the highest national production, and two PDOs can be found there [15]. The presence of the chestnut tree in Trás-os-Montes is millenary, having been one of the main sources of food in the region where the Padrela PDO is found [16]. The Judia is the principal variety of the “Castanha da Padrela PDO”, but contributions from the “Lada”, “Negral”, “Côta”, “Longal” and “Preta” varieties are also included [15]. The geographical production area (production, processing and packaging) is circumscribed to the municipality of Chaves, Murça, Valpaços and Vila Pouca de Aguiar [16]. The typical “Castanha da Padrela PDO” is very versatile and may be in peeled, frozen, candied or syrup form and have some special features, such as striped rind and a good peeling aptitude [16]. Similarly, to the previous PDO, the local rural community of the “Terra Fria PDO” has long depended on the chestnut trees that contribute significantly to the local economy and serve as a source of food [16]. The “Castanha da Terra Fria PDO” includes contribuitions of several varieties, such as the “Amarelal”, “Aveleira”, “Boa ventura”, “Côta”, “Judia”, “Lamela”, “Longal”, “Martaínha”, “Negral” and “Trigueira”, among others. Its production is concentrated in the municipalities of Alfândega da Fé, Bragança, Chaves, Macedo de Cavaleiros, Mirandela, Valpaços and Vimioso e Vinhais [15,16].
In the Beira Alta region, where the “Castanha de Soutos da Lapa PDO” is located, the chestnut tree stands out for its numerous applications in construction and handicraft, with the use of its wood and in the food sector, as well as for fruit consumption [16]. The geographical production area of this PDO is limited to the municipalities of Armamar, Aguiar da Beira, Lamego, Moimenta da Beira, Penedono, S. João da Pesqueira, Tabuaço, Tarouca, Trancoso and Sernancelhe [16]. The main varieties are the “Longal” and “Martaínha” [16].
The “Castanha de Marvão-Portalegre PDO”, in the inner south of Portugal, includes the municipalities of Marvão, Castelo de Vide and Portalegre [16]. Similarly, the Alentejo chestnut orchards represent a considerable source of subsistence for the populations of this area, which have limited economic resources [16]. The color of the “Castanha de Marvão-Portalegre PDO” ranges from dark dull brown in the “Bárea” variety to lighter brown in the “Clarinha” and “Enxerta” varieties and brilliant reddish-brown in the “Bravo” variety [16]. Additionally, the ability to peel varies from good to very good in the main varieties—“Clarinha”, “Enxerta” and “Bravo”—and regular in the “Bárea” variety [16].
3. Ecophysiology and Fruit Characterization
3.1. Plant Growth Requirements
The chestnut (C. sativa) tree is a species of great interest due to its ability to be associated with different agricultural and forestry crops systems [5,17]. This species has great variability in morphological and ecological traits, wide biodiversity and the capacity to adapt to different ecosystems due to its high frugality, reproductive and vegetative growth habits, production (fruit size and wood characteristics), adaptability and resistance to biotic and abiotic stresses [5,17]. However, it is vital to understand the most advantageous environmental conditions to grow them to extract the full productive potential of this crop [5,17].
All chestnut varieties are heliophytic, needing sun exposure [18], with hypogeous germination—deciduous, megaphanerophyte and mesothermic—a characteristic that makes them quite suitable for establishment in mountainous areas and valleys [1]. It is considered a species also adapted to semi-arid to extremely humid climates, tolerating some aridity levels if they last no more than two months [19].
The chestnut tree prefers sub-Atlantic climates with temperatures below minus 15 °C, between 400 to 1000 m altitude, on slightly acidic soils [20]. However, it adapts to many regions with different climates [20]. The optimal conditions for the growth and production of the chestnut tree are found in the fresh, light and well-drained soils based on granitic gravel and siliceous sand or from the decomposition of gneisses, schists, volcanic soils, sandstone and alluvium with moderately high molybdenum rates with the pH below 6–6.5 [20]. It also requires at least 180 days of cold exposure and a growth temperature of −6 °C [1]. The root system adjusts, encouraging extensive horizontal root development rather than deep and strong roots, if penetration challenges occur [19].
3.2. Morphological Characterization
Numerous studies have shown that leaves can adapt, in terms of their morphological and photochemical characteristics, in changing environments. A relationship between temperature variations and changes in the anatomy, chemistry and morphology of chestnut varieties’ leaves has been described [21]. The European chestnut tree has a long lifespan and displays a tremendous stature (it may grow to a height of 30–35 m) [22,23]. The deciduous leaves of the chestnut tree are characterized by their petiolate morphology, with a crenate-serrated or serrated marginal cut and an oblong-lanceolate limb [22,23]. On the extension of the secondary veins, they also have spatulate-aristate teeth that range in size from 5 to 8 cm in width and 10 to 25 cm in length and are subcordate to subtruncate at the base of the lamina [22,23]. This plant is monoicous, meaning that it has separate male and female flowers on the same plant [24]. The staminate flowers are arranged in glomerules of 5–6 flowers with yellow anthesis, from which emerge 10–20 stamens with yellow-greenish ovoid anthers [1]. The male flowers, usually gathered in catkins, either erect or subpendent, may be about 1 cm in diameter and 15–30 cm long and are inserted near the base of their leaves or in the terminal part of the year’s branches [1]. In contrast, the androgynous female flowers are protected by a protective coriaceous envelope of green spinescent bracts with 2–3 flowers, which will give rise to the cupule [25]. A single viable seed-containing ovule (monospermic) or many fertile ovules (polyspermic) from each female flower are used to create fruit [25]. The pollen is viscous, and pollination can be anemophilous or entomophilous [25]. The C. sativa species has a single reddish-brown dry pseudo-fruit usually consisting of three ovoid to subglobose achenes with a small and irregular insertion scar at the base [1,12,25]. Aside from the embryo, each seed contains two cotyledons, the part of the fruit that is edible [25]. The following table represents the morphological characterization of the principal chestnut varieties (Table 1).

Table 1.
Morphological characterization of the principal Portuguese chestnut varieties. Information in this table has been retrieved from [14].
3.3. Fruit Nutritional Characterization
Many works characterize the nutritional qualities and technological aptitude of each chestnut variety. Chestnut is a gluten free fruit, mostly composed of complex carbs, but it also contains a considerable number of proteins (with high biological value) and fibers, which help to control insulin response and cholesterol levels [26]. Compared to other nuts and dried fruits, it is a fruit with a low-fat content [26,27] and an excellent source of vitamins, namely B6, vitamin C and folic acid [28]. It also has a relevant quantity of minerals such as calcium, copper, iron, manganese, magnesium, potassium, phosphorus, selenium and zinc [29]. Indeed, the phenolic compounds it contains further contribute to its nutritional importance [30]. Given its nutritional value and beneficial effects on health, it is a fruit that can be chosen as part of a balanced diet throughout the year. In addition, it can be used in both meat meals and sweets [13,31].
3.4. Commercialization and Uses
In Portugal, chestnut trees have a dual purpose, being planted and cultivated for their fruit as well as for their high-quality wood, which makes them a relevant income source for rural communities’ economies [13]. The selection of the best varieties and the best trees was made through successive generations, choosing those that produced the best nuts and those that gave the best wood. The wood has a good density and is quite appreciated for furniture, cooperage and basketry [13]. Currently, the chestnut’s main markets in Portugal are its commercialization in fresh form for traditional (street roasters) and domestic consumption (boiled, raw or roasted), as well as exportation to the international market in either fresh or frozen form, which has been increasing, particularly to Canada, France, Italy, Spain, Switzerland and the United States [13,32,33]. According to Portuguese data available in [32,33], for July 2022, the trade balance was positive (14,299€ (2010) vs. 20,461€ (2021)), with average export prices higher (24,461€) than import prices (3934€).
4. The Castanea Genetic Resources, Genetic Diversity and Biotechnology Based Approaches for Improvement
The foundation of agricultural progress is represented by plant genetic resources, which also serve as a genetic adaption reserve that serves as a biodiversity repository for defense against environmental changes [34,35]. Due to environmental disruptions, intense parasite infestations and cultivation in recent decades, the genetic variability of the chestnut tree has been significantly diminished in both natural populations and cultivated stands [34,35]. When handled properly, the plant genetic resources supply the raw material that results in new and improved varieties and are an unreplaceable source of features including greater yields, environmental adaptation and pest and disease resistance [34,35]. A huge number of genetic resources [36] has been developed for the American chestnut (Castanea dentata L.) and the Chinese chestnut (Castanea mollissima) throughout several research projects in the scope of the American Chestnut Foundation [37]. Importantly, the genome sequence for these two species is already available at Phytozome [38]. These resources were a relevant basis for comparative genetic and genomic studies in C. sativa, supporting the development of marker-assisted breeding programs for this species [38].
In situ and ex situ gene bank repositories play a major role in the conservation of genetic resources. Some studies about the Castanea species highlight the value of cryopreservation as an approach to conserve genetic resources [39,40]. Throughout cryopreservation, several plant materials/explants, such as recalcitrant seeds, somatic embryos, cell lines, genetically transformed material and vegetatively propagated species, could be safely and long-term conserved [39]. These repositories include the collection and cataloguing of germplasm information, identification, application, description of accessions, molecular research and preservation as part of the backing-up strategy for germplasm [40]. According to Costa [41], Columbano Taveira Fernandes, a member of Vieira Natividade’s team, pioneered the controlled hybridization of chestnut trees in Portugal in the 1950s at Alcobaça. Consequently, a collection of clones that Columbano Fernandes identified has been hand-picked and was made available at the Polytechnic Institute of Bragança’s Escola Superior Agrária (ESA-IPB). Both Centro Nacional de Sementes Florestais (CENASEF, Amarante) and the University of Trás-os-Montes and Alto Douro (UTAD, Vila Real) received the same collection as a gift from IPB [41]. A collection of clones of the “Aveleira”, “Bária”, “Colarinha”, “Côta”, “Judia”, “Lada”, “Longal”, “Martaínha”, “Negral” and “Verdeal” varieties are maintained at the Agricultural Colony by Martim Rei. Clones of the “Martaínha”, “Longal”, “Judia” and “Verdeal” varieties and the hybrid “Marigoule” are maintained at the Agricultural Station of Viseu, while “Amarelal”, “Martaínha” and “Longal” varieties are maintained at the “Quinta de Sergude” [14].
4.1. Intra- and Inter-Varietal Variability
The genetic variability of the regional chestnut varieties differs greatly, with certain varieties being more stable than others [14]. The results of a preliminary study using single sequence repeats (SSR) has been successfully used for typing Portuguese traditional chestnut varieties [42]. These results evidenced a low genetic variability among the varieties, explained to some extent by reduced variability for fruit orchard populations compared to the populations explored for wood or mixed purposes [42]. Despite being mainly propagated by grafting, Portuguese chestnut varieties are polyclonal, which means that a single variety might have many genotypes, indicating the existence of multiple clones of the same variety, possibly due to mutations and cross-pollination between varieties [14]. According to the literature, there are varieties with highly repeated genotypes that may thus be regarded as the primary clone from which additional clones within the same variety have been descended or even having given rise to other varieties through seed [14]. The genetic variability and stability of the varieties are directly related to the common practice of exchanging plant material for grafting [14]. This practice is regularly used in the Northern regions for production purposes [14].
Intra- and inter-varietal diversity studies have been conducted in Portuguese chestnut populations. Dinis et al. [43] used SSR markers to assess the heterogeneity of the genotype of the cultivar “Judia” in Trás-os-Montes, and they describe some intra-varietal differences within the studied accessions. In another approach, seven different types of isoenzyme markers were utilized by Pereira and colleagues [44], allowing for the differentiation of 32 genotype classes in Portuguese varieties. Each class has a matching cultivar or varieties, which have various names (synonymy) and may be genetically extremely similar or genetically different and have the same name but belong to separate classes (homonymy) [44].
According to [42] and [44]’s molecular characterization investigations of Portuguese chestnut varieties, a heteronymy between the varieties “Martaínha” and “Verdeal” can exists, which is supported by the 82% similarity value observed between the two varieties. The varieties “Longal” and “Martaínha” are also mentioned in these articles as being genotypically unstable, meaning that they exhibit high intra-varietal variability. The sets of varieties that emphasize this quality can only be alternative names for the same genotype:
“Cancela” (Lamego) and “Negral” (Valpaços); “Cota” (Murça) and “Negral” (Moimenta da Beira); “Cota” (Valpaços) and “Demanda” (Tarouca); “Lamela” (Vinhais) and “Pelada” (Moimenta da Beira); “Pelada” (Boticas) and “Redonda” (Montalegre); “Riscal” and “Verdeal” (from Armamar).
4.2. Portuguese Breeding Programs and Candidate Gene Identification
Chestnut breeding in Portugal has been focused on the improvement of varieties and rootstocks through selection and hybridization with Asian species resistant to ink disease. Interspecific crosses between the European chestnut and the Asian species of C. crenata and C. mollissima have been the foundation of the Portuguese chestnut breeding programs [45]. The first interspecific crosses in Portugal were made in 1948 by Bernardino Gomes using C. crenata (Tamba variety) as the pollen donor [46,47,48]. At the University Trás-os-Montes e Alto Douro, in the 1990s, Professor Lopes Gomes launched a breeding program that resulted in the development of 53 genotypes resistant to ink disease [49,50]. One of the most well-documented outcomes of this breeding program was the selection of the COLUTAD (COLumbano + UTAD), a hybrid between Castanea sativa × Castanea crenata found resistant to PC and broadly used as rootstock [49].
Another breeding program was started more recently by Costa et al. [50], focused on development of C. sativa × C. crenata and C. sativa × C. mollissima hybrids. Four F1 hybrids were chosen for extensive propagation according to their potential to proliferate and root in vitro, ink disease resistance levels and field development [51,52,53]. The selection process began with the identification of genotypes, in vitro establishment, large-scale propagation and characterization of molecular basis behind susceptible and resistant genotypes [45,54]. The elucidation of the genetic basis and molecular mechanism of P. cinnamomi resistance is a need for the implementation of marker-assisted selection in the breeding programs for resistance, since they will allow the selection of molecular markers and candidate genes to support breeding purposes. Costa et al. [50] performed a DNA marker:trait association analysis to identify quantitative trait loci (QTLs) related to ink disease and also to identify putative resistance genes to P. cinnamomi using a transcriptomic approach. Since the genome of C. sativa is still not available, the authors took advantage of the genetic resources developed and genome sequence available for C. molissima. The genetic linkage map and QTLs developed in an interspecific cross between C. sativa and C. crenata by Santos et al. [51] constituted the first effort to map genomic regions (QTLs) associated with P. cinnamomi resistance. Serrazina et al. [55] compared the root transcriptome of the susceptible species C. sativa and the resistant species C. crenata after P. cinnamomi inoculation in an approach to elucidate chestnut defense mechanisms. These results evidenced that the C. crenata response triggered more relevant changes in expressed genes related with biotic stress upon pathogen inoculation than the same situation in C. sativa, suggesting that despite both species recognizing the pathogen attack, the resistant species (C. crenata) may involve more genes in the defense response than the susceptible species (C. sativa) [55].
Two distinct strategies, genetic mapping and transcriptomics, were combined to better understand the molecular and genetic pathways and to find molecular markers that enable the early selection of resistant genotypes from the ongoing breeding program. Indeed, the genetic linkage maps created allowed the identification of regions of the genome associated with resistance (Table 2) [45].

Table 2.
Summary of efforts to control Cryphonectria parasitica (CP) and Phytophthora cinnamomi (PC) using mapping and identification of Quantitative Trait Loci (QTL) in Castanea.
4.3. The Potential of Genetic Transformation and Genome Editing as a Tool for Pathogen Control
Genetic transformation and breeding have been combined by researchers due to their ability to speed up restoration when compared to standard backcross breeding. Chestnut genetic transformation systems have made tremendous advances, and, today, it is possible to evaluate genes for their capacity to confer pathogen resistance. In the 1990s, Carraway et al. [60] carried out the first attempt on Castanea spp. genetic transformation through embryogenic regeneration systems using microprojectile bombardment; however, they only obtained transgenic calli. Later, Seabra and Pais [61] successfully transformed and regenerated C. sativa shoots expressing the uidA gene encoding for the β-GLUCURONIDASE by Agrobacterium-mediated transformation and regeneration. Nevertheless, and despite many woody crops, the regeneration of transformed plants and their ex vitro acclimation remains a challenge to be addressed.
Since then, chestnut researchers have been dedicated to improving genetic Agrobacterium-mediated transformation of this recalcitrant species. The method involves co-culturing somatic embryos with liquid Agrobacterium suspension, while still in semi-solid multiplication media, and micropropagation of the plants. Several works were published on the Agrobacterium-mediated transformation in C. sativa with pathogen resistance genes as genes of interest, either as an attempt to validate gene function or modulate their resistance levels. Nowadays, cisgenes are the focus of increased investigation [2]. Corredoira et al. [62,63] obtained cisgene overexpressing lines with a thaumatin-like protein (CsTL1) gene and an endochitinase gene (CsCH3) (Table 3).

Table 3.
Genetic transformation studies performed in European and American chestnuts with the goal of developing pathogen control strategies.
The functional characterization of genes involved in plant–pathogen interaction now has new directions thanks to genome editing [68]. Two S-genes, POWDERY MILDEW RESISTANCE 4 (pmr4) and DOWNY MILDEW RESISTANCE 6 (dmr6), which are likely candidates for functional validation via CRISPR/Cas9 knockdown, were identified and chosen by the same research team in C. sativa after PC and CP infection [69]. The potentialities of the genome editing tools and the research on S-genes may enable us to determine if PC has been adapted to the vulnerable chestnuts, possibly inducing effector-triggered susceptibility, and how it is interfering with their immunity [69].
5. Conclusions
Chestnuts are under threat from several biotic pressures, yet numerous initiatives are being developed to overcome the obstacles and conserve this valuable species. In the context of climate change, the prevalence of Phytophthora cinnamomi and Cryphonectria parasitica infections might increase due to the potential emergence of new strains. Several breeding programs have been implemented to improve chestnut resistance, particularly to ink disease. The next phase of chestnut breeding may involve strengthening resistance to both pathogens and searching for long-lasting resistance. Exploring the pathogen’s virulence/avirulence variables specific to chestnut interactions may be beneficial for the future Castanea–pathogen study plan. The resistant genotypes, as a result of the successful approach to develop C. sativa × C. crenata and C. sativa × C. mollissima, are being mass-propagated using micropropagation and tested in the field conditions under various edaphoclimatic conditions, as well as for graft compatibility with Portuguese varieties for fruit production. Still, to overcome the lack of improved germplasm for plantation in Portugal and Europe, several biotechnology-based or conventional breeding approaches are being tested. Understanding the genetic basis of disease resistance, as well as the identification of candidate genes, will support the development of new tools and genotypes capable of coping with the threating scenarios. The use of modern techniques such as CRISPR/Cas systems in trees is opening up new avenues for the functional investigation and characterization of genes. Applying these technical advancements, along with biotic stressors, will enhance our capacity to respond to chestnut challenges and are expected to bring innovation and competitiveness to the productive chestnut sector.
Author Contributions
Conceptualization, J.N.; writing—original draft preparation, M.B.; writing—review and editing, M.B., S.d.S.A., H.S., R.P. and J.N.; supervision, S.d.S.A., H.S., R.P. and J.N.; funding acquisition, J.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the I-CERES project, NORTE-01-0145- FEDER-000082—Norte Portugal Regional Operational Program (NORTE2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF); RHAQ NORTE, NORTE-06-3559-FSE-000103—Norte Portugal Regional Operational Program (NORTE2020), under the PORTUGAL 2020 Partnership Agreement, through the European Social Fund (ESF) and RHAQ CENTRO, CENTRO-04-3559-FSE-000146—Centro Portugal Regional Operational Program (CENTRO2020), under the PORTUGAL 2020 Partnership Agreement, through the European Social Fund (ESF); the Centre Bio R&D Unit (UIDB/05083/2020) funded by Fundação para a Ciência e Tecnologia and the Interface Mission RE-C05-i02, under the Portuguese Recovery and Resilience Plan, through the European Union NextGenerationEU Fund.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article.
Acknowledgments
The authors would like to thank Marta Bobiano (BLC3) for her support during the constructive discussion made on the topic.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gomes-Laranjo, J.; Cardoso-Ferreira, J.; Portela, E.; Abreu, G.C. Castanheiros. 2007. Available online: https://www.cm-sabugal.pt/wp-content/uploads/Castanheiros-livro.pdf (accessed on 29 May 2023).
- Fernandes, P.; Colavolpe, M.B.; Serrazina, S.; Costa, R.L. European and American Chestnuts: An Overview of the Main Threats and Control Efforts. Front. Plant Sci. 2022, 13, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Lage, J. Castanea: Uma Dádiva Dos Deuses, 2nd ed.; Oficina São José: Braga, Portugal, 2006; pp. 1–325. [Google Scholar]
- Moura, A.R.; Gomes-Laranjo, J.C.; Cardoso, J.V. Seleção Clonal Na Variedade de Castanha “Longal” Visando a Sua Aptidão Agronómica e Agroalimentar. Master’s Thesis, University of Trás-os-Montes and Alto Douro, Vila Real, Portugal, 2018. [Google Scholar]
- Mellano, M.G.; Beccaro, G.L.; Donno, D.; Marinoni, D.T.; Boccacci, P.; Canterino, S.; Cerutti, A.K.; Bounous, G. Castanea Spp. Biodiversity Conservation: Collection and Characterization of the Genetic Diversity of an Endangered Species. Genet. Resour. Crop. Evol. 2012, 59, 1727–1741. [Google Scholar] [CrossRef]
- Bounous, G. The Chestnut: A Multipurpose Resource for New Millenium. Acta Hortic. 2005, 693, 33–40. [Google Scholar] [CrossRef]
- Gomes-Laranjo, J.; Peixoto, F.; Costa, R.; Ferreira-Cardoso, J. Portugal, Following Chestnut Footprints, Cultivation and Culture, Folklore and History, Traditions and Uses. Scr. Hortic. 2009, 9, 106–111. [Google Scholar]
- Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/faostat/en/#home (accessed on 29 May 2023).
- Serdar, U.; Saito, T.; Cuenca, B.; Akyüz, B.; Laranjo, J.G.; Beccaro, G.; Bounous, G.; Costa, R.L.; Fernandes, P.; Mellano, M.G. Advances in cultivation of Chestnuts. In Achieving Sustainable Cultivation of Tree Nuts, 1st ed.; Serdar, U., Fulbright, D., Eds.; Burleigh Dodds Science Publishing: Cambridge, UK, 2019; Volume 1, pp. 349–388. [Google Scholar] [CrossRef]
- Instituto Nacional de Investigação Agrária e Veterinária (INIAV). Novos Porta-Enxertos de Castanheiro Com Resistência à Doença Da Tinta. 2018, pp. 1–36. Available online: https://projects.iniav.pt/NewCastRootstocks/images/Manual_INIAV_web.pdf (accessed on 29 May 2023).
- Freitas, G.M.; Igrejas, G.; Santos, H. Caracterização de Cultivares Portuguesas de Castanea Sativa Mill: Uma Análise Proteómica. Master’s Thesis, University of Trás-os-Montes and Alto Douro, Vila Real, Portugal, 2017. [Google Scholar]
- Brito, D.; Gomes-Laranjo, J.; Álvaro, R.C. Produção de Novos Porta-Enxertos Híbridos Para Castanheiro. Caracterização Ecofisiológica Da Geração F1. Master’s Thesis, University of Trás-os-Montes and Alto Douro, Vila Real, Portugal, 2014. [Google Scholar]
- Silva, A.P. Castanha. Um Fruto Saudável. In Projeto Agro 939, Dinamização do Consumo da Castanha Com Denominação de Origem, 1st ed.; Mingo Gráfica: Braga, Portugal, 2007; Volume 1, pp. 1–178. [Google Scholar]
- Costa, R.; Ribeiro, C.; Valdiviesso, T.; Afonso, S.; Borges, O.; Soeiro de Carvalho, J.; Costa, H.A.; Assunção, A.; Fonseca, L.; Augusta, C.; et al. Variedades Portuguesas de Castanha das Regiões Centro e Norte de Portugal, 1st ed.; Instituto Nacional dos Recursos Biológicos, I.P. (INRB, I.P.): Oeiras, Portugal, 2008; pp. 1–78. [Google Scholar]
- Europeia, C. Jornal Oficial Das Comunidades Europeias; European Union: Maastricht, The Netherlands, 1996. [Google Scholar]
- Direção-Geral de Agricultura e Desenvolvimento Rural. Produtos Tradicionais Portugueses. 2018. Available online: https://tradicional.dgadr.gov.pt/pt/ (accessed on 29 May 2023).
- Queijeiro, J.M.; Diaz-Raviña, M.; de la Montaña, J. Edaphic Characterization of Chestnut Tree Orchards in Monterrei (Southeast Galicia, Spain). Ecol. Mediterr. 2000, 26, 163–167. [Google Scholar] [CrossRef]
- Rojo, D.; Santín, J.; Sánchez, J.A. Guía de Buenas Prácticas Para La Conservación y Custodia Del Território Del Castãno; Imprenta Manolete: Asturias, Spain, 2016; pp. 1–37. [Google Scholar]
- Suarez, P.C. Fitopatologia Del Castaño (Castanea Sativa Miller). In Boletin de Sanidad Vegetal; Secretaría General Técnica Ministerio de Agricultura, Pesca y Alimentación: Madrid, Spain, 1989; Volume 3, pp. 1–130. [Google Scholar]
- Gonçalves, J.C. Influência de Alguns Factores Na Micropropagação Do Castanheiro (Castanea Miller). Ph.D. Thesis, Higher Institute of Agronomy, Lisbon, Portugal, 1991. [Google Scholar]
- Dinis, L.; Peixoto, F.; Pinto, T.; Costa, R.; Bennett, R.N.; Gomes-Laranjo, J. Study of Morphological and Phenological Diversity in Chestnut Trees (“Judia” Variety) as a Function of Temperature Sum. Environ. Exp. Bot. 2011, 70, 110–120. [Google Scholar] [CrossRef]
- Bradford, M.; Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Irigoyen, J.J.; Einerich, D.W.; Sánchez-Díaz, M. Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol. Plant. 1992, 84, 55–60. [Google Scholar] [CrossRef]
- Valdiviesso, T.; Mederia, C.; Pinto de Abreu, C. Contribution for The Study of the Chestnut Floral Biology. In Proceedings of the International Congress on Chestnut, Spoleto, Italy, 20–23 October 1993; pp. 95–97. [Google Scholar]
- Giordano, E. Biology, Physiology and Ecology of Chestnut, Comunità Montana Monti Martani e Serano of Spoleto and Istituto di Coltivazioni Arboree; University of Perugia: Perugia, Italy, 1993. [Google Scholar]
- De Vasconcelos, M.C.; Bennett, R.N.; Rosa, E.A.; Ferreira-Cardoso, J.V. Composition of European Chestnut (Castanea Sativa Mill.) and Association with Health Effects: Fresh and Processed Products. J. Sci. Food Agric. 2010, 90, 1578–1589. [Google Scholar] [CrossRef]
- Sales, R.L.; Costa, N.M.B.; Monteiro, J.B.R.; Peluzio, M.D.C.G.; Coelho, S.B.; de Oliveira, C.G.; Mattes, R. Efeitos Dos Óleos de Amendoim, Açafrão e Oliva Na Composição Corporal, Metabolismo Energético, Perfil Lipídico e Ingestão Alimentar de Indivíduos Eutróficos Normolipidêmicos. Rev. Nutr. 2005, 18, 499–511. [Google Scholar] [CrossRef]
- Manela-Azulay, M.; Mandarim-De-Lacerda, C.A.; da Perez, M.A.; Filgueira, A.L.; Cuzzi, T. Vitamina C. An. Bras. Dermatol. 2003, 78, 265–272. [Google Scholar] [CrossRef]
- Borges, O.; Gonçalves, B.; de Carvalho, J.L.S.; Correia, P.; Silva, A.P. Nutritional Quality of Chestnut (Castanea Sativa Mill.) Cultivars from Portugal. Food Chem. 2008, 106, 976–984. [Google Scholar] [CrossRef]
- Dinis, L.R.; Gomes-Laranjo, J.; Peixoto, F.; Costa, R. Castanheiros: Seleção Clonal Na Variedade Judia. Ph.D Thesis, University of Trás-os-Montes and Alto Douro, Vila Real, Portugal, 2011. [Google Scholar]
- Montanhas de Investigação. A Castanha Da Terra Fria DOP Inclui 10 Variedades Do Castanheiro (Castanea Sativa Mill)? Available online: https://morecolab.pt/2020/06/15/castanha-da-terra-fria-10-variedades-do-castanheiro/ (accessed on 29 May 2023).
- Gabinete de Planeamento, Políticas e Administração Geral. Available online: https://www.gpp.pt/ (accessed on 29 May 2023).
- Instituto Nacional de Estatística. Available online: https://www.ine.pt/xportal/xmain?xpgid=ine_main&xpid=INE (accessed on 29 May 2023).
- Esquinas-Alcázar, J.T. Plant Breeding. In Plant Breeding: Principles and Prospects; Hayward, M.D., Bosemark, N.O., Romagosa, I., Cerezo, M., Eds.; Springer: Dordrecht, The Netherlands, 1993; pp. 33–51. [Google Scholar] [CrossRef]
- Carvalho, C. Detecção de Cromatina de Centeio e Análise Da Biodiversidade N landrace de Trigo “Barbela”, Através de Marcadores Genéticos. Ph.D Thesis, University of Trás-os-Montes and Alto Douro, Vila Real, Portugal, 2003. [Google Scholar]
- Kremer, A.; Abbott, A.G.; Carlson, J.E.; Manos, P.S.; Plomion, C.; Sisco, P.; Staton, M.E.; Ueno, S.; Vendramin, G.G. Genomics of Fagaceae. Tree Genet. Genomes 2012, 8, 583–610. [Google Scholar] [CrossRef]
- American Chestnut Foundation. Available online: https://acf.org/ (accessed on 29 May 2023).
- Goodstein, D.M.; Shu, S.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J.; Mitros, T.; Dirks, W.; Hellsten, U.; Putnam, N.; et al. Phytozome: A comparative platform for green plant genomics. Nucleic Acids Res. 2012, 40, D1178–D1186. [Google Scholar] [CrossRef]
- Vidal, N.; Vieitez, A.M.; Fernández, M.R.; Cuenca, B.; Ballester, A. Establishment of Cryopreserved Gene Banks of European Chestnut and Cork Oak. Eur. J. For. Res. 2010, 129, 635–643. [Google Scholar] [CrossRef]
- Guo-Tian, L.; Cheng-Xiang, A.; Li-Si, Z.; Hai-Rong, W.; Qing-Zhong, L. Chestnut Genebank In China National Clonal Plant Germplasm Repository. Acta Hortic. 2009, 199–206. [Google Scholar] [CrossRef]
- Costa, R. Variedades e porta-enxertos. Man. Boas Práticas Frutic. 2020, 6, 4. Available online: https://www.iniav.pt/images/publicacoes/livros-manuais/manual_de_fruticultura_castanheiro.pdf (accessed on 29 May 2023).
- Costa, R.; Valdiviesso, T.; Marum, L.; Fonseca, L.; Borges, O.; Soeiro, J.; Soares, F.M.; Sequeira, J.; Assunção, A.; Correia, P. Characterisation of Traditional Portuguese Chestnut Cultivars by Nuclear SSRS. Acta Hortic. 2005, 437–440. [Google Scholar] [CrossRef]
- Dinis, L.-T.; Peixoto, F.; Costa, R.; Gomes-Laranjo, J. Molecular Characterization Of “Judia” (Castanea Sativa Mill.) From Several Trás-Os-Montes Regions By Nuclear Microsatellite Markers. Acta Hortic. 2010, 225–232. [Google Scholar] [CrossRef]
- Pereira, M.; Castro, L.; Torres-Pereira, J.; Lorenzo, S. Isozyme Polymorphisms in Portuguese Chestnut Cultivars. Acta Hortic. 1999, 283–286. [Google Scholar] [CrossRef]
- Santos, C.; Machado, H.; Serrazina, S.; Gomes, F.; Gomes-Laranjo, J.; Correia, I.; Zhebentyayeva, T.; Duarte, S.; Bragança, H.; Fevereiro, P. Comprehension of Resistance to Diseases in Chestnut. Rev. Ciências Agrárias 2016, 39, 189–193. [Google Scholar] [CrossRef]
- Natividade, J.V. Quatro Anos Na Defesa Da Campanha e Reconstituição Dos Soutos; Boletim Junta Nacional das Frutas: Lisbon, Portugal, 1947; pp. 1–21. [Google Scholar]
- Gomes Guerreiro, M. Alguns Estudos Do Género Castanea. Relatório. Tomos I e II. In Alcobaça: Publicação da Direcção Geral dos Serviços Florestais e Aquícolas; Topografia Alcobacence lda: Alcobaça, Portugal, 1948; Volume XV. [Google Scholar]
- Gomes Guerreiro, M. Castanheiros Alguns Estudos Sobre a Sua Ecologia e o Seu Melhoramento Genético. Master’s Thesis, Higher Institute of Agronomy, Lisbon, Portugal, 1957. [Google Scholar]
- Martins, L.; Anjos, R.; Costa, R.; Gomes-Laranjo, J. Colutad. Um Clone de Castanheiro Resistente à Doença Da Tinta. In Castanheiros. Técnicas e Práticas; Gomes-Laranjo, J., Peixoto, F., Ferreira-Cardoso, J., Eds.; Indústria Criativa and University of Trás-os-Montes and Alto Douro: Vila Real, Portugal, 2009; pp. 135–142. [Google Scholar]
- Costa, R.; Santos, C.; Tavares, F.; Machado, H.; Gomes-Laranjo, J.; Kubisiak, T.; Nelson, C.D. Mapping and Transcriptomic Approches Implemented for Understanding Disease Resistance to Phytophthora Cinammomi in Castanea Sp. BMC Proc. 2011, 5, O18. [Google Scholar] [CrossRef]
- Santos, C.; Machado, H.; Correia, I.; Gomes, F.; Gomes-Laranjo, J.; Costa, R. Phenotyping Castanea Hybrids for Phytophthora Cinnamomi Resistance. Plant Pathol. 2015, 64, 901–910. [Google Scholar] [CrossRef]
- Fernandes, P.; Amaral, A.; Colavolpe, B.; Pereira, A.; Costa, R. Avaliação Agronómica de Genótipos de Castanheiro Selecionados Do Programa de Melhoramento Genético Para a Resistência à Tinta. Vida Rural 2021, 1, 76–82. Available online: https://www.researchgate.net/publication/352878064 (accessed on 29 May 2023).
- Fernandes, P.; Amaral, A.; Colavolpe, B.; Balonas, D.; Serra, M.; Pereira, A.; Costa, R.L. Propagation of New Chestnut Rootstocks with Improved Resistance to Phytophthora cinnamomi—New Cast Rootstocks. Silva Lusit. 2020, 28, 15–29. [Google Scholar] [CrossRef]
- Kubisiak, T.L.; Nelson, C.D.; Staton, M.E.; Zhebentyayeva, T.; Smith, C.; Olukolu, B.A.; Fang, G.-C.; Hebard, F.V.; Anagnostakis, S.; Wheeler, N.; et al. A Transcriptome-Based Genetic Map of Chinese Chestnut (Castanea Mollissima) and Identification of Regions of Segmental Homology with Peach (Prunus Persica). Tree Genet. Genomes 2013, 9, 557–571. [Google Scholar] [CrossRef]
- Serrazina, S.; Santos, C.; Machado, H.; Pesquita, C.; Vicentini, R.; Pais, M.S.; Sebastiana, M.; Costa, R. Castanea Root Transcriptome in Response to Phytophthora Cinnamomi Challenge. Tree Genet. Genomes 2015, 11, 6. [Google Scholar] [CrossRef]
- Zhang, J.; Bernatzky, R.; Anagnostakis, S.L.; Doudrick, R.L.; Kubisiak, F.V.H.T.L.; Ji, F.; Wei, W.; Liu, Y.; Wang, G.; Zhang, Q.; et al. Molecular Mapping of Resistance to Blight in an Interspecific Cross in the Genus Castanea. Phytopathology 1997, 87, 751–759. [Google Scholar] [CrossRef]
- Zhebentyayeva, T.; Chandra, A.; Abbott, A.; Staton, M.; Olukolu, B.; Hebard, F.; Georgi, L.; Jeffers, S.; Sisco, P.; James, J.; et al. Genetic and Genomic Resources For Mapping Resistance To Phytophthora Cinnamomi In Chestnut. Acta Hortic. 2014, 263–270. [Google Scholar] [CrossRef]
- Zhebentyayeva, T.N.; Sisco, P.H.; Georgi, L.L.; Jeffers, S.N.; Perkins, M.T.; James, J.B.; Hebard, F.V.; Saski, C.; Nelson, C.D.; Abbott, A.G. Dissecting Resistance to Phytophthora Cinnamomi in Interspecific Hybrid Chestnut Crosses Using Sequence-Based Genotyping and Qtl Mapping. Phytopathology 2019, 109, 1594–1604. [Google Scholar] [CrossRef]
- Santos, C.; Nelson, C.D.; Zhebentyayeva, T.; Machado, H.; Gomes-Laranjo, J.; Costa, R.L. First Interspecific Genetic Linkage Map for Castanea Sativa x Castanea Crenata Revealed QTLs for Resistance to Phytophthora Cinnamomi. PLoS ONE 2017, 12, e0184381. [Google Scholar] [CrossRef] [PubMed]
- Carraway, D.; Wilde, H.; Merkle, S. Somatic Embryogenesis and Gene Transfer in American Chestnut. J. Am. Chestnut 1994, 6, 29–33. [Google Scholar]
- Seabra, R.C.; Pais, M.S. Genetic transformation of European chestnut. Plant Cell Rep. 1998, 17, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Corredoira, E.; Valladares, S.; Allona, I.; Aragoncillo, C.; Vieitez, A.M. Ballester, Genetic Transformation of European Chestnut Somatic Embryos with a Native Thaumatin-like Protein (CsTL1) Gene Isolated from Castanea Sativa Seeds. Tree Physiol. 2012, 32, 1389–1402. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Corredoira, E.; José, M.C.S.; Vieitez, A.M.; Allona, I.; Aragoncillo, C.; Ballester, A. Agrobacterium-Mediated Transformation of European Chestnut Somatic Embryos with a Castanea Sativa (Mill.) Endochitinase Gene. New For. 2016, 47, 669–684. [Google Scholar] [CrossRef]
- Carlson, E.; Stewart, K.; Baier, K.; McGuigan, L.; Ii, T.C.; Powell, W. Pathogen-induced Expression of a Blight Tolerance Transgene in American Chestnut. Mol. Plant Pathol. 2022, 23, 370–382. [Google Scholar] [CrossRef]
- Polin, L.D.; Liang, H.; Rothrock, R.E.; Nishii, M.; Diehl, D.L.; Newhouse, A.E.; Nairn, C.J.; Powell, W.A.; Maynard, C.A. Agrobacterium-Mediated Transformation of American Chestnut (Castanea Dentata (Marsh.) Borkh.) Somatic Embryos. Plant Cell Tissue Organ Cult. (PCTOC) 2006, 84, 69–79. [Google Scholar] [CrossRef]
- Rothrock, R.E.; Polin-McGuigan, L.D.; Newhouse, A.E.; Powell, W.A.; Maynard, C.A. Plate Flooding as an Alternative Agrobacterium-Mediated Transformation Method for American Chestnut Somatic Embryos. Plant Cell Tissue Organ Cult. (PCTOC) 2007, 88, 93–99. [Google Scholar] [CrossRef]
- McGuigan, L.; Fernandes, P.; Oakes, A.; Stewart, K.; Powell, W. Transformation of American Chestnut (Castanea Dentata (Marsh.) Borkh) Using RITA® Temporary Immersion Bioreactors and We Vitro Containers. Forests 2020, 11, 1196. [Google Scholar] [CrossRef]
- Pavese, V.; Moglia, A.; Corredoira, E.; Martínez, M.T.; Marinoni, D.T.; Botta, R. First Report of CRISPR/Cas9 Gene Editing in Castanea Sativa Mill. Front. Plant Sci. 2021, 12, 1–11. [Google Scholar] [CrossRef]
- Pavese, V.; Moglia, A.; Gonthier, P.; Marinoni, D.T.; Cavalet-Giorsa, E.; Botta, R. Identification of Susceptibility Genes in Castanea sativa and Their Transcription Dynamics following Pathogen Infection. Plants 2021, 10, 913. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).