The Macroalgal Biostimulant Improves the Functional Quality of Tomato Fruits Produced from Plants Grown under Salt Stress
Abstract
1. Introduction
2. Material and Methods
2.1. Seaweed Collection and Extraction
2.2. Preparation of Treatment Solutions
2.3. Tomato Seed Preparation
2.4. Greenhouse Assay
2.5. Effect of Salinity and SWE on Tomato
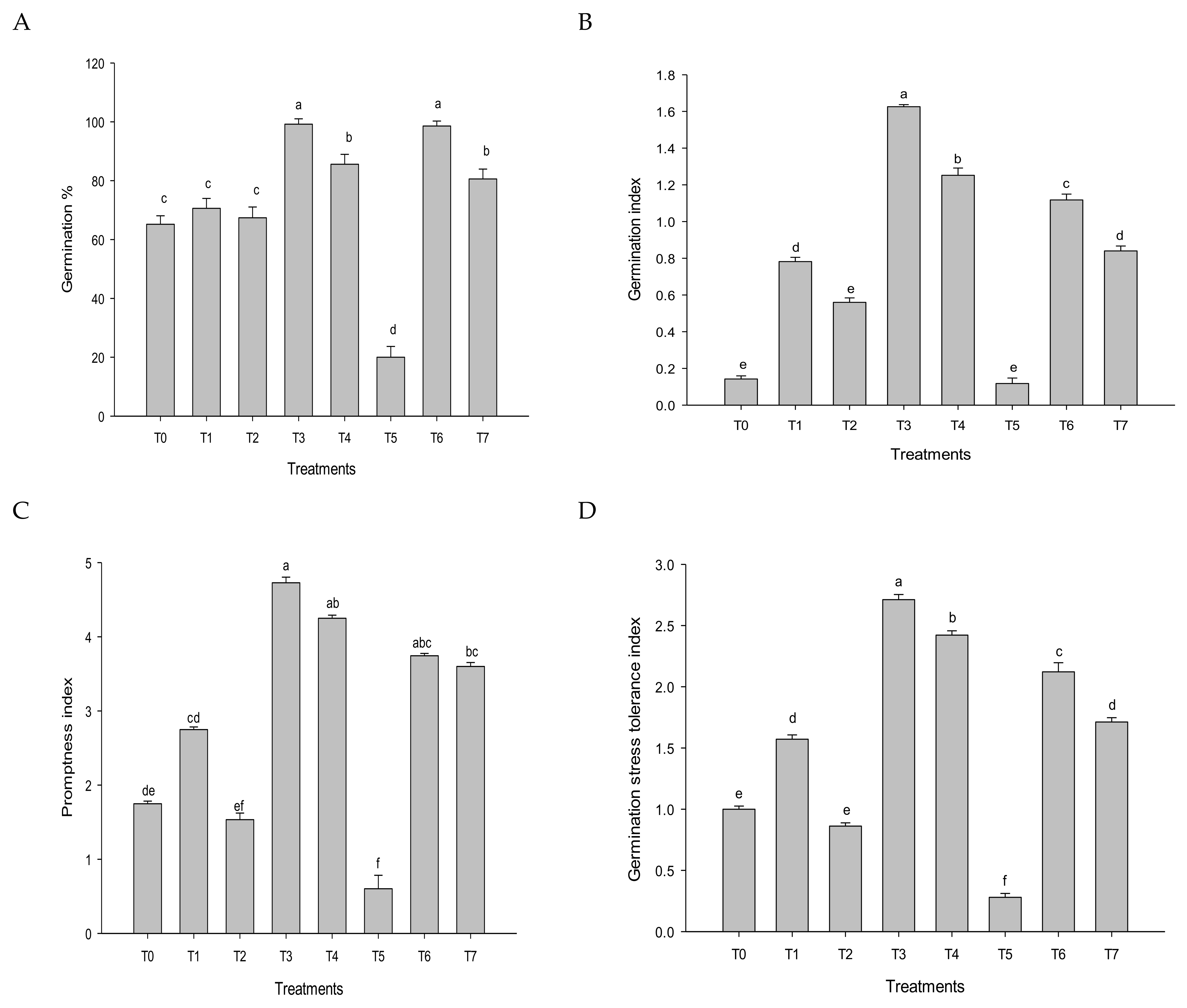
2.5.1. Seed Germination
2.5.2. Plant Growth
2.5.3. Plant Functionality
2.5.4. Nutraceutics
2.5.5. Fruit Organolepty
2.5.6. Metabolomics—GCMS
2.6. Statistical Analysis
3. Results
3.1. Germination Parameters
3.2. Growth Parameters
3.3. Physiological Parameters
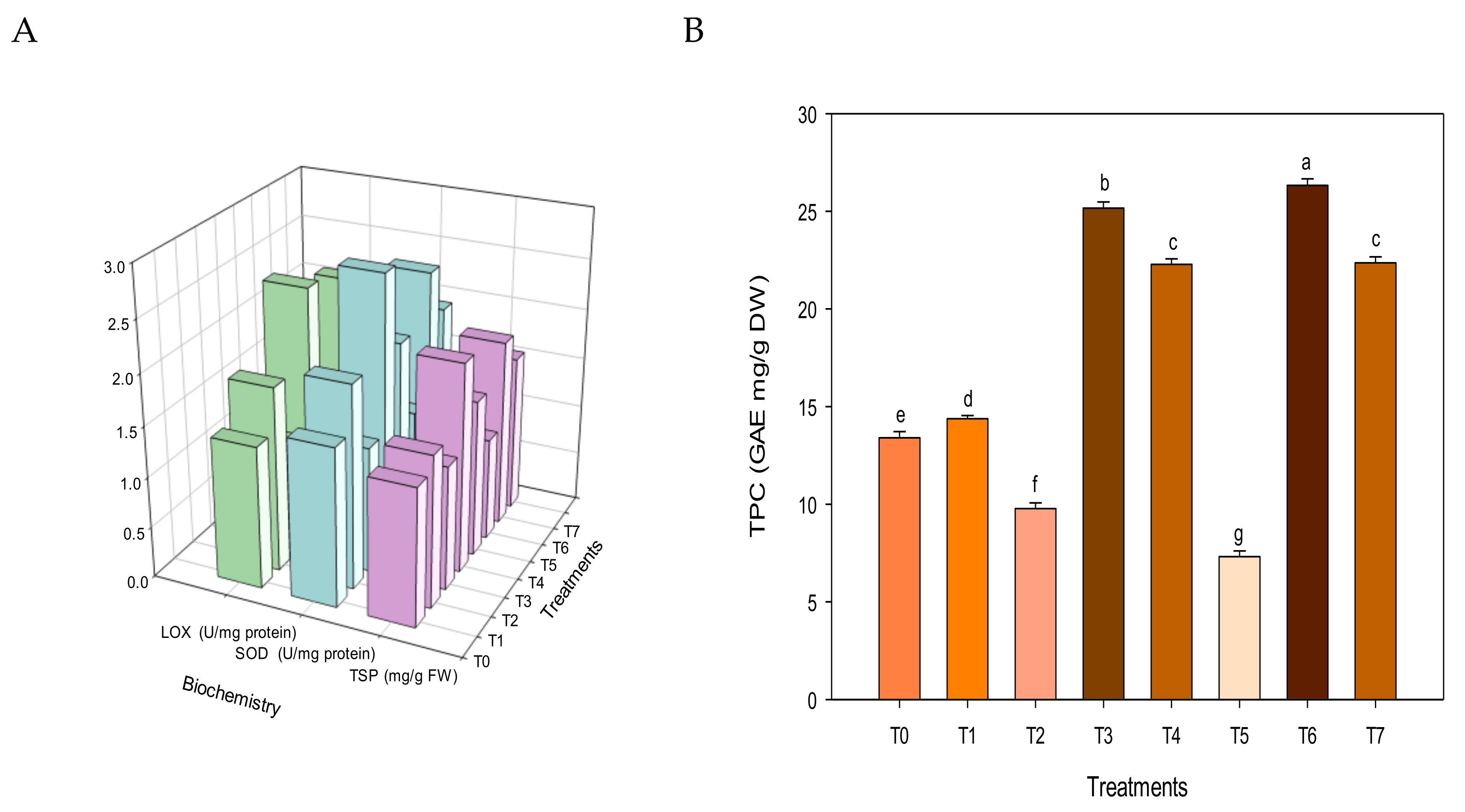
3.4. Plant Functionality
3.5. Nutritional Parameters
3.6. Fruit Organolepty
3.7. GCMS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maham, S.G.; Rahimi, A.; Subramanian, S.; Smith, D.L. The environmental impacts of organic greenhouse tomato production based on the nitrogen-fixing plant (Azolla). J. Clean. Prod. 2020, 245, 118679. [Google Scholar] [CrossRef]
- Szutowska, J. Functional properties of lactic acid bacteria in fermented fruit and vegetable juices: A systematic literature review. Eur. Food Res. Technol. 2020, 246, 357–372. [Google Scholar] [CrossRef]
- Branthôme, F.-X. Worldwide (Total Fresh) Tomato Production Exceeds 187 Million Tonnes in 2020. Available online: https://www.tomatonews.com/en/worldwide-total-fresh-tomato-production-exceeds-187-million-tonnes-in-2020_2_1565.html (accessed on 21 October 2022).
- Choi, S.H.; Kim, D.-S.; Kozukue, N.; Kim, H.J.; Nishitani, Y.; Mizuno, M.; Levin, C.E.; Friedman, M. Protein, free amino acid, phenolic, β-carotene, and lycopene content, and antioxidative and cancer cell inhibitory effects of 12 greenhouse-grown commercial cherry tomato varieties. J. Food Compos. Anal. 2014, 34, 115–127. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Phenols, Polyphenols and Tannins: An Overview. In Plant Secondary Metabolites: Occurrence, Structure and Role in the Human Diet; John Wiley & Sons: Hoboken, NJ, USA, 2006; pp. 1–25. [Google Scholar]
- Kalaivani, K.; Kalaiselvi, M.M.; Senthil-Nathan, S. Effect of Methyl Salicylate (MeSA) induced changes in rice plant (Oryza sativa) that affect growth and development of the rice leaffolder, Cnaphalocrocis medinalis. Physiol. Mol. Plant Pathol. 2018, 101, 116–126. [Google Scholar] [CrossRef]
- Shahzad, A.; Ullah, S.; Dar, A.A.; Sardar, M.F.; Mehmood, T.; Tufail, M.A.; Shakoor, A.; Haris, M. Nexus on climate change: Agriculture and possible solution to cope future climate change stresses. Environ. Sci. Pollut. Res. 2021, 28, 14211–14232. [Google Scholar] [CrossRef] [PubMed]
- Corwin, D.L. Climate change impacts on soil salinity in agricultural areas. Eur. J. Soil Sci. 2021, 72, 842–862. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef]
- Parađiković, N.; Teklić, T.; Zeljković, S.; Lisjak, M.; Špoljarević, M. Biostimulants research in some horticultural plant species—A review. Food Energy Secur. 2019, 8, e00162. [Google Scholar] [CrossRef]
- Stanley-Raja, V.; Senthil-Nathan, S.; Chanthini, K.M.-P.; Sivanesh, H.; Ramasubramanian, R.; Karthi, S.; Shyam-Sundar, N.; Vasantha-Srinivasan, P.; Kalaivani, K. Biological activity of chitosan inducing resistance efficiency of rice (Oryza sativa L.) after treatment with fungal based chitosan. Sci. Rep. 2021, 11, 20488. [Google Scholar] [CrossRef]
- El-Katony, T.M.; Deyab, M.A.; El-Adl, M.F.; Ward, F.M.E.-N. Extracts of the brown alga Dictyota dichotoma (Hudson) JV Lamouroux alleviate salt stress in rice (Oryza sativa L.) during germination. J. Plant Growth Regul. 2021, 40, 986–999. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Chanthini, K.M.P.; Stanley-Raja, V.; Thanigaivel, A.; Karthi, S.; Palanikani, R.; Shyam Sundar, N.; Senthil-Nathan, S. Sustainable agronomic strategies for enhancing the yield and nutritional quality of wild tomato, Solanum Lycopersicum (l) var Cerasiforme Mill. Agronomy 2019, 9, 311. [Google Scholar] [CrossRef]
- Abdelaziz, M.E.; Abdelsattar, M.; Abdeldaym, E.A.; Atia, M.A.M.; Mahmoud, A.W.M.; Saad, M.M.; Hirt, H. Piriformospora indica alters Na+/K+ homeostasis, antioxidant enzymes and LeNHX1 expression of greenhouse tomato grown under salt stress. Sci. Hortic. 2019, 256, 108532. [Google Scholar] [CrossRef]
- Chanthini, K.M.-P.; Senthil-Nathan, S.; Stanley-Raja, V.; Thanigaivel, A.; Karthi, S.; Sivanesh, H.; Sundar, N.S.; Palanikani, R.; Soranam, R. Chaetomorpha antennina (Bory) Kützing derived seaweed liquid fertilizers as prospective bio-stimulant for Lycopersicon esculentum (Mill). Biocatal. Agric. Biotechnol. 2019, 20, 101190. [Google Scholar] [CrossRef]
- Zarei, L.; Farshadfar, E.; Haghparast, R.; Rajabi, R.; Badieh, M.M.S. Evaluation of some indirect traits and indices to identify drought tolerance in bread wheat (Triticum aestivum L.). Asian J. Plant Sci. 2007, 6, 1204–1210. [Google Scholar] [CrossRef]
- Parvin, S.; Javadi, T.; Ghaderi, N. Proline, protein, RWC and MSI contents affected by paclobutrazol and water deficit treatments in strawberry cv. Paros. Cercet. Agron. Mold. 2015, 161, 107–114. [Google Scholar] [CrossRef][Green Version]
- Wang, S.; Bao, L.; Chen, B. Study on Estimation Method of Plant Leaf Area Based on Image Processing Technology. Int. J. Front. Sociol. 2020, 2, 76–84. [Google Scholar]
- Agra-Neto, A.C.; Napoleão, T.H.; Pontual, E.V.; Santos, N.D.D.L.; Luz, L.D.A.; de Oliveira, C.M.F.; de Melo-Santos, M.A.V.; Coelho, L.C.B.B.; Navarro, D.M.D.A.F.; Paiva, P.M.G. Effect of Moringa oleifera lectins on survival and enzyme activities of Aedes aegypti larvae susceptible and resistant to organophosphate. Parasitol. Res. 2014, 113, 175–184. [Google Scholar] [CrossRef]
- Tavallali, V.; Rahemi, M.; Eshghi, S.; Kholdebarin, B.; Ramezanian, A. Zinc alleviates salt stress and increases antioxidant enzyme activity in the leaves of pistachio (Pistacia vera L. ‘Badami’) seedlings. Turk. J. Agric. For. 2012, 34, 349–359. [Google Scholar] [CrossRef]
- Kalaivani, K.; Maruthi-Kalaiselvi, M.; Senthil-Nathan, S. Seed treatment and foliar application of methyl salicylate (MeSA) as a defense mechanism in rice plants against the pathogenic bacterium, Xanthomonas oryzae pv. oryzae. Pest. Biochem. Physiol. 2021, 171, 104718. [Google Scholar] [CrossRef]
- Suwanaruang, T. Analyzing lycopene content in fruits. Agric. Agric. Sci. Procedia. 2016, 11, 46–48. [Google Scholar] [CrossRef]
- Jantra, C.; Slaughter, D.C.; Roach, J.; Pathaveerat, S. Development of a handheld precision penetrometer system for fruit firmness measurement. Postharvest Biol. Technol. 2018, 144, 1–8. [Google Scholar] [CrossRef]
- Abraham, G.L.B.; Rafael, A.B.R.L.P.; Enrique, R.G.A.; Manuel, T.A.; Genaro, M.S. Tomato quality evaluation with image processing: A review. Afr. J. Agric. Res. 2011, 6, 3333–3339. [Google Scholar]
- Luengwilai, K.; Saltveit, M.; Beckles, D.M. Metabolite content of harvested Micro-Tom tomato (Solanum lycopersicum L.) fruit is altered by chilling and protective heat-shock treatments as shown by GC–MS metabolic profiling. Postharvest Biol. Technol. 2012, 63, 116–122. [Google Scholar] [CrossRef]
- Tanveer, K.; Gilani, S.; Hussain, Z.; Ishaq, R.; Adeel, M.; Ilyas, N. Effect of salt stress on tomato plant and the role of calcium. J. Plant Nutr. 2020, 43, 28–35. [Google Scholar] [CrossRef]
- Das, P.; Nutan, K.K.; Singla-Pareek, S.L.; Pareek, A. Understanding salinity responses and adopting ‘omics-based’ approaches to generate salinity tolerant cultivars of rice. Front. Plant Sci. 2015, 6, 712. [Google Scholar] [CrossRef]
- Jafarlou, M.B.; Pilehvar, B.; Modarresi, M.; Mohammadi, M. Performance of algae extracts priming for enhancing seed germination indices and salt tolerance in Calotropis procera (Aiton) WT. Iran J. Sci. Technol. Trans. A Sci. 2021, 45, 493–502. [Google Scholar] [CrossRef]
- Chanthini, K.M.-P.; Senthil-Nathan, S.; Stanley-Raja, V.; Karthi, S.; Sivanesh, H.; Ramasubramanian, R.; Abdel-Megeed, A.; El Maghraby, D.M.; Ghaith, A.; Alwahibi, M.S.; et al. Biologically active toxin from macroalgae Chaetomorpha antennina Bory, against the lepidopteran Spodoptera litura Fab. and evaluation of toxicity to earthworm, Eudrilus eugeniae Kinb. Chem. Biol. Techol. Agri. 2021, 8, 49. [Google Scholar] [CrossRef]
- Xia-Yu, G.; Meng, Z.; Ming-Dong, Z.; Ji-Rui, L.; Zhong-Wei, W.; Jian-Wu, L.; Bin, Z.; Zhi-Yong, A.; Hua-Feng, D. Comparative transcriptomic analysis of the super hybrid rice Chaoyouqianhao under salt stress. BMC Plant Biol. 2022, 22, 233. [Google Scholar] [CrossRef]
- Cisse, E.H.M.; Miao, L.-F.; Yang, F.; Huang, J.-F.; Li, D.-D.; Zhang, J. Gly Betaine surpasses melatonin to improve salt tolerance in Dalbergia Odorifera. Front. Plant Sci. 2021, 12, 588847. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef]
- Breś, W.; Kleiber, T.; Markiewicz, B.; Mieloszyk, E.; Mieloch, M. The Effect of NaCl Stress on the Response of Lettuce (Lactuca sativa L.). Agronomy 2022, 12, 244. [Google Scholar] [CrossRef]
- El-Sharkawy, M.S.; El-Beshsbeshy, T.; Al-Shal, R.; Missaoui, A. Effect of plant growth stimulants on alfalfa response to salt stress. Agric. Sci. 2017, 8, 267–291. [Google Scholar] [CrossRef][Green Version]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Abdurakhmonov, I.Y. (Ed.) Plant Breeding; IntechOpen: Lodon, UK, 2012. [Google Scholar]
- Kalaivani, K.; Kalaiselvi, M.M.; Senthil-Nathan, S. Effect of methyl salicylate (MeSA), an elicitor on growth, physiology and pathology of resistant and susceptible rice varieties. Sci. Rep. 2016, 6, 34498. [Google Scholar] [CrossRef]
- Michalak, I.; Górka, B.; Wieczorek, P.P.; Rój, E.; Lipok, J.; Łęska, B.; Messyasz, B.; Wilk, R.; Schroeder, G.; Dobrzyńska-Inger, A.; et al. Supercritical fluid extraction of algae enhances levels of biologically active compounds promoting plant growth. Eur. J. Phycol. 2016, 1, 243–252. [Google Scholar] [CrossRef]
- Mutale-joan, C.; Rachidi, F.; Mohamed, H.A.; Mernissi, N.E.; Aasfar, A.; Barakate, M.; Arroussi, H.E. Microalgae-cyanobacteria–based biostimulant effect on salinity tolerance mechanisms, nutrient uptake, and tomato plant growth under salt stress. J. Appl. Phycol. 2021, 33, 3779–3795. [Google Scholar] [CrossRef]
- Cao, K.; Yu, J.; Xu, D.; Ai, K.; Bao, E.; Zou, Z. Exposure to lower red to far-red light ratios improve tomato tolerance to salt stress. BMC Plant Biol. 2018, 18, 92. [Google Scholar] [CrossRef]
- Dehnadi, N.; Saeidisar, S.; Roudi, B. Ebadi, M; Masoudian, N. Roles of ascorbic acid on physiological, biochemical and molecular system of Lycopersicon esculentum Mill. against salt stress. Iran. J. Plant Physiol. 2020, 11, 3457–3464. [Google Scholar]
- Granrut, A.D.B.D.; Cacas, J.-L. How very-long-chain fatty acids could signal stressful conditions in plants? Front. Plant Sci. 2016, 7, 1490. [Google Scholar]
- Sytar, O.; Mbarki, S.; Zivcak, M.; Brestic, M. The Involvement of Different Secondary Metabolites in Salinity Tolerance of Crops. In Salinity Responses and Tolerance in Plants, Volume 2; Springer: Cham, Switzerland, 2018; pp. 21–48. [Google Scholar]
- Castellanos-Barriga, L.G.; Santacruz-Ruvalcaba, F.; Hernández-Carmona, G.; Ramírez-Briones, E.; Hernández-Herrera, R.M. Effect of seaweed liquid extracts from Ulva lactuca on seedling growth of mung bean Vigna radiata. J. Appl. Phycol. 2017, 29, 2479–2488. [Google Scholar] [CrossRef]
- Whapham, C.A.; Blunden, G.; Jenkins, T.; Hankins, S.D. Significance of betaines in the increased chlorophyll content of plants treated with seaweed extract. J. Appl. Phycol. 1993, 5, 231–234. [Google Scholar] [CrossRef]
- Kim, S.H.; Ahn, Y.O.; Ahn, M.-J.; Lee, H.-S.; Kwak, S.-S. Down-regulation of β-carotene hydroxylase increases β-carotene and total carotenoids enhancing salt stress tolerance in transgenic cultured cells of sweetpotato. Phytochemistry 2012, 74, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Uragami, C.; Cogdell, R.J. Carotenoids and photosynthesis. Carotenoids Nat. 2016, 79, 111–139. [Google Scholar]
- Maach, M.; Boudouasar, K.; Akodad, M.; Skalli, A.; Moumen, A.; Baghour, M. Application of biostimulants improves yield and fruit quality in tomato. Int. J. Veg. Sci. 2021, 27, 288–293. [Google Scholar] [CrossRef]
- Zhukov, A.V.; Shumskaya, M. Very-long-chain fatty acids (VLCFAs) in plant response to stress. Funct. Plant Biol. 2020, 47, 695–703. [Google Scholar] [CrossRef]
- Tigist, M.; Workneh, T.S.; Woldetsadik, K. Effects of variety on the quality of tomato stored under ambient conditions. J. Food Sci. Technol. 2013, 50, 477–486. [Google Scholar] [CrossRef]






| T0 | T1 | T2 | T3 | T4 | T5 | T6 | T7 |
|---|---|---|---|---|---|---|---|
| Control | NC | NC + S150mM | PC | PC + S150mM | S150mM | SWE 80% | T5+ T6 |
| Treatments | SVI | RWC (%) | Leaf Area (cm2) |
|---|---|---|---|
| T0 | 254.66 ± 3.97 g | 72.61 ± 0.0498 g | 8.44 ± 0.1817 b |
| T1 | 903.74 ± 2.59 d | 79.93 ± 0.302 e | 9.2 ± 0.2121 ab |
| T2 | 504.9 ± 3.51 f | 75.37 ± 0.038 f | 8.84 ± 0.1817 ab |
| T3 | 1506.97 ± 5.09 b | 93.66 ± 0.0522 a | 10.2 ± 0.2121 a |
| T4 | 1052.78 ± 3.96 c | 86.41 ± 0.0474 c | 9.72 ± 0.228 ab |
| T5 | 40.2 ± 5.07 h | 55.46 ± 0.0507 h | 4.6 ± 2.074 c |
| T6 | 1596.66 ± 5.23 a | 89.9 ± 0.264 b | 9.88 ± 0.239 ab |
| T7 | 685.62 ± 3.97 e | 82.69 ± 0.044 d | 9.04 ± 0.416 ab |
| Compounds | Peak Area % | ||
|---|---|---|---|
| T0 | T5 | T7 | |
| Antioxidants | |||
| 2-Ethylacridine | 20.18 | 4.34 | 8.38 |
| 4H-Pyran-4-one,2,3-dihydro-3,5- | -- | 4.27 | 26.37 |
| Alkanes | |||
| Cyclotrisiloxane, hexamethyl | 8.98 | 5.27 | 10.82 |
| Octasiloxane | 70.84 | 9.34 | 12.71 |
| Methyltris(trimethylsiloxy)silane | 5.88 | 7.71 | 16.52 |
| Aromatic benzene compounds | |||
| 1,2-Bis(trimethylsilyl)benzene | -- | 3.03 | 10.82 |
| 1,2-Benzisothiazol-3-amine tbdms | 20.18 | 6.62 | 19.76 |
| Fatty acid | |||
| Benzo [h] quinoline, 2,4-dimethyl | -- | 14.06 | -- |
| Phenols | |||
| Ethylene glycol phenyl ether methacrylate | -- | -- | 17.91 |
| Indole acetic acid derivative | |||
| 1H-Indole, 1-methyl-2-phenyl- | -- | 1.45 | 4.06 |
| Treatments | pH | Firmness (kg/cm2) | Fruit Fresh Weight (g/fruit) |
|---|---|---|---|
| T0 | 4.04 ± 0.1537 ab | 2.21 ± 0.1228 c | 19.94 ± 0.305 g |
| T1 | 4.20 ± 0.255 ab | 3.08 ± 0.0377 d | 24.04 ± 0.365 e |
| T2 | 4.04 ± 0.261 ab | 2.30 ± 0.1188 d | 22 ± 0.474 f |
| T3 | 4.37 ± 0.432 a | 4.46 ± 0.321 a | 32 ± 0.316 a |
| T4 | 4.21 ± 0.2151 ab | 4.17 ± 0.2046 a | 29.64 ± 0.78 b |
| T5 | 3.78 ± 0.228 b | 1.72 ± 0.327 e | 15 ± 0.316 h |
| T6 | 4.24 ± 0.364 ab | 3.67 ± 0444 b | 28.04 ± 0.365 c |
| T7 | 4.07 ± 0.0825 ab | 3.17 ± 0.2046 c | 25.06 ± 0.397 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chanthini, K.M.-P.; Senthil-Nathan, S.; Pavithra, G.-S.; Asahel, A.-S.; Malarvizhi, P.; Murugan, P.; Deva--Andrews, A.; Sivanesh, H.; Stanley-Raja, V.; Ramasubramanian, R.; et al. The Macroalgal Biostimulant Improves the Functional Quality of Tomato Fruits Produced from Plants Grown under Salt Stress. Agriculture 2023, 13, 6. https://doi.org/10.3390/agriculture13010006
Chanthini KM-P, Senthil-Nathan S, Pavithra G-S, Asahel A-S, Malarvizhi P, Murugan P, Deva--Andrews A, Sivanesh H, Stanley-Raja V, Ramasubramanian R, et al. The Macroalgal Biostimulant Improves the Functional Quality of Tomato Fruits Produced from Plants Grown under Salt Stress. Agriculture. 2023; 13(1):6. https://doi.org/10.3390/agriculture13010006
Chicago/Turabian StyleChanthini, Kanagaraj Muthu-Pandian, Sengottayan Senthil-Nathan, Ganesh-Subbaraja Pavithra, Arul-Selvaraj Asahel, Pauldurai Malarvizhi, Ponnusamy Murugan, Arulsoosairaj Deva--Andrews, Haridoss Sivanesh, Vethamonickam Stanley-Raja, Ramakrishnan Ramasubramanian, and et al. 2023. "The Macroalgal Biostimulant Improves the Functional Quality of Tomato Fruits Produced from Plants Grown under Salt Stress" Agriculture 13, no. 1: 6. https://doi.org/10.3390/agriculture13010006
APA StyleChanthini, K. M.-P., Senthil-Nathan, S., Pavithra, G.-S., Asahel, A.-S., Malarvizhi, P., Murugan, P., Deva--Andrews, A., Sivanesh, H., Stanley-Raja, V., Ramasubramanian, R., Ghaith, A., Abdel-Megeed, A., & Krutmuang, P. (2023). The Macroalgal Biostimulant Improves the Functional Quality of Tomato Fruits Produced from Plants Grown under Salt Stress. Agriculture, 13(1), 6. https://doi.org/10.3390/agriculture13010006








